Abstract
Effects of the in ovo administration of vitamin D3 (D3) and 25-hydroxyvitamin D3 (25OHD3) on broiler intestinal lesion incidence, performance and breast meat yield after a coccidiosis challenge were investigated. On each of 10 incubator tray levels, 10 Ross 708 broiler hatching eggs were randomly assigned to each of the following 5 in ovo injection treatments administrated at 18 d of incubation (doi): 1) noninjected; 2) diluent; diluent containing either 3) 2.4 μg D3 (D3), 4) 2.4 μg 25OHD3 (25OHD3), or 5) 2.4 μg D3 + 2.4 μg 25OHD3 (D3+25OHD3). A 50 μL solution volume was injected into each egg using an Inovoject multi-egg injector. Four male chicks were randomly assigned to each of 80 battery cages in each of 2 rooms. Half of the treatment-replicate cages (8) in each room were challenged with a 20× live coccidial vaccine at 14 d of age (doa). One randomly selected bird from each of 4 treatment-replicate cages was scored for coccidiosis lesions before and 2 wk after challenge. Mean BW, BW gain (BWG), feed intake, and feed conversion ratio were determined for all birds from 0 to 14, 15 to 28, and 29 to 41 doa. Carcass weight, and the absolute and relative (% of carcass weight) weights of carcass parts were determined in 3 birds per treatment-replicate cage at 42 doa. Hatchability of live embryonated injected eggs and hatch residue were not affected by treatment. Across challenge treatment, birds in the 25OHD3 treatment group experienced an increase in BWG between 29 and 41 doa when compared to the D3 or diluent-injected birds. Furthermore, pectoralis major muscle percentage tended (P = 0.059) to increase in birds belonging to the 25OHD3 treatment in comparison to birds in the D3 or diluent-injected treatments. These results indicate that regardless of challenge treatment, 2.4 μg of 25OHD3 may increase the BWG and breast meat yield of birds relative to those that only received an injection of commercial diluent.
Key words: vitamin D source, in ovo injection, coccidiosis, broiler performance, breast meat yield
INTRODUCTION
Coccidiosis is the major parasitic disease affecting poultry and results in severe economic loss due to severe reductions in feed utilization and BW gain (Ritzi et al., 2014). Increased oxidative stress has been reported in birds infected by coccidiosis (Georgieva et al., 2006) which can lead to a reduction in the fat-soluble vitamin status, including that of vitamin D (Lee et al., 2018). Vitamin D3 is mainly absorbed via diffusion into enterocytes residing in the duodenum and upper jejunum (Borel, 2003), and is facilitated by the formation of aggregates called micelles, along with other lipophilic food components. These are then transported to the liver as portomicrons (Elaroussi et al., 1994; Cooke and Haddad, 1989). Vitamin D3 must undergo 2 sequential hydroxylation steps to become active. The first hydroxylation occurs through 25-hydroxylase activity in liver microsomes and mitochondria. This first hydroxylation produces 25-hydroxycholecalciferol (25OHD3). Later, 25OHD3 is hydroxylated to 1,25-dihydroxyvitamin D3 (1,25(OH)2 D3) by 1α-hydroxylase in the kidney (Henry, 1980).
Vitamin D3 sources are capable of accelerating calcium (Ca) absorption through increased calbindin activity (Bikle and Munson, 1985). Calbindin is involved in intestinal intracellular Ca transport and its expression occurs in the intestine and kidney. Calbindin activity in chickens is regulated by 1,25(OH)2 D3 (Hall and Norman, 1990; Ferrari et al., 1992). In comparison to vitamin D3 at the same level of inclusion, dietary 25OHD3 provided at a dosage of 69 μg/kg has been shown to lead to a greater increase in the expression of calbindin after 6 h (Hsiao et al., 2018). Although the enzyme 1 α-hydroxylase is mainly expressed in renal cells, it is also expressed in muscle and macrophage cells (Shanmugasundaram and Selvaraj, 2012), and its activity is known to cause the inhibition of Eimeria tenella replication (Morris and Selvaraj, 2014). It is well documented that dietary 25OHD3 reduces the proinflammatory response (IL-1β) and increases anti-inflammatory (IL-10) cytokine levels, leading to increases in the BW gain of layers during a coccidiosis infection (Morris et al., 2014). These results indicate that vitamin D3 sources have the potential to reduce the negative effects caused by a coccidiosis infection. Although the dietary effects of various forms of vitamin D3 on broiler performance during a coccidiosis infection are well understood, the influence of the in ovo injection of various vitamin D3 sources on the physiological attributes of broilers subjected to a coccidiosis infection have to-date not been investigated. Therefore, the objective of this study was to determine the effects of the in ovo administration of D3 and its metabolite, 25OHD3, on the incidence of intestinal lesions, and the performance and breast meat yield of broilers after a coccidiosis challenge.
MATERIAL AND METHODS
Experiment Design and Egg Incubation
This study was conducted according to a protocol (IACUC# 17-406) approved by the Institutional Animal Care and Use Committee of Mississippi State University. Fifty eggs were assigned to each of 5 preassigned treatment groups (trays) on each of 10 incubator tray levels (replicate blocks) in a single stage Chick Master Incubator (Chick Master Incubator Company, Medina, OH) set at 37.5°C dry bulb and 29°C wet bulb temperatures. The same incubator served as both a setter and hatcher unit. Positional effects were removed by re-randomizing all treatments between each incubator tray level. Incubator air temperature and relative humidity were recorded every 15 min using HOBO ZW Series wireless data loggers (Onset Computer Corporation, Bourne, MA) during the 21 d of incubation (doi) period. All eggs were candled at 12 and 18 doi, and percentage egg weight loss (PEWL) between 0 and 12 doi was determined. At 18 doi, 50 μL solution volumes of prespecified treatments were injected into eggs using a Zoetis Inovoject m (Zoetis Animal Health, Research Triangle Park, NC) in ovo injection machine. The in ovo injection treatments were: 1) noninjected; 2) diluent (control; 50 μL of commercial diluent); 3) D3 (50 μL of commercial diluent containing 2.4 μg D3); 4) 25OHD3 (50 μL of commercial diluent containing 2.4 μg 25OHD3); and 5) D3+25OHD3 (50 μL of commercial diluent containing 2.4 μg of D3 and 25OHD3). All in ovo injection solutions were prepared and injected according to the procedure of Fatemi et al. (2020a,b).
After injection, eggs were transferred to hatching baskets that were arranged in the hatcher unit to coincide with the arrangement of the trays for each respective treatment replicate in the setter unit. At hatch, all chicks belonging to a replicate basket in each treatment group were counted and weighed together to determine mean hatchling BW. Also, hatch residue analysis and the hatchability of injected live embryonated eggs (HI) were determined at 21 doi (502 h of incubation). Hatch residue was analyzed as described by Avakian (2006). Postinjection late, pip, post-pip, and hatchling mortalities were defined respectively, as those mortalities that occurred between 18 doi (432 h of incubation) and 21 doi (502 h of incubation) prior to pip, during the pipping process, after the pipping process, and immediately after complete emergence from the shell. All chicks were feather-sexed to select for male broilers in their prespecified treatment, and then male chicks from each replicate basket were pooled within their respective treatment group. Four male chicks were randomly selected from each pooled treatment group, and were weighed and placed in each of 8 replicate isolated wire-floored battery cages belonging to each treatment group in each of 2 separate rooms of a light-controlled research facility (320 total birds). Each battery cage measured 0.76 m × 0.46 m (0.35 m2). All birds received ad libitum access to water and a Mississippi State University basal corn-soybean diet formulated to meet Ross 708 commercial guidelines (Aviagen, 2014) throughout the 41 d of age (doa) period (Fatemi et al., 2021a; Table 1).
Table 1.
Feed composition of the experimental diets from 0 to 41 d of age (doa).
| Feed composition | Commercial diet |
|---|---|
| Starter (0–14 doa) | |
| Item | |
| Ingredient (%) | Pct |
| Yellow corn | 53.23 |
| Soybean meal | 38.23 |
| Animal fat | 2.6 |
| Dicalcium phosphate | 2.23 |
| Limestone | 1.27 |
| Salt | 0.34 |
| Choline chloride 60% | 0.10 |
| Lysine | 0.28 |
| DL-methionine | 0.37 |
| L-threonine | 0.15 |
| Premix1 | 0.25 |
| BMD2 | 0.05 |
| Total | 100 |
| Calculated nutrients | |
| Crude protein | 23 |
| Calcium | 0.96 |
| Available phosphorus | 0.48 |
| Apparent metabolizable energy (AME; Kcal/kg) | 3,000 |
| Digestible methionine | 0.51 |
| Digestible lysine | 1.28 |
| Digestible threonine | 0.86 |
| Digestible total sulfur amino acids (TSAA) | 0.95 |
| Sodium | 0.16 |
| Choline | 0.16 |
| Grower (15–28 doa) | |
| Item | |
| Ingredient (%) | Pct |
| Yellow corn | 57.13 |
| Soybean meal | 34.8 |
| Animal fat | 3.5 |
| Dicalcium phosphate | 2 |
| Limestone | 1.17 |
| Salt | 0.34 |
| Choline chloride 60% | 0.10 |
| Lysine | 0.21 |
| DL-methionine | 0.32 |
| L-threonine | 0.16 |
| Premix | 0.25 |
| BMD | 0.05 |
| Total | 100 |
| Calculated nutrients | |
| Crude protein | 21.5 |
| Calcium | 0.87 |
| Available phosphorus | 0.435 |
| AME (Kcal/kg) | 3,100 |
| Digestible methionine | 0.47 |
| Digestible lysine | 1.15 |
| Digestible threonine | 0.77 |
| Digestible TSAA | 0.87 |
| Sodium | 0.16 |
| Choline | 0.16 |
| Finisher (29–45 doa) | |
| Item | |
| Ingredient (%) | Pct |
| Yellow corn | 54.23 |
| Soybean meal | 38.23 |
| Animal fat | 2.5 |
| Dicalcium phosphate | 2.23 |
| Limestone | 1.27 |
| Salt | 0.34 |
| Choline chloride 60% | 0.10 |
| Lysine | 0.28 |
| DL-methionine | 0.37 |
| L-threonine | 0.15 |
| Premix | 0.25 |
| BMD | 0.05 |
| Total | 100 |
| Calculated nutrients | |
| Crude protein | 19.5 |
| Calcium | 0.78 |
| Available phosphorus | 0.39 |
| AME (Kcal/kg) | 3,200 |
| Digestible methionine | 0.43 |
| Digestible lysine | 1.02 |
| Digestible threonine | 0.68 |
| Digestible TSAA | 0.8 |
| Sodium | 0.16 |
| Choline | 0.16 |
The broiler premix provided per kilogram of diet: vitamin A (retinyl acetate), 10,000 IU; cholecalciferol, 250 IU; vitamin E (DL-α-tocopheryl acetate), 50 IU; vitamin K, 4.0 mg; thiamine mononitrate (B1), 4.0 mg; riboflavin (B2), 10 mg; pyridoxine HCL (B6), 5.0 mg; vitamin B12 (cobalamin), 0.02 mg; D-pantothenic acid, 15 mg; folic acid, 0.2 mg; niacin, 65 mg;biotin, 1.65 mg; iodine (ethylene diamine dihydroiodide), 1.65 mg; Mn (MnSO4H2O), 120 mg; Cu, 20 mg; Zn, 100 mg, Se, 0.3 mg; Fe (FeSO4.7H2O), 800 mg.
Bacitracin methylene disalicylate (BMD 110; Zoetis, Parsippany, NJ): containing 55 mg of BMD per kg.
Growth Performance
All birds were fed a starter diet from 0 to 14 doa, a grower diet from 15 to 28 doa, and a finisher diet from 29 to 41 doa. The BW, BW gain, average daily gain (ADG), feed intake (FI), and average daily FI (ADFI) of the birds on a pen basis were determined in each dietary phase. Percentage mortality and feed conversion ratio (FCR; g feed/g gain) adjusted for bird mortality, were calculated for the same time periods.
Challenge, and Lesion Score and Oocyst Counts
At 14 doa, the chicks that belonged to the diluent, D3, 25OHD3, and D3 + 25OHD3 treatment groups were challenged by oral gavage with a 20 × dose of a commercial coccidial vaccine containing live oocysts of Eimeria acervulina, maxima, mivati, and tenella (Coccivac-B52, Intervet Inc. Omaha, NE), that was diluted in 1 mL of distilled water. Coccidial lesions from E. acervulina and maxima in the duodenum, jejunum, and ileum, and overall lesion incidence in the ceca were determined at 14 and 28 doa as described by Johnson and Reid (1970). Fecal samples from each of the 8 replicate cages in each treatment group of each room that belonged to the diluent, D3, 25OHD3, and D3 + 25OHD3 treatment groups were collected for oocyst count analysis at 7 and 14 d post-coccidiosis challenge (21 and 28 doa, respectively). The sporulated oocysts were counted in each 1.0 mL of solution using the hemocytometer method described by Holdsworth et al. (2004). Fecal samples from 4 replicate cages in each treatment group of each room were also randomly collected at 21 and 28 doa from the noninjected and unchallenged treatment group for oocyst count analysis for comparative purpose in order to confirm success of the coccidiosis challenge.
Processing
The birds that remained in each pen (approximately 47 birds/treatment) were processed at 42 doa according to the method described by Wang et al. (2018). Weights of the whole carcass, and carcass parts including the pectoralis (P) major and P. minor muscles, and leg, thigh, and wing were determined. Parts yields were calculated as percentages of cold carcass weight.
Statistical Analysis
The experimental design was a randomized complete block for both the hatch and rearing periods. Incubator level in the setter and hatcher served as the unit of treatment replication for the hatch data, and battery cage as the unit of treatment replication for the performance, meat yield, and coccidiosis lesion scoring data. Room was the blocking factor for the grow out phase of the experiment. The noninjected control group was not subjected to a coccidiosis challenge at 14 doa, as were the 4 in ovo-injected treatment groups. Therefore, there were 5 in ovo injection treatments for the incubation and grow out periods from 0 to 14 doa, but there were only 4 in ovo injection treatments for the grow out period from 15 to 42 doa. Although a noninjected treatment group was kept in a separate part of the battery cages to eliminate their exposure to coccidial oocysts, we were not able to provide another replicate unit to simultaneously determine the effects of in ovo injection along with coccidial challenge. Therefore, for that reason, the noninjected treatment was not included in the statistical section for any analysis after coccidiosis challenge. All data were analyzed by one-way ANOVA using the procedure for general linear mixed models (PROC GLIMMIX) of SAS 9.4 (SAS Institute, Cary, NC). Differences were considered significant at P < 0.05. The following model was used for analysis of the incubation and posthatch data:
where μ was the population mean; Bi was the block factor (i = 1 or 2); Ti was the effect of each in ovo injection treatments (j = number of treatments); and Eij was the residual error.
RESULTS
No significant treatment differences (P > 0.05) were observed for egg weight, 0 to 12 doi PEWL, HI, hatchling BW, and hatch residue analysis (Table 2). There were also no significant (P > 0.05) treatment effects on the broiler performance variables of the coccidiosis-challenged broilers in the 0 to 14, 15 to 28, and 0 to 41 doa intervals (Table 3). However, coccidiosis-challenged birds injected in ovo with 2.4 μg of 25OHD3 had a higher (P > 0.05) BWG and ADG between 29 and 41 doa in comparison to those injected with diluent or D3 alone (Table 3).
Table 2.
Effects of treatment (noninjected; diluent-injected; injected with 2.4 μg of vitamin D3 (D3) or 25-hydroxycholecalciferol (25OHD3); and 2.4 μL of D3 and 25OHD3) on egg weight; percentage egg weight loss (PEWL) from 0 to 12 d of incubation (doi); hatchability of injected live embryonated (HI) eggs; hatchling BW; late, pip, and post-pip embryo mortalities; and hatchling mortality at 21 doi.
| In ovo injection treatment | N | Egg weight (g) | PEWL (%) | HI (%) | Hatchling BW (g) | Late embryo mortality1 (%) | Pip embryo mortality 2 (%) | Post-pip embryo mortality 3 (%) | Hatchling mortality 4 (%) |
|---|---|---|---|---|---|---|---|---|---|
| Noninjected5 | 10 | 60.8 | 6.7 | 96.3 | 42.9 | 1.1 | 1.7 | 0 | 0.6 |
| Diluent6 | 10 | 60.9 | 6.4 | 92.3 | 43.2 | 3.7 | 1.3 | 0 | 2.5 |
| D37 | 10 | 61.1 | 6.3 | 97.3 | 43.5 | 2.3 | 0 | 0 | 1.1 |
| 25OHD38 | 10 | 61.4 | 6.4 | 94.9 | 43.2 | 2.9 | 1.8 | 0.6 | 0 |
| D3+25OHD39 | 10 | 61.8 | 7.0 | 95.5 | 43.0 | 2.5 | 1.9 | 0 | 0 |
| Pooled SEM | 0.30 | 0.26 | 1.75 | 0.27 | 1.93 | 0.73 | 0.29 | 1.65 | |
| P-value | 0.171 | 0.243 | 0.304 | 0.427 | 0.747 | 0.340 | 0.445 | 0.555 |
Mortality between 18 doi (432 h of incubation) and 21 doi (502 h of incubation) prior to pip.
Mortality during the pipping process at 21 doi.
Mortality after the pipping process at 21 doi.
Mortality immediately after complete emergence of hatchlings from the shell at 21 doi.
Eggs that were not injected with diluent.
Eggs injected with 50 μL of commercial diluent at 18 doi.
Eggs injected with 50 μL of commercial diluent containing 2.4 μg of vitamin D3 at 18 doi.
Eggs injected with 50 μL of commercial diluent containing 2.4 μg of 25OHD3 at 18 doi.
Eggs injected with 50 μL of commercial diluent containing 2.4 μg of D3 and 2.4 μg of 25OHD3 at 18 doi.
Table 3.
Effects of treatment (noninjected; diluent-injected; injected with 2.4 μg of vitamin D3 (D3) or 25-hydroxycholecalciferol (25OHD3); and 2.4 μL of D3 and 25OHD3) BW, BW gain (BWG), average daily gain (ADG), feed intake (FI), average daily feed intake (ADFI), and total mortality through 41 d of age (doa).
| N | BW (g) | BWG1 (g) | ADG (g) | FI (g) | ADFI (g) | FCR (g/g) | ||
|---|---|---|---|---|---|---|---|---|
| In ovo injection treatment | Starter (0–14 doa) | |||||||
| Noninjected1 | 8 | 427 | 385 | 27.5 | 496 | 35.4 | 1.29 | |
| Diluent2 | 8 | 410 | 368 | 26.3 | 485 | 34.6 | 1.34 | |
| D33 | 8 | 405 | 363 | 25.9 | 488 | 34.8 | 1.35 | |
| 25OHD34 | 8 | 421 | 378 | 27.0 | 497 | 35.5 | 1.32 | |
| D3+25OHD35 | 8 | 419 | 376 | 26.9 | 513 | 36.6 | 1.37 | |
| Pooled SEM | 9.5 | 14.2 | 1.01 | 15.2 | 1.08 | 0.042 | ||
| P-value | 0.508 | 0.489 | 0.490 | 0.332 | 0.330 | 0.288 | ||
| BW (g) | BWG (g) | ADG (g) | FI (g) | ADFI (g) | FCR (g/g) | |||
| Grower (15–28 doa) | ||||||||
| Diluent | 8 | 1,390 | 980 | 70.0 | 1,623 | 116 | 1.67 | |
| D3 | 8 | 1,398 | 993 | 70.9 | 1,746 | 125 | 1.79 | |
| 25OHD3 | 8 | 1,469 | 1048 | 74.8 | 1,702 | 122 | 1.63 | |
| D3+25OHD3 | 8 | 1,434 | 1014 | 72.5 | 1,750 | 125 | 1.74 | |
| Pooled SEM | 31.5 | 28.1 | 2.00 | 67.2 | 4.8 | 0.081 | ||
| P-value | 0.282 | 0.353 | 0.353 | 0.513 | 0.512 | 0.518 | ||
| BW (g) | BWG (g) | ADG (g) | FI (g) | ADFI (g) | FCR (g/g) | |||
| Finisher (29–41 doa) | ||||||||
| Diluent | 8 | 2,925 | 1,523b | 117b | 2,870 | 122 | 1.93 | |
| D3 | 8 | 2,943 | 1,544b | 119b | 2,954 | 127 | 1.91 | |
| 25OHD3 | 8 | 3,096 | 1,665a | 128a | 3,032 | 128 | 1.83 | |
| D3+25OHD3 | 8 | 3,054 | 1,620ab | 125ab | 3,041 | 129 | 1.88 | |
| Pooled SEM | 80.4 | 51.2 | 3.9 | 115.2 | 3.7 | 0.091 | ||
| P-value | 0.098 | 0.030 | 0.030 | 0.578 | 0.578 | 0.730 | ||
| BW (g) | BWG (g) | ADG (g) | FI (g) | ADFI (g) | FCR (g/g) | |||
| 0–41 doa | ||||||||
| Diluent | 8 | 2,925 | 2,881 | 70.3 | 4977 | 226 | 1.70 | |
| D3 | 8 | 2,943 | 2,900 | 70.7 | 5188 | 227 | 1.76 | |
| 25OHD3 | 8 | 3,096 | 3,053 | 74.5 | 5232 | 233 | 1.68 | |
| D3+25OHD3 | 8 | 3,054 | 3,011 | 73.4 | 5304 | 234 | 1.74 | |
| Pooled SEM | 80.4 | 83.0 | 1.43 | 145.5 | 5.0 | 0.063 | ||
| P-value | 0.098 | 0.100 | 0.100 | 0.431 | 0.578 | 0.741 | ||
Treatment means within the same column within effect with no common superscripts are significantly different (P < 0.05).
Eggs that were not injected with diluent and that were also not challenged with coccidiosis at 14 doa.
Eggs injected with 50 μL of commercial diluent at d 18 of incubation (doi) and the subsequent coccidiosis challenge of chicks at 14 doa.
Eggs injected with 50 μL of commercial diluent containing 2.4 μg of vitamin D3 at 18 doi and the subsequent coccidiosis challenge of chicks at 14 doa.
Eggs injected with 50 μL of commercial diluent containing 2.4 μg of 25OHD3 at 18 doi and the subsequent coccidiosis challenge of chicks at 14 doa.
Eggs injected with of 50 μL of commercial diluent containing 2.4 μg of D3 and 2.4 μg of 25OHD3 at 18 doi and the subsequent coccidiosis challenge of chicks at 14 doa.
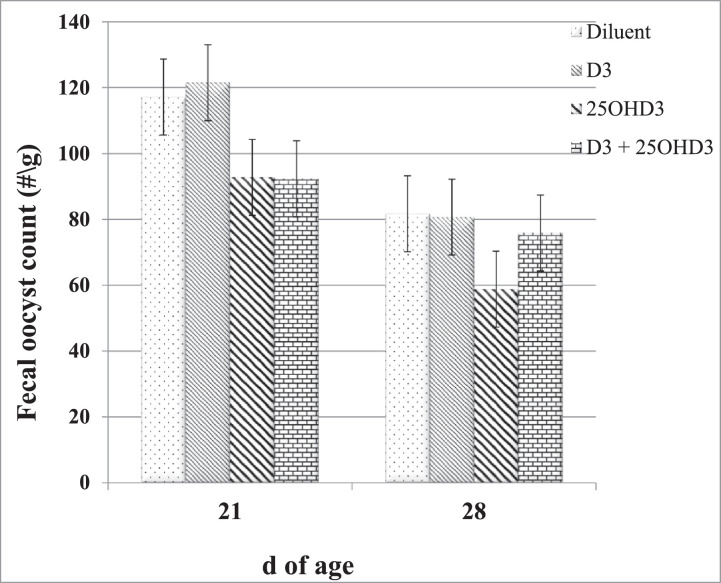
No coccidiosis lesions (P > 0.05) were observed in all intestinal sections before challenge at 14 doa. There were also no lesions in the ceca and no E. acervulina lesions in the ileum at 28 doa. Furthermore, at 28 doa, there were no significant (P > 0.05) treatment differences for E. acervulina and maxima lesion scores in the duodenum and jejunum, and there were no significant (P > 0.05) treatment differences for E. acervulina lesion scores in the ileum (Table 4). No coccidia oocysts were observed (P > 0.05) in the fecal samples taken from the noninjected and unchallenged birds at 21 (7 d post-coccidiosis challenge) and 28 doa (14 d post-coccidiosis challenge). Conversely, in challenged birds, fecal oocyst counts ranged from 93 to 121 per g of feces at 21 doa and from 59 to 81 per g of feces at 28 doa, depending on in ovo injection treatment (Figure 1). Nevertheless, there were no significant (P > 0.05) in ovo injection treatment differences for the fecal coccidia oocyst counts at both 7 and 14 d post-coccidiosis challenge (Figure 1).
Table 4.
Effects of treatment (noninjected; diluent-injected; injected with 2.4 μg of vitamin D3 (D3) or 25-hydroxycholecalciferol (25OHD3); and 2.4 μL of D3 and 25OHD3) on Emeria acervulina and maxima lesion scores in the duodenum (D), jejunum (J), and ileum (I), and overall lesion scores in the ceca at 28 d of age (doa).
| In ovo injection treatment | N | Acervulina-D1 | Maxima-D2 | Acervulina-J3 | Maxima-J4 | Acervulina-I5,7 | Maxima-I6 | Ceca7 |
|---|---|---|---|---|---|---|---|---|
| Diluent8 | 8 | 2.50 | 0 | 0.13 | 2.88 | - | 0.25 | - |
| D39 | 8 | 3.00 | 0.13 | 0.25 | 2.38 | - | 0.13 | - |
| 25OHD310 | 8 | 1.75 | 0.13 | 0.25 | 1.25 | - | 0.50 | - |
| D3+25OHD311 | 8 | 1.75 | 0 | 0.13 | 1.75 | - | 0.38 | - |
| Pooled SEM | 0.895 | 0.096 | 0.201 | 0.628 | - | 0.194 | - | |
| P-value | 0.520 | 0.546 | 0.942 | 0.304 | - | 0.566 | - |
Eimeria acervulina lesion score in the duodenum at 14 d of post-coccidiosis challenge.
Eimeria maxima lesion score in the duodenum at 14 d of post-coccidiosis challenge.
Eimeria acervulina lesion score in the jejunum at 14 d of post-coccidiosis challenge.
Eimeria maxima lesion score in the jejunum at 14 d of post-coccidiosis challenge.
Eimeria acervulina lesion score in the ileum at 14 d of post-coccidiosis challenge.
Eimeria maxima lesion score in the ileum at 14 d of post-coccidiosis challenge.
No coccidiosis lesions were observed.
Eggs injected with 50 μL of commercial diluent at d 18 of incubation (doi) and the subsequent coccidiosis challenge of chicks at 14 doa.
Eggs injected with 50 μL of commercial diluent containing 2.4 μg of vitamin D3 at 18 doi and the subsequent coccidiosis challenge of chicks at 14 doa.
Eggs injected with 50 μL of commercial diluent containing 2.4 μg of 25OHD3 at 18 doi and the subsequent coccidiosis challenge of chicks at 14 doa.
Eggs injected with of 50 μL of commercial diluent containing 2.4 μg of D3 and 2.4 μg of 25OHD3 at 18 doi and the subsequent coccidiosis challenge of chicks at 14 doa.
Figure 1.
Effects of 50 μL volume treatment (noninjected; diluent-injected; injected with 2.4 μg of vitamin D3 (D3) or 25-hydroxycholecalciferol (25OHD3); and 2.4 μL of D3 and 25OHD3 on fecal oocyst counts at 7 and 14 d post-coccidiosis challenge (21 and 28 d of age [doa]). P values: 21 doa, P = 0.153; 28 doa, P = 0.235.
Absolute carcass weight and the relative weights of the P. major and P. minor muscles, as well as the breast, wings, legs, thighs, and abdominal fat pad process parts of birds challenged with coccidiosis, were not significantly affected (P > 0.05) by in ovo injection treatment. However, the effects of in ovo injection treatment approached significance for absolute carcass weight (P = 0.058) and relative P. major muscle weight (P = 0.059). Coccidiosis-challenged birds that received 25OHD3 alone tended to have a higher carcass weight in comparison to those that were injected with diluent or D3 alone. Additionally, in ovo injection of 25OHD3 alone tended to increase relative P. major muscle weight when compared to the injection of diluent or D3 alone (Table 5).
Table 5.
Effects of treatment (noninjected; diluent-injected; injected with 2.4 μg of vitamin D3 (D3) or 25-hydroxycholecalciferol (25OHD3); and 2.4 μL D3 and 25OHD3) on absolute carcass weight, and weights of pectoralis major (P-major) and minor (P-minor) muscle, breast, wing, leg, thighs, and abdominal fat pad parts relative to carcass weight at 42 d of age (doa).
| In ovo injection treatment | N | Carcass (kg) | P-major (%) | P-minor (%) | Breast (%) | Wings (%) | Legs (%) | Thighs (%) | Fat (%) |
|---|---|---|---|---|---|---|---|---|---|
| Diluent1 | 47 | 2,062 | 28.2 | 5.72 | 33.9 | 10.7 | 13.5 | 17.4 | 1.54 |
| D32 | 47 | 2,072 | 28.1 | 5.78 | 33.9 | 10.5 | 13.4 | 17.4 | 1.61 |
| 25OHD33 | 47 | 2,168 | 30.0 | 5.85 | 35.9 | 10.6 | 13.4 | 17.3 | 1.55 |
| D3+25OHD34 | 47 | 2,153 | 29.2 | 5.64 | 34.8 | 10.3 | 13.1 | 16.7 | 1.58 |
| Pooled SEM | 48.0 | 2.87 | 0.130 | 0.89 | 0.19 | 0.25 | 0.36 | 0.083 | |
| P-value | 0.058 | 0.059 | 0.540 | 0.085 | 0.563 | 0.632 | 0.434 | 0.937 |
Eggs injected with 50 μL of commercial diluent at d 18 of incubation (doi) and the subsequent coccidiosis challenge of chicks at 14 doa.
Eggs injected with 50 μL of commercial diluent containing 2.4 μg of vitamin D3 at 18 doi and the subsequent coccidiosis challenge of chicks at 14 doa.
Eggs injected with 50 μL of commercial diluent containing 2.4 μg of 25OHD3 at 18 doi and the subsequent coccidiosis challenge of chicks at 14 doa.
Eggs injected with of 50 μL of commercial diluent containing 2.4 μg of D3 and 2.4 μg of 25OHD3 at 18 doi and the subsequent coccidiosis challenge of chicks at 14 doa.
DISCUSSION
The fat absorption in the small intestine is reduced during a coccidiosis infection (Adams et al., 1996). In addition, liver functionality is reduced in response to severe Emeria infections (Ali, 1997). Vitamin D3 is categorized as a fat soluble vitamin whose absorption is facilitated by the formation of micelles and the presence of bile salts (Garrett and Young, 1975). Vitamin D3 is predominantly converted to 25OHD3 in the hepatic cells (Booth et al., 1985), with smaller rates of conversion occurring in the intestine, kidney (Norman, 1987), and skin (Hansdottir et al., 2008), in response to 25-hydroxlase. This information indicates that fat soluble vitamin requirements may increase during a coccidiosis infection. Coccidiosis is a parasitic disease, mainly affecting the intestinal tract of many species, including chickens. Subclinical coccidiosis results in decreases in BW and feed intake, and increases in the FCR of broiler chickens (Amerah and Ravindran, 2015). Coccidiosis has also been shown to inhibit small intestine morphological development (Sharma et al., 2015), decease cellular immunity (Morris et al., 2015), and increase inflammatory responses (Morris et al., 2014) in chickens. A decline in small intestine morphological development in response to a coccidiosis infection is associated with impaired broiler performance (Wang et al., 2019). In addition to this, a lower BWG due to a coccidiosis infection has been linked to an increase in the inflammatory response of broilers (Morris et al., 2014).
Comparison of the fecal oocyst counts between the unchallenged and challenged birds showed that under the housing conditions in this study, fecal oocysts were only observed in those birds that received a coccidiosis vaccine challenge, and that overall counts across injection treatment were reduced between 21 and 28 doa, indicating a lack of oocyst cycling. These current results reflect those of Shanmugasundaram et al. (2019), whose likewise observed similar fecal oocyst counts in turkeys that had received on oral coccidiosis vaccine and were housed in suspended cages. Conversely, Sokale et al. (2016) observed that the fecal oocyst shedding continued when birds were house in floor pens containing used litter.
Shanmugasundaram et al. (2019) further reported that 25OHD3 at a 110 μg/kg level reduced fecal oocyst counts 5 d after a coccidial vaccine challenge in turkeys. The occurrence of fewer oocysts in the feces has been shown to be associated with a decrease in coccidiosis lesion scores and improved broiler performance (Ritzi et al., 2014). Nevertheless, vitamin D-injection treatment did not significantly affect fecal oocyst counts at 7 or 14 d post-challenge. More specifically, the in ovo injection of either D3 or 25OHD3 did not affect oocyst shedding at both 7 and 14 doi. The differing results between the current and previous studies could be due the different methods of 25OHD3 administration (in ovo injection vs. dietary supplementation), differences in the levels of administered of 25OHD3 (5 μg vs. 110 μg), and differences in the species of bird (broilers vs. turkeys) used. Among the various vitamin D3 sources, 25OHD3 has been reported to be the more potent and safer form for chickens, because it has a longer half-life (approximately 15 d) in comparison to the other forms of vitamin D3 (Mawer et al., 1969; Jones et al., 2014). It is also less toxic in comparison to 1,25(OH)2 D3 (Pesti and Shivaprasad, 2010), and does not require liver hydroxylation. In comparison to D3, 25OHD3 is more efficiently absorbed due to its greater polarity (Bar et al., 1980), and at the same level of inclusion, 25OHD3 has been shown to better promote performance (Yarger et al., 1995), protein synthesis, and breast muscle yield (Vignale et al., 2015; Fatemi, 2016) in broilers. Furthermore, dietary 25OHD3 has been reported to increase the BWG of broilers challenged with coccidiosis (Morris et al., 2014; Leyva-Jimenez et al., 2019). In addition, when compared to the in ovo injection of D3 or diluent alone, the in ovo injection of 2.4 of μg of 25OHD3 has been shown to increase the breast meat yield (Fatemi et al., 2021a,b) and improve the live performance (Fatemi et al., 2021a) of Ross 708 broilers. This same treatment has also been shown to comparatively improve small intestine morphology (Fatemi et al., 2021c) and immunity (Fatemi et al., 2021a,c) of the broilers. In the current study, the in ovo injection of 25OHD3 resulted in an increase in the BWG and ADG of Ross 708 broilers when compared to the injection of diluent alone. Therefore, improvements in the performance of the Ross 708 broilers in response to the in ovo injection of 25OHD3 may be due to its moderation of the negative effects caused by coccidiosis.
In agreement with the results of this study, a coccidiosis challenge has been shown to result in impaired broiler performance (Amerah and Ravindran, 2015; Wang et al., 2019). In addition to its effects on performance, a reduction in breast meat yield of coccidiosis-challenged birds was observed in this study. Wang et al. (2019) reported that reductions in the breast meat yield of coccidiosis-challenged broilers can be linked to decreases in their intestinal villus height to crypt depth ratios (VCR). The small intestine morphological findings observed in studies in which coccidiosis-unchallenged (Fatemi et al., 2021c) and coccidiosis-challenged (unpublished data) birds were used, revealed that the in ovo injection of 25OHD3 increased their VCR in comparison to the in ovo injection of diluent or D3 alone. Thus, the improvement in small intestine morphology might have been a partial reason for the increase in the BWG and breast meat yield of the broilers that received 25OHD3 alone during a coccidiosis challenge. In addition to small intestine morphology, a reduction in meat yield caused by coccidiosis can be due to changes in breast muscle histomorphology. A subclinical Eimeria infection has been observed to result in a decrease in muscle fiber cross-sectional area (Chodová et al., 2018) and an increase in plasma levels of 3-methyl histidine, which is associated with muscle breakdown (Fetterer and Allen, 2001). Dietary 25OHD3 has been shown to increase muscle fiber cross-sectional area (Hutton et al., 2014), which can subsequently result in an increase in breast meat yield (Vignale et al., 2015) in broilers. In chickens, 1α-hydroxylase and 24-hydroxylase are both expressed in high amounts (Shanmugasundaram and Selvaraj, 2012), with 1α-hydroxylase converting 25OHD3 to the active hormone, 1,25(OH)2 D3. Subsequently, 1,25(OH)2 D3 is converted to the inactive form of vitamin D, 24,25-dihydroxyvitamin D3, by 24-hydroxylase (Jones et al., 2012). Jones et al. (2012) further reported that the expression of 1α-hydroxylase remained constant, whereas the expression of 24-hydroxylase was reduced in chicken breast muscle during an inflammatory response. These results indicated that 25OHD3 has a greater impact on breast meat yield in comparison to the in ovo injection of D3 alone.
In conclusion, the impact of the in ovo injection of 2.4 μg of D3 and 25OHD3 on broiler performance and meat yield of Ross 708 broilers before and after a coccidiosis challenge was investigated. Our findings revealed that a coccidiosis challenge resulted in a decline in broiler performance and to some extend a decrease in breast meat yield. Nevertheless, regardless of challenge treatment, 2.4 μg of 25OHD3 exhibited a potential to increase the BWG and breast meat yield of birds relative to those that only received an injection of commercial diluent or D3 alone. The improvement in breast meat yield and performance observed in response to the in ovo injection of 2.4 μg of 25OHD3 may be due to its longer half-life, the greater expression of 1α-hydroxylase than 24-hydroxylase in breast meat tissue, and improvements in Ross 708 broiler immunity and small intestine morphology during a coccidiosis challenge. Further research is required to determine effects of the in ovo injection of vitamin D3 sources on immunity, small intestine morphology and gene expression of broilers during a coccidiosis challenge.
ACKNOWLEDGMENTS
We express our appreciation for the financial support of the United States Department of Agriculture (USDA grant no. 58-6406-4-016), DSM Nutritional Products Inc., Zoetis Animal Health Co., Merial Select Inc., and for the assistance of the graduate and undergraduate students of the Mississippi State University Poultry Science Department . Special thanks to Dr. Bradley Turner and Dr. April Levy for their invaluable assistance.
DISCLOSURES
There is no conflict of interest.
Footnotes
This publication is contribution of the Mississippi Agriculture and Forestry Experiment Station.
2This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under accession number 1011797.
3Use of trade names in this publication does not imply endorsement by Mississippi Agricultural and Forestry Experiment Station of these products, nor similar ones not mentioned.
REFERENCES
- Adams C., Vahl H.A., Veldman A. Interaction between nutrition and Eimeria acervulina infection in broiler chickens: development of an experimental infection model. Br. J. Nutr. 1996;75:867–873. doi: 10.1079/bjn19960192. [DOI] [PubMed] [Google Scholar]
- Ali B.H. The hepatic and duodenal activities of some drug metabolizing enzymes in chickens: influence of infection with Escherichia coli endotoxin and coccidiosis. Eur. J. Drug Metab. Pharmacokinet. 1997;22:223–227. doi: 10.1007/BF03189811. [DOI] [PubMed] [Google Scholar]
- Amerah A.M., Ravindran V. Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poult. Sci. 2015;94:673–680. doi: 10.3382/ps/pev022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avakian A.P. Understanding in ovo vaccination. Int. Hatchery Pract. 2006;20:15–17. [Google Scholar]
- Aviagen . Aviagen Ltd.; Newbridge, UK: 2014. Ross 708 Broiler Nutrition Specification. [Google Scholar]
- Bar A., Sharvit M., Noff D., Edelstein S., Hurwitz S. Absorption and excretion of cholecalciferol and of 25-hydroxycholecalciferol and metabolites in birds. J. Nutr. 1980;110:1930–1934. doi: 10.1093/jn/110.10.1930. [DOI] [PubMed] [Google Scholar]
- Bikle D.D, Munson S. 1,25-Dihydroxyvitamin D increases calmodulin binding to specific proteins in the chick duodenal brush border membrane. J. Clin. Invest. 1985;76:2312–2316. doi: 10.1172/JCI112241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B.E., Tsai H.C., Morris R.C., Jr. Vitamin D status regulates 25-hydroxyvitamin D3-1 alpha-hydroxylase and its responsiveness to parathyroid hormone in the chick. J. Clin. Invest. 1985;75:155–161. doi: 10.1172/JCI111668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel P. Factors affecting intestinal absorption of highly lipophilic food microconstituents (fat-soluble vitamins, carotenoids and phytosterols) Clin. Chem. Lab. Med. 2003;41:979–994. doi: 10.1515/CCLM.2003.151. [DOI] [PubMed] [Google Scholar]
- Chodová D., Tůmová E., Sládková K., Langrová I., Jankovská I., Vadlejch J., Čadková Z., Krejčířová R. Effects of subclinical Eimeria tenella infection on pectoralis major muscle in broiler chickens. Ital. J. Anim. Sci. 2018;17:18–21. [Google Scholar]
- Cooke N.E., Haddad J.G. Vitamin D binding protein (Gc-Globulin) Endocrinol. Rev. 1989;10:294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- Elaroussi M.A., Prahl J.M., DeLuca H.F. The avian vitamin D receptors: primary structures and their origins. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11596–11600. doi: 10.1073/pnas.91.24.11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi, S. A. 2016. Effects of dietary 25-hydroxycholecalciferol and vitamin D3 on performance, meat yield, bone characteristics, innate immune response and gene expression of Ross 308 broilers grown on reused or fresh litter. M.Sc. Diss. Univ. Alberta, Edmonton, Canada.
- Fatemi S.A., Elliott K.E.C., Bello A., Durojaye O., Zhang H., Turner B., Peebles E.D. Effects of different levels of in ovo-injected vitamin D sources on the hatchability and serum 25-hydroxycholecalciferol concentrations of Ross 708 broilers. Poult. Sci. 2020;99:3877–3884. doi: 10.1016/j.psj.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.A., Elliott K.E.C., Bello A., Durojaye O., Zhang H., Turner B., Peebles E.D. Effects of the in ovo injection of vitamin D3 and 25-hydroxyvitamin D3 in Ross 708 broilers subsequently fed commercial or calcium and phosphorous-restricted diets: I. Performance, carcass characteristics, and incidence of woody breast myopathy. Poult. Sci. 2020;99:1357–1362. doi: 10.1016/j.psj.2021.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.A., Alqhtani A., Elliott K.E.C., Bello A., Zhang H., Levy A.W., Peebles E.D. Improvement in the performance and inflammatory reaction of Ross 708 broilers in response to the in ovo injection of 25-hydroxyvitamin D3. Poult. Sci. 2021;100:138–146. doi: 10.1016/j.psj.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.A., Elliott K.E.C., Bello A., Zhang H., Alqhtani A., A., Peebles E.D. Effects of the in ovo injection of vitamin D3 and 25-hydroxyvitamin D3 in Ross 708 broilers subsequently fed commercial or calcium and phosphorous-restricted diets: I. Performance, carcass characteristics, and incidence of woody breast myopathy. Poult. Sci. 2021;100:101220. doi: 10.1016/j.psj.2021.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.A., Elliott K.E.C., Bello A., Zhang H., Peebles E.D. Effects of the in ovo injection of vitamin D3 and 25-hydroxyvitamin D3 in Ross 708 broilers subsequently fed commercial or calcium and phosphorous-restricted diets: II. Immunity and small intestine morphology. Poult. Sci. 2021;100:101240. doi: 10.1016/j.psj.2021.101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Molinari S., Battini R., Cossu G., Lamon-Fava S. Induction of calbindin-D28k by 1,25-dihydroxyvitamin D3 in cultured chicken intestinal cells. Exp. Cell Res. 1992;200:528–531. doi: 10.1016/0014-4827(92)90205-m. [DOI] [PubMed] [Google Scholar]
- Fetterer R.H., Allen P.C. Eimeria tenella infection in chickens: effect on plasma and muscle 3-methylhistidine. Poult. Sci. 2001;80:1549–1553. doi: 10.1093/ps/80.11.1549. [DOI] [PubMed] [Google Scholar]
- Garrett R.L., Young R.J. Effect of micelle formation on the absorption of neutral fat and fatty acids by the chicken. J. Nutr. 1975;105:827–838. doi: 10.1093/jn/105.7.827. [DOI] [PubMed] [Google Scholar]
- Georgieva N.V., Koinarski V., Gadjeva V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J. 2006;172:488–492. doi: 10.1016/j.tvjl.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Hall A.K., Norman A.W. Regulation of calbindin-D28K gene expression in the chick intestine: effects of serum calcium status and 1,25-dihydroxyvitamin D. J. Bone Mineral Res. 1990;5:331–336. doi: 10.1002/jbmr.5650050405. [DOI] [PubMed] [Google Scholar]
- Hansdottir S., Monick M.M., Hinde S.L., Lovan N., Look D.C., Hunninghake G.W. Respiratory epithelial cells covert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry H.L. Measurement of the chicken kidney 25-hydroxyvitamin D3 1-hydroxylase and 25-hydroxyvitamin D3 24-hydroxylase. Methods Enzymol. 1980;67:445–449. doi: 10.1016/s0076-6879(80)67054-2. [DOI] [PubMed] [Google Scholar]
- Holdsworth P.A., Conway D.P., McKenzie M.E., Dayton A.D., Chapman H.D., Mathis G.F., Skinner J.T., Mundt H.C., Williams R.B. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet. Parasitol. 2004;121:189–212. doi: 10.1016/j.vetpar.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hsiao F.Shih-Hsiang, Cheng Yeong-His., ang Jin-Cheng.Han, Chang Ming-Huang., Yu Yu-Hsiang. Effect of different vitamin D3 metabolites on intestinal calcium homeostasis-related gene expression in broiler chickens. R. Bras. Zootec. 2018;47 [Google Scholar]
- Hutton K.C., Vaughn M.A., Litta G., Turner B.J., Starkey J.D. Effect of vitamin D status improvement with 25-hydroxycholecalciferol on skeletal muscle growth characteristics and satellite cell activity in broiler chickens. J. Anim. Sci. 2014;92:3291–3299. doi: 10.2527/jas.2013-7193. [DOI] [PubMed] [Google Scholar]
- Johnson J.K., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Jones K.S., Assar S., Harnpanich D., Bouillon R., Lambrechts D., Prentice A., Schoenmakers I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J. Clin. Endocrinol. Metab. 2014;99:3373–3381. doi: 10.1210/jc.2014-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G., Prosser D.E., Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Lee W.C., Mokhtar S.S., Munisamy S., Yahaya S., Rasool A.H.G. Vitamin D status and oxidative stress in diabetes mellitus. Cell. Mol. Biol. 2018;64:60–69. [PubMed] [Google Scholar]
- Leyva-Jimenez H., Gardner K., AL-Jumaa Y., Padgett J.C., Bailey C.A. Partial replacement of dietary cholecalciferol with 25-hydroxycholecalciferol on broiler chickens subjected to a coccidiosis vaccine challenge. J. Appl. Poult. Res. 2019;0:1–12. [Google Scholar]
- Mawer E.B., Lumb G.A., Stanbury S.W. Long biological half-life of vitamin D3 and its polar metabolites in human serum. Nature. 1969;222:482–483. doi: 10.1038/222482a0. [DOI] [PubMed] [Google Scholar]
- Morris A., Shanmugasundaram R., Lilburn M.S., Selvaraj R.K. 25 Hydroxycholecalciferol supplementation improves growth performance and decreases inflammation during an experimental lipopolysaccharide injection. Poult. Sci. 2014;93:1951–1956. doi: 10.3382/ps.2014-03939. [DOI] [PubMed] [Google Scholar]
- Morris A., Shanmugasundaram R., McDonald J., Selvaraj R.K. Effect of in vitro and in vivo 25-hydroxyvitamin D treatment on macrophages, T cells, and layer chickens during a coccidia challenge. J Anim. Sci. 2015;93:2894–2903. doi: 10.2527/jas.2014-8866. [DOI] [PubMed] [Google Scholar]
- Norman A.W. Studies on the vitamin D endocrine system in the avian. J. Nutr. 1987;117:797–807. doi: 10.1093/jn/117.4.797. [DOI] [PubMed] [Google Scholar]
- Pesti G.M., Shivaprasad H.L. The influence of excessive levels of 1α-hydroxycholecalciferol on the growth and tissue appearance of market weight chickens. J. Appl. Poult. Res. 2010;19:349–353. [Google Scholar]
- Ritzi M.M., Abdelrahman W., Mohnl M., Dalloul R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014;93:2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram R., Morris A., Selvaraj R.K. Effect of 25-hydroxycholecalciferol supplementation on turkey performance and immune cell parameters in a coccidial infection model. Poult Sci. 2019;98:1127–1133. doi: 10.3382/ps/pey480. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram R., Selvaraj R.K. Vitamin D-1alpha-hydroxylase and vitamin D-24-hydroxylase mRNA studies in chickens. Poult. Sci. 2012;91:1819–1824. doi: 10.3382/ps.2011-02129. [DOI] [PubMed] [Google Scholar]
- Sharma S., Azmi S., Iqbal A., Nasirudullah N., Mushtaq I. Pathomorphological alterations associated with chicken coccidiosis in Jammu division of India. J. Parasit. Dis. 2015;39:147–151. doi: 10.1007/s12639-013-0302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokale A.O., Zhai W., Pote L.M., Williams C.J., Peebles E.D. Effects of coccidiosis vaccination administered by in ovo injection on the hatchability and hatching chick quality of broilers. Poult. Sci. 2016;96:541–547. doi: 10.3382/ps/pew370. [DOI] [PubMed] [Google Scholar]
- Vignale K., Greene E.S., Caldas J.V., England J., Boonsinchai N., Sodsee P., Pollock E.D., Dridi S., Coon C.N. 25-Hydroxycholecalciferol enhances male broiler breast meat yield through the mTOR pathway. J. Nutr. 2015;145:855–863. doi: 10.3945/jn.114.207936. [DOI] [PubMed] [Google Scholar]
- Wang X., Peebles E.D., Kiess A.S., Wamsley K.G.S., Zhai W. Effects of coccidial vaccination and dietary antimicrobial alternatives on the growth performance, internal organ development, and intestinal morphology of Eimeria-challenged male broilers. Poult. Sci. 2019;98:2054–2065. doi: 10.3382/ps/pey552. [DOI] [PubMed] [Google Scholar]
- Wang X., Kiess A.S., Peebles E.D., Wamsley K.G.S., Zhai W. Effects of Bacillus subtilis and zinc on the growth performance, internal organ development, and intestinal morphology of male broilers with or without subclinical coccidia challenge. Poult. Sci. 2018;97:3947–3956. doi: 10.3382/ps/pey262. [DOI] [PubMed] [Google Scholar]
- Yarger J.G., Quarles C.L., Hollis B.W., Gray R.W. Safety of 25-hydroxycholecalciferol as a source of cholecalciferol in poultry rations. Poult. Sci. 1995;74:1437–1446. doi: 10.3382/ps.0741437. [DOI] [PubMed] [Google Scholar]