Abstract
This study was conducted to evaluate the effect of flavor on reproductive performance and fecal microbiota of sows during late gestation and lactation. A total of 20 healthy Yorkshire sows were fed a corn-soybean basal diet unsupplemented or supplemented with 0.1% flavor compound from d 90 of gestation to 25 d post-farrowing, and then the piglets were weaned. The reproductive performance and the fecal microbiota of sows were analyzed. Compared with the controls, flavor supplementation in maternal diets increased (P < 0.05) weaning litter weight, litter weight gain, weaning body weight, and average daily gain of piglets. There was a trend of increase in the average daily feed intake of sows (P = 0.09) by maternal dietary flavor addition. The backfat thickness and litter size were not affected by flavor supplementation (P > 0.05). The 16S rRNA analysis showed that flavor supplementation significantly increased the abundance of Phascolarctobacterium (P < 0.05), but significantly decreased genera Terrisporobacter, Alloprevotella, Clostridium_sensu_stricto_1, and Escherichia-shigella (P < 0.05). Spearman correlation analysis showed that Phascolarctobacterum was positively correlated with the average daily feed intake of sows (P < 0.05), the litter weight gain and average daily gain of piglets (P < 0.05). In contrast, Clostridium_sensu_stricto_1 and unclassified_f__Lachnospiraceae were negatively correlated with the litter weight gain and average daily gain of piglets (P < 0.05). Taken together, dietary flavor supplementation improved the reproductive performance of the sows, which was associated with enhanced beneficial microbiota and decreased potentially pathogenic bacteria in the sows.
Keywords: Correlation, Flavor, Fecal microbiota, Reproductive performance
1. Introduction
It is well-known that the performance of sows plays an important role in the productivity of the whole swine industry. Modern genetic selection for high growth rate and lean tissue accretion results in lower appetite in sows (Wang et al., 2014), which might exert a negative effect on the maintenance of body weight, body condition, and lactation performance of the sows (Lundgren et al., 2014). A lower feed intake during the lactation period results in decreased milk production (Zijlstra et al., 1996; Kim et al., 2004), which could limit the growth and development of nursery pigs (He et al., 2017). So, increasing feed intake of the lactational sows might be beneficial to maternal condition, and growth of nursery pigs. Since the 1960s, feed flavors have been widely used in nursery pig diets as palatability enhancers and feed attractants to increase feed intake (Seabolt et al., 2010; Sulabo et al., 2010). However, the effect of flavor addition on the performance of sows has seldom been reported. Therefore, the effect of flavors supplementation on maternal performance still needs to be further investigated.
It is generally believed that intestinal microbiota interacts with host cells through multiple levels of mechanisms, therefore regulating the metabolism, immunity, development, and behavior of the host (Erkosar et al., 2013; Valeriano et al., 2017; Zhang et al., 2017). Gut microbiota composition is affected by developmental stage, physiological status of animals, as well as various environmental factors, such as nutritional composition, pathogen infection, antibiotic application, and others (Ji et al., 2017). It has been reported that gut microbiota undergoes a remarkable shift during pregnancy and lactation periods (Santacruz et al., 2010; Koren et al., 2012a), which might be passed onto the developing fetus or newborn piglets through the placenta or maternal milk, respectively (Everaert et al., 2017; Macpherson et al., 2017). Recent studies indicate that gut microbiota of the sows plays an important role in the performance of both the maternal and offspring pigs (Wang et al., 2018; Li et al., 2019; Xiong et al., 2019). Feed flavor, such as palatability enhancers and feed attractants, was widely used in swine production. But to the best of our knowledge, there has been no research on the effect of dietary flavor on gut microbiota. Thus, it was necessary to determine the effect of flavor addition on the gut microbiota community.
Considering the critical role of intestinal microbiota on physiology, metabolism, and immune response of the host (Hollister et al., 2014), as well as the functional role of maternal intestinal bacteria on the progeny gut microbiota colonization and shaping of the immune response in newborn animals (Macpherson et al., 2017), we hypothesized that flavor supplementation during late gestation and lactation periods would improve reproductive performance by improving feed intake and regulating gut microbiota of the sows. In the present study, sows were unsupplemented or supplemented with flavor compounds from d 90 of pregnancy to 25 d post-farrowing. Reproductive performance, fecal microbiota of the sows, and growth performance of the piglets were determined.
2. Materials and methods
2.1. Animals, treatment, housing, and sample collection
This study was approved by the Institutional Animal Care and Use Committee of China Agricultural University. A total of 20 healthy Yorkshire sows with second parity were assigned into a control group (CON) or flavor supplementary group (FLA) based on backfat thickness. The individual sow was considered as an experimental unit, and there were 10 replicates per treatment group. All the sows were artificially inseminated with pooled semen from Landrace boars and were fed a soy-bean basal diet unsupplemented or supplemented with 1.0 g of flavor/kg diet (milk flavor; DadHank Biotechnology Corporation, Chengdu, China) from d 90 of gestation to 25 d post-farrowing. The chemical composition of milk flavor is presented in Table 1. The basal diets for the gestational or lactational sows were formulated according to NRC (2012). The composition of the basal diets is presented in Table 2.
Table 1.
Chemical composition of milk flavor.
| Chemical composition | Content, % |
|---|---|
| Coconut aldehyde | 4.25 |
| Propyl octyl lactone | 2.13 |
| Benzyl alcohol | 1.95 |
| Isoamyl acetate | 1.60 |
| Strawberry aldehyde | 1.07 |
| Peach aldehyde | 1.00 |
| Ethyl butyrate | 0.98 |
| Piperonyl aldehyde | 0.88 |
| Butyric acid | 0.75 |
| Ethyl vanillin | 0.75 |
| Ethyl acetoacetate | 0.40 |
| Benzyl butyrate | 0.33 |
| Eugenol | 0.33 |
| Isoamyl isovalerate | 0.33 |
| Benzyl acetate | 0.28 |
| Ethyl acetate | 0.26 |
| 4-Hydroxy-2-butanone | 0.25 |
| Decalactone | 0.25 |
| Anisic aldehyde | 0.20 |
Table 2.
Ingredients and chemical composition of diets (as-fed basis, %).
| Item | Content |
|
|---|---|---|
| Gestation | Lactation | |
| Ingredients | ||
| Corn | 71.76 | 63.07 |
| Wheat bran | 3.76 | 5.67 |
| Soybean meal | 14.85 | 20.01 |
| Extruded soybean | 3.00 | 5.00 |
| Soybean oil | 2.50 | 2.50 |
| Premix 1 | 1.00 | 1.00 |
| Salt | 0.50 | 0.50 |
| Limestone | 1.13 | 0.84 |
| Calcium hydrophosphate | 1.50 | 1.41 |
| Total | 100.00 | 100.00 |
| Chemical composition 2 | ||
| Metabolizable energy, kcal/kg | 3,357.00 | 3,360.00 |
| Crude protein | 14.69 | 17.21 |
| SID lysine | 0.58 | 0.73 |
| SID methionine + cysteine | 0.47 | 0.53 |
| SID threonine | 0.44 | 0.52 |
| SID tryptophan | 0.13 | 0.17 |
| SID valine | 0.56 | 0.65 |
| Calcium | 0.87 | 0.77 |
| Phosphorus | 0.62 | 0.65 |
| STTD phosphorus | 0.37 | 0.38 |
SID = standardized ileal digestible; STTD = standardized total tract digestible.
Premix provide the following per kilogram of diets: vitamin A 4,000 IU, vitamin D3 800 IU, vitamin E 44 IU, vitamin K 0.5 mg, biotin 0.2 mg, choline 1250 mg, folacin 1.3 mg, niacin 10 mg, pantothenic 12 mg, riboflavin 3.75 mg, thiamin 1 mg, vitamin B6 15 mg, Cu 10 mg, I 0.14 mg, Fe 80 mg, Mn 25 mg, Se 0.15 mg, Zn 100 mg.
Calculated value.
Pregnant sows were housed individually in crates until d 108 of pregnancy and then were housed in farrowing crates to 25 d post-farrowing. The birth weight of piglets and litter size were recorded. Cross-fostering was performed within 24 h post-farrowing and litters of piglets were standardized to 10 to 12 piglets. Creep feed was not offered. Sows were fed 2 times (i.e. 07:30 and 14:00) on gestation with a total of 2.7 kg/d gestational diet, and they were fed 3 times (i.e. 07:30, 11:30 and 16:00) on lactation with a total of 1.5 kg/d lactational diet on d 1 and 2.5 kg/d on d 2. Sows were fed 0.5 kg more feed each day from d 3 to 7, and then sows were fed ad libitum from d 8 to 25. Sows and piglets had free access to water. The average daily feed intake of sows was calculated from d 8 to 25 during lactation. The numbers of litter after cross-fostering and weaning were recorded. Nursing pigs were weighed on d 1 (birth) and d 25 (weaning) of age. On d 90 of gestation and d 25 of lactation, the backfat thickness of each sow was measured at 6.5 cm off the midline in the last rib level (P2) by using an ultrasonography (Lean-meter; Renco Corporation, Minneapolis, MN, USA). Litter weight, litter weight gain and average daily gain (ADG) were calculated. Fresh feces of the sows (n = 5 per treatment) were individually collected from the rectum on d 100 of gestation (G100) and d 14 of lactation (L14), and then were transported to the laboratory and stored at −80 °C until later analysis.
2.2. DNA extraction, PCR amplification, and bacterial 16S ribosomal RNA (rRNA) gene sequencing
The total genomic DNA of fecal bacteria was extracted by using a DNA Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The quality of isolated DNA was determined by agarose gel electrophoresis, and then the genomic DNA was used as a template for PCR amplification. The V3–V4 gene region of 16S rRNA was amplified by using the primers F341 (5′-CCTACGGGRSGCAGCAG-3′) and 806R (5′-GGACTACVVGGGTATCTAATC-3′) (Sun et al., 2017) and the 16S rRNA gene was sequenced on the Illumina HiSeq sequencing platform at the Realbio Genomics Institute (Shanghai, China). Sequences were quality filtered and clustered into operational taxonomic unit (OTU) at 97% identity (Wang et al., 2007).
2.3. Statistical analysis
An individual sow was considered as an experimental unit in all of the statistical analyses. Beyond analysis, the data were tested for normality and homoscedasticity using the Kolmogorov–Smirnov and Levene tests (with the significance level set at 5%). The t-test was conducted to analyze the reproductive performance data by SPSS statistical software (SPSS, Inc., Chicago, IL, USA). For the analysis of 16S rRNA gene sequencing data, data were normalized by copy number. Phylum and genus at <1.0% relative abundance for both diets at different stages were excluded from all analyses. Alpha diversity (Shannon, Simpson, ACE, Chao, and Coverage) was assessed by Mothur (Version 1.35.0) (Schloss et al., 2009). The differences of alpha diversity indices were determined by two-way ANOVA. Principal coordinate analysis (PCoA) was conducted based on Bray–Curtis distance of OTU relative abundance in sow fecal microbiota. The difference between groups was tested by the analysis of similarities (ANOSIM). The differences between groups on the phylum and genus levels were analyzed by the Wilcoxon rank-sum test. Spearman correlation analysis was applied to assess the correlations between differential genera and sows’ reproductive performance. Data are reported as means ± pooled SEM. Differences were considered as statistically significant at P < 0.05, and a tendency was considered to exist at 0.05 ≤ P < 0.10.
3. Results
3.1. Effect of flavor supplementation on performance of the sows and piglets
The performance of the sows and piglets are showing in Table 3. Compared with the controls, flavor supplementation in maternal diets increased (P < 0.05) weaning litter weight, litter weight gain, weaning body weight, and average daily gain of piglets. There was a trend of increase in the daily feed intake of sows (P = 0.09) by maternal dietary flavor addition. The backfat thickness, litter size, and weaning survival rate were not affected by flavor supplementation (P > 0.05).
Table 3.
The effect of flavor supplementation on the performance of lactating sows and suckling piglets.1
| Item | CON | FLA | SEM | P-value |
|---|---|---|---|---|
| Average daily feed intake, kg/d | 7.11 | 7.44 | 0.10 | 0.09 |
| Backfat thickness, mm | ||||
| Initial backfat thickness | 21.10 | 20.60 | 0.82 | 0.77 |
| Weaning backfat thickness | 15.80 | 15.00 | 0.72 | 0.59 |
| Backfat thickness change, mm | 5.30 | 5.60 | 0.49 | 0.77 |
| Litter weight, kg | ||||
| Cross-fostering litter weight | 18.52 | 19.36 | 0.37 | 0.26 |
| Weaning litter weight | 77.04 | 90.09 | 2.48 | <0.01 |
| Litter weight gain, kg | 58.52 | 70.72 | 2.43 | 0.01 |
| Mean body weight, kg | ||||
| Cross-fostering body weight | 1.59 | 1.70 | 0.04 | 0.14 |
| Weaning body weight | 6.77 | 7.91 | 0.21 | <0.01 |
| Average daily gain, g/d | 207.21 | 248.22 | 8.01 | 0.01 |
| Litter size | ||||
| Cross-fostering litter size | 11.70 | 11.40 | 0.14 | 0.28 |
| Weaning litter size | 11.40 | 11.40 | 0.15 | 1.00 |
| Weaning survival rate, % | 97.50 | 100.00 | 0.91 | 0.19 |
CON, control group, basal diet; FLA, flavor supplementary group, basal diet + feed flavor supplement at 1 g/kg; n = 10 for each group; P < 0.05 means a significant difference.
3.2. Effect of flavor supplementation on sequence data, alpha-diversity, and beta-diversity
A total of 721,063 sequences were obtained, with an average of 36,053 sequences per sample, and the average length of the sequence was 416 bp. Overall, 1,146 OTU were detected according to a nucleotide sequence identity of 97% between sequences. Bacterial diversity (Shannon and Simpson), richness estimators (Chao and ACE), and the Coverage (good's coverage estimator) are shown in Table 4. Shannon index was significantly decreased (P < 0.05) by the reproductive stage, and there was a tendency to decrease (0.05 ≤ P < 0.10) by the diet × stage interaction. The richness of bacteria as evidenced by Chao and ACE was decreased (P < 0.05) by the reproductive stage, but not affected by the diet or the diet × stage interaction. A similar result was observed for the Coverage, neither the diet nor the reproductive stage affected the Coverage of the fecal bacteria of the sows.
Table 4.
Sequencing data and the alpha diversity in each group of sows.1
| Item | G100 |
L14 |
SEM |
P-values |
||||
|---|---|---|---|---|---|---|---|---|
| CON | FLA | CON | FLA | Diet | Stage | Diet × Stage | ||
| Seq_num | 37,236.40 | 35,306.80 | 36,122.60 | 35,546.80 | 425.43 | 0.16 | 0.61 | 0.44 |
| OTU_num | 701.80 | 708.80 | 621.60 | 597.00 | 12.58 | 0.73 | <0.01 | 0.54 |
| Shannon | 4.84 | 4.97 | 4.80 | 4.58 | 0.04 | 0.59 | 0.02 | 0.05 |
| Simpson | 0.020 | 0.017 | 0.020 | 0.024 | 0.001 | 0.87 | 0.26 | 0.24 |
| ACE | 809.51 | 820.79 | 713.66 | 700.84 | 16.61 | 0.98 | 0.01 | 0.72 |
| Chao | 820.16 | 823.74 | 715.00 | 704.77 | 16.56 | 0.92 | <0.01 | 0.84 |
| Coverage | 0.996 | 0.995 | 0.996 | 0.996 | <0.001 | 0.31 | 0.05 | 0.76 |
Seq_num = sequence number; OTU_num = operational taxonomic unit number; ACE = abundance-based coverage estimator.
Sows were regarded as the experimental units, n = 5 for each group. G100: d 100 of gestation; L14: d 14 of lactation; CON, control group, basal diet; FLA, flavor supplementary group, basal diet + feed flavor supplement at 1 g/kg. When significant main effects or interactive effects were observed, the means were compared using the least significant difference method with a P < 0.05 indicating significance.
To evaluate overall differences in beta-diversity, PCoA was used to identify discrepancies between groups. Principal coordinate analysis was conducted based on Bray–Curtis distance of OTU relative abundance in sow fecal microbiota. As shown in Fig. 1A, the fecal microbiota of CON and FLA were similar on G100 (ANOSIM: R = 0.11, P = 0.22), but it tended to be separate from each group (ANOSIM: R = 0.20, P = 0.09) on L14 (Fig. 1B). From Fig. 1C and D, we can know the fecal microbiota of G100 and L14 in CON group were not separated (ANOSIM: R = 0.11, P = 0.74), but they separated in FLA group (ANOSIM: R = 0.36, P = 0.03).
Fig. 1.
Beta-diversity analysis among experimental groups. Principal coordinates analysis (PCoA) between the control group (CON) and flavor supplementary group (FLA) on d 100 of gestation (G100) (A) and on d 14 of lactation (L14) (B). PCoA between G100 and L14 in the CON (C) and in the FLA (D). Sows were regarded as the experimental units, n = 5 for each group.
3.3. Effects of flavor administration on community composition of microbiota at phyla or genera level
The relative abundance of bacteria at the phylum level of all samples are presented in Fig. 2A. As shown, the top 5 dominant phyla (>1% at least in 1 of the 4 groups) were Firmicutes (45.19%), Bacteroidetes (41.19%), Spirochaetes (7.83%), Proteobacteria (2.60%), and unclassified_k_norank_d_Bacteria (1.44%). At the genus level, 34 dominant genera were identified (> 1% at least in 1 of the 4 groups). The top 5 dominant genera are norank_f_Muribaculaceae, Treponema_2, Prevotellaceae_NK3B31_group, Lactobacillus, and Rikenellaceae_RC9_gut_group, with an average percentage of 8.03%, 7.60%, 5.18%, 4.86%, and 4.42% respectively (Fig. 2B).
Fig. 2.
Fecal microbiota composition in sows at different levels. Relative abundance of fecal microbiota in each group at the phylum level (A) and genus level (B). Sows were regarded as the experimental units, n = 5 for each group. CON_G100: d 100 of gestation of control sows; FLA_G100: d 100 of gestation of flavor group sows; CON_L14: d 14 of lactation of control group sows; FLA_L14: d 14 of lactation of flavor group sows.
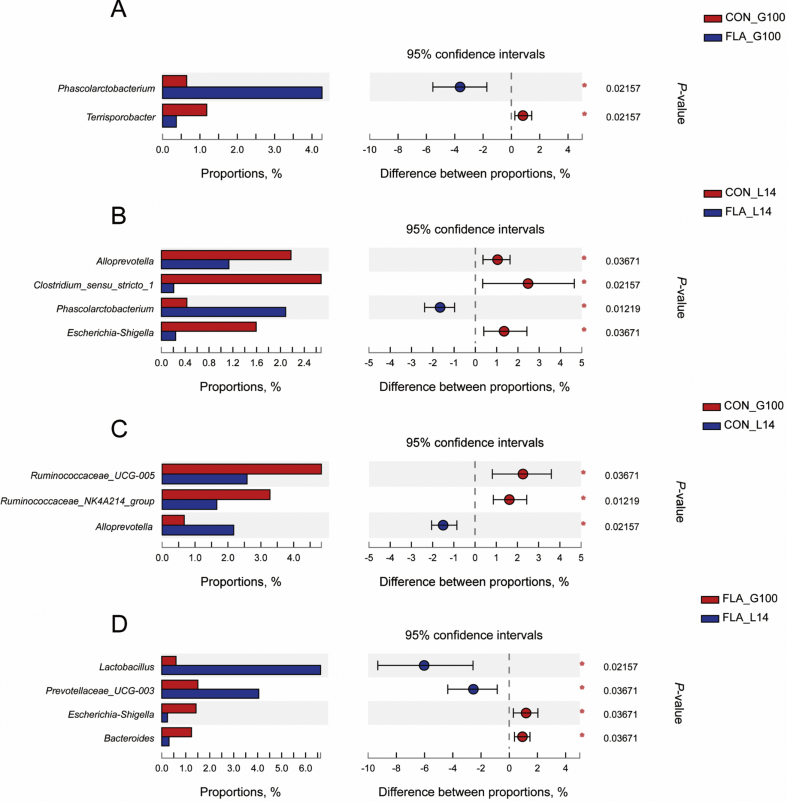
The difference of fecal microbiota between CON and FLA on phylum and genus level were analyzed. There was no significant difference (P > 0.05) between CON and FLA on the phylum level on G100 and L14 (Fig. 3A and B). As shown in Fig. 4A and B, compared with CON, genus Phascolarctobacterium was increased (P < 0.05) by flavor supplementation on G100 and L14, whereas genera Terrisporobacter, Alloprevotella, Clostridium_sensu_stricto_1, and Escherichia-shigella were significantly decreased in response to flavor addition (P < 0.05). Changes in the fecal microbiota were also analyzed from G100 to L14. In CON, genera Ruminococcaceae_UCG_005 and Ruminococcaceae_NK4A214_group were significantly reduced (P < 0.05) from G100 to L14, but the genus Alloprevotella was increased (P < 0.05) (Fig. 4C). Phylum Proteobacteria, genera Escherichia-shigella, and Bacteroides were found to be less abundant (P < 0.05) from G100 to L14, whereas genera Prevotellaceae_UCG_003 showed more abundance (P < 0.05) (Fig. 3, Fig. 4D).
Fig. 3.
Differences in fecal microbiota at phylum level among experimental groups. Fecal microbiota differed in sows between the control group (CON) and flavor supplementary group (FLA) on d 100 of gestation (G100) (A) and on d 14 of lactation (L14) (B). Fecal microbiota differed in sows between G100 and L14 in the CON (C) and in the FLA (D). Sows were regarded as the experimental units, n = 5 for each group.
Fig. 4.
Differences in fecal microbiota at genus level among experimental groups. Fecal microbiota differed in sows between the control group (CON) and flavor supplementary group (FLA) on d 100 of gestation (G100) (A) and on d 14 of lactation (L14) (B). Fecal microbiota differed in sows between G100 and L14 in the CON (C) and in the FLA (D). Sows were regarded as the experimental units, n = 5 for each group.
3.4. Correlation between gut microbiota at genera level and the sows’ reproductive performance
As shown in Fig. 5, the Spearman correlation matrix illustrated that the relative abundance of Phascolarctobacterum was positively correlated with the average daily feed intake of sows (P < 0.05), the litter weight gain and average daily gain of piglets (P < 0.05). In contrast, Clostridium_sensu_stricto_1 and unclassified_f__Lachnospiraceae were negatively correlated with the litter weight gain and average daily gain of piglets (P < 0.05), and Alloprevotella was negatively correlated with the average daily gain of nursing piglets (P < 0.05).
Fig. 5.
Spearman correlation analysis between differential genera and sows' performance. Significant correlations are noted by: ∗ 0.01 < P ≤ 0.05, ∗∗ 0.001 < P ≤ 0.01, ∗∗∗P ≤ 0.001.
4. Discussion
In the present study, we investigated the effects of flavor supplementation on reproductive performance and fecal microbiota of sows during lactation. To the best of our knowledge, this might be the first study to evaluate the difference in the fecal microbiota between the unsupplementation and supplementation of dietary flavor and explore the correlation of fecal microbiota with the reproductive performance of sows.
Insufficient feed intake during lactation resulted in a decrease in the provision of nutrients for milk production, which could limit the growth and development of piglets (Zijlstra et al., 1996; Kim et al., 2004). It has been reported that flavor compounds as palatability enhancers and feed attractants increase the feed intake of the sow, which is correlated with increased milk production as previously described (Strathe et al., 2017), and support piglet growth (Laws et al., 2018; Miao et al., 2019). In the current study, flavor supplementation at a level of 0.1% in the maternal diet increased the average daily feed intake of the sow, litter weight gain, and average daily gain of the piglet. This result was consistent with previous reports showing that flavor addition in maternal feed increased average daily feed intake, digestibility of dry matter, gross energy, crude protein of sow, as well as average daily gain of piglets (Wang et al., 2014; He et al., 2017).
The mammalian gastrointestinal tract is inhabited by trillions of microbes which are approximately 10 times the number of body cells, and play a significant role in physiology, metabolism, immunity, development, and the behavior of the host through active interactions between bacteria and host cells. Growing evidence demonstrates that various factors, including developmental stage, gut environment, nutritional and non-nutritional dietary components, and antibiotics, are implicated in and affect the composition of gut microbiota (Ji et al., 2017). Of interest, maternal gut microbiota can subsequently be passed onto the developing fetus or neonates through the placenta, maternal milk, or other routes (DiGiulio et al., 2015; Macpherson et al., 2017), and ultimately, directly or indirectly, affects fetal growth, survival, and offspring development (Turnbaugh et al., 2009; Houghteling and Walker, 2015). Considering the animal welfare of the sows and piglets, as well as the correlation between microbial communities in fecal samples and that in the gut microbiota (Koren et al., 2012b; Falony et al., 2016), fecal microbiota has been used as an indicator of the gut flora of sows in animal nutrition related studies (Vandeputte et al., 2017; Zhao et al., 2018; Li et al., 2019).
In the present study, we examined the microbiota of the fecal samples by using 16S rRNA sequencing analysis and found that the abundance of Phascolarctobacterium was increased by flavor addition on G100 and L14. Phascolarctobacterium is one of the short-chain fatty acid (SCFA) producers (Zhang et al., 2015). The increase of Phascolarctobacterium by flavor supplementation might produce more SCFA by fermentation (He et al., 2017), which can be absorbed and used as a source of energy by enterocytes and peripheral tissue, affecting lipogenesis and gluconeogenesis (Zhang et al., 2018). We found that the abundance of Phascolarctobacterium was positive associated with the average daily feed intake of sows, litter weight gain, and average daily gain of the piglet. Flavor addition increased the abundance of Phascolarctobacterium, which improves the energy supplementation of the sows therefore enhances the maternal condition, milk production, and the growth performance of piglets.
During late gestation and lactation, sows experience substantial immunological and metabolic changes (Cheng et al., 2018). Increased metabolic burdens cause elevated systemic oxidative stress during the specific periods (Tan et al., 2016). Terrisporobacter is an anaerobic pathogen (Cheng et al., 2016). The increased abundance of it contributes to an increased oxidative stress as observed in animals (Cai et al., 2019). In this study, flavor supplementation in the maternal diet decreased the abundance of Terrisporobacter on d 100 of gestation, indicating that dietary flavor may alleviate maternal oxidative stress during gestation and improve maternal health. Moreover, the abundance of genera Clostridium_sensu_stricto_1 and Escherichia-shigella, 2 potentially pathogenic bacteria associated with intestinal disorders (Wells and Wilkins, 1996; Fukuda et al., 2011) were decreased following flavor administration. It has been reported that eugenol and butyric acid, 2 components of the flavor, could inhibit biofilm formation and attenuate the virulence of Escherichia (Kim et al., 2016) or Clostridium (Hsiao and Siebert, 1999; Salsali et al., 2008), respectively. The unclassified_f__Lachnospiraceae, which belongs to the Lachnospiraceae family, is also involved in intra- and extraintestinal diseases (Vacca et al., 2020). In our study, unclassified_f__Lachnospiraceae and Clostridium_sensu_stricto_1 were negatively correlated with litter weight gain and average daily gain. Dietary flavor addition reduced maternal pathogenic bacteria and contributed to improve the performance of sows and piglets. The genus Alloprevotella is considered to be beneficial bacteria, which can produce SCFA (Kong et al., 2019). Unexpectedly, we observed a decreased abundance of Alloprevotella following flavor administration on d 14 of lactation. The reason for this result is not clear at present, and needs to be further investigated.
5. Conclusion
We found that flavor supplementation to maternal diet during gestation and lactation increased feed intake of sows during lactation, average daily gain, weaning body weight, and litter weight gain of piglets. This beneficial effect of flavor was associated with enhanced beneficial microbiota and decreased potentially pathogenic bacteria in the gastrointestinal tract of the sow.
Author contributions
Z. Wu and D. Li designed the research. R. Wang, N. Liu, Y. Yang, Y. Lei, and J. Lyu performed the research. N. Liu, Z. Dai, J. Li, Z. Wu, and D. Li analyzed the data. R. Wang, I. H. Kim, Z. Wu, and D. Li wrote the paper. Z. Wu and D. Li had primary responsibility for the final content. All authors read and approved the final manuscript.
Conflict of interest
Authors Y. Lei and J. Lyu are employed by DadHank Biotechnology Corporation. The remaining authors declare that they have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31625025, 31572412, 31272451), the Zhengzhou 1125 Talent Program, Jingxinnong Animal Science Development Foundation, and the “111 Project” (B16044).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Cai C., Zhang Z., Morales M., Wang Y., Khafipour E., Friel J. Feeding practice influences gut microbiome composition in very low birth weight preterm infants and the association with oxidative stress: a prospective cohort study. Free Radic Biol Med. 2019;142:146–154. doi: 10.1016/j.freeradbiomed.2019.02.032. [DOI] [PubMed] [Google Scholar]
- Cheng C., Wei H., Yu H., Xu C., Jiang S., Peng J. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.01989. 1989-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.P., Domingo M.-C., Lévesque S., Yansouni C.P. A case report of a deep surgical site infection with Terrisporobacter glycolicus/T. Mayombei and review of the literature. BMC Infect Dis. 2016;16(1) doi: 10.1186/s12879-016-1865-8. 529-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiulio D.B., Callahan B.J., McMurdie P.J., Costello E.K., Lyell D.J., Robaczewska A., Sun C.L., Goltsman D.S., Wong R.J., Shaw G., Stevenson D.K., Holmes S.P., Relman D.A. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A. 2015;112(35):11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkosar B., Storelli G., Defaye A., Leulier F. Host-intestinal microbiota mutualism: “learning on the fly”. Cell Host Microbe. 2013;13(1):8–14. doi: 10.1016/j.chom.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Everaert N., Van Cruchten S., Weström B., Bailey M., Van Ginneken C., Thymann T., Pieper R. A review on early gut maturation and colonization in pigs, including biological and dietary factors affecting gut homeostasis. Anim Feed Sci Technol. 2017;233:89–103. [Google Scholar]
- Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., Kurilshikov A., Bonder M.J., Valles-Colomer M., Vandeputte D., Tito R.Y., Chaffron S., Rymenans L., Verspecht C., De Sutter L., Lima-Mendez G., D'Hoe K., Jonckheere K., Homola D., Garcia R., Tigchelaar E.F., Eeckhaudt L., Fu J., Henckaerts L., Zhernakova A., Wijmenga C., Raes J. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., Taylor T.D., Itoh K., Kikuchi J., Morita H., Hattori M., Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- He L., Zang J., Liu P., Fan P., Song P., Chen J., Ma Y., Ding W., Ma X. Supplementation of milky flavors improves the reproductive performance and gut function using sow model. Protein Pept Lett. 2017;24(5):449–455. doi: 10.2174/0929866524666170223144728. [DOI] [PubMed] [Google Scholar]
- Hollister E.B., Gao C., Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 2014;146(6):1449–1458. doi: 10.1053/j.gastro.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghteling P.D., Walker W.A. Why is initial bacterial colonization of the intestine important to infants' and children's health? J Pediatr Gastroenterol Nutr. 2015;60(3):294–307. doi: 10.1097/MPG.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C.P., Siebert K.J. Modeling the inhibitory effects of organic acids on bacteria. Int J Food Microbiol. 1999;47(3):189–201. doi: 10.1016/s0168-1605(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Ji Y., Kong X., Li H., Zhu Q., Guo Q., Yin Y. Effects of dietary nutrient levels on microbial community composition and diversity in the ileal contents of pregnant Huanjiang mini-pigs. PloS One. 2017;12(2) doi: 10.1371/journal.pone.0172086. e0172086-e0172086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., McPherson R.L., Wu G.Y. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr. 2004;134(3):625–630. doi: 10.1093/jn/134.3.625. [DOI] [PubMed] [Google Scholar]
- Kim Y.G., Lee J.H., Gwon G., Kim S.I., Park J.G., Lee J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci Rep. 2016;6:36377. doi: 10.1038/srep36377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C., Gao R., Yan X., Huang L., Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2019;60:175–184. doi: 10.1016/j.nut.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Koren O., Goodrich J.K., Cullender T.C., Spor A., Laitinen K., Backhed H.K., Gonzalez A., Werner J.J., Angenent L.T., Knight R., Backhed F., Isolauri E., Salminen S., Ley R.E. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O., Goodrich Julia K., Cullender Tyler C., Spor A., Laitinen K., Kling Bäckhed H., Gonzalez A., Werner Jeffrey J., Angenent Largus T., Knight R., Bäckhed F., Isolauri E., Salminen S., Ley Ruth E. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws J., Juniper D.T., Lean I.J., Amusquivar E., Herrera E., Dodds P.F., Clarke L. Supplementing sow diets with palm oil during late gestation and lactation: effects on milk production, sow hormonal profiles and growth and development of her offspring. Animal. 2018;12(12):2578–2586. doi: 10.1017/S1751731118000885. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu H., Zhang L., Yang Y., Lin Y., Zhuo Y., Fang Z., Che L., Feng B., Xu S., Li J., Wu Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int J Mol Sci. 2019;21(1):31. doi: 10.3390/ijms21010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren H., Fikse W.F., Grandinson K., Lundeheim N., Canario L., Vangen O., Olsen D., Rydhmer L. Genetic parameters for feed intake, litter weight, body condition and rebreeding success in primiparous Norwegian Landrace sows. Animal. 2014;8(2):175–183. doi: 10.1017/S1751731113002000. [DOI] [PubMed] [Google Scholar]
- Macpherson A.J., de Aguero M.G., Ganal-Vonarburg S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol. 2017;17(8):508–517. doi: 10.1038/nri.2017.58. [DOI] [PubMed] [Google Scholar]
- Miao J., Adewole D., Liu S., Xi P., Yang C., Yin Y. Tryptophan supplementation increases reproduction performance, milk yield, and milk composition in lactating sows and growth performance of their piglets. J Agric Food Chem. 2019;67(18):5096–5104. doi: 10.1021/acs.jafc.9b00446. [DOI] [PubMed] [Google Scholar]
- Salsali H., Parker W.J., Sattar S.A. The effect of volatile fatty acids on the inactivation of Clostridium perfringens in anaerobic digestion. World J Microbiol Biotechnol. 2008;24(5):659–665. [Google Scholar]
- Santacruz A., Collado M.C., Garcia-Valdes L., Segura M.T., Martin-Lagos J.A., Anjos T., Marti-Romero M., Lopez R.M., Florido J., Campoy C., Sanz Y. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104(1):83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabolt B.S., van Heugten E., Kim S.W., Ange-van Heugten K.D., Roura E. Feed preferences and performance of nursery pigs fed diets containing various inclusion amounts and qualities of distillers coproducts and flavor. J Anim Sci. 2010;88(11):3725–3738. doi: 10.2527/jas.2009-2640. [DOI] [PubMed] [Google Scholar]
- Strathe A.V., Bruun T.S., Hansen C.F. Sows with high milk production had both a high feed intake and high body mobilization. Animal. 2017;11(11):1913–1921. doi: 10.1017/S1751731117000155. [DOI] [PubMed] [Google Scholar]
- Sulabo R.C., Tokach M.D., Derouchey J.M., Dritz S.S., Goodband R.D., Nelssen J.L. Influence of feed flavors and nursery diet complexity on preweaning and nursery pig performance. J Anim Sci. 2010;88(12):3918–3926. doi: 10.2527/jas.2009-2724. [DOI] [PubMed] [Google Scholar]
- Sun W., Qian X., Gu J., Wang X.J., Zhang L., Guo A.Y. Mechanisms and effects of arsanilic acid on antibiotic resistance genes and microbial communities during pig manure digestion. Bioresour Technol. 2017;234:217–223. doi: 10.1016/j.biortech.2017.03.025. [DOI] [PubMed] [Google Scholar]
- Tan C., Wei H., Ao J., Long G., Peng J. Inclusion of konjac flour in the gestation diet changes the gut microbiota, alleviates oxidative stress, and improves insulin sensitivity in sows. Appl Environ Microbiol. 2016;82(19):5899–5909. doi: 10.1128/AEM.01374-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., Egholm M., Henrissat B., Heath A.C., Knight R., Gordon J.I. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca M., Celano G., Calabrese F.M., Portincasa P., Gobbetti M., De Angelis M. The controversial role of human gut lachnospiraceae. Microorganisms. 2020;8(4):573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriano V.D.V., Balolong M.P., Kang D.-K. Probiotic roles of Lactobacillus sp. in swine: insights from gut microbiota. J Appl Microbiol. 2017;122(3):554–567. doi: 10.1111/jam.13364. [DOI] [PubMed] [Google Scholar]
- Vandeputte D., Falony G., Vieira-Silva S., Wang J., Sailer M., Theis S., Verbeke K., Raes J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66(11):1968–1974. doi: 10.1136/gutjnl-2016-313271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ji Y., Yin C., Deng M., Tang T., Deng B., Ren W., Deng J., Yin Y., Tan C. Differential analysis of gut microbiota correlated with oxidative stress in sows with high or low litter performance during lactation. Front Microbiol. 2018;9:1665. doi: 10.3389/fmicb.2018.01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang M., Xu S., Lin Y., Che L., Fang Z., Wu D. Comparative effects of sodium butyrate and flavors on feed intake of lactating sows and growth performance of piglets. Anim Sci J. 2014;85(6):683–689. doi: 10.1111/asj.12193. [DOI] [PubMed] [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C.L., Wilkins T.D. 4th ed. University of Texas Medical Branch at Galveston; Galveston (TX): 1996. Clostridia: sporeforming anaerobic bacilli. Medical microbiology. [PubMed] [Google Scholar]
- Xiong Y., Pang J., Lv L., Wu Y., Li N., Huang S., Feng Z., Ren Y., Wang J. Effects of maternal supplementation with rare earth elements during late gestation and lactation on performances, health, and fecal microbiota of the sows and their offspring. Animals (Basel) 2019;9(10):738. doi: 10.3390/ani9100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Yu H., Xiao X., Hu L., Xin F., Yu X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ. 2018;6:e4446. doi: 10.7717/peerj.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhao Y., Xu J., Xue Z., Zhang M., Pang X., Zhang X., Zhao L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lu T., Han L., Zhao L., Niu Y., Chen H. L-Glutamine supplementation alleviates constipation during late gestation of mini sows by modifying the microbiota composition in feces. BioMed Res Int. 2017;2017:9. doi: 10.1155/2017/4862861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zhang F., Ding X., Wu G., Lam Y.Y., Wang X., Fu H., Xue X., Lu C., Ma J., Yu L., Xu C., Ren Z., Xu Y., Xu S., Shen H., Zhu X., Shi Y., Shen Q., Dong W., Liu R., Ling Y., Zeng Y., Wang X., Zhang Q., Wang J., Wang L., Wu Y., Zeng B., Wei H., Zhang M., Peng Y., Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- Zijlstra R.T., Whang K.Y., Easter R.A., Odle J. Effect of feeding a milk replacer to early-weaned pigs on growth, body composition, and small intestinal morphology, compared with suckled littermates. J Anim Sci. 1996;74(12):2948–2959. doi: 10.2527/1996.74122948x. [DOI] [PubMed] [Google Scholar]