Summary
Background
There are limited data on healthcare-associated infections (HAI) from African countries like Malawi.
Aim
We undertook a point prevalence survey of HAI and antimicrobial use in the surgery department of Queen Elizabeth Central Hospital (QECH) in Malawi and ascertained the associated risk factors for HAI.
Methods
A cross-sectional point prevalence survey (PPS) was carried out in the surgery department of QECH. The European Centre for Disease Prevention and Control PPS protocol version 5.3 was adapted to our setting and used as a data collection tool.
Findings
105 patients were included in the analysis; median age was 34 (IQR: 24–47) years and 55.2% patients were male. Point prevalence of HAI was 11.4% (n=12/105) (95% CI: 6.0%–19.1%), including four surgical site infections, four urinary tract infections, three bloodstream infections and one bone/joint infection. We identified the following risk factors for HAI; length-of-stay between 8 and 14 days (OR=14.4, 95% CI: 1.65–124.7, p=0.0143), presence of indwelling urinary catheter (OR=8.3, 95% CI: 2.24–30.70, p=0.003) and history of surgery in the past 30 days (OR=5.11, 95% CI: 1.46–17.83, p=0.011). 29/105 patients (27.6%) were prescribed antimicrobials, most commonly the 3rd-generation cephalosporin, ceftriaxone (n=15).
Conclusion
The prevalence rates of HAI and antimicrobial use in surgery wards at QECH are relatively high. Hospital infection prevention and control measures need to be strengthened to reduce the burden of HAI at QECH.
Keywords: Healthcare-associated infection, Infection prevention and control, Point-prevalence survey, Antimicrobial use, Surgery, Malawi
Introduction
Healthcare-associated infections (HAI) constitute a worldwide public health concern affecting hospitalised patients, hospitals and health systems [[1], [2], [3]]. They increase healthcare costs by prolonging the hospital stay and requiring the use of expensive broad-spectrum antibiotics and they are associated with high morbidity and mortality [[4], [5], [6], [7]].
Increasing antimicrobial resistance (AMR) is associated with antimicrobial consumption, suggesting that optimising antimicrobial prescription may curb the development of AMR [5,8]. The use of broad-spectrum antibiotics in the treatment of HAI before the results of culture may drive the emergence and spread of multidrug-resistant (MDR) pathogens in hospital settings and their dissemination to the community. However, in a context where the microbiology service is limited, HAI are diagnosed clinically and treated empirically. This makes the treatment of HAI less effective and more costly [4,5,9]. In Europe, about 1%–35% of pathogens isolated in HAI are resistant to antimicrobials [[10], [11], [12], [13], [14]]. Meanwhile, the AMR rates reported among isolates from HAI in Africa are of 10%–100% [4,15].
Data on HAI from low and middle income countries (LMICs), like Malawi, are limited, posing challenges on assessing the impact of control interventions and surveillance strategies [1,5,9,16]. Where studied, prevalence of HAI in Africa has been reported in up to 15.5% of patients admitted to general wards and 50% of patients admitted to intensive care units (ICUs) [1,9,16,17]. High rates of HAI in Africa are due to the paucity of infection prevention and control (IPC) policies and guidelines, exacerbated by the lack of personnel, lack of antimicrobial policies resulting in the emergence of MDR pathogens, poor laboratory support, limited funding, and suboptimal adherence to safe practices by health workers and typically limited compulsion to report HAI [4,9,15,18,19]. In addition to these, the structure of hospitals (including the fixed components within the facility with which health care workers, patients, and families touch or interact as a part of the health care process) plays a role in risk of acquiring HAI [20].
It has been estimated that evidence-based interventions can prevent about 50% of HAI [1], and clinical and microbiological surveillance of HAI is a major component of assessing strategies to reduce HAI within hospitals [18]. Moreover, these data are useful in prioritizing further areas for interventions in the prevention and control of HAI [4,18,21]. Whilst prospective longitudinal clinical surveillance is optimal, it is resource-intense. Consequently, cross-sectional point prevalence surveys (PPS) are pragmatic alternatives that can provide valuable snapshots of the HAI burden and epidemiology as they can be conducted quickly and without sophisticated techniques [5,[22], [23], [24]]. In HAI PPS, the McCabe score is used as a subjective score of underlying illness severity. It is an important tool for risk stratification in infection prevention and control [25].
Currently, there is no HAI surveillance system in Malawi. Therefore, this study aimed at estimating the point prevalence of HAI and antimicrobial use in the surgery department of Queen Elizabeth Central Hospital (QECH) in Malawi and ascertained the associated risk factors for HAI.
Methods
Study setting
The study was conducted at QECH, a large urban government central hospital in Blantyre, which has a capacity of about 1,300 beds but frequently operates above its capacity. Its surgery department provides care in general surgery, orthopaedics, neurosurgery, and paediatric surgery. The 4 surgical wards at QECH, with about 190 inpatient beds were surveyed in this study. The accident and emergency wards and ear, nose and throat department were excluded and wards under the Mercy James Centre (MJC) for paediatric surgery and intensive care were not included.
Study design
A single-day cross-sectional point prevalence survey (PPS) was conducted in different wards of the surgery department of QECH on 9th June 2020, using an adapted version of the European Centre for Disease Prevention and Control (ECDC) tool for PPS on HAI and antimicrobial use, protocol version 5.3 [26].
Inclusion and exclusion criteria
Patients admitted to surgical wards before or at 8 a.m. on the day of the survey and not discharged at the time of the survey were included in the study. Patients who were transferred in/out after 8 a.m. from/to another unit were also excluded. In addition, all day cases patients (patients undergoing same-day treatment or surgery, patients seen at the outpatient department, patients in an emergency room and dialysis patients) were excluded. Patients admitted in gynaecology and obstetrics department were excluded. All patients hospitalised for less than 48 hours were also excluded in the study.
Data collection
Data were collected by a clinical microbiologist, and by nurses trained in use of the data collection tool and the case report form. Before data collection, a competency-based evaluation was undertaken. For all eligible patients with or without a HAI, a case report form was used for collecting demographic data, clinical history (length of hospital stay (LOS), surgical procedure, indwelling devices, comorbidities), information on antimicrobial use, data on HAI if present, results of routine microbiological tests performed if available, and calculating the McCabe score. The McCabe score categorizes the severity of underlying medical conditions into non-fatal disease (expected survival of at least five years), ultimately fatal disease (expected survival between one and five years), rapidly fatal disease (expected death within one year) and unknown [26].
Case definitions
The ECDC criteria and definitions for HAI [26] were used and these are based on the presence of signs and symptoms of a particular HAI on the day of the survey, with or without microbiological results. Briefly, an infection was considered to be HAI when the onset of the signs and symptoms occurred >48 hours after the current admission or became apparent within 48 hours of admission, but the patient had been discharged from an acute care hospital <48 hours before the current admission [26]. Infections were categorized into surgical site infection, bone/joint infection, urinary tract infection, sepsis or bloodstream infection, pneumonia, and other infections including skin and soft tissue infection, nervous system infections, and gastrointestinal tract infection [5,26]. For surgical site infections (SSIs), the definition included infections that occurred up to 30 days after a surgical procedure and affected either the incision or deep tissue at the surgical site, or infections related to an implant that occurred within one year. Moreover, device-associated HAI was recorded for urinary tract infections (urinary catheter in place within seven days preceding HAI onset), sepsis or bloodstream infections (vascular catheter in place within 48 hours before HAI onset) and pneumonia (intubation within 48 hours before HAI onset) [26].
Data analysis
Data were entered into MS Excel 2010, double-checked for coding errors, cleaned and exported into SPSS version 24 (IBM Corp., Armonk, NY, USA) and Stata 16 (StataCorp., USA) for analysis. The point prevalence of HAI was reported as percentage of the patients with at least one clinically identified HAI divided by the total number of included patients. Prevalence rates were calculated with the exact binomial 95% confidence intervals (95% CI). Arithmetic means, median and interquartile range (IQR) were calculated to summarise continuously measured variables. Effect sizes of associations of risk factors with the outcome of HAI were reported using odds ratios (ORs). For categorical and binary variables, Fisher's exact test was used to test the null hypothesis of no association with HAI. Statistical significance was determined by p-values <0.05. Due to the small number of HAI in our data, multivariable regression model of most variables was not reported as this would have likely overfit and resulted in overinterpretation of the results and unstable coefficient estimates. However, for avoiding loss of information in categorising the continuous variable LOS, we fit a logistic regression model for HAI against LOS using restricted cubic splines (RCS), using R v4.0.2 [27] and the rms package v6.0.0. [28]. We used Akaike's Information Criterion (AIC) to select the number of knots, with a model with 3 knots having the lowest AIC and having therefore been selected. To test the null hypothesis of no association between HAI and LOS we performed a likelihood ratio test, comparing the RCS model to an intercept-only model.
Ethical considerations
The study was approved by the College of Medicine Research and Ethics Committee (COMREC), protocol number P.10/19/2834. Informed written consent was obtained from patients, who were free to withdraw their consent at any time.
Results
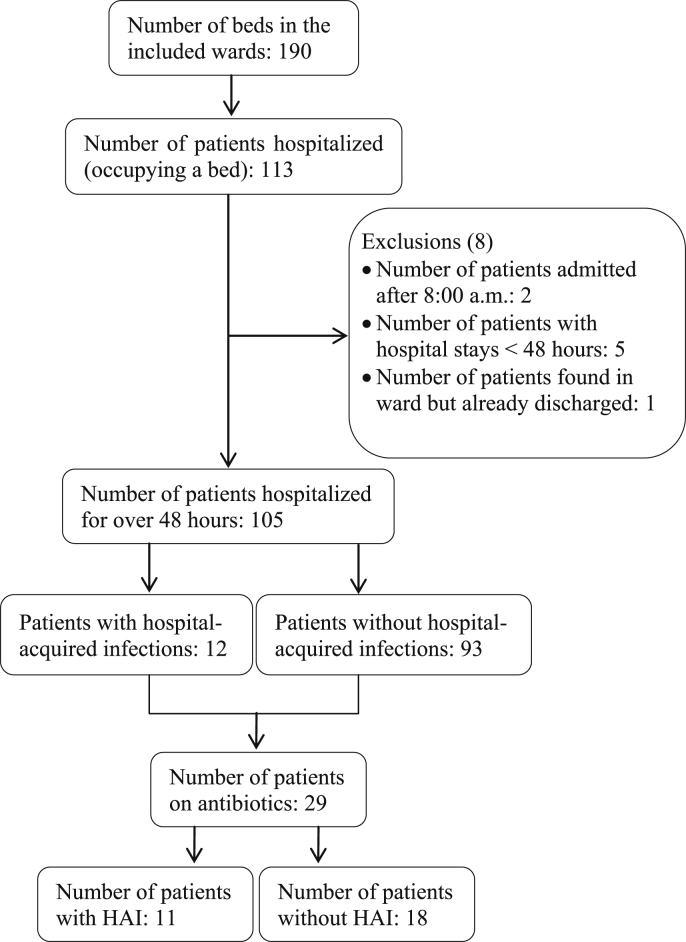
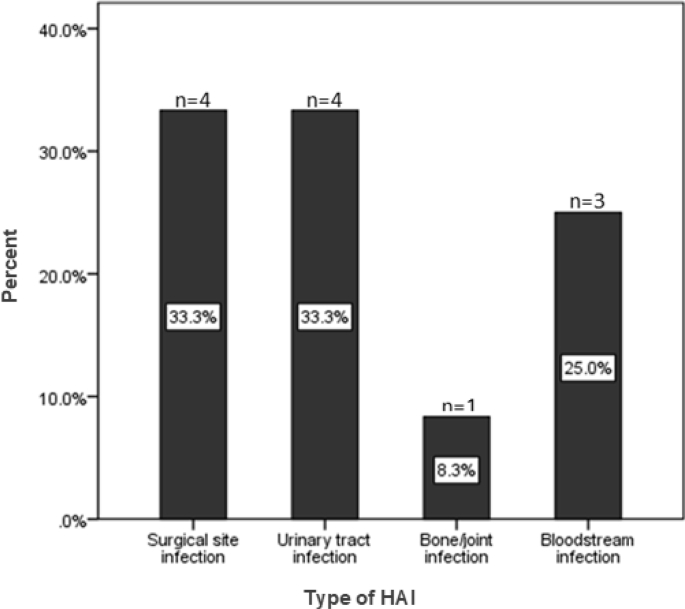
A total of 113 patients were screened on the survey day (Figure 1). The bed occupancy rate was 59.5%. Of the 113 screened patients, 105 were hospitalised for >48 hours and were hence included in the study. Eight of the 113 screened patients were excluded because they were admitted after 8:00 a.m. on the day of the survey (2), and/or with a hospital stay <48 hours (5), and/or found in a ward but already discharged (1). Of the 105 included patients, 12 (11.4%) (95% CI: 6.0%–19.1%) had HAI and 29 (27.6%) (95% CI: 19.3%–37.2%) were prescribed at least one antimicrobial drug. The most frequently reported HAI were surgical site infections (n=4, 33.3%) and urinary tract infections (n=4, 33.3%), followed by bloodstream infections (n=3, 25.0%) and bone/joint infections (n=1, 8.3%) (Figure 2). Of the 12 HAI identified, six (50.0%) were device-associated HAI (four urinary tract infections and two bloodstream infections).
Figure 1.
Flow chart of patients' recruitment.
Figure 2.
Distribution of different hospital-acquired infections among admitted patients in surgical wards at QECH (N=12).
Patients' characteristics and risk factors for HAI
Of the 105 patients included in the survey, 58 (55.2%) were male and 47 (44.8%) were female (Table I). The median age of the patients was 34 (IQR: 24–47) years. 77 (73.3%) patients had non-fatal diseases in the McCabe scoring. The median hospital length of stay (LOS) was eight (IQR: 5–20) days. Of the patients surveyed, 32 (30.5%) had a peripheral venous catheter, 16 (15.2%) had an indwelling urinary catheter, 42 (40.0%) had documented comorbidities, and 27 (25.7%) had undergone surgery in the past 30 days.
Table I.
Demographic, clinical characteristics, and exposures of surveyed patients
| Variables | All patients, N=105 | Patients without HAI, N=93 | Patients with HAI, N=12 | OR (95% CI) | p-value∗ | Patients on AM, N=29 |
|---|---|---|---|---|---|---|
| Patients' characteristics, n (%) | ||||||
| Gender | 0.764 | |||||
| Female | 47 (44.8) | 41 (44.1) | 6 (50.0) | Ref. | 16 (55.2) | |
| Male | 58 (55.2) | 52 (55.9) | 6 (50.0) | 0.79 (0.20–3.20) | 13 (44.8) | |
| Age (years), Median (IQR) | 34 (24–47) | 0.4901 | ||||
| <20 | 20 (19.0) | 17 (18.3) | 3 (25.0) | Ref. | 5 (17.2) | |
| 20–39 | 47 (44.8) | 40 (43.0) | 7 (58.3) | 29.8 (6.46–201.4) | 16 (55.2) | |
| 40–59 | 25 (23.8) | 23 (24.7) | 2 (16.7) | 0.50 (0.038–4.88) | 6 (20.7) | |
| ≥60 | 13 (12.4) | 13 (14.0) | 0 (0.0) | 0.0 (0.0–3.68) | 2 (6.9) | |
| McCabe score∗∗ | 0.0731∗ | |||||
| Non-fatal diseases | 77 (73.3) | 68 (73.1) | 9 (75.0) | Ref. | 23 (79.3) | |
| Ultimately fatal disease | 16 (15.3) | 16 (17.2) | 0 (0.0) | 0.0 (0.0–2.43) | 2 (6.9) | |
| Rapidly fatal disease | 4 (3.8) | 4 (4.3) | 0 (0.0) | 0.0 (0.0–13.10) | 0 (0.0) | |
| Unknown | 8 (7.6) | 5 (5.4) | 3 (25.0) | 4.53 (0.59–27.65) | 4 (13.8) | |
| Exposure, n (%) | ||||||
| Length of stay (days), Median (IQR) | 8 (5–20) | 0.0143 | ||||
| ≤7 | 42 (40.0) | 41 (44.1) | 1 (8.3) | Ref. | 11 (37.9) | |
| 8–14 | 27 (25.7) | 20 (21.5) | 7 (58.3) | 14.4 (1.65–124.7) | 11 (37.9) | |
| 15–21 | 12 (11.4) | 10 (10.8) | 2 (16.7) | 8.20 (0.67–99.70) | 4 (13.8) | |
| ≥22 | 24 (22.9) | 22 (23.6) | 2 (16.7) | 3.73 (0.32–43.44) | 3 (10.4) | |
| Peripheral venous catheter | 1.000 | |||||
| No | 73 (69.5) | 65 (69.9) | 8 (66.7) | Ref. | 10 (34.5) | |
| Yes | 32 (30.5) | 28 (30.1) | 4 (33.3) | 0.86 (0.21–4.24) | 19 (65.5) | |
| Indwelling urinary catheter | 0.003 | |||||
| No | 89 (84.8) | 83 (89.2) | 6 (50.0) | Ref. | 20 (69.0) | |
| Yes | 16 (15.2) | 10 (10.8) | 6 (50.0) | 8.30 (2.24–30.70) | 9 (31.0) | |
| Documented comorbidities | 0.537 | |||||
| No | 63 (60.0) | 57 (61.3) | 6 (50.0) | Ref. | 19 (65.5) | |
| Yes | 42 (40.0) | 36 (38.7) | 6 (50.0) | 1.58 (0.47–5.29) | 10 (34.5) | |
| Surgery in past 30 days | 0.011 | |||||
| No | 78 (74.3) | 73 (78.5) | 5 (41.7) | Ref. | 14 (48.3) | |
| Yes | 27 (25.7) | 20 (21.5) | 7 (58.3) | 5.11 (1.46–17.83) | 15 (51.7) | |
| Patients on AM | ||||||
| Yes | 29 (27.6) | 18 (19.4) | 11 (91.7) | - | - | - |
| No | 76 (72.4) | 75 (80.6) | 1 (8.3) | - | - | - |
HAI: healthcare-associated infections, IQR: interquartile range, AM: antimicrobial agent.
∗∗After removing the unknown category in the McCabe score, the p-value is 0.5625.
P-values obtained using Fisher's exact test.
The following risk factors were significantly associated with HAI: presence of indwelling urinary catheter (OR=8.3, 95% CI: 2.24–30.70, p=0.003), history of surgery in the past 30 days (OR=5.11, 95% CI: 1.46–17.83, p=0.011) and LOS between 8-14 days as associated risk with an OR=14.4 (95% CI: 1.65–124.7, p=0.0143) (Table I). Given the non-linear association between the probability of an HAI and LOS, we have repeated this last analysis using a logistic regression model with restricted cubic spline terms [29,30] for LOS and assessing the association between HAI and LOS using a likelihood ratio test. This confirmed our finding (p=0.0035 with this model).
Antimicrobial use
Of the 29 patients that received antimicrobials, 13 (44.8%) received one and 16 (55.2%) received two antimicrobial agents (Table II). The purposes of prescribing antimicrobial agents were for prophylaxis in three (10.3%) cases, therapeutic in 14 (48.3%) cases and both prophylaxis and therapeutic in 12 (41.4%) cases. The third-generation cephalosporin (ceftriaxone) was used in 15 (51.7%) cases, metronidazole in 13 (44.8%) cases, amoxicillin in seven (24.1%) cases, doxycycline in four (13.8%) cases, ciprofloxacin in four (13.8%) cases and flucloxacillin in two (6.9%) cases.
Table II.
Antimicrobial use in surgery department at QECH, Malawi
| Variables | All patients, N=29 n (%) |
Patients with HAI n (%)∗∗ |
Patients without HAI n (%)∗∗ |
|---|---|---|---|
| Number of AM prescribed | |||
| 1 | 13 (44.8) | 4 (30.8) | 9 (69.2) |
| 2 | 16 (55.2) | 7 (43.8) | 9 (56.2) |
| Purpose of AM use | |||
| Prophylactic | 3 (10.3) | 0 (0.0) | 3 (100) |
| Therapeutic | 14 (48.3) | 4 (28.6) | 10 (71.4) |
| Both | 12 (41.4) | 7 (58.3) | 5 (41.7) |
| AM prescribed∗ | |||
| Ceftriaxone | 15 (51.7) | 7 (46.7) | 8 (53.3) |
| Metronidazole | 13 (44.8) | 4 (30.8) | 9 (69.2) |
| Amoxicillin | 7 (24.1) | 3 (42.9) | 4 (57.1) |
| Doxycycline | 4 (13.8) | 2 (50.0) | 2 (50.0) |
| Ciprofloxacin | 4 (13.8) | 2 (50.0) | 2 (50.0) |
| Flucloxacillin | 2 (6.9) | 0 (0.0) | 2 (100) |
HAI: healthcare-associated infections, AM: antimicrobial agent.
There are patients who received more than one antibiotic.
Percentages of these columns are calculated by taking corresponding lines of the first columns as the total.
Discussion
In this single-day cross-sectional PPS, we observed an estimated HAI point prevalence of 11.4%. This survey focused on surgical wards in an urban teaching hospital in Malawi. Similar studies reported HAI prevalence rates of 16.4% in Burkina Faso [31], 14.3% in Nigeria [4] and 11.9% in Ethiopia [32].
The most frequent HAI were surgical site infections (SSI) and urinary tract infections (UTI) (33.3% each), which is comparable to other settings [1,7,19]. Half of the reported infections were device-associated HAI, and thus preventable. Prevention of HAI in surgical patients requires integrated IPC measures before, during and after surgery [33,34]. This particularly applies while using medical devices, however, a previous study has reported a low adherence to hand hygiene practice by clinicians and medical students at QECH [35]. A recent report has proposed IPC among top priorities for patient-centred surveillance of drug-resistant infections and we echo this call [36].
The rate of antimicrobial prescribing (27.6%) found in this survey is relatively higher than that reported in Iran (9.4%) among patients with documented infections [22]. However, Labi et al. reported 61% of patients on antibiotics in Ghana and 89.5% of them had HAI [2]. Our survey considered only the surgery wards, while these other studies were conducted within all the hospital wards and on a large scale including several hospitals. The most frequently prescribed antimicrobial was ceftriaxone. Since January 2020, QECH has recommended the use of cefazolin in surgical prophylaxis. The exceptional use of ceftriaxone is only indicated for established infection prior to surgery is done or in cases where patients were already on ceftriaxone before the surgery, suggesting much of this use was contrary to QECH guidelines, however this study did not analyse the appropriateness of the use of antibiotics nor availability of cefazolin. A recent study from the Democratic Republic of the Congo reported the use of ceftriaxone in non-compliance with surgical antimicrobial prophylaxis guidelines [37].
In many sub-Saharan African healthcare facilities, third-generation cephalosporins (3GCs) are the first choice for antibiotics used in the empiric treatment of acute and severe infections [38]. In Malawi, lack of alternatives has been reported as a reason for preventing the broad use of 3GCs in most hospitals [39]. An antimicrobial stewardship program implemented in adult medical wards at QECH was effective in reducing the use of 3GCs [40]. Such a program should be extended to other departments for promoting the rational use of antibiotics as inappropriate use of 3GCs may facilitate the emergence of multi-drug resistant pathogens. Certainly, increased incidence of extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae has been reported since the introduction of ceftriaxone into the Malawian formulary [41].
We report hospital length of stay, presence of indwelling urinary catheter and history of surgery in the past 30 days as risk factors significantly associated with HAI, consistent with other reports [2,9,22,24,[42], [43], [44], [45]]. Indwelling urinary catheter exposure is a well-established risk factor for UTI, associated with extra hospital length of stay and healthcare-cost [3,42,44,45]. Recent surgery may be a proxy for high-risk procedures such as central/peripheral vein catheter, urinary catheter and endotracheal intubation during the surgical procedure. Placement of these invasive medical devices requires strict hygiene measures because they are key risk factors for HAI.
There were some limitations to our study; first, numbers were small and the survey was not repeated. Second, as the survey was conducted during the COVID-19 pandemic, infection prevention and control (IPC) measures had recently been reinforced in hospital, which might have influenced the HAI prevalence. Further, the admission rate during this period was decreased so nursing staff had fewer patients to care for. Lastly, we could not assess the appropriateness of antimicrobial use due to poor documentation of compliance measures, although this is typically a marker of poor practice in antimicrobial prescribing.
Conclusions
To the best of our knowledge, this is the first survey reporting on the epidemiology of HAI and antimicrobial use in surgery wards in Malawi. The prevalence rates of HAI and antimicrobial use in surgery units at QECH are relatively high compared with other LMICs. Acquiring HAI was significantly associated with length of stay, presence of indwelling urinary catheter, and history of surgery in the past 30 days. Hospital infection prevention and control measures should be strengthened for reducing HAI burden at QECH. Interventions supporting improved IPC should be implemented at QECH. In addition, study on antimicrobial resistance patterns of bacteria isolated from HAI should be conducted.
CrediT author statement
Gabriel Kambale Bunduki: Conceptualised and designed the study, Data curation, analysis and interpretation, Writing-Original draft preparation, Funding acquisition.
Nicholas Feasey: Supervision, Data interpretation, Reviewing and editing the manuscript.
Marc Y.R. Henrion: Data analysis and interpretation, Reviewing and editing the manuscript.
Patrick Noah: Literature search.
Janelisa Musaya: Supervision, Data interpretation, Reviewing and editing the manuscript.
Funding
Authors are thankful to the Africa Centre of Excellence in Public Health and Herbal Medicine (ACEPHEM) and the Malawi-Liverpool-Wellcome Trust (MLW) core funding (Wellcome Trust strategic award 206545/Z/17/Z). GKB received funding from the ACEPHEM through the College of Medicine, University of Malawi. The funding bodies had no role in the study design, data collection, analysis and interpretation of results, writing the manuscript or decision to publish this paper.
Conflict of interest statement
None declared.
Acknowledgements
The authors would like to thank the nursing staff of the Surgery department at Queen Elizabeth Central Hospital for their cooperation during the data collection.
References
- 1.Nejad S.B., Allegranzi B., Syed S.B., Ellis B., Pittet D. Health-care-associated infection in Africa : a systematic review. Bull World Health Organ. 2011;89:757–765. doi: 10.2471/BLT.11.088179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labi A., Obeng-Nkrumah N., Owusu E., Bjerrum S., Bediako-Bowan A., Sunkwa-Mills G. Multi-centre point-prevalence survey of hospital- acquired infections in Ghana. J Hosp Infect. 2019;101:60–68. doi: 10.1016/j.jhin.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 3.De Angelis G., Murthy A., Beyersmann J., Harbarth S. Estimating the impact of healthcare-associated infections on length of stay and costs. Clin Microbiol Infect. 2010;16(12):1729–1735. doi: 10.1111/j.1469-0691.2010.03332.x. [DOI] [PubMed] [Google Scholar]
- 4.Abubakar U. Point-prevalence survey of hospital acquired infections in three acute care hospitals in Northern Nigeria. Antimicrob Resist Infect Control. 2020;9:63. doi: 10.1186/s13756-020-00722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Zhong Z., Chen S., Zhou D., Li Z., Meng Y. Prevalence of healthcare-associated infections and antimicrobial use in China: Results from the 2018 point prevalence survey in 189 hospitals in Guangdong Province. Int J Infect Dis. 2019;89:179–184. doi: 10.1016/j.ijid.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Aliki M., Miriam V., Rami S., Jonas M., Cathy V., Nicolas T. Point prevalence of healthcare-associated infections and antibiotic use in three large Swiss acute-care hospitals. Swiss Med Wkly. 2018;148 doi: 10.4414/smw.2018.14617. w14617. [DOI] [PubMed] [Google Scholar]
- 7.Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Du M., Chang Y., Chen L., Zhang Q. Incidence, clinical characteristics, and outcomes of nosocomial Enterococcus spp. bloodstream infections in a tertiary-care hospital in Beijing, China: A four-year retrospective study. Antimicrob Resist Infect Control. 2017;6:73. doi: 10.1186/s13756-017-0231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali S., Birhane M., Bekele S., Kibru G., Teshager L., Yilma Y. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: longitudinal study. Antimicrob Resist Infect Control. 2018;7:2. doi: 10.1186/s13756-017-0298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sax H., Clack L., Touveneau S. Jantarada F da L, Pittet D, Zingg W. Implementation of infection control best practice in intensive care units throughout Europe: a mixed-method evaluation study. Implement Sci. 2013;8:24. doi: 10.1186/1748-5908-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allerberger F., Küenburg B. Organization of control of nosocomial infections in Central Eastern European countries. Wiener Medizinische Wochenschrift. 2019;169(Suppl 1):S1–S2. doi: 10.1007/s10354-018-0671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clack L., Zingg W., Saint S., Casillas A., Touveneau S., Jantarada L. Implementing infection prevention practices across European hospitals: an in-depth qualitative assessment. BMJ Qual Saf. 2018:1–10. doi: 10.1136/bmjqs-2017-007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malobicka E., Roskava D., Svihrva V., Hudeckova H. Point prevalence survey of nosocomial infections in University Hospital in Martin. Acta Medica Martiniana. 2013;13(2):34–41. [Google Scholar]
- 14.Gori F. Point prevalence study of hospital acquired infection in an Italian hospital. Eur J Public Health. 2018;28(Suppl 4) 2018. [Google Scholar]
- 15.Ahoyo T.A., Bankolé H.S., Adéoti F.M., Gbohoun A.A., Assavedo S., Amoussou-Guenou M. Prevalence of nosocomial infections and anti-infective therapy in Benin: results of the first nationwide survey in 2012. Antimicrob Resist Infect Control. 2014;3:17. doi: 10.1186/2047-2994-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittet D. Burden of endemic healthcare-associated infection in Africa. 16th ICID Abstracts. Int J Infect Dis. 2014;21S:1–460. [Google Scholar]
- 17.Yallew W.W., Kumie A., Yehuala F.M. Risk factors for hospital-acquired infections in teaching hospitals of Amhara regional state, Ethiopia: A matched-case control study. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181145. e0181145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mpinda-joseph P., Paramadhas B.D.A., Reyes G., Maruatona M.B., Chise M., Monokwane-Thupiso B.B. Healthcare-associated infections including neonatal bloodstream infections in a leading tertiary hospital in Botswana. Hosp Pract. 2019;47(4):203–210. doi: 10.1080/21548331.2019.1650608. [DOI] [PubMed] [Google Scholar]
- 19.Yallew W.W., Kumie A., Yehuala F.M. Point prevalence of hospital-acquired infections in two teaching hospitals of Amhara region in Ethiopia. Drug, Healthc Patien Saf. 2016;8:71–76. doi: 10.2147/DHPS.S107344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alessandro D.D., Fara G.M. Indoor air quality in healthcare facilities. 2017. Hospital Environments and Epidemiology of Healthcare-Associated Infections; pp. 41–52. [Google Scholar]
- 21.Tao X., Qian L., Li Y., Wu Q., Ruan J., Cai D. Hospital-acquired infection rate in a tertiary care teaching hospital in China: a cross-sectional survey involving 2434 inpatients. Int J Infect Dis. 2014;27:7–9. doi: 10.1016/j.ijid.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Askarian M., Yadollahi M., Assadian O. Point prevalence and risk factors of hospital acquired infections in a cluster of university-affiliated hospitals in Shiraz. Iran. J Infect Public Health. 2012;5:169–176. doi: 10.1016/j.jiph.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Marani A., Napoli C., Berdini S., Montesano M., Ferretti F., Di Ninno F. Point prevalence surveys on healthcare acquired infections in medical and surgical wards of a teaching hospital in Rome. Ann Ig. 2016;28:274–281. doi: 10.7416/ai.2016.2106. [DOI] [PubMed] [Google Scholar]
- 24.Razine R., Azzouzi A., Barkat A., Khoudri I., Hassouni F., Chefchaouni A.C. Prevalence of hospital-acquired infections in the university medical center of Rabat, Morocco. Int Arch Med. 2012;5:26. doi: 10.1186/1755-7682-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly J.S., Coignard B., Price L., Godwin J., Cairns S., Hopkins S. The reliability of the McCabe score as a marker of co-morbidity in healthcare-associated infection point prevalence studies. J Infect Prev. 2016;17(3):127–129. doi: 10.1177/1757177415617245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Centre for Disease Prevention and Control . ECDC; Stockolm: 2016. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals-protocol version 5.3. [Google Scholar]
- 27.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A language and environment for statistical computing.https://www.r-project.org/ [Google Scholar]
- 28.Harrell F.E., Jr. 2020. rms: regression Modeling Strategies. R package version 6.0-0.https://cran.r-project.org/package=rms [Google Scholar]
- 29.Perperoglou A., Sauerbrei W., Abrahamowicz M., Schmid M. A review of spline function procedures in R. BMC Med Res Methodol. 2019;19:46. doi: 10.1186/s12874-019-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd B.E., Rebeiro P.F. Assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J Acquir Immune Defic Syndr. 2017;74(3):e60–e63. doi: 10.1097/QAI.0000000000001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanou J., Traore S.S., Lankoande J., Ouedraogo R.M., Sanou A. Survey of nosocomial infection prevalence in the surgery department of the Central National Hospital of Ouagadougou. Dakar Med. 1999;44:105–108. [PubMed] [Google Scholar]
- 32.Messele G., Woldemedhin Y., Demissie M., Mamo K., Geyid A. Common causes of nosocomial infections and their susceptibility patterns in two hospitals in Addis Ababa. Ethiop J Heal Biomed Sci. 2009;2:3–8. [Google Scholar]
- 33.Allegranzi B., Zayed B., Bischoff P., Kubilay N.Z., de Jonge S., de Vries F. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet. 2016;3099(16):30402–30409. doi: 10.1016/S1473-3099(16)30402-9. [DOI] [PubMed] [Google Scholar]
- 34.Uçkay I., Hoffmeyer P., Lew D., Pittet D. Prevention of surgical site infections in orthopaedic surgery and bone trauma: state-of-the-art update. J Hosp Infect. 2013;84:5–12. doi: 10.1016/j.jhin.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Kalata N.L., Kamange L., Muula A.S. Adherence to hand hygiene protocol by clinicians and medical students at Queen Elizabeth Central Hospital, Blantyre-Malawi. Malawi Med J. 2013;25(2):50–52. [PMC free article] [PubMed] [Google Scholar]
- 36.Ashley E.A., Mclean A., Chiara F., Feasey N., Jaoko W., Opintan J.A. Setting priorities for patient-centered surveillance of drug-resistant infections. Int J Infect Dis. 2020;97:60–65. doi: 10.1016/j.ijid.2020.05.121. [DOI] [PubMed] [Google Scholar]
- 37.Bunduki G.K., Mukululi M.P., Masumbuko C.K., Uwonda S.A. Compliance of antibiotics used for surgical site infection prophylaxis among patients undergoing surgery in a Congolese teaching hospital. Infect Prev Pract. 2020;2(3):100075. doi: 10.1016/j.infpip.2020.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lester R., Musicha P., Van Ginneken N., Dramowski A., Hamer D.H., Garner P. Prevalence and outcome of bloodstream infections due to third-generation cephalosporin-resistant Enterobacteriaceae in sub-Saharan Africa: a systematic review. J Antimicrob Chemother. 2020;75:492–507. doi: 10.1093/jac/dkz464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haigh K., Dube Q., Kasambara W., Feasey N.A., Lester R. Cephalosporin resistance in Malawi. Lancet Infect Dis. 2020;20(3):285–286. doi: 10.1016/S1473-3099(20)30047-5. [DOI] [PubMed] [Google Scholar]
- 40.Lester R., Haigh K., Wood A., Macpherson E.E., Maheswaran H., Bogue P. Sustained Reduction in Third-generation Cephalosporin Usage in Adult Inpatients Following Introduction of an Antimicrobial Stewardship Program in a Large, Urban Hospital in Malawi. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musicha P., Cornick J.E., Bar-zeev N., French N., Masesa C., Denis B. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi ( 1998 – 2016 ): a surveillance study. Lancet Infect Dis. 2017;17(10):1042–1052. doi: 10.1016/S1473-3099(17)30394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uckay I., Sax H., Gayet-Ageron A., Muhlemann K., Troillet N., Petignat C. High proportion of healthcare-associated urinary tract infection in the absence of prior exposure to urinary catheter: a cross-sectional study. Antimicrob Resist Infect Control. 2013;2:5. doi: 10.1186/2047-2994-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang G., Huang Q., Zhang G., Jiang H., Lin Z. Point-prevalence surveys of hospital-acquired infections in a Chinese cancer hospital: from 2014 to 2018. J Infect Public Health. 2020;13(12):1981–1987. doi: 10.1016/j.jiph.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Strasheim W., Kock M.M., Ueckermann V., Hoosien E., Dreyer A.W., Ehlers M.M. Surveillance of catheter-related infections: the supplementary role of the microbiology laboratory. BMC Infect Dis. 2015;15:5. doi: 10.1186/s12879-014-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tolera M., Marami D., Abate D., Dheresa M. Are Invasive Procedures and a Longer Hospital Stay Increasing the Risk of Healthcare-Associated Infections among the Admitted Patients at Hiwot Fana Specialized University Hospital. Eastern Ethiopia? Adv Prev Med. 2020;2020 doi: 10.1155/2020/6875463. [DOI] [PMC free article] [PubMed] [Google Scholar]