Highlights
-
•
This is the first study on LODDS and ENE together. The current study showed that LODDS and ENE are liable prognostic parameters of CRC or CC.
-
•
ENE is an independent influencing factor on the prognosis of both CRC and CC, and the prognostic impact of ENE was observed in both CRC and CC.
-
•
The frequency of ENE increases from the proximal (right) to the distal (left) colon as well as the rectum.
Keywords: Colorectal cancer, Extranodal extension, Log odds of positive lymph nodes, Lymph node-positive, Tumor location
Abstract
Background
Extranodal extension (ENE) and log odds of positive lymph nodes (LODDS) are associated with the aggressiveness of both colon and rectal cancers. The current study evaluated the clinicopathological significance and the prognostic impact of ENE and LODDS in the colon and rectal patients independently.
Methods
The clinical and histological records of 389 colorectal cancer (CRC) patients who underwent curative surgery were reviewed.
Results
For the ENE system, 244 patients were in the ENE1 group and 145 in the ENE2 system. Compared with the ENE1 system, the patients included in the ENE2 system were prone to nerve invasion (P < 0.001) and vessel invasion (P < 0.001) with higher TNM (P = 0.009), higher T category (P = 0.003), higher N category (P < 0.001), advanced differentiation (P = 0.013), more number of positive lymph nodes (NPLN) (P < 0.001), more lymph node ratio (LNR) (P < 0.001), and a higher value of LODDS (P < 0.001). ENE was more frequent in patients with left and rectal than right cancer. For the LODDS system, 280 patients were in the LODDS1 group, and 109 in the LODDS2 group. Compared to the LODDS1 group, the patients included in the LODDS2 group were more prone to nerve invasion (P = 0.0351) and vessel invasion (P < 0.001) with a higher rate of N2 stage, less NDLN (P < 0.001), more NPLN (P < 0.001), more LNR (P < 0.001), and a higher value of ENE (P < 0.001). Based on the results in the univariable analysis, the N, NPLN, LNR, LODDS, and ENE were separately incorporated into five different Cox regression models combined with the same confounders. The multivariable Cox regression analysis demonstrated that all the five staging systems were independent prognostic factors for overall survival.
Conclusion
The current study confirmed that the LODDS stage is an independent influence on the prognosis of both CRC and CC patients. ENE is an independent influencing factor on the prognosis of both CRC and CC patients, and the prognostic impact of extracapsular lymph node was observed in both CRC and CC. The frequency of ENE increases from the proximal (right) to the distal (left) colon as well as the rectum. Therefore, combining ENE and LODDS into the current TNM system to compensate for the inadequacy of pN staging needs further investigation.
Introduction
Colorectal cancer (CRC) is the leading cause of cancer-related deaths worldwide because of its poor prognosis and many related complications. Also, it is the third highest incidence rate and the second highest mortality rate among all tumors worldwide [1]. In China, the incidence of CRC is increasing every year [2]. In clinical practice, lymph node metastasis is one of the major metastases of CRC and a major indicator for evaluating recurrence and survival of CRC patients, as well as a key prognostic factor in determining the development of postoperative treatment plans and follow-up of patients with CRC [3,4].
Nodal status in surgical oncology is used to assist in prognostication [5] and guide decision-making regarding adjuvant chemotherapy [6], the number of lymph nodes examined or the lymph node yield (LNY) could be used as a marker for the quality of oncological resection [7]. The current lymph node staging is the pN staging proposed by the 8th American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) staging system. The staging is influenced by the number of lymph nodes dissected and the prognosis of patients with the same number of metastatic lymph nodes, thus affecting the accuracy of judging the prognosis of CRC patients. The National Comprehensive Cancer Network (NCCN) guidelines recommend a minimum of 12 lymph nodes to be examined for staging, but the number of dissected lymph nodes (NDLN) is greatly influenced by the surgeon's skill, the extent of lymph node dissection or pathologist's thoroughness in retrieving the pNs, and individual patient variation [8]. Tsai et al. found that NDLN was significantly more effective in determining the accuracy of negative lymph nodes in CRC showing that the 5-year survival rate of CRC patients with NDLN ≥18 was significantly higher than that of patients with NDLN<18, indicating that the number of NDLN affects the accuracy of detecting pN [9].
Lymph node ratio (LNR) (defined as the number of positive lymph nodes divided by the total number of lymph nodes removed, pN as a percentage of NDLN) has been investigated as an adjunct parameter to conventional nodal staging. It aids in prognosis and identifying high-risk patients [10]. In the node-negative colon cancer(CC) that accounts for approximately 75% of the patients who have surgery for CC, LNR is zero and is the same as the pN0 classification and therefore does not provide any additional prognostic information [11]. Huh et al. demonstrated that LNR was statistically significant for the overall survival (OS) in CRC patients irrespective of the number of NDLNs and an independent prognostic factor for CRC patients that compensates for the pN staging bias when NDLN is not adequate [12]. It has been shown that the rate of lymph node metastasis as the factor reflecting the status of lymph nodes is advantageous in predicting the prognosis of patients with gastric [13], colorectal [14]and thyroid cancers [15]. Previous studies have demonstrated that higher values of the number of positive lymph nodes (NPLNs) and LNR were associated with poor survival outcomes in CRC [16,17].
Recent studies found that tumor cells in lymph node metastases of malignant tumors have both extracapsular and intracapsular growth patterns. The extranodal extension (ENE) of nodal metastasis growth refers to the proliferation of tumor cells within the lymph node capsule and breaking through the capsule wall into the surrounding perinodal adipose tissues. Intracapsular growth refers to the proliferation of tumor cells only within but without breaking through the lymph node capsule [18]. Reportedly, ENE of lymph node metastasis is significantly associated with the poor prognosis of CRC patients [19]. In the latest edition of the AJCC malignancy staging manual, extracapsular growth is a major indicator of head and neck cancer lymph node staging [20]. Link et al. compared the expression of SOX9 in gastric cancer lymph node metastases with extracapsular growth and intracapsular growth tumor cells and found that the former had significantly higher expression than the latter, suggesting that enhanced SOX9 expression is involved in driving an extracapsular growth pattern with high invasive potential [21].
Traditionally, clinicians have relied solely on nodal disease involvement (including the total number of positive lymph nodes) while determining patient prognosis in CRC [22]. However, biologically aggressive tumors can initially be placed in the same stage as less clinically aggressive tumors, irrespective of the nodal disease. The log odds of positive lymph nodes (LODDS) is a logistic transformation formula that uses pathological lymph node data to stratify survival differences among patients within a single stage of the disease. This formula allows clinicians to identify whether patients with clinically aggressive tumors fall into higher-risk groups regardless of nodal positivity and can potentially guide adjuvant treatment modalities. Recently, LODDS has been proposed as a novel prognostic index in colonic and non-colonic cancers [23], [24], [25]. The previous studies also showed that the classification of lymph node status by LODDS is a robust prognostic indicator with a strong ability to identify patients with a homogeneous prognosis, irrespective of lymph node status and count. Herein, we investigated the prognostic impact of LODDS and compared the survival of patients in LNR and LODDS groups who underwent a colonic cancer resection.
Based on the Shanghai General Hospital (SGH) database, our first objective was to evaluate the prognostic role of ENE and LODDS staging system for predicting the long-term prognosis of postoperative node-positive colon and rectal cancer patients. Next, we aimed to comprehensively compare the prognostic performance of the AJCC N classification, NPLN, LNR, ENE, and LODDS systems and identify a superior staging system for predicting the survival outcomes of patients with colon and rectal cancer. Finally, we attempted to establish two nomograms to predict the long-term OS for patients with CRC and CC.
Methods
Patients
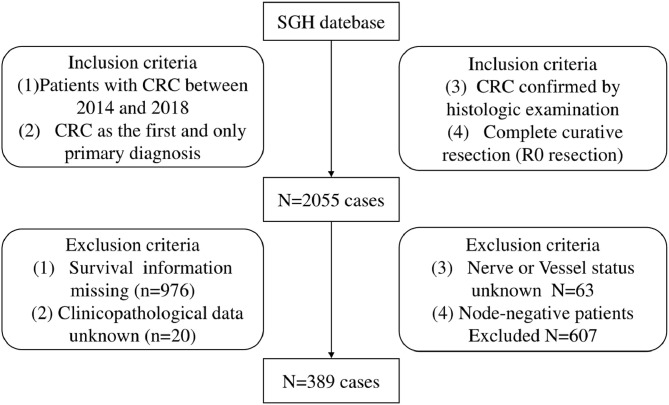
The data from this study were collected from the SGH database between January 2014 and December 2018. The patient eligibility criteria were as follows: (1) Patients with CRC between 2014 and 2018, (2) CRC as the first and only primary diagnosis, (3) CRC confirmed by histological examination, and (4) Complete curative resection (R0 resection). The exclusion criteria were as follows: (1) Survival information missing, (2) Unknown clinicopathological data, nerve status, and vessel status, (3) Lymph node-negative. Finally, 389 eligible patients with CRC were selected from the database. The patient selection is illustrated in Fig. 1. All CRC patients underwent standardized postoperative follow-up regularly after radical surgery with respect to clinical examinations, complete blood counts, blood chemistry tests, estimation of s-CEA levels, and chest radiography. Typically, the patients were followed up every 3 months for the first 2 postoperative years and every 6 months thereafter for 3–5 years. In the current study, the colorectal OS was used to evaluate the prognosis. It was defined as the duration from the date of surgery to the date of death from any cause or the last follow-up (July 31, 2019) for censored observations.
Fig. 1.
Flow chart of patient selection.
Histological evaluation
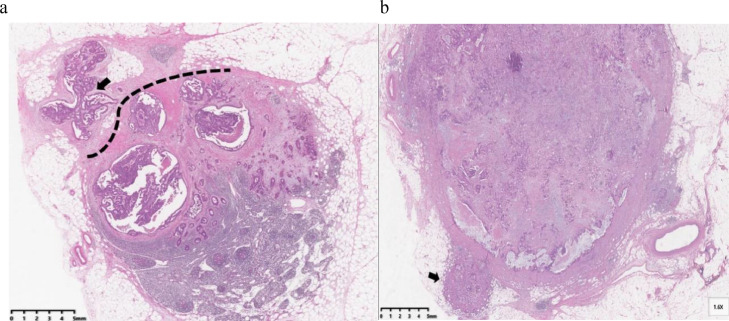
The ENE status of all specimens was examined by two pathologists, and the final diagnosis was based on intradepartmental consultations with staff specialized in CRC. ENE was defined as cancer cells infiltrating the extranodal adipose tissue beyond the capsule of the lymph node (Fig. 2a). The tumor cells outside the LNs, continuous with the primary tumor (Fig. 2b), were not considered ENE. A tumor was considered ENE-positive when one or more of the metastatic LNs showed ENE, as described previously [26].
Fig. 2.
Examples of ENE in lymph nodes of patients with CRC (hematoxylin and eosin stain, original magnification × 10). (a) Tumor cells infiltrating adipose tissue (arrow) beyond the capsule of the lymph node (dotted line) (ENE-positive). (b) Tumor cells identified in the lymphatic channels (arrows) outside the capsule of the lymph node were classified as ENE-negative.
Optimal cutoff values of the Variables
The optimal cutoff points of NDLN, NPLN, LNR, ENE, and LODDS with the highest sensitivity and specificity were calculated using X-tile3.6.1 software (Yale University School of Medicine, New Haven, CT, USA) [27]. The study calculated LODDS by log (NPLN +0.050)/(NDLN − NPLN + 0.050). We added 0.050 to both the numerator and denominator to avoid an undefined number [28]. NDLN was divided into NDLN1 (1 ≤ NDLN1 ≤ 10) and NDLN2 (11 ≤ NDLN2 ≤ 87), and NPLN was grouped into NPLN1 (1 ≤ NPLN1 ≤ 3) and NPLN2 (4 ≤ NPLN2 ≤ 33). In addition, LNR was categorized into LNR1 (0.01 ≤ LNR1≤ 0.028) and LNR2 (0.28 < LNR2 ≤ 1.00), ENE was classified into ENE1 (ENE1 was ENE-negative) and ENE2 (ENE2 was ENE-positive), and LODDS was classified into LODDS1 (−1.70 ≤ LODDS1 ≤ −0.37) and LODDS2 (−0.37 < LODDS2 ≤ 1.40). The notation “−” indicates that the value is less than zero. The notation“≤” means less or equal to the value, “<” means less than the value.
Construction and validation of the ENE- and LODDS-based nomogram
We used univariate Cox regression analysis to determine whether N status, NPLN, LNR, ENE, and LODDS served as independent factors for predicting the OS of CRC and CC. We estimated the 95% confidence interval (CI) and the adjusted hazard ratio (HR). The predictive efficiency of N status, NPLN, LNR, ENE, and LODDS was compared using the concordance index (C-index), the Akaike information criterion (AIC), and the area under the receiver operating characteristic (ROC) curve (AUC).
The LN staging system with maximal accuracy was further identified by the multivariate Cox proportional hazards model together with other independent prognostic factors. The results of the multivariate analysis were used to construct a nomogram for predicting 1-year OS in the CRC cohort, and verified in the CC cohort.
The predictive performance of the nomogram was evaluated based on the C-index, AUC and calibration plots (comparing the survival probability predicted by the nomogram with the observed value by Kaplan–Meier analysis). Additionally, the decision curve analysis (DCA) confirmed the threshold probability range of the nomogram and compared to the 8th AJCC TNM staging system.
Statistical analysis
Categorical variables were compared using chi-square tests, and continuous variables were compared using independent sample t-tests and were were presented with the mean [standard deviation (SD)] or the median [interquartile range (IQR)]. The Kaplan–Meier method was used to compare OS. The OS was defined as the time from the date of death from any cause or the last follow-up. Univariate and multivariate analyses of factors associated with the OS rates were performed using Cox proportional hazard regression models to estimate the HRs and 95% CIs. First, univariate analyses were performed to determine which of these factors were statistically significant (p < 0.050). The statistically significant variables were subsequently included in multivariate Cox regression analyses. Second, we included N, NPLN, LNR, LODDS, and ENE in five different multivariate Cox regression models (Table 2). Third, all five staging systems and other potential predictors from the univariate analysis were entered into the Cox regression models simultaneously (Tables 3 and 4). Linear trend χ2 scores were used to assess the discriminatory power and monotonicity of each model. Likelihood ratios were used to assess homogeneity between groups using the χ2 test. The Akaike information criterion (AIC) was applied to test the goodness of fit. The Harrell consistency index (C index) was calculated to assess the accuracy of the predictions. correlations between N, NPLN, LNR, LODDS and ENE were shown by scatter plots and assessed by Pearson correlation coefficients. All statistical tests were two-sided, and P < 0.05 indicated statistical significance. All statistical analyses were performed using SPSS ver. 21.0 for Windows (IBM Corp., Armonk, NY, USA).
Table 2.
Univariable Cox regression analysis of potential prognostic predictors for OS in patients with CRC and CC.
| Variable | CRC-OS | CC-OS | |||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p value | HR | (95% CI) | p value | ||
| Age(years) | |||||||
| <65 | 1 | 1 | |||||
| ≥65 | 1.741 | 1.151–2.633 | 0.009 | 2.699 | 1.389–5.242 | 0.003 | |
| Gender | |||||||
| Male | 1 | 1 | |||||
| Female | 0.742 | 0.489–1.126 | 1.161 | 0.742 | 0.489–1.126 | 1.161 | |
| Stage | |||||||
| I+II | 1 | 1 | |||||
| III+IV | 1.209 | 0.444–3.291 | 0.71 | 0.799 | 0.249–2.561 | 0.705 | |
| Depth of invasion | |||||||
| T1+T2 | 1 | 1 | |||||
| T3+T4 | 1.117 | 0.542–2.302 | 0.764 | 2.194 | 0.303–15.897 | 0.437 | |
| Lymph node metastasis | |||||||
| N1 | 1 | 1 | |||||
| N2 | 1.912 | 1.297–2.846 | 0.001 | 2.636 | 1.523–4.563 | 0.001 | |
| Nerve invasion | |||||||
| NO | 1 | 1 | |||||
| YES | 2.232 | 1.505–3.311 | <0.001 | 1.994 | 1.161–3.424 | 0.012 | |
| Vessel invasion | |||||||
| NO | 1 | 1 | |||||
| YES | 1.58 | 1.060–2.356 | 0.025 | 1.828 | 1.053–3.175 | 0.032 | |
| Differentiation status | |||||||
| Well | 1 | 1 | |||||
| Moderate+poor | 3.504 | 0.863–14.225 | 0.079 | 4.723 | 0.652–34.192 | 0.124 | |
| size | |||||||
| <5cm | 1 | 1 | |||||
| ≥5cm | 1.382 | 0.913–2.052 | 0.109 | 1.544 | 0.901–2.648 | 0.114 | |
| Tumor location | |||||||
| Right | 1 | 1 | |||||
| Left and rectal cancer | 0.796 | 0.527–1.200 | 0.276 | 0.557 | 0.315–0.983 | 0.044 | |
| NPLN | |||||||
| NPLN1 | 1 | 1 | |||||
| NPLN2 | 1.949 | 1.315–2.888 | <0.001 | 2.698 | 1.552–4.691 | <0.001 | |
| NDLN | |||||||
| NDLN1 | 1 | 1 | |||||
| NDLN2 | 1.376 | 0.694–2.730 | 0.361 | 1.377 | 0.429–4.415 | 0.591 | |
| LNR | |||||||
| LNR1 | 1 | 1 | |||||
| LNR2 | 1.854 | 1.233–2.786 | 0.003 | 3.475 | 2.010–6.008 | <0.001 | |
| LODDS | |||||||
| LODDS1 | 1 | 1 | |||||
| LODDS2 | 1.782 | 1.186–2.677 | 0.005 | 3.475 | 2.010–6.008 | <0.001 | |
| ENE | |||||||
| ENE1 | 1 | 1 | |||||
| ENE2 | 1.716 | 1.157–2.546 | 0.007 | 1.698 | 0.987–2.921 | 0.056 | |
Abbreviations: LNM, Lymph node metastasis; LNR, lymph node ratio; NPLN, number of positive lymph nodes; NDLN, number of dissected lymph nodes; LODDS, log ODDS; ENE, Extranodal extension.
Table 3.
Multivariable Cox regression analysis of prognostic predictors for OS in patients with CRC
| Variable | N | NPLN | LNR | LODDS | ENE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age(years) | ||||||||||
| <65 | 1 | 1 | 1 | 1 | 1 | |||||
| ≥65 | 1.801(1.186–2.734) | 0.006 | 1.817(1.197–2.760) | 0.005 | 1.742(1.150–2.641) | 0.009 | 1.758(1.159–2.666) | 0.008 | 1.687(1.114–2.555) | 0.014 |
| Nerve invasion | ||||||||||
| NO | 1 | 1 | 1 | 1 | 1 | |||||
| YES | 1.958(1.261–3.309) | 0.003 | 1.914(1.255–3.027) | 0.003 | 1.988(1.279–3.092) | 0.002 | 1.985(1.276–3.087) | 0.002 | 1.907(1.216–2.990) | 0.005 |
| Vessel invasion | ||||||||||
| NO | 1 | 1 | 1 | 1 | 1 | |||||
| YES | 1.120(0.716–1.751) | 0.62 | 1.118(0.714–1.749) | 0.627 | 1.503(0.665–1.668) | 0.824 | 1.057(0.667–1.676) | 0.814 | 1.087(0.683–1.730) | 0.752 |
| N stage | ||||||||||
| N1 | 1 | |||||||||
| N2 | 1.950(1.279–2.837) | 0.002 | ||||||||
| NPLN | ||||||||||
| NPLN1 | 1 | |||||||||
| NPLN2 | 1.934(1.297–2.883) | 0.001 | ||||||||
| LNR | ||||||||||
| LNR1 | 1 | |||||||||
| LNR2 | 1.714(1.123–2.615) | 0.013 | ||||||||
| LODDS | ||||||||||
| LODDS1 | 1 | |||||||||
| LODDS2 | 1.654(1.082–2.530) | 0.02 | ||||||||
| ENE | ||||||||||
| ENE1 | 1 | |||||||||
| ENE2 | 1.429(0.938–2.177) | 0.097 | ||||||||
Abbreviations: LNM, Lymph node metastasis; LNR, lymph node ratio; NPLN, number of positive lymph nodes; NDLN, number of dissected lymph nodes; LODDS, log ODDS; ENE, Extranodal extension.
Table 4.
Multivariable Cox regression analysis of prognostic predictors for OS in patients with CC.
| Variable | N | NPLN | LNR | LODDS | ENE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age(years) | ||||||||||
| <65 | 1 | 1 | 1 | 1 | 1 | |||||
| ≥65 | 2.909(1.481–5.713) | 0.002 | 2.979(1.515–5.857) | 0.002 | 2.716(1.411–5.403) | 0.003 | 2.716(1.411–5.403) | 0.003 | 2.559(1.310–5.001) | 0.006 |
| Nerve invasion | ||||||||||
| NO | 1 | 1 | 1 | 1 | 1 | |||||
| YES | 11.602(0.871–2.946) | 0.129 | 1.605(0.872–2.954) | 0.129 | 1.828(0.981–3.406) | 0.057 | 1.828(0.981–3.406) | 0.057 | 1.423(0.762–2.659) | 0.268 |
| Vessel invasion | ||||||||||
| NO | 1 | 1 | 1 | 1 | 1 | |||||
| YES | 1.434(0.767–2.681) | 0.269 | 1.441(0.770–2.695) | 0.253 | 1.090(0.561–2.118) | 0.8 | 1.090(0.561–2.118) | 0.8 | 1.224(0.595–2.519) | 0.583 |
| Tumor location | ||||||||||
| Right | 1 | 1 | 1 | |||||||
| Left and rectal | 0.570(0.321–1.011) | 0.055 | 0.566(0.319–1.005) | 0.052 | 0.523(0.293–0.931) | 0.028 | 0.523(0.293–0.931) | 0.028 | 0.432(0.227–0.822) | 0.011 |
| N stage | ||||||||||
| N1 | 1 | |||||||||
| N2 | 2.916(1.660–5.121) | 0.001 | ||||||||
| NPLN | ||||||||||
| NPLN1 | 1 | |||||||||
| NPLN2 | 3.062(1.735–5.406) | 0.001 | ||||||||
| LNR | ||||||||||
| LNR1 | 1 | |||||||||
| LNR2 | 3.808(2.111–6.869) | 0.001 | ||||||||
| LODDS | ||||||||||
| LODDS1 | 1 | |||||||||
| LODDS2 | 3.808(2.111–6.869) | 0.001 | ||||||||
| ENE ENE1 ENE2 |
1 1.998(0.987–4..47) |
0.0055 |
||||||||
Abbreviations: LNM, Lymph node metastasis; LNR, lymph node ratio; NPLN, number of positive lymph nodes; LODDS, log ODDS; ENE, Extranodal extension.
Results
Patients characteristics
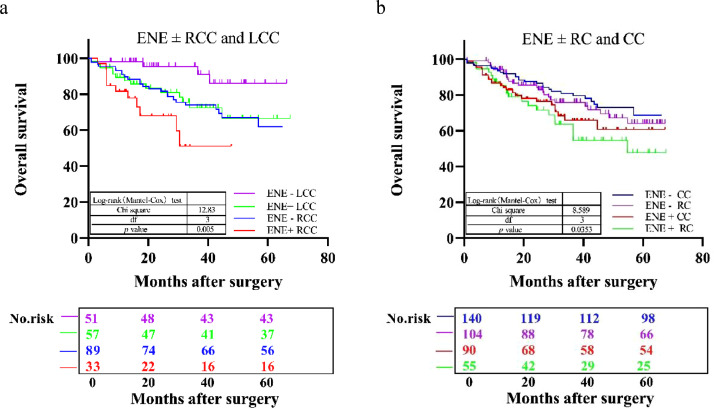
The study consisted of 389 eligible CRC patients. Table 1 summarizes the demographic and clinicopathological characteristics stratified by the ENE and LODDS system. Among the 389 enrolled patients, 236(60.67%) were diagnosed with N1 stage, and 153(39.37%) were diagnosed with N2 stage; the mean age was 65 ± 11.50 years. Moreover, the mean and median NDLN was 19.28 ± 10.14 and 17.00 (interquartile range (IQR): 7.00–27.00), the mean and median NPLN was 4.29 ± 5.45 and 3.00 (IQR: −1.00 to 7.00), the mean and median LNR were 0.24 ± 0.22 and 0.167 (IQR: -0.093 to 0.427), the mean and median LODDS were −0.57 ± 0.51 and −0.623 (IQR: −1.303 to 0.057), and the mean and median ENE were 0.28 ± 0.45 and 0 (IQR: −1 to 1), respectively. For the LODDS system, 280(71.98%) patients constituted the LODDS1 group, and 109(28.02%) patients were in the LODDS2 group. Compared to the LODDS1 group, the patients included in the LODDS2 group were more prone to nerve invasion (P = 0.0351) and vessel invasion (P < 0.001) with a higher rate of N2 stage, less NDLN (P < 0.001), more NPLN (P < 0.001), more LNR (P < 0.001), and a higher value of ENE (P < 0.001). For the ENE system, 244(62.72%) patients were in the ENE1 group, and 145(37.28%) patients comprised the ENE2 system. Compared to the ENE1 system, the patients included in the ENE2 system were more likely to experience nerve invasion (P < 0.001) and vessel invasion (P < 0.001) with higher TNM (P = 0.009), higher T category (P = 0.003), higher N category (P < 0.001), advanced differentiation (P = 0.013), more NPLN (P < 0.001), more LNR (P < 0.001), and a higher value of LODDS (P < 0.001). Furthermore, we found that ENE was more frequent in patients with left and rectal cancer than right cancer. Therefore, we classified CRCs into the right colon, left colon, and rectal cancers and evaluated the clinicopathological significance and prognostic impact of ENE in these patients. ENE in a metastatic LN detected in 90(39.13%) CC patients, including 33(27.0%) patients with right colon cancer(RCC), 57(52.8%) with left colon cancer(LCC), and 55(34.6%) with rectal cancer(RC) (Supplementary Tables 1 and 2). The 5-year OS rate in patients with RCC and LCC differed significantly by ENE status: highest in patients with ENE-negative LCC (85.9%), followed by patients with ENE-positive LCC (66.4%), and ENE-positive RCC (51.1%) (P = 0.005) (Fig. 3a). However, the 5-year OS rate in patients with colon and rectal cancer differed significantly with respect to the tumor site and ENE status: the values were 69.6%, 64.1%, 60.8%, and 47.7% in patients with ENE-negative CC, ENE-negative RC, ENE-positive CC, and ENE-positive RC, respectively (P = 0.0353) (Fig. 3b).
Table 1.
Baseline characteristics of the study population stratified by LODDS and ENE systems.
| Variable | Colorectal cancer(n=389) | |||||||
|---|---|---|---|---|---|---|---|---|
| N | LODDS1 | LODDS2 | p value | N | ENE1 | ENE2 | p value | |
| Age(years) | 0.332 | 0.911 | ||||||
| <65 | 181 | 126 (45.0%) | 55 (50.5%) | 181 | 113(46.3%) | 68(46.9%) | ||
| ≥65 | 208 | 154 (55.0%) | 54 (49.5%) | 208 | 131(53.7%) | 77(53.1%) | ||
| Gender | 0.925 | 0.772 | ||||||
| Male | 237 | 171(61.1%) | 66(60.6%) | 237 | 147(60.2%) | 90(62.1%) | ||
| Female | 152 | 109(38.9%) | 43(39.4%) | 152 | 97(39.8%) | 55(37.9%) | ||
| Stage | 0.769 | 0.009 | ||||||
| I+II | 16 | 11(3.9%) | 5(4.6%) | 16 | 15(6.1%) | 1(0.7%) | ||
| III+IV | 373 | 269(96.1%) | 104(95.4%) | 373 | 229(93.9%) | 144(99.3%) | ||
| Depth of invasion | 0.614 | 0.003 | ||||||
| T1+T2 | 28 | 19(6.8%) | 9(8.3%) | 28 | 25(10.2%) | 3(2.1%) | ||
| T3+T4 | 361 | 261(93.2%) | 100(91.7%) | 361 | 219(89.9%) | 142(97.9%) | ||
| LNM | <0.001 | <0.001 | ||||||
| N1 | 236 | 223(79.6%) | 13(11.9%) | 236 | 215(88.1%) | 21(14.5%) | ||
| N2 | 153 | 57(20.4%) | 96(88.1%) | 153 | 29(11.9%) | 124(85.5%) | ||
| Nerve invasion | 0.035 | <0.001 | ||||||
| NO | 153 | 101(36.1%) | 52(47.7%) | 153 | 98(38.9%) | 108(74.5%) | ||
| YES | 236 | 179(63.9%) | 57(52.3%) | 236 | 149(61.1%) | 37(25.5%) | ||
| Vessel invasion | <0.001 | <0.001 | ||||||
| NO | 203 | 122(43.6%) | 81(74.3%) | 203 | 95(38.9%) | 108(74.5%) | ||
| YES | 186 | 158(56.4%) | 28(25.7%) | 186 | 149(61.1%) | 37(25.5%) | ||
| Differentiation status | 0.024 | 0.013 | ||||||
| Well | 19 | 18(6.4%) | 1(0.9%) | 19 | 17(7.0%) | 2(1.4%) | ||
| Moderate+poor | 370 | 262(93.6%) | 108(99.1%) | 370 | 227(93.0%) | 143(98.6%) | ||
| size | 0.862 | 0.907 | ||||||
| <5cm | 240 | 172(61.4%) | 68(62.4%) | 240 | 150(61.5%) | 90(62.1%) | ||
| ≥5cm | 149 | 108(38.6%) | 41(37.6%) | 149 | 94(38.5%) | 55(37.9%) | ||
| Tumor location | 0.773 | 0.005 | ||||||
| Right | 122 | 89(31.8%) | 33(30.3%) | 122 | 89(36.5%) | 33(22.8%) | ||
| Left and rectal | 267 | 191(68.2%) | 76(69.7%) | 267 | 155(63.5%) | 112(77.2%) | ||
| NPLN | <0.001 | <0.001 | ||||||
| NPLN1 | 233 | 223(79.6%) | 10(9.2%) | 233 | 213(87.3%) | 20(13.8%) | ||
| NPLN2 | 156 | 57(20.4%) | 99(90.8%) | 156 | 31(12.7%) | 125(86.2%) | ||
| NDLN | 0.003 | 0.215 | ||||||
| NDLN1 | 48 | 26(9.3%) | 22(20.2%) | 48 | 34(13.9%) | 14(9.7%) | ||
| NDLN2 | 341 | 254(90.7%) | 87(79.8%) | 341 | 210(86.1%) | 131(90.3%) | ||
| LNR | <0.001 | <0.001 | ||||||
| LNR1 | 282 | 280(100%) | 2(1.8%) | 282 | 224(91.8%) | 58(40.0%) | ||
| LNR2 | 107 | 0(0%) | 107(98.2%) | 107 | 20(8.2%) | 87(60.0%) | ||
| ENE | <0.001 | |||||||
| ENE1 | 244 | 222(79.3%) | 22(20.2%) | |||||
| ENE2 | 145 | 58(20.7%) | 87(79.8%) | |||||
| LODDS | <0.001 | |||||||
| LODDS1 | 280 | 222(91.0%) | 58(40.0%) | |||||
| LODDS2 | 109 | 22(9.0%) | 87(60.0%) | |||||
Abbreviations: LNM, Lymph node metastasis; LNR, lymph node ratio; NPLN, number of positive lymph nodes; NDLN, number of dissected lymph nodes; LODDS, log ODDS; ENE, Extranodal extension.
Fig. 3.
(a) OS curves in patients with CC according to the tumor location (right colon vs. left colon) and extranodal extension (ENE) status (n = 230). (b) OS curves in patients with colon and rectal cancer according to the tumor location (colon vs. rectum) and ENE status (n = 398).
Survival analysis
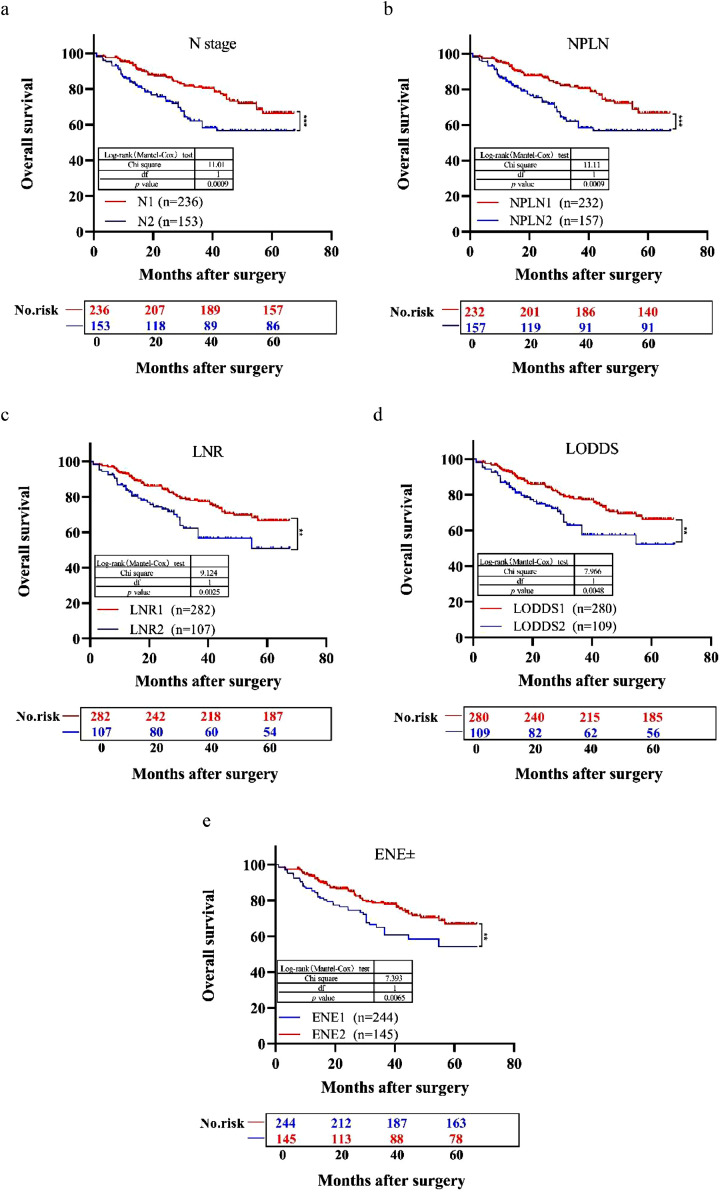
The median follow-up time for the entire cohort was 31 months (IQR, 1–61 months). As shown in Fig. 4, patients with N2 stage, a large number of positive LNs dissected, or high values of LNR, LODDS, and ENE were significantly correlated with poor OS (all log-rank P < 0.001). Univariable Cox regression model suggested that age, N stage, nerve invasion, vessel invasion, NPLN, LNR, LODDS, and ENE were potential prognostic factors for OS in both CRC and CC. (all P < 0.05; Table 2). Based on these results in the univariable analysis, the N, NPLN, LNR, LODDS, and ENE were independently incorporated into five Cox regression models combined with the same confounders. Multivariable Cox regression analysis demonstrated that these five staging systems except ENE were all independent prognostic factors for OS in CRC (P < 0.05; Table 3) and these five staging systems were all independent prognostic factors for OS in CC (P < 0.05; Table 4) . The HRs and 95% CIs were as follows: for N (CRC: HR=1.950; 95% CI: 1.279–2.837; CC: HR=2.916; 95% CI: 1.660–5.121); for NPLN (CRC: HR=1.934; 95% CI: 1.297–2.883; CC: HR=3.062; 95% CI: 1.735–5.406); for LNR (CRC: HR=1.714; 95% CI: 1.123–2.615; CC: HR=3.808; 95% CI: 2.111–6.869); for LODDS (CRC: HR =1.654; 95% CI, 1.082-2.530; CC: HR =3.808; 95% CI: 2.111–6.869); for ENE (CRC: HR=1.429; 95% CI: 0.938–2.177; CC: HR=1.998; 95% CI: 0.987–4.47).
Fig. 4.
Kaplan–Meier estimates of the OS for patients with N1/N2 stage CRC after surgery according to (a) N, (b) NPLN, (c) LNR, (d) LODDS, and (e) ENE staging systems. CRC, colorectal cancer; NPLN, number of positive lymph nodes; LNR, lymph node ratio; LODDS, log odds of positive lymph nodes; ENE, extranodal extension.
Correlation of LODDS with the clinicopathological characteristics of CRC
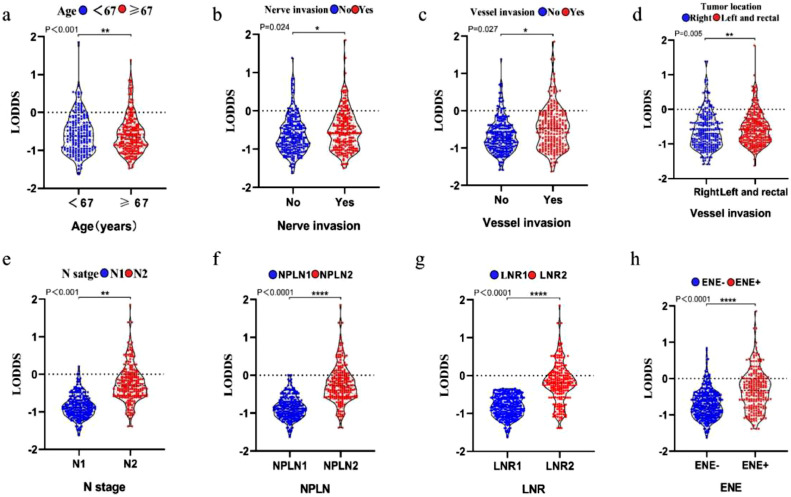
Age, nerve invasion, vessel invasion, tumor location, N stage, NPLN, LNR, and ENE were significantly correlated with LODDS in CRC patients after surgery. The older patients, especially those≥65-years-old at diagnosis, yielded notably higher LODDS values than younger patients (P < 0.001, Kruskal–Wallis H test; Fig. 5a). Patients with nerve invasion, vessel invasion, and those with left and right colon cancer tended to have higher LODDS values than other patients (P < 0.05, Wilcoxon test; Fig. 5b–d). The patients with high N stage, NPLN, and LNR had high LODDS points (P < 0.001, Kruskal–Wallis H test; Fig. 5e–g). Moreover, the LODDS value differed significantly among ENE-positive patients (P < 0.001, Kruskal–Wallis H test; Fig. 5h).
Fig. 5.
Correlation of LODDS with age (a), nerve invasion (b), vessel invasion (c), tumor location (d), N stage (e), NPLN (f), LNR (g), and ENE (h).
Correlation between LODDS and N, NPLN, LNR, and ENE
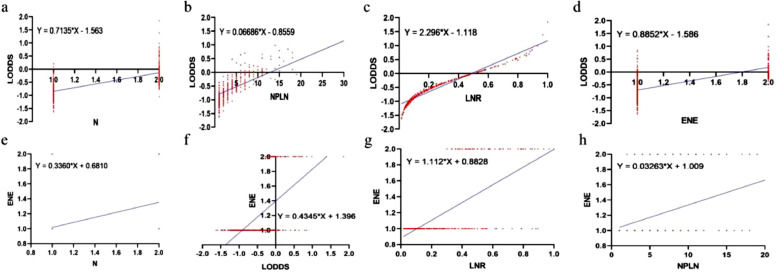
To illustrate the reason for the superiority of LODDS over the other systems, scatter plots were used to observe the correlation between LODDS and the other four staging systems. LODDS was positively correlated with N, NPLN, ENE, and LNR (r = 0.4699, 0.5123, 0.3846, and 0.9418, respectively; P < 0.001 for all four variables) (Fig. 6a–d). To elucidate the superiority of ENE over other systems, scatter plots were used to visualize the correlation between ENE and the other four staging systems. ENE was positively correlated with the N, NPLN, LNR, and LODDS (r = 0.2123, 0.2486, 0.4503, and 0.4503, respectively; P < 0.001 for all four variables)(Fig. 6e–h). Typically, the LODDS value increased with the increasing LNR value, while the correlation was not completely linear. When LNR was ≤0.1 or ≥0.9, the curves of LNR increased at a slower rate compared to LODDS, especially when the value of LNR was to 0 or 1, indicating that the LODDS system could detect heterogeneity. In addition, when NPLN was ≤10, the LODDS system was heterogeneous, and it could distinguish different survival outcomes for patients with the same NPLN.
Fig. 6.
Scatter plots of the correlation between LODDS and ENE. LODDS vs. (a) N, (b) NPLN and (c) LNR and (d) ENE in patients with N1/N2 stage CRC after surgery. ENE vs. (e) N, (f) LODDS, (g) LNR and (h) NPLN in patients with N1/N2 stage CRC after surgery. CRC, colorectal cancer; NPLN, number of positive lymph nodes; LNR, lymph node ratio; LODDS, log odds of positive lymph nodes. ENE, extranodal extension.
Sensitivity analysis and subgroup analysis
The evaluation of survival according to lymph node metastasis status is shown in Tables 3 and 4. In the multivariable analysis adjusted for confounding variables, LODDS2 was associated with poor outcomes (HR=1.654; 95% CI: 1.082–2.530) compared to the LODDS1 group (P = 0.02) in CRC (Table 3). Compared to ENE1, ENE2 exhibited a two-fold hazard of death (HR=1.998; 95% CI: 0.987–4.47) (P = 0.0055) in CC (Table 4). Among the CC cases, LODDS2 status was associated with more than three-fold risk of death (HR=3.808; 95% CI: 2.111–6.869) compared with LODDS1 (P < 0.0001) in CC (Table 4). These results suggested that the hazard of death of LODDS2 is higher in CC than CRC. Together, these findings indicated that the ENE status was significant in CC (P = 0.0055) but not in CRC (P = 0.097). Thus, tumors in different locations exhibit marked differences.
Construction and validation of the prognostic nomogram for OS in CRC and CC
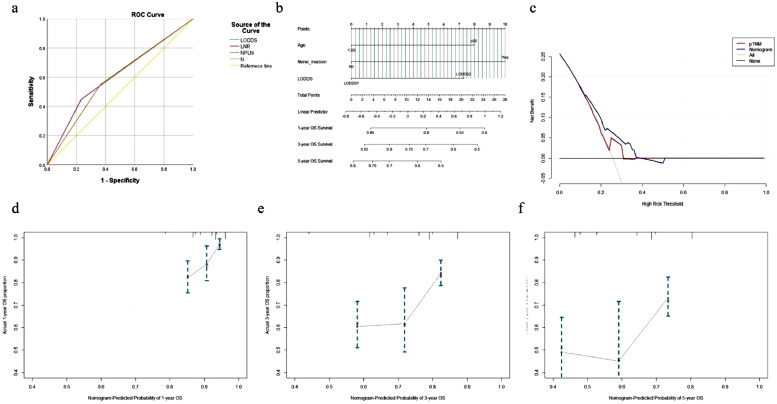
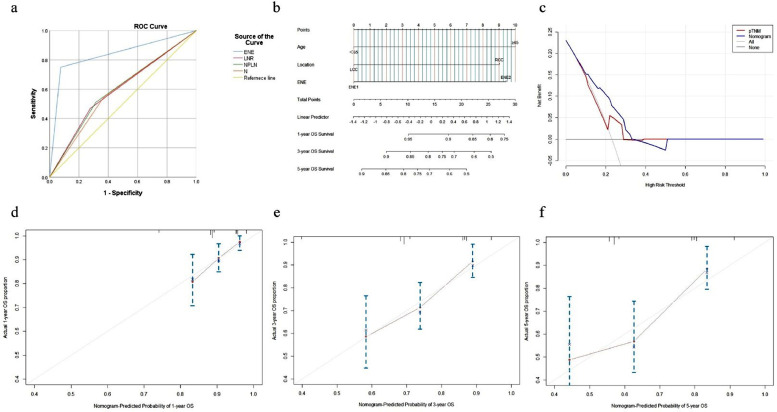
We selected LODDS over N status and NPLN as the LN staging system in the multivariate Cox analysis due to its satisfactory performance in predicting OS for CRC. On the other hand, ENE was used as the staging system in the multivariate Cox analysis due to its satisfactory performance in predicting OS for CC. The results showed that age, nerve invasion, and the LODDS system were independent prognostic factors for OS in CRC (Table 3) and that age, tumor location, and the ENE system were independent prognostic factors for OS in CC (Table 4). The prognostic performance of the four classification systems in CRC and CC. we compared the power of discrimination and found that AUROC of LODDS (0.609) and ENE (0.837) was higher than pN (0.591,0.588), respectively, (Figs. 7a, 8a and Table 5) .Two nomograms for predicting 1-, 3-, and 5-year OS were constructed based on the three key factors (Figs. 7b and 8b).
Fig. 7.
ROC analysis of AJCC pN, LNR, NPLN and LODDS for CRC (a);Nomogram to predict 1-, 3-, and 5-year OS for CRC (b); The DCA of the nomogram and the AJCC TNM staging system to OS in CRC (c);The calibration curves to predict 1-, 3-, and 5-year OS in CRC (d–f). AJCC,American Joint Committee on Cancer; LNR, lymph node ratio; LODDS, log odds; OS, overall survival; DCA, decision curve analysis.
Fig. 8.
ROC analysis of AJCC pN, LNR, NPLN and ENE for CC (a);Nomogram to predict 1-, 3-, and 5-year OS for CC (b); The DCA of the nomogram and the AJCC TNM staging system to OS in CC (c);The calibration curves to predict 1-, 3-, and 5-year OS in CC (d-f). AJCC,American Joint Committee on Cancer; LNR, lymph node ratio; ENE, extranodal extension; OS, overall survival; DCA, decision curve analysis.
Table 5.
Comparison of discriminatory ability and AUROC in CRC and CC.
| AUROC-CRC | |||||
|---|---|---|---|---|---|
| Classifications | AUROC | SE | Lower bound | Upper bound | p value |
| LODDS | 0.609 | 0.037 | 0.536 | 0.682 | 0.003 |
| LNR | 0.607 | 0.037 | 0.534 | 0.68 | 0.003 |
| NPLN | 0.592 | 0.036 | 0.521 | 0.664 | 0.012 |
| N | 0.591 | 0.036 | 0.519 | 0.662 | 0.013 |
| AUROC-CC | |||||
|---|---|---|---|---|---|
| Classifications | AUROC | SE | Lower bound | Upper bound | p value |
| ENE | 0.837 | 0.028 | 0.783 | 0.892 | 0.001 |
| LNR | 0.596 | 0.034 | 0.529 | 0.663 | 0.005 |
| NPLN | 0.598 | 0.034 | 0.531 | 0.664 | 0.004 |
| N | 0.588 | 0.034 | 0.521 | 0.654 | 0.01 |
Abbreviations: AUROC, area under receiver operator curve.
The respective C-index values for the CRC using the nomogram was 0.674 (95% CI: 0.727–0.621). Similar results were obtained in the CC; the C-index of the nomogram was 0.680 (95% CI: 0.748–0.611). A high AUC confirmed the favorable sensitivity and specificity of the nomogram in both CRC (0.609) and the CC (0.837), respectively, for 5-year OS (Table 5).
The novel nomogram obtained a net clinical benefit than the 8th AJCC TNM staging system and showed better performance in prognostic predictions for CRC and CC (Figs. 7c and 8c). In addition,the calibration plot demonstrated the agreement between the optimal bootstrap-predicted values and the actual observed values of CRC and CC, indicating appreciable prognostic reliability of the nomogram (Figs. 7d–f and 8d–f), and the DCA results showed a wide field of the threshold probabilities, supporting the clinical applicability of the nomogram system to predict OS for CRC and CC.
Discussion
Lymph node metastasis is the most common metastatic route for CRC and a critical factor affecting patients’ prognosis, and accurate lymph node staging is the key to determine postoperative adjuvant therapy. Lymph node status predicts the prognosis of patients with CRC, rendering it as the key factor post-surgery [29]. Currently, the 8th edition of the AJCC/UICC N-stage is the most widely used method for staging lymph nodes, but the accuracy of this staging method is questionable [30,31]. However, the prognostic value of LODDS and ENE for patients with CRC is unclear. In this study, for the first time, we studied LODDS and ENE together and identified them as independent prognostic factors for predicting long-term OS among N1/N2 stage CRC patients after undergoing surgery. In addition, the current study demonstrated that LODDS or ENE is an independent prognostic indicator for CRC or CC, showing better prognostic performance than the N, NPLN, and LNR staging systems.
Moreover, a significant correlation was established between pN and NDLN, while NDLN is influenced by the surgeon's skills, the actual number of regional lymph nodes and the number of lymph nodes dissected and retrieved [32]. The LNR staging system integrates pLNs and NDLN and is considered a critical prognostic factor for patients with rectal cancer after neoadjuvant radiotherapy [33,34]. In addition to lymph node status, the adequacy of lymph node detection is crucial in the prognosis of patients with rectal cancer [35].
LNR is a ratio-based lymph node status evaluation method that takes into account both the number of positive and negative lymph nodes. Some findings suggested that LNR is a better independent prognostic parameter for rectal cancer compared to the 8th AJCC N staging system and can classify patients with the same N stage into significantly different prognostic groups [36].
The results of the study by Rausei et al. comprising 444 CRC patients showed that LNR (NR0 0, NR1 1–19%, NR2≥20%) was a simpler, reliable predictive tool and more valuable than the current pN staging system because it was less dependent on the extent of lymph node dissection [37]. LNR was a better predictor of OS and disease-free survival (DFS) in patients with rectal cancer compared with N stage. For example, patients with 1 NPLN and 4 NDLN had an LNR of 0.25 compared to those with 4 NPLN and 16 NDLN. However, several studies have questioned the prognostic accuracy of LNR when all retrieved lymph nodes metastasized, as survival outcomes were inconsistent in patients with identical LNR values [38]. The new LODDS system is based on the findings of LNR researchers, and therefore can be further graded for patients with identical LNR values but inconsistent survival outcomes.
The predictive performance of LODDS was better than that of N-stage and rN-stage, and when the overall number of metastatic lymph nodes was 0, only the LODDS stage had predictive performance. The lymph node status was applied as a continuous variable to assess prognosis. For each pN stage, significant survival differences were observed among patients with different LODDS stages, deeming it as a more reliable prognostic indicator of OS than LNR. Kwon et al. found that LODDS was an independent prognostic factor for DFS after adjuvant treatment post-radical surgery in high-risk patients with cervical cancer, high LODDS value, the low survival rate in patients [39]. LODDS is a novel prognostic predictor that improves the accuracy of lymph node assessment for prognostic assessment and is superior to LNR in many malignancies.
Nevertheless, the present study has some limitations. First, the population was not universal and clinically representative in this single-center retrospective study. Second, there is no standard staging method for LNR and LODDS, and the staging of our group was based on the retrospective analysis of 2561 lung squamous cell carcinoma patients in the Surveillance, Epidemiology, and End Results (SEER) database by Yu et al. [40]. The findings of this study on the prognosis assessment of LODDS in lymph node-positive CRC patients are consistent with those of Yu et al., but the basis of LODDS grouping deserves further investigation. Finally, only lymph node-positive CRC patients were studied in this group and the expected effect on lymph node-negative CRC patients should be investigated in the future.
Since the 8th AJCC TNM classification relies on only three pathological indicators (T, N, and M status) and neglects other vital factors, its ability to evaluate the prognosis of CRC is limited. Based on LODDS, ENE, and other prognosis-related parameters, we established two novel prognostic models to predict OS for CRC or CC. Herein, we displayed the nomogram, which integrates multiple prognostic parameters. As newly added information in the nomogram, LODDS and ENE provide an in-depth insight for clinicians to analyze the postoperative LN status and evaluate the prognosis for CRC. To the best of our knowledge, this is the first study on LODDS and ENE together. The current study showed that LODDS and ENE are liable prognostic parameters of CRC or CC. Based on the relevant literature studies, combined with our own conclusions it is known that LODDS and ENE showed the superior ability of CRC or CC in predicting OS compared to the N status and the NPLN system. The proposed nomogram based on LODDS and ENE had superior predictive accuracy, calibration, and clinical applicability compared to the 8th AJCC TNM staging system. Interestingly, this study showed that ENE rates increased significantly from patients with right CC (27.0%) to left CC (52.8%) to rectal cancer (34.6%) (right vs. left, P = 0.005; left vs. rectum, P = 0.0353). These findings indicated that ENE was a common aggressive feature of CRC; however, it might be related to the distal part of CRC. Considering the continuum hypothesis of tumorigenesis, the frequency of ENE increases from the proximal (right) to the distal (left) colon as well as the rectum. This conclusion needs to be verified by future experiments at the genetic and molecular levels.
Taken together, LODDS stage is an independent influence on the prognosis of both CRC and CC patients, and higher the LODDS stage, the lower the 5-year survival rate of patients. ENE is an independent influence on the prognosis of both CRC and CC patients; the more the prognostic impact of extracapsular lymph node involvement in both CRC and CC and the more positive ENE, the lower the 5-year survival rate of patients. Therefore, combining ENE and LODDS into the current TNM system to compensate for the inadequacy of pN staging should be considered in future studies.
Ethics approval and consent to partcipate
The use of human hematoxylin & eosin(H&E) stained sections of specimens were approved by the Ethical Committee for Clinical Research of Shanghai General Hospital.
CRediT authorship contribution statement
Tengfei Li: Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. Yan Yang: Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. Weidong Wu: Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. Zhongmao Fu: Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. Feichi Cheng: Data curation, Formal analysis, Software, Validation, Writing – original draft, Writing – review & editing. Jiahui Qiu: Data curation, Formal analysis, Software, Validation, Writing – original draft, Writing – review & editing. Qi Li: Formal analysis, Resources, Software, Validation, Writing – review & editing. Kundong Zhang: Formal analysis, Resources, Validation, Writing – review & editing. Zai Luo: Formal analysis, Resources, Validation, Writing – review & editing. Zhengjun Qiu: Investigation, Project administration, Resources, Supervision, Writing – review & editing. Chen Huang: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors have declared no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [Grant No. 81772526 to Chen Huang], National Natural Science Foundation of China [Grant No. 82072662 to Chen Huang], Shanghai Municipal Education Commission-Gaofeng Clinical Medicine [Grant No. 20161425 to Chen Huang], Shanghai Jiaotong University Medical Cross Fund [Grant No. YG2017MS28 to Chen Huang], Three-year Action Plan for Clinical Skills and Clinical Innovation in Shanghai-level Hospitals [Grant No.SHDC2020CR4022 to Chen Huang].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101190.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68(1):11. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Ichimasa K., Kudo S.E., Miyachi H., Kouyama Y., Misawa M., Mori Y. Risk stratification of T1 colorectal cancer metastasis to lymph nodes: current status and perspective. Gut Liver. 2020 doi: 10.5009/gnl20224. [published online ahead of print, 2020 Dec 24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu H.B., Zhang L.Y., Li Y.F., Zhou Z.W., Keshari R.P., Xu RH. Ratio of metastatic to resected lymph nodes enhances to predict survival in patients with stage III colorectal cancer. Ann Surg Oncol. 2011;18(6):1568–1574. doi: 10.1245/s10434-010-1528-8. [DOI] [PubMed] [Google Scholar]
- 5.Lee H.Y., Choi H.J., Park K.J. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14:1712–1717. doi: 10.1245/s10434-006-9322-3. [DOI] [PubMed] [Google Scholar]
- 6.Carrato A. Adjuvant treatment of colorectal cancer. Gastrointest. Cancer Res. 2008;2:S42–SS6. [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald J.R., Renehan A.G., O'Dwyer S.T., Haboubi N.Y. Lymph node harvest in colon and rectal cancer: current considerations. World J. Gastrointest. Surg. 2012;4:9–19. doi: 10.4240/wjgs.v4.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger A.C., Sigurdson E.R., LeVoyer T. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J. Clin. Oncol. 2005;23(34):8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 9.Tsai H.L., Lu C.Y., Hsieh J.S. The prognostic significance of total lymph node harvest in patients with T2-4N0M0 colorectal cancer. J. Gastrointest. Surg. 2007;11(5):660–665. doi: 10.1007/s11605-007-0119-x. [DOI] [PubMed] [Google Scholar]
- 10.Galizia G., Orditura M., Ferraraccio F. The lymph node ratio is a powerful prognostic factor of node-positive colon cancers undergoing potentially curative surgery. World J. Surg. 2009;33:2704–2713. doi: 10.1007/s00268-009-0207-z. [DOI] [PubMed] [Google Scholar]
- 11.Ricciardi R., Madoff R.D., Rothenberger D.A., Baxter NN. Population-based analyses of lymph node metastases in colorectal cancer. Clin. Gastroenterol. Hepatol. 2006;4:1522–1527. doi: 10.1016/j.cgh.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Huh J.W., Kim Y.J., Kim HR. Ratio of metastatic to resected lymph nodes as a prognostic factor in node-positive colorectal cancer. Ann. Surg. Oncol. 2010;17(10):2640–2646. doi: 10.1245/s10434-010-1015-2. [DOI] [PubMed] [Google Scholar]
- 13.Kano K., Yamada T., Yamamoto K. Evaluation of lymph node staging systems as independent prognosticators in remnant gastric cancer patients with an insufficient number of harvested lymph nodes. Ann. Surg. Oncol. 2021 doi: 10.1245/s10434-020-09433. [published online ahead of print, 2021 Jan 3] [DOI] [PubMed] [Google Scholar]
- 14.Priolli D.G., Cardinalli I.A., Pereira J.A., Alfredo C.H., Margarido N.F., Martinez CA. Metastatic lymph node ratio as an independent prognostic variable in colorectal cancer: study of 113 patients. Tech. Coloproctol. 2009;13(2):113–121. doi: 10.1007/s10151-009-0467-5. [DOI] [PubMed] [Google Scholar]
- 15.Schneider D.F., Chen H., Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann. Surg. Oncol. 2013;20(6):1906–1911. doi: 10.1245/s10434-012-2802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baqar A.R., Wilkins S., Wang W., Oliva K., McMurrick P. Log odds of positive lymph nodes is prognostically equivalent to lymph node ratio in non-metastatic colon cancer. BMC Cancer. 2020;20(1):762. doi: 10.1186/s12885-020-07260-y. Published 2020 Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei J.P., Zhang C.D., Fan Y.C., Dai DQ. Comparison of different lymph node staging systems in patients with resectable colorectal cancer. Front. Oncol. 2019;8:671. doi: 10.3389/fonc.2018.00671. Published 2019 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nottegar A., Veronese N., Senthil M. Extra-nodal extension of sentinel lymph node metastasis is a marker of poor prognosis in breast cancer patients: A systematic review and an exploratory meta-analysis. Eur. J. Surg. Oncol. 2016;42(7):919–925. doi: 10.1016/j.ejso.2016.02.259. [DOI] [PubMed] [Google Scholar]
- 19.Veronese N., Nottegar A., Pea A. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann. Oncol. 2016;27(1):42–48. doi: 10.1093/annonc/mdv494. [DOI] [PubMed] [Google Scholar]
- 20.Lydiatt W.M., Patel S.G., O'Sullivan B. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67(2):122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 21.Link H., Angele M., Schüller M. Extra-capsular growth of lymph node metastasis correlates with poor prognosis and high SOX9 expression in gastric cancer. BMC Cancer. 2018;18(1):483. doi: 10.1186/s12885-018-4413-7. Published 2018 Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee C.H.A., Wilkins S., Oliva K., Staples M.P., McMurrick PJ. Role of lymph node yield and lymph node ratio in predicting outcomes in non-metastatic colorectal cancer. BJS Open. 2019;3:95–105. doi: 10.1002/bjs5.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arslan N.C., Sokmen S., Canda A.E., Terzi C., Sarioglu S. The prognostic impact of the log odds of positive lymph nodes in colon cancer. Colorectal Dis. 2014;16:O386–O392. doi: 10.1111/codi.12702. [DOI] [PubMed] [Google Scholar]
- 24.Calero A., Escrig-Sos J., Mingol F. Usefulness of the log odds of positive lymph nodes to predict and discriminate prognosis in gastric carcinomas. J. Gastrointest. Surg. 2015;19:813–820. doi: 10.1007/s11605-014-2728-5. [DOI] [PubMed] [Google Scholar]
- 25.Chen L.J., Chung K.P., Chang Y.J., Chang Y.J. Ratio and log odds of positive lymph nodes in breast cancer patients with mastectomy. Surg. Oncol. 2015;24:239–247. doi: 10.1016/j.suronc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Kim C.W., Kim J., Yeom S.S. Extranodal extension status is a powerful prognostic factor in stage III colorectal cancer. Oncotarget. 2017;8(37):61393–61403. doi: 10.18632/oncotarget.18223. Published 2017 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camp R.L., Dolled-Filhart M., Rimm D.L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y.Y., Du X.J., Zhang C.H. Comparison of three lymph node staging schemes for predicting the outcome in patients with small bowel adenocarcinoma: a population-based cohort and international multicentre cohort study. EBioMedicine. 2019;41:276–285. doi: 10.1016/j.ebiom.2019.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 30.Lykke J., Jess P., Roikjaer O. Danish Colorectal Cancer Group. The prognostic value of lymph node ratio in a national cohort of rectal cancer patients. Eur. J. Surg. Oncol. 2016;42(4):504–512. doi: 10.1016/j.ejso.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg R., Friederichs J., Schuster T. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann. Surg. 2008;248(6):968–978. doi: 10.1097/SLA.0b013e318190eddc. [DOI] [PubMed] [Google Scholar]
- 32.Berger A.C., Sigurdson E.R., LeVoyer T. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J. Clin. Oncol. 2005;23(34):8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 33.Park I.J., Yu C.S., Lim S.B. Ratio of metastatic lymph nodes is more important for rectal cancer patients treated with preoperative chemoradiotherapy. World J. Gastroenterol. 2015;21(11):3274–3281. doi: 10.3748/wjg.v21.i11.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q.G., Li D.W., Zhuo C.H., Cai G.X., Cai SJ. Metastatic lymph node ratio can further stratify prognosis in rectal cancer patients treated with preoperative radiotherapy: a population-based analysis. Tumour Biol. 2014;35(7):6389–6395. doi: 10.1007/s13277-014-1817-0. [DOI] [PubMed] [Google Scholar]
- 35.Kidner T.B., Ozao-Choy J.J., Yoon J., Bilchik AJ. Should quality measures for lymph node dissection in colon cancer be extrapolated to rectal cancer? Am. J. Surg. 2012;204(6):843–848. doi: 10.1016/j.amjsurg.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Park I.J., Yu C.S., Lim S.B. Ratio of metastatic lymph nodes is more important for rectal cancer patients treated with preoperative chemoradiotherapy. World J. Gastroenterol. 2015;21(11):3274–3281. doi: 10.3748/wjg.v21.i11.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rausei S., Iovino D., Tenconi S. Impact of lymph node ratio on survival of colorectal cancer patients. Int. J. Surg. 2013;11 Suppl 1:S95–S99. doi: 10.1016/S1743-9191(13)60026-6. [DOI] [PubMed] [Google Scholar]
- 38.Peschaud F., Benoist S., Julié C. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann. Surg. 2009;249(4):701. doi: 10.1097/SLA.0b013e31818842ec. [published correction appears in. Apr. [DOI] [PubMed] [Google Scholar]
- 39.Kwon J., Eom K.Y., Kim I.A. Prognostic value of log odds of positive lymph nodes after radical surgery followed by adjuvant treatment in high-risk cervical cancer. Cancer Res. Treat. 2016;48(2):632–640. doi: 10.4143/crt.2015.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y., Zhang P., Yao R. Prognostic value of log odds of positive lymph nodes in node-positive lung squamous cell carcinoma patients after surgery: a SEER population-based study. Transl. Lung Cancer Res. 2020;9(4):1285–1301. doi: 10.21037/tlcr-20-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.