Highlights
-
•
The predictive model used preoperatively accessible clinical parameters and radiographic features developed and validated by us to predict micro vascular invasion (MVI), based on a large sample, two Liver Transplantation (LT) centers observed 5 years among Hepatocellular Carcinoma (HCC) patients who underwent LT.
-
•
This is the first study to report preoperative clinical variables and radiographic features for preoperative prediction of MVI among HCC patients undergoing LT.
-
•
Prediction of the presence of MVI can help surgical decision-making and improve surgical management for HCC to further distinguish clinical outcomes.
Keywords: Microvascular Invasion (MVI), Liver Transplantation (LT), Hepatocellular Carcinoma (HCC)
Abstract
Purpose
The prediction of microvascular invasion (MVI) has increasingly been recognized to reflect prognosis involving local invasion and distant metastasis of hepatocellular carcinoma (HCC). The aim of this study was to assess a predictive model using preoperatively accessible clinical parameters and radiographic features developed and validated to predict MVI. This predictive model can distinguish clinical outcomes after liver transplantation (LT) for HCC patients.
Methods
In total, 455 HCC patients who underwent LT between January 1, 2015, and December 31, 2019, were retrospectively enrolled in two centers in China as a training cohort (ZFA center; n = 244) and a test cohort (SLA center; n = 211). Univariate and multivariate backward logistic regression analysis were used to select the significant clinical variables which were incorporated into the predictive nomogram associated with MVI. Receiver operating characteristic (ROC) curves based on clinical parameters were plotted to predict MVI in the training and test sets.
Results
Univariate and multivariate backward logistic regression analysis identified four independent preoperative risk factors for MVI: α-fetoprotein (AFP) level (p < 0.001), tumor size ((p < 0.001), peritumoral star node (p = 0.003), and tumor margin (p = 0.016). The predictive nomogram using these predictors achieved an area under curve (AUC) of 0.85 and 0.80 in the training and test sets. Furthermore, MVI could discriminate different clinical outcomes within the Milan criteria (MC) and beyond the MC.
Conclusions
The nomogram based on preoperatively clinical variables demonstrated good performance for predicting MVI. MVI may serve as a supplement to the MC.
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of death from cancer in the Asia-Pacific region [1], and its incidence is growing worldwide. In the clinic, malignant tumor lesions are treated by intervention therapy and/or removed by surgical resection [2,3]. Liver transplantation (LT) is the most effective treatment when it comes to liver failure, which refers to an irreversible and advanced stage of disease [4]. Unfortunately, almost 70% of patients suffer from recurrence when surgical resection performed[5,6]. Post-transplant HCC recurrence is found in 3% to 18% of patients even after gaining a healthy liver by LT, with a 5-year overall survival (OS) rate of approximately 10–20% [7].
Microvascular invasion (MVI) is aligned with a poor prognosis for HCC after LT, which has become an independent risk factor for early recurrence [8]. Hence, to obtain a better prognosis after LT for HCC, obtaining an adequate preoperative insight of MVI is necessary, can help identifying high-risk patients for postoperative recurrence, and more importantly, can lead to providing earlier treatment for a better outcome [9]. In fact, MVI in combination with hidden features can only be diagnosed postoperatively with a surgical specimen in most cases. MVI is hard to detect with diagnostic imaging and to distinguish it from macrovascular invasion [10]. There is still a long way to go in terms of applying particular methods preoperatively to predict MVI in any case.
Serum inflammatory indices and some radiographic findings have been illustrated and have variable diagnostic efficiency. They can be simply acquired for any patients using regular preoperative noninvasive tests. Some studies have proven that serum inflammatory indices or tumor features are related to MVI in patients with HCC [11]. However, these attractive findings used to predict MVI suffer from limitations. Importantly, interobserver variability has not been assessed, and external validation has not been performed [12]. Overall, it has not yet been widely acknowledged that these findings provided a preoperative diagnosis of MVI for HCC patients undergoing LT . Moreover, preoperative clinical characteristics including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) in HCC patients have been shown to be associated with MVI and prognosis [13,14]. Unfortunately, this research only evaluated patients with HCC and MVI but not in HCC patients undergoing LT.
In this study, we aimed to examine whether clinical parameters and radiographic features assessed by univariate and multivariate backward logistic regression analysis and a radiomics nomogram integrating large-scale clinical and imaging modalities could be useful for predicting MVI and the long-term clinical outcomes of LT for HCC patients.
Materials and methods
Patients
This retrospective study involved standard care performed at two medical institutions, the First Affiliated Hospital at the School of Medicine at Zhejiang University and Shulan (Hangzhou) Hospital. The ethics committees approved this retrospective study at the two hospitals, and the requirement for patient written informed consent was waived.
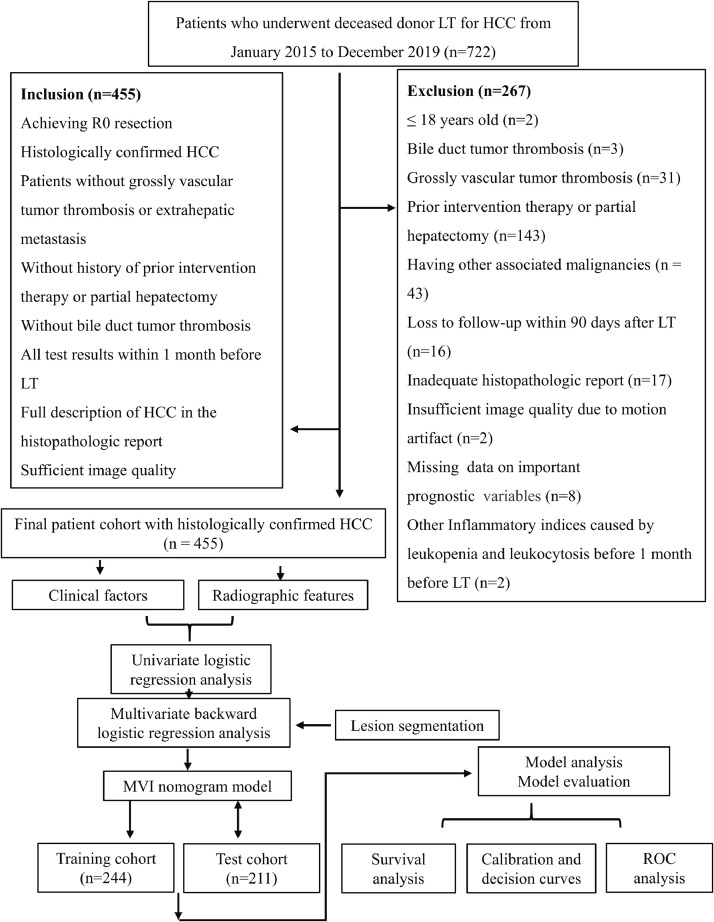
The China Liver Transplant Registry (CLTR) was searched for all patients who underwent LT for HCC from January 2015 to December 2019, and 722 patients were identified. Among these, a total of 455 consecutive patients (413 males and 42 females; mean age, 52.76 ± 9.60 years; range, 26–75 years) constituted the final cohort according to the patient recruitment pathway and the inclusion and exclusion criteria (Fig. 1).
Fig. 1.
Flowchart for the study. LT, liver transplantation; HCC, hepatocellular carcinoma; MVI, microvascular invasion; ROC, receiver operating characteristics.
Hangzhou criteria: (1) Total tumor diameter less than or equal to 8 cm; (2) total tumor diameter more than 8 cm, with histopathologic grade I or II and preoperative α-fetoprotein (AFP) level less than or equal to 400 ng/mL, simultaneously. Milan criteria: single lesion ≤ 5 cm or up to three separate lesions, none larger than 3 cm.
Clinicopathologic variables
This study investigated demographic information and medical history including age at LT, gender and body mass index (BMI). Preoperative clinical variables included liver and renal function tests, hepatitis B and C immunology, preoperative serum AFP level, Child-Pugh score, Model for End-stage Liver Disease (MELD) score, preoperative serum total bilirubin, preoperative serum albumin, preoperative routine blood tests including the white blood cell (WBC) count, neutrophil count, monocyte count, lymphocyte count, platelet count, LMR, NLR and PLR, preoperative international normalized ratio (INR), and preoperative serum creatinine, liver disease etiology, albumin-to-bilirubin ratio (ABR), preoperative clinical features with or without intractable ascites, treatment with or without transcatheter arterial chemoembolization (TACE) and underlying diseases.
For the inflammatory markers, all test results were gained within 1 month preoperatively and demonstrated concomitant white blood cell counts within the normal range of (4–10) *109/L. Inflammatory indices in patients with leukopenia and leukocytosis were excluded in the analysis given the likelihood of alterations from an acute infectious or inflammatory process rather than chronic cancer-related effects. In this study, the NLR was calculated by the ratio of the absolute count of neutrophils to lymphocytes, the PLR was calculated by the ratio of the count of platelets to lymphocytes, LMR was calculated by the ratio of the absolute count of lymphocytes to monocytes, ABR was calculated by the ratio of the absolute count of albumin to bilirubin [15]. Moreover, we examined preoperative abdominal imaging and pathology from the resected tumor with recurrence-free survival (RFS) and OS. The tumor radiographic features ordinarily included the presence of a pseudo-capsule or a peritumoral star node, tumor size, tumor margins and number of tumors. The preoperative diagnosis of HCC was based on the criteria of the American Association for the Study of Liver Diseases (AASLD) [16].
The cohort was divided into a training set (ZFA center) from the First Affiliated Hospital, School of Medicine, Zhejiang University (n = 244; 221 men and 23 women; mean age, 52.9 ± 9.1 years from January 2015 to December 2019), and a time-independent test set (SLA center) from Shulan (Hangzhou) Hospital (n = 211; 192 men and 19 women; mean age, 52.6 ± 10.1 years from June 2017 to December 2019).
Histopathology
Based on R0 resection, all patients experienced surgical radical resection treatment. All surgical specimens were histologically estimated to confirm HCC by agreement from two pathologists belonging to a team of experienced abdominal pathologists. MVI was defined as the presence of a tumor in a portal vein, hepatic vein, or a large capsular vessel of the surrounding hepatic tissue lined with endothelium that was visible only on microscopy.
Follow-up
Patients were routinely followed-up after LT at intervals of 2 to 4 years with routine blood tests and imaging examinations, and the time of disease-specific progression (local recurrence or distant organ metastasis) or death was recorded.
Clinicoradiological risk factors
Individual variables were analyzed for significant differences in the training and test cohorts using Student's t-test to analyze parametric data of component numerical variable, the Mann-Whitney U test to analyze non-parametric data, chi square test or Fisher's exact test to compare the categorical variables, as appropriate. The univariate analysis and multivariate backward logistic regression analysis were used to assess single factors for discriminating significant variables with the presence of MVI in the primary cohort, and these significant variables were enrolled into the predictive nomogram to determine potential predictive factors for MVI.
Radiographic evaluation and analysis
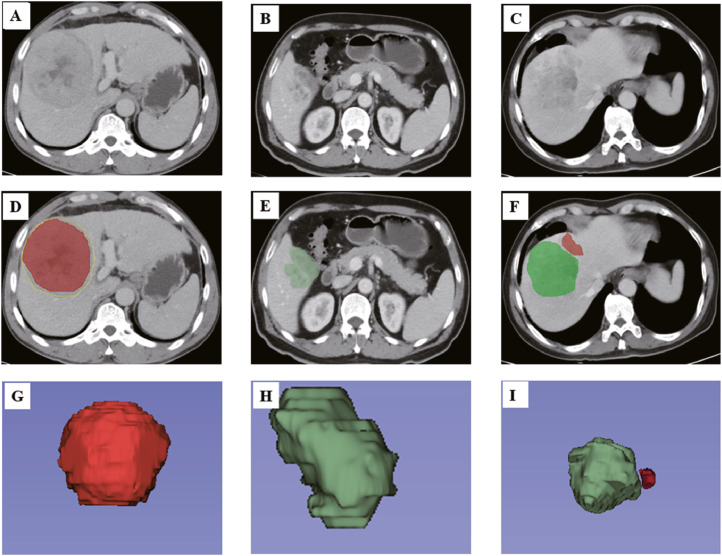
Considering previous references [17], five primary radiographic features were included: (1) number of tumors; (2) tumor size; (3) tumor margin; (4) pseudo-capsule; and (5) presence of a peritumoral star node. Three-dimensional tumor segmentation of patients with HCC undergoing LT was performed by using software written in 3D slicer 4.11 (https://www.slicer.org/) on computed tomography (CT) images (Fig. 2).
Fig. 2.
Imaging features of 3D visualization. First, radiologists found the primary CT images for HCC patients undergoing LT (A), (B) and (C). Then, the seed region that enclosed the contour of the lesion was manually drawn (D), (E) and (F); then, the computer automatically segmented the lesion contour with a dichotomic classification algorithm slice-by-slice, and a semiautomatic VOI entire of the tumor was identified. On the bases of the VOI entire of the tumor, regions with a pseudo-capsule (D), non-smooth tumor margins (E) and peritumoral star node (F) were automatically reconstructed. (G), (H) and (I) show 3D views of the VOI entire. 3D, 3-dimensional; CT, computed tomography; LT, liver transplantation; HCC, hepatocellular carcinoma; VOI entire, entire-volumetric interest.
Construction of a predictive classifier based on MVI
Twenty-nine risk factors were identified as predictive factors for MVI in HCC patients after LT. Multivariate backward logistic regression identified four factors (preoperative AFP level, tumor size, presence of a peritumoral star node, and tumor margins) that were included in the classifier.
Nomogram Construction and Evaluation
As a graphical presentation, a nomogram was constructed from the predictive model. The diagnostic performance of the nomogram in both the training and test cohorts can be analyzed by plotting calibration curves. Using the Hosmer-Lemeshow test analyzed the agreement between nomogram-predicted MVI and actual MVI from the calibration curves. Calculating the net benefit of individuals and clinical efficacy of the prediction model was by using decision curve analysis (DCA). In DCA, the decision curve was compared with extreme cases of treating all or none patients. And greater net benefit showed its clinical utility that put benefits and harms on a certain model [18].
Evaluation of the Predictive MVI Model
Identifying MVI risk factors and constructing a MVI predictive model from multiscale clinicoradiological and radiographic features were realized by univariate and multivariate backward logistic regression analysis with a predictive nomogram. Receiver operating characteristic (ROC) curves were displayed, and area under curves (AUCs) were used to quantify the discriminative efficacy for predicting MVI in the training set and internally validated in the independent test set.
Statistical analysis
RFS and OS were defined as the interval between surgery and radiographic detection of recurrence, last follow-up, or death. Survival curves were plotted by the Kaplan-Meier method and compared by a two-sided log-rank test. Variables that reached statistical significance in the univariate and multivariate backward logistic regression analysis with the predictive nomogram were considered for the MVI predictive model. All the statistical analyses were performed at R version 3.6.1 (www.R-project.org, 2019). A two-tailed p value of less than 0.05 was considered statistically significant.
Results
Clinicoradiological characteristics
Histologic MVI was diagnosed in explanted tissue from 204 patients that out of 455 patients were incorporated (44.8%). The etiology for most of the cases was hepatitis B virus (HBV) (422, 92.7%) followed by infection with HCV (2, 0.4%), alcohol (11, 2.4%) and others (20, 4.4%). The age range of these patients was 26–75 years. In our results, there were 413 males and 42 females. The majority of patients had more than two lesions (56.1%), a small tumor size (63.9%), peritumoral star node (55.3%) and smooth tumor margins (74.6%). The baseline clinicopathological characteristics of all HCC patients with MVI-positive and MVI-negative were included in Table 1. Patients with MVI were younger and had higher platelet, INR and AFP levels than those without MVI. Patient characteristics and tumor features were presented and compared between the training group and test group (Table 1). The two groups were similar in their distribution of age, gender, BMI, liver disease etiology, platelet, WBC, neutrophil, lymphocyte, PLR, NLR, HBsAb, HBsAg, HBeAb, HBeAg, TACE, history of diabetes and number of tumors.
Table 1.
The clinical and histologic characteristics of primary cohort
| Characteristics | Training cohort (n = 244) |
Test cohort (n = 211) |
p Value | ||||
|---|---|---|---|---|---|---|---|
| Total | MVI (+) | MVI (-) | Total | MVI (+) | MVI (-) | ||
| Age (year) | 52.9 ± 9.1 | 52.6 ± 9.8 | 53.1 ± 8.5 | 52.6 ± 10.1 | 49.8 ± 10.7 | 55.5 ± 8.7 | 0.739 |
| Gender | |||||||
| male | 221 | 93 | 128 | 192 | 100 | 92 | 0.877 |
| female | 23 | 6 | 17 | 19 | 5 | 14 | |
| BMI (kg/m2) | |||||||
| ≥ 21.5 | 174 | 66 | 108 | 151 | 74 | 77 | 0.953 |
| < 21.5 | 70 | 33 | 37 | 60 | 31 | 29 | |
| Liver disease etiology | |||||||
| HBV | 230 | 97 | 133 | 192 | 97 | 95 | 0.176 |
| HCV | 2 | 0 | 2 | 0 | 0 | 0 | |
| Alcohol | 5 | 2 | 3 | 6 | 2 | 4 | |
| Others | 7 | 0 | 7 | 13 | 6 | 7 | |
| Platelet (*109/L) | |||||||
| ≥ 69.5 | 132 | 66 | 66 | 130 | 74 | 56 | 0.209 |
| < 69.5 | 112 | 33 | 79 | 87 | 36 | 51 | |
| WBC (*109/L) | |||||||
| ≥ 4.0 | 137 | 64 | 73 | 124 | 71 | 53 | 0.573 |
| < 4.0 | 107 | 35 | 72 | 87 | 34 | 53 | |
| Neutrophil (*109/L) | |||||||
| ≥ 3.4 | 80 | 39 | 41 | 86 | 48 | 38 | 0.078 |
| < 3.4 | 164 | 60 | 104 | 125 | 57 | 68 | |
| Monocyte (*109/L) | |||||||
| ≥ 0.3 | 149 | 68 | 81 | 167 | 88 | 79 | < 0.001 |
| < 0.3 | 95 | 31 | 64 | 44 | 17 | 27 | |
| Lymphocyte (*109/L) | |||||||
| ≥ 0.5 | 183 | 80 | 103 | 171 | 87 | 84 | 0.122 |
| < 0.5 | 61 | 19 | 42 | 40 | 18 | 22 | |
| PLR | |||||||
| ≥ 75.4 | 167 | 78 | 89 | 155 | 87 | 68 | 0.241 |
| < 75.4 | 77 | 21 | 56 | 56 | 18 | 38 | |
| NLR | |||||||
| ≥ 4.7 | 75 | 36 | 39 | 67 | 41 | 26 | 0.816 |
| < 4.7 | 169 | 63 | 106 | 144 | 64 | 80 | |
| LMR | |||||||
| ≥ 2.3 | 128 | 44 | 84 | 90 | 36 | 54 | 0.037 |
| < 2.3 | 116 | 55 | 61 | 121 | 69 | 52 | |
| Albumin (g/dL) | |||||||
| ≥ 35 | 145 | 50 | 95 | 43 | 27 | 16 | < 0.001 |
| < 35 | 99 | 49 | 50 | 168 | 78 | 90 | |
| Bilirubin (μmol/L) | |||||||
| ≥ 123 | 52 | 28 | 24 | 191 | 95 | 96 | < 0.001 |
| < 123 | 192 | 71 | 121 | 20 | 10 | 10 | |
| ABR | |||||||
| ≥ 0.1 | 222 | 84 | 138 | 79 | 42 | 37 | < 0.001 |
| < 0.1 | 22 | 15 | 7 | 132 | 63 | 69 | |
| INR | |||||||
| ≥ 2.6 | 25 | 15 | 10 | 162 | 78 | 84 | < 0.001 |
| < 2.6 | 219 | 84 | 135 | 49 | 27 | 22 | |
| Creatinine (μmol/L) | |||||||
| ≥ 91.5 | 49 | 24 | 25 | 169 | 83 | 86 | < 0.001 |
| < 91.5 | 195 | 75 | 120 | 42 | 22 | 20 | |
| Child-Pugh score | |||||||
| ≥ 9.5 | 55 | 25 | 30 | 183 | 88 | 95 | < 0.001 |
| < 9.5 | 189 | 74 | 115 | 28 | 17 | 11 | |
| MELD score | |||||||
| ≥ 30.5 | 29 | 18 | 11 | 149 | 74 | 75 | < 0.001 |
| < 30.5 | 215 | 81 | 134 | 62 | 31 | 31 | |
| HBsAb | |||||||
| Positive | 30 | 17 | 13 | 24 | 10 | 14 | 0.762 |
| Negative | 214 | 82 | 132 | 187 | 95 | 92 | |
| HBsAg | |||||||
| Positive | 214 | 88 | 126 | 173 | 90 | 83 | 0.088 |
| Negative | 30 | 11 | 19 | 38 | 15 | 23 | |
| HBeAb | |||||||
| Positive | 127 | 54 | 73 | 97 | 53 | 44 | 0.196 |
| Negative | 117 | 45 | 72 | 114 | 52 | 62 | |
| HbeAg | |||||||
| Positive | 58 | 22 | 36 | 42 | 20 | 22 | 0.321 |
| Negative | 186 | 77 | 109 | 169 | 85 | 84 | |
| TACE | |||||||
| Yes | 115 | 50 | 65 | 85 | 42 | 43 | 0.142 |
| No | 129 | 49 | 80 | 126 | 63 | 63 | |
| Diabetes | |||||||
| Yes | 30 | 13 | 17 | 28 | 9 | 19 | 0.756 |
| No | 214 | 86 | 128 | 183 | 96 | 87 | |
| Intractable ascites | |||||||
| Yes | 43 | 18 | 25 | 80 | 44 | 36 | < 0.001 |
| No | 201 | 81 | 120 | 131 | 61 | 70 | |
| AFP (ng/mL) | |||||||
| ≥ 355 | 56 | 44 | 12 | 68 | 55 | 13 | 0.027 |
| < 355 | 188 | 55 | 133 | 143 | 50 | 93 | |
| Tumor size (cm) | |||||||
| ≥ 7.5 | 88 | 61 | 27 | 102 | 67 | 35 | 0.008 |
| < 7.5 | 156 | 38 | 118 | 109 | 38 | 71 | |
| Peritumoral star node | |||||||
| Yes | 109 | 70 | 39 | 47 | 38 | 9 | < 0.001 |
| No | 135 | 29 | 106 | 164 | 67 | 97 | |
| Tumor margin | |||||||
| Non-smooth | 182 | 88 | 94 | 139 | 79 | 60 | 0.042 |
| Smooth | 62 | 11 | 51 | 72 | 26 | 46 | |
| Number of tumors | |||||||
| ≥ 2 | 137 | 71 | 66 | 120 | 73 | 47 | 0.877 |
| =1 | 107 | 28 | 79 | 91 | 32 | 59 | |
| Pseudo-capsule | |||||||
| Yes | 143 | 53 | 90 | 64 | 38 | 26 | < 0.001 |
| No | 101 | 46 | 55 | 147 | 67 | 80 | |
Abbreviations: MVI-/+, micro vascular invasion negative/positive; BMI, body mass index; WBC, white blood count; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; ABR, albumin to bilirubin ratio; INR, international normalized ratio; MELD Score, Model for End-stage Liver Disease score; TACE, transcatheter arterial chemoembolization; AFP, α-fetoprotein.
Preoperative clinical parameters for MVI prediction in patients with HCC undergoing LT
In this research, 99 patients (40.6%) from the ZFA center and 105 patients (49.8%) from SLA center had tumors with MVI. Those with MVI had high AFP levels, longer tumor size, non-smooth tumor margins and peritumoral star nodes.
A risk coefficient estimated by the univariate analysis from the demographic, tumor, and clinical characteristics of HCC patients undergoing LT is summarized in Table 2. AFP [odds ratio (OR)=8.87, (p < 0.001)], platelet (OR = 2.39, p = 0.001), PLR (OR = 2.20, p = 0.008), bilirubin (OR = 1.99, p = 0.030), peritumoral star node (OR = 6.56, p < 0.001), tumor size (OR = 7.02, p < 0.001), tumor margin (OR = 4.34, p < 0.001) and number of tumors (OR = 3.04, p < 0.001) were associated with the presence of MVI in the training cohort.
Table 2.
Univariate logistic regression analysis of MVI presence based on preoperative data in the training cohort.
| Variable | OR | 95% CI | Z-score | p value |
|---|---|---|---|---|
| Age (year) | 0.99 | 0.97–1.02 | 0.40 | 0.688 |
| Gender | 0.49 | 0.17–1.22 | 1.46 | 0.144 |
| BMI (kg/m2) | 0.97 | 0.90–1.04 | 0.86 | 0.390 |
| Liver disease etiology | 1.09 | 0.19–8.42 | 0.10 | 0.922 |
| Platelet (*109/L) | 2.39 | 1.42–4.10 | 3.23 | 0.001 |
| WBC (*109/L) | 1.10 | 0.99–1.22 | 1.79 | 0.074 |
| Neutrophil (*109/L) | 1.10 | 0.98–1.24 | 1.56 | 0.118 |
| Monocyte (*109/L) | 1.79 | 0.81–4.41 | 1.38 | 0.166 |
| Lymphocyte (*109/L) | 1.19 | 0.78–1.84 | 0.80 | 0.424 |
| PLR | 2.20 | 1.25–3.99 | 2.67 | 0.008 |
| NLR | 1.01 | 0.97–1.06 | 0.63 | 0.529 |
| LMR | 0.96 | 0.84–1.07 | 0.68 | 0.499 |
| Albumin (g/dL) | 1.10 | 0.36–3.73 | 0.16 | 0.873 |
| Bilirubin (μmol/L) | 1.99 | 1.07–3.72 | 2.18 | 0.030 |
| ABR | 0.87 | 0.73–1.02 | 1.66 | 0.096 |
| INR | 1.19 | 0.85–1.66 | 1.01 | 0.312 |
| Creatinine (μmol/L) | 1.00 | 0.10–1.01 | 0.97 | 0.333 |
| Child-Pugh score | 1.08 | 0.97–1.23 | 1.36 | 0.174 |
| MELD Score | 1.02 | 0.99–1.05 | 1.44 | 0.149 |
| HBsAb | 2.11 | 0.98–4.64 | 1.89 | 0.059 |
| HBsAg | 1.21 | 0.56–2.74 | 0.47 | 0.642 |
| HBeAb | 1.18 | 0.71–1.98 | 0.65 | 0.519 |
| HBeAg | 0.87 | 0.47–1.58 | 0.47 | 0.639 |
| TACE | 1.26 | 0.753–2.100 | 0.87 | 0.383 |
| Diabetes | 1.14 | 0.52–2.46 | 0.33 | 0.743 |
| Intractable ascites | 1.07 | 0.54–2.07 | 0.19 | 0.850 |
| AFP (ng/mL) | 8.87 | 4.48–18.76 | 6.01 | <0.001 |
| Tumor size (cm) | 7.02 | 3.96–12.73 | 6.56 | <0.001 |
| Peritumoral star node | 6.56 | 3.76–11.72 | 6.50 | <0.001 |
| Tumor margin | 4.34 | 2.20–9.26 | 4.03 | <0.001 |
| Number of tumors | 3.04 | 1.77–5.30 | 3.99 | <0.001 |
| Pseudo-capsule | 0.70 | 0.420–1.18 | 1.33 | 0.185 |
Abbreviations: MVI, microvascular invasion; OR, odds ratio; CI, confidence interval; BMI, body mass index; WBC, white blood count; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; ABR, albumin to bilirubin ratio; INR, international normalized ratio; MELD Score, Model for End-stage Liver Disease score; TACE, transcatheter arterial chemoembolization; AFP, α-fetoprotein.
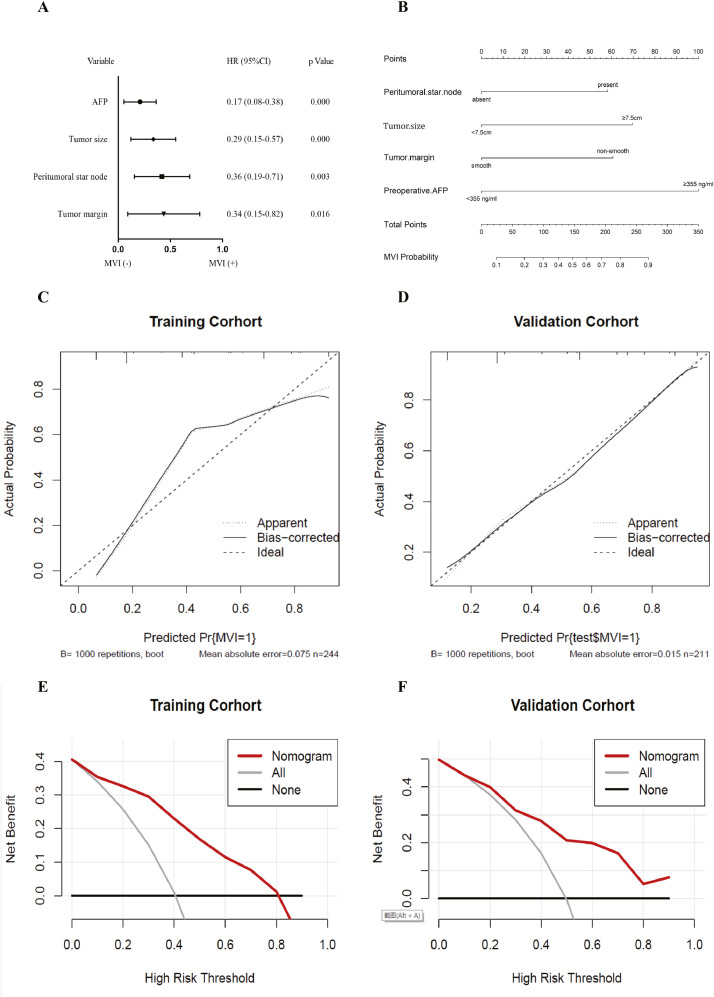
Moreover, the above variables were further entered into multivariate backward logistic regression model according to the cutoff p value < 0.05 in univariate logistic regression analysis. Multivariate backward logistic regression analysis identified four factors (preoperative AFP level, tumor size, peritumoral star node, and tumor margin) that were included in the classifier (Fig. 3).
Fig. 3.
Forest plot of independent predictors of MVI by multivariate backward logistic regression analysis (A). Nomogram for predicting MVI probabilities and calibration of the nomogram. A nomogram integrating clinical characteristics and tumor features (B). Calibration curves of the nomogram in the training and test datasets (C, D). The X-axis is the nomogram-predicted probability of MVI. The Y-axis is the observed MVI, and the diagonal dashed line indicates the ideal prediction by a perfect model. DCA for the nomogram in the training group (E) and the validation group (F), respectively. The black solid lines hypothesized that all patients were MVI positive or negative, respectively. The dotted-line represented the net benefit of the nomogram at different threshold probabilities. MVI, microvascular invasion; DCA, decision curve analysis; HR, hazard ratio; CI, confidence interval.
Development and validation of the nomogram
A forest plot of independent predictors of MVI with hazard ratios (HRs) from the multivariate backward regression model are shown in Fig. 3A. The nomogram based on the predictive model is presented in Fig. 3B. Satisfactory predictive performance of the nomogram was obtained in the training and test cohorts. Calibration curves (Fig. 3C and D) showed that the predicted probabilities of the nomogram were closely aligned with the actual MVI estimating in both the training [mean absolute error (MAE)=0.075] and test cohorts (MAE = 0.015). The decision curves were exhibited in Fig. 3E and F. According to the DCA results, the present nomogram model provided a greater standardized net benefit compared to “treat-all” and “treat-none” strategies when the risk threshold ranged approximately from 0.4 to 0.8 in both cohorts. This nomogram suggests that basing therapy strategy on our nomogram to identify MVI accurately will improve clinical outcome.
Performance for MVI prediction using the predictive model for HCC patients undergoing LT
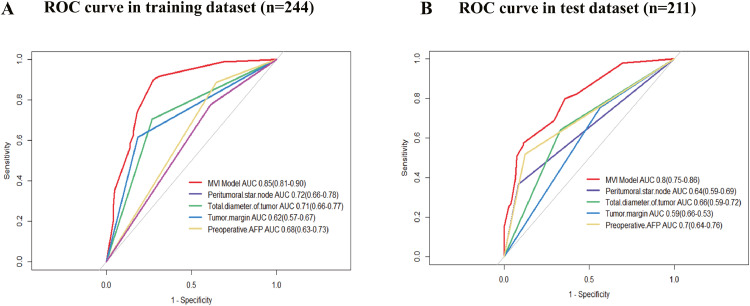
The AUCs of the models combining the four predictors were 0.85 (95% CI 0.81–0.90) in the training cohort and 0.80 (95% CI 0.75–0.86) in the test cohort, which achieved better predictive efficacy for MVI (Fig. 4).
Fig. 4.
ROC curves for prediction of MVI (A and B). ROC, receiver operating characteristics; MVI, microvascular invasion; AUC, area under curve; AFP, α-fetoprotein.
The presence of a peritumoral star node performed better than tumor size, tumor margin and preoperative AFP level in the training cohort (AUC 0.72; 95% CI 0.66–0.78), but the preoperative AFP level showed the best performance in the test cohort (AUC 0.70; 95% CI 0.64–0.76). An optimal threshold was used to maximize the Youden index of the ROC curve analysis from the training/test sets.
Association of MVI and different LT criteria with postoperative RFS and OS in patients with HCC undergoing LT
As of December 31, 2019, 244/455 (53.6%) patients had completed the RFS follow-up (loss to follow-up at Shulan Hospital), and 455/455 (100.0%) had finished the OS follow-up. The overall recurrence rate was 27.0% (66/244), and the overall death rate was 28.4% (129/455). The median RFS of the patients was 12.53 (range, 0.03–56.78) months, and the median OS of the patients was 28.41 (range, 0.03–56.78) months. MVI positive/negative obviously distinguished posttransplant outcomes at the ZFA center or SLA center regardless of the transplantation criteria (p < 0.001) (Fig. S1).
At the ZFA center, these results showed significant difference in RFS for the patients within/beyond the Milan criteria (MC) with MVI compared with those within/beyond the MC without MVI (log-rank p = 0.046 vs p = 0.033; Fig. S2A). Rather, there was no significant difference in OS for patients within/beyond the MC with MVI compared with those within/beyond the MC without MVI (p > 0.05; Fig. S2B). At SLA center, there was no significant difference in OS for the patients within the MC with MVI in contrast to the patients within the MC without MVI (p > 0.05; Fig. S2C). Interestingly, survival curves showed significant difference in OS for the patients beyond MC with MVI compared with those beyond MC without MVI (p = 0.038; Fig. S2C).
Kaplan–Meier survival analysis showed that among 244 HCC patients undergoing LT at the ZFA center, those beyond the MC with MVI had a significantly poorer RFS and OS than those within the MC with MVI (median OS undefined vs. 17.40 months, p = 0.074; median RFS undefined vs. 9.84 months, p = 0.021). The group beyond the MC without MVI also had a poorer RFS and OS than the group within the MC without MVI (median OS undefined vs. 30.59 months, p = 0.002; median RFS undefined vs. 41.78 months, p < 0.001) (Fig. S2A and 2B). Kaplan-Meier survival curves of HCC patients undergoing LT at SLA center stratified by the MC and MVI demonstrated a higher OS in the group within the MC without MVI group than in the group beyond the criteria without MVI (p = 0.003) (Fig. S2C). Likewise, the groups based on the Hangzhou criteria (HC) showed similar results (Fig. S3).
Discussion
The aim of this study was to investigate prognostic aspects derived from large-scale clinical variables and radiographic features for preoperative prediction of histologic MVI status and clinical outcomes in a cohort of 455 HCC patients undergoing LT. The predictive model demonstrated good performance for successfully preoperatively predicting MVI in patients undergoing LT for HCC. Additionally, the predictive model for MVI was independently associated with disease-specific recurrence and long-term mortality, indicating that our findings can help surgical decision-making and further promote surgical management for HCC. In all, the combination of these clinical preoperative characteristics provided a truthful, noninvasive and robust approach for personalized prediction of MVI before LT. Moreover, this multicenter study incorporated two LT centers equipped with abundant clinical parameters and sample resources.
As is known to all, pre-operative prediction of MVI presence has been summarized in a variety of studies, this prediction can support clinicians in making pre-operative clinical decisions, [19], [20], [21]. The major variables included imaging examinations, inflammatory markers and tissue features that have been incorporated into different prediction models of MVI risk [22,23]. In a radiomic analysis enrolling 495 observational studies, Xun Xu et al. [24] demonstrated that higher AFP level, non-smooth tumor margin and incomplete tumor capsule were associated with higher risk of MVI presence in advanced-stage HCC. Here, in our study by including 455 patients with HCC, higher AFP level and non-smooth tumor margin were also significantly associated with MVI risk. Additionally, other prognostic factors included AFP level, tumor size and peritumoral star node consistent with the findings of previous reports [23,25]. AFP is as the basis for screening for malignant tumors. Predicting MVI risk independently associated with worse RFS and OS by higher AFP level in serum markers can be the most widely-recognized, which was similar to the findings of previous reports [26,27]. The presence of MVI was also associated with preoperative non-smooth tumor margin on CT[28,29]. And Hidetoshi Nitta et al. [30] has demonstrated that the larger tumor size was associated with higher risks of MVI presence. Peritumoral star node can distinctly indicate poor prognosis in HCC [31]. The presence of peritumoral star node is associated with HCC recurrence and poor survival, possibly because peritumoral star node means the degree of malignancy of HCC [31]. Our study has revealed that some tumor features are independent predictive factors of MVI in HCC patients undergoing LT. Prior large studies have also concluded that some tumor features observed on CT or magnetic resonance imaging (MRI) could predict potential MVI in HCC patients [24,32,33].
MVI-positive patients generally had a poor prognosis after LT whether they were within the MC or beyond the MC, which has also been reported in many reports [30,34]. The OS and RFS of patients who exceeded the MC were lower than those of patients who were within the MC [35,36]. The RFS and OS of patients without MVI within the MC were the best survival [35,36]. Moreover, the HC can provide an ideal prognosis in comparison to the MC, give more patients access to LT, and serve as a demarcation for better or worse outcomes [37]. Further analysis of our results found that survival curves of RFS at the ZFA center proved that there was a significant difference in/beyond the MC with/without MVI in terms of different post-transplant outcomes. This indicated that MVI may assist the MC to distinguish prognostic stratification. It is worth mentioning that OS analysis presents several confounders in the post-LT setting (for example immunosuppressant regimen, post-recurrence treatments, and so forth) not taken into account in our analysis.
Some limitations of this study should be addressed. First, approximately 8% of the patients within 1 month preoperatively for acute inflammatory states or unavailable data were not evaluated and excluded. All the patients included in the study had a normal WBC count within 1 month of surgery to erase effects of acute inflammatory changes and to capture cancer-related inflammatory changes. Because of this, the reproducibility and comparability of the results may be hindered by potential selection bias of this retrospective study, and the value of this model still needs to improve and independent to validate in further studies with large samples. Moreover, further sufficient experimental evidences are needed to support the correlation between some risk factors and MVI. The second limitation is that there was no significant difference in the OS at the ZFA center for patients who were within/beyond the HC with or without MVI. This suggests that MVI cannot assist the MC in distinguishing post-transplant outcomes. Another disadvantage of this research is that due to the incomplete follow-up data from SLA center, there was no significant difference in RFS among different groups of HCC patients undergoing LT (p > 0.05). Furthermore, we had a small sample size of the test cohort even smaller than the training series, but further a far bigger test cohort was required to predict MVI.
In conclusion, satisfactory preoperative prediction of the individualized risk assessment of MVI both the training and test cohorts can be gained by our model in HCC patients undergoing LT. MVI combined with the MC may discriminate post-transplant outcomes and serve as a biomarker for prognostic stratification. Thus, it may be useful in preoperative individual prediction of MVI and help surgical decision-making and further improve surgical management for HCC.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Funding
This work was supported by the Key Program, National Natural Science Foundation of China [Grant number 81930016]; National Natural Science Foundation of China [Grant number 81802889]; and Key Research & Development Plan of Zhejiang Province [Grant number 2019C03050].
CRediT authorship contribution statement
Wenhui Zhang: Conceptualization, data curation, writing and original draft, writing & review & editing; Zhikun Liu: Conceptualization, data curation, funding acquisition, writing-original draft, writing-review & editing; Junli Chen: Analysis and interpretation of the data; Siyi Dong: Analysis of the data; Beini Cen: Analysis and interpretation of the data; Shusen Zheng: Conceptualization; Xiao Xu: Conceptualization, funding acquisition, writing-review & editing; All authors read and approved the final manuscript.
Statement
Informed consent was waived which approved by the ethics committees for each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution's human research committee. Consent was obtained to the use of donor organs for transplantation, either from the donor in life or from the family of the donor. No organs/tissues were obtained from prisoners and these organs/tissues were obtained by the First Affiliated Hospital at the School of Medicine, Zhejiang University and Shulan (Hangzhou) Hospital.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101200.
Contributor Information
Shusen Zheng, Email: shusenzheng@zju.edu.cn.
Xiao Xu, Email: zjxu@zju.edu.cn.
Appendix. Supplementary materials
References
- 1.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kibe Y, Takeda A. Methodological concerns for investigating the effects of mid-treatment break of stereotactic body radiotherapy (SBRT) for hepatocellular carcinoma (HCC) Radiother. Oncol. 2020;147:234. doi: 10.1016/j.radonc.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Wettstein D, Tóth SJ, Máthé Z. [New challenges of liver transplantation] Orv. Hetil. 2019;160:1127–1135. doi: 10.1556/650.2019.31465. [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Yang L, Shi J, Liu C, Zhang X, Chai Z. Postoperative adjuvant IMRT for patients with HCC and portal vein tumor thrombus: An open-label randomized controlled trial. Radiother. Oncol. 2019;140:20–25. doi: 10.1016/j.radonc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Kwong A, Mehta N. Expanding the limits of liver transplantation for hepatocellular carcinoma: is there a limit? Clin. Liver Dis. 2021;25:19–33. doi: 10.1016/j.cld.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Wu J, Cheng S, Wang L, Rong W, Wu F. Phase II study of adjuvant radiotherapy following narrow-margin hepatectomy in patients with hepatocellular carcinoma. Hepatology (Baltimore, Md) 2021 doi: 10.1002/hep.31993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Xing H, Han J, Li Z, Lau W, Zhou Y. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA surgery. 2019;154:209–217. doi: 10.1001/jamasurg.2018.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta N, Dodge J, Grab J, Yao F. National experience on down-staging of hepatocellular carcinoma before liver transplant: influence of tumor burden, Alpha-Fetoprotein, and Wait Time. Hepatology (Baltimore, Md) 2020;71:943–954. doi: 10.1002/hep.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaguchi T, Shimose S, Torimura T. Challenges and prospects in prediction and treatment for hepatocellular carcinoma with micro vascular invasion. Hepatobiliary Surg. Nutr. 2019;8:651–654. doi: 10.21037/hbsn.2019.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K, Tao C, Siqin T, Wu J, Rong W. Establishment, validation and evaluation of predictive model for early relapse after R0 resection in hepatocellular carcinoma patients with micro vascular invasion. J. Transl. Med. 2021;19:293. doi: 10.1186/s12967-021-02940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min J, Lee M, Park H, Lee D, Park H, Lim S. Interobserver variability and diagnostic performance of Gadoxetic Acid-enhanced MRI for predicting micro vascular invasion in hepatocellular carcinoma. Radiology. 2020;297:573–581. doi: 10.1148/radiol.2020201940. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Wang M, Tian T, Huang J, Yuan S, Liu L. A retrospective study. a high preoperative Platelet-Lymphocyte ratio is a negative predictor of survival after liver resection for Hepatitis B Virus-Related Hepatocellular Carcinoma. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.576205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan S, Wong L, Chan K, Chow C, Tong J, Yip T. Development of a novel inflammation-based index for hepatocellular carcinoma. Liver Cancer. 2020;9:167–181. doi: 10.1159/000504252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren A, Li Z, Zhang X, Deng R, Ma Y. Inflammation-based prognostic scores in patients with Hepatitis B virus-related hepatocellular carcinoma after liver transplantation. J Hepatocellular Carcinoma. 2020;7:101–106. doi: 10.2147/jhc.S259992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee S, Wang DS, Kim HJ, Sirlin CB, Chan MG, Korn RL. A computed tomography radio genomic biomarker predicts micro vascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology. 2015;62:792–800. doi: 10.1002/hep.27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ. Reporting and interpreting decision curve analysis: a guide for investigators. Eur. Urol. 2018;74:796–804. doi: 10.1016/j.eururo.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Tian W, Zhang L, Huang Q, Lin S, Ding Y. Preoperative prediction power of imaging methods for micro vascular invasion in hepatocellular carcinoma: a systemic review and meta-analysis. Front. Oncol. 2020;10:887. doi: 10.3389/fonc.2020.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong Y, Zhou L, Xia W, Zhao XY, Zhang Q, Jian JM. Preoperative prediction of micro vascular invasion in hepatocellular carcinoma: initial application of a radiomic algorithm based on grayscale ultrasound images. Front. Oncol. 2020;10:353. doi: 10.3389/fonc.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan Y, Zhou Q, Zhang M, Liu H, Lin J, Liu Q. Integrated nomograms for preoperative prediction of micro vascular invasion and Lymph Node metastasis risk in hepatocellular Carcinoma Patients. Ann. Surg. Oncol. 2020;27:1361–1371. doi: 10.1245/s10434-019-08071-7. [DOI] [PubMed] [Google Scholar]
- 22.Nebbia G, Zhang Q, Arefan D, Zhao X, Wu S. Pre-operative micro vascular invasion prediction using multi-parametric Liver MRI Radiomics. J Digit Imaging. 2020;33:1376–1386. doi: 10.1007/s10278-020-00353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Min JH, Lee MW, Park HS, Lee DH, Park HJ, Lim S. Inter observer variability and diagnostic performance of Gadoxetic Acid-enhanced MRI for predicting micro vascular invasion in hepatocellular carcinoma. Radiology. 2020;297:573–581. doi: 10.1148/radiol.2020201940. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Zhang HL, Liu QP, Sun SW, Zhang J, Zhu FP. Radiomic analysis of contrast-enhanced CT predicts micro vascular invasion and outcome in hepatocellular carcinoma. J. Hepatol. 2019;70:1133–1144. doi: 10.1016/j.jhep.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Beumer B, Takagi K, Vervoort B, Buettner S, Umeda Y, Yagi T, et al. Prediction of Early Recurrence After Surgery for Liver Tumor (ERASL): An International Validation of the ERASL Risk Models. Ann. Surg. Oncol. 2021. doi: 10.1245/s10434-021-10235-3. [DOI] [PMC free article] [PubMed]
- 26.Ryu T, Takami Y, Wada Y, Tateishi M, Hara T, Yoshitomi M. A clinical scoring system for predicting micro vascular invasion in patients with Hepatocellular Carcinoma Within the Milan Criteria. J. Gastrointest. Surg. 2019;23:779–787. doi: 10.1007/s11605-019-04134-y. [DOI] [PubMed] [Google Scholar]
- 27.Hu HT, Wang Z, Huang XW, Chen SL, Zheng X, Ruan SM. Ultrasound-based radiomics score: a potential biomarker for the prediction of micro vascular invasion in hepatocellular carcinoma. Eur. Radiol. 2019;29:2890–2901. doi: 10.1007/s00330-018-5797-0. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Zhang Y, Fang Q, Zhang X, Hou P, Wu H. Radiomics analysis of [(18)F]FDG PET/CT for micro vascular invasion and prognosis prediction in very-early- and early-stage hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:2599–2614. doi: 10.1007/s00259-020-05119-9. [DOI] [PubMed] [Google Scholar]
- 29.Sun SW, Liu QP, Xu X, Zhu FP, Zhang YD, Liu XS. Direct Comparison of Four Pre-surgical Stratifying Schemes for Prediction of Micro vascular Invasion in Hepatocellular Carcinoma by Gadoxetic Acid-Enhanced MRI. J. Magn. Reson. Imaging. 2020;52:433–447. doi: 10.1002/jmri.27043. [DOI] [PubMed] [Google Scholar]
- 30.Nitta H, Allard MA, Sebagh M, Ciacio O, Pittau G, Vibert E. Prognostic value and prediction of extra tumoral micro vascular invasion for hepatocellular carcinoma. Ann. Surg. Oncol. 2019;26:2568–2576. doi: 10.1245/s10434-019-07365-0. [DOI] [PubMed] [Google Scholar]
- 31.Jiang YQ, Cao SE, Cao S, Chen JN, Wang GY, Shi WQ. Preoperative identification of micro vascular invasion in hepatocellular carcinoma by XGBoost and deep learning. J. Cancer Res. Clin. Oncol. 2021;147:821–833. doi: 10.1007/s00432-020-03366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Gu D, Wei J, Yang C, Rao S, Wang W. A Radiomics Nomogram for preoperative prediction of micro vascular invasion in Hepatocellular Carcinoma. Liver Cancer. 2019;8:373–386. doi: 10.1159/000494099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Zhang Y, Fang Q, Zhang X, Hou P, Wu H, et al. Radiomics analysis of [F]FDG PET/CT for micro vascular invasion and prognosis prediction in very-early- and early-stage hepatocellular carcinoma. European journal of nuclear medicine and molecular imaging. 2021;48:2599–614. doi: 10.1007/s00259-020-05119-9. [DOI] [PubMed]
- 34.Zhang XP, Wang K, Wei XB, Li LQ, Sun HC, Wen TF. An Eastern Hepatobiliary Surgery Hospital Micro vascular Invasion Scoring System in Predicting Prognosis of Patients with Hepatocellular Carcinoma and Micro vascular Invasion After R0 Liver Resection: a large-scale. Multicenter Study. Oncol. 2019;24 doi: 10.1634/theoncologist.2018-0868. e1476–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/s0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 36.Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat. Rev. Gastroenterol. Hepatol. 2017;14:203–217. doi: 10.1038/nrgastro.2016.193. [DOI] [PubMed] [Google Scholar]
- 37.Zhan QF, Ling SB, Deng YN, Shan QN, Ye QW, Xu SJ. Hangzhou criteria as down staging criteria in hepatocellular carcinoma before liver transplantation: a multicenter study from China. Hepatobiliary Pancreat. Dis. Int. 2020;19:349–357. doi: 10.1016/j.hbpd.2020.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.