Abstract
Background
HIV self-testing (HIVST) is recommended by the WHO as an innovative strategy to reach UNAIDS targets to end HIV by 2030. HIVST with digital supports is defined as the use of digital interventions (e.g., website-based, social media, mobile HIVST applications (apps), text messaging (SMS), digital vending machines (digital VMs)) to improve the efficiency and impact of HIVST. HIVST deployment and integration in health services is an emerging priority. We conducted a systematic review aiming to close the gap in evidence that summarizes the impact of digitally supported HIVST and to inform policy recommendations.
Methods
We searched PubMed and Embase for articles and abstracts on HIVST with digital supports published during the period February 1st, 2010 to June 15th, 2021, following Cochrane guidelines and PRISMA methodology. We assessed feasibility, acceptability, preference, and impact outcomes across all populations and study designs. Metrics reported were willingness to use HIVST, preferences for HIVST delivery, proportion of first-time testers, HIVST uptake, HIVST kit return rate, and linkage to care. Heterogeneity of the interventions and reported metrics precluded us from conducting a meta-analysis.
Findings
46 studies were narratively synthesized, of which 72% were observational and 28% were RCTs. Half of all studies (54%, 25/46) assessed web-based innovations (e.g., study websites, videos, chatbots), followed by social media (26%, 12/46), HIVST-specific apps (7%, 3/46), SMS (9%, 4/46), and digital VMs (4%, 2/46). Web-based innovations were found to be acceptable (77–97%), preferred over in-person and hybrid options by more first-time testers (47–48%), highly feasible (93–95%), and were overall effective in supporting linkage to care (53–100%). Social media and app-based innovations also had high acceptability (87–95%) and linkage to care proportions (80–100%). SMS innovations increased kit return rates (54–94%) and HIVST uptake among hard-to-reach groups. Finally, digital VMs were highly acceptable (54–93%), and HIVST uptake was six times greater when using digital VMs compared to distribution by community workers.
Interpretation
HIVST with digital supports was deemed feasible, acceptable, preferable, and was shown to increase uptake, engage first-time testers and hard-to-reach populations, and successfully link participants to treatment. Findings pave the way for greater use of HIVST interventions with digital supports globally.
Keywords: Digital, HIV, Self-testing, Online, Mhealth, Intervention
Research in context.
Evidence before this study
Previous systematic reviews have assessed evidence on technology-assisted HIV testing without a focus on self-test interventions in the past decade. Self-test interventions present unique point-of-care possibilities for digital interventions to improve timely HIV detection and entry to the HIV care cascade. An initial PubMed search from February 1st, 2010, to December 1st, 2020 using the search terms “HIV”, “self-testing”, and “digital” indicated a lack of systematic reviews summarizing evidence on digital self-test strategies for HIV.
Added value of this study
Supported by FIND, this systematic review is the first to assess existing evidence on all published digital HIV self-test innovations. A diverse range of studies were included in this review without limits on outcomes, geographic region, or study population. Existing reviews on technology-assisted interventions did not focus on self-testing and were limited to interventions published in the past five years.
Implications of all the available evidence
HIVST with digital supports strategies reported successes in uptake, participant preference, and linkage to care in key and general populations. Digital innovations were highly acceptable across diverse settings and particularly effective in reaching first-time testers and hard-to-reach populations. Leveraging existing social media platforms to promote HIVST demonstrates promise, and further research evaluating HIVST with digital supports innovations among specific subgroups is necessary.
Alt-text: Unlabelled box
1. Introduction
In recent years, HIV self-testing (HIVST) has garnered interest in the HIV testing space as a last mile solution to achieve the UNAIDS’ 95–95–95 goals by 2030. With evolving guidelines that favor the use of HIVST in over 40 countries, conversations have shifted towards an integration of HIVST as a strategy for entry to the HIV care continuum [1]. In 2019, the WHO called for evidence to respond to the many needs in service delivery including innovations and a greater use of frontline health care workers [2].
The COVID-19 pandemic led to service disruptions in the supply chain for HIV diagnostics and treatment, which contributed to falling short of the UNAIDS 90–90–90 targets [3]. However, COVID-19 also accelerated the digitization of health services with incorporation of digital tools by governments for communication and notification. Digital health is defined as the use of digital, mobile, and wireless technologies to support the achievement of health objectives [4]. Digital health technology is gaining traction in high- and middle-income countries, and telemedicine has allowed continuity of care for many patients. COVID-19 may pave the way for an evolution of HIVST with digital supports to close gaps in HIV health service delivery. HIVST with digital supports is the use of digital interventions (e.g., website-based, social media, mobile HIVST applications (apps), text messaging (SMS), digital vending machines (digital VMs)) to improve the efficiency and impact of HIVST. Interest in digital tools to support HIVST has grown since 2012, when the US Food and Drug Administration (FDA) approved OraQuick, the first true over the counter self-administered test for HIV [5]. Digitally supported HIVST innovations offer an important opportunity to reduce stigma and confidentiality concerns among hard-to-reach populations [6].
With upfront implementation costs and lower recurring fees downstream, digital health innovations have expanded the ability to reach diverse audiences with the potential for widespread scale-up, increased access to testing, and efficient linkage to health services [7]. Existing reviews of digital health innovation literature have focused on conventional HIV testing [8], on Mhealth, or HIVST technologies restricted to a defined population, such as men who have sex with men (MSM) or transgender women [9]. While digital supports for HIVST are expanding, a knowledge gap persists regarding which digital support strategies are effective, for which populations, and in which settings they are best suited for scale-up. To close this knowledge gap, we set out to systematically review global evidence on HIVST with digital supports in the context of implementation research.
Our primary objective was to synthesize evidence that summarizes all digital innovations for HIVST with digital supports across all populations and outcomes and across all settings. We also focused, secondarily, on implementation research evidence on effectiveness where the outcome of an offer of HIVST with digital supports was compared against conventional reference standard or a non-digital offer of HIVST. Given that an offer of digital supports is not yet the norm, we analyzed all studies, including interventional studies with a comparator, as well as observational research studies (cohort studies and cross sectional).
2. Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Cochrane guidelines. The protocol was registered with PROSPERO on September 18th, 2020 (CRD42020205025).
2.1. Eligibility criteria
Randomized controlled trials (RCTs) or observational studies that evaluated any digital technologies used for HIVST in any global setting that reported quantitative outcomes were included. Studies that did not focus on HIV as a primary sexually transmitted infection (STI) of interest, did not have a self-testing component, did not include a digital intervention, or used a qualitative design were excluded. Modeling studies, trial protocols, reviews, narratives, or editorials were also excluded. All studies required the use of a substantive digital component in HIVST delivery, administration, or interpretation of results, and interventions were excluded if the digital component was only implemented during recruitment (e.g., via apps or social media, and not related to the HIVST process itself).
2.2. Information sources
PubMed and Embase were searched for full-text articles and conference abstracts published in a 10-year period from February 1st, 2010 until June 15th, 2021, with no language restrictions. Abstracts published in the proceedings of the 29th Annual Canadian Conference on HIV/AIDS Research (CAHR) 2020, Infectious Diseases Society of America (IDSA) IDWeek 2020, and the 23rd International AIDS Conference (AIDS 2020) were searched for additional eligible records.
2.3. Search strategy
The search strategy used for PubMed was as follows: ((hiv[Text Word]) AND (self-testing[Text Word] OR self testing[Text Word] OR self-sampling[Text Word] OR self sampling[Text Word])) AND (Mhealth[Text Word] OR mobile health[Text Word] OR digital[Text Word] OR online[Text Word] OR web[Text Word])).
2.4. Study selection
Two reviewers (MM, ADW) evaluated and assessed citations for eligibility, and assessed the quality. A senior reviewer (AK) was consulted on citations for resolution of discordance.
2.5. Data abstraction
Two reviewers (MM, ADW) independently abstracted all data on the following items: study design, study population, sample size, type of digital innovation, outcome measures (acceptability, preference, feasibility, and impact), and associated metrics (e.g., willingness to use HIVST with digital support and ease of use, participant preferences for HIVST delivery, uptake of HIVST, proportion of first-time testers, HIVST kit return rate, and proportion linked to care). A senior reviewer (AK) was consulted for resolution of discrepancies in data abstraction.
2.6. Summary measures and narrative synthesis of results
Heterogeneity in study designs, outcomes, metrics, and insufficient data, precluded the conduct of a meta-analysis. The primary outcomes used to assess HIVST with digital supports were: (1) acceptability, (2) feasibility, (3) preference, and (4) impact.
Acceptability was defined as the ease of use and willingness of participants to use digital innovations for HIVST [10]. Feasibility was defined as the convenience of using HIVST with digital supports, and it was reported with various metrics: HIVST uptake, retention, cost-effectiveness, visits to web-based HIVST providers, and response rate [10,11]. Preference was defined as the proportion of study participants who preferred HIVST with digital supports over conventional HIV testing [12].
Impact in controlled trials was defined as a statistically significant improvement in measured outcomes compared with a comparator group, or a net change in outcomes amongst a particular group that can be attributed to a specific intervention [12]. Impact metrics were evaluated according to their effect size and precision. The metrics used to evaluate impact were: (1) proportion of first-time testers, (2) detection of new cases, (3) HIVST kit return rate, (4) proportion of participants linked to care. Linkage to care was defined as any way of linking recently diagnosed HIV+ patients to healthcare services and/or initiating ART with a rapid turnaround time [10].
2.7. Quality assessment
Cochrane Risk of Bias Tool [13] was used to assess the quality of clinical trials, and Newcastle-Ottawa Scale [14] was used for observational studies.
2.8. Role of funding source
The funder of the study (FIND) framed the study and designed the original concept in conjunction with the principal investigator (PI) but had no role in the study design, data collection, data analysis and interpretation, and the initial writing of the paper. FIND-affiliated authors provided critical final reviews. The work was commissioned with the intention that it be published. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
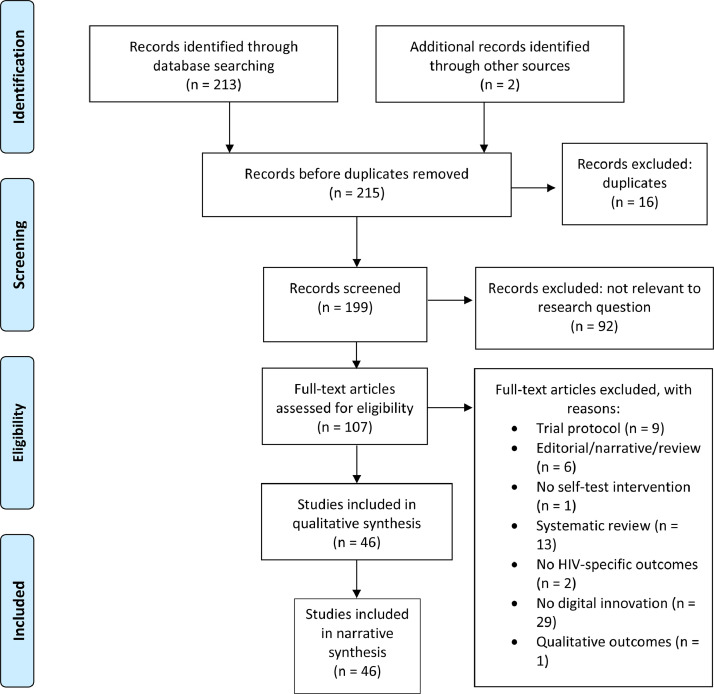
Of 215 studies identified in our initial search, 107 records were assessed for eligibility, and 46 citations met our inclusion criteria to be included in this review for evidence synthesis (Fig. 1). A summary of all included studies can be found in Supplementary Table 1 (Summary of Study Characteristics) and Supplementary Table 2 (Summary of Key Findings).
Fig. 1.
Study Selection. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.
3.1. Study characteristics
According to geographical location, 37% (17/46) of the studies were conducted in Asia, 28% (13/46) in North America, 24% (11/46) in Europe, 7% (3/46) in Africa, and 4% (2/46) in South America.
By study design, a majority were observational studies (e.g., cross-sectional and cohort studies) (72%, 33/46), followed by RCTs (28%, 13/46). All studies in this review reported findings from quantitative studies, including quantitative results from mixed methods studies.
By study population, most studies focused on MSM (83%, 38/46), followed by ethnic minorities (4%, 2/46), residents of high HIV-prevalence settings (4%, 2/46), transgender youth (2%, 1/46), female sex workers (2%, 1/46), male truckers (2%, 1/46), and online government sexual health service users (2%, 1/46) (Supplementary Table 1).
3.2. HIVST innovations with digital supports
HIVST with website-based innovations (e.g., study websites, chatbots, online video counseling) were evaluated in half 54% (25/46) of all studies. Of the 25 website-based innovations, 32% (8/25) offered access to or requests for HIVST on a website, 36% (9/25) provided access to HIVST results on a website, and 48% (12/25) offered counseling, coaching, and linkage to care features on a website (Supplementary Table 1).
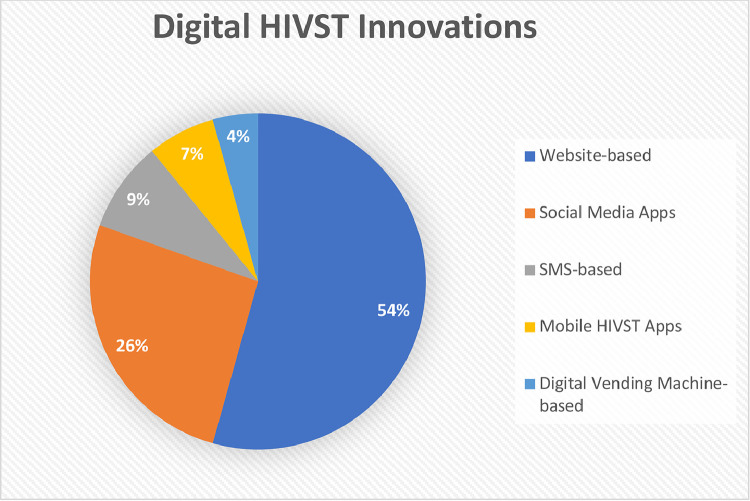
Other digital innovations (HIVST delivered with social media, mobile apps, SMS, and digital VMs were evaluated in 21/46 studies. Social media-based innovations, including geospatial dating apps, were assessed in 26% (12/46), SMS-based innovations in 9% (4/46), mobile HIVST-specific apps in 7% (3/46), and digital VMs in 4% (2/46) of studies (Fig. 2).
Fig. 2.
Distribution of HIVST with digital supports innovations. Dark blue indicates web-based innovations (25/46, 54%), orange indicates social media-based innovations (12/46, 25%). gray indicates SMS-based innovations (4/46, 9%), yellow indicates mobile HIVST-specific apps (3/46, 7%), and light blue indicates digital VM-based innovations (2/46, 4%).
3.3. Measures
Acceptability outcomes were reported in 19% (9/46) of studies, preference in 7% (3/46), feasibility in 41% (19/46), and impact in 33% (15/46).
Feasibility was reported as HIVST uptake in 79% (15/19) of feasibility studies, return rate in 5% (1/19), cost-effectiveness in 5% (1/19), visits to web-based HIVST providers in 5% (1/19), and response rate in 5% (1/19) of studies. Within impact measures, metrics such as proportion of first-time testers were reported in 33% (5/15), detection of new cases in 7% (1/15), HIVST kit return rate in 20% (3/15), and linkage to care in 40% (6/15) of studies. Definitions of metrics and measures were reported in summary measures and narrative synthesis of results.
3.4. Narrative syntheses
We have classified reported metrics according to category of digital support innovation for HIVST.
3.5. Website-based HIVST innovations
Half of all studies (54%, 25/46) deployed website-based interventions. Results were largely in favor of digital strategies including e-testing websites [15], [16], [17], [18], [19], study websites [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], online counselling [33], [34], [35], [36], [37], [38], and chatbots [39].
3.5.1. Acceptability
Eight (32%, 8/25) studies assessing web-based interventions reported acceptability outcomes [18,21,[26], [27], [28], [29],37]. Willingness to use web-based HIVST interventions (61%−79%) [18,21,26,27,29,37,38] and ease of use (97%) [28] was high across studies, and among high-risk groups such as MSM, transgender women, men with multiple sexual partners, and men who were less likely to have previously sought out HIV testing [28,29,37]. An RCT conducted among MSM in China found the intervention group receiving HIVST with promotion of online real-time instructions and counseling expressed greater willingness to take up HIV testing than a control group receiving promotion only for conventional HIV testing (69.0 vs 43.9%, p<0.001) [37].
3.5.2. Preference
Three studies (12%, 3/25) reported on participant preference for online HIVST and counselling rather than conventional HIV testing and counselling [15,20,34]. When provided a choice between HIVST performed using online services, a hybrid option (online pretest counselling and private clinic-based HIV testing), or a clinic-based option, the majority of participants preferred the online option [34]. In two studies conducted in Thailand, the highest proportion of first-time testers was in the online group (47%−48%) [15,34].
3.5.3. Feasibility
Feasibility was reported as HIVST kit uptake by three studies delivering oral self-tests through study websites, and was found to be high at 90%−96% [28], [29], [30]. Conversely, due to a relatively low uptake of 48%, a telehealth intervention offering home-based HIVST and web-based video counselling may not represent the ideal HIVST strategy for transgender and non-binary youth; voice call or text message-based counselling may be a preferrable option for this population [36]. HIVST delivered with online services was found to reach individuals who likely would have been missed by traditional facility or clinic-based testing. In a study assessing an online social entrepreneurship model, 38% of MSM who purchased an HIVST kit had never tested at a facility [29].
3.5.4. Impact
Impact measures were reported in 36% (9/25) of web-based innovation studies. Impact was reported in two studies as the proportion of new HIV infections detected, with rates of 1.9% and 2.2% [23,33]. Five (20%, 5/25) studies reported kit return rates, which ranged between 55% and 98% [16,[20], [21], [22], [23]]. Seven (28%, 7/25) web-based interventions evaluated impact on linkage to treatment/care, and these overall indicated success with proportions ranging between 53%−100% [19,[21], [22], [23], [24],29,35]. Linkage to care in 6/7 studies included confirmatory testing, 4/7 included referral to a clinic, 3/7 included post-test counselling, and 1/7 included ART initiation. A study conducted in Thailand indicated challenges in linking online HIVST participants to conventional HIV treatment facilities among those with reactive results (53% linkage in online group, compared to the hybrid (78%), or offline (85%) groups) [35].
3.6. Social media and app-based HIVST innovations
Social media or app-based innovations were evaluated in 33% (15/46) of studies [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]. Three studies assessed mobile HIVST-specific apps (7%, 3/46) [41,50,54]. Social media apps (e.g., Facebook, WeChat) and geospatial dating apps (e.g., Blued™, Grindr™) to promote HIVST were assessed in 26% (12/46) of studies [40,[42], [43], [44], [45], [46], [47], [48], [49],[51], [52], [53]].
3.6.1. Acceptability
A number of studies supported willingness to use HIV self-tests distributed through geospatial dating apps or ease of use, with acceptability ranging from 87% to 95% [42,44,45,49]. A social media campaign promoting peer-delivered HIVST found nearly all MSM participants reported satisfaction with HIVST (94•8%; n = 761/803) [42]. In a survey assessing willingness to use geosocial sexual networking apps to notify partners of a positive HIV test, 93% reported they would likely obtain counselling and testing if anonymously notified via an app, and 93% would engage in HIVST if provided with a voucher for free testing [45]. Findings from a study promoting HIVST through sexual networking apps indicated that the intention to self-test was positively associated with stigma or fear (8·61; 95% CI 2·50–29·68), and negatively associated with higher social support (0·48; 95% CI 0·33–0·72) [46]. HIVST-specific apps also had high acceptability, with a majority of participants in all three studies desiring to use an app for HIVST in the future (66%−99%) [41,50] or finding it very helpful to order HIVST kits through the apps (87%) [54].
3.6.2. Preference
With respect to preference, a California-based study conducted among Black and Hispanic MSM found that a majority (77%) of participants preferred using HIVST delivered by mail through a self-test campaign on Grindr™ to HIV testing in a clinical setting, which may be attributed to the anonymity and convenience provided by online options [48].
3.6.3. Feasibility
Social media and app-based interventions demonstrated success in increasing HIVST uptake and were found to be highly feasible. Participants in a social media-based HIVST messaging campaign had significantly higher rates of self-testing (oral HIVST) relative to a control group (RR = 2.17, 95% confidence interval (CI) [1.08–4.37] [53].
3.6.4. Impact
The impact of social media or app-based innovations was reported as first-time tester proportions in 40% (6/15) of studies, and these were overall successful in reaching those who had never tested for HIV (9%−51%) [42,[47], [48], [49],51,52]. Two studies conducted among MSM reported newly detected HIV infection proportions among 4% and 11% of participants [42,48]. Linkage to care outcomes were reported in 33% (5/15) of studies assessing HIVST facilitated by social media or apps, where a majority of participants (80%−100%) received confirmatory testing and initiated ART [42,44,48,51,52]. Two studies reported on the impact of social media-based secondary distribution of HIVST, and these indicated high HIVST kit return rate (99%) [51] and linkage to care proportions (80%−93%) [42,51].
3.7. SMS-based HIVST innovations and digital VMs
Four studies (9%, 4/46) assessed SMS-based interventions and two studies (4%, 2/46) assessed HIVST delivery via digital VM [55], [56], [57], 58, [59], [60].
3.7.1. Acceptability
Both studies assessing HIVST delivery via digital VM demonstrated high overall acceptability (54%−93%) [59,60]. A UK study reported lower willingness to use digital VM-delivered HIVST among the Black Caribbean subgroup (54%) than Black African (78%), Latin American (82%), or other non-white ethnicity (80%) participants [59]. Primary concerns related to digital VM use in this study included being seen using a digital VM to access HIVST kits (27%−60%), not being able to use the self-test kits correctly (30%−48%), and not being able to use the digital VM correctly (30%−50%) [59].
3.7.2. Feasibility
Two studies (50%, 2/4) in Kenya led to significant increases in HIVST uptake [57,58]. Both interventions promoted HIVST with three text message reminders a week apart, which increased testing among male truckers (31 participants vs. 10 controls, p = 0·002) [58] and female sex workers (119 participants vs. 46 controls, p = 0·001) [57]. However, very low testing rates (1·8% for male truckers and 7·5% for female sex workers) hamper the feasibility of this approach, which may have been due to intentional selection of irregular testers [57,58]. Uptake of HIVST was also six times greater (34 vs 6 tests per month) for HIVST distributed by digital VM free of charge to high-risk MSM in a sex-on-premises venue vs. tests distributed by community outreach workers at the same venue [60].
3.7.3. Impact
One study provided SMS-based behavioral insight reminders, which prompted a higher kit return rate than those who received standard text messages (OR = 1·13; 95% CI [1·04–1·23]; p = 0·003) [56]. Another reminder-based SMS intervention accompanying HIVST was implemented during hurricanes in Puerto Rico, and 100% of participants were able to communicate sexual behavior and HIV testing within 30 days [55].
3.8. Quality
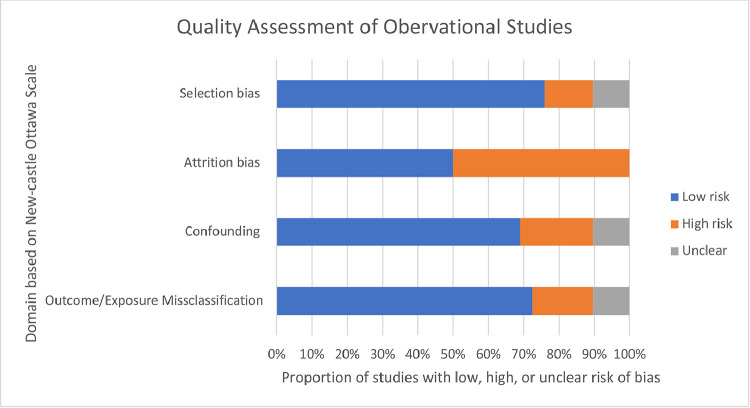
Scores from the observational studies on the Newcastle-Ottawa Scale ranged from 4 to 9 (Fig. 3). Three conference abstracts were evaluated with this scale, but a final score could not be calculated due to a lack of detail in methods and reporting of outcome metrics. Of the studies where full-text articles were available, potential for confounding, selection bias, or outcome/exposure misclassification was found in a minority (21%, 14%, and 17%) of observational studies respectively. Of the 10 studies with follow-up time, 50% had risk for attrition bias. Several observational studies (22%) had small samples, low power, or insufficient follow-up time.
Fig. 3.
Risk of bias assessment for observational studies. The Newcastle-Ottawa Scale was used to assess potential sources of bias in included observational studies, rating each as low, high, or unclear risk of bias.
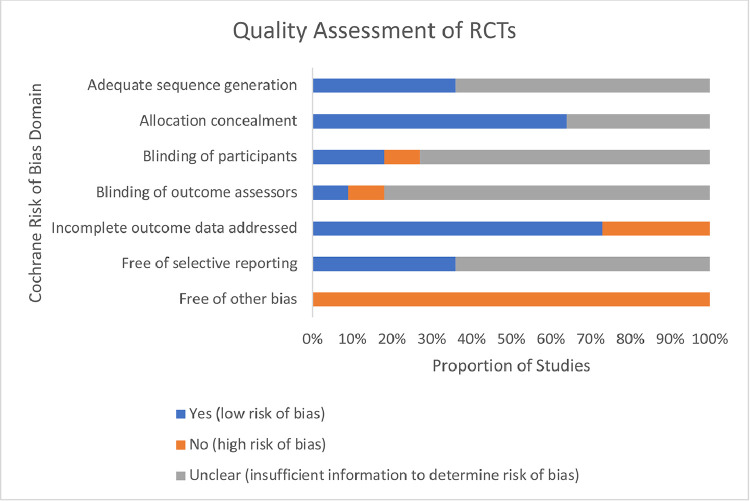
A majority of RCTs demonstrated appropriate allocation concealment and adequately addressed incomplete outcome data, and other biases (e.g., recall bias, social desirability bias) were of concern (Fig. 4). While blinding of participants and outcome assessors was clearly described in only 10%−20% of RCTs, blinding is not possible in open-label trials.
Fig. 4.
Risk of bias assessment for RCTs. The Cochrane Risk of Bias tool was used to assess potential sources of bias in included RCTs, rating each as low, high, or unclear risk of bias. ‘Yes’ indicates the Cochrane RoB domain is satisfied, and presents a low risk of bias; ‘No’ indicates the Cochrane RoB domain is not satisfied and may present a high risk of bias; ‘Unclear’ indicates that insufficient information was presented to determine risk of bias.
4. Discussion
The studies included in this review assessed vastly different interventions in a range of settings and on a global scale. Diverse interventions were deployed across studies, including websites, mobile apps, text messaging, combined technologies, digital VMs, and online counselling. While certain interventions leveraged existing social media platforms to promote messaging or deliver HIVST kits, others provided mixed (online and offline) HIVST options to their participants. Heterogeneity in the metrics collected and types of innovations presented challenges in making direct comparisons between the included studies.
An overall pattern was observed: firstly, acceptability was high, with 54%−99% of participants expressing willingness to use HIVST with digital supports in the future. While further research comparing acceptability of digitally supported HIVST to HIVST without digital supports is necessary, evidence from a health promotion RCT indicates greater willingness to take up HIV testing among those receiving online instructions and counselling than conventional HIV testing [37]. Social media and app-based delivery of HIVST had overall greater acceptability than web-based, SMS, or digital VM delivery of HIVST. Across studies, most participants expressed willingness to use HIVST with digital supports [42,45,59], or reported high rates of interest [18,37,39]. Apps and web-based interventions have significant engagement potential, and evidence supports their role in catalyzing pre-exposure prophylaxis (PrEP) and non-occupational post-exposure prophylaxis (nPEP) uptake [36,50]. However, further research is needed to quantify their uptake in different contexts, populations and settings.
Second, HIVST uptake increased with the implementation of HIVST strategies with digital supports. The incorporation of websites and social media increased HIVST uptake compared to a non-digital or offline offer of HIVST [35,53]. Social media-based distribution of HIVST kits was identified as an effective strategy to reach high-risk MSM populations [40]. Effective interventions often used digital platforms to provide video demonstrations [18,31,33,37,53] or real-time video chatting [34,36] to guide self-administration of HIVST and interpretation of results, which in some cases was followed by app-based messages providing referrals to HIV health services [53]. Website, social media, and SMS-based interventions also demonstrated high HIVST kit return rates, which points to their increasing role in improving uptake of health communication and services [20,51,56].
Third, overall results indicate that linkage to care with digital interventions shows a promising trend towards higher proportions of HIV positive participants being linked to confirmatory testing and ART initiation compared to conventional HIV testing. In addition, high proportions of HIV negative participants were linked to or considered accessing prevention care, indicating improved engagement with PrEP [18,22,23,50]. HIVST with digital supports innovations, potentially in combination with other program components such as in-person or online post-test counselling, promoted the use of HIV services such as ART initiation and PrEP [50,52]. The positive impact of HIVST with digital supports can therefore extend beyond uptake of testing or diagnostic confirmation to facilitate next steps of linkage and retention in the continuum of care.
Finally, digital interventions that offered private and discreet options via mobile apps or web-based counselling were effective in reaching vulnerable populations and appreciated by end users. Mail delivery of HIVST may also be preferred as it removes the need to commute to a clinic setting for testing [48]. Smartphone-based prompts are useful in engaging and communicating with end users for prevention and to initiate care, in contrast to strategies where there is no communication from healthcare providers. This highlights the importance of ensuring that the integration of digital self-testing into the continuum of care and existing healthcare infrastructure is user-centered.
Understanding the needs and preferences of end users is critical, as these should guide the design of effective HIVST interventions with digital supports. Culturally sensitive training in health service delivery is equally important to the co-creation and successful delivery of effective interventions for high-risk sub-populations. For example, in the UK, an online service providing HIVST indicated that Black and other minority ethnic group participants were less likely to return an HIVST kit, indicating that clinics may remain an important point of access to testing and related treatment services for certain high-risk sub-populations [20]. A recommendation that emerged was to deploy a hybrid online/offline HIVST strategy for such sub-populations that prefer engagement with a healthcare professional, either pre-test or post-test.
Customization of the digital package to support HIVST is highly relevant for users who are digitally savvy and conscious of social visibility. The location of digital VM interventions may also be important, as high social visibility due to placement of VM in common locations might deter certain communities due to stigma and discrimination concerns. For example, health facilities such as sexual health clinics were rated as acceptable venues for digital VMs dispensing self-tests [59]. These settings provide privacy when using the digital VM in contrast to more public areas not related to sexual health or healthcare in general [59]. This was apparent in the digital VM-based intervention that was not well received by the Black Caribbean populations due to concerns about being able to use the HIVST correctly and the visibility of the location they were offered in [59]. Digital VMs may not be an optimal strategy for communities of color for sole distribution of test kits, as they may face additional race-related stigma and discrimination.
Online health service provision and the use of apps has increased during the COVID-19 pandemic, and HIVST with digital supports incorporating these solutions may present an important opportunity to address unmet HIV testing needs. Offering free and discreet HIVST through geospatial networking apps and community partner websites demonstrated success in reaching first-time testers [42,47]. This trend will likely continue in the coming years, and communication platforms may facilitate end user engagement with clinical settings.
The COVID-19 pandemic has highlighted the need for health care interventions that involve minimal interpersonal contact. Furthermore, the availability of self-tests for HIV and COVID-19 that allow for interpretation by end users presents an important opportunity to address unmet testing needs. While preference for an in-clinic visit was expressed in several studies, engagement and communication were facilitated by technology. These results suggest the relevance to offer HIVST as a complementary strategy to facility-based testing in order to reach as many untested and undiagnosed at-risk sub-populations as possible. The settings of use and solutions varied between studies; it is important to understand the contextual placement of these solutions according to the resources available and the populations that are most likely to avail them.
Data on digitally supported HIVST interventions from low- and middle-income countries (LMICs) remain limited, in part due to resource constraints and availability of technology. Therefore, the uptake of digital innovations in low resource settings may be hampered by costs associated with data or digital tools themselves [41]. It is pertinent to explore partnerships with mobile phone providers to reduce these costs for the envisioned implementation of the One Health concept proposed by the World Health Organization (WHO) [61]. With that, the end users will have greater ability to learn from digital technologies as data packages become more available and decrease in cost across LMICs. Digital platforms are being used in many businesses (financial, agriculture, consumer goods), and perhaps, these could be optimized or expanded to include health products and services. In parallel, increase in smartphone connectivity is facilitating the potential scope of HIVST with digital supports interventions as growing 4 G and 5 G networks will improve accessibility to healthcare services in many African and Asian countries [62]. However, for global applicability, connectivity must be optimized for all platforms and phones (Android, iPhone, Microsoft) and for all bandwidths.
This systematic review is the first to assess extensive evidence to date from published literature that focuses on HIVST exclusively with digital support innovations. It includes a broad range of studies without limiting outcomes, geographic regions, or study populations.
Due to the heterogeneity of interventions, outcomes, populations, and settings, we were unable to perform a meta-analysis. Inconsistent terminologies used in the published literature increased the difficulty in pooling results. Existing literature has described misclassification and misuse of metrics within measures in the HIVST field, particularly for feasibility definitions [12]. The heterogeneity in reporting of metrics presented challenges in summarizing findings across studies according to hard outcomes. The definitions and interpretations for acceptability, preference, feasibility, and impact were obtained from a published framework for monitoring and evaluation of HIV diagnostic devices [12]. There is a need for a framework in digital health focused on HIV diagnostics. Furthermore, diversity in both innovation type and metrics presented challenges in making direct comparisons between the included studies. Our review also identified limited evidence from LMICs, indicating a need for further research in high-burden settings to provide insight to the acceptability, feasibility, and impact of digitally supported HIVST among vulnerable populations that may have limited access to or uptake of certain technologies. In addition, given the novelty of the field of HIVST with digital supports, there is a lack of data from RCTs to draw evidence from. While PubMed and Embase are recognized as comprehensive biomedical literature databases, our search strategy was restricted to these two databases alongside abstracts from relevant conferences, and consequently may have missed certain studies. As most of our included studies were observational, there is potential for confounding and selection bias to influence our results. Given that most of the literature in diagnostics are observational in nature, this alone is unlikely to influence our findings. The need for large RCTs has been acknowledged as a limitation of digital health [63].
Given the diversity of digital innovations that have been studied and that only a small number of studies focus on each, reporting of clear metrics is necessary. The co-creation of interventions with target communities will be important in designing effective future HIVST strategies with digital supports. Furthermore, quasi-controlled trials, RCTs, and cohort studies with larger sample sizes will be valuable to assess the efficacy of promising digital interventions. Research evidence must guide implementation policy for innovations across diverse populations in high, middle-, and low-income countries. Implementation of HIVST with digital supports innovations in practice will require close collaboration with practitioners for sustainability.
HIVST interventions with digital supports offer an important strategy to reach at-risk populations, particularly in the current context of the COVID-19 pandemic and to align our progress with one digital health initiative. A wide variety of HIVST interventions with digital supports reported successes in feasibility, acceptability, preference, and impact to increase linkage to care and ART initiation. The overall evidence suggests that HIVST with digital supports is well poised to become the new paradigm to improve efficiency and increase impact in the delivery of HIVST services worldwide.
Declaration of Competing Interest
Dr Pant Pai reports a copyright for HIVSmart! (an open access mobile application), issued by McGill University (#1105598) in 2013, and co-owned by Grand Challenges Canada and McGill University. The other authors have no conflicts of interest to declare.
Acknowledgements
Funding
This work was funded by the Foundation for Innovative New Diagnostics. The agency had no role in the decision to submit it for publication, however the funders contributing to the writing of the manuscript. NPP also acknowledges support from the Fonds de recherche du Québec – Santé (Senior scientist scholar award), The Canadian Institutes of Health Research (PJT 153149 and HBR 422155), Grand Challenges Canada (Transition to Scale award, 0710–05), the India-Canada centre for Innovative Multidisciplinary Partnerships to Accelerate Community Transformation and Sustainability (IC-IMPACTS), and the MUHC Foundation. The agencies had no role in the writing of the manuscript or the decision to submit it for publication.
Contributors
MM, AdW, and NPP designed, drafted and reviewed the initial manuscript, while the remaining authors (AK, RJ, NE, MFS, SC, RS, AAZ) provided further review. The search strategy was developed and executed by MM, AdW, NPP, and AK. The quality assessment was performed by MM and AdW. Critique of the final manuscript was provided by all authors. All authors approved and contributed to the final written manuscript.
Data sharing statement
All data presented in this study were extracted from published original data. The data that support the findings of this study are available in Supplementary Tables 1 and 2.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101059.
Appendix. Supplementary materials
References
- 1.World Health Organization. Number of countries adopting HIV self-testing policies rises sharply. July 25, 2017. https://www.who.int/hiv/mediacentre/news/hiv-self-testing-increases/en/#:~:text=A%20total%20of%2040%20countries,national%20HIV%20self%2Dtesting%20policies (accessed May 10, 2021).
- 2.World Health Organization. Innovative WHO HIV testing recommendations aim to expand treatment coverage. November 27, 2019. https://www.who.int/news/item/27-11-2019-innovative-who-hiv-testing-recommendations-aim-to-expand-treatment-coverage#:~:text=Innovative%20WHO%20HIV%20testing%20recommendations%20aim%20to%20expand%20treatment%20coverage,-27%20November%202019&text=The%20World%20Health%20Organization%20(WHO,unable%20to%20obtain%20lifesaving%20treatment. (accessed May 10, 2021).
- 3.UNAIDS. UNAIDS report on the global AIDS epidemic shows that 2020 targets will not be met because of deeply unequal success; COVID-19 risks blowing HIV progress way off course. July 6, 2020. https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2020/july/20200706_global-aids-report. (accessed May 10, 2021).
- 4.World Health Organization. Monitoring and evaluating digital health interventions: a practical guide to conducting research and assessment. 2016. https://saluddigital.com/wp-content/uploads/2019/06/WHO.-Monitoring-and-Evaluating-Digital-Health-Interventions.pdf. (accessed May 10, 2021).
- 5.Roehr B. FDA approves “instant” HIV home test. BMJ. 2012;345:e4636. doi: 10.1136/bmj.e4636. [DOI] [PubMed] [Google Scholar]
- 6.Qin Y., Han L., Babbitt A. Experiences using and organizing HIV self-testing. AIDS. 2018;32(3):371–381. doi: 10.1097/QAD.0000000000001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw J., Agarwal P., Desveaux L. Beyond “implementation”: digital health innovation and service design. NPJ Digit Med. 2018;1(1):1–5. doi: 10.1038/s41746-018-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvath K.J., Walker T., Mireles L., Bauermeister J.A., Hightow-Weidman L., Stephenson R. A systematic review of technology-assisted HIV testing interventions. Curr HIV/AIDS Rep. 2020;17(4):269–280. doi: 10.1007/s11904-020-00506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veronese V., Ryan K.E., Hughes C., Lim M.S., Pedrana A., Stoové M. Using digital communication technology to increase HIV testing among men who have sex with men and transgender women: systematic review and meta-analysis. J Med Internet Res. 2020;22(7):e14230. doi: 10.2196/14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dave S., Peter T., Fogarty C., Karatzas N., Belinsky N., Pant Pai N. Which community-based HIV initiatives are effective in achieving UNAIDS 90-90-90 targets? A systematic review and meta-analysis of evidence (2007–2018) PLoS ONE. 2019;14(7) doi: 10.1371/journal.pone.0219826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daher J., Vijh R., Linthwaite B. Do digital innovations for HIV and sexually transmitted infections work? Results from a systematic review (1996–2017) BMJ Open. 2017;7(11) doi: 10.1136/bmjopen-2017-017604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pant Pai N., Chiavegatti T., Vijh R. Measures and metrics for feasibility of proof-of-concept studies with human immunodeficiency virus rapid point-of-care technologies: the evidence and the framework. Point Care. 2017;16(4):141–150. doi: 10.1097/POC.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J.P., Thomas J., Chandler J. John Wiley & Sons; Chichester: 2019. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 14.Wells G.A., Shea B., O'Connell Da. Oxford; Ottawa: 2000. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 15.Anand T., Nitpolprasert C., Kerr S.J. Implementation of an online HIV prevention and treatment cascade in Thai men who have sex with men and transgender women using Adam's love electronic health record system. J Virus Erad. 2017;3(1):15–23. doi: 10.1016/S2055-6640(20)30293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Boni R.B., Veloso V.G., Fernandes N.M. An internet-based HIV self-testing program to increase HIV testing uptake among men who have sex with men in brazil: descriptive cross-sectional analysis. J Med Internet Res. 2019;21(8):e14145. doi: 10.2196/14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y., Ni Y., Li X. Proceedings of the 23rd international AIDS conference. 2020. Financial incentives and peer referral in promoting digital network-based secondary distribution of HIV self-testing among MSM in China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao J., Li M.Y., Qian H.Z. Home-based HIV testing for men who have sex with men in China: a novel community-based partnership to complement government programs. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0102812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day S., Khan K., Kelly A.M., Jones S., Kinsella R. Characteristics of newly diagnosed HIV-positive service users using a pan-London e-sexually transmitted infection screening service. Int J STD AIDS. 2021 doi: 10.1177/09564624211014729. [DOI] [PubMed] [Google Scholar]
- 20.Barnard S., Free C., Bakolis I., Turner K.M.E., Looker K.J., Baraitser P. Comparing the characteristics of users of an online service for STI self-sampling with clinic service users: a cross-sectional analysis. Sex Transm Infect. 2018;94(5):377. doi: 10.1136/sextrans-2017-053302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliot E., Rossi M., McCormack S., McOwan A. Identifying undiagnosed HIV in men who have sex with men (MSM) by offering HIV home sampling via online gay social media: a service evaluation. Sex Transm Infect. 2016;92(6):470–473. doi: 10.1136/sextrans-2015-052090. [DOI] [PubMed] [Google Scholar]
- 22.Jin X., Xu J., Smith M.K. An internet-based self-testing model (easy test): cross-sectional survey targeting men who have sex with men who never tested for HIV in 14 Provinces of China. J Med Internet Res. 2019;21(5):e11854. doi: 10.2196/11854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platteau T., Fransen K., Apers L. Swab2know: an HIV-testing strategy using oral fluid samples and online communication of test results for men who have sex with men in Belgium. J Med Internet Res. 2015;17(9):e213. doi: 10.2196/jmir.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loos J., Manirankunda L., Platteau T. Acceptability of a community-based outreach HIV-testing intervention using oral fluid collection devices and web-based HIV test result collection among sub-saharan african migrants: a mixed-method study. JMIR Public Health Surveill. 2016;2(2):e33. doi: 10.2196/publichealth.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang W., Wei C., Cao B. Crowdsourcing to expand HIV testing among men who have sex with men in China: a closed cohort stepped wedge cluster randomized controlled trial. PLoS Med. 2018;15(8) doi: 10.1371/journal.pmed.1002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X., Tang Z., Wu Z., Nong Q., Li Y. Promoting oral HIV self-testing via the internet among men who have sex with men in China: a feasibility assessment. HIV Med. 2020;21(5):322–333. doi: 10.1111/hiv.12830. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A., Chavez P.R., MacGowan R.J. Willingness to distribute free rapid home HIV test kits and to test with social or sexual network associates among men who have sex with men in the United States. AIDS Care. 2017;29(12):1499–1503. doi: 10.1080/09540121.2017.1313386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witzel T.C., Gabriel M.M., McCabe L. Pilot phase of an internet-based RCT of HIVST targeting MSM and transgender people in England and Wales: advertising strategies and acceptability of the intervention. BMC Infect Dis. 2019;19(1):699. doi: 10.1186/s12879-019-4247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong F., Tang W., Cheng W. Acceptability and feasibility of a social entrepreneurship testing model to promote HIV self-testing and linkage to care among men who have sex with men. HIV Med. 2017;18(5):376–382. doi: 10.1111/hiv.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yun K., Chu Z., Zhang J. Mobile phone intervention based on an HIV risk prediction tool for HIV prevention among men who have sex with men in China: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9(4):e19511. doi: 10.2196/19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan P.S.F., Chidgey A., Lau J., Ip M., Lau J.T.F., Wang Z. Effectiveness of a novel HIV self-testing service with online real-time counseling support (HIVST-Online) in increasing HIV testing rate and repeated HIV testing among men who have sex with men in Hong Kong: results of a pilot implementation project. Int J Environ Res Public Health. 2021;18(2):729. doi: 10.3390/ijerph18020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agustí C., Muñoz R., González V. Outreach HIV testing using oral fluid and online consultation of the results: pilot intervention in Catalonia. Enferm Infecc Microbiol Clin (Engl Ed) 2021;39(1):3–8. doi: 10.1016/j.eimc.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 33.MacGowan R.J., Chavez P.R., Borkowf C.B. Effect of internet-distributed HIV self-tests on HIV diagnosis and behavioral outcomes in men who have sex with men: a randomized clinical trial. JAMA Intern Med. 2020;180(1):117–125. doi: 10.1001/jamainternmed.2019.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phanuphak N., Anand T., Jantarapakde J. What would you choose: online or offline or mixed services? Feasibility of online HIV counselling and testing among Thai men who have sex with men and transgender women and factors associated with service uptake. J Int AIDS Soc. 2018;21(Suppl 5):e25118. doi: 10.1002/jia2.25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phanuphak N., Jantarapakde J., Himmad L. Linkages to HIV confirmatory testing and antiretroviral therapy after online, supervised, HIV self-testing among Thai men who have sex with men and transgender women. J Int AIDS Soc. 2020;23(1):e25448. doi: 10.1002/jia2.25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephenson R., Todd K., Kahle E. Project moxie: results of a feasibility study of a telehealth intervention to increase HIV testing among binary and nonbinary transgender youth. AIDS Behav. 2020;24(5):1517–1530. doi: 10.1007/s10461-019-02741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Lau J.T., Ip M. A randomized controlled trial evaluating efficacy of promoting a home-based HIV self-testing with online counseling on increasing HIV testing among men who have sex with men. AIDS Behav. 2018;22(1):190–201. doi: 10.1007/s10461-017-1887-2. [DOI] [PubMed] [Google Scholar]
- 38.Hoagland B., Torres T.S., Bezerra D.R.B. High acceptability of PrEP teleconsultation and HIV self-testing among PrEP users during the COVID-19 pandemic in Brazil. Braz J Infect Dis. 2021;25(1) doi: 10.1016/j.bjid.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vermey K., Daas C.D., Zweers W., Bergen J.V., Bos H. P046 Ensuring quality-assured and personalized online self-testing within a market-driven context. Sex Transm Infect. 2019;2019:A99. (abstr) [Google Scholar]
- 40.Chiu C.J., Young S.D. Correlates of requesting home HIV self-testing kits on online social networks among African-American and Latino men who have sex with men. AIDS Care. 2016;28(3):289–293. doi: 10.1080/09540121.2015.1090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gous N., Fischer A.E., Rhagnath N., Phatsoane M., Majam M., Lalla-Edward S.T. Evaluation of a mobile application to support HIV self-testing in Johannesburg, South Africa. S Afr J HIV Med. 2020;21(1):1–7. doi: 10.4102/sajhivmed.v21i1.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green K.E., Vu B.N., Phan H.T. From conventional to disruptive: upturning the HIV testing status quo among men who have sex with men in Vietnam. J Int AIDS Soc. 2018;21(Suppl 5):e25127. doi: 10.1002/jia2.25127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang E., Marlin R.W., Medline A., Young S.D., Daniels J., Klausner J.D. P17.09 cost-effectiveness of hiv self-testing promotion through grindr™, a smartphone social networking application. Sex Transm Infect. 2015;91(Suppl 2):A226. (abstr) [Google Scholar]
- 44.Huang E., Marlin R.W., Young S.D., Medline A., Klausner J.D. Using Grindr, a smartphone social-networking application, to increase HIV self-testing among Black and Latino men who have sex with men in Los Angeles, 2014. AIDS Educ Prev. 2016;28(4):341–350. doi: 10.1521/aeap.2016.28.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John S.A., Starks T.J., Rendina H.J., Parsons J.T., Grov C. High willingness to use novel HIV and bacterial sexually transmitted infection partner notification, testing, and treatment strategies among gay and bisexual men. Sex Transm Infect. 2020;96(3):173–176. doi: 10.1136/sextrans-2019-053974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koblin B.A., Nandi V., Hirshfield S. Informing the development of a mobile phone HIV testing intervention: intentions to use specific HIV testing approaches among young black transgender women and men who have sex with men. JMIR Public Health Surveill. 2017;3(3):e45. doi: 10.2196/publichealth.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menza T.W., Garai J., Ferrer J., Hecht J. Rapid uptake of home-based HIV self-testing during social distancing for SARS-CoV2 infection in oregon. AIDS Behav. 2020;25:167–170. doi: 10.1007/s10461-020-02959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosengren A.L., Huang E., Daniels J., Young S.D., Marlin R.W., Klausner J.D. Feasibility of using Grindr(TM) to distribute HIV self-test kits to men who have sex with men in Los Angeles, California. Sex Health. 2016 doi: 10.1071/SH15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samoh N., Peerawaranun P., Jonas K.J., Lim S.H., Wickersham J.A., Guadamuz T.E. Willingness to Use HIV self-testing with online supervision among app-using young men who have sex with men in Bangkok. Sex Transm Dis. 2021;48(3):e41. doi: 10.1097/OLQ.0000000000001271. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan P.S., Driggers R., Stekler J.D. Usability and acceptability of a mobile comprehensive HIV prevention app for men who have sex with men: a pilot study. JMIR Mhealth Uhealth. 2017;5(3):e26. doi: 10.2196/mhealth.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu D., Zhou Y., Yang N. Social media-based secondary distribution of human immunodeficiency virus/syphilis self-testing among Chinese men who have sex with men. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang W., Hu Q., Tang W. HIV self-testing programs to men who have sex with men delivered by social media key opinion leaders and community-based organizations are both effective and complementary: a national pragmatic study in China. JAIDS J Acquir Immune Defic Syndr. 2020;84(5):453–462. doi: 10.1097/QAI.0000000000002375. [DOI] [PubMed] [Google Scholar]
- 53.Zhu X., Zhang W., Operario D. Effects of a mobile health intervention to promote HIV self-testing with MSM in China: a randomized controlled trial. AIDS Behav. 2019;23(11):3129–3139. doi: 10.1007/s10461-019-02452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biello K.B., Horvitz C., Mullin S. HIV self-testing and STI self-collection via mobile apps: experiences from two pilot randomized controlled trials of young men who have sex with men. Mhealth. 2021;7:26. doi: 10.21037/mhealth-20-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown W., Lopez Rios J., Sheinfil A. Text messaging and disaster preparedness aids engagement, re-engagement, retention, and communication among Puerto Rican participants in a human immunodeficiency virus (HIV) self-testing study after hurricanes Irma and Maria. Disaster Med Public Health Prep. 2020:1–8. doi: 10.1017/dmp.2020.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown L.J., Tan K.S., Guerra L.E., Naidoo C.J., Nardone A. Using behavioural insights to increase HIV self-sampling kit returns: a randomized controlled text message trial to improve England's HIV self-sampling service. HIV Med. 2018;19(9):585–596. doi: 10.1111/hiv.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelvin E.A., George G., Mwai E. A randomized controlled trial to increase HIV testing demand among female sex workers in Kenya through announcing the availability of HIV self-testing via text message. AIDS Behav. 2019;23(1):116–125. doi: 10.1007/s10461-018-2248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelvin E.A., George G., Kinyanjui S. Announcing the availability of oral HIV self-test kits via text message to increase HIV testing among hard-to-reach truckers in Kenya: a randomized controlled trial. BMC Public Health. 2019;19(1):7. doi: 10.1186/s12889-018-6345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee M.J., Onyango D., Hamza H. Surveying testing preferences in Black, Latin American, and other minorities for the co-design of digital vending machines for HIV self-testing. Int J STD AIDS. 2020;31(2):158–165. doi: 10.1177/0956462419887042. [DOI] [PubMed] [Google Scholar]
- 60.Vera J.H., Soni S., Pollard A. Acceptability and feasibility of using digital vending machines to deliver HIV self-tests to men who have sex with men. Sex Transm Infect. 2019;95(8):557. doi: 10.1136/sextrans-2018-053857. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization. One Health. September 21, 2017. https://www.who.int/news-room/q-a-detail/one-health. (accessed June 10, 2021).
- 62.Mwangama J., Malila B., Douglas T., Rangaka M. What can 5G do for healthcare in Africa? Nat Electron. 2020;3(1):7–9. [Google Scholar]
- 63.Topol E.J. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.