Abstract
Microlevel properties such as mineralogical and chemical compositions greatly control the macro behaviour of expansive soils. In this paper, the combined effect of mineral (i.e. montmorillonite, MMC) and chemical contents (i.e. Ca and Na in their total (T), leachable (L) and exchangeable form (CEC)) on swelling behaviour is investigated in a comprehensive way. Several 3-dimensional (3D) graphs correlating MMC and Ca/Na ratio, together, with swelling property (swelling potential, Sa, and swelling pressure, Sp) are developed. 3D plots, in general, portrayed a non-linear relationship of Sa and Sp with MMC and Ca/Na ratio, together. It is hypothesized that swelling initially is triggered by chemical parameters due to their quick and rapid ionization capability, but the overall swelling phenomenon is largely controlled by MMC. It is importantly found that expansive soils are dominant with divalent Ca++ ions up to MMC of 67% and beyond this percentage, monovalent Na+ ions are prevalent. From the interpretation of results, the maximum Sa of 18% and Sp of 93 kPa is measured at MMC of 43%, (Ca/Na)T of 10–14 and (Ca/Na)L of 2–7. It is concluded from study that total CEC + MMC for determining Sa and (Ca/Na)T + MMC for determining Sp are superior parameters to be considered. The findings of the study also excellently endorsed the results of Foster32, who stated that ionization of Na or Ca depends on the constituent mineral contents. The findings presented herein are unique, interesting and bear very practical significance, as no earlier research work reported such findings by accounting for chemical and mineralogical parameters impact, in tandem, on swelling properties.
Subject terms: Civil engineering, Geochemistry
Introduction
Accurate prediction of expansive soils behaviour when hydrated with water is yet a major challenge faced by geotechnical engineers for long time. Expansive clays undergo volumetric deformations (viz., swelling and shrinkage) which in turn induce differential settlements leading to failure of a structure built in/on them or when they are used as geomaterials for construction purposes1–7. Numerous studies focused on understanding and evaluating the swelling behaviour by establishing interrelations with properties of physical, chemical and mineralogical8–11. However, most of the relationships so far developed are only 2-dimensional as they considered alone of physical or chemical or mineralogical features.
Demonstrably, swelling is the combined effect of chemical and mineralogical properties of a clay12. This statement, in general, is valid true for all natural soils, but prominently key for expansive soils because of the presence of montmorillonite mineral, which documented to be a principal cause for aberrant behaviour such as volume change characteristic. Minerals such as montmorillonite, kaolinite and illite are major compositions of clay soils. Lambe and Whitman13 documented the influence of different minerals on consistency limits in the order of: montmorillonite > attapulgite > illite > kaolinite. Generally, bentonite soils are rich in montmorillonite mineral, which is normally absent in natural soils12,14. The montmorillonite mineral upon imbibing of water, usually in significant quantity because of its remarkable surface area, tends to separate the clay sheets resulting in the formation of thicker diffuse double layer. Whereas in case of kaolinite, the absorption of water is limited to surface only, and thereby, effect is confined to marginal to nil.
Quantification of chemical and mineralogical parameters and relating their combined effect bears a practical significance for accurate prediction and precise determination of swelling behaviour. The quantification of chemical compositions, which would be achieved with the help of X-ray florescence (XRF) and inductively coupled plasma-optical emission spectrometry (ICP-OES) techniques, is relatively easy. However, the mineralogical quantification is a complex process and requires highly sophisticated equipment such as X-ray Diffraction (XRD), which is a commonly employed technique for establishing the diffraction patterns of soils. By giving the obtained diffraction pattern as input data, mineral content can be quantified using software’s like Topas. Methodologies such as External Standard Method15, Reference Intensity Ratio (RIR)16, Rietveld Method17, No-standard Method18, Mineral Intensity Factor (MIF)19, and Full Pattern Summation Methods20 are widely used for such purpose. Among the above methods, Rietveld method is reported to be the most reliable method for quantifying the minerals content21.
Several studies reported that the montmorillonite content is the prime factor behind the higher consistency and swelling behaviour of expansive soils22–27. In connection to this, Sun et al.28 predicted the swelling pressure of bentonite and sand mixes by developing empirical relationship between void ratio and swelling pressure. In similar lines, Chittoori et al.29 studied the effect of clay mineral montmorillonite on the performance of chemically stabilized soils, and Mehta and Sachan30 investigated the influence of mineralogy on mechanical behaviour of expansive soils. More deeply, Tahasildar et al.11 studied the influence of montmorillonite on swelling behaviour with limited set of data. Recently, Reddy et al.31 quantified the montmorillonite content and established relationships with swelling behaviour determined by free swell index test.
Besides the noteworthy influence of mineralogy, the effect of chemical parameters on swelling behaviour cannot be simply ignored. Foster32 reported that the soils dominated with Na tends to swell more than those Ca does. As per Chen12, soils dominated with sodium (Na) and calcium (Ca) can greatly affect the swelling behaviour of expansive soils. This occurs as these chemicals exhibit affinity to adsorb substantial amount of water that in turn inflates the thickness of diffuse double layer33,34. Many studies also reported that Na and Ca contents could stimulus the swelling behaviour35–37. Shainberg and Letey38 stated that, higher is the concentration of monovalent cations (i.e. Na), greater is the swelling in soils. Most of the literature, however, confined to highlighting a fact that there is an influence of chemical parameters on swelling, but very a few efforts are oriented to quantify and establish generalized relationships.
From the foregoing discussion, it is evident that both mineralogical and chemical parameters have a combined impact on the swelling behaviour. But studies pivoted on analyzing the combined effect of these parameters, which are crucial factors controlling the macro behaviour such as volume change of clayey soils, are teensy. The main objective of this paper is to understand the combined impact of mineralogical and chemical parameters on the swelling behaviour. For experimentation purpose, several expansive soil samples were collected from different locations across India. Sodium (Na), calcium (Ca) and montmorillonite content (MMC) of these soil samples were quantified. For vivid understanding, 3-D graphs relating chemical and mineralogical parameters with swelling parameters were developed. All total, leachable and exchangeable forms of Na and Ca were considered for developing the 3D graphs. Evidently, such combined analysis is indispensable for accurately determining the swelling characteristics as well as to validate the empirical equations proposed by various researchers. The results presented in the study can also serve as guiding factors for practical applications to assess swelling potential based on input of Ca/Na ratio and MMC values, to decide upon whether or not to go for construction on expansive soils, and importantly, as a tool to practical engineers for the selection of proper remedial measure which is more compatible to the media with which it is implemented. Overall, the study portrays that it is important to merge the effect of chemical and mineralogical parameters for reliable understanding on the swelling behaviour of expansive soils.
Materials and test methodology
For the study purpose, total 46 soil samples of expansive in nature from nine different locations across India were collected following the guidelines of ASTM D145239, and were used. Figure 1 depicts the geographic status showing the locations of soil samples from where they were collected. Whereas, Table 1 presents the latitude and longitude of the exact location of samples collection. All the soil samples were primarily observed to be blackish grey color and they were generally termed as “black cotton soils” in Indian context40. Soil environment of all the regions was found predominantly semi-arid type, which experiences climates of hot during the summer and precipitation during the rainy season. As regards temperature, the minimum and maximum was recorded in the range of 10.2–19.2 °C and 38–48.5 °C41–43, and that of humidity in the range of 21–84%41–43. These regions were reported to be receiving an annual average rainfall from 776.7 to 1637 mm. The geological formations of these regions include granite, gneiss, schist, basalt, and sandstone. A typical chemical weathering of the rocks dominated with fines content seems to be a fundamental cause for soil formations in these regions.
Figure 1.
Geographical map of India showing regions from where soil samples were collected for the study purpose.
Table 1.
Latitude and longitude of soil samples collection region and their designation.
| Sl. No | Sample Region | Sample Designation | Latitude | Longitude |
|---|---|---|---|---|
| 1 | Bhopal | B1 | 23° 13′ 01.35″ | 77° 24′ 13.52″ |
| B2 | 23° 12′ 20.90″ | 77° 26′ 44.89″ | ||
| B3 | 23° 14′ 46.23″ | 77° 30′ 39.32″ | ||
| B4 | 23° 14′ 51.10″ | 77° 30′ 36.68″ | ||
| B5 | 23° 14′ 55.33″ | 77° 15′ 45.95″ | ||
| B6 | 23° 18′ 23.81″ | 77° 19′ 56.12″ | ||
| B7 | 23° 19′ 46.14″ | 77° 24′ 09.19″ | ||
| B8 | 23° 06′ 33.45″ | 77° 30′ 31.85″ | ||
| 2 | Guntur | G1 | 16° 16′ 25.29″ | 80° 28′ 15.07″ |
| G2 | 16° 16′ 25.29″ | 80° 28′ 15.07″ | ||
| G3 | 16° 18′ 17.10″ | 80° 28′ 58.38″ | ||
| G4 | 16° 18′ 37.48″ | 80° 28′ 27.75″ | ||
| G5 | 16° 19′ 09.82″ | 80° 27′ 01.86″ | ||
| G6 | 16° 19′ 42.28″ | 80° 24′ 23.03″ | ||
| G7 | 16° 19′ 12.30″ | 80° 23′ 41.06″ | ||
| 3 | Kakinada | K1 | 16° 58′ 53.74″ | 82° 12′ 59.65″ |
| K2 | 16° 57′ 38.28″ | 82° 15′ 42.06″ | ||
| K3 | 17° 00′ 36.79″ | 82° 13′ 31.09″ | ||
| 4 | Nagpur | N1 | 21° 03′ 39.82″ | 79° 04′ 24.52″ |
| N2 | 21° 03′ 54.54″ | 79° 07′ 33.57″ | ||
| N3 | 21° 03′ 55.79″ | 78° 57′ 24.19″ | ||
| N4 | 21° 08′ 44.04″ | 78° 59′ 12.58″ | ||
| N5 | 21° 11′ 02.49″ | 79° 02′ 01.82″ | ||
| N6 | 21° 15′ 31.20″ | 79° 05′ 11.50″ | ||
| N7 | 21° 11′ 38.33″ | 79° 07′ 51.29″ | ||
| N8 | 21° 08′ 33.62″ | 79° 10′ 59.73″ | ||
| 5 | Raipur | R1 | 21° 12′ 01.53″ | 81° 38′ 43.15″ |
| R2 | 21° 12′ 20.00″ | 81° 36′ 58.98″ | ||
| R3 | 21° 18′ 19.52″ | 81° 41′ 38.72″ | ||
| 6 | Vijayawada | V1 | 16° 28′ 45.92″ | 80° 41′ 43.00″ |
| V2 | 16° 30′ 00.49″ | 80° 42′ 14.07″ | ||
| V3 | 16° 31′ 40.29″ | 80° 40′ 51.18″ | ||
| V4 | 16° 33′ 11.00″ | 80° 39′ 43.29″ | ||
| V5 | 16° 32′ 59.68″ | 80° 38′ 00.92″ | ||
| V6 | 16° 32′ 53.56″ | 80° 36′ 23.34″ | ||
| V7 | 16° 28′ 48.00″ | 80° 36′ 24.50″ | ||
| 7 | Warangal | W1 | 17° 58′ 07.12″ | 79° 30′ 22.63″ |
| W2 | 17° 57′ 05.08″ | 79° 35′ 09.70″ | ||
| W3 | 17° 59′ 57.95″ | 79° 36′ 26.24″ | ||
| 8 | Mysore | M1 | 12° 16′ 32.20″ | 76° 39′ 29.08″ |
| 9 | Kendrapara | KP1 | 20° 41′ 27.95″ | 86° 09′ 38.17″ |
| KP2 | 20° 40′ 17.68″ | 86° 10′ 08.10″ | ||
| KP3 | 20° 35′ 26.50″ | 86° 16′ 52.63″ | ||
| KP4 | 20° 34′ 33.39″ | 86° 17′ 36.67″ | ||
| KP5 | 20° 30′ 36.43″ | 86° 22′ 45.35″ | ||
| KP6 | 20° 25′ 24.66″ | 86° 29′ 47.82″ |
The samples collected were dried and pulverized as per the test standard ASTM D221644. The processed samples were later subjected to extensive experimental investigations, including particle size distribution45, consistency limits46, swelling properties, mineralogical and chemical compositions. As per the Unified Soil Classification System (USCS)47, the soils were classified as Inorganic clays of high compressibility (CH) based on gradation and index properties. The physical properties of soil samples used in the study can be seen in Table 2.
Table 2.
Physical properties of soil samples used in the study.
| State/Region | Sample ID | % Fraction | Consistency limits (%) | USCS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gravel (> 4.75 mm) | Sand (4.75 mm–75 µm) | Silt (75 µm–2 µm) | Clay (< 2 µm) | wL | wP | wPI | |||
| Bhopal | B1 | – | 6 | 43 | 51 | 68 | 27.52 | 40.48 | CH |
| B2 | – | 6 | 50 | 44 | 57.5 | 23.54 | 33.96 | CH | |
| B3 | – | 3 | 51 | 46 | 62 | 27 | 35 | CH | |
| B4 | – | 7 | 53 | 40 | 63.9 | 21.42 | 42.48 | CH | |
| B5 | – | 17 | 44 | 39 | 43 | 19.25 | 23.75 | CL | |
| B6 | 1 | 23 | 43 | 33 | 48 | 18.52 | 29.48 | CL | |
| B7 | 1 | 21 | 42 | 36 | 46 | 16.9 | 29.1 | CL | |
| B8 | 1 | 17 | 40 | 42 | 50.8 | 23.5 | 27.3 | CL | |
| Guntur | G1 | – | 11 | 31 | 58 | 93 | 36 | 57 | CH |
| G2 | – | 26 | 37 | 37 | 78 | 25.78 | 52.22 | CH | |
| G3 | 1 | 40 | 29 | 30 | 43 | 19.35 | 23.65 | CL | |
| G4 | – | 8 | 47 | 45 | 68 | 24 | 44 | CH | |
| G5 | – | 32 | 32 | 36 | 44.83 | 21.23 | 23.6 | CL | |
| G6 | – | 10 | 33 | 57 | 80 | 29.53 | 50.47 | CH | |
| G7 | – | 13 | 28 | 59 | 88 | 27.63 | 60.37 | CH | |
| Kakinada | K1 | – | 5 | 46 | 49 | 82 | 31 | 51 | CH |
| K2 | – | 3 | 42 | 55 | 73.6 | 29.78 | 43.82 | CH | |
| K3 | – | 4 | 40 | 56 | 70.2 | 27.65 | 42.55 | CH | |
| Nagpur | N1 | 2 | 14 | 43 | 41 | 61 | 21.92 | 39.08 | CH |
| N2 | 4 | 16 | 38 | 42 | 58 | 23 | 35 | CH | |
| N3 | 3 | 26 | 38 | 33 | 48 | 20.85 | 27.15 | CL | |
| N4 | 1 | 22 | 30 | 53 | 57.7 | 22.52 | 35.18 | CH | |
| N5 | 3 | 19 | 29 | 51 | 69 | 24.68 | 44.32 | CH | |
| N6 | 2 | 21 | 28 | 49 | 61.9 | 29.76 | 32.14 | CH | |
| N7 | 1 | 14 | 32 | 53 | 74 | 27.24 | 46.76 | CH | |
| N8 | 3 | 13 | 30 | 54 | 66 | 27.16 | 38.84 | CH | |
| Raipur | R1 | 1 | 15 | 39 | 45 | 76.7 | 25.39 | 51.31 | CH |
| R2 | 0 | 10 | 43 | 47 | 62 | 23 | 39 | CH | |
| R3 | 0 | 14 | 38 | 48 | 57 | 25.54 | 31.46 | CH | |
| Vijayawada | V1 | – | 12 | 33 | 55 | 92 | 34.32 | 57.68 | CH |
| V2 | 2 | 18 | 35 | 45 | 43 | 22 | 21 | CL | |
| V3 | 2 | 20 | 32 | 46 | 46 | 17.7 | 28.3 | CL | |
| V4 | 3 | 22 | 33 | 42 | 46.1 | 18.93 | 27.17 | CL | |
| V5 | 1 | 17 | 39 | 43 | 52 | 15.54 | 36.46 | CH | |
| V6 | – | 9 | 40 | 47 | 82.8 | 26.43 | 56.37 | CH | |
| V7 | – | 11 | 39 | 50 | 79.8 | 26.20 | 53.6 | CH | |
| Warangal | W1 | 2 | 26 | 30 | 42 | 70 | 26 | 44 | CH |
| W2 | – | 27 | 29 | 44 | 51.5 | 23.27 | 28.23 | CH | |
| W3 | 1 | 43 | 28 | 28 | 53.45 | 22.89 | 30.56 | CH | |
| Mysore | M1 | – | 21 | 26 | 53 | 72 | 28.35 | 43.65 | CH |
| Kendrapara | KP1 | 4 | 15 | 38 | 43 | 60 | 28.25 | 31.75 | CH |
| KP2 | 3 | 17 | 33 | 47 | 53 | 29.54 | 23.46 | CH | |
| KP3 | 2 | 18 | 29 | 51 | 70 | 32 | 38 | CH | |
| KP4 | 3 | 21 | 31 | 45 | 42.5 | 19.5 | 23 | CH | |
| KP5 | 4 | 20 | 29 | 47 | 51 | 23.73 | 27.27 | CH | |
| KP6 | 2 | 19 | 35 | 44 | 68 | 22.87 | 45.13 | CH | |
Swelling characteristics
Swelling characteristics (i.e. Sa and Sp) of each soil sample were measured in accordance with the guidelines of ASTM D243548 standard. Samples of oven-dried and passing 425 microns sieve were used for the preparation of test specimen. Swelling tests were carried out with the help of a conventional oedometer apparatus, which houses an oedometer ring of 60 mm internal diameter and 20 mm height for accommodating the specimen. Each soil sample was compacted to maximum dry density at optimum moisture content inside the ring up to the height of 12 mm. Three LVDTs (with least count of 0.001 mm) to measure the change in height (i.e. deformation) of specimen with time in three different directions were mounted and an average of them was considered representative value. The test was commenced by flooding the sample with water, supplied from an overhead reservoir, and it is continued until the time taken to attain three consecutive values of deformations were identical. The final deformation when divided with initial height yields the swelling potential, Sa, of a soil sample. From the stage of final deformation, the sample was compressed by gradually applying compressive pressure with initially from zero and in steps of 10 kPa until the deformation reaches to 0 or negative49. The pressure corresponding to zero deformation was determined, which yields the swelling pressure, Sp.
Quantification of mineral and chemical parameters
The objective of the study is to investigate the combined effect of mineral (i.e. montmorillonite) and chemical parameters (sodium and calcium contents) on swelling property. Accordingly, efforts were devoted to quantify, first, these parameters in 46 numbers of soil samples. However, chemical parameters in soils can present in different forms such as total (designated as NaT for sodium and CaT for calcium), leachable (designated as NaL for sodium and CaL for calcium), and exchangeable (designated as NaCEC for sodium and CaCEC for calcium)50, in disproportionate quantity. The total form is the direct image of structural chemical element of a given soil sample51, whereas the leachable form is the quantity of structural chemical element that leaches out under the influence of concentric acids52. On the other hand, exchangeable form is the quantity of cations hold by clay particles and are ready to exchange with other cations when fluid comes in contact53.
XRF and ICP-OES are widely used analytical techniques to determine elemental compositions of soil samples. XRF is a rapid technique for analyzing the total form of an element (i.e. NaT and CaT) in dry soil samples accurately. Whereas, ICP-OES technique is basically meant for analyzing aqueous samples to determine major and trace elements in leachable form (i.e. NaL and CaL), specifically in natural soils, polluted soils, and biological samples. These chemical parameters, prima facie, represent mean structural chemical parameters of different mineral components. However, the swelling is attributed the exchange of cations (i.e. cation exchange capacity, CEC), which broadly manifests external chemical environment. Therefore, besides the quantification of Na and Ca contents in their total and leachable form, the exchangeable form of these individual elements (i.e. NaCEC, CaCEC), their ratio (i.e. (Ca/Na)CEC) and combination (i.e. total CEC) were also picked up for the study purpose. In this regard, an additional data pertinent to exchangeable form of Ca, Na and total contents for expansive soils were collected from the literature.
Holistically, it is incorrect to consider the effect of individual element/ion. Therefore, their ratio (i.e. (Ca/Na)T, (Ca/Na)L, and (Ca/Na)CEC) was chosen as studying chemical parameters to derive a vivid understanding of them along with MMC.
Mineralogical characteristics
Separation of clay size particles
Orientation of clay particles has a dominant impact on quantification of clay minerals54,55. Errors in the quantification of clay minerals can be pronounced if there are no preferred orientations, which can severely be disturbed in the presence of non-clay fine particles54. In order to increase the rate of accuracy in quantification, the planes (001) should be oriented parallel to the surface56. For achieving this, the clay fraction in the soil sample needs to be segregated. To accomplish this, wet sieve analysis was performed using sieve with aperture size of 20 microns. The procedure postulated by Brandt et al.57 was followed for this purpose. The suspension derived was collected in a container and was oven dried at 105 ± 0.5 °C for 24 h. Specimens obtained subsequent to drying were pulverized to down size the particles with the help of masher. 2 to 3 g of fine soil was grabbed from this pulverized fraction and was transferred into the centrifuge tube after adding 1% calgon solution. The mixture was ultra-sonicated for 1 min to disperse the particles prior to the centrifugation. The suspension was centrifuged at a speed of 1000 rpm for 5 min, so that larger particles settle at the bottom of the tube. After centrifugation, calgon solution was decanted into another centrifuge tube and the same was centrifuged again at 1500 rpm for 15 min. After decanting the calgon solution again, the residue deposited at the bottom of the tube was washed with distilled water. The above steps of decantation and centrifugation were repeated for 3 to 5 times. Finally, 2 to 3 drops of the suspension were placed on a glass slide by uniformly spreading with glass rod and allowed to dry overnight at temperature of 60–70 °C in the desiccator containing ethyl glycol solution, which was already filled to 1 cm deep from the bottom. Ethylene glycol expands the clay minerals such as montmorillonite, smectite, and other mixed minerals for easy identification and their quantification57. Another advantage of this treatment is it causes fewer disturbances in orientations along with the amorphous scattering of X-rays by excess liquid than other methods57. Then the dried sample over glass slide is fed to the equipment for further analysis.
Quantification of montmorillonite content
The mineralogical composition of a soil was determined with the help of D8 Advanced X-ray powder diffraction device (make, BRUKER, USA). The sample from the desiccator was directly mounted on the equipment platform and scanned for reflections with a voltage and current of 40 kV and 40 mA, respectively, with 2θ ranging from 5 to 80° with a step size of 0.025° and time interval of 0.5 s for each step using a copper X-ray tube (i.e. Cu-Kα radiation). X-ray beams were directed to hit the sample such that process scatters the atoms in their path. Post scattering, structural characteristics of the sample were detected by applying Bragg’s law58. The presence of minerals in each sample was identified with the help of DIFFRAC.SUITE EVA V.3.058 software. The software performs automatic search on raw data, which was background subtracted automatically, and on the peak list to identify the most appropriate mineral phase.
Montmorillonite content, MMC, in each soil sample was quantified using TOPAS 4.2 software, which takes whole pattern as input data. Different minerals in each soil sample was identified by comparing the obtained diffraction data with the International Centre for Diffraction Data base (ICDD). Once the phases have been identified, the necessary atomic information was extracted from the database to produce a computed profile. However, quantitative clay mineralogy requires mineral standards with XRD properties similar to those of mineral phases in unknown samples59. For this purpose, Inorganic Crystal Structure Database (ICSD) reference patterns were employed to match with the measured pattern. TOPAS 4.260 is a graphics based profile analysis program and it integrates various types of X-ray and neutron diffraction analyses by supporting all profile fit methods currently employed in powder diffractometry. The software by resorting to the Rietveld technique computed the mineralogical quantitative analysis61. For the sake of clarity, diffraction pattern established on soil sample from each region is presented in Fig. 2.
Figure 2.
X-ray diffraction pattern of a soil sample designated with dominant minerals identified.
Rietveld refinement performs on whole pattern basis which is a powerful approach for quantifying the identified minerals. In this method, the reflections are replicated with the help of calibrated crystallographic parameters17,62–64. Whereas in the case of single reflection method, individual peak is considered for the quantification of clay minerals, which requires mineral standards21. Generally, there are two types of internal standards such as ZnO (Zincite) and α-Al2O3 (corundum) for quantifying the minerals in clay based soil samples. The former and latter standards are normally used in Mineral Intensity Factor65 and Reference Intensity Ratio methods19. Conforming this fact, Srodon et al.65 and Hillier66 employed Zincite and Corundum as internal standards for quantifying the minerals. The software performs the least square method for quantitative phase analysis on the data extracted and known data from ICSD. The difference between the observed and known structural factors such as site occupancy information, cell dimension, inter-atomic distance, temperature, and magnetic factors was minimized by multiple iterations. Later on successful completion of scale factor (i.e. atomic positions, thermal vibrations), different quantities of minerals were obtained67.
Quantification of NaT and CaT by X-ray fluorescence spectroscopy (XRF)
Total form of sodium and calcium contents in each soil sample was determined with the help of X-ray fluorescence device (make, PANalytical, The Netherland). The soil sample used for analysis purpose was in pellet form. The palletization was accomplished by filling 32 mm diameter aluminium cup with boric acid powder at the bottom first and then with dry soil sample. The boric acid is selected because it doesn’t consist of trace elements. Approximately 5–8 g of oven-dry soil passing sieve size of 75 μm was used. The cup filled up with boric acid and soil sample was transferred to automatic press apparatus and a load of 20 ton was applied for the duration of 20 s. This process transforms the powder sample into pellet, which was then utilized for XRF analysis to measure calcium and sodium contents. Each sample was scanned for reflections with a voltage and current of 60 kV and 50 mA, respectively, using a copper X-ray tube (i.e. Cu-Kα radiation). Soil sample when exposed to X-ray source, elements in the sample produce a specific sign/emission, which is distinct for every element. By counting the intensity of sign and wavelength, quantification of different elements shall be made68. The analysis quantifies range of each element accurately from 0.01–100% of total content by its dry weight basis69. The results in elemental form were then converted into their oxides form of soils70 .
Quantification of NaL and CaL by inductively coupled plasma-optical emission spectrometry (ICP-OES)
ICP analysis gives leachable form of chemical elements and it is generally performed on solutions derived by digesting soil samples with high concentrated acids, as per the guidelines of USEPA 3050B71 standard. The digestion involves addition of 0.5 g of soil to 9 ml of HNO3, 3 ml of HF, 2 ml of HCl, and 5 ml of distilled water. In case of soil sample containing any organic matter, 2 ml of H2O2 was added additionally. The solution mix was then transferred to vessels made of Polytetrafluoroethylene (PTFE). The vessels capped to ensure air or water tightness were then placed inside the microwave digester and the soils mixtures were digested for the duration of 60 min. The resultant mixture was filtered through 42 micron Whatman filter paper to segregate liquor and residue. The filtrate solution thus obtained was fed to ICP-OES device (make, PerkinElmer, Model: Avio 200) for the analysis purpose. The liquid when exposed to plasma energy, constituent elements i.e. atoms, gets excited. The type of element was determined by employing plasma energy and its quantity is obtained from the intensity of photon rays. The chemical compositions determined from XRF and ICP analysis can be seen in Table 3.
Table 3.
Chemical properties of soil samples used in the study.
| State/Region | Sample ID | XRF Analysis | ICP Analysis | ||
|---|---|---|---|---|---|
| CaT (%) | NaT (%) | CaL (mg/kg) | NaL (mg/kg) | ||
| Bhopal | B1 | 2.602 | 0.37 | 63.71 | 75.57 |
| B2 | 10.303 | 0.402 | 100.5 | 135.3 | |
| B3 | 3.03 | 0.42 | 61.29 | 88.9 | |
| B4 | 4.081 | 0.518 | 74.31 | 160.6 | |
| B5 | 3.886 | 0.417 | 82.47 | 104.3 | |
| B6 | 5.065 | 0.38 | 84.29 | 125.3 | |
| B7 | 10.515 | 0.382 | 79.8 | 97.3 | |
| B8 | 4.201 | 0.298 | 70.6 | 129 | |
| Guntur | G1 | 5.796 | 0.517 | 76.9 | 108.4 |
| G2 | 8.675 | 0.811 | 123.1 | 200.4 | |
| G3 | 5.032 | 0.719 | 98.25 | 207.9 | |
| G4 | 3.442 | 0.258 | 73.22 | 81.72 | |
| G5 | 5.253 | 0.634 | 68.08 | 222.9 | |
| G6 | 4.51 | 0.427 | 62.13 | 224.5 | |
| G7 | 2.5 | 0.314 | 110.5 | 123.6 | |
| Kakinada | K1 | 3.846 | 0.463 | 66.19 | 70.84 |
| K2 | 3.04 | 0.338 | 39.69 | 170.1 | |
| K3 | 3.182 | 0.409 | 50.03 | 74.73 | |
| Nagpur | N1 | 6.858 | 0.334 | 89.94 | 48.26 |
| N2 | 5.229 | 0.401 | 77.95 | 67.17 | |
| N3 | 3.502 | 0.287 | 55.71 | 108 | |
| N4 | 5.25 | 0.213 | 96.85 | 57.5 | |
| N5 | 3.792 | 0.228 | 69.74 | 129.9 | |
| N6 | 5.369 | 0.267 | 62.28 | 47.18 | |
| N7 | 5.252 | 0.231 | 60.68 | 47.07 | |
| N8 | 4.636 | 0.225 | 114.1 | 52.38 | |
| Raipur | R1 | 4.271 | 0.181 | 46.94 | 53.4 |
| R2 | 4.286 | 0.249 | 43 | 41.02 | |
| R3 | 2.059 | 0.154 | 25.32 | 112 | |
| Vijayawada | V1 | 7.067 | 0.626 | 185.3 | 164.4 |
| V2 | 5.532 | 0.891 | 149.4 | 189.8 | |
| V3 | 4.713 | 0.753 | 96.6 | 148.8 | |
| V4 | 5.494 | 0.738 | 63.02 | 105.8 | |
| V5 | 4.476 | 0.681 | 68.31 | 197.5 | |
| V6 | 4.271 | 0.677 | 103.7 | 136.5 | |
| V7 | 4.746 | 0.725 | 74.38 | 99.82 | |
| Warangal | W1 | 3.906 | 1.12 | 63.17 | 103 |
| W2 | 3.301 | 0.648 | 50.98 | 106.7 | |
| W3 | 3.859 | 1.405 | 71.95 | 277 | |
| Mysore | M1 | 2.599 | 0.349 | 101 | 143.5 |
| Kendrapara | KP1 | 1.161 | 0.178 | 27.82 | 120.2 |
| KP2 | 1.56 | 0.427 | 36.44 | 120.7 | |
| KP3 | 2.226 | 0.464 | 26.08 | 154.3 | |
| KP4 | 1.327 | 0.293 | 21.91 | 75.8 | |
| KP5 | 1.322 | 0.432 | 18.68 | 136.7 | |
| KP6 | 1.294 | 0.435 | 26.71 | 132.1 | |
Results
Based on the results obtained from extensive experimentations, 3D graphs demonstrating the combined effect of chemical and mineralogical parameters on swelling parameters were developed, as depicted in Figs. 3, 4, 5, 6 and 7 respectively. Please note that chemical parameters effect includes separately for structural chemical parameters (i.e. (Ca/Na)T and (Ca/Na)L) and external chemical environment (i.e. (Ca/Na)CEC). To delineate the exact impact, 3D polynomial surface curve fits relating (Ca/Na)T/L/CEC and MMC with Sa and Sp were employed to the data, as shown in Figs. 3, 4, 5, 6 and 7.
Figure 3.
Combined influence of MMC and (Ca/Na)T on (a) swelling potential and (b) swelling pressure of expansive soils.
Figure 4.
Combined influence of MMC and (Ca/Na)L on swelling potential of expansive soils.
Figure 5.
Combined influence of MMC and (Ca/Na)Lon the swelling pressure of expansive soils (a) Sp < 120 kPa and (b) Sp > 120 kPa.
Figure 6.
Combined influence of MMC and (Ca/Na)CEC on (a) swelling potential and (b) swelling pressure of expansive soils.
Figure 7.
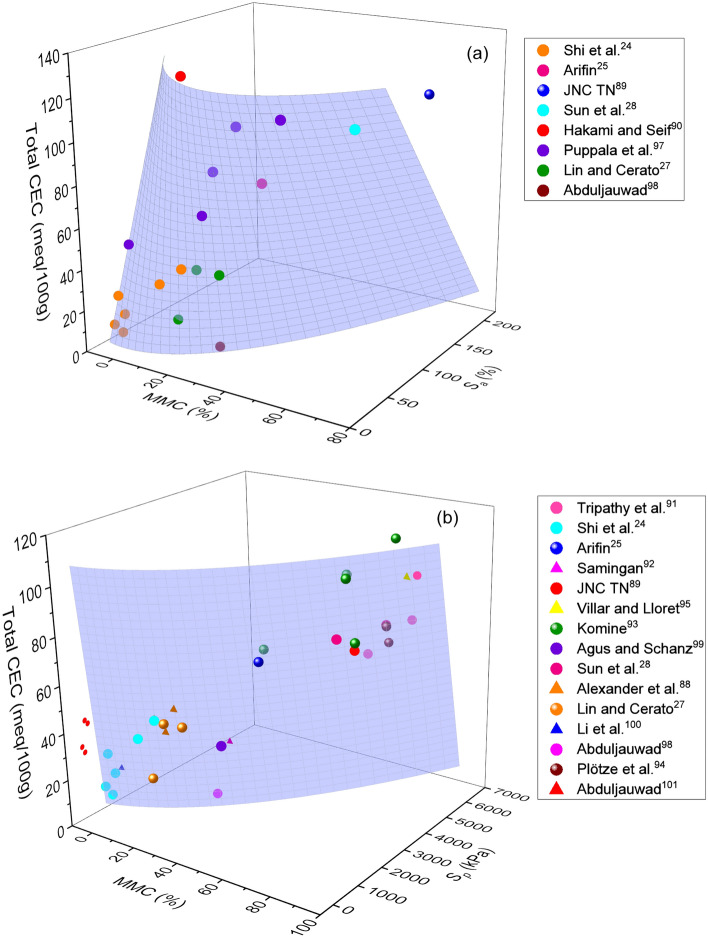
Combined influence of MMC and total CEC on (a) swelling potential and (b) swelling pressure of expansive soils.
A clear concave shape polynomial surface profile, which extends towards higher values of MMC, corresponding to Sa and Sp can be viewed from Fig. 3. It is obvious that the combined effect of Ca/Na ratio and MMC on Sa and Sp appears to be non-linear in nature. It is seen from Fig. 3 that with an increase in MMC and (Ca/Na)T, both Sa and Sp increased (diagonally upward), reached a peak and then receded. It can be noticed that when MMC and (Ca/Na)T ratio is least that is near the origin of the graph, the values of Sa and Sp are also trivial. As evident from Fig. 3, the influence of MMC and (Ca/Na)T on Sa is well defined for Sa, as it is increased in proportion to increment of MMC and (Ca/Na)T parameter, in comparison with Sp. Understandably on the other hand, the ratio of (Ca/Na)T seems increases with an increase in MMC. Similarly, Figs. 4 and 5 show the polynomial surface fits employed to correlate Sa and Sp with (Ca/Na)L and MMC parameters, in tandem. Two distinct correlations for Sp < 120 kPa and Sp > 120 kPa were formulated depending upon the trend revelations when data is drawn for Sp against (Ca/Na)L and MMC. Thus, for the sake of clarity, two independent plots were developed, as depicted in Fig. 5. Please note that data used in Fig. 5b is explicitly collected from the literature. From Figs. 4 and 5a, it can be observed that both Sa and Sp increased linearly with MMC and (Ca/Na)L. Evidently, the ratio of (Ca/Na)L is mostly confined to unity, and when it is exceeded this value, Sa is found to be significantly ascended although MMC is minimal (Fig. 4). It is also seen from Fig. 5, a reversal in trend for Sp above and below 120 kPa.
When (Ca/Na)T exceeded 20, surface profile is apparently seen sloping towards MMC (Fig. 3). Similar such observation of surface profile inclining towards MMC can be noticed from Figs. 4 and 5b. In fact, a complete dominance of MMC over (Ca/Na)L can be seen from Fig. 5b. These statements substantiate a likely supremacy of MMC over chemical parameters (i.e. (Ca/Na)T,L) on Sa and Sp. From these statements, it can also be inferred that initial swelling might be triggered by chemical parameters and the subsequent volume change seems completely controlled by MMC. This is an interesting and important finding by the study, as no earlier research work reported such findings with a bearing on chemical and mineralogical parameters together on swelling properties.
A close observation of Figs. 3 and 4 reveals a wide variation of total form of chemical data vis-à-vis that of leachable form, which remains largely confined to less than unity. Conversely, a noticeable difference in effect can be perceived between total and leachable forms from Figs. 3, 4 and 5. It is interesting to note their distinct impact on Sa and Sp, although, total and leachable forms are inherent constituents of a given soil. Contrary to the total form response that showed non-linearity with swelling parameters, leachable form depicts linear variation with Sa and Sp. Furthermore, the influence of leachable form is remained same on both Sa and Sp up to 120 kPa, as profile curve fits have closely resembled each other between Figs. 4 and 5a. Based on the pronounced and marked influence of total form chemical parameters, it can be interpreted from Figs. 3 and 4 that resorting to total form is beneficial for precise determination of swelling behaviour, over leachable form. From Figs. 3, 4, and 5, the maximum Sa of 18% and Sp of 93 kPa is measured at MMC of 43%, (Ca/Na)T of 10–14, and (Ca/Na)L of 2–7. The results obtained in the present study are in well agreement with the studies reported by Shi et al.24, She et al.72, and Abd-Allah et al.73, who have reported properties of MMC, chemical and swelling parameters that are found to be falling in the range of data produced by the presented study.
Figures 6 and 7 show the variations of Sa and Sp with (Ca/Na)CEC, total CEC, and MMC parameters. Obviously, an increase in Sa and Sp with an increase in MMC, (Ca/Na)CEC, and total CEC, together, as well as a decrease and an increase in (Ca/Na)CEC and total CEC respectively, with an increase in MMC can be noticed from these figures. In particular, lesser values of Sa and Sp corresponding to lower values of MMC and greater values of (Ca/Na)CEC and vice versa can be visualized from these figures. A closer examination of surface profile of Fig. 6a further reveals that Sa becomes constant at (Ca/Na)CEC of approximately 30, for the whole range of MMC. On the contrary, Sp attained peak value and then became constant only when the ratio of (Ca/Na)CEC exceeds 30 and MMC lies above 50% (Fig. 6b). However, when MMC is less than 40%, steady vertical rise in surface profile of Sp with an increase in (Ca/Na)CEC ratio is noticeable. On the other hand, a remarkable lower values of (Ca/Na)CEC and exorbitantly higher values of MMC can be noticed when Sp exceeds 5000 kPa, as is seen from Fig. 6b. From the surface profile sloping towards MMC, it can be inferred that (a) Sa is largely governed by MMC for its whole range, not the ratio of (Ca/Na)CEC, and (b) Sp is seemed controlled by MMC only when it is above 50%.
Similarly, steady increase in Sa and Sp (diagonally upward) with an increase in total CEC and MMC can be witnessed from Fig. 7a. The observation made from figure is in well agreement with the results of Pedarla et al.74 and Tahasildar et al.11, who have reported an increment of swelling properties with MMC. As such, surface profile nearly vertical for Sp, but leaned towards total CEC for Sa. Inclination of surface profile towards chemical parameter may be an indication that Sa is more impacted by total CEC, rather than MMC. Understandably, perfect vertical rise substantiates equal influence of both total CEC and MMC of a given soil on Sp parameter. It can also be concluded from Fig. 7b that the combination of total CEC and MMC are best parameters to predict the swelling pressure of expansive soils.
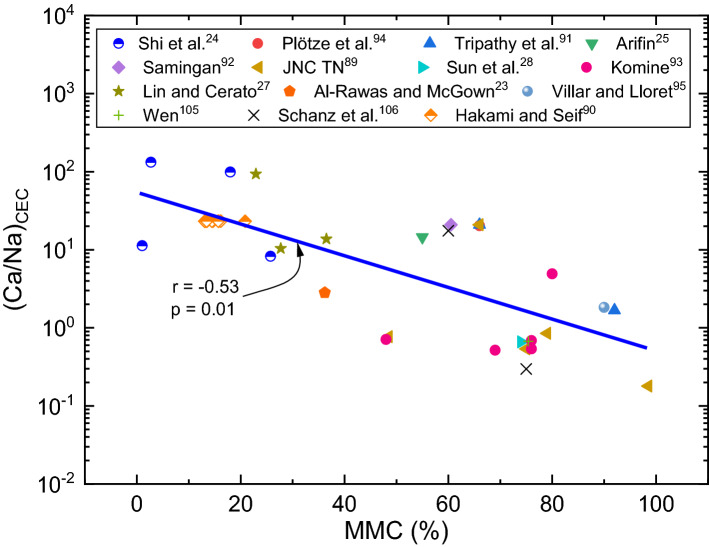
As evident from Figs. 3, 4, 5, 6 and 7, surface profiles are not similar among total, leachable, and exchangeable forms of chemical parameters, though MMC range is bracketed. This demonstrates a distinct impact of chemical parameters on Sa and Sp, though the degree of disparity appears to be indifferent. Conversely, surface profiles are reasonably similar between Sa and Sp irrespective of total, leachable or exchangeable forms of chemical parameters. It portrays a fact that there is a significant variance in chemical compositions and even the availability of parameters form corresponding to a particular MMC value. In order to verify and confirm the above facts, variations of (Ca/Na)T, (Ca/Na)L, (Ca/Na)CEC, and total CEC versus MMC are plotted, as shown in Figs. 8, 9, 10 and 11. Please note that the maximum MMC quantified for the soils used in the study is 43%. Therefore, relevant data for MMC beyond this value were collected from the literature and superimposed on Figs. 8, 9, 10 and 11. It is obvious from Fig. 8 that as MMC increases, (Ca/Na)T and (Ca/Na)L increases, reaches a peak at MMC of 67% for total form and 50% for leachable form, and thereafter, decreases with further increment of MMC. Incidentally, this observation excellently corroborates with the inferences made with Fig. 3, wherein it is pinpointed that Sa and Sp decreases when (Ca/Na)T exceeds 20. The decrease in (Ca/Na)T and (Ca/Na)L indicates a fact that with an increase in MMC in a given soil so does total and leachable Na content in it, which in turn highlights that MMC increases soils become rich in total and leachable Na content, not the total Ca content.
Figure 8.

Relationship between MMC and Ca/Na Ratio of (a) total and (b) Leachable forms.
Figure 9.
Variation of NaCEC and CaCEC with MMC for expansive soils.
Figure 10.
Variation of (Ca/Na)CEC with MMC for expansive soils.
Figure 11.
Variation of total CEC with MMC of expansive soils.
Many studies have reported significantly higher values of CEC for mineral rich soils such as bentonite (60–150 meq/100 g) vis-à-vis natural expansive soils (0–70 meq/100 g)12,14. Based on these disclosures, it is prudent to conclude that there should be an inherent relationship between mineral content and exchangeable capacity of cations. To verify and confirm this fact, efforts are devoted to develop relationships for NaCEC, CaCEC, (Ca/Na)CEC ratio and total CEC with MMC, as shown in Figs. 9, 10 and 11 respectively. Trends shown in these figures bear very practical significance in a sense that it is important to know the relationship between chemical and mineralogical parameter as both these parameters are key to predict volume change behaviour of expansive soils. From the above observations, it can be deduced that contents of Ca and Na, say in total, leachable or exchangeable form, are depended upon constituent minerals content. These findings well substantiate the results of Foster32, who stated that ionization of Na or Ca depends on the constituent minerals content.
Trends of these figures manifest a fact that individual NaCEC and CaCEC displays an exponential relationship, while the ratio (Ca/Na)CEC and total CEC portrays linear relationship with MMC for expansive soils. It can be observed from Figs. 9, 10 and 11 that NaCEC, CaCEC and total CEC increases, and (Ca/Na)CEC decreases with an increase in MMC. As striking from Fig. 8, the value of CaCEC is higher in comparison with NaCEC for a given MMC up to 65% and beyond this value, NaCEC exhibited prominence over its counterpart ion. The same statement might be valid true from Figs. 10 and 11, but linear fitting seems masking the effect. A close review of Fig. 10 makes it noticeable that (Ca/Na)CEC is clearly less than unity when MMC exceeds 65%. The decrement of (Ca/Na)CEC versus MMC (Fig. 10) is quite analogous to Fig. 6 response. Incidentally, MMC of 67% is identical between the intersection point of NaCEC and CaCEC (Fig. 9) and peak of (Ca/Na)T (Fig. 8). These inferences further remarkably substantiate the results of Fig. 6b, wherein it is found that surface profile slopes towards MMC when it is above 50%. Similarly, the increment of total CEC with MMC is identical between Figs. 7 and 10. The authors opine that the explanation given in the context of Figs. 6 and 7 holds true here as well.
The following inferences were further made from Figs. 3, 4, 5, 6 and 7: (a) Sa of natural expansive soils is largely confined to 30%, while the same for bentonite soils has reached as high as 150%, (b) Sp of natural expansive soils is found to be less than 120 kPa, while the same for bentonite soils is found to be exorbitantly greater (i.e. above 5000 kPa), (c) MMC in natural expansive soils is mostly found below 45%, while the same in bentonite soils is reported above 90%, (d) the effect of (Ca/Na)CEC ratio and total CEC are predominant on Sp than on Sa, and (e) total form of Ca/Na ratio is attracted merit over leachable form in predicting Sa and Sp.
From the comprehensive test results, it has been noticed that the minimal amount of Sa and Sp can be envisioned at MMC < 10%, (Ca/Na)T > 15, (Ca/Na)L > 1, and (Ca/Na)CEC > 10 respectively. In order to constrain the swelling behaviour of expansive clays within the range that would pose no potential threat to structure, it is recommended to ensure that soils comprise of mineral and chemical compositions not exceeding the above specified limits.
Discussion
Montmorillonite among minerals and Na and Ca contents among chemical parameters are chief constituents of expansive soils. Furthermore, Na and Ca contents are also the structural constituents of montmorillonite mineral, which is evident from its empirical formula (i.e. Na0.2Ca0.1Al2SiO10(OH)2(H2O)10. As per the Foster32, variances in swelling characteristics are associated with chemical parameters, degree of ionization, and type and amount of associated exchangeable cations. The results presented herein evidently prove the same fact.
Many studies report that MMC and cations concentration enhance the swelling behaviour by forming diffuse double layer upon hydration8,33,34. Hydration is the prime cause for swelling soils to exhibit volume change behaviour. Incidentally, both mineral (MMC) and chemical (Na and Ca) parameters prone to undergo hydration phenomenon, and thereby accentuate swelling in expansive soils32,75. As discussed during the explanation of Fig. 2 about triggering mechanism of swelling between chemical and mineral components, it is sensible to hypothesize that chemical effect occurs first at microscale level and the same gets manifested at macroscale level, essentially replicating as mineral behaviour75. Revisiting the hydration phenomenon, Na or Ca contents in soils acts as a wedge between the mineral layers, so does the swelling behaviour. It certifies that the swelling is primarily induced by the chemical parameters and followed by MMC. Generally, hydration of cations is quick and rapid in comparison with minerals76,77. For ex: there is a 20 fold difference in ionization between Na and Ca ions in the case of bentonite soil32,78,79. Similarly, Zhang et al.80 postulated that Na-montmorillonite hydrates more than Ca-montmorillonite does at the same water content. Differing in the ionization capabilities between Na and Ca ions can be reasoned out for the disparity in the values of swelling properties, as shown in Figs. 3, 4 and 5. The increase in ionization of either Na or Ca with an increase in MMC could be a logical hypothesis for the increase in swelling property as the MMC increases, of response depicted in Figs. 3, 4 and 5. Furthermore, the cations that exist between sheets of clay minerals generally act as bridges76,81. During the hydration phenomenon, these links/bridges result in the formation of thicker diffuse double layer around the clay particles. It is obvious to expect that as the MMC increases so does thickness of diffuse layer and hence, swelling in soils. Validating these statements, continues increase in swelling property with an increase in MMC can be witnessed from Figs. 3, 4 and 5. On the other hand, there is an increase and decrease of Ca/Na ratio with MMC, as apparent from Fig. 8. This trend of Sa and Sp increase and decrease with (Ca/Na)T (Fig. 3) very well mimics the variation of (Ca/Na)T with MMC (Fig. 8).
As is true from Fig. 9, there is a disparity in the quantity of exchangeable cations for a given MMC. It can be interpreted from trends in Fig. 9 that divalent Ca++ cations are dominant in expansive soils up to MMC of 65% and beyond this percentage, prevalence of monovalent Na+ is clear corresponding to bentonite soils. It is thus judicious to conclude from the figure that the soils with MMC below 65% can be categorized as natural expansive soils, which as per Fig. 9 are evidently dominated with Ca++ cations. Results presented in Fig. 6 are in perfect agreement with this fact, as it is seen that Sa values are appreciably low even when (Ca/Na)CEC values are markedly high at MMC of negligible value. With the dominance of Ca over Na, monovalent cations will be replaced with divalent Ca++ ions, resulting in the condensation of thickness of diffuse double layer32. Moreover, the atomic radius of Na is lesser than that of Ca. This has tremendous implication as it directly impacts affinity to water absorption, which in turn affects the thickness of diffuse double layer over the clay platelets.
It is understood that Sa of soils is influenced by the hydration of cations content, whereas the same is not prevalent in case of Sp due to the applied loading condition. Under no hydration condition the effect of chemical parameters is less, which is logical to link differences in behaviour of Sa and Sp, as evident from Fig. 6. Certifying this, the surface profile of Sp remained vertical, although (Ca/Na)CEC and MMC varied. The vertical profile is due to the variance of (Ca/Na)CEC with MMC of soils. From this, it is evident that there is an inherent relationship exists between exchangeable cations and MMC. The same is evident in the case of total and leachable forms as well (refer to Figs. 3, 4 and 5). Based on this understanding, it can be discerned from Fig. 6 that Sa and Sp values are significantly lower when MMC is below 40%, though wider variance in the values of (Ca/Na)CEC is explicit. Conversely, more affinity to water by Na ions obviously results in greater volume change or swelling24, as the same is true that greater values of swelling property at higher content of MMC from Fig. 6. These statements well corroborate with the results of Liu et al.82, who have reported that Sa would be reduced when Na-montmorillonite is mixed with the solution rich in Ca ions. Reduction in ionization strength by the replacement of Na ions with Ca ions, which in turn abates the water adsorption capacity83, may be a cause for waning of trend with further increase of (Ca/Na)CEC as shown in Fig. 6. The above findings excellently endorse the results of Foster32, who have stated that ionization of Na or Ca depends on the constituent mineral contents.
It is important to highlight here that the interlayer space for Ca saturated montmorillonite can be higher than Na; this however depends on the relative humidity of the environment81. Acknowledging this fact, Watanabe and Sato84 investigated the same and reported that the basal spacing of Ca saturated montmorillonite is greater than Na until relative humidity of 90%. At relative humidity of 100% (i.e. third water layer), the basal spacing found to be same (i.e. 18.8 A°)85. But under water saturated conditions, both Na and Ca saturated montmorillonites do not exhibit similar interlayer spacing corresponding to three water layers. It is in confirmation with the studies of Teich-McGoldrick et al.86, who have reported an increment in interlayer spacing with an increment in water content. This is due to the fact that Ca cations provide stronger bonding force within the interlayers, and thereby, allow lesser water molecules into these interlayers. As appraised earlier, the ionization capacity of Na is 20 folds greater than Ca32. This may be a reason why Na-montmorillonite is selected as a barrier material in hazardous landfills. From the ongoing discussion, it is clear that the Na and Ca saturated soils possess same interlayer space at 100% relative humidity, but not for the water condition. The above inferences validating to the present study, samples in the present study were analyzed at an ambient temperature, and at that condition, the interlayer spacing of Ca-saturated will be greater than Na as per Watanabe and Sato84. Whereas in the case of water saturated, which is the current tested environment, the interlayer spacing with Na is greater than Ca, so does the swelling behaviour.
It can be noted that total CEC is the sum effect of Na, Ca and other cations. As evident from Fig. 11, sum effect is found to be increased with an increase in MMC. Such analogy validates the results presented in Fig. 7, wherein it can be seen a continuous increase in Sa and Sp with an increase in MMC and total CEC. Studies also reported that, in addition to montmorillonite, the exchange phenomenon in kaolinite soil group can also exhibit minor amount of swelling upon on hydration, which might be a reason behind the scatter of data in Fig. 7. From the foregoing discussion, it can be understood that constituent different chemical elements and their quantities might vary with the mineralogy in soils. In this context, it can be stated that natural expansive soils are generally composed with different minerals with no dominance to particular mineral, so does the quantity of other cations. Also evident from the formula mentioned above, montmorillonite composes of other elements such as Al, Si, Fe and Mg. Because of their trivial quantity, these elements may not affect the swelling behaviour largely alike Na and Ca does, but surely effect the total CEC of soils. From this, it is obvious that as MMC increase, so does the other elements as well, which may be a reason behind the linearity observed in Fig. 11.
Conclusions
In the present study, 46 numbers of different expansive type soil samples were collected from diverse locations across India. Mineralogical and chemical contents of these soil samples were analyzed in order to investigate their combined impact on swelling property. The results presented are unique and bear a practical significance for field engineers, as they can predict the swelling behaviour based on the measured chemical and mineral parameters. From the extensive experimentation and interpretation of obtained results, the following salient conclusions were made:
The analysis and interpretation of exhaustive results presented in 3D graphical forms and substantiated further with 2D graphs clearly demonstrate the merit and necessity of the combined impact of chemical and mineralogical parameters on swelling properties.
The various results vividly establish that all three forms including total, leachable and exchangeable of Ca and Na contents have had an influence and it is distinct among them on swelling properties of expansive soils.
The maximum value of MMC, Sa, and Sp of expansive soils used in the study are measured as: 43%, 18%, and 93 kPa respectively. Further, the maximum Sa and Sp is measured at (Ca/Na)T of 11.2 and (Ca/Na)L of 1.1.
The study recommends (Ca/Na)T + MMC combination for accurate measurement of Sa and total CEC + MMC combination for accurate measurement of Sp parameters.
It is in general noticed from the trends that (Ca/Na)T and total CEC along with MMC have a non-linear and (Ca/Na)L and (Ca/Na)CEC along with MMC have linear correlation with Sa and Sp parameters.
It has been confirmed that Ca and Na contents, either in total or leachable or exchangeable form, are depended on constituent minerals content, which is in conformation with the statement of Foster32 that ionization of Na or Ca depends on the constituent minerals content.
It is evident from the study that divalent Ca++ cations are dominant in expansive soils up to MMC of 65% and beyond this percentage, prevalence of monovalent Na+ is clear.
From the comprehensive analysis of results, it is advocated to confine the MMC, (Ca/Na)T, and (Ca/Na)L values to < 10%, > 15, and > 1 respectively. In order to restrict the swelling, it is advised to avoid the expansive soils with (Ca/Na)CEC below 10, which will be dominated with Na content and susceptible to unpredictable volume change behaviour.
Author contributions
P.S.R. conducted the experiments and produced the relevant data. B.H.R. and B.M. conceptualized the project, provided the funding and facilities, analyzed and interpreted the data, and prepared the original manuscript. K.R.R. reviewed the data analysis and edited the manuscript.
Funding
Funding was provided by CSIR-India/12/EMR-II(23(0025)).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zumrawi MME. Prediction of swelling characteristics of expansive soils. Sudan Eng. Society J. 2012;58:55–62. [Google Scholar]
- 2.Chen FH. Foundations on Expansive Soils. 2. Elsevier Science; 1988. [Google Scholar]
- 3.Sharma AK, Sivapullaiah PV. Ground granulated blast furnace slag amended fly ash as an expansive soil stabilizer. Soils Found. 2016;56:205–212. doi: 10.1016/j.sandf.2016.02.004. [DOI] [Google Scholar]
- 4.Al-Mukhtar M, Lasledj A, Alcover JF. Behaviour and mineralogy changes in lime-treated expansive soil at 20 °C. Appl. Clay Sci. 2010;50:191–198. doi: 10.1016/j.clay.2010.07.023. [DOI] [Google Scholar]
- 5.Pedarla A, Chittoori S, Puppala AJ. Influence of mineralogy and plasticity index on the stabilization effectiveness of expansive clays. Transport. Res. Rec. 2011;2212:91–99. doi: 10.3141/2212-10. [DOI] [Google Scholar]
- 6.Puppala AJ, Kadam R, Madhyannapu RS, Hoyos LR. Small-strain shear moduli of chemically stabilized sulfate-bearing cohesive soils. J. Geotech. Geoenviron. Eng. 2006;132:322–336. doi: 10.1061/(ASCE)1090-0241(2006)132:3(322). [DOI] [Google Scholar]
- 7.Uzundurukan S, Keskin SN, Yıldırım H, Göksan TS, Çimen Ö. Suction and swell characteristics of compacted clayey soils. Arab. J. Sci. Eng. 2014;39:747–752. doi: 10.1007/s13369-013-0852-2. [DOI] [Google Scholar]
- 8.Yǐlmaz I. Relationships between liquid limit, cation exchange capacity, and swelling potentials of clayey soils. Eurasian Soil Sci. 2004;37:506–512. [Google Scholar]
- 9.Rao BH, Venkataramana K, Singh DN. Studies on the determination of swelling properties of soils from suction measurements. Can. Geotech. J. 2011;48:375–387. doi: 10.1139/T10-076. [DOI] [Google Scholar]
- 10.Thakur VKS, Singh DN. Rapid determination of swelling pressure of clay minerals. J. Test. Eval. 2005;33:239–245. doi: 10.1520/JTE11866. [DOI] [Google Scholar]
- 11.Tahasildar J, Rao BH, Shukla SK. Mineralogical compositions of some indian expansive soils and their influence on swelling properties. Int. J. Geosynth. Gr. Eng. 2017;3:1–10. doi: 10.1007/s40891-016-0079-x. [DOI] [Google Scholar]
- 12.Chen FH. Foundations on Expansive Soils. Elsevier Science; 1975. [Google Scholar]
- 13.Lambe, T. W. & Whitman, R.V. Soil Mechanics. (1969).
- 14.Mitchell JK, Soga K. Fundamentals of Soil Behaviour. 3. Wiley; 2005. [Google Scholar]
- 15.Leroux J, Lennox DH, Kay K. Direct quantitative X-ray analysis: by diffraction–absorption technique. Anal. Chem. 1953;25:740–743. doi: 10.1021/ac60077a017. [DOI] [Google Scholar]
- 16.de Woolf PM, Visser JW. Absolute intensities—outline of a recommended practice. Powder Diffr. 1988;3:202–204. doi: 10.1017/S0885715600013488. [DOI] [Google Scholar]
- 17.Rietveld HM. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969;2:65–71. doi: 10.1107/S0021889869006558. [DOI] [Google Scholar]
- 18.Salyn, A. L. & Drits, V. A. Method of quantitative phase analysis of clays. in Proc. Int. Clay Conf., National Research Council of Spain and the Univ. of Madrid, Spain, 797–806 (1972).
- 19.Moore DM, Reynolds RC., Jr . X-Ray Diffraction and the Identification and Analysis of Clay. Oxford University Press; 1989. [Google Scholar]
- 20.Chipera SJ, Bish DL. Fitting full X-ray diffraction patterns for quantitative analysis: A method for readily quantifying crystalline and disordered phases. Adv. Mater. Phys. Chem. 2013;3:47–53. doi: 10.4236/ampc.2013.31A007. [DOI] [Google Scholar]
- 21.Zhou X, et al. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2017;3:16–29. doi: 10.1016/j.sesci.2017.12.002. [DOI] [Google Scholar]
- 22.Basma AA, Al-Homoud AS, Husein A. Laboratory assessment of swelling pressure of expansive soils. Appl. Clay Sci. 1995;9:355–368. doi: 10.1016/0169-1317(94)00032-L. [DOI] [Google Scholar]
- 23.Al-Rawas AA, McGown A. Microstructure of Omani expansive soils. Can. Geotech. J. 1999;36:272–290. doi: 10.1139/t98-111. [DOI] [Google Scholar]
- 24.Shi B, Jiang H, Liu Z, Fang HY. Engineering geological characteristics of expansive soils in China. Eng. Geol. 2002;67:63–71. doi: 10.1016/S0013-7952(02)00145-X. [DOI] [Google Scholar]
- 25.Arifin YF. Thermo-Hydro-Mechanical Behavior of Compacted Bentonite-Sand Mixtures: An Experimental Study. Bauhaus-University; 2008. [Google Scholar]
- 26.Sudjianto AT, Cakrawala M, Aditya C. The effects of water contents on free swelling of expansive soil. Int. J. Civ. Environ. Eng. 2012;12:13–17. [Google Scholar]
- 27.Lin B, Cerato AB. Prediction of expansive soil swelling based on four micro-scale properties. Bull. Eng. Geol. Environ. 2012;71:71–78. doi: 10.1007/s10064-011-0410-7. [DOI] [Google Scholar]
- 28.Sun D, Sun W, Fang L. Swelling characteristics of Gaomiaozi bentonite and its prediction. J. Rock Mech. Geotech. Eng. 2014;6:113–118. doi: 10.1016/j.jrmge.2014.01.001. [DOI] [Google Scholar]
- 29.Chittoori BCS, Puppala AJ, Pedarla A. Addressing clay mineralogy effects on performance of chemically stabilized expansive soils subjected to seasonal wetting and drying. J. Geotech. Geoenviron. Eng. 2018;144:04017097. doi: 10.1061/(ASCE)GT.1943-5606.0001796. [DOI] [Google Scholar]
- 30.Mehta B, Sachan A. Effect of mineralogical properties of expansive soil on its mechanical behavior. Geotech. Geol. Eng. 2017;35:2923–2934. doi: 10.1007/s10706-017-0289-6. [DOI] [Google Scholar]
- 31.Reddy PS, Mohanty B, Rao BH. Influence of clay content and montmorillonite content on swelling behavior of expansive soils. Int. J. Geosynth. Ground Eng. 2020;6:1. doi: 10.1007/s40891-020-0186-6. [DOI] [Google Scholar]
- 32.Foster MD. The relation between composition and swelling in clays. Clays Clay Miner. 1954;3:205–220. doi: 10.1346/CCMN.1954.0030117. [DOI] [Google Scholar]
- 33.Norrish K, Quirk JP. Crystalline swelling of montmorillonite: Use of electrolytes to control swelling. Nature. 1954;173:255–256. doi: 10.1038/173255a0. [DOI] [Google Scholar]
- 34.Olson RE, Mesri G. Mechanisms controlling compressibility of clays. J. Soil Mech. Found. Div. 1970;96:1863–1878. doi: 10.1061/JSFEAQ.0001475. [DOI] [Google Scholar]
- 35.Pusch R. Mineral-water interactions and their influence on the physical behavior of highly compacted Na bentonite. Can. Geotech. J. 1982;19:381–387. doi: 10.1139/t82-041. [DOI] [Google Scholar]
- 36.Daniel DE. Predicting hydraulic conductivity of clay liners. J. Geotech. Eng. 1984;110:285–300. doi: 10.1061/(ASCE)0733-9410(1984)110:2(285). [DOI] [Google Scholar]
- 37.Kenney TC, Van Veen WA, Swallow MA, Sungaila MA. Hydraulic conductivity of compacted bentonite–sand mixtures. Can. Geotech. J. 1992;29:364–374. doi: 10.1139/t92-042. [DOI] [Google Scholar]
- 38.Shainberg I, Letey J. Response of soils to sodic and saline conditions. Hilgardia. 1984;52:1–57. doi: 10.3733/hilg.v52n02p057. [DOI] [Google Scholar]
- 39.ASTM D1452 Standard practice for soil exploration and sampling by auger borings. (ASTM International, West Conshohocken, PA, 2016).
- 40.Oza JB, Gundaliya PJ. Study of black cotton soil characteristics with cement waste dust and lime. Procedia Eng. 2013;51:110–118. doi: 10.1016/j.proeng.2013.01.017. [DOI] [Google Scholar]
- 41.Central Ground Water Board. Ministry of Water Resources, Government of India. (2012)
- 42.Central Ground Water Board. Ministry of Water Resources, Government of India. (2013)
- 43.Central Ground Water Board. Ministry of Water Resources, Government of India. (2020)
- 44.ASTM D2216 Standard test methods for laboratory determination of water (moisture) content of soil and rock by mass. (ASTM International, West Conshohocken, PA, 2019).
- 45.ASTM D7928 Standard test method for particle-size distribution (Gradation) of fine-grained soils using the sedimentation (Hydrometer) analysis. (ASTM International, West Conshohocken, PA, 2017).
- 46.ASTM D4318 Standard test methods for liquid limit, plastic limit, and plasticity index of soils. (ASTM International, West Conshohocken, PA, 2000).
- 47.ASTM D2487 Standard practice for classification of soils for engineering purposes (Unified Soil Classification System). (ASTM International, West Conshohocken, PA, 2017).
- 48.ASTM D2435/D2435M-11 Standard test methods for one-dimensional consolidation properties of soils using incremental loading. (ASTM International, West Conshohocken, PA, 2020).
- 49.Thompson, R. W., Perko, H. A. & Rethamel, W. D. Comparison of constant volume swell pressure and oedometer load-back pressure. in Proc. of Fourth Int. Conf. on Unsaturated Soils American Society of Civil Engineers (ASCE), Carefree, Arizona, United States, 1787–1798 (2006).
- 50.Patel, A. Geotechnical investigations and improvement of ground conditions Woodhead Publishing Series in Civil and Structural Engineering (Eds. Jones, G.) 1–201 (Woodhead Publishing, 2019).
- 51.Tuncer ER, Demirel T, Lohnes RA. Quantitative analysis of elements in sediments and soils by X-ray fluorescence. Clays Clay Miner. 1977;25:73–77. doi: 10.1346/CCMN.1977.0250201. [DOI] [Google Scholar]
- 52.de Oliveira MW, Trivelin PCO, Boaretto AE, Muraoka T, Mortatti J. Leaching of nitrogen, potassium, calcium and magnesium in a sandy soil cultivated with sugarcane. Pesq. Agropec. Bras. 2002;37:861–868. doi: 10.1590/S0100-204X2002000600016. [DOI] [Google Scholar]
- 53.Schoonheydt RA, Johnston CT, Bergaya F. 1 - Clay minerals and their surfaces. Developments in Clay Science. 2018;9:1–21. doi: 10.1016/B978-0-08-102432-4.00001-9. [DOI] [Google Scholar]
- 54.Środoń, J. Identification and quantitative analysis of clay minerals in Developments in clay science (Eds. Bergaya, F., Theng, B. K. G. & Lagaly, G.) 765–787 (Elsevier, 2006).
- 55.Dohrmann R, Rüping KB, Kleber M, Ufer K, Jahn R. Variation of preferred orientation in oriented clay mounts as a result of sample preparation and composition. Clays Clay Miner. 2009;57:686–694. doi: 10.1346/CCMN.2009.0570602. [DOI] [Google Scholar]
- 56.Al-Ani T, Sarapää O. Clay and clay mineralogy. Ind. Eng. Chem. Res. 2008;31:1937–1946. [Google Scholar]
- 57.Brandt, L., Barnes, H. & Bronk, K. X-Ray Diffraction (XRD): Quick User Guide. International Ocean Discovery Program. (2013).
- 58.User Manual. Diffrac. Evaluation Package. Federal Republic of Germany. (2013)
- 59.Raval, N. et al. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development in Basic fundamentals of drug delivery (ed. Tekade, R. K.) 369–400 (Academic Press, 2019).
- 60.User Manual. Topas 4.2. Federal Republic of Germany. (2009).
- 61.Brindley, G. W. & Brown, G. Crystal Structures of Clay Minerals and their X-Ray Identification. (Mineralogical Society of Great Britain and Ireland, 1980).
- 62.Post, J. E. & Bish, D. L. Rietveld refinement of crystal structures using powder X-ray diffraction data in Modern powder diffraction reviews in mineralogy & geochemistry 277–308 (De Gruyter, 1989).
- 63.Lutterotti L, Scardi P. Simultaneous structure and size-strain refinement by the Rietveld method. J. Appl. Cryst. 1990;23:246–252. doi: 10.1107/S0021889890002382. [DOI] [Google Scholar]
- 64.Bish DL, Post JE. Quantitative mineralogical analysis using the Rietvetd full-pattern fitting method. Am. Miner. 1993;78:932–940. [Google Scholar]
- 65.Środoń J, Drits VA, McCarty DK, Hsieh JCC, Eberl DD. Quantitative X-ray diffraction analysis of clay-bearing rocks from random preparations. Clays Clay Miner. 2001;49:514–528. doi: 10.1346/CCMN.2001.0490604. [DOI] [Google Scholar]
- 66.Hillier S. Accurate quantitative analysis of clay and other minerals in sandstones by XRD: Comparison of a Rietveld and a reference intensity ratio (RIR) method and the importance of sample preparation. Clay Miner. 2000;35:291–302. doi: 10.1180/000985500546666. [DOI] [Google Scholar]
- 67.Connolly, J. R. Introduction quantitative X-ray diffraction methods in Introduction to X-ray powder diffraction for EPS400 1–15 (2012).
- 68.Hazardous Waste Consultant Using XRF technologies to monitor and measure inorganic elements in soil and sediment. Tech. Res. 2007;25:1.4–1.9. [Google Scholar]
- 69.ALS. Schedule of Services & Fees: Geochemistry. (2021).
- 70.Baranowski R, Rybak A, Baranowska I. Speciation analysis of elements in soil samples by XRF. Pol. J. Environ. Stud. 2002;11:473–482. [Google Scholar]
- 71.US EPA 3050B Acid digestion of sediments, sludges and soils. (U.S. Environmental Protection Agency (USEPA), Washington DC, 1996).
- 72.She J, Lu Z, Yao H, Fang R, Xian S. Experimental study on the swelling behavior of expansive soil at different depths under unidirectional seepage. Appl. Sci. 2019;9:1233. doi: 10.3390/app9061233. [DOI] [Google Scholar]
- 73.Abd-Allah AMA, Dawood YH, Awad SA, Agila WA. Mineralogical and chemical compositions of shallow marine clays, East of Cairo, Egypt: A geotechnical perception. J. King Abdulaziz Univ. Earth Sci. 2009;20:141–166. doi: 10.4197/Ear.20-1.8. [DOI] [Google Scholar]
- 74.Pedarla A, Acharya R, Bheemasetti T, Puppala AJ, Hoyos LR. Influence of mineral montmorillonite on soil suction modeling parameters of natural expansive clays. Indian Geotech. J. 2016;46:291–298. doi: 10.1007/s40098-015-0175-1. [DOI] [Google Scholar]
- 75.Grims RE. Properties of Clay in Recent Marine Sediments. Thos. Murby & Co.; 1935. [Google Scholar]
- 76.Grim RE. Modern concepts of clay materials. J. Geol. 1942;50:225–275. doi: 10.1086/625050. [DOI] [Google Scholar]
- 77.Wiegner G. Some physico-chemical properties of clays: I. Base exchange or ionic exchange. J. Soc. Chem. Ind. Trans. Commun. 1931;50:65T–74T. doi: 10.1002/jctb.5000500816. [DOI] [Google Scholar]
- 78.Bernal JD, Fowler RH. A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J. Chem. Phys. 1933;1:515–548. doi: 10.1063/1.1749327. [DOI] [Google Scholar]
- 79.Hendricks SB, Nelson RA, Alexander LT. Hydration mechanism of the clay mineral montmorillonite saturated with various cations. J. Am. Chem. Soc. 1940;62:1457–1464. doi: 10.1021/ja01863a037. [DOI] [Google Scholar]
- 80.Zhang X, Yi H, Zhao Y, Min F, Song S. Study on the differences of Na- and Ca-montmorillonites in crystalline swelling regime through molecular dynamics simulation. Adv. Powder Technol. 2016;27:779–785. doi: 10.1016/j.apt.2016.03.005. [DOI] [Google Scholar]
- 81.Houwink R. On the structure of the hydration hull of inorganic soil colloids: Zeitschr. Koll. 1937;93:110–114. [Google Scholar]
- 82.Liu J, Song S, Chen T, Li H, Zhao Y. Swelling capacity of montmorillonite in the presence of electrolytic ions. J. Dispers. Sci. Technol. 2016;37:380–385. doi: 10.1080/01932691.2015.1028070. [DOI] [Google Scholar]
- 83.Craster, B. Studies of the modification of ion and water movement through clays and shales (PhD Thesis, Sheffield Hallam University). http://shura.shu.ac.uk/19509/ (1999).
- 84.Watanabe T, Sato T. Expansion characteristics of montmorillonite and saponite under various relative humidity conditions. Clay Sci. 1988;7:129–138. [Google Scholar]
- 85.Bradley WF, Grim RE, Clark GL. A study of the behavior of montmorillonite upon wetting. Zeitschrift für Krist. Cryst. Mater. 2015;97:216–222. [Google Scholar]
- 86.Teich-McGoldrick SL, Greathouse JA, Jové-Colón CF, Cygan RT. Swelling properties of montmorillonite and beidellite clay minerals from molecular simulation: Comparison of temperature, interlayer cation, and charge location effects. J. Phys. Chem. C. 2015;119:20880–20891. doi: 10.1021/acs.jpcc.5b03253. [DOI] [Google Scholar]
- 87.Zha F, Liu S, Du Y, Cui K. Behavior of expansive soils stabilized with fly ash. Nat. Hazards. 2008;47:509–523. doi: 10.1007/s11069-008-9236-4. [DOI] [Google Scholar]
- 88.Alexander WR, et al. Assessing the long-term behaviour of the industrial bentonites employed in a repository for radioactive wastes by studying natural bentonites in the field. Geoscience. 2017;7:5. doi: 10.3390/geosciences7010005. [DOI] [Google Scholar]
- 89.JNC TN. Project to Establish the Scientific and Technical Basis for HLW Disposal in Japan. Japan Nuclear Cycle Development Institute, H12, JNC TN1410 2000-003. (2000).
- 90.Hakami BA, Seif ESSA. Expansive potentiality of sabkha soils of Rabigh Lagoon, Saudi Arabia: A case study. Arab. J. Geosci. 2019;12:107. doi: 10.1007/s12517-019-4271-x. [DOI] [Google Scholar]
- 91.Tripathy S, Sridharan A, Schanz T. (2004) Swelling pressures of compacted bentonites from diffuse double layer theory. Can. Geotech. J. 2004;41:437–450. doi: 10.1139/t03-096. [DOI] [Google Scholar]
- 92.Samingan AS. An Experimental Study on Hydro-Mechanical Characteristics of Compacted Bentonite-Sand Mixtures. Bauhaus-University; 2005. [Google Scholar]
- 93.Komine H. Simplified evaluation for swelling characteristics of bentonites. Eng. Geol. 2004;71:265–279. doi: 10.1016/S0013-7952(03)00140-6. [DOI] [Google Scholar]
- 94.Plötze M, Kahr G, Hermanns Stengele R. Alteration of clay minerals—gamma-irradiation effects on physicochemical properties. Appl. Clay Sci. 2003;23:195–202. doi: 10.1016/S0169-1317(03)00103-0. [DOI] [Google Scholar]
- 95.Villar MV, Lloret A. Influence of temperature on the hydro-mechanical behaviour of a compacted bentonite. Appl. Clay Sci. 2004;26:337–350. doi: 10.1016/j.clay.2003.12.026. [DOI] [Google Scholar]
- 96.Komine H, Yasuhara K, Murakami S. Swelling characteristics of bentonites in artificial seawater. Can. Geotech. J. 2009;46:177–189. doi: 10.1139/T08-120. [DOI] [Google Scholar]
- 97.Puppala AJ, Manosuthikij T, Chittoori BCS. Swell and shrinkage strain prediction models for expansive clays. Eng. Geol. 2014;168:1–8. doi: 10.1016/j.enggeo.2013.10.017. [DOI] [Google Scholar]
- 98.Abduljauwad SN. Study on the performance of calcareous expansive clays. Bull. Assoc. Eng. Geol. 1993;30:481–498. [Google Scholar]
- 99.Agus SS, Schanz T. A method for predicting swelling pressure of compacted bentonites. Acta Geotech. 2008;3:125–137. doi: 10.1007/s11440-008-0057-0. [DOI] [Google Scholar]
- 100.Li J, Cameron DA, Ren G. Case study and back analysis of a residential building damaged by expansive soils. Comput. Geotech. 2014;56:89–99. doi: 10.1016/j.compgeo.2013.11.005. [DOI] [Google Scholar]
- 101.Abduljauwad SN. Characteristics and chemical treatment of expansive clay in Al-Qatif, Saudi Arabia. Eng. Geol. 1991;31:143–158. doi: 10.1016/0013-7952(91)90003-4. [DOI] [Google Scholar]
- 102.Ufer K, et al. Quantitative phase analysis of bentonites by the Rietveld method. Clays Clay Miner. 2008;56:272–282. doi: 10.1346/CCMN.2008.0560210. [DOI] [Google Scholar]
- 103.Karnland, O. Chemical and Mineralogical Characterization of the Bentonite Procedure in a KBS-3 Repository. (2010).
- 104.Al-Rawas AA, Taha R, Nelson JD, Al-Shab BT, Al-Siyabi H. A comparative evaluation of various additives used in the stabilization of expansive soils. Geotech. Test. J. 2002;25:199–209. doi: 10.1520/GTJ11363J. [DOI] [Google Scholar]
- 105.Wen Z. Physical property of China’s buffer material for high-level radioactive waste repositories. J. Rock Mech. Eng. 2006;25:794–800. [Google Scholar]
- 106.Schanz T, Nguyen-Tuan L, Datcheva M. A column experiment to study the thermo-hydro-mechanical behaviour of expansive soils. Rock Mech. Rock Eng. 2013;46:1287–1301. doi: 10.1007/s00603-012-0361-8. [DOI] [Google Scholar]
- 107.Tang AM, Cui YJ, Barnel N. Thermo-mechanical behaviour of a compacted swelling clay. Géotechnique. 2008;58:45–54. doi: 10.1680/geot.2008.58.1.45. [DOI] [Google Scholar]
- 108.Majeed ZH, Taha MR, Jawad IT. Stabilization of soft soil using nanomaterials. Res. J. Appl. Sci. Eng. Technol. 2014;8:503–509. doi: 10.19026/rjaset.8.999. [DOI] [Google Scholar]