Abstract
This study is the first investigation of parasites and other pathogens present in Sunda leopard cats (Prionailurus javanensis) in Aborlan, Palawan, Philippines. With the nature of wild carnivore sampling, four (4) wild Sunda leopard cats were captured in Aborlan, Palawan, Philippines for a period of nine (9) months. Of these, three (3) were considered for blood and fecal examination due to the poor condition of one animal. Rapid diagnostic kits were used to detect the presence of selected pathogens in blood samples while fecal samples were examined for parasite fauna and diet contents. Nine (9) parasite species were identified namely: Toxoplasma gondii, Ancylostoma sp., Capillaria hepatica, Echinostoma sp., Hymenolepis nana, Isospora felis, Physaloptera sp., Trichostrongylus sp., and a fasciolid. Chlamydophila felis, a bacterial pathogen was also detected in the blood. No individuals were found to be positive for feline immunodeficiency virus, feline infectious peritonitis virus, and feline leukemia virus antibodies. Six (6) small mammal prey species were identified from the feces of Sunda leopard cats namely: Palawan spiny rat (Maxomys panglima), Asian house rat (Rattus tanezumi), Polynesian rat (Rattus exulans), house mouse (Mus musculus), Southern Palawan tree squirrel (Sundasciurus steerii), and Palawan treeshrew (Tupaia palawanensis). Sunda leopard cats in Aborlan, Palawan, may be highly infected by parasites primarily due to their diet of small mammals such as rodents. Transmission is also possible through environmental contact with contaminated water or soil or direct physical contact with infected domestic animals. This paper contributes to the knowledge on host-parasite systems in wildlife ecosystem in the Philippines which is extremely poorly understood.
Keywords: Leopard cat, Diet, Parasites, Palawan island, Wildlife parasitology
Introduction
There is a dearth of information regarding infectious agents harbored by wildlife and their impact on wildlife health. Environmental changes and anthropogenic disturbances also have impacts to host-pathogen interactions in wildlife ecosystems (Cunningham et al. 2017; Osofsky et al. 2005). Recently, wildlife has also been implicated in the emergence of infectious diseases (Aguirre 2010; Karesh et al. 2005; Huong et al. 2020). Thus, there is a need to do surveillance and monitoring of host-parasite systems in wildlife animals of the Philippines as it is a critical component of the epidemiological triad similar to humans and domestic animals.
The Sunda leopard cat (Prionailurus javanensis) is a small wild feline with a brownish-gray to reddish-fawn fur color and brown body spots and facial stripes. This species was previously grouped under the Asian leopard cat (Prionailurus bengalensis) as a subspecies but has now been elevated as a separate species with populations occurring in the Southeast Asian islands of Java, Bali, Borneo, Sumatra, Panay, Cebu, Negros, and Palawan (Bellani 2020). There is currently no conservation status assessment for the Sunda leopard cat due to its recent establishment as a separate species and the paucity of data regarding its biology and ecology. However, under the Updated National List of Threatened Philippine Fauna and Their Categories (DENR-DAO 2019-09), the species P. bengalensis is listed as Vulnerable. The species has been known to travel through forests as well as open areas in and around human-dominated landscapes (Fernandez et al. 2018). The changes in wildlife ecosystem landscape due to environmental changes and anthropogenic impacts have consequences in the transmission dynamics of parasites and other pathogens. Wildlife parasitology is poorly understood in the Philippines due to lack of experts in this field and the difficulty in wildlife sampling. This is especially true for carnivores which are often nocturnal, elusive, and occur in low densities (Boitani et al. 2012). Elucidating the parasites and other possible pathogens present in Sunda leopard cats in Aborlan, Palawan, Philippines could contribute to the knowledge on host-parasite systems in wildlife ecosystem in the Philippines which is extremely poorly understood.
Materials and methods
Study area
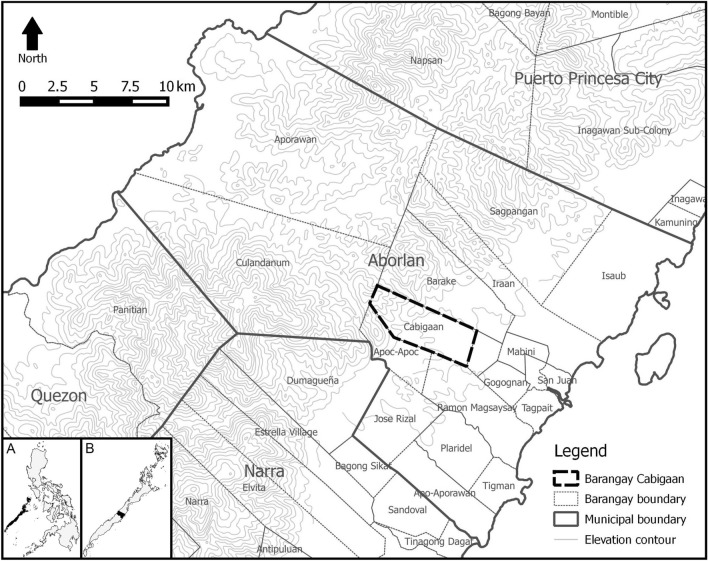
The study was conducted from May 2013 to February 2014 in Barangay Cabigaan, Aborlan Municipality, Palawan Province, Philippines located at 9°27′57″ N 118°28′37″ E (Fig. 1). The study site lies in a closed-canopy secondary-growth tropical lowland evergreen rainforest within the Aborlan-Guba Watershed along the eastern slope of the Victoria-Anapahan Mountain Range, with adjacent cropland, mixed brushland and residential areas. At the time of study, there were 58 households in Barangay Cabigaan consisting of 1951 residents, many of which are dependent on forest and agricultural resources for their daily needs. This site was chosen since leopard cats are known to dwell in mountain forests and locals claim to encounter the species here frequently. The climate in the area is tropical, specifically Type III in the Modified Coronas Classification wherein the dry and wet seasons are somewhat indistinct.
Fig. 1.
Location of Barangay Cabigaan in Aborlan Municipality, Palawan Province, Philippines
Trapping and sample collection
Target animals were wild and unowned Sunda leopard cats. Twelve (12) cage traps measuring 50 x 25 x 25 cm were set along various elevational gradients in Barangay Cabigaan for 155 days for a total of 1860 trap-nights. Each trap was baited with live juvenile chickens placed in a smaller separate secure cage at the end of each cage-trap. Upon capture, a licensed veterinarian gave each leopard cat a subcutaneous injection of atropine sulfate. After 15 min, tiletamine hypochloride-zolazepam hypochloride (Zoletil®, 50 milligram/milliliter, Virbac Laboratories, Carros, France) was injected intramuscularly (0.1 mg/kg) for immobilization. Once completely sedated (5–10 min), each leopard cat was sexed, aged, measured, weighed, and photographed. Blood samples were collected via cephalic vein puncture using a 1 cc disposable syringe with a 24-gauge needle and stored in a 1 mL Eppendorf tube. Each leopard cat was monitored until full recovery then released at their respective capture sites. Fecal samples were collected within each cage-trap to ensure correct attribution of specimens to each individual.
Sample processing
Capillary tubes were used to deposit 10 microliters of each blood sample into various rapid diagnostic test kits: (a) dot-ELISA antibody tests kits (ImmunoComb®, Biogal Galed Laboratories, Israel) to test for the presence of Toxoplasma gondii, Chlamydophila felis, immunodeficiency virus (FIV), and feline infectious peritonitis virus (FIPV) antibodies; (b) antigen test cassettes (ImmunoRun®, Biogal Galed Laboratories, Israel) for the detection of feline leukemia virus (FeLV) and Giardia duodenalis antigens; and (c) antibody test cassettes (ImmunoRun®, Biogal Galed Laboratories, Israel) to test for the presence of feline immunodeficiency virus (FIV) antibodies. All assays were performed according to manufacturer’s instructions at 20–25 °C as soon as each blood sample was collected in the field. Selected tests for pathogens were those that were commonly detected in domestic cats in the Philippines since pathogen transmission between domestic and wild cats is possible (Lima et al. 2020). All tests used have a specificity level of not less than 99.7% and a sensitivity level of not less than 92.3% (Table 1).
Table 1.
Specificity and sensitivity levels (in percentage) of test kits used to serologically detect selected pathogens
| Test kit | Specificity (%) | Sensitivity (%) |
|---|---|---|
| Toxoplasma gondii (Antibody) | 100 | 92.3 |
| Chlamydophila felis (Antibody) | 100 | 94.7 |
| Feline Infectious Peritonitis Virus (Antibody) | 99 | 93.4 |
| Feline Immunodeficiency Virus (Antibody) | 99.7 | 96.8 |
| Feline Leukemia Virus (Antigen) | 99.7 | 94.7 |
| Giardia duodenalis (Antigen) | 100 | 100 |
A 2 gram subsample of each fecal sample was processed using formalin-ether concentration technique. Parasites were examined under a compound microscope (Nikon HFX-DX, Japan), identified with the use of keys and other references, and confirmed by an expert (Baker 2007; Zajac and Conboy 2012). The rest of each fecal sample was washed in 95% ethanol and air dried for 48 h. Prey items were identified from hair, bones, feathers, and other remains with the use of keys and other references (Fernandez and de Guia 2011, 2013).
Results
Four (4) wild Sunda leopard cats were collected in Aborlan, Palawan, Philippines. However, one trapped animal was not sampled for blood and feces due to its weak condition at the time of trapping. The remaining three (3) Sunda leopard cats examined have tested positive for T. gondii antibodies and one (1) tested positive for C. felis antibodies. However, the presence of antibodies for these pathogens does not automatically signify an active infection at the time of examination. No individual was found to be positive for FIPV antibody, FIV antibody, FeLV antigen, or Giardia duodenalis antigen.
Nine (9) parasite species were identified from three (3) fecal samples collected (Fig. 2, Table 2). Strongylid larvae, Ancylostoma sp., and Capillaria hepatica were the most prevalent pathogens and were found in all three (3) samples, while Echinostoma sp., Hymenolepis nana, Isospora felis, Physaloptera sp., Trichostrongylus sp., and a fasciolid ova were found in one (1) out of three (3) samples. Strongylid larvae demonstrated the highest mean intensity (217 larvae per gram) followed by Ancylostoma sp. (30 epg), Capillaria sp. (26 epg), Echinostoma sp. (13 epg), H. nana (13 epg), I. felis (13 oocyst per gram ), Physaloptera sp. (13 epg), Trichostrongylus sp. (13 epg), and the fasciolid (13 epg).
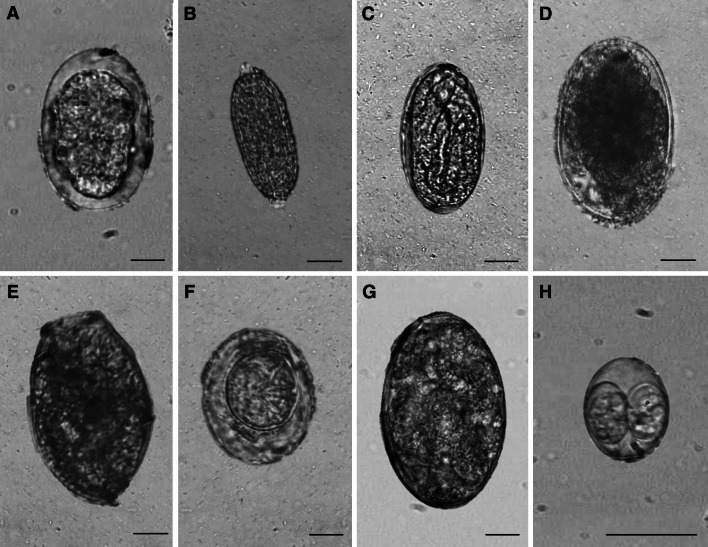
Fig. 2.
Parasites of Sunda leopard cats in Aborlan, Palawan Island, Philippines. (Photos A-G: 400x, Photo H: 1000x; scale bar=10 μm). a Ancylostoma sp. b Capillaria hepatica. c Physaloptera sp. d Trichostrongylus sp. e Echinostoma sp. f Hymenolepis nana. g Fasciolid. h Toxoplasma gondii.
Table 2.
Parasite fauna of Sunda leopard cat (Prionailurus javanensis) scats in Aborlan, Palawan Island, Philippines (n = 3)
| Parasite group | Parasites | Stage | Number of positive samples (% prevalence) | Mean intensity (eggs/oocyst/larvae per gram) [min, max] |
|---|---|---|---|---|
| Nematoda | Ancylostoma sp. | Ova | 3 (100%) | 30 [26, 39] |
| Capillaria hepatica | Ova | 3 (100%) | 26 [13, 39] | |
| Physaloptera sp. | Ova | 1 (33.33%) | 13* | |
| Trichostrongylus sp. | Ova | 1 (33.33%) | 13* | |
| Strongylids | Larva | 3 (100%) | 217 [13, 429] | |
| Platyhelminthes | Echinostoma sp. | Ova | 1 | 13* |
| Hymenolepis nana | Ova | 1 | 13* | |
| Fasciolid | Ova | 1 | 13* | |
| Apicomplexa | Isospora sp. | Oocyst | 1 | 13* |
*Mean intensity cannot be computed because only one sample was positive
Three (3) fecal samples were analyzed and were found to contain the remains of small mammals including the Palawan spiny rat, Maxomys panglima (100.0%), Asian house rat, Rattus tanezumi (66.7%), Polynesian rat, Rattus exulans (66.7%), house mouse, Mus musculus (33.3%), Southern Palawan tree squirrel, Sundasciurus steerii (33.3%), and Palawan treeshrew, Tupaia palawanensis (33.3%), as well as an unidentified bird (33.3%).
Discussion
This is the first report of parasites present in Sunda leopard cats and the first report of C. felis in leopard cats. Three (3) Sunda leopard cats from Aborlan were found highly infected by parasites which could be due to their diet of small mammals such as rodents. Several studies found leopard cats to be mainly carnivorous, preying on rodents as well as birds, reptiles, and insects with seasonal variation (Austin et al. 2007; Fernandez and De Guia 2011; Grassman et al. 2005; Inoue 1972; Khan 2004; Lee et al. 2013; Lorica and Heaney 2013; Rajaratnam et al. 2007; Sakaguchi and Ono 1994; Shehzad et al. 2012; Watanabe 2015; Xiong et al. 2016). Rodents are known to be effective disease reservoirs (Han et al. 2015), but parasites may also be transmitted to leopard cats and other wildlife via environmental contact such as exposure to the feces of other free-ranging wild or domestic animals or exposure to contaminated water or soil (Lélu et al. 2010; Miller et al. 2002).
T. gondii was the most prevalent pathogen, a common coccidian parasite among felines. The rodents, which are part of the diet of Sunda leopard cats, complete the life cycle of this parasite in wildlife ecosystems, and transmission is also possible via ingestion of oocysts from domestic cat feces or in contaminated water (Miller et al. 2002). Toxoplasmosis is usually asymptomatic in felines, but immunocompromised individuals may succumb to clinical symptoms such as ocular inflammation, lethargy, fever, anorexia, neurologic disorders, and abdominal pain (Vollaire et al. 2005). This pathogen was also detected in the Tsushima leopard cat (Prionailurus bengalensis euptilurus) in a zoo in Czech Republic (Lukešová and Literák 1998).
Other detected parasites that may have been transmitted via ingestion of rodents or through environmental contact include Echinostoma sp., Hymenolepis sp., Capillaria hepatica., and Physaloptera sp. Studies of parasites of rodents in the Philippines reported the occurrence of E. ilocanum, E. malayanum, H. nana, H. diminuta, C. hepatica, Angiostrongylus cantonensis, and P. praeputialis (Castillo and Paller 2018; Estaño et al. 2020; Quilla and Paller 2020; Tongson and San Pablo 1979; Tujan et al. 2016). Hookworms such as Ancylostoma sp. may have been acquired via direct contact with domestic cat or dog feces or through contaminated soil (Otranto and Deplazes 2019). This parasite, specifically Ancylostoma tubaeforme, is also present in Tsushima leopard cats (Yasuda et al. 1993). Meanwhile, Trichostrongylus sp. is a common herbivore parasite transmitted via the ingestion of contaminated soil, and Isospora felis is a common coccidian parasite which can cause diarrhea in cats (Zajac and Conboy, 2012).
Other pathogens were also tested in the study such as Chlamydophila felis, FIV, FIPV, FeLV, and Giardia duodenalis, but antibodies of these pathogens were not detected using the ELISA test kits except for C. felis. This is the first record of C. felis in leopard cats and could be transmitted by close contact with ocular secretions from an infected domestic cat (Gruffydd-Jones et al. 2009). Infection with this bacteria could lead to conjunctivitis, pneumonia, fever, weight reduction, lethargy, and lameness (Sykes et al. 1999). This pathogen was also detected from an Iberian lynx (Lynx pardinus) in Sierra Morena, Spain (Meli et al. 2009). Other studies also did not find FIV and FeLV in leopard cats in Vietnam, Taiwan, and Japan (Ikeda et al. 1999; Miyazawa et al. 1998; Mochizuki et al. 1990). False negative results could be possible such as in the case of a very recent infection with yet undetectable antibodies in the bloodstream at the time of testing (Little et al. 2020). However, it must be kept in mind that there is a low chance of false negatives for the samples since the sensitivity of the test kit used is 94.7% and the specificity is 99.7% (Table 1).
The parasites identified from Sunda leopard cats are common in felines (Lima et al. 2020; Patton and Rabinowitz 1994). These parasites may not be life-threatening; however, environmental disturbances such as climate change, land use change, biodiversity loss, and pollution may serve as animal stressors and may pose threats especially to debilitated wild animals or those that have weakened immune system which are more likely to suffer due to severe effects of parasitism (Aguirre 2010; Brearley et al. 2013; Cunningham et al. 2017; Okulewicz 2017). Intestinal worms, for example, can be a serious problem in young animals since hookworms and roundworms can cause anemia and can lead to poor growth and development (Seguel and Gottdenker 2017; Traversa 2012). A concurrent spatial ecology study by Fernandez et al. (2018) found that leopard cats in the site used not only forests but also disturbed habitats in and around human settlements where they may have range overlaps with free-ranging domestic animals. Furthermore, their study showed that the small mammal population of the area is dominated by commensal rodents in habitats outside the forest areas. Through radio-tracking, they found that some Sunda leopard cats may occupy larger home ranges during periods of decreased small mammal abundance, presumably in search of food, which may lead to direct physical contact with infected domestic animals or their fecal matter.
In the current study, four leopard cat individuals were captured during a total of 20 weeks of trapping. This emphasizes the difficulty of capturing and studying cryptic carnivores. Wildlife studies by their nature, and especially those involving species with sparse distributions, are often characterized by small sample size although exceptions may exist. However, studies with small sample size may still be considered useful, particularly for detection of parasites and other pathogens.
Several factors affect the population dynamics of wildlife species such as its pathogen load, prey-predator relationship, habitat, environmental disturbances, and human encroachment (Adams et al. 2006). Pathogen infections can affect the behavior of an animal and may influence its overall fitness, movement, social interactions, and home range (Herrera and Nunn 2019). Understanding the synergistic effects of anthropogenic disturbances and parasitism to animal hosts could lead to a more in-depth understanding of pathogen transmission dynamics and the regulation of host populations in natural habitats, which in turn may have implications to ecosystem health and public health (Aguirre 2010; Brearley et al. 2013; Cunningham et al. 2017; Osofsky et al. 2005).
Author contributions
CLCL, DAPF, APODG, MCTDL and VGVP conceived and designed the study. CLCL, DAPF, and MCTDL collected samples in the field. CLCL performed laboratory and data analyses. CLCL and DAPF drafted the manuscript. APODG, MCTDL, and VGVP revised the manuscript critically. All authors read and approved the final manuscript.
Funding
This work was funded primarily by the Nagao Natural Environment Foundation (NEF) and supported by the Accelerated Science and Technology Human Resource Development Program of the Philippine Department of Science and Technology. The authors would also like to thank the Cabigaan Barangay Council, Aborlan Municipal Government, Palawan Provincial Government, and the Palawan Council for Sustainable Development Staff (PCSDS) for their support.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no competing interests. Disclosure forms included.
Consent to participate
The authors declare that they give their consent to participate in the review process.
Consent for publication
The authors declare that they give their consent to publish this manuscript.
Ethical approval
Prior to the conduct of the study, the protocol was approved by the research ethical panel of the Institute in accordance with Department of Agriculture Administrative Order No. 40 series of 1999 otherwise known as “Rules and Regulations on the Conduct of Scientific Procedures Using Animals” pursuant to Republic Act No. 8485 or the “Animal Welfare Act of 1998” and Republic Act No. 9147 or the “Wildlife Resources Conservation and Protection Act”. Wildlife Gratuitous Permit No. 2013-04 was granted to the authors by the Palawan Council for Sustainable Development.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams CE, Lindsey KJ, Ash SJ. Urban wildlife management. Florida: CRC Press; 2006. [Google Scholar]
- Aguirre AA. Parasitic diseases in wildlife and domestic animals: new trends of disease emergence. In: Lefèvre P-C, Blancou J, Chermette R, Uilenberg G, editors. Infectious and parasitic diseases of livestock. Lavosier: France; 2010. pp. 73–79. [Google Scholar]
- Austin SC, Tewes ME, GrassmanJ Lon I, Silvy NJ. Ecology and conservation of the leopard cat Prionailurus bengalensis and clouded leopard Neofelis nebulosa in Khao Yai National Park, Thailand. Acta Zool Sin. 2007;7:1–14. [Google Scholar]
- Baker DG. Flynn's parasites of laboratory animals. 2. Iowa: Blackwell Publishing; 2007. [Google Scholar]
- Bellani GG. Felines of the World: discovery in taxonomic classification. 1. London, United Kingdom: Elsevier; 2020. Sunda leopard cat; pp. 254–257. [Google Scholar]
- Boitani L, Ciucci P, Mortelliti A. Designing carnivore surveys. In: Boitani L, Powell R, editors. Carnivore ecology and management: a handbook of techniques. Oxford, United Kingdom: Oxford University Press; 2012. pp. 8–29. [Google Scholar]
- Brearley G, Rhodes J, Bradley A, Baxter G, Seabrook L, Lunney D, Liu Y, McAlpine C. Wildlife disease prevalence in human-modified landscapes. Biol Rev. 2013;88(2):427–442. doi: 10.1111/brv.12009. [DOI] [PubMed] [Google Scholar]
- Castillo DSC, Paller VGV. Occurrence of Angiostrongylus cantonensis in rodents from the rice granary of the Philippines and associated risk factors for zoonotic transmission. J Parasit Dis. 2018 doi: 10.1007/s12639-018-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AA, Daszak P, Wood JLN. One health emerging infectious diseases and wildlife: two decades of progress? Phil Trans R Soc B. 2017;372:20160167. doi: 10.1098/rstb.2016.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENR (2019) Administrative Order 2019-09: Updated National List of Threatened Philippine Fauna and Their Categories. Philippines: Department of Environment and Natural Resources. Retrieved on 07 Oct 2020 from http://www.bmb.gov.ph/index.php/e-library/laws-and-policies/denr-administrative-orders/dao-2017-2019?download=383:denr-administrative-order-2019-09
- Estaño LA, Bordado AMD, Paller VGV. Angiostrongylus cantonensis infection of non-native rats in mount makiling forest reserve, the Philippines. Parasitol. 2020 doi: 10.1017/S0031182020001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DAP, de Guia APO, Dimalibot JC, Bantayan NC. Spatial ecology of a male and a female leopard cat (Prionailurus bengalensis heaneyi Groves 1997) in Aborlan, Palawan, Philippines. Sylvatrop. 2018;28:1–16. [Google Scholar]
- Fernandez DAP, De Guia APO. Feeding habits of Visayan leopard cats (Prionailurus bengalensis rabori) in sugarcane fields of Negros Occidental, Philippines. Asia Life Sci. 2011;20:141–152. [Google Scholar]
- Fernandez DAP, De Guia APO (2013) Morphology of guard hair from the dorsal body region of selected non-volant terrestrial small mammals of Palawan Island, Philippines. University of the Philippines Los Baños, Laguna, Philippines. pp 34
- Grassman LI, Jr, Tewes ME, Silvy NJ, Kreetiyutanont K. Spatial organization and diet of the leopard cat (Prionailurus bengalensis) in north-central Thailand. J Zool Lond. 2005;266(1):45–54. doi: 10.1017/S095283690500659X. [DOI] [Google Scholar]
- Gruffydd-Jones T, Addie D, Belák S, Boucraut-Baralon C, Egberink H, Frymus T, et al. Chlamydophila felis infection: ABCD guidelines on prevention and management. J Feline Med Surg. 2009;11:605–609. doi: 10.1016/j.jfms.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BA, Schmidt JP, Bowden SE, Drake JM. Rodent reservoirs of future zoonotic diseases. Proc Natl Acad Sci USA. 2015;112(22):7039–7044. doi: 10.1073/pnas.1501598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera J, Nunn CL. Behavioural ecology and infectious disease: implications for conservation of biodiversity. Philos Trans R Soc Lond B. 2019;374(1781):20180054. doi: 10.1098/rstb.2018.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huong NQ, Nga NTT, Long NV, Luu BD, Latinne A, Pruvot M, et al. Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Viet Nam, 2013–2014. PLoS ONE. 2020;15(8):e0237129. doi: 10.1371/journal.pone.0237129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Miyazawa T, Nakamura K, Naito R, Inoshima Y, Tung KC, Lee WM, Chen MC, Kuo TF, Lin JA, Mikami T. Serosurvey for selected virus infections of wild carnivores in Taiwan and Vietnam. J Wildl Dis. 1999;35(3):578–581. doi: 10.7589/0090-3558-35.3.578. [DOI] [PubMed] [Google Scholar]
- Inoue T. The food habit of Tsushima leopard cat, Felis bengalensis ssp., analysed from their scats. J Mammal Soc Japan. 1972;5:155–169. [Google Scholar]
- Karesh WB, Cook RA, Bennett EL, Newcomb J. Wildlife trade and global disease emergence. Emerg Infect Dis. 2005;11(7):1000–1002. doi: 10.3201/eid1107.050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MMH. Food habit of the Leopard cat Prionailurus bengalensis (Kerr 1792) in the Sundarbans East Wildlife Sanctuary, Bangladesh. Zoos’ Print J. 2004;19:1475–1476. doi: 10.11609/JoTT.ZPJ.1101.1475-6. [DOI] [Google Scholar]
- Lee O, Lee S, Nam DH, Lee HY. Molecular analysis for investigating dietary habits: Genetic screening of prey items in scat and stomach contents of leopard cats Prionailurus bengalensis euptilurus. Zool Stud. 2013;52:1–6. doi: 10.1186/1810-522X-52-45. [DOI] [Google Scholar]
- Lélu M, Langlais M, Poulle ML, Gilot-Fromont E. Transmission dynamics of Toxoplasma gondii along an urban–rural gradient. Theor Popul Biol. 2010;78(2):139–147. doi: 10.1016/j.tpb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Lima TA, Salgado PAB, Chagas CRF, Ramos PL, Adriano PL, Adriano EA, Gonzalez IHL. Feral cats: parasitic reservoirs in our zoos? Open J Vet Med. 2020;10:126–138. doi: 10.4236/ojvm.2020.108011. [DOI] [Google Scholar]
- Little S, Levy J, Hartmann K, Hofmann-Lehmann R, Hosie M, Olah G, Denis KS. 2020 AAFP feline retrovirus testing and management guidelines. J Feline Med Surg. 2020;22(1):5–30. doi: 10.1177/1098612X19895940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorica MRP, Heaney LR. Survival of a native mammalian carnivore, the leopard cat Prionailurus bengalensis Kerr 1792 (Carnivora: Felidae), in an agricultural landscape on an oceanic Philippine island. J Threat Taxa. 2013;5:4451–4460. doi: 10.11609/JoTT.o3352.4451-60. [DOI] [Google Scholar]
- Lukešová D, Literák I. Shedding of Toxoplasma gondii oocysts by Felidae in zoos in the Czech Republic. Vet Parasitol. 1998;74:1–7. doi: 10.1016/S0304-4017(97)00155-6. [DOI] [PubMed] [Google Scholar]
- Meli ML, Cattori V, Martínez F, López G, Vargas A, Simón MA, et al. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus) PLoS One. 2009;4:e4744. doi: 10.1371/journal.pone.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Gardner IA, Kreuder C, Paradies DM, Worcester KR, Jessup DA, et al. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) Int J Parasitol. 2002;32:997–1006. doi: 10.1016/S0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Ikeda Y, Maeda K, Horimoto T, Tohya Y, Mochizuki M, et al. Seroepidemiological survey of feline retrovirus infections in domestic and leopard cats in northern Vietnam in 1997. J Vet Med Sci. 1998;60(11):1273–1275. doi: 10.1292/jvms.60.1273. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Akuzawa M, Nagatomo H. Serological survey of the Iriomote cat (Felis iriomotensis) in Japan. J Wildl Dis. 1990;26(2):236–245. doi: 10.7589/0090-3558-26.2.236. [DOI] [PubMed] [Google Scholar]
- Okulewicz A. The impact of global climate change on the spread of parasitic nematodes. Ann Parasitol. 2017;63(1):15–20. doi: 10.17420/ap6301.79. [DOI] [PubMed] [Google Scholar]
- Osofsky SA, Kock RA, Kock MD, Kalema-Zikusoka G, Grahn R, Leyland T, Karesh WB. Building support for protected areas using a “One Health” perspective. In: McNeely JA, editor. Friends for life: new partners in support of protected areas. Gland, Switzerland and Cambridge, UK: IUCN; 2005. pp. 65–79. [Google Scholar]
- Otranto D, Deplazes P. Zoonotic nematodes of wild carnivores. Int J Parasitol Parasites Wildl. 2019;9:370–383. doi: 10.1016/j.ijppaw.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton S, Rabinowitz AR. Parasites of wild Felidae in Thailand: a coprological survey. J Wildl Dis. 1994;30(3):472–475. doi: 10.7589/0090-3558-30.3.472. [DOI] [PubMed] [Google Scholar]
- Quilla MHDP, Paller VGV. Histopathological features and prevalence of Capillaria hepatica infection in Rattus spp. in Philippine mount makiling forest reserve and its adjacent areas. J Parasit Dis. 2020;44:338–348. doi: 10.1007/s12639-019-01189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaratnam R, Sunquist M, Rajaratnam L, Ambu L. Diet and habitat selection of the leopard cat (Prionailurus bengalensis borneoensis) in an agricultural landscape in Sabah, Malaysian Borneo. J Trop Ecol. 2007;23:209–217. doi: 10.1017/S0266467406003841. [DOI] [Google Scholar]
- Sakaguchi N, Ono Y. Seasonal change in the food habits of the Iriomote cat Felis iriomotensis. Ecol Res. 1994;9:167–174. doi: 10.1007/BF02347492. [DOI] [Google Scholar]
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6(3):177–94. doi: 10.1016/j.ijppaw.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad W, Riaz T, Nawaz MA, et al. Carnivore diet analysis based on next-generation sequencing: application to the leopard cat (Prionailurus bengalensis) in Pakistan. Mol Ecol. 2012;21:1951–1965. doi: 10.1111/j.1365-294X.2011.05424.x. [DOI] [PubMed] [Google Scholar]
- Sykes JE, Anderson GA, Studdert VP, Browning GF. Prevalence of feline Chlamydia psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease. J Vet Intern Med. 1999;13:153–162. doi: 10.1111/j.1939-1676.1999.tb02172.x. [DOI] [PubMed] [Google Scholar]
- Tongson MS, San Pablo FG. A study on the prevalence of gastro-intestinal worms of cats in Metropolitan Manila. Philipp J Vet Med. 1979;18(2):1–15. [Google Scholar]
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vect. 2012;5:91. doi: 10.1186/1756-3305-5-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tujan MAA, Fontanilla IKC, Paller VGV. Vectors and spatial patterns of Angiostrongylus cantonensis in selected rice-farming villages of Muñoz. Nueva Ecija, Philippines: J Parasitol Res; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollaire MR, Radecki SV, Lappin MR. Seroprevalence of Toxoplasma gondii antibodies in clinically ill cats in the United States. Am J Vet Res. 2005;66:874–877. doi: 10.2460/ajvr.2005.66.874. [DOI] [PubMed] [Google Scholar]
- Watanabe S. Ecological flexibility of the top predator in an Island ecosystem—The iriomote cat changes feeding patterns in relation to prey availability. Biodivers Ecosys-Link Struct Funct. 2015 doi: 10.5772/59502. [DOI] [Google Scholar]
- Xiong M, Shao X, Long Y, et al. Molecular analysis of vertebrates and plants in scats of leopard cats (Prionailurus bengalensis) in southwest China. J Mammal. 2016;97:1054–1064. doi: 10.1093/jmammal/gyw061. [DOI] [Google Scholar]
- Yasuda N, Akuzawa M, Maruyama H, Izawa M, Doi T. Helminths of the Tsushima leopard cat (Felis bengalensis euptilura) J Wildl Dis. 1993;29(1):153–155. doi: 10.7589/0090-3558-29.1.153. [DOI] [PubMed] [Google Scholar]
- Zajac AM, Conboy GA. Veterinary clinical parasitology. 8. West Sussex: John Wiley; 2012. [Google Scholar]