Abstract
Determining the cause of gastrointestinal bleeding is critical to determining appropriate treatment. Upper gastrointestinal bleeding from the pancreas, referred to hemosuccus pancreaticus, is a rare entity that can cause massive and life-threatening bleeding. Diagnosis remains challenging, and the mortality rate of hemosuccus pancreaticus remains high, ranging from 9.6%–90%. In this article, we present a case that was successfully diagnosed and treated at the Department of General Surgery, Hanoi Medical University Hospital, and a review of the available literature regarding this rare disease.
Keywords: Intraductal papillary mucinous neoplasm, Hemosuccus pancreaticus, Upper gastrointestinal bleeding
Introduction
Hemosuccus pancreaticus (HP) is a very rare cause of upper gastrointestinal (GI) bleeding and accounts for only 1/1,500 cases; however, HP can lead to massive bleeding events that are life-threatening [1]. HP is defined as bleeding that occurs through the pancreatic duct into the duodenum. Due to its rarity, diagnosis is often difficult. Only approximately 150 clinical cases have been reported in the English-language literature to date [2]. HP secondary to pancreatic tumor is extremely rare. We present a case of HP associated with an intraductal papillary mucinous tumor (IPMN) located in the head of the pancreas.
Case report
A 61-year-old male patient was admitted to the hospital because of melena. The patient had a history of chronic obstructive pulmonary disease for two years and experienced melena 1–2 times/day for 3 days without hematemesis. At the time of admission, his vital signs were stable, with a heart rate of 75 beats/min and blood pressure of 120/70 mmHg. The patient did not present with anemia, fever, dyspnea, enlarged lymph nodes, subcutaneous hemorrhage, abdominal pain, or jaundice. Physical abdominal examination showed that the abdomen was soft, with no palpable mass, no enlarged gallbladder, and no hepatomegaly or splenomegaly. Rectal examination showed black stools. No other abnormalities were noted.
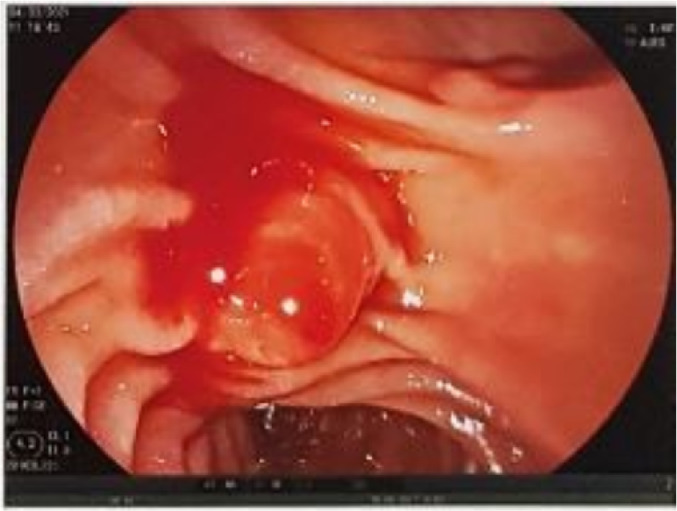
Complete blood count showed hematocrit 0.36%, hemoglobin (Hb) 123 g/L, white blood cell (WBC) 7.44 G/L, and platelets 239 G/L. Laboratory studies showed total bilirubin 8.5 µmol/L and CA19-9 12.3 U/mL. Upper GI endoscopy revealed fresh blood flowing out of the major papilla. The papilla surface was not ulcerative (Fig. 1). After the procedure, the patient's hemodynamic status remained stable, and the patient experienced melena once per day.
Fig. 1.
Upper GI endoscopy revealed fresh blood oozing from the papilla of the duodenum
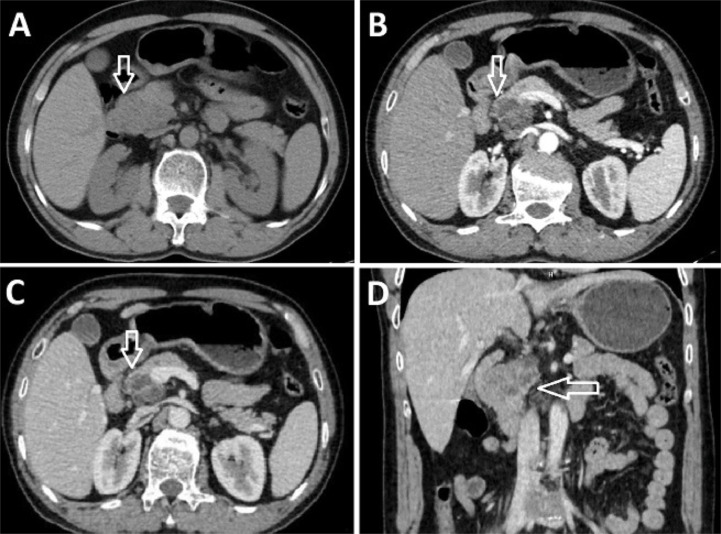
Ultrasound showed a hypoechoic mass in the head of the pancreas. Computed tomography (CT) examination of the abdomen showed a pancreatic head tumor, sized 45 × 33 mm, with poorly defined borders (Fig. 2). The tumor contained cystic and solid components, and the solid component enhanced poorly. No calcification or bleeding was observed within the tumor. The tumor had ill-defined borders and descended into the (D2) duodenum. No abdominal lymphadenopathy, pseudoaneurysm, or active bleeding was observed. The biliary and pancreatic ducts were normal.
Fig. 2.
CT images of the patient's abdomen. Axial images of the pancreatic head tumor in the non-contrast (A), arterial (B), and venous (C), and coronal venous phases (D) showed a tumor of the pancreatic head with solid and cystic components. The solid component showed poor enhancement
The CT findings suggested a pancreatic tumor with malignant transformation, leading to invasion of the duodenum. The patient was diagnosed with upper GI bleeding due to pancreatic head tumor invading the D2 duodenum and underwent pancreaticoduodenal resection.
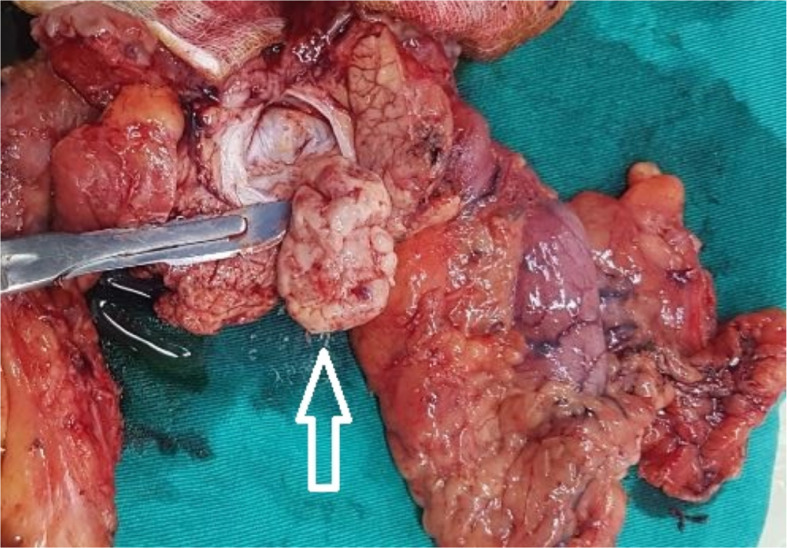
Intraoperative results revealed a small amount of free intraperitoneal fluid, smooth liver surface, and normal common bile duct and gallbladder. The head of the pancreas was adhesive to the adjacent tissue, with a tumor sized approximately 4 × 4 cm that did not invade the superior mesenteric vessels (Fig. 3). Abdominal lymph nodes were not enlarged. Pancreaticoduodenectomy was successfully performed.
Fig. 3.
Macroscopic image of the pancreatic duodenal mass specimen. The tumor appeared white in the head of the pancreas
On gross examination of the pancreaticoduodenectomy specimen, the transversal section through the papilla revealed a cystic 4 × 3 cm tumor that was continuous with the pancreatic duct, containing little soft white matter. Serial sectioning of the remaining pancreatic parenchyma showed no abnormalities. Microscopically, the cyst wall was lined with complex branching papillary structures, consisting of columnar mucin-secreting cells. The tumor cells showed a striking loss of polarity, with significant atypical nuclei. Associated invasive adenocarcinoma was also present in some small foci. All surgical margins and resected lymph nodes were negative. The pathological diagnosis was high-grade pancreatic IPMN with associated moderately differentiated invasive adenocarcinoma.
After surgery, the patient was treated with third-generation cephalosporins (cefoperazone 3 g/day), octreotide 200 µg/day, analgesia (fentanyl followed by paracetamol), and parenteral nutrition, with electrolyte balance maintenance. Clinical and hemodynamic parameters were stable, and the stool returned to a normal color on the fifth day after surgery. Abdominal drainages were removed 10 and 14 days after surgery, and the patient was discharged 15 days after surgery.
Discussion
Although HP was first reported in 1931, the first comprehensive description of HP was reported by Van Kemmel in 1969, who referred to this phenomenon as Wirsung ductal bleeding (Wirsungorrhagia, hemowirsungia) [3,4]. The term HP was coined 1 year later by Sandblom [3,4].
HP occurs more commonly in males, with a male/female ratio of 7:1, and is especially frequent in cases of prolonged alcohol consumption [5]. Clinical manifestations include upper GI bleeding (more commonly associated with melena than hematemesis) and upper abdominal pain due to the formation of blood clots that result in increased pressure on the pancreatic duct [5]. Bleeding is usually intermittent, repetitive, and most often not severe enough to cause hemodynamic changes [4]. Jaundice may occur due to blood reflux into the bile duct, in addition to vomiting and weight loss [4]. Patients may present with the signs and symptoms of anemia due to GI bleeding [5]. Most patients had a history of pancreatic disease, with pancreatitis being the most common [1]. According to Sul, a differential diagnosis of HP should be established in patients with intermittent upper GI bleeding who have a history of chronic alcohol abuse [6]. Without treatment, the mortality rate of HP can reach as high as 90% [4].
The source of pancreatic bleeding can be the pancreatic parenchyma, the pancreatic duct, or structures adjacent to the pancreas, such as the splenic artery or the arteries feeding the stomach [4]. The most common cause of pancreatic bleeding is pancreatitis [7]. Pancreatic tumors or trauma are rarer causes [5]. Pseudoaneurysms are common and are often associated with a more severe clinical presentation [5].
Pseudoaneurysm is a well-recognized cause of HP. The injured artery may be intrapancreatic or extrapancreatic (adjacent). The most common site of pseudoaneurysm in the context of pancreatitis is the splenic artery (60%–65%) [5]. Injuries to the hepatic artery, gastroduodenal artery, and pancreaticoduodenal artery have also been reported in the literature [5].
Pancreatitis can result in the corrosion of blood vessel walls by pancreatic fluid, leading to bleeding into the pancreatic duct [7,8]. Rarely, pancreatic pseudocysts or pancreatic stones can develop, causing an inflammatory reaction of the pancreatic duct, resulting in the sporadic bleeding of the pancreatic duct [8].
Pancreatic tumor is a rare cause of HP [9], and both benign and malignant tumors can cause bleeding in the pancreatic duct [5,10]. According to a meta-analysis by Ru, which included 153 patients, up to 83% of HP patients had pancreatitis, and only 16% had pancreatic tumors. Cystadenoma was the most common tumor, identified in 8 patients, whereas 4 patients (3.5%) were diagnosed with IPMN [2]. Carcinoma, lymphoma, neuroendocrine tumors (NETs), and pancreatic metastases can also cause HP [5,11].
IPMN is a cystic epithelial tumor of the pancreas that develops from the pancreatic duct [12]. IPMN tumors can be divided into two types: the main duct type and the branch duct type. The main duct type is often associated with chronic pancreatitis, tends to diffuse, is associated with dilated pancreatic ducts (containing mucin in the lumen), and has a high risk of malignancy (57%–92%)[13,14]. The branch duct type is often localized, especially within the head of the pancreas, and only 6%–46% are malignant [13,14]. The mean age of IPMN patients was 63.4 years among those with the branch duct form versus 57.2 years among those with the main duct form [13]. The tumor has a papillary structure and often secretes mucus [13]. When small, the lesion is usually brought to clinical attention as an incidental finding [13]. IPMN occurs more commonly in men than in women [14], and the most common site for IPMN the head of the pancreas [15].
The diagnosis of HP is often difficult because the clinical and laboratory tests are nonspecific. Upper GI endoscopy is important for excluding other causes of GI bleeding. In HP, bleeding from the duodenal papilla can be observed during endoscopy, as in our case; however, this symptom is only observable in 30% of patients because the bleeding is often intermittent [5]. Therefore, some authors recommend that endoscopy be repeated if no bleeding point is detected during the first procedure [5]. Endoscopic retrograde cholangiopancreatography (ERCP) is rarely indicated today but may show a pancreatic duct-filling defect, which is suggestive of a blood clot in the lumen of the pancreatic duct, or may reveal the leakage of contrast material outside of the pancreatic duct [5]. Imaging methods, especially abdominal CT, have high value for the evaluation of the pancreas, vascular lesions, and determining the cause of bleeding [1]. The imaging findings of a blood clot in the pancreatic duct (sentinel clot) are quite specific, but the sensitivity is low, and our case did not present with this sign [4]. Magnetic resonance imaging can be useful for the diagnosis of pancreatic tumor lesions, especially cystic pancreatic tumors, and the evaluation of relationships between tumors and the pancreatic duct [14]. Angiography is the gold standard when examining blood vessels, during which endovascular interventions can be performed [5].
Eradicating the source of bleeding is the only cure for HP. Interventional radiological procedures and surgery remain the primary approaches to HP. Vascular intervention is preferred when vascular damage is detected, such as pseudoaneurysm or active bleeding, and preoperative embolization is one possibility when vascular damage is detected on the background of a pancreatic tumor [1]. Surgery is indicated when a tumor lesion is detected, when a vascular lesion cannot be embolized or when bleeding recurs after embolization [1]. For tumors located in the body or tail of the pancreas, surgery is typically recommended to remove the body or tail of the pancreas [15]. Pancreatic head tumors are surgically removed by performing a pancreaticoduodenectomy [15,16]. Central pancreatectomy with pancreatic body tumors is rarely performed due to technical difficulties and the high risk of postoperative pancreatic fistula complications [16]. Intraoperative ultrasound can be useful in some cases. Pancreatic pseudocysts can be treated with less-invasive procedures, such as percutaneous drainage or surgery [5]. Overall, the recurrence rate of HP, especially after surgical treatment, is low [5].
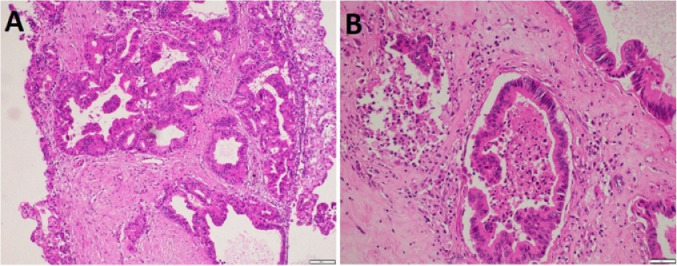
The patient we encountered presented with upper GI bleeding due to an IPMN, which is an extremely rare complication [12]. In our case, no pancreatitis or vascular lesions were detected. Although no blood clots were observed in the pancreatic duct, the pancreatic head tumor was thought to have been the cause of HP. The tumor occurred in a middle-aged male, located in the head of the pancreas, and contained both cystic and solid components, with ill-defined borders consistent with a malignant transformation from IPMN. No evidence of a dilated pancreatic duct was observed, and the tumor was localized, resulting in the preoperative diagnosis of branch duct type IPMN, which was confirmed by the postoperative pathological results (Fig. 4).
Fig. 4.
Microscopic images of the tumor with hematoxylin and eosin stain. (A) The proliferative glands are structurally complex, distorted, or cystic, infiltrating the fibrous stroma. Tumor cells lost polarity and appeared polymorphic (×100). (B) Intraluminal cystic tumor cells contained necrotic tumor cells or disrupted glands that infiltrated the hyaline stroma, resulting in fibrosis. Tumor cells proliferated in the ductal lumen, lost polarity, and were polymorphic (×200)
Conclusion
HP due to pancreatic tumor is an extremely rare cause of upper GI bleeding that can be life-threatening. The diagnosis is challenging, and upper GI endoscopy and imaging modalities greatly aided in the diagnosis. An accurate and timely diagnosis improves treatment efficiency and significantly reduces mortality.
Ethical statement
Appropriate written informed consent was obtained for the publication of this case report and accompanying images.
Author contributions
Tran BL, Nguyen TB, and Nguyen MD contributed equally to this article as co-first authors. All authors have read the manuscript and agree to the contents.
Footnotes
Acknowledgements: Self-financed.
competing interests: The authors do not report any conflicts of interest.
Hemosuccus pancreaticus by intraductal papillary mucinous neoplasm
Contributor Information
Nguyen Thai Binh, Email: nguyenthaibinh@hmu.edu.vn.
Nguyen Minh Duc, Email: bsnguyenminhduc@pnt.edu.vn.
References
- 1.Mandaliya R., Krevsky B, Sankineni A, Walp K, Chen O. Hemosuccus pancreaticus: a mysterious cause of gastrointestinal bleeding. Gastroenterology res. 2014;7(1):32. doi: 10.14740/gr596w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ru N, Zou WB, Qian YY, Tang XY, Zhu JH, Hu LH. A systematic review of the etiology, diagnosis, and treatment of hemosuccus pancreaticus. Pancreas. 2019;48(5):e47–e49. doi: 10.1097/MPA.0000000000001278. [DOI] [PubMed] [Google Scholar]
- 3.Etienne S, Pessaux P, Tuech JJ, Lada P, Lermite E, Brehant O. Hemosuccus pancreaticus: a rare cause of gastrointestinal bleeding: a series of 9 cases. Gastroentérol clin biol. 2005;29(3):237–242. doi: 10.1016/s0399-8320(05)80755-9. [DOI] [PubMed] [Google Scholar]
- 4.Yu P, Gong J. Hemosuccus pancreaticus: A mini-review. Ann med surg. 2018;28:45–48. doi: 10.1016/j.amsu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han B, Song ZF, Sun B. Hemosuccus pancreaticus: a rare cause of gastroirtestiral bleeding. Hepatobiliary Pancreat Dis Int. 2012;11(5):479–488. doi: 10.1016/S1499-3872(12)60211-2. [DOI] [PubMed] [Google Scholar]
- 6.Sul HR, Lee HW, Kim JW, Cha SJ, Choi YS, Kim GH. Endovascular management of hemosuccus pancreaticus, a rare case report of gastrointestinal bleeding. BMC gastroenterol. 2016;16(1):1–4. doi: 10.1186/s12876-016-0418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray S, Das K, Ray S, Khamrui S, Ahammed M, Deka U. Hemosuccus pancreaticus associated with severe acute pancreatitis and pseudoaneurysms: a report of two cases. JOP. 2011;12(5):469–472. [PubMed] [Google Scholar]
- 8.Heath DI, Reid AW, Murray W. Bleeding pseudocysts and pseudoaneurysms in chronic pancreatitis. Br J Surg 1992;79(3):281-281. doi:10.1002/bjs.1800790338. [DOI] [PubMed]
- 9.Shinzeki M, Hori Y, Fujino Y, Matsumoto I, Toyama H, Tsujimura T. Mucinous cystic neoplasm of the pancreas presenting with hemosuccus pancreaticus: report of a case. Surg Today. 2010;40(5):470–473. doi: 10.1007/s00595-009-4079-5. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto Y, Miyamoto H, Fukuya A, Nakamura F, Goji T, Kitamura S. Hemosuccus pancreaticus caused by a mucinous cystic neoplasm of the pancreas. Clin J gastroenterol. 2017;10(2):185–190. doi: 10.1007/s12328-016-0711-2. [DOI] [PubMed] [Google Scholar]
- 11.Inoue H, Katurahara M, Hamada Y, Ninomiya K, Tano S, Takayama R. Hemosuccus pancreaticus caused by in situ carcinoma of the pancreas. Endoscopy. 2012;44(S 02):E336–E337. doi: 10.1055/s-0032-1309863. [DOI] [PubMed] [Google Scholar]
- 12.Kuruma S, Kamisawa T, Tu Y, Egawa N, Tsuruta K, Tonooka A. Hemosuccus pancreaticus due to intraductal papillary-mucinous carcinoma of the pancreas. Clin J gastroenterol. 2009;2(1):27–29. doi: 10.1007/s12328-008-0040-1. [DOI] [PubMed] [Google Scholar]
- 13.Procacci C, Megibow AJ, Carbognin G, Guarise A, Spoto E, Biasiutti C. Intraductal papillary mucinous tumor of the pancreas: a pictorial essay. Radiographics. 1999;19(6):1447–1463. doi: 10.1148/radiographics.19.6.g99no011447. [DOI] [PubMed] [Google Scholar]
- 14.Grützmann R, Post S, Saeger HD, Niedergethmann M. Intraductal papillary mucinous neoplasia (IPMN) of the pancreas: its diagnosis, treatment, and prognosis. Dtsch Ärztebl Int. 2011;108(46):788. doi: 10.3238/arztebl.2011.0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waters JA, Schmidt CM. Intraductal papillary mucinous neoplasm—when to resect? Adv Surg. 2008;42:87–108. doi: 10.1016/j.yasu.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Hackert T, Hinz U, Fritz S, Strobel O, Schneider L, Hartwig W. et al.Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbecks Arch Surg. 2011;396(8):1197–1203. doi: 10.1007/s00423-011-0801-z. [DOI] [PubMed] [Google Scholar]