Abstract
Purpose of review
Review recent literature on role of indirect calorimetry (IC) in critical care nutrition management.
Recent findings
Critical illness demands objective, targeted nutritional therapy to prevent adverse effects of under-/over feeding. Thus, all recent societal guidelines recommend IC use to determine energy needs. Very recently, IC technology has finally evolved to allow for accurate, simple, and routine utilization in a wider range of ICU patients. Recent data continues to confirm poor correlation between measured and equation-predicted energy expenditure (EE) emphasizing need for IC to be standard of care. This may be particularly true in COVID-19, where significant progressive hypermetabolism and variability in EE has been shown. Metabolic physiology can change frequently during ICU stay in response to changes in clinical condition or care. Thus, repeated longitudinal IC measures are needed throughout ICU stay to optimize care, with initial data showing improved clinical outcomes when IC-targets are utilized.
Summary
Personalized ICU care demands objective data to guide therapy. This includes use of IC to determine EE and guide ICU nutrition therapy. Long-awaited new innovations in IC technology should finally lead to IC to becoming a fundamental component of modern ICU standard of care and clinical research moving forward.
Keywords: Indirect calorimetry, Metabolism, Nutrition, ICU, COVID-19
Introduction
The use of indirect calorimetry (IC) or the metabolic cart as a monitor for resting energy expenditure (EE) and a guide for caloric dosing in critically ill patients is undergoing a “rebirth” and rapid growth from both a scientific (PubMed results on “indirect calorimetry AND ICU” increased with 263% in the last 10 years) and clinical recommendation perspective (stimulated by recommendations by European, American and Canadian nutrition societies) [1**, 2]. An excellent recent narrative review on IC principles and modern routine use was recently published by Achamrah et al entitled “Indirect calorimetry: The 6 main issues”. This review demonstrated rapidly evolving knowledge on technical IC procedures and interpretation is now available to ensure safe use of IC as a routine monitor in ICU[3**]. As an example, the COVID-19 pandemic of 2020 obliged the ICU nutrition world to launch new targeted guidelines for nutrition therapy in COVID-19 ICU patients. Throughout the year ICU nutrition protocols were launched, most all of which included the key role of indirect calorimetry (Table 1). COVID-19 guideline authors confirm the essential role of indirect calorimetry but suggest key safety precautions be taken to optimally use in this new pandemic illness.
Table 1.
List of examples of nutritional guidelines on COVID-19 patients referring to indirect calorimetry
| Title | Authors Journal |
Publication Online/Final | Statement about Indirect Calorimetry |
|---|---|---|---|
| ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection | Barazzoni et al. Clinical Nutrition |
March 2020 June 2020 |
Energy needs can be assessed using indirect calorimetry if safely available with ensured sterility of the measurement system |
| Nutrition Therapy in Critically Ill Patients with Coronavirus Disease (COVID-19) | Martindale et al. JPEN |
May 2020 Sept 2020 |
While energy requirements can ideally be determined by indirect calorimetry, this technology would involve contamination of equipment and additional exposure to healthcare providers. Thus, we recommend utilizing weight-based equations instead of indirect calorimetry to estimate energy requirements as a practical matter for the COVID-19 patients. |
| Nutrition Support in the ICU—A Refresher in the Era of COVID-19 | Micic et al. Am J Gastroenterol |
July 2020 Sept 2020 |
Although energy expenditure is best measured by indirect calorimetry in critically ill patients, the prolonged time needed for these measures increases clinician risk for viral exposure and is contrary to the principle of “clustering care,” in which patient care is bundled to limit provider exposures. Consider indirect calorimetry if prolonged intubation (>7 days) |
| Nutrition of the COVID-19 patient in the intensive care unit (ICU): a practical guidance | Thibault et al. Crit Care |
July 2020 July 2020 |
IC is the reference method to assess the energy requirements in the non-COVID-19 ICU patients Indirect calorimetry should be proposed only for patients staying for more than 10 days in the ICU or those on full parenteral nutrition (PN) to avoid overfeeding. |
| Easy-to-prescribe nutrition support in the intensive care in the era of COVID-19 | De Watteville et al Clin Nutr Espen |
July 2020 Oct 2020 |
Due to the lack of resources and the high risk of contagion, in- direct calorimetry (IC) measurements were not used to measure patients’ energy expenditure. |
| Practical guidance for the use of indirect calorimetry during COVID 19 pandemic | Singer P Clin Nutr Exp |
July 2020 Oct 2020 |
It is mandatory to ensure health professional safety while assessing resting energy expenditure using metabolic monitors. Indirect calorimetry (IC) remains the best tool to assess resting energy expenditure in critically ill patients and ESPEN as well as ASPEN societies recommend its use. |
A New Innovation in ICU Metabolic and Nutrition Care: The Creation of a New Generation Metabolic Cart
Predictive equations for measured resting energy expenditure (REE) have repeatedly failed to show reasonable correlation with IC measured values [4,5,6*,7*,8**], This data continues to grow and has recently been shown again by the work of Singer et al [8**] and others [6*, 7*]. It continues to reinforce the inaccuracies of predictive equations to determine ICU nutrition targets as well as the need for routine IC use [6*, 7* 8**]. The ventilator-derived carbon dioxide consumption (EEVCO2) method to calculate EE seemed promising as an alternative to a separate measurement by IC. In a large prospective cohort study, the mean EE by IC and by EEVCO2 was 511 kcal. This unfortunately is clinical unacceptable and indicates it is not an valid alternative to true IC measures. EEVCO2 overestimates EE and the introduction of the food quotient did not improve performance [9*]. Thus, it is clear that longitudinal IC measures are needed to accurately target nutrition therapy in the ICU setting.
Unfortunately, recent studies have shown current commercially available IC’s are often inaccurate [10, 11] and the inconveniences and challenges of routine ICU IC measurements (i.e. complex maintenance, challenging calibration, long warm up duration, large device size, and limitation of Fraction of Inspired Oxygen (FiO2) etc.) have led to significant challenges to routine IC use in ICU practice[12],[13]. To address this critical need for a next generation IC device, an ambitious undertaking was launched uniting academic ICU nutrition leaders with industry innovation experts to address this vital deficiency in ICU nutrition care. This International Multicentric Study Group for Indirect Calorimetry (ICALIC) set out to develop an accurate, user-friendly, reasonable cost, reliable metabolic cart (IC) to measure energy targets and metabolic measures in critically ill and other hospitalized patients. The result of this endeavor was the development of the innovative next-generation Q-NRG® IC device (Baxter, USA and COSMED Inc, Italy), which has received U.S. Food and Drug Administration (FDA) approval and has recently become available worldwide [13].
The new device was rigorously validated versus the gold-standard of mass spectroscopy for analytical performance and accuracy. It allows accurate IC measurements in a much wider range of patients as it showed accurate measurements at FiO2 delivery of up to 70%, extending the longstanding traditional ranges of most existing IC devices where use is limited to FiO2 ≤ 60% [14**]. A comparison of the performance of the new generation Q-NRG IC device versus existing IC devices in clinical practice was recently described in a new publication [15**]. The study examined real-world IC device performance between the new Q-NRG IC and existing IC devices in six academic ICU centers across three continents. The new metabolic cart demonstrated much shorter measurement periods to yield accurate steady state EE results in mechanically ventilated ICU patients compared to existing IC devices. (The Q-NRG was able to deliver accurate, steady state measures in 5–10 minutes versus > 35 minutes in most other IC devices). Current data indicates the new Q-NRG device fills a longstanding void in ICU and clinical nutrition care as the only commercially available IC device tested against mass spectrometry to ensure gas accuracy, while being simple and easy-to use for longitudinal IC measures in a range of patients in and out of the ICU environment. These characteristics finally allow for wide-spread implementation of IC for the critical ill patients to optimize prescription of nutrition therapy via objective measurement of energy targets, thus potentially limiting poor clinical outcomes due to the common risk of under- or overfeeding.
New Data for Use of Indirect Calorimetry in CRRT and ECMO:
Effects of Continuous Renal Replacement Therapy (CRRT) such as CO2 extraction, citrate use and pre- and/or post-dilution fluid(s) can effect IC measurements and/or mREE [16**]. The role of CO2 extraction on mREE has recently been determined to be quite minor, leading to a difference of 34 to 44 kcal/day (only 2 to 3% of REE) depending on dilution fluids[17]. As this is a minimal effect, a correction factor for REE during CRRT should not be required [16**], [17**]. Citrate used in CRRT, is known to alter metabolism, thus IC is indicated to detect metabolic changes and adapt nutritional therapy [16**]. Assessing accurate energy targets via IC in extracorporeal membrane oxygenation (ECMO), has also been addressed successfully by both a German approach based via blood gas analysis and IC measurement [18] and the double IC-measurement technique of De Waele et al. [19*]. The technical details of IC measurement on ECMO are thoughtfully explained in the recent narrative review of Moonen et al [20**].
Use of Indirect Calorimetry in Severe COVID-19 ICU Patients
As described above, the recent worldwide COVID-19 pandemic [21*] has led to an increased emphasis on the need for accurate longitudinal IC-measurements to guide nutrition care in this challenging new ICU condition. To assess the metabolic phenotype of this new pandemic disease, Dr. Wischmeyer and the LEEP-COVID study team recently utilized the new-generation Q-NRG IC device (Figure 1) to conduct the first longitudinal study of mREE and other metabolic measures in COVID-19 ICU patients (the LEEP-COVID study- ClincalTrials.Gov NCT04350073) [22**]. This study was the first to demonstrate that longitudinal IC measures can be routinely and safely obtained in mechanically ventilated COVID-19 ICU patients [22**]. Initial results from the LEEP-COVID study show that in the first ICU week following intubation mREE was between 15–20 kcal/kg (for Actual body weight (ABW) in BMI<30 and Adjusted BW (AdjBW) in obese subjects) in COVID-19 ICU patients. A significant and persistent increase in energy needs (hypermetabolism) and marked variability in mREE values was observed following the first week post-intubation. Distinct from data in smaller studies of other ICU populations [23], the hypermetabolism and mREE in COVID-19 patients following the first week of intubation persisted, and actually continued to rise during the second and third ICU weeks (often with a mean mREE=150% predicted REE (pREE) by 3rd ICU week post-intubation). Some patients were observed to have mREE of greater than two-fold that pREE by commonly utilized predictive equations (i.e., Harris-Benedict equation (HBE)). This finding is consistent with another small trial of with a median mREE was 4044 Kcal/day which was 235.7% ± 51.7% of pREE [24*].
Figure 1. Conduct of indirect calorimetry (IC) in COVID-19 ICU patients.

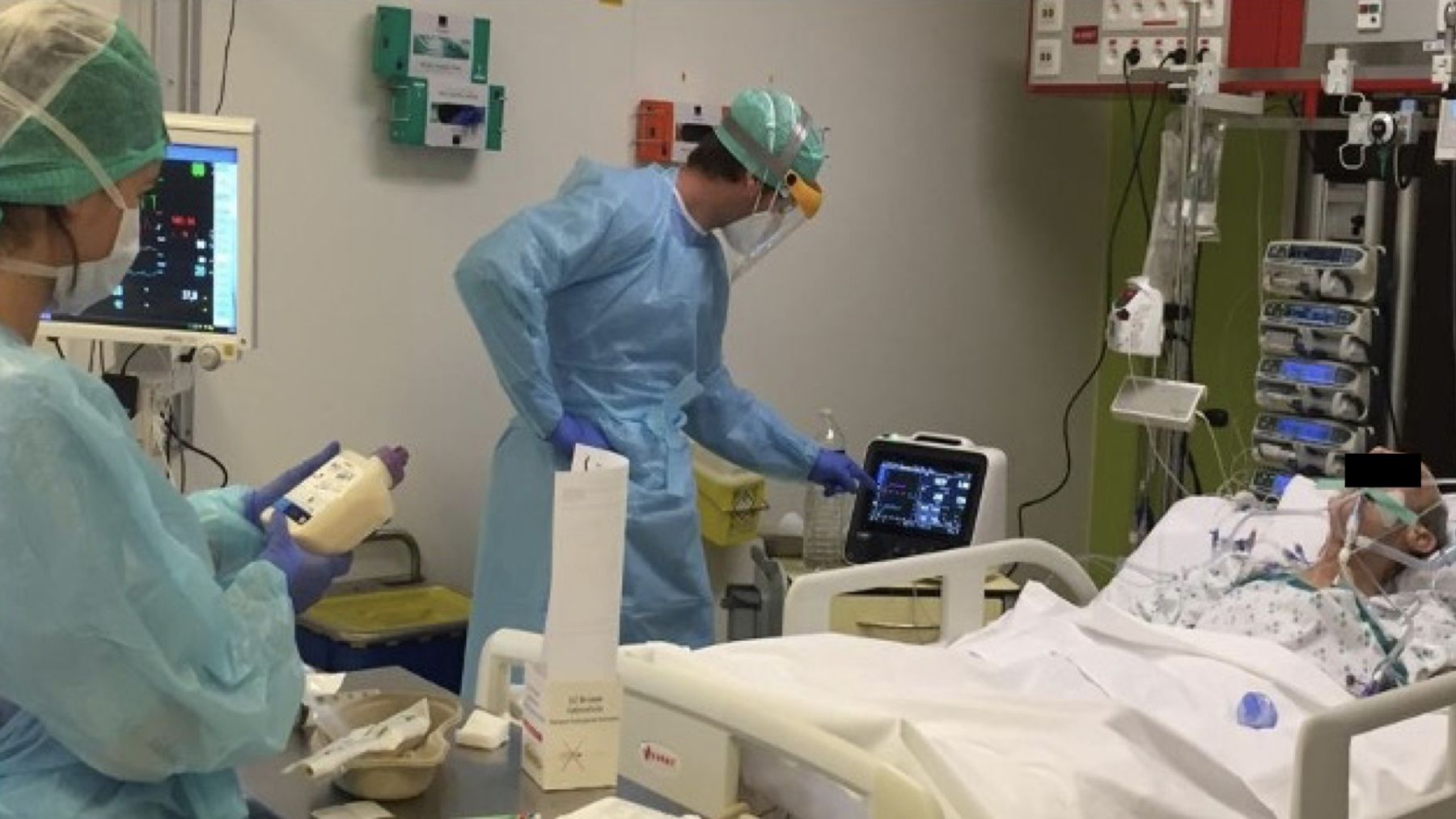
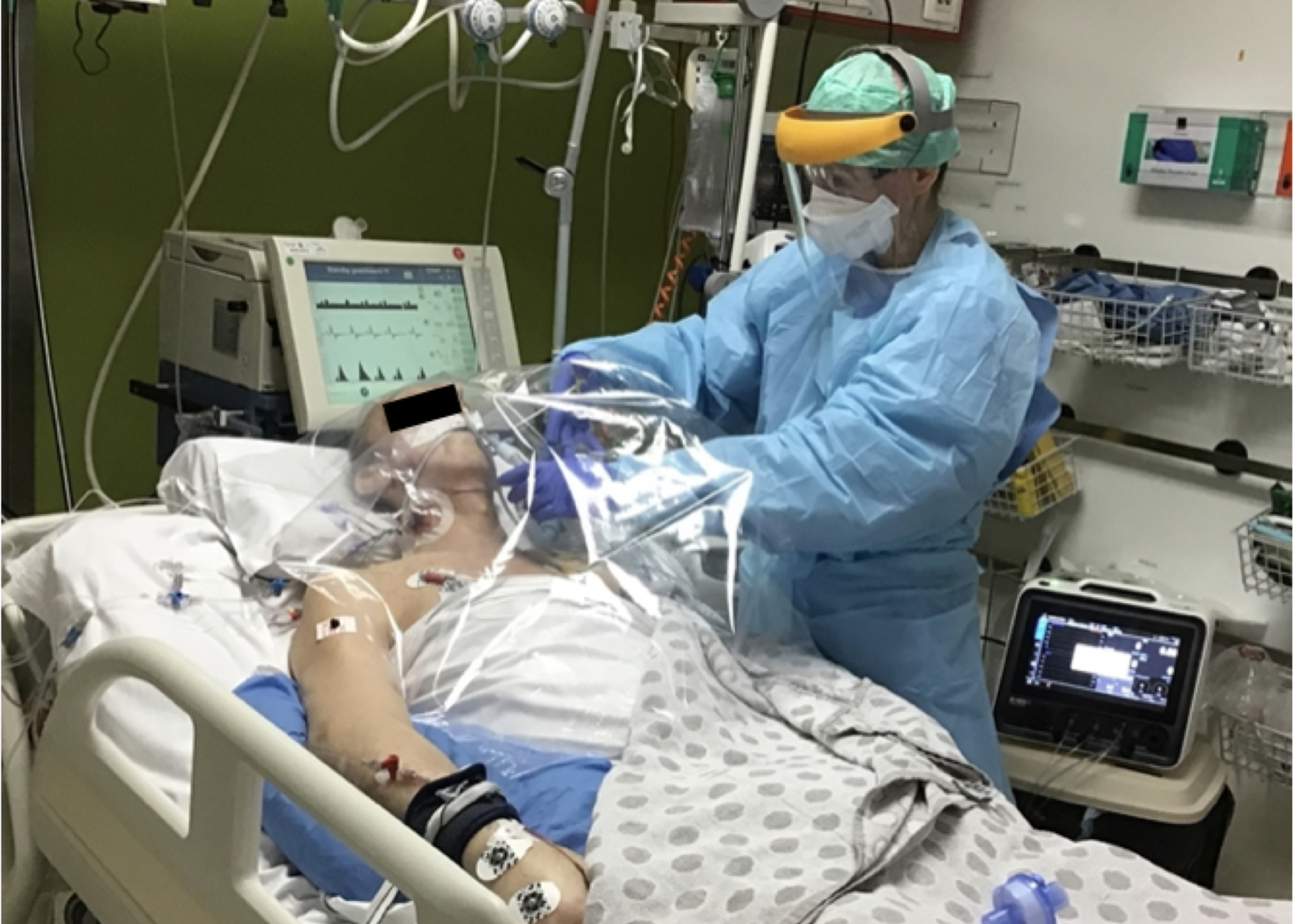
. 2.A.) Jeroen Molinger preparing to perform IC measurements using new Q-NRG IC device in COVID-19 ICU patients at Duke University. 2.B.) Dr. Joop Jonckheer and Dietitian Miss Joy Demol developing nutritional strategy guided by IC at Brussels ICU. 2.C.) Prof. Dr. Elisabeth De Waele performing IC in a ventilated COVID19 ICU patient using safety first approach at Brussels ICU
Consistent with aforementioned studies showing the inaccuracies of predictive energy equations in ICU populations [8**], the HBE routinely and markedly underpredicted mREE following the first ICU week. Interestingly, the HBE often overpredicted energy targets in the first ICU week post-intubation in COVID-19 patients. This is another examples showing current utilized predictive equations do not accurately predict energy needs in ICU patients [4,5,6*,7*,8**], and predictive equations appear to be leading to significant over- and under-feeding in COVID-19 throughout their ICU stays as well. Initial LEEP-COVID data demonstrate that mREE does not appear to be affected by paralysis or sedation and does not show a relationship to severity of organ failure. This is consistent with previously published data demonstrating that neuromuscular blockade appears to have a very minor effect on mREE [25*].
Dr. De Waele and Dr. Jonckheer also began to use IC in COVID-19 patients in March 2020 to guide optimal nutritional therapy (Figure 1). Original retrospective analysis of IC data in COVID-19 collected in Brussel ICU reveals a wide variation of correlation between measured and predicative equation calculated EE. This variability in mREE was consistently observed in the first and second COVID-19 waves in the Brussels ICU (Figure 2). A mean mREE of 21 kcal/kg/day over 19 measurements was presented in September 2020 at the European Society of Parenteral and Enteral Nutrition (ESPEN) congress (Figure 3).
Figure 2.
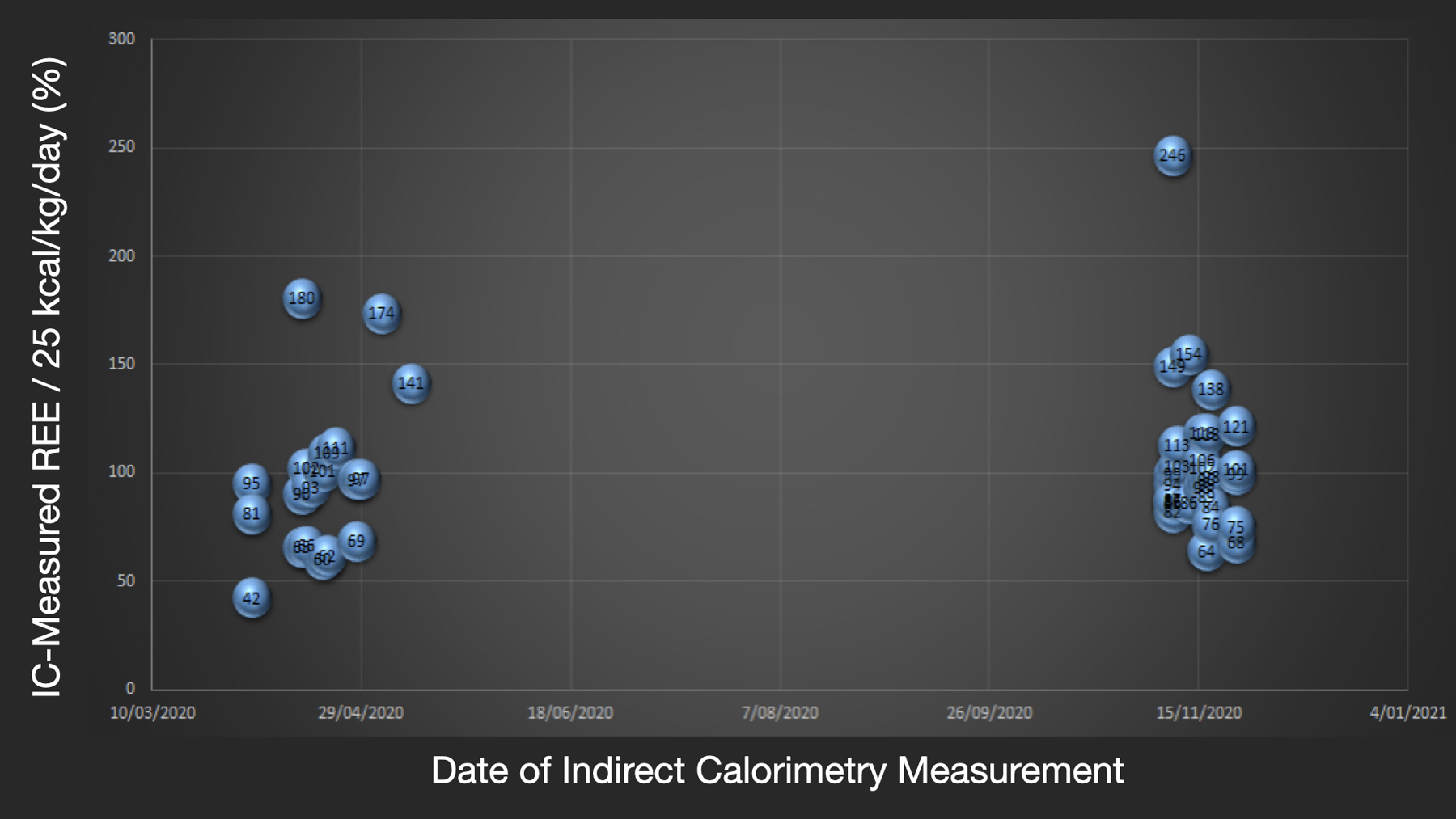
Resting Energy Expenditure (REE) in COVID-19 ICU patients measured by indirect calorimetry (IC) in first and second wave of COVID-19 in Brussels ICU.
Figure 3.
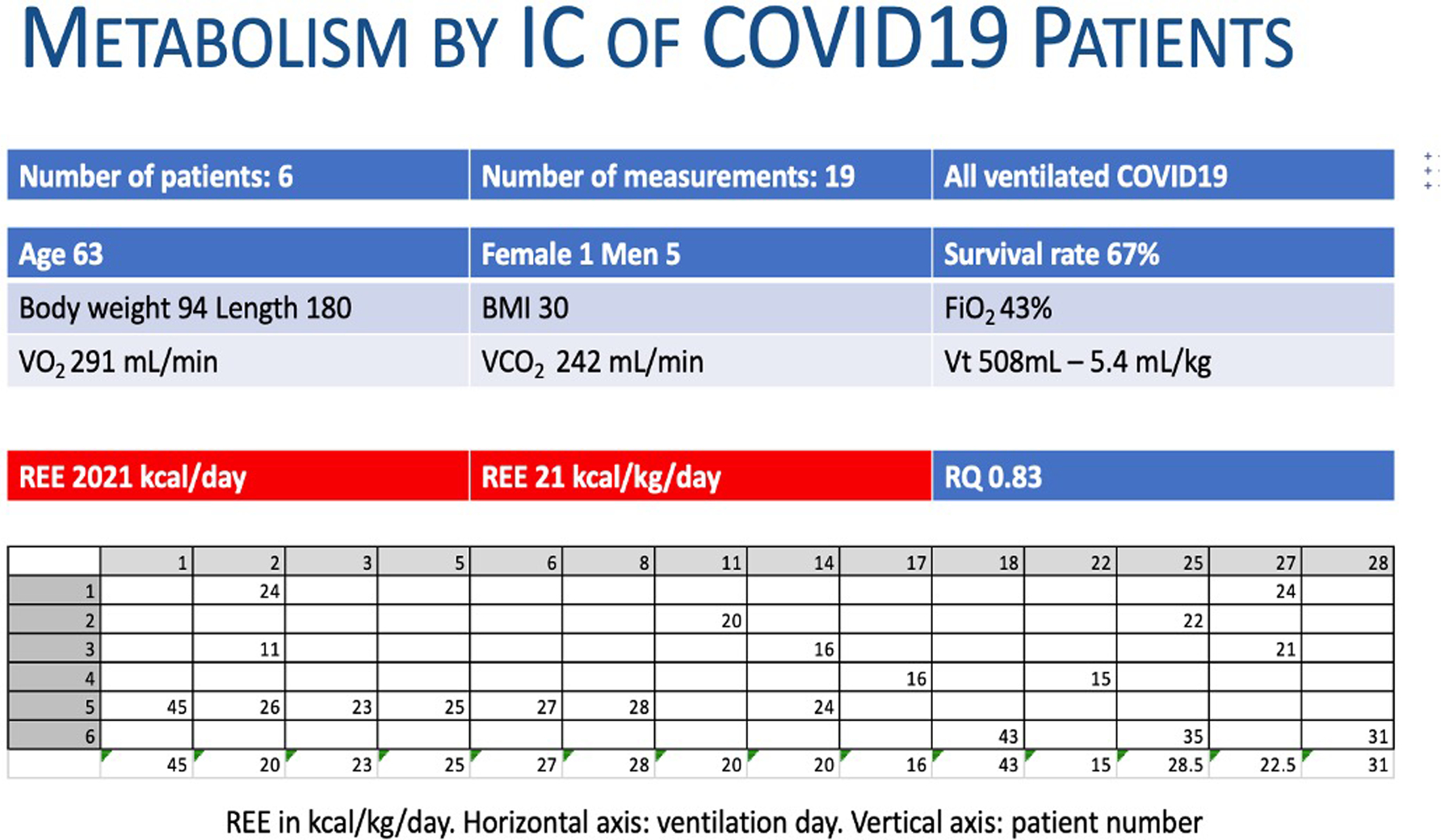
Resting Energy Expenditure (REE) of 19 first indirect calorimetry (IC) measurements in COVID-19 patients.
Additional data on mREE in the severe COVID-19 patient in the ICU prior to intubation is urgently needed as many patients are now being managed for considerable periods on non-invasive respiratory support, such as Bilevel Positive Airway Pressure (Bi-PAP) and high flow nasal cannula oxygen delivery. Further, an understanding of the metabolic needs and mREE in the post-ICU COVID survivor is also a critical area for future research to optimize recovery of patients from this ongoing pandemic. Overall, the LEEP-COVID study [22**] and other initial data reported here demonstrates that routine, longitudinal IC use to accurately assess EE [1**],[15**] should become the standard of care to personalize nutrition therapy in COVID-19 and improve patient care in these challenging patients.
The Physiology of REE Throughout Phases of Care in the ICU and Need for Repeated Longitudinal IC Measures
REE during the ICU journey is driven by fundamental metabolic physiology. Different phases during the stay of the patient have been described and influence the caloric delivery. The acute phase, which starts with ICU admission disturbs metabolic homeostasis and is accompanied by rapid catabolism during which well-nourished patients can endogenously generate a significant portion of required non-protein calories [1**, 2]. Although it is currently impossible to measure this initial early endogenous nutrient production, the current ESPEN/ASPEN ICU guidelines suggest hypocaloric (~70% REE) feeding during the early acute phase to prevent the risk of overfeeding [1**, 2]. This has been the subject of a recent review citing the lack of studies and evidence supporting permissive underfeeding in sepsis and need for additional high quality trials in this area [26*].
Various predictive equations have been proposed to calculate predicted REE (pREE) in the absence of gold-standard IC-measured REE, but as mentioned these have been found to be consistently inaccurate leading to harmful over- and under-feeding[1**, 4,5,6*,7*,8**],. The reason for these inaccuracies is these predictive equations are not able to account for the rapidly changing physiology of the ICU patient. Indeed, as shown in Figure 4 multiple factors have been found to influence REE [3**, 16**, 27**]. Endogenous physiologic changes such as increased temperature, increased minute volume, and increased heart rate all can elevate metabolic rate and increase mREE [27**]. In addition to these physiological parameters, clinical interventions such as the use of citrate during renal replacement (CRRT) therapy, caloric intake, vasopressor/inotrope use and/or rehabilitation activity will also increase mREE. [16**, 17**] [28**]. Metabolism can be minorly reduced (~6.6%) by paralysis [25] and possibly with deep sedation and lower core temperature (hypothermia) if compensating mechanism like shivering are disabled. [3**, 16**, 27**]. The only tool to assess the effect of these ever-evolving modulators of metabolism and REE is the metabolic cart (IC).
Figure 4. Key factors affecting resting energy expenditure (REE).
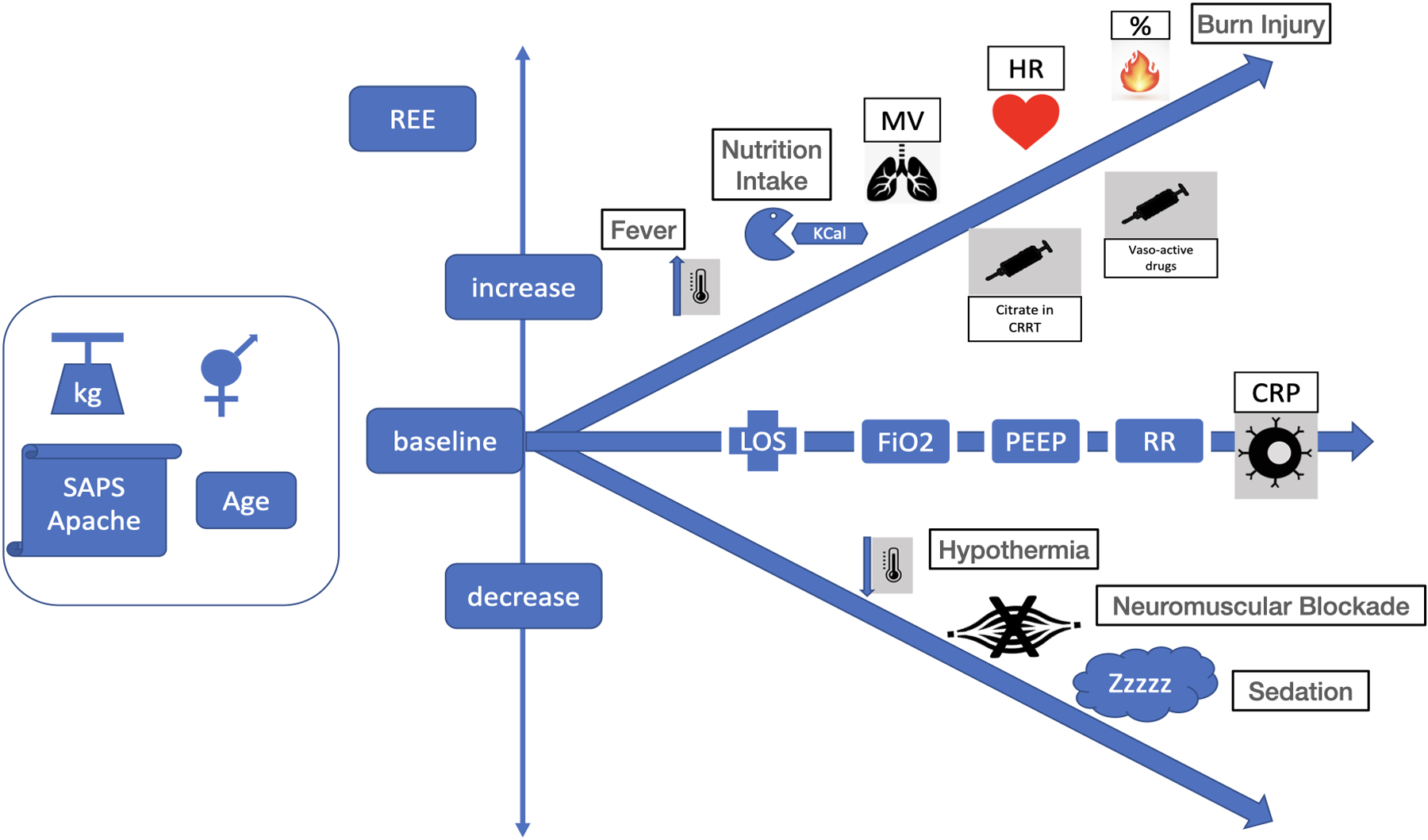
Legend: kg: kilogram, REE: resting energy expenditure, Kcal: kilocalories, MV: minute volume, HR: heart rate, CRRT: continuous renal replacement therapy, LOS: length of stay, FiO2: inhaled oxygen concentration, PEEP: positive end expiratory pressure, RR: respiratory rate, CRP: c-reactive protein,
The continuous changes in physiology and clinical care of the ICU patient also demands that repeat, longitudinal IC measurements should be performed when any significant change in clinical condition (i.e., new infection or surgery) or clinical care of the patient occurs. Indeed, the simplified time based model proposed by authors such as van Zanten and Wischmeyer [29**, 30] in previous publications does not take into account the rapidly evolving and ever-changing clinical condition of the majority of ICU patients. This is exemplified by recent data in critically ill COVID-19 patients, where individual metabolism has been shown to vary greatly day-to-day (by as much as 1000 kcal/day) during ICU stay [22**]. This was commonly related to changes in clinical condition, new fever, new septic episodes, and increased energy expenditure due to increased physical activity (such as ventilator weaning). Therefore, we propose an evolution of the existing simplified timeline models of nutrition delivery that currently exist [29**, 30] in Figure 5. This new evolved care nutrition care schema includes longitudinal IC measures when changes in metabolism could occur to guide energy targets and delivery. Indeed, time since admission alone has not found to be associated with REE[27]. Thus, it is key to repeat REE measurements via metabolic cart (IC) when changes during the patient’s journey in the ICU occur. More-over, a new catabolic event (i.e., septic shock event) should trigger the nutritional therapist to make new measurements with the metabolic cart and caloric prescription should probably be tailored back to 70% of REE during this acute phase
Figure 5. Personalized Indirect Calorimetry-Guided Critical Care Nutrition Algorithm.
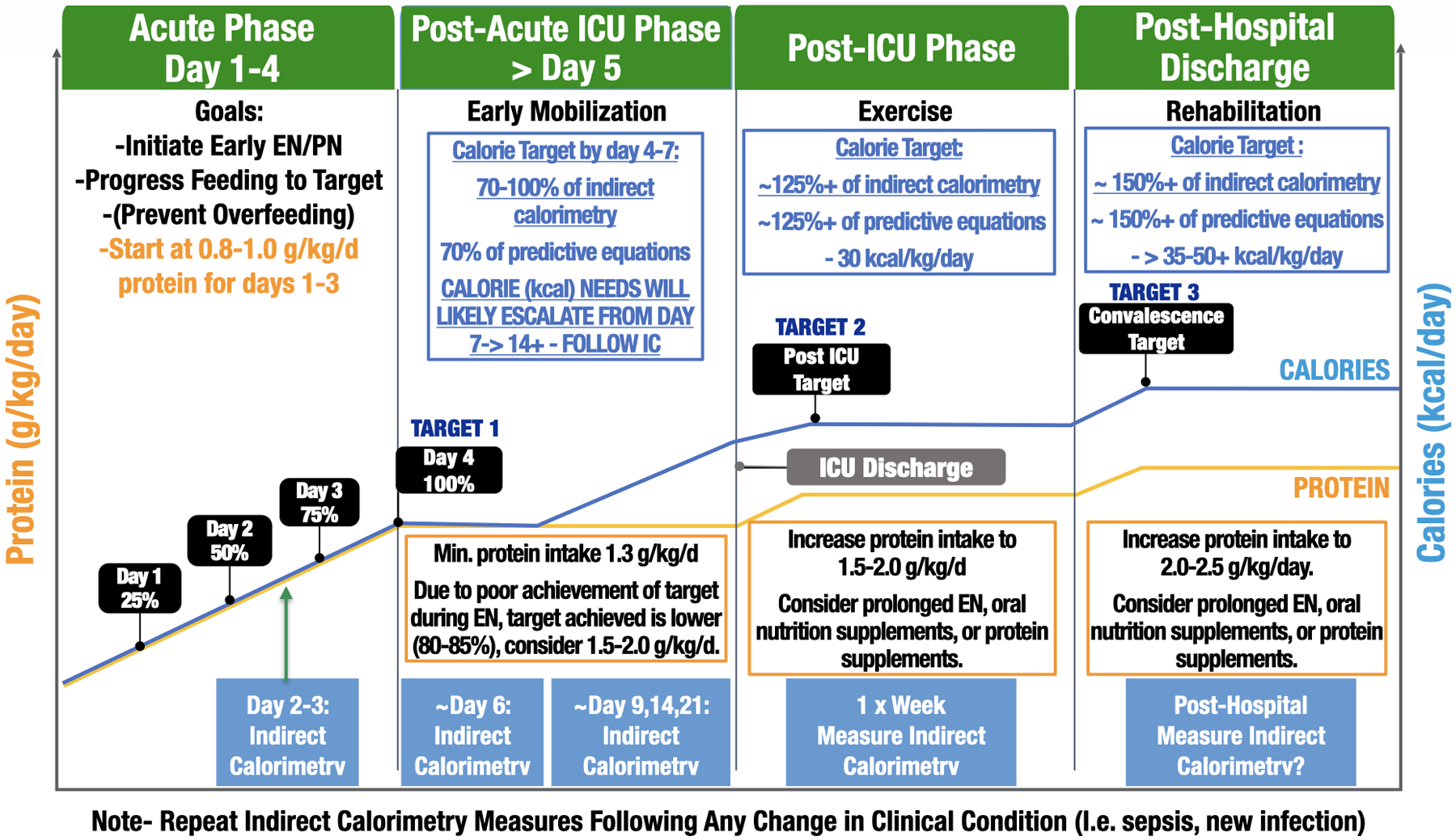
(derived from recent evidenced-based ICU nutrition reviews [29, 30, 37]. Please note: Suggested IC measurement days are intended as general guidelines to create consistency in measurement throughout patient stay. Ideally, IC measurements should be performed 2–3 times/week and when there is a significant clinical change patient status, such as a new infection, sepsis episode, or increased physical activity/rehabilitation.
Role of Personalized Nutrition via Use of Indirect Calorimetry to Improve Outcome in the ICU?
Review of recent data for the use of IC to improve clinical and functional outcomes includes a recent meta-analysis in 8 randomized controlled trials (RCTs) enrolling 991 subjects that demonstrated IC-targeted nutrition delivery reduced ICU mortality [31**]. An additional study by Fetterplace et al showed that minimization of nutrition delivery deficits may decrease ICU-AW when IC was used to set energy targets[32**]. To this point, evidence supporting clinical outcomes benefits of IC-use has been limited by long-standing practical challenges to routine IC-use and concerns around accuracy of previously existing IC-devices. Thus, large-scale clinical evidence utilizing IC to improve clinical and ultimately functional outcomes is urgently needed. Given the new wide availability of an accurate, simple, and practical next generation IC-device, we hope larger-scale trials exploring the role of IC-targeted ICU nutrition delivery to improve clinical and functional outcomes will be initiated. Further, we propose that all future clinical trials of nutrition delivery in critical illness should be conducted with objectively-defined nutrition targets guided by longitudinal metabolic cart (IC) measures.
As metabolic cart technology has recently evolved, the design of future ICU nutrition trials also must evolve to move beyond mortality as a primary endpoint. The use of IC-guided targets to adequate deliver caloric needs has been shown to support reduction of catabolism and protein breakdown, which in turn should theoretically increase muscle preservation and should enhance functional recovery [33*]. Thus, it is essential that future clinical trials of ICU nutrition therapy should focus on muscle function and quality of life as primary endpoints rather than mortality. These should include measures of ICU acquired weakness (ICU-AW), such as muscle strength, 6-minute walk distance, EQ-5D, and activities of daily life as described by the National Institutes of Health (NIH) funded Improving Long-Term Outcomes Research for Acute Respiratory Failure initiative to standardize long term outcome reporting in ICU trials. (see project website for details on evidenced-based core outcome set of assessments for ICU-AW and ICU survivorship- www.IMPROVELTO.com). Indeed, recent literature and ICU clinical trials groups have indicated mortality may no longer be a useful primary outcome in for future ICU trials [34]. Thus, we should heed this call for a focus on QoL-based primary outcomes in ICU nutrition trials. Examples of the challenges of mortality as primary endpoint in other specialties include that craniectomy in ischemic brain injury decreases mortality, but may concomitantly increase morbidity which is not an optimal goal. Therefore, trials based on functional outcomes are needed to guide individual therapy for these neurologic affected critical ill patients[35]. Hence the quote “Are we creating victims or survivors” is of crucial importance not only in how we deliver care, but also how we design our future clinical trials[36].
Conclusion:
Given recent innovations in IC technology and wide availability of a new generation IC device, it is essential that longitudinal IC measures before, during and after ICU care become the new worldwide standard of care to guide nutrition care. This position is well-described and advocated for in the recent position paper by Wischmeyer et al advocating that metabolic cart measures should become the new standard of care in the ICU[37**]. We as the authors of this review agree and conclude that longitudinal IC measures should become as ubiquitous in their use and reporting on ICU rounds as blood pressures and heart rates are reported and used to guide vasopressor therapy and other ICU care. As we have often said on rounds, we would not give vasopressors without measuring blood pressure, neither should we be blindly delivering nutrition without objective IC measures to guide its optimal administration. It is only with increased implementation of objective nutrition and metabolic measurement data, such as via longitudinal IC measures and routine bedside ultrasound-derived muscle mass/energy state measures[28], that we will ensure each ICU patient receives optimal personalized nutrition care that delivers the right nutrition, in the right patient, at the right time to best optimize clinical outcomes.
Key messages.
Indirect calorimetry is the gold standard by which to measure energy expenditure and is universally recommended for use in the ICU by all existing societal nutrition guidelines
New innovations in metabolic cart technology have occurred recently, including the development of a new generation indirect calorimeter that is accurate, self-calibrating, and simple to operate providing mREE measurements rapidly in a wider range of ICU patients
Indirect calorimetry is safe and feasible in COVID-19 patients, who demonstrate progressive hypermetabolism and marked variability in energy needs when measured via IC
Indirect calorimetry derived REE should always be interpreted within the framework of the physiological condition of the patient and repeated longitudinal IC measures are needed during ICU stay to account for the ever-changing physiology of the critically ill patient
Given data for inaccuracies of predictive equations and wide availability of new generation metabolic cart device, longitudinal indirect calorimetry should become the new standard of care to personalize and optimize ICU nutrition therapy in clinical care and future ICU nutrition research trials
Acknowledgements:
Jeroen Molinger for extraordinary commitment to improving care of COVID-19 patients worldwide by spending thousands of hours in COVID-19 patient ICU rooms performing indirect calorimetry and other metabolic measures for LEEP-COVID study. Entire LEEP-COVID research team at Duke University for all their work on collection and analysis of IC and other metabolic measures in COVID-19 ICU patients.
Disclosures:
Dr. Wischmeyer reports receiving investigator-initiated grant funding related to this work from National Institutes of Health, Canadian Institutes of Health Research, Abbott, Baxter, and Fresenius. Dr. Wischmeyer has served as a consultant to Abbott, Fresenius, Baxter, Cardinal Health, and Nutricia, for research related to this work. Dr. Wischmeyer has received unrestricted gift donation for nutrition research from Musclesound. Dr. Wischmeyer has received honoraria or travel expenses for CME lectures on improving nutrition care from Abbott, Baxter, Danone-Nutricia and Nestle.
Prof. Dr. De Waele reports receiving investigator-initiated grant funding related to this work from National Institutes of Health, Baxter, Nutricia and Fresenius. Prof. Dr. De Waele has served as a consultant to Baxter, Nutricia, Fresenius and Cardinal Health, and for research related to this work. Prof. Dr. De Waele has received honoraria or travel expenses for CME lectures on improving nutrition care from Baxter, Danone-Nutricia and Fresenius.
Footnotes
Conflict of interest:
JJ declares hereby to have no conflict of interest.
References and recommended reading:
- **1.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C et al. : ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019, 38(1):48–79. [DOI] [PubMed] [Google Scholar]; Recent comprehensive guidelines on nutrition delivery in critically ill patients.
- 2.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA et al. : Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016, 40(2):159–211. [DOI] [PubMed] [Google Scholar]
- **3.Achamrah N, Delsoglio M, De Waele E, Berger MM, Pichard C: Indirect calorimetry: The 6 main issues. Clin Nutr 2021, 40(1):4–14. [DOI] [PubMed] [Google Scholar]; Excellent recent review of key issues in utilization of indirect calorietry in clinical practice
- 4.Fraipont V, Preiser JC: Energy estimation and measurement in critically ill patients. JPEN J Parenter Enteral Nutr 2013, 37(6):705–713. [DOI] [PubMed] [Google Scholar]
- 5.Guttormsen AB, Pichard C: Determining energy requirements in the ICU. Curr Opin Clin Nutr Metab Care 2014, 17(2):171–176. [DOI] [PubMed] [Google Scholar]
- *6.Israfilov E, Kir S: Comparison of Energy Expenditure in Mechanically Ventilated Septic Shock Patients in Acute and Recovery Periods via Indirect Calorimetry. JPEN J Parenter Enteral Nutr 2020. [DOI] [PubMed] [Google Scholar]; Retropective study of indirect calorimetry measurements in 28 septici shock patients showing measuerd resting energy expenditure (mREE) increases in septic shock. Significant differences existed between mREE and equation predicted energy expenditure (pREE) which were not correlated. The mREE was higher in the acute period. Despite the increasing energy requirement throughout ICU stay, the pREE was consistently well below mREE.
- *7.Smetana KS, Hannawi Y, May CC: Indirect Calorimetry Measurements Compared With Guideline Weight-Based Energy Calculations in Critically Ill Stroke Patients. JPEN J Parenter Enteral Nutr 2020. [DOI] [PubMed] [Google Scholar]; Study of of indirect calorimetry measurements in stroke patients in ICU comparing IC-derived mREE and two weight based predictive feeding doses (25 kcal/kg and 30 kcal/kg/d). The low weight based strategy (25 kcal/kg/d) consistently underpredicted IC-determined mREE in intracerebral hemorrhage and subarachnoid hemorrhage patients, but not in acute ischemic stroke. 30 kcal/kg/day better approximated needs in intracerebral hemorrhage and subarachnoid hemorrhage patients.
- **8.Zusman O, Kagan I, Bendavid I, Theilla M, Cohen J, Singer P: Predictive equations versus measured energy expenditure by indirect calorimetry: A retrospective validation. Clin Nutr 2019, 38(3):1206–1210. [DOI] [PubMed] [Google Scholar]; The performance of IC-measured mREE was compared with predictive nutrition equations in a total of 3573 REE measurements in 1440 ICU patients. The Faisy equation had the least mean difference (90 Kcal); Harris-Benedict had the highest correlation (only 52%) and agreement (only 50%) and Jolliet the highest concordance (only 62%). Agreement within 10% of caloric needs was met only in a third of patients. This paper showed predictive equations have low performance when compared to IC-measured mREE in ICU patients and IC is needed for accurate estimation of REE in the ICU.
- *9.Koekkoek WAC, Xiaochen G, van Dijk D, van Zanten ARH: Resting energy expenditure by indirect calorimetry versus the ventilator-VCO(2) derived method in critically ill patients: The DREAM-VCO(2) prospective comparative study. Clin Nutr ESPEN 2020, 39:137–143. [DOI] [PubMed] [Google Scholar]; Large prospective cohort study comparing EE via IC and ventilator-derived carbon dioxide consumption (EEVCO2) method to calculate Energy Expenditure.. The mean difference between 2 techniques was large (511 kcal). EEVCO2 overestimates EE and the introduction of the food quotient did not improve performance. This large clinical unacceptable difference indicates EEVCO2 is not a valid alternative to gold-standard IC measures.
- 10.Sundstrom M, Tjader I, Rooyackers O, Wernerman J: Indirect calorimetry in mechanically ventilated patients. A systematic comparison of three instruments. Clin Nutr 2013, 32(1):118–121. [DOI] [PubMed] [Google Scholar]
- 11.Graf S, Karsegard VL, Viatte V, Heidegger CP, Fleury Y, Pichard C, Genton L: Evaluation of three indirect calorimetry devices in mechanically ventilated patients: which device compares best with the Deltatrac II((R))? A prospective observational study. Clin Nutr 2015, 34(1):60–65. [DOI] [PubMed] [Google Scholar]
- 12.De Waele E, Spapen H, Honore PM, Mattens S, Van Gorp V, Diltoer M, Huyghens L: Introducing a new generation indirect calorimeter for estimating energy requirements in adult intensive care unit patients: feasibility, practical considerations, and comparison with a mathematical equation. J Crit Care 2013, 28(5): 884 e881–886. [DOI] [PubMed] [Google Scholar]
- 13.Oshima T, Berger MM, De Waele E, Guttormsen AB, Heidegger C-P, Hiesmayr M, Singer P, Wernerman J, Pichard C: Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clinical Nutrition 2017, 36(3):651–662. [DOI] [PubMed] [Google Scholar]
- **14.Delsoglio M, Dupertuis YM, Oshima T, van der Plas M, Pichard C: Evaluation of the accuracy and precision of a new generation indirect calorimeter in canopy dilution mode. Clin Nutr 2020, 39(6):1927–1934. [DOI] [PubMed] [Google Scholar]; Key study evaluating study the accuracy and intra- and inter-unit precision of the new Q-NRG IC device in canopy dilution mode in vitro and in spontaneously breathing adults versus gold-standard mass spectroscopy. Results showed both in vitro and in vivo measurements of VO2, VCO2, RQ and EE on multiple Q-NRG units showed minimal differences compared to expected values and mass spectroscopy with very low intra- and inter-unit variability. These results confirm the very high accuracy and precision of the Q-NRG indirect calorimeter.
- **15.Oshima T, Delsoglio M, Dupertuis YM, Singer P, De Waele E, Veraar C, Heidegger C-P, Wernermann J, Wischmeyer PE, Berger MM et al. : The clinical evaluation of the new indirect calorimeter developed by the ICALIC project. Clinical Nutrition 2020. [DOI] [PubMed] [Google Scholar]; This multicenter study evaluated and validated use of the new indirect calorimeter (IC) device in ICU patients. Results of the study showed the Q-NRG® IC required a much shorter time (with reliable measurements in ~10 minutes) to determine energy expenditure in mechanically ventilated ICU patients versus other ICs. The authors concluded new Q-NRG® is only commercially available IC tested against mass spectrometry to ensure gas accuracy, while being easy-to use. These characteristics should allow for a much broader use of IC in order to optimize the prescription of nutritional support by limiting the risk of under- or overfeeding.
- **16.Jonckheer J, Spapen H, Malbrain M, Oschima T, De Waele E: Energy expenditure and caloric targets during continuous renal replacement therapy under regional citrate anticoagulation. A viewpoint. Clin Nutr 2020, 39(2):353–357. [DOI] [PubMed] [Google Scholar]; Key review on use of metabolc cart (IC) in patients on continuous renal replacement therapy (CRRT). Paper discusses effects of CRRT factors such as CO2 extraction, citrate use and pre- and/or postdilution fluid(s) on IC measurements and/or mREE values.
- **17.Jonckheer J, Demol J, Lanckmans K, Malbrain M, Spapen H, De Waele E: MECCIAS trial: Metabolic consequences of continuous veno-venous hemofiltration on indirect calorimetry. Clin Nutr 2020, 39(12):3797–3803. [DOI] [PubMed] [Google Scholar]; Until recently continuous renal replacement therapy (CRRT) precluded the use of IC due to several perceived limitations. This key new study investigated the impact of CRRT on , and REE to help facilitate indirect calorimetry use during CRRT. Data showed a minimal effect of CO2 removal on mREE measures. Study concluded: 1) CO2 alterations due to CRRT are clinically of no importance so no correction factor of REE is needed on CRRT; 2) IC must be performed during CVVH as CVVH seems to alter metabolism; 3) These changes may be mainly explained by the use of citrate predilution.
- 18.Wollersheim T, Frank S, Müller MC, Skrypnikov V, Carbon NM, Pickerodt PA, Spies C, Mai K, Spranger J, Weber-Carstens S: Measuring Energy Expenditure in extracorporeal lung support Patients (MEEP) - Protocol, feasibility and pilot trial. Clin Nutr 2018, 37(1):301–307. [DOI] [PubMed] [Google Scholar]
- *19.De Waele E, Jonckheer J, Pen JJ, Demol J, Staessens K, Puis L, La Meir M, Honoré PM, M LNGM, Spapen HD: Energy expenditure of patients on ECMO: A prospective pilot study. Acta Anaesthesiol Scand 2019, 63(3):360–364. [DOI] [PubMed] [Google Scholar]; Key paper describing 2 metabolic cart (ICs) device technique to measure IC values in extracorporeal membrane oxygenation (ECMO) patients.
- **20.Moonen H, Beckers KJH, van Zanten ARH: Energy expenditure and indirect calorimetry in critical illness and convalescence: current evidence and practical considerations. J Intensive Care 2021, 9(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent review of IC use in ICU and post-ICU period covering recent evidence and practical IC use considerations
- *21.Berlin D, Gulick R, Martinez F: Severe Covid-19. The New England journal of medicine 2020. [DOI] [PubMed] [Google Scholar]; Definitive early review of pathophysiology and initial treatment strategies in severe COVID-19 in critically ill patients
- **22.Whittle J, Molinger J, MacLeod D, Haines K, Wischmeyer PE, Group L-CS: Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID-19. Crit Care 2020, 24(1):581. [DOI] [PMC free article] [PubMed] [Google Scholar]; First description of longitudinal metabolic phenotype and energy expenditure in critically ill COVID-19 patients. Data shows COVID-19 ICU patients display a unique metabolic response of normo-metabolism in 1st week post-ICU admission and become markedly hypermetabolic in the 2nd and 3rd week post-ICU admission, further study showed consistent inaccuracy of predictive equations for energy need throughout ICU stay
- 23.Uehara M, Plank LD, Hill GL: Components of energy expenditure in patients with severe sepsis and major trauma: a basis for clinical care. Crit Care Med 1999, 27(7):1295–1302. [DOI] [PubMed] [Google Scholar]
- *24.Yu PJ, Cassiere H, DeRosa S, Bocchieri K, Yar S, Hartman A: Hypermetabolism and Coronavirus Disease 2019. JPEN J Parenter Enteral Nutr 2020, 44(7):1234–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]; Small study of seven intubated COVID-19 patients showing significanthypermetabolism when REE is measured by IC.
- *25.Koekkoek WAC, Menger YA, van Zanten FJL, van Dijk D, van Zanten ARH: The effect of cisatracurium infusion on the energy expenditure of critically ill patients: an observational cohort study. Critical Care 2020, 24(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]; Key study showing minimal effect of neuromuscular blockade on measured REE (via IC) of approxaimately 6.6% in ventilated, paralyzed ICU patients
- *26.van Niekerk G, Meaker C, Engelbrecht AM: Nutritional support in sepsis: when less may be more. Crit Care 2020, 24(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent review examining role of permissive underfeeding in sepsis that cites lack of clinical studies supporting this often recommended practice and discusses need for additional high quality trials in this area
- **27.Mtaweh H, Soto Aguero MJ, Campbell M, Allard JP, Pencharz P, Pullenayegum E, Parshuram CS: Systematic review of factors associated with energy expenditure in the critically ill. Clin Nutr ESPEN 2019, 33:111–124. [DOI] [PubMed] [Google Scholar]; Excellent review of key factors that influence energy expenditure in critically ill patients.
- *28.Molinger J, Pastva AM, Whittle J, Wischmeyer PE: Novel approaches to metabolic assessment and structured exercise to promote recovery in ICU survivors. Curr Opin Crit Care 2020, 26(4):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent review of new appraoches to assessment of muscle-mass and metabolism via new muscle-specific ultrasound device and cardiopumonary exercise testing (CPET) in critically ill and recovering post-ICU patients. Discusses use of these new technologies in ICU to direct structured,objective exercise and nurtrtition interventions.
- **29.van Zanten ARH, De Waele E, Wischmeyer PE: Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit Care 2019, 23(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent and comprehensive review of ICU nutrition delivery across all phases of ICU and post-ICU care
- 30.Wischmeyer PE: Nutrition Therapy in Sepsis. Crit Care Clin 2018, 34(1):107–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **31.Duan JY, Zheng WH, Zhou H, Xu Y, Huang HB: Energy delivery guided by indirect calorimetry in critically ill patients: a systematic review and meta-analysis. Crit Care 2021, 25(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]; Key recent meta-analysis of energy delivery guided by indirect calorimetry in critically ill patients. 8 randomized controlled trials (RCTs) enrolling 991 subjects were evaluated and demonstrated IC-targeted nutrition delivery reduces short-term ICU mortality. IC-guided nutrition strategies did not significantly prolong or effect the duration of mechanical ventilation, length of stay in ICU or hospital.
- **32.Fetterplace K, Beach LJ, MacIsaac C, Presneill J, Edbrooke L, Parry SM, Rechnitzer T, Curtis R, Berney S, Deane AM et al. : Associations between nutritional energy delivery, bioimpedance spectroscopy and functional outcomes in survivors of critical illness. J Hum Nutr Diet 2019, 32(6):702–712. [DOI] [PubMed] [Google Scholar]; A prospective observational study of 60 intensive care unit (ICU) patients showing cumulative energy deficit based on IC-measured energy targets was associated with increased ICU-acquired weakness, reduced functional outcomes and greater loss of fat-free mass in ventilated ICU patients. Specifically, cumulative energy deficit (per 1000 kcal) was independently associated with greater odds of ICU-acquired weakness [odds ratio (OR) = 2.1, P = 0.001], reductions in fat-free mass (−1.3 kg; 95% P = 0.02) and physical function scores (−0.6 points; P = 0.001).
- *33.Sundström Rehal M, Liebau F, Wernerman J, Rooyackers O: Whole-body protein kinetics in critically ill patients during 50 or 100% energy provision by enteral nutrition: A randomized cross-over study. PLoS One 2020, 15(10):e0240045. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clinical trial looking at effect of different levels of energy provision on whole body protein kinetics in critically ill patients. Data showed during feeding (determined by IC-targets) where all patients received >1.2 g/kg/day of protein that mean whole-body protein balance increased significantly when patients received 100% of IC-determined EN targets as compared to 50% of EN targets. Results suggest a better whole-body protein balance during full dose as compared to half dose EN.
- 34.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R: Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. Jama 2014, 311(13):1308–1316. [DOI] [PubMed] [Google Scholar]
- *35.Lazaridis C, Mansour A: To Decompress or Not? An Expected Utility Inspired Approach To Shared decision-making For Supratentorial Ischemic Stroke. Neurocrit Care 2021. [DOI] [PubMed] [Google Scholar]; Editorial paper on illustrating challenges of mortality alone as primary endpoint of clinical trials. Discusses data showing craniectomy in ischemic brain injury decreases mortality, but may concomitantly increase morbidity which may be not an optimal goal and creates challenges with mortality as a primary endpoint.
- 36.Wischmeyer PE: Are we creating survivors...or victims in critical care? Delivering targeted nutrition to improve outcomes. Curr Opin Crit Care 2016, 22(4):279–284. [DOI] [PubMed] [Google Scholar]
- **37.Wischmeyer PE, Molinger J, Haines K: Indirect Calorimetry is Essential for Optimal Nutrition Therapy in the ICU. Nutrition in Clinical Practice 2021, [DOI] [PMC free article] [PubMed] [Google Scholar]; Accpeted for publication. Recent excellent review advocating that with development/availability of a new generation indirect calorimeter and new data supporting its use that IC is essential for optimal ICU nutrition care and should now be standard of care in the ICU. This paper was one of two point-counterpoint papers on the routine use of indirect calorimetry in the ICU in Nutrition in Clinical Practice journal.


