Abstract
Global bottled water consumption has largely increased (14.35 billion gallons in 2020) [1], [2], [3], [4], [5] during the last decade since consumers are demanding healthier and safer forms of rehydration. Bottled water sources are normally labeled as mountainous and pristine mineral springs (fed by rainfall and snow/glacier melting processes), deep groundwater wells or industrial purified water. The advent of numerous international and national-based bottled water brands has simultaneously raised a worldwide awareness related to the water source and chemical content traceability [6]. Here, we present the first database of stable isotope compositions and reported chemical concentrations from imported and national-based bottled waters in Costa Rica. In total, 45 bottled waters produced in Costa Rica and 31 imported from USA, Europe, Oceania, and other countries of Central America were analyzed for δ18O, δ2H, and d-excess. Chemical compositions were obtained from available bottle labels. National-based bottle waters ranged from -2.47‰ to -10.65‰ in δ18O and from -10.4‰ to -78.0‰ in δ2H, while d-excess varied from +4.2‰ up to +17.0‰. International bottle waters ranged between -2.21‰ and -11.03‰ in δ18O and from -11.3‰ up to -76.0‰ in δ2H, while d-excess varied from +5.0‰ up to +19.1‰. In Costa Rica, only 19% of the brands reported chemical parameters such as Na+, K+, Ca+2, Mg+2, F−, Cl−, NO3−, SO4−2, CO3−2, SiO2, dry residue, and pH; whereas 27% of the international products reported similar parameters. The absence of specific geographic coordinates or water source origin limited a spatial analysis to validate bottled water isotope compositions versus available isoscapes in Costa Rica [7]. This database highlights the potential and relevance of the use of water stable isotope compositions to improve the traceability of bottled water sources and the urgent need of more robust legislation in order to provide detailed information (i.e., water source, chemical composition, purification processes) to the final consumers.
Keywords: Imported and national-based bottled waters, Water stable isotopes, Chemical compositions, Water sources, Recharge elevations, Traceability
Specifications Table
| Subject | Analytical Chemistry, Environmental Chemistry, Hydrology, Water Resources Management. |
| Specific subject area | Stable isotope compositions in bottled waters |
| Type of data | Graphs and Tables |
| How data were acquired | Laser spectroscopy for water stable isotopes analysis with an IWA-45EP water analyzer (Los Gatos Research, Inc., California, USA). |
| Data format | Raw Analyzed |
| Parameters for data collection | Imported and national-based bottled waters were classified as natural mineral water, spring water, purified water and not specified waters. Chemical compositions were extracted from available bottle labels, the parameters included: Na+, K+, Ca+2, Mg+2, F−, Cl−, NO3−, SO4−2, CO3−2, SiO2, dry residue, and pH. National-based samples were coded from CR1 to CR45; while imported samples were coded from FW1 to FW31. |
| Description of data collection | Bottled waters were purchased across a broad range of commercial stores in Costa Rica, Honduras, Panamá, Guatemala, and El Salvador. Samples were classified as national-based (Costa Rica), imported from USA, Europe, Oceania, and other countries of Central America. All samples were sealed and refrigerated at 5 °C until analysis at the Stable Isotopes Research Group, Universidad Nacional (Heredia, Costa Rica). |
| Data source location | Institution: Universidad Nacional City/Town/Region: Heredia Country: Costa Rica Latitude and longitude (and GPS coordinates) for collected samples/data: 10.094533, −84.058700 Elevation: 1153m asl. |
| Data accessibility | Repository name: https://www.hydroshare.org/ Data identification number: 10.4211/hs.86225d08252747d5a78477c1f74eb158 Direct URL to data: https://www.hydroshare.org/resource/86225d08252747d5a78477c1f74eb158/ |
| Related research article | Sánchez‐Murillo, R. and C. Birkel, C, Groundwater recharge mechanisms inferred from isoscapes in a complex tropical mountainous region. Geophysical Research Letters. 43 (2016) 5060–5069. https://doi.org/10.1002/2016GL068888 |
Value of the Data
-
•
Our information provides the first database of stable isotope compositions and reported (from bottled water labels) chemical concentrations from imported and national-based bottled waters in Costa Rica.
-
•
Bottled water isotopic compositions may be used in concert with available isoscapes to assist with the traceability of water sources used in the industry of bottled waters across the Central America region.
-
•
Our data revealed a large degree of inconsistency in reporting chemical compositions both in national-based and imported bottled waters.
-
•
This database highlights the urgent need of more robust legislation for the bottled water industry worldwide in order to provide detailed information to the final consumers related-but not limited-to water sources (origin and geographic coordinates), chemical compositions (a complete spectrum of major ion contents), and clarity of the purification processes used.
1. Data Description
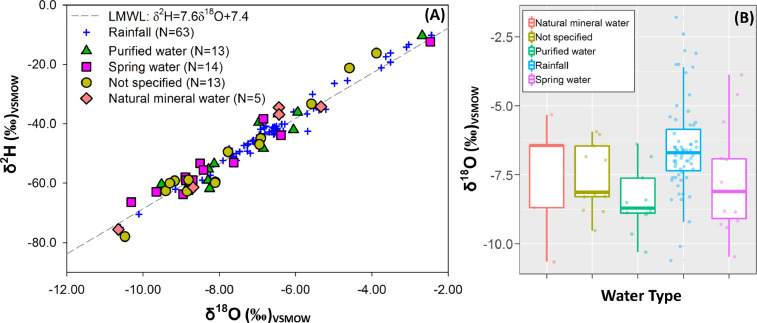
Fig. 1A shows a dual water isotope diagram including bottled waters classified as Purified Water, Spring Water, Natural Mineral Water, and samples in which the water type was Not Specified (i.e., unclear potential source). Overall, all samples exhibited a strong meteoric origin [7,8]. Based on available isoscapes of Costa Rica and recent monitoring efforts [7], [8], [9], only 4 bottled waters were in the range of typically enriched Caribbean-type water -2‰ to -4‰ in δ18O; Fig 1A), while 41 samples revealed Pacific-type δ18O compositions (ranging from -6 up to -11‰; Fig. 1B). Purified, Spring, and Not Specified bottled waters exhibited depleted δ18O values, confirming the strong bias and industry preference towards Pacific-type water sources [9]. Deuterium excess values ranged from + 4.2‰ up to + 17.0‰, with a mean value of + 11.0‰, which are in the range of rainfall across Costa Rica [7], [8], [9], [10], [11]. Potential source elevation (in m asl) were evaluated using an available isotopic lapse rate for Costa Rica derived from precipitation weighted mean annual δ18O compositions. Based on precipitation amounts, average annual weighted ratios were calculated across 63 monitoring stations [7,8]. The exisiting isotope monitoring network in precipitation provides a reliable spatial distibution across different climatic zones, elevation gradient, and biomes. For δ18O in rainfall, the altitude effect across the country averaged −1.4 ‰ per 1 km increased for stations above ∼340 m of elevation (r2 = 0.43, P < 0.001). . In general, potential source elevations ranged from 233 up to 3386 m asl, with a mean value of 2020 m asl, which in turn reflects the large dependency of high elevation recharge processes and spring discharges for the bottled water industry in Costa Rica (Fig. 2).
Fig. 1.
(A) Dual water isotope diagram including bottled waters classified as purified water (triangles), spring water (squares), natural mineral water (rhombi), and samples in which the water type appeared as not specified (circles). The LMWL of Costa Rica [8] was included as reference. Blue crosses denote the precipitation-weighted mean annual composition across 63 monitoring stations in Costa Rica [7,8]. (B) δ18O (in ‰) scattered/box plots per water type, including rainfall values of 63 monitoring stations in Costa Rica [7,8] as a reference. δ18O (in ‰) scattered/box plots include 25th, 75th, median, and outliers for each water type.
Fig. 2.
Potential elevation source (in m asl) for all national-based bottled waters in Costa Rica. The δ18O lapse rate (-1.4‰/km) was obtained from 63 monitoring stations in Costa Rica [7,8].
Fig. 3A shows a dual water isotope diagram including imported bottled waters from Europe (Norway, Italy, Spain, and France), North America (USA and Mexico), Oceania (Fiji Islands), and Central America (Guatemala, El Salvador, Honduras, and Panama). In general, imported bottled waters covered a similar δ18O range (from -2‰ up to -12‰). Deuterium excess values of imported bottled waters varied from + 5.0‰ to + 19.1‰, with mean of + 12.4‰. As expected, bottled waters from the mountainous regions of Europe exhibited lower δ18O compositions due to strong latitudinal and temperature effects (Fig. 3B) [13,14]. The median δ18O composition of bottled waters from Central America corresponded to rainfall/groundwater isotope compositions in the Pacific domain of this region (Fig. 3B) [12].
Fig. 3.
(A) Dual water isotope diagram including imported bottled waters classified as Purified Water (triangles), Spring Water (squares), and Natural Mineral Water (rhombi). The LMWL of Costa Rica [7,8] was included as reference. (B) δ18O (in ‰) scattered/box plots per region (one sample from Oceania was excluded). δ18O (in ‰) scattered/box plots include 25th, 75th, median, and outliers for each region.
The traceability of chemical compositions in bottled waters has been a matter of debate worldwide [15], [16], [17], [18]. Fig. 4 shows major ion concentrations, dry residue, and pH reported in bottled water labels. In Costa Rica, only 19% of the brands reported a combination of chemical parameters such as Na+, K+, Ca+2, Mg+2, Fe+2/+3, F−, Cl−, NO3−, SO4−2, CO3−2, SiO2, dry residue, and pH; whereas 27% of the international products reported similar parameters (Fig. 4). Imported bottled waters reported greater concentrations in Na+, K+, F−, Cl−, NO3−, SO4−2, CO3−2, and dry residue. Major recharge/discharge processes in mountainous volcanic aquifers in Costa Rica are represented by high silicate and low pH values (Fig. 4). In general, there is a large degree of inconsistency in reporting chemical compositions both in national-based and imported bottled waters. However, it is important to highlight that each country has different acceptable limits of the chemical compositions in bottled waters.
Fig. 4.
Reported chemical compositions of national-based and imported bottled waters in Costa Rica. All concentrations are expressed in mg/L (Si refers to SiO2). Scattered/box plots include 25th, 75th, median, and outliers for each group.
2. Experimental design, materials and methods
2.1. Sample collection
National-based (N = 45) and imported (N = 31) bottled water samples were purchased between 2019–2020 across a broad range of commercial stores (all bottles were in perfect condition) in Costa Rica, Honduras, Panamá, Guatemala, and El Salvador. Samples were classified as national-based (Costa Rica), imported from USA, Europe, Oceania, and other countries of Central America. As this type of sampling is opportunistic, seasonal effects are not included in our analysis. All samples were sealed with parafilm and refrigerated at 5 °C until analysis at the Stable Isotopes Research Group, Universidad Nacional (Heredia, Costa Rica). Chemical compositions were extracted from available bottle labels, the parameters included: Na+, K+, Ca+2, Mg+2, Fe+2/+3, F−, Cl−, NO3−, SO4−2, CO3−2, SiO2, dry residue, and pH. National-based samples were coded from CR1 to CR45; while imported samples were coded from FW1 to FW31. All information is available at https://www.hydroshare.org/resource/86225d08252747d5a78477c1f74eb158/ [11]. CUASI´s hydrological repository Hydroshare (https://www.hydroshare.org/) is an online platform to share data, models, and code.
2.2. Stable Isotopes analysis
Samples were analyzed at the Stable Isotopes Research Group laboratory at the Universidad Nacional (Heredia, Costa Rica) using an IWA-45EP water analyzer (Los Gatos Research, Inc., California, USA) with a precision of ± 0.5‰ for δ2H and ± 0.1‰ for δ18O (1σ). Stable isotope compositions are expressed as δ18O or δ2H = (Rs/Rstd - 1)•1000, where R is the 18O/16O or 2H/1H ratio in a sample (s) or standard (std) and reported in the delta-notation (‰) relative to V-SMOW/SLAP scale. The instrument accuracy was assessed with a combination of in-house and primary international water standards (SMOW and SLAP). Deuterium excess was calculated as d-excess = δ2H - 8•δ18O [19].
CRediT Author Statement
Ricardo Sánchez-Murillo: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft; Germain Esquivel-Hernández: Writing – review & editing, Writing – original draft; Christian Birkel: Writing – review & editing, Writing – original draft; Lucía Ortega: Writing – review & editing, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that have, or could be perceived to have, influenced the work reported in this article.
Acknowledgments
This work was partially supported by International Atomic Energy Agency grants to R.S.M: Stable isotopes in precipitation and paleoclimatic archives in tropical areas to improve regional hydrological and climatic impact models (CRP-19747) and Isotope Techniques for the Evaluation of Water Sources for Domestic Water Supply in Urban Areas (CRP- F33024). Analytical instrumental support from the IAEA Technical Cooperation Project (COS7005: Ensuring water security and sustainability of Costa Rica) is also acknowledged. Support from the Research Office of the Universidad Nacional of Costa Rica through grants SIA-0482-13, SIA-0378-14, SIA-0101-14, and SIA-0332-18 was also fundamental. The authors thank various helping hands that contributed to bottled water sampling across Costa Rica and Central America.
References
- 1.Beverage Marketing Corporation. Bottled Water in the US Through 2024. https://www.beveragemarketing.com/shop/bottled-water-reports.aspx, 2021 (accessed 21 May 2021).
- 2.Guo R., Wang S., Zhang M., Argiriou A.A., Liu X., Su B., Qiu X., Jiao R., Shi M., Zhou S., Zhang Y. Stable hydrogen and oxygen isotope characteristics of bottled water in china: a consideration of water source. Water. 2019;11:1065. doi: 10.3390/w11051065. [DOI] [Google Scholar]
- 3.Porowski A., Romanova A., Gebus-Czupyt B., Wach B., Radzikowska M. Stable hydrogen and oxygen isotopic composition of bottled waters in Poland: characterization in the context of different market categories and implications for the origin authentication and natural isotopic quality preservation. J. Geochem. Explor. 2021;220 doi: 10.1016/j.gexplo.2020.106684. [DOI] [Google Scholar]
- 4.W. Al-Basheer, A. Al-Jalal, K. Gasmi, Isotopic composition of bottled water in Saudi Arabia, Isotopes Environ. Health Stud. 54 (2018) 106–112. doi: 10.1080/10256016.2017.1377195. [DOI] [PubMed]
- 5.Nakano T., Yamashita K., Ando A., Kusaka S., Saitoh Y. Geographic variation of Sr and S isotope ratios in bottled waters in Japan and sources of Sr and S. Sci. Total Environ. 2020;704 doi: 10.1016/j.scitotenv.2019.135449. [DOI] [PubMed] [Google Scholar]
- 6.Olsen P., Borit M. The components of a food traceability system. Trends Food Sci. Technol. 2018;77:143–149. doi: 10.1016/j.tifs.2018.05.004. [DOI] [Google Scholar]
- 7.Sánchez-Murillo R., Birkel C. Groundwater recharge mechanisms inferred from isoscapes in a complex tropical mountainous region. Geophys. Res. Lett. 2016;43:5060–5069. doi: 10.1002/2016GL068888. [DOI] [Google Scholar]
- 8.Sánchez-Murillo R., Esquivel-Hernández G., Welsh K., Brooks E., Boll J., Alfaro-Solís R., Valdés-González J. Spatial and temporal variation of stable isotopes in precipitation across costa rica: an analysis of historic GNIP records. Open J. Mod. Hydrol. 2013;3:226–240. doi: 10.4236/ojmh.2013.34027. [DOI] [Google Scholar]
- 9.Sánchez-Murillo R., Esquivel-Hernández G., Birkel C., Correa A., Welsh K., Durán-Quesada A.M., Sánchez-Gutiérrez R., Poca M. Tracing water sources and fluxes in a dynamic tropical environment: from observations to modeling. Front. Earth Sci. 2020;8 doi: 10.3389/feart.2020.571477. [DOI] [Google Scholar]
- 10.Sánchez-Murillo R., Durán-Quesada A.M., Birkel C., Esquivel-Hernández G., Boll J. Tropical precipitation anomalies and d-excess evolution during El Niño 2014-16. Hydrol. Process. 2017;31:956–967. doi: 10.1002/hyp.11088. [DOI] [Google Scholar]
- 11.R. Sánchez-Murillo, (2021). Isotopic composition and major ion concentrations of national and international bottled waters in Costa Rica, HydroShare, doi: 10.4211/hs.86225d08252747d5a78477c1f74eb158. [DOI] [PMC free article] [PubMed]
- 12.Sánchez-Murillo R., Esquivel-Hernández G., Corrales-Salazar J.L., Castro-Chacón L., Durán-Quesada A.M., Guerrero-Hernández M., Delgado V., Barberena J., Montenegro-Rayo K., Calderón H., Chevez C. Tracer hydrology of the data-scarce and heterogeneous Central American isthmus. Hydrol. Process. 2020;34:2660–2675. doi: 10.1002/hyp.13758. [DOI] [Google Scholar]
- 13.Stefan T.W., Wassenaar L., Welker J., Araguás L. Improved high-resolution global and regionalized isoscapes of δ18O, δ2H and d-excess in precipitation. Hydrol. Process. 2021;35:e14254. [Google Scholar]
- 14.Nelson D.B., Basler D., Kahmen A. Proceedings of the National Academy of Sciences. Vol. 118. 2021. Precipitation isotope time series predictions from machine learning applied in Europe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levêque J.G., Burns R.C. Drinking water in West Virginia (USA): tap water or bottled water–what is the right choice for college students? J. Water Health. 2018;16:827–838. doi: 10.2166/wh.2018.129. [DOI] [PubMed] [Google Scholar]
- 16.Daniele L., Cannatelli C., Buscher J.T., Bonatici G. Chemical composition of Chilean bottled waters: anomalous values and possible effects on human health. Sci. Total Environ. 2019;689:526–533. doi: 10.1016/j.scitotenv.2019.06.165. [DOI] [PubMed] [Google Scholar]
- 17.Yilkal E., Zewge F., Chandravanshi B.S. Assessment of the quality of bottled water marketed in Addis Ababa, Ethiopia. Bull. Chem. Soc. Ethiop. 2019;33:21–41. doi: 10.4314/bcse.v33i1.3. [DOI] [Google Scholar]
- 18.Zuliani T., Kanduč T., Novak R., Vreča P. Characterization of bottled waters by multielemental analysis, stable and radiogenic isotopes. Water. 2020;12:2454. doi: 10.3390/w12092454. [DOI] [Google Scholar]
- 19.Dansgaard W. Stable isotopes in precipitation. Tellus. 1964;16:436–468. doi: 10.1111/j.2153-3490.1964.tb00181.x. [DOI] [Google Scholar]