Abstract
Cellular therapy exerts profound therapeutic potential for curing a broad spectrum of diseases. Adult stem cells reside within a specified dynamic niche in vivo, which is essential for continuous tissue homeostatic maintenance through balancing self-renewal with lineage selection. Meanwhile, adult stem cells may be multipotent or unipotent, and are present in both quiescent and actively dividing states in vivo of the mammalians, which may switch to each other state in response to biophysical cues through mitochondria-mediated mechanisms, such as alterations in mitochondrial respiration and metabolism. In general, stem cells facilitate tissue repair after tissue-specific homing through various mechanisms, including immunomodulation of local microenvironment, differentiation into functional cells, cell “empowerment” via paracrine secretion, immunoregulation, and intercellular mitochondrial transfer. Interestingly, cell-source-specific features have been reported between different tissue-derived adult stem cells with distinct functional properties due to the different microenvironments in vivo, as well as differential functional properties in different tissue-derived stem cell-derived extracellular vehicles, mitochondrial metabolism, and mitochondrial transfer capacity. Here, we summarized the current understanding on roles of mitochondrial dynamics during stem cell homeostasis and aging, and lineage-specific differentiation. Also, we proposed potential unique mitochondrial molecular signature features between different source-derived stem cells and potential associations between stem cell aging and mitochondria–endoplasmic reticulum (ER) communication, as well as potential novel strategies for anti-aging intervention and healthy aging.
Subject terms: Energy metabolism, Stem-cell research
Facts
Stem cell niche is essential for cell-fate decisions via regulation of stem cell homeostasis and mitochondrial dynamics of fusion and fission through balancing self-renewal and lineage-specific differentiation, as well as stem cell quiescence and activation.
Circulating cell-free mitochondria exist within the peripheral blood, which may be involved in various pathophysiological processes.
Mitochondria are highly dynamic organelles that change their morphology in response to cellular signals and differentiation states.
Mitochondria–endoplasmic reticulum crosstalk is implicated in aging progression.
Asymmetrically sort and distribution of aged and young mitochondria are critically involved in stemness regulation of stem cells.
Open questions
Is mitochondrial functional decline involved in aging progression of stem cells through age-dependent subcellular localization and redistribution of the mitochondria, thereby causing loss of stem cell properties?
Are mitochondrial connections and transfer implicated in cell-based therapies via sharing and receiving of energetic and young mitochondria of cells of damaged tissues from functional stem cells?
Is the coupling of endoplasmic reticulum (ER) stress and cell differentiation associated with the interplay between mitochondrial–ER crosstalk?
Is it possible to alleviate stem cell aging or achieve rejuvenation of aging stem cells through switching prolonged or excessive endoplasmic reticulum (ER) stress to adaptive ER stress via regulation of mitochondrial function and stem cell niche?
Introduction
Stem cell-based therapies exert profound therapeutic potential for curing a broad spectrum of diseases [1, 2]. Adult stem cells reside in a specified dynamic niche that is essential for continuous tissue homeostatic maintenance through balancing self-renewal with lineage selection [3, 4]. Meanwhile, adult stem cells may be multipotent or unipotent, and are present in both quiescent and actively dividing states in vivo of the mammalians, which may switch to each other state in response to various intrinsic or extrinsic signals through mitochondria-mediated mechanisms, such as alterations in mitochondrial respiration and metabolism [4–9]. In addition, the co-incidence of endoplasmic reticulum (ER) and mitochondria, and their dynamic interconnections and crosstalk are involved in a series of cellular processes, including mitochondrial homeostasis in fusion and fission, autophagy, and inflammasome formation [10, 11].
Emerging novel techniques during recent years, such as single-cell transcriptomics for analysis of spatial and temporal turnover of certain cellular processes, may enable advancing our understanding of dynamic gene regulation in stem cell maintenance within stem cell niche, stem cell activation and mobilization, lineage specification, tissue-specific molecular phenotypes in adult stem cells, identification of major cell types and their localization, as well as cellular and spatial sources of key growth factors and cytokines [12–18].
Diversity and heterogeneity of mitochondria
Mitochondria are complex organelles existing in a network undergoing continuous morphological dynamic changes through fission and fusion, which is crucial for the maintenance of pluripotency and differentiation capacity of stem cells [19–24]. Mitochondria usually undergo continuous morphologic dynamic changes through fission and fusion events controlled by the large GTPases Drp1, Mfn1, Mfn2, and Opa1 (fusion), which are important for mitochondrial function, and their imbalance would cause cell dysfunction and various diseases [25–28]. Metabolic changes are essential for cell-lineage commitment during mesenchymal stem cell differentiation, accompanied with alterations in mitochondrial morphology and dynamics [21, 22, 29]. Distinct morphological characteristics of mesenchymal stem cells mitochondria occur during different lineage-specification state (Fig. 1).
Fig. 1. Representative images of mitochondrial morphology.
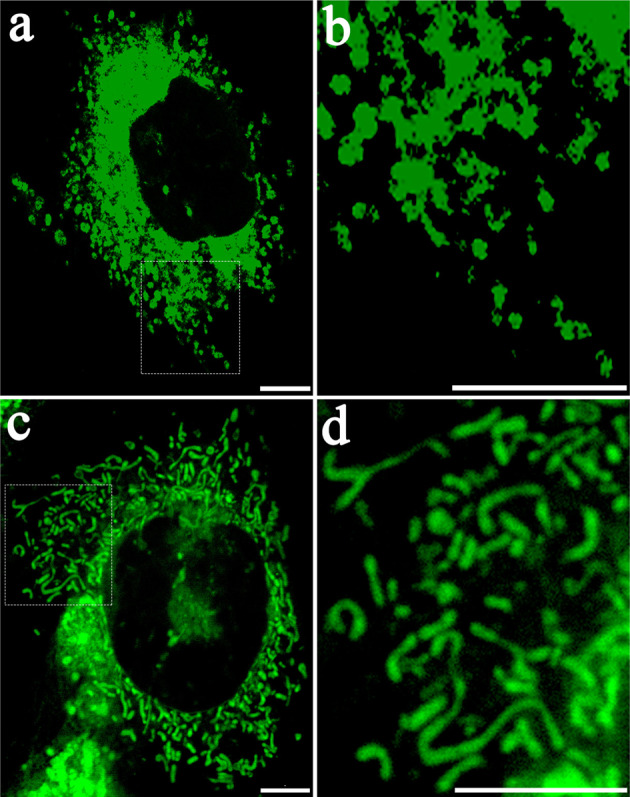
Observation of mitochondria of murine peripheral blood-derived mesenchymal stem cells (mPB-MSCs) under confocal microscope through Mito-Tracker Green staining. a, b Representative images of mitochondria of undifferentiated mPB-MSCs. c, d Representative images of mitochondria during osteogenic differentiation. Scale bar: 10 µm.
In general, stem cells facilitate tissue repair after tissue-specific homing through various mechanisms, such as immunomodulation of local microenvironment, differentiation into functional cells [30, 31], cell “empowerment” via paracrine secretion [32–34], immunomodulation [35, 36], and intercellular mitochondrial transfer [37–40]. Recent studies have demonstrated intercellular mitochondrial transfer within osteocyte-dendritic network [41]. Intriguingly, potential mitochondrial connections and communications were also observed between co-cultured mature chondrocytes and stem cells ex vivo (Fig. 2A). Meanwhile, cell-source-specific features have been reported between different tissue-derived adult stem cells with distinct functional properties [5, 42], as well as differential functional properties in different tissue-derived stem cell-derived extracellular vehicles [43, 44], mitochondrial metabolism [45], and mitochondrial transfer capacity [46]. Strikingly, recent studies have reported the presence of circulating cell-free mitochondria within the peripheral blood, suggesting the diversity of existing forms of mitochondria [47, 48]. Therefore, it is highly possible that distinct mitochondrial gene expression patterns may exist between different tissue-derived stem cells, owing to the heterogeneity of stem cells and diverse populations of mitochondrial DNA (mt-DNA) [48–55]. Simultaneously, visualization of replicating mt-DNA nucleoids has suggested the physical linkage between the ER and mitochondria (Fig. 2B) [56–58]. Therefore, manipulation of mt-DNA within cells may represent a powerful approach for the development of therapeutic interventions to treat mitochondrial diseases.
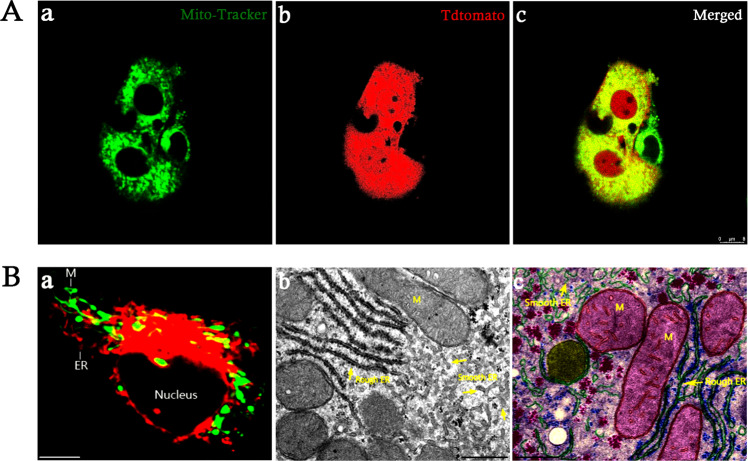
Fig. 2. Endoplasmic reticulum-mitochondrial localization.
A Representative live mitochondrial images of tdTomato-labeled joint progenitor cells co-cultured with mature chondrocytes through Mito-Tracker Green staining under confocal microscope. a Representative live-cell imaging of mitochondria through Mito-Tracker Green staining; b representative live-cell imaging of tdTomato fluorescence; c merged images. Scale bar: 8 µm. B Structural features of mitochondria (M) and endoplasmic reticulum (ER) within cells. a Representative immunocytochemical images of M (green) and ER (red); scale bar: 10 µm. b Representative electron micrographs of M and ER; scale bar: 500 nm. c Representative images of ultrastructure of M and ER. Scale bar: 500 nm (adapted from netterimages.com and chegg.com).
Mitochondrial metabolic regulation on stem cell fates
It is generally believed that stem cells fuel tissue development and tissue repair, and these activities are controlled by the local stem cell microenvironment or niche. Highly heterogeneous populations of resident stem/progenitor cells have been demonstrated residing within adult organs and tissues [55, 59–62]. An appropriate balance between self-renewal and differentiation is essential for stem cell function during both development and tissue homeostasis throughout life [63]. At steady state, adult stem cells are quiescent cells within niche. Both cell-intrinsic and -extrinsic signaling networks, such as mitochondrial dynamic-associated signaling, have been reported to fine-tune the self-renewal and differentiation of stem cells, and are involved in tissue homeostasis and tissue repair [64, 65].
Notably, mitochondrial plasticity, such as mitochondrial metabolism and mitochondrial respiratory chain, is vital for cell-fate decisions and function of stem cells [66–69]. The metabolic switch of mitochondria is required for stem cell activation and cell cycle activity [70]. Meanwhile, accumulating evidence has suggested a causative association between mitochondrial dysfunction and major phenotypes associated with aging. The self-renewal of tissues and organs in aging organisms requires stem cells, which have the unusual ability to divide asymmetrically into one daughter cell that retains stem cell properties and another that differentiates into a particular tissue type. Further, mitochondria have been reported to distribute passively during mitosis upon their release from microtubules [71]. Importantly, subcellular localization and distribution of young and old mitochondria determine stemness properties in the progeny stem cells during asymmetric cell divisions. Subsequently, the daughter cells that retains a stem cell nature inherits young mitochondria, whereas older mitochondria are inherited by the more differentiated cells [72]. Accumulation of aged mitochondria would lead to cell aging and cellular functional decline [70, 73]. Mohrin et al. [74] further elucidated a regulatory branch of mitochondrial unfolded protein response (UPRmt) that is coupled to cellular energy metabolism and proliferation in stem cells. Mitochondrial protein-folding stress triggered a metabolic checkpoint regulating cell cycle, whereas deregulation of this pathway interfered with stem cell quiescence and compromised regenerative potential [74]. Therefore, mitochondrial function may represent an important determinant of the regenerative potential of stem cells.
Stem cells possess multi-differentiation potential into various cell types, making them medically relevant for the treatment of a variety of diseases and injuries. However, there remains a major hurdle of stem cell therapy into the clinics, namely the limited efficiency to create fully functional and specialized terminally differentiated cells. Mitochondrial dynamics is crucial for cell-fate determination of stem cells [75, 76]. Importantly, different cell states require specific metabolic demands to support specialized functions [77]. Thus, efficient mitochondrial oxidative metabolism and dynamics are required for efficient specific lineage commitment [21, 78–83].
Strikingly, recent studies report that chaperone-mediated autophagy and a related metabolite in embryonic stem cells, also known as the self-eating process, have emerged as promising novel therapeutics for regeneration of damaged tissues and organs [84]. Simultaneously, accumulating evidence has indicated tight associations between mitochondrial metabolism and stem cell differentiation [85, 86]. Studies have demonstrated that mouse embryonic stem cells sorted for low- and high-resting mitochondrial membrane potential (ΔΨmL and ΔΨmH) are indistinguishable in terms of morphology and expression levels of pluripotency markers, whereas differing markedly in metabolic rates, suggesting that a coupling between intrinsic metabolic parameters and stem cell fate may provide clues for novel enrichment strategies and therapeutic approaches of stem cell therapy [87, 88]. Furthermore, a recent study demonstrated lactate mobilization of intracellular Mg2+, indicating potential links between mitochondrial Mg2+ transportation with major metabolic feedback circuits and mitochondrial bioenergetics (Fig. 3) [89].
Fig. 3. Mitochondrial regulation of stem cell homeostasis and aging.
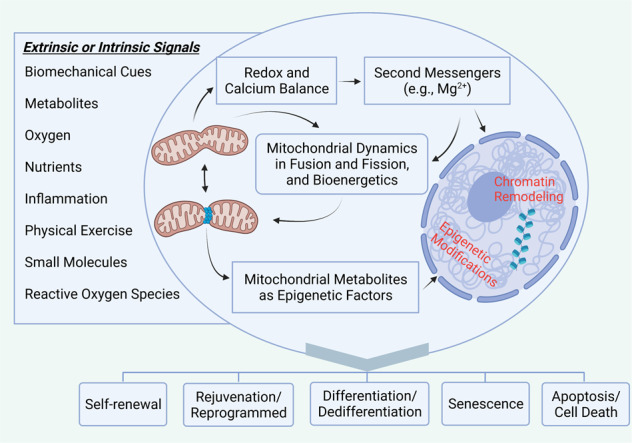
Regulation of stem cell homeostasis in response to environmental cues and epigenetic factors. Upon exposure to various extrinsic or intrinsic signals, mitochondria respond through modulation of morphological network and bioenergetics, the redox and calcium balance, and epigenetic modifications and chromatin remodeling within stem cells. Cell-fate decisions occur following mitochondria-based cellular response, mainly including self-renewal, rejuvenation/cell reprogrammed, differentiation/dedifferentiation, and senescence, cell death, or apoptosis [65, 134–142]. Created with BioRender.com.
Correlations between mitochondrial functions and aging
Adult stem cells are essential for tissue homeostasis and regeneration, yet are susceptible to senescence during aging [90–92], accompanied with aging microenvironments around adult stem cells [93, 94]. The main hallmarks of aging in mammalian organisms include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication [95]. The degeneration or dysfunction of aging tissues and organs is attributed to the deterioration of adult stem cells [95–97], which may result from disordered mitochondrial dynamics and declined mitochondrial functions [98–100]. Mitochondrial activity and metabolism are important determinants for specification of stem cell fate [87, 101].
Studies have demonstrated the importance of oxidized form of cellular nicotinamide adenine dinucleotide on mitochondrial activity as a pivotal switch to modulate muscle adult stem cell senescence [102, 103]. SIRT3, a mammalian sirtuin that regulates the global acetylation landscape of mitochondrial proteins and reduces oxidative stress, is suppressed during aging. In addition, the upregulation of SIRT3 in aged hematopoietic stem cells (HSCs) improved the regenerative capacity of HSCs [104]. Also, maintenance of self-renewal of a purified Tie2+ HSC population relies on mitochondrial clearance [105]. Further studies have identified a regulatory branch of the UPRmt, which mediated through the interplay between SIRT7 and NRF1, which is coupled to cellular energy metabolism and proliferation. Deregulation of a UPRmt-mediated metabolic checkpoint as a reversible main factor for HSC aging [74]. Further, systemic chronic inflammation has been reported as an important feature of aging, which is critically implicated in the process of stem cell aging [106–108]. A recent study has uncovered mitochondrial stress-initiated aberrant activation of the NLRP3 inflammasome as a reversible driver of functional decline during HSC aging [109]. In the meanwhile, studies have documented the crucial roles of PTPMT1 (a PTEN-like mitochondrial phosphatase) in the metabolic regulation of self-renewal and differentiation of HSCs [110].
Mitochondria–ER crosstalk and aging
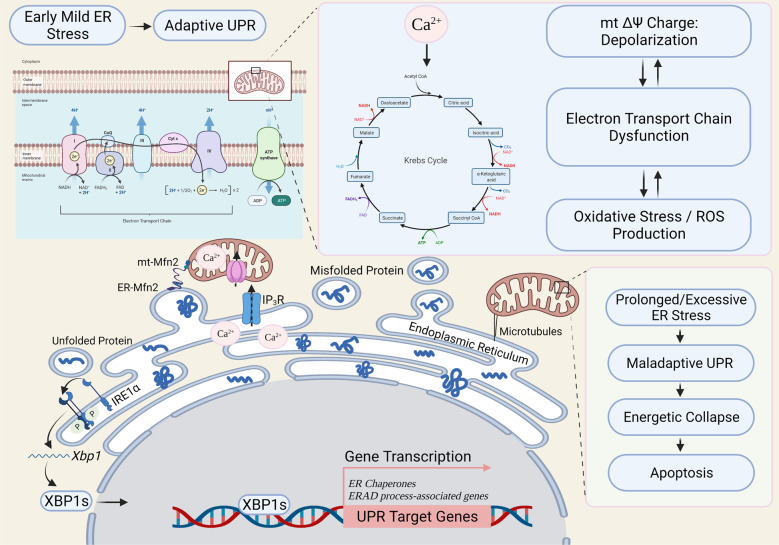
Notably, surviving ER stress has been demonstrated coupling to altered chondrocyte differentiation and functioning, which facilitates survival and recovery through adaptive unfolded protein response (UPR) during pathophysiology of chondrodysplasia [111, 112]. Also, studies have suggested the intimate linkage between stress adaptation and aging process [113]. Stress responses and the aging process may share common features and mechanisms initially arising from studies in model organisms [114, 115], where various molecular pathways have been demonstrated to implicate in the progression of aging process, including insulin/insulin-like growth factor, sirtuins, targets of rapamycin (TORs), and AMP-activated kinase. Thus, intrinsic induction of stress defense programs and the resulting adaptation may become a potential strategy to increase life expectancy [114]. Usually, mild ER stress activates the adaptive UPR on the one hand. Adaptive UPR is conducive to stress alleviation in response to cellular stress, which has recently been reported to preserve self-renewal of hematopoietic and pre-leukemic HSCs via inositol-requiring enzyme 1α/X-box-binding protein 1 signaling [116]. On the other hand, however, beyond a certain degree of ER damage, namely prolonged UPR response, would trigger apoptotic pathways (Fig. 4) [117–120]. Studies have suggested that depletion of the proteins involved in the regulation of mitochondrial–ER crosstalk, such as mammalian TOR, would lead to increased apoptosis, autophagy, and cellular dysfunction [121, 122]. In contrast, artificially increasing ER–mitochondria contacts in cells would restore cell viability [89, 119, 123, 124]. Studies have further identified critical roles of ER–mitochondria contacts in the biogenesis of mitochondrial-derived compartments [125–127], which may play essential roles in cellular adaptation to environmental stress conditions [127].
Fig. 4. Proposed working model of communication between mitochondria and endoplasmic reticulum stress.
Endoplasmic reticulum (ER) stress triggers an increase in mitochondrial metabolism, which mainly relies on organelle coupling and Ca2+ transfer. The onset of ER stress is accompanied with redistribution of reticular and mitochondrial networks towards the perinuclear region and a microtubule-dependent increase in connection. Physical interaction is mainly achieved by anchoring proteins, such as Mitofusin 2 (Mfn2), which allows buffering of intracellular Ca2+ from ER to mitochondria through its endoplasmic reticulum–mitochondria tethering activity, enhancing mitochondrial bioenergetics and ATP production consequently. The unfolded protein response (UPR) is a cellular self-defense adaptive mechanism to restore ER homeostasis. Crosstalk between the UPR pathways could facilitate a coordinated response to conditions of ER stress. During early mild ER stress, activated IRE1α then removes a 26-base intron from Xbp1 mRNA to generate a potent transcription factor XBP1s (Xbp1 spliced) that translocates into the nucleus and regulates a diverse array of genes, such as ER folding chaperones and ER-associated degradation (ERAD) process-associated genes. However, prolonged or excessive ER stress (e.g., induced by aging) would cause mitochondrial collapse and apoptotic cell death. ER: endoplasmic reticulum; UPR: unfolded protein response; ROS: reactive oxygen species; Mfn2: mitofusin 2; mt: mitochondrial; IRE1α: inositol-requiring kinase 1α (ER stress sensor); XBP1: X-box-binding protein 1; XBP1s: spliced form of XBP1; Krebs cycle: also known as TCA cycle (tricarboxylic acid cycle); IP3R: inositol trisphosphate receptor (Ca2+ channels); mt ΔΨ: mitochondrial membrane potential; ERAD: ER-associated degradation; ATP: adenosine 5´-triphosphate [74, 116,117,134, 142–145]. Created with BioRender.com.
Importantly, aging is one of the main causing factors of the increased prolonged ER stress, accompanied with mitochondrial dysfunction consequently [128]. Thus, attenuation of ER stress is a potential approach for the improvement and restoration of mitochondrial function in aging organisms. In addition, studies have suggested the correlation between spatial re-organization of mitochondria and increased ATP levels, oxygen consumption, reductive power, and increased mitochondrial Ca2+ uptake [129, 130]. However, uncoupling of the organelles or blocking Ca2+ transfer impaired the metabolic response, rendering cells more vulnerable to ER stress [129, 131]. Consequently, ER stress induces an early increase in mitochondrial metabolism that depends crucially upon organelle coupling and Ca2+ transfer, which, by enhancing cellular bioenergetics, establishes the metabolic basis for the adaptation to this response [129, 132]. As aging is one of the main factors causing increased ER stress and mitochondrial dysfunction, attenuation of ER stress is conducive to anti-aging [128]. Therefore, enhanced mitochondrial biogenesis has been reported associated with improved efficiency of the electron-transport chain, which may become a potential therapeutic anti-aging approach to block reactive oxygen species accumulation and promote cell survival through alleviation of ER stress [133].
Conclusions
All together, mitochondrial plasticity plays central roles in regulation of activity and functions of stem cells. Intrinsic and extrinsic signaling networks are responsible for dynamic regulation in mitochondrial function and adaptation to intrinsic and extrinsic signals for ultimate cell fate decisions. Interplay and crosstalk among aging microenvironments, ER stress, and inter- and intracellular mitochondrial dynamics are implicated in the progression of stem cell aging and functionally declined tissues and organs. Further extensive investigations on mitochondria–ER communication-associated stem cell aging, and changes of chromatin states and mitochondrial dynamics within the regenerative niche will not only boost the development of novel pharmaceutical targets for the cure of age-related disorders through targeting mitochondria–ER associated signaling pathways, but also provide novel insights into mitochondria-mediated stem cell activation during tissue regeneration. Identification of specific mitochondrial molecular signatures between different source-derived stem cells may advance our understanding of stem cell biology and shed light on novel strategies for healthy longevity and improved therapeutic outcomes of cellular therapy.
Acknowledgements
Figure 2B was adapted from netterimages.com and chegg.com. Figure 3 and 4 were created through content and elements of BioRender.com (2021) with a granted license (https://app.biorender.com/biorender-templates).
Author contributions
G.L., X.J., and W.Y.-W.L.: conceptualization, framework design, manuscript editing, and funding support. W.L., S.C., Y.W., and M.W.: collection of relevant literature and research data, manuscript writing, and figure preparation. All authors contributed to substantial discussion of content and approval of the final version.
Funding
This work was funded by Hong Kong Government Research Grants Council, Collaborative Research Fund (C7030-18G to G.L.), General Research Fund (19-093-GRF, 14120118, 9054014, N_CityU102/15, and 14119115 to G.L.), Hong Kong Innovation Technology Commission Funds (PRP/050/19FX and ITS/448/18 to G.L. and X.J.), Natural Science Foundation of Guang Dong (2018B030311065 to X.J.), National Natural Science Foundation of China (NSFC No. 31970815 to X.J.), Hong Kong UGC/GRF (14165217 to X.J.), Start‐up Fund of The Chinese University of Hong Kong, Hong Kong SAR (Reference number 4930991 to W.Y.-W.L.). This study was also supported by the research funds from Health@InnoHK Program launched by Innovation Technology Commission of the Hong Kong SAR, P. R. China, and Sports Medicine and Regenerative Technology Program, Lui Che Woo Institute of Innovative Medicine, The Chinese University of Hong Kong.
Ethics statement
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Edited by P. Pinton
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Weiping Lin, Email: weiping.lin@crmh-cas.org.hk.
Gang Li, Email: gangli@cuhk.edu.hk.
References
- 1.Cable J, Fuchs E, Weissman I, Jasper H, Glass D, Rando T, et al. Adult stem cells and regenerative medicine—a symposium report. Ann. N. Y. Acad. Sci. 2020;1462:27. doi: 10.1111/nyas.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27:523–31. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Adam RC, Yang H, Rockowitz S, Larsen SB, Nikolova M, Oristian DS, et al. Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature. 2015;521:366–70. doi: 10.1038/nature14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–5. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visvader JE, Clevers H. Tissue-specific designs of stem cell hierarchies. Nat. Cell Biol. 2016;18:349–55. doi: 10.1038/ncb3332. [DOI] [PubMed] [Google Scholar]
- 6.Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17:385–95. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ransom RC, Carter AC, Salhotra A, Leavitt T, Marecic O, Murphy MP, et al. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature. 2018;563:514–21. doi: 10.1038/s41586-018-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng M, Kim SK, Joe Y, Back SH, Cho HR, Kim HP, et al. Sensing endoplasmic reticulum stress by protein kinase RNA-like endoplasmic reticulum kinase promotes adaptive mitochondrial DNA biogenesis and cell survival via heme oxygenase-1/carbon monoxide activity. FASEB J. 2012;26:2558–68. doi: 10.1096/fj.11-199604. [DOI] [PubMed] [Google Scholar]
- 9.Feng J, Lu S, Ding Y, Zheng M, Wang X. Homocysteine activates T cells by enhancing endoplasmic reticulum-mitochondria coupling and increasing mitochondrial respiration. Protein Cell. 2016;7:391–402. doi: 10.1007/s13238-016-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys. Acta. 2014;1837:461–9. doi: 10.1016/j.bbabio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Genovese I, Vezzani B, Danese A, Modesti L, Vitto VAM, Corazzi V, et al. Mitochondria as the decision makers for cancer cell fate: from signaling pathways to therapeutic strategies. Cell Calcium. 2020;92:102308. doi: 10.1016/j.ceca.2020.102308. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JB, Sedgewick AJ, Finnegan AI, Harirchian P, Lee J, Kwon S, et al. Transcriptional programming of normal and inflamed human epidermis at single-cell resolution. Cell Rep. 2018;25:871–83. doi: 10.1016/j.celrep.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng C, Chan WCW, Lam Y, Wang X, Chen P, Niu B, et al. Lgr5 and Col22a1 mark progenitor cells in the lineage toward juvenile articular chondrocytes. Stem Cell Rep. 2019;13:713–29. doi: 10.1016/j.stemcr.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pallafacchina G, Francois S, Regnault B, Czarny B, Dive V, Cumano A, et al. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res. 2010;4:77–91. doi: 10.1016/j.scr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Hussenet T, Dembele D, Martinet N, Vignaud JM, du Manoir S. An adult tissue-specific stem cell molecular phenotype is activated in epithelial cancer stem cells and correlated to patient outcome. Cell Cycle. 2010;9:321–7. doi: 10.4161/cc.9.2.10421. [DOI] [PubMed] [Google Scholar]
- 16.Liang R, Ghaffari S. Mitochondria and FOXO3 in stem cell homeostasis, a window into hematopoietic stem cell fate determination. J. Bioenerg. Biomembr. 2017;49:343–6. doi: 10.1007/s10863-017-9719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baccin C, Al-Sabah J, Velten L, Helbling PM, Grunschlager F, Hernandez-Malmierca P, et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 2020;22:38–48. doi: 10.1038/s41556-019-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finnegan A, Cho RJ, Luu A, Harirchian P, Lee J, Cheng JB, et al. Single-cell transcriptomics reveals spatial and temporal turnover of keratinocyte differentiation regulators. Front Genet. 2019;10:775. doi: 10.3389/fgene.2019.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Duan S, Yi F, Ocampo A, Liu GH, Izpisua Belmonte JC. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 2013;18:325–32. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Archer SL. Mitochondrial dynamics-mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013;369:2236–51. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 21.Forni MF, Peloggia J, Trudeau K, Shirihai O, Kowaltowski AJ. Murine mesenchymal stem cell commitment to differentiation is regulated by mitochondrial dynamics. Stem Cells. 2016;34:743–55. doi: 10.1002/stem.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin W, Xu L, Pan Q, Lin S, Feng L, Wang B, et al. Lgr5-overexpressing mesenchymal stem cells augment fracture healing through regulation of Wnt/ERK signaling pathways and mitochondrial dynamics. FASEB J. 2019;33:8565–77. doi: 10.1096/fj.201900082RR. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Weaver D, Shirihai O, Hajnoczky G. Mitochondrial ‘kiss-and-run’: interplay between mitochondrial motility and fusion-fission dynamics. EMBO J. 2009;28:3074–89. doi: 10.1038/emboj.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasahara A, Cipolat S, Chen Y, Dorn GW, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science. 2013;342:734–7. doi: 10.1126/science.1241359. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl Acad. Sci. USA. 2008;105:19318–23. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter J, Bolognin S, Antony PMA, Nickels SL, Poovathingal SK, Salamanca L, et al. Neural stem cells of Parkinson’s disease patients exhibit aberrant mitochondrial morphology and functionality. Stem Cell Rep. 2019;12:878–89. doi: 10.1016/j.stemcr.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–7. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angelova PR, Barilani M, Lovejoy C, Dossena M, Vigano M, Seresini A, et al. Mitochondrial dysfunction in Parkinsonian mesenchymal stem cells impairs differentiation. Redox Biol. 2018;14:474–84. doi: 10.1016/j.redox.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo J, Ren R, Yao X, Ye Y, Sun K, Lin J, et al. PKM2 suppresses osteogenesis and facilitates adipogenesis by regulating beta-catenin signaling and mitochondrial fusion and fission. Aging (Albany NY) 2020;12:3976–92. doi: 10.18632/aging.102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gojo S, Gojo N, Takeda Y, Mori T, Abe H, Kyo S, et al. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp. Cell Res. 2003;288:51–9. doi: 10.1016/S0014-4827(03)00132-0. [DOI] [PubMed] [Google Scholar]
- 31.Su J, Guo L, Wu C. A mechanoresponsive PINCH-1-Notch2 interaction regulates smooth muscle differentiation of human placental mesenchymal stem cells. Stem Cells 2021;39:650–68. [DOI] [PubMed]
- 32.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc. Natl Acad. Sci. USA. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roefs MT, Sluijter JPG, Vader P. Extracellular vesicle-associated proteins in tissue repair. Trends Cell Biol. 2020;30:990–1013. doi: 10.1016/j.tcb.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Hou Y, Lin W, Li Y, Sun Y, Liu Y, Chen C, et al. De-osteogenic-differentiated mesenchymal stem cells accelerate fracture healing by mir-92b. J. Orthop. Transl. 2021;27:25–32. doi: 10.1016/j.jot.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–55. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 37.Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, le Coz O, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29:812–24. doi: 10.1002/stem.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Rehman R, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018;25:31. doi: 10.1186/s12929-018-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luz-Crawford P, Hernandez J, Djouad F, Luque-Campos N, Caicedo A, Carrere-Kremer S, et al. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res. Ther. 2019;10:232. doi: 10.1186/s13287-019-1307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Qin A, Liu D, Ruan R, Wang Q, Yuan J, et al. Endoplasmic reticulum mediates mitochondrial transfer within the osteocyte dendritic network. Sci. Adv. 2019;5:eaaw7215. doi: 10.1126/sciadv.aaw7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu LL, Liu YM, Sun YX, Wang B, Xiong YP, Lin WP, et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017;8:1–11. doi: 10.1186/s13287-016-0461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katsuda T, Ochiya T. Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res. Ther. 2015;6:212. doi: 10.1186/s13287-015-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang ZG, He ZY, Liang S, Yang Q, Cheng P, Chen AM. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020;11:511. doi: 10.1186/s13287-020-02032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wanet A, Caruso M, Domelevo Entfellner JB, Najar M, Fattaccioli A, Demazy C, et al. The transcription factor 7-like 2-peroxisome proliferator-activated receptor gamma coactivator-1 alpha axis connects mitochondrial biogenesis and metabolic shift with stem cell commitment to hepatic differentiation. Stem Cells. 2017;35:2184–97. doi: 10.1002/stem.2688. [DOI] [PubMed] [Google Scholar]
- 46.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Human tissue-specific MSCs demonstrate differential mitochondria transfer abilities that may determine their regenerative abilities. Stem Cell Res. Ther. 2018;9:298. doi: 10.1186/s13287-018-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiu RW, Chan LY, Lam NY, Tsui NB, Ng EK, Rainer TH, et al. Quantitative analysis of circulating mitochondrial DNA in plasma. Clin. Chem. 2003;49:719–26. doi: 10.1373/49.5.719. [DOI] [PubMed] [Google Scholar]
- 48.Al Amir Dache Z, Otandault A, Tanos R, Pastor B, Meddeb R, Sanchez C, et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 2020;34:3616–30. doi: 10.1096/fj.201901917RR. [DOI] [PubMed] [Google Scholar]
- 49.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011;12:126–31. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker DJ, Iyer A, Shah S, Moran A, Hjelmeland AB, Basu MK, et al. A new mitochondrial pool of cyclin E, regulated by Drp1, is linked to cell-density-dependent cell proliferation. J. Cell Sci. 2015;128:4171–82. doi: 10.1242/jcs.172429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.St John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum. Reprod. Update. 2010;16:488–509. doi: 10.1093/humupd/dmq002. [DOI] [PubMed] [Google Scholar]
- 52.Kelly RD, Rodda AE, Dickinson A, Mahmud A, Nefzger CM, Lee W, et al. Mitochondrial DNA haplotypes define gene expression patterns in pluripotent and differentiating embryonic stem cells. Stem Cells. 2013;31:703–16. doi: 10.1002/stem.1313. [DOI] [PubMed] [Google Scholar]
- 53.Lin W, Xu L, Li G. A novel protocol for isolation and culture of multipotent progenitor cells from human urine. J. Orthop. Transl. 2019;19:12–7. doi: 10.1016/j.jot.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson A, Hodgson-Garms M, Frith JE, Genever P. Multiplicity of mesenchymal stromal cells: finding the right route to therapy. Front Immunol. 2019;10:1112. doi: 10.3389/fimmu.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J. Exp. Med. 2010;207:1173–82. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ban-Ishihara R, Ishihara T, Sasaki N, Mihara K, Ishihara N. Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome c. Proc. Natl Acad. Sci. USA. 2013;110:11863–8. doi: 10.1073/pnas.1301951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–62. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewis SC, Uchiyama LF, Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science. 2016;353:aaf5549. doi: 10.1126/science.aaf5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J. Cell Mol. Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O’Connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–98. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 61.Godwin S, Ward D, Pedone E, Homer M, Fletcher AG, Marucci L. An extended model for culture-dependent heterogenous gene expression and proliferation dynamics in mouse embryonic stem cells. NPJ Syst. Biol. Appl. 2017;3:19. doi: 10.1038/s41540-017-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rennert RC, Januszyk M, Sorkin M, Rodrigues M, Maan ZN, Duscher D, et al. Microfluidic single-cell transcriptional analysis rationally identifies novel surface marker profiles to enhance cell-based therapies. Nat. Commun. 2016;7:11945. doi: 10.1038/ncomms11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–18. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–13. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- 65.Lisowski P, Kannan P, Mlody B, Prigione A. Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep. 2018;19:e45432. doi: 10.15252/embr.201745432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11:678–84. doi: 10.1038/embor.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bahat A, Gross A. Mitochondrial plasticity in cell fate regulation. J. Biol. Chem. 2019;294:13852–63. doi: 10.1074/jbc.REV118.000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maryanovich M, Zaltsman Y, Ruggiero A, Goldman A, Shachnai L, Zaidman SL, et al. An MTCH2 pathway repressing mitochondria metabolism regulates haematopoietic stem cell fate. Nat. Commun. 2015;6:7901. doi: 10.1038/ncomms8901. [DOI] [PubMed] [Google Scholar]
- 69.Anso E, Weinberg SE, Diebold LP, Thompson BJ, Malinge S, Schumacker PT, et al. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat. Cell Biol. 2017;19:614–25. doi: 10.1038/ncb3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hinge A, He J, Bartram J, Javier J, Xu J, Fjellman E, et al. Asymmetrically segregated mitochondria provide cellular memory of hematopoietic stem cell replicative history and drive HSC attrition. Cell Stem Cell. 2020;26:420–30 e6. doi: 10.1016/j.stem.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung JY, Steen JA, Schwarz TL. Phosphorylation-induced motor shedding is required at mitosis for proper distribution and passive inheritance of mitochondria. Cell Rep. 2016;16:2142–55. doi: 10.1016/j.celrep.2016.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katajisto P, Dohla J, Chaffer CL, Pentinmikko N, Marjanovic N, Iqbal S, et al. Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science. 2015;348:340–3. doi: 10.1126/science.1260384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347:1374–7. doi: 10.1126/science.aaa2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iwata R, Casimir P, Vanderhaeghen P. Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science. 2020;369:858–62. doi: 10.1126/science.aba9760. [DOI] [PubMed] [Google Scholar]
- 76.Ren L, Chen X, Chen X, Li J, Cheng B, Xia J. Mitochondrial dynamics: fission and fusion in fate determination of mesenchymal stem cells. Front Cell Dev. Biol. 2020;8:580070. doi: 10.3389/fcell.2020.580070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agathocleous M, Love NK, Randlett O, Harris JJ, Liu J, Murray AJ, et al. Metabolic differentiation in the embryonic retina. Nat. Cell Biol. 2012;14:859–64. doi: 10.1038/ncb2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen CT, Shih YRV, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–8. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 79.Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovascular Med. 2007;4:S60–S7. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells. 2011;29:486–95. doi: 10.1002/stem.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khacho M, Clark A, Svoboda DS, Azzi J, MacLaurin JG, Meghaizel C, et al. Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell. 2016;19:232–47. doi: 10.1016/j.stem.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 83.Hoque A, Sivakumaran P, Bond ST, Ling NXY, Kong AM, Scott JW, et al. Mitochondrial fission protein Drp1 inhibition promotes cardiac mesodermal differentiation of human pluripotent stem cells. Cell Death Discov. 2018;4:39. doi: 10.1038/s41420-018-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu Y, Zhang Y, Garcia-Canaveras JC, Guo L, Kan M, Yu S, et al. Chaperone-mediated autophagy regulates the pluripotency of embryonic stem cells. Science. 2020;369:397–403. doi: 10.1126/science.abb4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.TeSlaa T, Chaikovsky AC, Lipchina I, Escobar SL, Hochedlinger K, Huang J, et al. alpha-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 2016;24:485–93. doi: 10.1016/j.cmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu V, Dahan P, Ahsan FM, Patananan AN, Roy IJ, Torres A, Jr, et al. Mitochondrial metabolism and glutamine are essential for mesoderm differentiation of human pluripotent stem cells. Cell Res. 2019;29:596–8. doi: 10.1038/s41422-019-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schieke SM, Ma M, Cao L, McCoy JP, Jr, Liu C, Hensel NF, et al. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J. Biol. Chem. 2008;283:28506–12. doi: 10.1074/jbc.M802763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma MS, Kannan V, de Vries AE, Czepiel M, Wesseling EM, Balasubramaniyan V, et al. Characterization and comparison of osteoblasts derived from mouse embryonic stem cells and induced pluripotent stem cells. J. Bone Min. Metab. 2017;35:21–30. doi: 10.1007/s00774-015-0730-y. [DOI] [PubMed] [Google Scholar]
- 89.Daw CC, Ramachandran K, Enslow BT, Maity S, Bursic B, Novello MJ, et al. Lactate elicits ER-mitochondrial Mg(2+) dynamics to integrate cellular metabolism. Cell. 2020;183:474–89 e17. doi: 10.1016/j.cell.2020.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, et al. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8:215–25. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Berger E, Rath E, Yuan D, Waldschmitt N, Khaloian S, Allgauer M, et al. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat. Commun. 2016;7:13171. doi: 10.1038/ncomms13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zong Z, Zhang X, Yang Z, Yuan W, Huang J, Lin W, et al. Rejuvenated ageing mesenchymal stem cells by stepwise preconditioning ameliorates surgery-induced osteoarthritis in rabbits. Bone Jt. Res. 2021;10:10–21. doi: 10.1302/2046-3758.101.BJR-2020-0249.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–60. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maryanovich M, Zahalka AH, Pierce H, Pinho S, Nakahara F, Asada N, et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 2018;24:782–91. doi: 10.1038/s41591-018-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;53:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014;20:870–80. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baker N, Boyette LB, Tuan RS. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. 2015;70:37–47. doi: 10.1016/j.bone.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 98.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 2008;205:2397–408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–98. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–47. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vannini N, Girotra M, Naveiras O, Nikitin G, Campos V, Giger S, et al. Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nat. Commun. 2016;7:13125. doi: 10.1038/ncomms13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–43. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 103.Jin C, Li J, Green CD, Yu X, Tang X, Han D, et al. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metab. 2011;14:161–72. doi: 10.1016/j.cmet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3:319–27. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ito K, Turcotte R, Cui J, Zimmerman SE, Pinho S, Mizoguchi T, et al. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science. 2016;354:1156–60. doi: 10.1126/science.aaf5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Z, Amro EM, Becker F, Holzer M, Rasa SMM, Njeru SN, et al. Cohesin-mediated NF-kappaB signaling limits hematopoietic stem cell self-renewal in aging and inflammation. J. Exp. Med. 2019;216:152–75. doi: 10.1084/jem.20181505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–32. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, et al. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012;1:56–68. doi: 10.1016/j.celrep.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luo H, Mu WC, Karki R, Chiang HH, Mohrin M, Shin JJ, et al. Mitochondrial stress-initiated aberrant activation of the NLRP3 inflammasome regulates the functional deterioration of hematopoietic stem cell aging. Cell Rep. 2019;26:945–54 e4. doi: 10.1016/j.celrep.2018.12.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu WM, Liu X, Shen J, Jovanovic O, Pohl EE, Gerson SL, et al. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 2013;12:62–74. doi: 10.1016/j.stem.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsang KY, Chan D, Cheslett D, Chan WC, So CL, Melhado IG, et al. Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol. 2007;5:e44. doi: 10.1371/journal.pbio.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 113.Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 2011;30:2520–31. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haigis MC, Yankner BA. The aging stress response. Mol. Cell. 2010;40:333–44. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taormina G, Ferrante F, Vieni S, Grassi N, Russo A, Mirisola MG. Longevity: lesson from model organisms. Genes (Basel). 2019;10:518. [DOI] [PMC free article] [PubMed]
- 116.Liu L, Zhao M, Jin X, Ney G, Yang KB, Peng F, et al. Adaptive endoplasmic reticulum stress signalling via IRE1alpha-XBP1 preserves self-renewal of haematopoietic and pre-leukaemic stem cells. Nat. Cell Biol. 2019;21:328–37. doi: 10.1038/s41556-019-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, et al. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int. J. Biochem. Cell Biol. 2012;44:16–20. doi: 10.1016/j.biocel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chan WCW, Tsang KY, Cheng YW, Ng VCW, Chik H, Tan ZJ, et al. Activating the unfolded protein response in osteocytes causes hyperostosis consistent with craniodiaphyseal dysplasia. Hum. Mol. Genet. 2017;26:4572–87. doi: 10.1093/hmg/ddx339. [DOI] [PubMed] [Google Scholar]
- 119.Abdullahi A, Barayan D, Vinaik R, Diao L, Yu N, Jeschke MG. Activation of ER stress signalling increases mortality after a major trauma. J. Cell Mol. Med. 2020;24:9764–73. doi: 10.1111/jcmm.15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ricci D, Marrocco I, Blumenthal D, Dibos M, Eletto D, Vargas J, et al. Clustering of IRE1alpha depends on sensing ER stress but not on its RNase activity. FASEB J. 2019;33:9811–27. doi: 10.1096/fj.201801240RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bravo-Sagua R, Parra V, Ortiz-Sandoval C, Navarro-Marquez M, Rodriguez AE, Diaz-Valdivia N, et al. Caveolin-1 impairs PKA-DRP1-mediated remodelling of ER-mitochondria communication during the early phase of ER stress. Cell Death Differ. 2019;26:1195–212. doi: 10.1038/s41418-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–93. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 123.Cortez L, Sim V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion. 2014;8:97–202. doi: 10.4161/pri.28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J. Cell Biol. 2010;190:363–75. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.English AM, Schuler MH, Xiao T, Kornmann B, Shaw JM, Hughes AL. ER-mitochondria contacts promote mitochondrial-derived compartment biogenesis. J. Cell Biol. 2020;219:e202002144. [DOI] [PMC free article] [PubMed]
- 126.Goodrum JM, Lever AR, Coody TK, Gottschling DE, Hughes ALRsp5. and Mdm30 reshape the mitochondrial network in response to age-induced vacuole stress. Mol. Biol. Cell. 2019;30:2141–54. doi: 10.1091/mbc.E19-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schuler M-H, English AM, Campbell TJ, Shaw JM, Hughes AL. Mitochondrial-derived compartments facilitate cellular adaptation to amino acid stress. [Preprint]. 2020. Available from 10.1101/2020.03.13.991091. [DOI] [PMC free article] [PubMed]
- 128.Chen Q, Samidurai A, Thompson J, Hu Y, Das A, Willard B, et al. Endoplasmic reticulum stress-mediated mitochondrial dysfunction in aged hearts. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165899. doi: 10.1016/j.bbadis.2020.165899. [DOI] [PubMed] [Google Scholar]
- 129.Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J. Cell Sci. 2011;124:2143–52. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–73. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Honrath B, Metz I, Bendridi N, Rieusset J, Culmsee C, Dolga AM. Glucose-regulated protein 75 determines ER-mitochondrial coupling and sensitivity to oxidative stress in neuronal cells. Cell Death Discov. 2017;3:17076. doi: 10.1038/cddiscovery.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu X, Hajnoczky G. Ca2+-dependent regulation of mitochondrial dynamics by the Miro-Milton complex. Int. J. Biochem. Cell Biol. 2009;41:1972–6. doi: 10.1016/j.biocel.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Knupp J, Arvan P, Chang A. Increased mitochondrial respiration promotes survival from endoplasmic reticulum stress. Cell Death Differ. 2019;26:487–501. doi: 10.1038/s41418-018-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mironov SL, Ivannikov MV, Johansson M. [Ca2+]i signaling between mitochondria and endoplasmic reticulum in neurons is regulated by microtubules. From mitochondrial permeability transition pore to Ca2+-induced Ca2+ release. J. Biol. Chem. 2005;280:715–21. doi: 10.1074/jbc.M409819200. [DOI] [PubMed] [Google Scholar]
- 135.Shan X, Roberts C, Lan Y, Percec I. Age alters chromatin structure and expression of SUMO proteins under stress conditions in human adipose-derived stem cells. Sci. Rep. 2018;8:11502. doi: 10.1038/s41598-018-29775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu H, Huang B, Xue S, U KP, Tsang LL, Zhang X, et al. Functional crosstalk between mTORC1/p70S6K pathway and heterochromatin organization in stress-induced senescence of MSCs. Stem Cell Res. Ther. 2020;11:279. doi: 10.1186/s13287-020-01798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu D, Wu X, Zhou J, Li X, Huang X, Li J, et al. NuRD mediates mitochondrial stress-induced longevity via chromatin remodeling in response to acetyl-CoA level. Sci. Adv. 2020;6:eabb2529. doi: 10.1126/sciadv.abb2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pietrangelo L, D’Incecco A, Ainbinder A, Michelucci A, Kern H, Dirksen RT, et al. Age-dependent uncoupling of mitochondria from Ca2(+) release units in skeletal muscle. Oncotarget. 2015;6:35358–71. doi: 10.18632/oncotarget.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miyamoto K, Tajima Y, Yoshida K, Oikawa M, Azuma R, Allen GE, et al. Reprogramming towards totipotency is greatly facilitated by synergistic effects of small molecules. Biol. Open. 2017;6:415–24. doi: 10.1242/bio.023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oliverio S, Beltran JSO, Occhigrossi L, Bordoni V, Agrati C, D’Eletto M, et al. Transglutaminase type 2 is involved in the hematopoietic stem cells homeostasis. Biochemistry. 2020;85:1159–68. doi: 10.1134/S0006297920100041. [DOI] [PubMed] [Google Scholar]
- 141.Cakouros D, Gronthos S. The changing epigenetic landscape of mesenchymal stem/stromal cells during aging. Bone. 2020;137:115440. doi: 10.1016/j.bone.2020.115440. [DOI] [PubMed] [Google Scholar]
- 142.Betto RM, Diamante L, Perrera V, Audano M, Rapelli S, Lauria A, et al. Metabolic control of DNA methylation in naive pluripotent cells. Nat. Genet. 2021;53:215–29. doi: 10.1038/s41588-020-00770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Terasaki M, Chen LB, Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J. Cell Biol. 1986;103:1557–68. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Choi GE, Oh JY, Lee HJ, Chae CW, Kim JS, Jung YH, et al. Glucocorticoid-mediated ER-mitochondria contacts reduce AMPA receptor and mitochondria trafficking into cell terminus via microtubule destabilization. Cell Death Dis. 2018;9:1137. doi: 10.1038/s41419-018-1172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu X, Zhang Y, Ni M, Cao H, Signer RAJ, Li D, et al. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat. Cell Biol. 2017;19:626–38. doi: 10.1038/ncb3527. [DOI] [PMC free article] [PubMed] [Google Scholar]