Abstract
Revaluing agri-food waste to offer consumers bioactive compounds for a healthy diet is an important issue. In the present work, the antioxidant capacity (AC), total phenolic content (TPC) and phenolic compounds of pulp and bagasse of four Peruvian berries with UHPLC-DAD was determined. Elderberry (Sambucus peruviana Kunth) bagasse had a greater amount of TPC (4.87 ± 0.02 mg GAE/100 gfw) and AC (7.66 ± 0.04 and 7.51 ± 0.24 μmol TE/gfw in DPPH and ABTS, respectively) than the bagasse of the other berries, with a strong positive correlation between TPC and AC. Blueberry (Vaccinium floribundum Kunth) bagasse contains the highest amount of gallic acid (103.26 ± 1.59 μg/gfw), chlorogenic acid (1276.55 ± 1.86 μg/gfw), caffeic acid (144.46 ± 1.78 μg/gfw), epicatechin (1113.88 ± 1.82 μg/gfw) and p-coumaric acid (77.82 ± 1.92 μg/gfw). Elderberry (Sambucus peruviana Kunth) bagasse contains the highest amount of catechin (153.32 ± 0.79 μg/gfw). No significant differences were found in the content of chlorogenic acid and epicatechin of blackberry (Rubus roseus Poir). It was shown that the wastes of the four Amazonian berries have higher values of bioactive properties than their pulp, being the elderberry bagasse the one with the best properties.
Keywords: Berry bagasse, Antioxidant capacity, Total phenolic content, Epicatechin, Chlorogenic acid
Berry bagasse, Antioxidant capacity, Total phenolic content, Epicatechin, Chlorogenic acid.
1. Introduction
The reuse of waste from agro-industrial processing has become an important issue for the economy, sustainability of the processes (Buratto et al., 2021), and the world's population growth (Mokhtar et al., 2018). In parallel, consumer preferences towards a healthy diet that is rich in natural bioactive compounds, motivate researchers to look for bioactive compounds in agri-food products (Mokhtar et al., 2018). There is evidence that shows that native fruits have a high content of bioactive compounds that generate their high antioxidant capacity (AC) (Pfukwa et al., 2020). However, the largest amount of bioactive compounds is found in the non-edible fraction of fruits which is generally discarded (Cádiz-Gurrea et al., 2020). The poor management of this by-product is reflected in a substantial loss of bioactive compounds (Vázquez-González et al., 2020). Fruits such as berries can be an important part of a healthy diet due to their content of bioactive compounds. Important berries such as: blackberry, blueberry, cranberry, raspberry and strawberry are important sources of bioactive compounds and are consumed as a fresh or processed product (Jimenez-Garcia et al., 2013). In Peru, the production of aguaymanto is 12 tons per ha (Instituto Nacional de Innovación Agraria, 2021), and by 2021, it is estimated that Peru would have 14789 ha of blueberries and in a few years we will reach 20 thousand hectares (Agencia Agraria de Noticias, 2021).
Phenolic compounds are bioactive molecules present throughout the plant kingdom, including non-edible materials remaining of the industrial fruit processing, which are considered by-products, waste or bagasse (Machado et al., 2021). This bagasse still contains a large amount of bioactive compounds of great interest to the food industry (Vázquez-González et al., 2020). In the case of goldenberry, it has been found that most studies have focused only on its properties, rather than on the by-products obtained from the industrial process (Nocetti et al., 2020). The manufacture of juices and derivatives of blueberry produces around 20–30 % of by-products that are discarded (Luchese et al., 2018).
Blackberries and blueberries are excellent sources of bioactive compounds and have high AC (Gowd et al., 2019; Koca and Karadeniz, 2009). Local communities use the extracts of these berries to treat diseases such as diabetes and inflammation (Prencipe et al., 2014). Goldenberry, is recognized for having high content of bioactive compounds (Dag et al., 2017), its bioactive compounds are responsible for the high AC of the products derived from this berry (Olivares-Tenorio et al., 2017; Ramadan, 2011; Puente et al., 2011). However, most reports have focused on the properties of goldenberry fruit only, rather than on its byproducts (Nocetti et al., 2020). Elderberry is a berry commonly used for its medicinal and nutritional properties (Kiprovski et al., 2021). In Europe, elderberries are used in the preparation of desserts and yogurts; and as a medicine thanks to its antioxidant, anticancer, immunostimulating, antiallergic, antiviral and antibacterial properties (Domínguez et al., 2020; Ferreira et al., 2020).
The wild berries are getting more attention due to the presence of an enormous range of phytoconstituents encompassing considerable antioxidant efficacy and health benefits (Umdale et al., 2020). In elderberries, chlorogenic acid is reported as being the major cinnamic acid present (Ferreira et al., 2020). In freeze-dried goldenberry juice, chlorogenic acid was found as the main phenolic acid followed by p-coumaric acid and ferulic acid (Dag et al., 2017). In selected cultivar blueberries, caffeic acid, chlorogenic acid, p-coumaric acid, and quercetin was found (You et al., 2011). In blackberry cultivar, five anthocyanins, five phenolic acids, and ten non-anthocyanin flavonoids were identified between they found catechin, epicatechin, chlorogenic acid, kaempferol and ellagic acid (Moraes et al., 2020). Various studies have reported that the by-products of various fruits (skin and seed) have high amounts of phytochemicals and antioxidants (Bora et al., 2019); however, studies on bioactive compounds, total phenolic content (TPC) and AC of Amazon berries are scarce. Then, the objective of this research was to determinate the AC, TPC and phenolic compounds of pulp and bagasse of four Peruvian berries.
2. Materials and methods
2.1. Materials
Before harvesting the fruits, their maturity was determined according to the experience of the local farmer. Then, mature and healthy fruits of elderberry (Sambucus peruviana Kunth), blackberry (Rubus roseus Poir), goldenberry (Physalis peruviana L.) and blueberry (Vaccinium floribundum Kunth) were harvested from the towns María y Cuelcho (Amazonas Region), during January to March 2019. One kilogram of each fruit was stored at -20 °C until its chemical analysis. Leaves, flowers and fruit of each berry were send to the “Herbarium Truxillense (HUT)” of the Universidad Nacional de Trujillo-Perú for its taxonomic identification.
2.2. Chemicals
Folin-Ciocalteu phenol reagent (Merck, Darmstadt, Germany), sodium carbonate (Spectrum, New Brunswick, USA), potassium persulphate, gallic acid, ethanol, HPLC standards (chlorogenic acid, caffeic acid, p-coumaric acid, (+)-catechin, (−)-epicatechin), (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (trolox), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-Diphenyl-1-picrylhydrazyl (DPPH), and methanol were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.3. Aqueous extract of polyphenols
According to (Fu et al., 2011) with some modifications, 200 g of berry were weighed, homogenized in an electric homogenizer for 2 min. Bagasse and pulp were separated using a sieve. One gram of bagasse or pulp was mixed with 9 mL of ultrapure water, separately. Each mixture was stirred for 30 min at room temperature and then centrifuged at 5000 rpm for 30 min. The supernatant was collected in a 15 mL amber glass flasks and they were stored under refrigeration until analysis.
2.4. Determination of total phenolic content
Total phenolic content (TPC) in aqueous extracts was determined according to the Folin–Ciocalteu's procedure (Singleton et al., 1999) (de Souza et al., 2014). The extracts (0.5 mL) were mixed with 2.5 mL of Folin–Ciocalteu reagent (10 %) and 2 mL of sodium carbonate solution (4 %). The mixture was stirred and kept at room temperature for 2 h in the dark. The absorbance was measured at 750 nm against a blank using an spectrophotometer (Unico, S2100, USA). TPC was estimated from a standard curve of gallic acid ( ) and results expressed as mg gallic acid equivalents (GAE)/100g fresh weight (mg GAE/100 gfw).
2.5. Antioxidant capacity
According to de Souza et al. (2014), the AC was determined using the ABTS, and DPPH methods. For the ABTS assay, the procedure followed the method of Re et al. (1999) with minor modifications. The ABTS radical cation (ABTS•+) was generated by the reaction of 5 mL of aqueous ABTS solution (7 mM) with 88 μL of 140 mM (2.45 mM final concentration) potassium persulphate. The mixture was kept in the dark for 16 h before use and then diluted with ethanol to obtain an absorbance of 0.7 ± 0.06 units at 734 nm using a spectrophotometer (Unico, S2100, USA). The berry extracts (30 μL) or a reference substance (Trolox) were allowed to react with 3 mL of the resulting blue–green ABTS radical solution in the dark. The decrease of absorbance at 734 nm was measured after 6 min. Ethanolic solutions of known Trolox concentrations were used for calibration ( ). The results are expressed as micromoles of Trolox equivalents (TEs) per gram of fresh weight (μmol of TE/gfw). The DPPH free radical-scavenging capacity was estimated using the method of Cardeñosa et al. (2016) and Brand-Williams et al. (1995). Briefly, the solution of DPPH (600 μM) was diluted with ethanol to obtain an absorbance of 0.7 ± 0.04 units at 517 nm. The fruit extracts (0.1 mL) were allowed to react with 3.9 mL of the DPPH radical solution for 30 min in the dark, and the decrease in absorbance from the resulting solution was monitored. The absorbance of the reaction mixture was measured at 517 nm. The results were expressed as μmol of TE/gfw.
2.6. UHPLC-DAD analyses of phenolic compounds profile
Quantification of phenolic compounds was performed according to Coklar and Akbulut (2017) and Demir et al. (2014) with some modifications. For that, an Ultra High Performance Liquid Chromatographic system (UHPLC) Agilent 1290 Infinity Series equipped with a G7167B mutisampler, G7104A flexible pump, G7116B column oven, and a G7117B diode array detector was used. Before the injection, the extracts were filtered through a 0.45 μm pore size x 33 mm syringe filter (Merck, Millex, Germany). The separation was achieved using a reversed-phase C18 column (5 μm, 250 × 4.6 mm i.d.). The mobile phase was (A) water/acetic acid (98:2) and (B) water/acetonitrile/acetic acid (78:20:2). The flow rate was 0.75 mL/min and the gradient was as follows: 10–14 % B (5 min), 14–23 % B (11 min), 23–35 % B (5 min), 35–40 % B (14 min), 40–100 % B (3 min), 100 % B isocratic (3 min), 100–10 % B (3 min) and 10 % B isocratic (4 min). The detector was set to 280 and 320 nm. The column temperature was 40 °C. According to Sasmaz et al. (2020) and Kelebek (2016), the phenolic compounds were quantified by comparison with peak areas and retention time (Ferreira et al., 2020) of each standard. The coefficient of correlation (R2) value of the standards phenolic compounds were above 0.9799 in all cases. The data was analyzed using Chemstation software.
2.7. Statistical analysis
One-way analysis of variance was performed using Rstudio. Tukey's test was used as a post-hoc multiple comparisons at a significance level of p < 0.05 (Wang et al., 2015). All the experiments were performed in quadruplicate, and the results were expressed as mean ± standard deviation (SD). The Pearson's correlation analysis between TPC and AC was performed with CurveExpert Professional software.
3. Results and discussions
3.1. Total phenolic content and antioxidant capacity
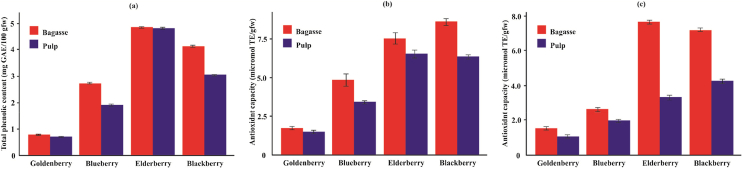
The AC of a fruit is related to the synergistic effects between its phenolic compounds (Acosta-Montoya et al., 2010). In addition, the TPC of the same species can vary significantly according to the geographical origin and different methods of obtaining its phenolic extracts (Jiang et al., 2021). Table 1 shows the results obtained in the quantification of TPC of bagasse and pulp of berries from the Amazon Region. The blueberry obtained a TPC of 2.71 ± 0.02 mg GAE/100 gfw in the bagasse and 1.91 ± 0.02 mg GAE/100 gfw in the pulp. This values are below those found by Koca and Karadeniz (2009), who reports values in a range of 77–82 mg GAE/100 gfw for blueberry grown in the Black Sea Region of Turkey, and de Souza et al. (2014) reports TPC values of 305.38 ± 5.09 mg GAE/100 gfw for blueberry produced in the subtropical areas of Brazil. Koca and Karadeniz (2009) and de Souza et al. (2014) reports 850.52 ± 4.77 and 173–305 mg GAE/100 gfw for blackberry, respectively. On the other hand, Acosta-Montoya et al. (2010) reports TPC of 520 ± 20 mg GAE/100 gfw in blackberry from Costa Rica, we must consider that these values have been reported for whole berries, that is, pulp and bagasse. In the present work it is shown that the TPC values found in pulp and bagasse of blackberry and blueberry were very low, because the berry extracts were obtained with pure water. For example, Pasquel Reátegui et al. (2014) used supercritical carbon dioxide extraction to obtain extracts from blackberry industrial residues, they found TPC of 2413 ± 162 mg GAE/100 g extract. For goldenberry, Ozturk et al. (2017) reports 42.16–48.60 mg GAE/100 g in Turkish goldenberry, and Ordóñez-Santos et al. (2017) reports 80.15 ± 1.09 mg GAE/100 g in goldenberry from Colombia, values higher than those found in the present work (0.77 ± 0.01 and 0.69 ± 0.01 mg GAE/100 gfw in bagasse and pulp, respectively). For elderberry (Sambucus nigra) from Portugal, Ferreira et al. (2020) reports 820 ± 45 mg GAE/100 gfw, values much higher than those found in our samples. Knowing that water is the solvent with the lowest extraction performance, the results found were as expected.
Table 1.
Total phenolic content and antioxidant capacity of bagasse and pulp of Peruvian berries.
| Sample | TPC (mg GAE/100 gfw) | Antioxidant capacity (μmol TE/gfw) |
|
|---|---|---|---|
| DPPH | ABTS | ||
| Bagasse | |||
| Blueberry | 2.71 ± 0.02c | 2.64 ± 0.09c | 4.85 ± 0.28c |
| Elderberry | 4.87 ± 0.02a | 7.66 ± 0.04a | 7.51 ± 0.24b |
| Blackberry | 4.11 ± 0.01b | 7.19 ± 0.06b | 8.61 ± 0.14a |
| Goldenberry |
0.77 ± 0.01d |
1.46 ± 0.04d |
1.69 ± 0.07d |
| Pulp | |||
| Blueberry | 1.91 ± 0.02c | 1.97 ± 0.05c | 3.39 ± 0.06c |
| Elderberry | 4.87 ± 0.12a | 3.35 ± 0.05b | 6.57 ± 0.14a |
| Blackberry | 3.07 ± 0.01b | 4.3 ± 0.09a | 6.34 ± 0.04b |
| Goldenberry | 0.69 ± 0.01d | 1.06 ± 0.09d | 1.5 ± 0.08d |
Results were given as mean ± standard deviation (n = 4). Different letters in the same column indicate statistically significant differences (p < 0.05). Tukey's test was apply separately on bagasse and pulp.
In general, the presence of TPC contributes to the AC of the berry (Nocetti et al., 2020). In the present work, the bagasse of the berries had the highest AC than pulp both in DPPH and ABTS (Table 1). In the case of AC measured in DPPH, 2.64 ± 0.09 μmol TE/gfw were found in blueberry bagasse, much lower value than reported by de Souza et al. (2014) in Brazilian blueberry (7775.45 ± 1009.60 μmol TE/gfw). The blackberry bagasse had AC of 7.19 ± 0.06 μmol TE/gfw, value lower than that reported by de Souza et al. (2014) (2142.42 ± 125.64 μmol TE/gfw). In elderberry bagasse, 7.66 ± 0.04 μmol TE/gfw was found, value much lower than that found by Jakobek et al. (2007) in elderberry from Croatia (100.16 μmol TE/gfw), and in goldenberry, 1.46 ± 0.04 μmol TE/gfw was founded, value lower than found by Ozturk et al. (2017) in goldenberry from Turkey (6.05–5.14 μmol TE/gfw). The AC results obtained by both methods are not directly comparable because the radical capture mechanisms are different for each method (Chaves et al., 2018; Martín-Gómez et al., 2020). However, both showed the same trend, the results obtained by the ABTS and DPPH methods agree that blackberry and elderberry have higher AC than other berries, both in their bagasse and in their pulp. The descending order of the AC of the berries is:
DPPH method: elderberry > blackberry > blueberry > goldenberry for bagasse and blackberry elderberry> >blueberry > goldenberry for pulp.
ABTS method: blackberry > elderberry > blueberry > goldenberry for bagasse and elderberry > blackberry > blueberry > goldenberry for pulp.
TPC can have a synergistic or antagonistic effect on AC (Jiang et al., 2021). If we make a comparison between the bioactive properties of the analyzed berries, we can notice that the elderberry bagasse has a higher amount of TPC (Figure 1a) than the other berries, as a consequence, it also has the highest AC measured in DPPH (Figure 1c), both in pulp and bagasse. Thus, we can also affirm that those berries that have a higher amount of TPC, also have a higher AC, so there is a direct proportion between both properties. In the case of AC measured in ABTS, an inverse proportion occurs (Figure 1b). This is corroborated with what Nocetti et al. (2020) affirms. It is important to consider that when using the Folin-Ciocalteu reagent, not only TPC is measured but also the total reducing substances in the sample (Sério et al., 2014).
Figure 1.
Bioactive compounds of berries from Amazonas Region. (a) TPC (b) ABTS (c) DPPH.
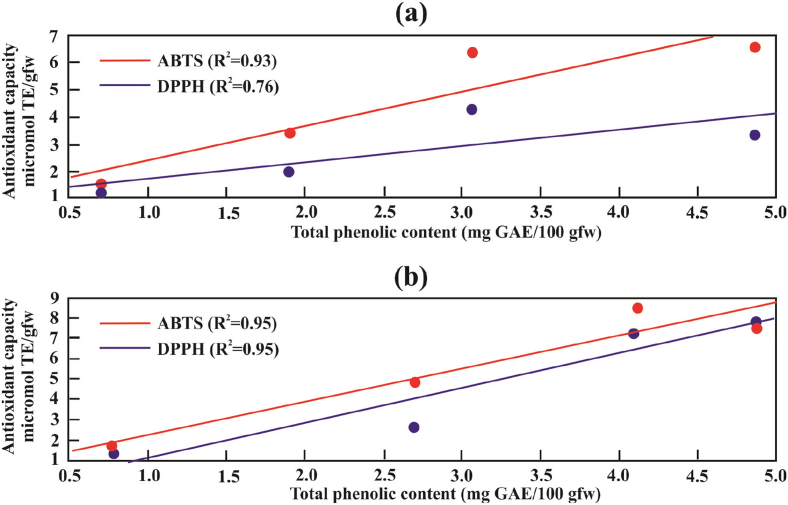
The AC depends on the structural factors of the phenolic compounds such as the number and position of the hydroxyl or methoxyl groups in the phenolic ring (Giovanelli and Buratti, 2009). Also, other researchers state that the AC could depend on the type of solvent used in the extraction (Sariburun et al., 2010). To demonstrate the influence of TPC on AC, the correlation between both values was established. Positive linear correlations shown in Figure 2 demonstrate that the TPC and AC values are better correlated positively to bagasse (R2 = 0.95 in ABTS and DPPH) rather than to pulp (R2 = 0.93 and R2 = 0.76 in ABTS and DPPH), of them, the best correlation was with AC measured in ABTS. These results agree with those reported by de Souza et al. (2014), Giovanelli and Buratti (2009), and Sariburun et al. (2010). Vasco et al. (2008) obtained coefficients of correlation of 0.66 in Ecuadorian berries. Martín-Gómez et al. (2020) obtained a coefficient of correlation of 0.81 for the DPPH method and 0.57 for the ABTS method in blueberry.
Figure 2.
Linear correlations of TPC versus the AC determined for pulp (a) and bagasse (b) of four Amazon berries.
3.2. Phenolic compound profile by UHPLC-DAD
Phenolic compounds are distributed throughout the plant kingdom and are thus form an integral part of the human diet (Denardin et al., 2015). Table 2 shows the phenolic compounds profile found in berry pulp and bagasse. Among the phenolic compounds of berries from the Amazon region identified and quantified with UHPLC-DAD, goldenberry pulp contains the highest amount of gallic acid (178.71 ± 3.88 μg/gfw). This is important if we consider the Corazza et al. (2018) findings, who reported that Brazilian goldenberry had a higher AC due to its high content of gallic acid. Respect to catechin and epicatechin, goldenberry bagasse and pulp contain 178.71 ± 3.88 and 144.69 ± 0.46 μg/gfw, respectively. Olivares-Tenorio et al. (2017) reports 16 ± 0.3 and 23 ± 0.4 μg/gfw of catechin and epicatechin, respectively, in goldenberry from Colombia, which are much lower than those found in Amazonian goldenberry. Elderberry bagasse contains the highest amount of catechin (153.32 ± 0.79 μg/gfw), chlorogenic acid (509.71 ± 1.11 μg/gfw) caffeic acid (44.50 ± 1.23 μg/gfw) and p-coumaric acid (58.13 ± 0.12 μg/gfw) (Table 2). These values are higher than the reported by Jakobek et al. (2007) in elderberry form Croatia (15.84 ± 0.2 and 10.80 ± 0.2 μg/gfw for caffeic acid and p-coumaric acid, respectively). Ferreira et al. (2020) and Mudge et al. (2016), report chlorogenic acid values of 200 ± 0.1 μg/gfw and 107 μg/gfw for Portuguese and American elderberry, respectively. These values are lower than the found in all berries of the present work, both in bagasse and pulp.
Table 2.
Phenolic compounds profile in berries from Amazonas Region by UPLC-DAD expressed as μg/gfw.
| Sample | Gallic acid | Catechin | Chlorogenic acid | Caffeic acid | Epicatechin | p-coumaric acid |
|---|---|---|---|---|---|---|
| Bagasse | ||||||
| Blueberry | 103.26 ± 1.59a | 89.96 ± 1.76b | 1276.55 ± 1.86a | 144.46 ± 1.78a | 1113.88 ± 1.82a | 77.82 ± 1.92a |
| Elderberry | Nd | 153.32 ± 0.79a | 509.71 ± 1.11b | 44.50 ± 1.23b | 984.14 ± 1.55b | 58.13 ± 0.12b |
| Blackberry | 72.44 ± 0.21b | Nd | 111.11 ± 0.12c | Nd | Nd | 60.01 ± 0.62b |
| Goldenberry |
Nd |
Nd |
108.44 ± 0.83d |
Nd |
144.69 ± 0.46c |
59.60 ± 0.33b |
| Pulp | ||||||
| Blueberry | 95.26 ± 1.46a | 89.45 ± 0.78b | 1071.90 ± 1.35a | 67.26 ± 0.99a | 970.16 ± 1.41a | 71.20 ± 0.40a |
| Elderberry | Nd | 129.67 ± 1.54a | 429.50 ± 1.34b | 27.43 ± 1.46b | 597.90 ± 1.65b | 58.94 ± 0.39c |
| Blackberry | Nd | Nd | 111.17 ± 0.95c | Nd | Nd | 60.39 ± 0.61b |
| Goldenberry | Nd | Nd | Nd | Nd | 112.20 ± 1.65c | 59.12 ± 0.86c |
Means with different letters within the same column are significantly different at p < 0.05. Tukey's test was apply separately on bagasse and pulp. Nd, not detected.
Blueberry bagasse contains higher amounts of gallic acid (103.26 ± 1.59 μg/gfw), chlorogenic acid (1276.55 ± 1.86 μg/gfw), caffeic acid (144.46 ± 1.78 μg/gfw), epicatechin (1113.88 ± 1.82 μg/gfw) and p-coumaric acid (77.82 ± 1.92 μg/gfw) (Table 2). Cesa et al. (2017) reports 18.2 ± 0.6 μg/gfw of chlorogenic acid in blueberry, lower values than those reported in this work. Sellappan et al. (2002) reports that blueberries (Vaccinium ashei Reade) from Georgia have gallic acid (15.3–2589 μg/gfw), caffeic acid (24–63.2 μg/gfw), p-coumaric acid (37.8–157.8 μg/gfw), catechin (145.3–3874.8 μg/gfw), epicatechin (342.3–1295.1 μg/gfw). Then the values of phenolic compounds found in the blueberry bagasse are within the parameters reported by Sellappan et al. (2002). The opposite occurs with blackberry, because the values found in the present work for gallic acid and p-coumaric acid are higher than those reported by Sellappan et al. (2002) (41.2–64.2 and 0.4–2.08 μg/gfw, respectively). Gündoğdu et al. (2016) reports for Turkish blackberry catechin values (1115.99 ± 14.72 to 4389.70 ± 50.74 μg/gfw) higher than those found in the blackberry bagasse of the present work and lower values (5.04 ± 0.07 to 11.75 ± 0.24 μg/gfw) of chlorogenic acid.
The difference between the TPC of berries can be due to several factors, one of them is the extraction, which can cause degradation of phenolic compounds (Domínguez et al., 2020). Also, several intrinsic (genetic factors and the degree of fruit ripeness) and extrinsic (environmental and climatic conditions that include exposure to different levels of radiation and wind, temperature, water availability, soil composition, and other agronomic factors) factors of plants influence the amount and type of phenolic compounds (Ferreira et al., 2020; Dag et al., 2017; Salvador et al., 2015). This is the reason why variability was found between the amount and type of phenolic compounds in the berries analyzed (Table 2). Our results coincide with those obtained by (Ferreira et al., 2020), who reports 0.02 ± 0.01 g/100 gdw of chlorogenic acid in Portuguese elderberry harvested in the first year and 0.10 ± 0.08 g/100 gdw of the same elderberry in the third year.
4. Conclusion
Results obtained in this study show that the four berries grown in the Amazonas region of Peru represent a very interesting source of antioxidant compounds contained mainly in bagasse. Elderberry bagasse had a greater amount of total phenolic content and antioxidant capacity than the bagasse and pulp of the other berries, with a strong positive correlation between both bioactive properties. Elderberry bagasse contains the highest amount of catechin, and blueberry bagasse contains the highest amount of gallic acid, chlorogenic acid, caffeic acid, epicatechin and p-coumaric acid. No significant differences were found in the content of chlorogenic acid and epicatechin of blackberry.
Declarations
Author contribution statement
Elizabeth Rojas-Ocampo: Performed the experiments; Wrote the paper.
Llisela Torrejón-Valqui, Marleni Medina-Mendoza: Performed the experiments.
Lucas D. Muñóz-Astecker: Contributed reagents, materials, analysis tools or data.
Diner Mori-Mestanza: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Efraín M. Castro-Alayo: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the World Bank Group through the Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica (Fondecyt) of the Peruvian government, and the Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank to Giuliana Díaz Pérez and Pamela Roxana Pizango Corman, monitors of the Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica (Fondecyt), for providing us with facilities in the execution of the Berries project.
References
- Acosta-Montoya Ó., Vaillant F., Cozzano S., Mertz C., Pérez A.M., Castro M.V. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chem. 2010;119:1497–1501. [Google Scholar]
- Agencia Agraria de Noticias . 2021. En Perú se instalan cada año 2 mil hectáreas de arándanos.https://agraria.pe/noticias/en-peru-se-instalan-cada-ano-2-mil-hectareas-de-arandanos-22151 [WWW Document]. Agrar. Agencia Agrar. Not. URL. (accessed 6.12.21) [Google Scholar]
- Bora P., Ragaee S., Abdel-Aal E.-S.M. Effect of incorporation of goji berry by-product on biochemical, physical and sensory properties of selected bakery products. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2019;112:108225. [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 1995;28:25–30. [Google Scholar]
- Buratto R.T., Cocero M.J., Martín Á. Characterization of industrial açaí pulp residues and valorization by microwave-assisted extraction. Chem. Eng. Process. - Process Intensif. 2021;160:108269. [Google Scholar]
- Cádiz-Gurrea de la L M., Villegas-Aguilar del C M., Leyva-Jiménez F.J., Pimentel-Moral S., Fernández-Ochoa Á., Alañón M.E., Segura-Carretero A. Revalorization of bioactive compounds from tropical fruit by-products and industrial applications by means of sustainable approaches. Food Res. Int. 2020;138:109786. doi: 10.1016/j.foodres.2020.109786. [DOI] [PubMed] [Google Scholar]
- Cardeñosa V., Girones-Vilaplana A., Muriel J.L., Moreno D.A., Moreno-Rojas J.M. Influence of genotype, cultivation system and irrigation regime on antioxidant capacity and selected phenolics of blueberries (Vaccinium corymbosum L.) Food Chem. 2016;202:276–283. doi: 10.1016/j.foodchem.2016.01.118. [DOI] [PubMed] [Google Scholar]
- Cesa S., Carradori S., Bellagamba G., Locatelli M., Casadei M.A., Masci A., Paolicelli P. Evaluation of processing effects on anthocyanin content and colour modifications of blueberry (Vaccinium spp.) extracts: comparison between HPLC-DAD and CIELAB analyses. Food Chem. 2017;232:114–123. doi: 10.1016/j.foodchem.2017.03.153. [DOI] [PubMed] [Google Scholar]
- Chaves V.C., Boff L., Vizzotto M., Calvete E., Reginatto F.H., Simões C.M. Berries grown in Brazil: anthocyanin profiles and biological properties: chemical composition of berries grown in Brazil. J. Sci. Food Agric. 2018;98:4331–4338. doi: 10.1002/jsfa.8959. [DOI] [PubMed] [Google Scholar]
- Coklar H., Akbulut M. Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J. Funct. Foods. 2017;35:166–174. [Google Scholar]
- Corazza G.O., Bilibio D., Zanella O., Nunes A.L., Bender J.P., Carniel N., dos Santos P.P., Priamo W.L. Pressurized liquid extraction of polyphenols from Goldenberry: influence on antioxidant activity and chemical composition. Food Bioprod. Process. 2018;112:63–68. [Google Scholar]
- Dag D., Kilercioglu M., Oztop M.H. Physical and chemical characteristics of encapsulated goldenberry (Physalis peruviana L.) juice powder. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;83:86–94. [Google Scholar]
- de Souza V.R., Pereira P.A.P., da Silva T.L.T., de Oliveira Lima L.C., Pio R., Queiroz F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014;156:362–368. doi: 10.1016/j.foodchem.2014.01.125. [DOI] [PubMed] [Google Scholar]
- Demir N., Yildiz O., Alpaslan M., Hayaloglu A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2014;57:126–133. [Google Scholar]
- Denardin C.C., Hirsch G.E., da Rocha R.F., Vizzotto M., Henriques A.T., Moreira J.C.F., Guma F.T.C.R., Emanuelli T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J. Food Drug Anal. 2015;23:387–398. doi: 10.1016/j.jfda.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez R., Zhang L., Rocchetti G., Lucini L., Pateiro M., Munekata P.E.S., Lorenzo J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020;330:127266. doi: 10.1016/j.foodchem.2020.127266. [DOI] [PubMed] [Google Scholar]
- Ferreira S.S., Silva P., Silva A.M., Nunes F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: a three-year study. Food Chem. 2020;302:125366. doi: 10.1016/j.foodchem.2019.125366. [DOI] [PubMed] [Google Scholar]
- Fu L., Xu B.-T., Xu X.-R., Gan R.-Y., Zhang Y., Xia E.-Q., Li H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- Giovanelli G., Buratti S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009;112:903–908. [Google Scholar]
- Gowd V., Bao T., Chen W. Antioxidant potential and phenolic profile of blackberry anthocyanin extract followed by human gut microbiota fermentation. Food Res. Int. 2019;120:523–533. doi: 10.1016/j.foodres.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Gündoğdu M., Kan T., Canan İ. Bioactive and antioxidant characteristics of blackberry cultivars from East Anatolia. Turk. J. Agric. For. 2016;40:344–351. [Google Scholar]
- Instituto Nacional de Innovación Agraria . 2021. Agricultores de Piura mejoran producción de aguaymanto con uso de semillas de alta calidad – Instituto Nacional de Innovación Agraria.https://www.inia.gob.pe/2020-nota-054/ URL. (accessed 6.12.21) [Google Scholar]
- Jakobek L., Seruga M., Novak I., Medvidovic-Kosanovic M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensm.-Rundsch. 2007;103(8):369–377. [Google Scholar]
- Jiang Y., Fang Z., Leonard W., Zhang P. Phenolic compounds in Lycium berry: composition, health benefits and industrial applications. J. Funct. Foods. 2021;77:104340. [Google Scholar]
- Jimenez-Garcia S.N., Guevara-Gonzalez R.G., Miranda-Lopez R., Feregrino-Perez A.A., Torres-Pacheco I., Vazquez-Cruz M.A. Functional properties and quality characteristics of bioactive compounds in berries: biochemistry, biotechnology, and genomics. Food Res. Int. 2013;54:1195–1207. [Google Scholar]
- Kelebek H. LC-DAD–ESI-MS/MS characterization of phenolic constituents in Turkish black tea: effect of infusion time and temperature. Food Chem. 2016;204:227–238. doi: 10.1016/j.foodchem.2016.02.132. [DOI] [PubMed] [Google Scholar]
- Kiprovski B., Malenčić Đ., Ljubojević M., Ognjanov V., Veberic R., Hudina M., Mikulic-Petkovsek M. Quality parameters change during ripening in leaves and fruits of wild growing and cultivated elderberry (Sambucus nigra) genotypes. Sci. Hortic. 2021;277:109792. [Google Scholar]
- Koca I., Karadeniz B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea Region of Turkey. Sci. Hortic. 2009;121:447–450. [Google Scholar]
- Luchese C.L., Uranga J., Spada J.C., Tessaro I.C., de la Caba K. Valorisation of blueberry waste and use of compression to manufacture sustainable starch films with enhanced properties. Int. J. Biol. Macromol. 2018;115:955–960. doi: 10.1016/j.ijbiomac.2018.04.162. [DOI] [PubMed] [Google Scholar]
- Machado da A.P.F., Geraldi M.V., do Nascimento de R.P., Moya A.M.T.M., Vezza T., Diez-Echave P., Gálvez J.J., Cazarin C.B.B., Maróstica Júnior M.R. Polyphenols from food by-products: an alternative or complementary therapy to IBD conventional treatments. Food Res. Int. 2021;140:110018. doi: 10.1016/j.foodres.2020.110018. [DOI] [PubMed] [Google Scholar]
- Martín-Gómez J., Varo M.Á., Mérida J., Serratosa M.P. Influence of drying processes on anthocyanin profiles, total phenolic compounds and antioxidant activities of blueberry (Vaccinium corymbosum) Lebensm. Wiss. Technol. 2020;120:108931. [Google Scholar]
- Mokhtar S.M., Swailam H.M., Embaby H.E.-S. Physicochemical properties, nutritional value and techno-functional properties of goldenberry ( Physalis peruviana) waste powder concise title: composition of goldenberry juice waste. Food Chem. 2018;248:1–7. doi: 10.1016/j.foodchem.2017.11.117. [DOI] [PubMed] [Google Scholar]
- Moraes D.P., Lozano-Sánchez J., Machado M.L., Vizzotto M., Lazzaretti M., Leyva-Jimenez F.J.J., da Silveira T.L., Ries E.F., Barcia M.T. Characterization of a new blackberry cultivar BRS Xingu: chemical composition, phenolic compounds, and antioxidant capacity in vitro and in vivo. Food Chem. 2020;322:126783. doi: 10.1016/j.foodchem.2020.126783. [DOI] [PubMed] [Google Scholar]
- Mudge E., Applequist W.L., Finley J., Lister P., Townesmith A.K., Walker K.M., Brown P.N. Variation of select flavonols and chlorogenic acid content of elderberry collected throughout the Eastern United States. J. Food Compos. Anal. 2016;47:52–59. doi: 10.1016/j.jfca.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocetti D., Núñez H., Puente L., Espinosa A., Romero F. Composition and biological effects of goldenberry byproducts: an overview. J. Sci. Food Agric. 2020;100:4335–4346. doi: 10.1002/jsfa.10386. [DOI] [PubMed] [Google Scholar]
- Olivares-Tenorio M.-L., Verkerk R., van Boekel M.A.J.S., Dekker M. Thermal stability of phytochemicals, HMF and antioxidant activity in cape gooseberry (Physalis peruviana L .) J. Funct. Foods. 2017;32:46–57. [Google Scholar]
- Ordóñez-Santos L.E., Martínez-Girón J., Arias-Jaramillo M.E. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017;233:96–100. doi: 10.1016/j.foodchem.2017.04.114. [DOI] [PubMed] [Google Scholar]
- Ozturk A., Ozdemi̇r Y., Albayrak B., Si̇msek M., Yildirim K.C. Some nutrient characteristics of goldenberry (Physalis peruviana L.) cultivar candidate from Turkey. Horticulture LXI. 2017:293–297. [Google Scholar]
- Pasquel Reátegui J.L., Machado da A.P.F., Barbero G.F., Rezende C.A., Martínez J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids. 2014;94:223–233. [Google Scholar]
- Pfukwa T.M., Chikwanha O.C., Katiyatiya C.L.F., Fawole O.A., Manley M., Mapiye C. Southern African indigenous fruits and their byproducts: prospects as food antioxidants. J. Funct. Foods. 2020;75:104220. [Google Scholar]
- Prencipe F.P., Bruni R., Guerrini A., Rossi D., Benvenuti S., Pellati F. Metabolite profiling of polyphenols in Vaccinium berries and determination of their chemopreventive properties. J. Pharmaceut. Biomed. Anal. 2014;89:257–267. doi: 10.1016/j.jpba.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Puente L.A., Pinto-Muñoz C.A., Castro E.S., Cortés M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: a review. Food Res. Int. 2011;44:1733–1740. [Google Scholar]
- Ramadan M.F. Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): an overview. Food Res. Int. 2011;44:1830–1836. [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Salvador Â.C., Rocha S.M., Silvestre A.J.D. Lipophilic phytochemicals from elderberries (Sambucus nigra L.): influence of ripening, cultivar and season. Ind. Crop. Prod. 2015;71:15–23. [Google Scholar]
- Sariburun E., Şahin S., Demir C., Türkben C., Uylaşer V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010;75:C328–C335. doi: 10.1111/j.1750-3841.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- Sasmaz H.K., Uzlasir T., Kelebek H. Effect of infusion time on the phenolic profile and some physicochemical properties of Lavandula x intermedia cv. “SUPER. J Raw Mater Process Foods. 2020;1:55–71. [Google Scholar]
- Sellappan S., Akoh C.C., Krewer G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002;50:2432–2438. doi: 10.1021/jf011097r. [DOI] [PubMed] [Google Scholar]
- Sério S., Rivero-Pérez M.D., Correia A.C., Jordão A.M., González-San José M.L. Analysis of commercial grape raisins: phenolic content, antioxidant capacity and radical scavenger activity. Ciência Técnica Vitivinícola. 2014;29:1–8. [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Methods in Enzymology. Elsevier; 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent; pp. 152–178. [Google Scholar]
- Umdale S., Ahire M., Aiwale V., Jadhav A., Mundada P. Phytochemical investigation and antioxidant efficacy of wild, underutilized berries of economically important Indian Sandalwood (Santalum album L.) Biocatal. Agric. Biotechnol. 2020;27:101705. [Google Scholar]
- Vasco C., Ruales J., Kamal-Eldin A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008;111:816–823. [Google Scholar]
- Vázquez-González M., Fernández-Prior Á., Bermúdez Oria A., Rodríguez-Juan E.M., Pérez-Rubio A.G., Fernández-Bolaños J., Rodríguez-Gutiérrez G. Utilization of strawberry and raspberry waste for the extraction of bioactive compounds by deep eutectic solvents. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2020;130:109645. [Google Scholar]
- Wang L.-J., Wu J., Wang H.-X., Li S.-S., Zheng X.-C., Du H., Xu Y.-J., Wang L.-S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods. 2015;16:295–304. [Google Scholar]
- You Q., Wang B., Chen F., Huang Z., Wang X., Luo P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011;125:201–208. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.