Abstract
Purpose
Development and validation of an animal model of labral healing would facilitate translation of novel surgical and biological strategies to improve glenolabral healing. The purpose of this study was to characterize the anatomic and histological properties of the shoulder labrum in rat, rabbit, dog, pig, goat, and humans. Given the demonstrated similarities in size and structural morphology in other joints, it was hypothesized that the goat glenoid with surrounding capsulolabral complex would most closely resemble that of humans in terms of dimensions and structure, as observed grossly and histologically.
Methods
Cadaveric glenohumeral joints from rats (n = 8), New Zealand white rabbits (n = 13), Mongrel dogs (n = 9), Spanish goats (n = 10), Yorkshire pigs (n = 10), and humans (n = 9) were freshly harvested. Photographs were taken of the glenoid with its surrounding capsulolabral complex. Linear dimensions of the glenoid articular surface were measured. It was determined where the capsulolabral complex was continuous with, or recessed from, the articular glenoid surface. The glenoid was divided into 6 equal segments radiating out toward 12, 2, 4, 6, 8, and 10 o’clock positions. Samples were sectioned and stained with Safranin O/Fast green and Mallory Trichrome. Insertion of the capsulolabral tissue onto the glenoid was qualitatively assessed and compared with gross morphology.
Results
Dimensions of the goat glenoid most closely paralleled dimensions of the human glenoid. A capsulolabral complex was continuous with the glenoid surface from ~ 9 to 12 o’clock in the rats, 7 to 12 o’clock in rabbits, 5 to 12 o’clock in the dogs, and 9 to 12 o’clock in goats, 6 to 12 o’clock in pigs, and 2 to 8 o’clock in humans. In contrast to humans, no other species demonstrated an organized fibrocartilaginous labrum either macroscopically or histologically.
Conclusion
The animals in the present study did not possess a discrete fibrocartilaginous labrum by gross or histological evaluation, as directly compared to humans. While models using these animals may be acceptable for examining other shoulder pathologies, they are not adequate to evaluate labral pathology.
Level of evidence
Basic Science Study; Anatomy and Histology; Cadaveric Animal Model.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40634-021-00383-6.
Keywords: Shoulder, Glenoid labrum, Animal model, Tissue engineering
Introduction
Given the inherent instability of the glenohumeral joint, traumatic shoulder dislocation is particularly common and can cause damage to the soft tissues surrounding the joint [13, 24]. The glenoid labrum is damaged in more than 70% of traumatic shoulder dislocations and often fails to heal without intervention [4, 7]. Similarly, the joint capsule is routinely stretched during instability events, with residual laxity permitting excessive humeral translation and recurrent subluxation [16]. Recurrent dislocation is most commonly treated with surgical repair to prevent continued instability and subsequent joint degeneration [2, 6, 12, 18, 22, 29]. However, high rates of recurrent instability have been reported after labral repair [20, 23, 30]. Repair failure may in part be due to insufficient labral healing, either within the labral body or at the glenoid-labrum interface. Tissue engineering strategies have shown promise in preclinical studies in restoring structure and function following injury of the meniscus and rotator cuff [32, 33], but similar strategies have been seldom applied to capsulolabral injuries [25, 27, 36].
The development of improved surgical techniques and tissue engineering strategies for enhanced capsulolabral repair would be greatly facilitated by the use of animal models. Unfortunately, most model animals are quadrupeds that use their forelimbs for weight-bearing during locomotion and have limited overhead activity, differing greatly from humans. Additionally, some quadrupeds rarely use their upper limbs for functional tasks (i.e. goat and pig), while others use their arms to gather and eat food (i.e. rat and rabbit) [22]. Despite these functional variations within quadrupeds and between quadrupeds and humans, it remains unclear whether common model animals can simulate capsulolabral pathology that occurs in humans. While the human labrum and capsule have been extensively characterized [3, 9, 10, 17, 26, 37, 38], similar analyses in animal shoulders are scarce and incomplete [1, 5, 27, 34, 35]. Before novel strategies for improved capsulolabral healing can be translated from animal models to human patients, an understanding of the similarities and differences in the native glenolabral structure and function across species is needed. Therefore, the purpose of this study was to characterize the anatomic and histological properties of the shoulder labrum in species commonly employed as animal models, including rat, rabbit, dog, pig, and goat. Given the demonstrated similarities in size and structural morphology in other joints [11, 31], it was hypothesized that the goat glenoid with surrounding capsulolabral complex would most closely resemble that of humans in terms of dimensions and structure, as observed grossly and histologically.
Methods
Approval was obtained from the Committee for Oversight of Research and Clinical Training Involving Decedents (BLINDED) from BLINDED. Cadaveric glenohumeral joints were freshly harvested from skeletally mature animals from five species commonly utilized as animal models for shoulder injury and repair, including rats (n = 8), New Zealand white rabbits (n = 13), Mongrel dogs (n = 9), Spanish goats (n = 10), Yorkshire pigs (n = 10), and humans (n = 9). All soft tissue surrounding the joint was dissected without violating the joint capsule. Thereafter, the capsule was sharply dissected free from its humeral insertions.
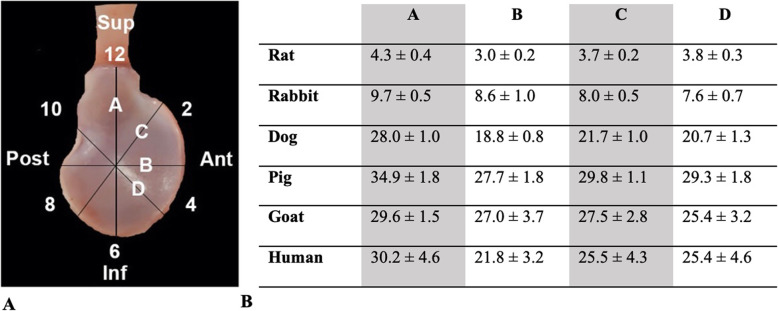
Photographs were taken of the glenoid en face with its surrounding capsulolabral complex, defined as the soft tissue inserting immediately into the glenoid (Fig. 1). Linear dimensions of the articular surface of the glenoid were determined using digital calipers along four lines dissecting the glenoid (Fig. 2A). According to a clock face orientation (Fig. 2A), it was determined where the capsulolabral complex was continuous with, or recessed from (i.e., soft tissue inserted towards the glenoid neck), the articular glenoid surface (Fig. 3A). The insertion of the long head of the biceps tendon into the supraglenoid tubercle designated the 12 o’clock position, as it was present in all specimens of all species. The glenoid was then divided into six equal segments radiating out from the glenoid center toward 12, 2, 4, 6, 8, and 10 o’clock positions in order to prepare samples for histology.
Fig. 1.
Gross anatomy of the glenoid with attached capsulolabral complex. Anterior (A) on right, posterior (P) on left, in each panel. Superior is top of panel, with 12 o’clock position indicated by the inserting long head of biceps. Glenolabral and capsulolabral junctions in the human shoulder indicated by arrowheads and arrows, respectively; absent in other animals
Fig. 2.
A Glenoid clockface positions with B linear dimensions (in mm)
Fig. 3.
A Delineation of continuous and recessed capsulolabral complex from the articular glenoid surface. Black arrows indicate continuous complex; arrowheads indicate recessed complex; yellow arrow indicates transition point between continuous and recessed complex. B Percent of specimens (by species) in which the capsulolabral complex was continuous with the glenoid articular surface
Samples were fixed in 10% formalin. Each glenoid was then decalcified in formic acid with ethylenediaminetetraacetic acid (Formical2000, Thermo Fisher, Pittsburgh, PA, USA), serially dehydrated in a graded ethanol series, and embedded in paraffin. Each glenoid segment was sectioned in a plane orthogonal to the glenoid surface and capturing the interface between the glenoid articular surface and labrum/capsule. The segments were cut into 7 μm slices by a Leica RM255 microtome (Leica Biosystems, Buffalo Grove, IL, USA). Histological sections were stained with either Mallory Trichrome (Electron Microscopy Sciences, Hatfield, PA, USA) or Safranin O/Fast Green (Electron Microscopy Sciences) according to standard histological protocols and photographed using an Olympus SZX16 stereoscope mounted with a SPOTFlex FX1520 camera (Olympus, Center Valley, PA, USA) [32, 33]. Insertion of the capsulolabral tissue onto the glenoid was qualitatively assessed and compared with gross morphology.
Unless otherwise indicated, data are displayed as mean ± standard deviation. Statistical analyses were performed using SPSS 27.0 software (IBM, Armonk, NY, USA). A two-way analysis of variance with post-hoc Tukey correction was performed to evaluate for differences among glenoid dimensions between and within species. Significance was set at P < .05.
Results
On gross inspection, the posterior capsule was qualitatively thicker than the anterior for all species (Fig. 1). When comparing dimensions within species, the superior-inferior (line A) and anterior-posterior (line B) dimensions demonstrated that the glenoid was taller than wide (A > B) in all animals (p < .005) except goats (p = .20). The anterosuperior-posteroinferior (line C) and anteroinferior-posterosuperior (line D) dimensions were equivalent (C = D) in all animals (Fig. 2). When comparing dimensions across species, line A was a similar length in dogs and goats (p = .58), dogs and humans (p = .27), and dogs and goats (p = .99). Line B was a similar length among dogs and humans (p = .07) and goats and pigs (p = .97). Line C was similar between pigs and goats (p = .15) as well as goats and humans (p = .35). Line D was similar between goats and humans (p = 1.0). All other combinations were significantly different across species (p < .05).
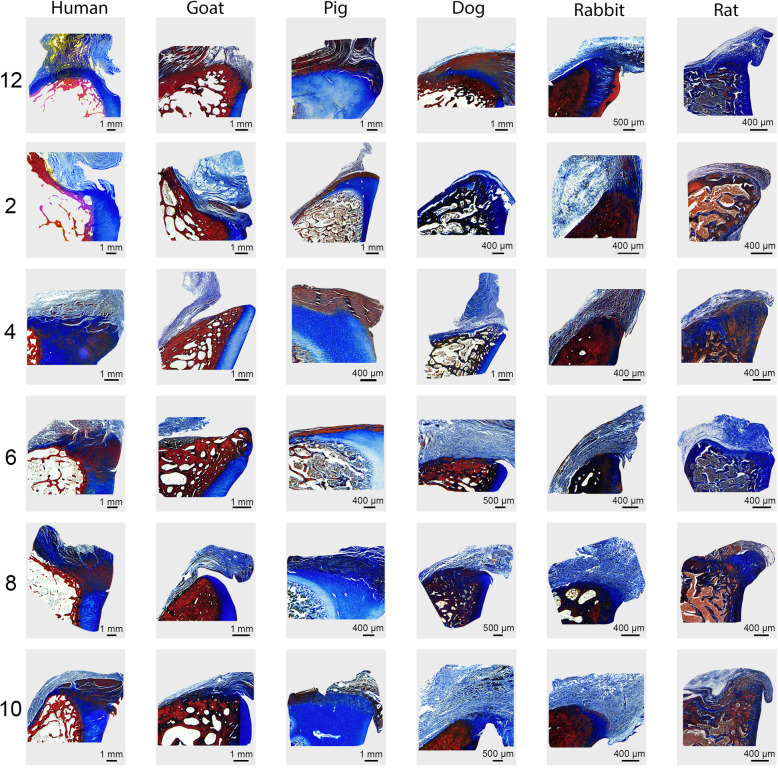
The capsulolabral complex was continuous with the glenoid surface from ~ 2 to 8 o’clock in humans, 6 to 12 o’clock in pigs, 9 to 12 o’clock in goats, 5 to 12 o’clock in the dogs, 7 to 12 o’clock in rabbits, and 9 to 12 o’clock in the rats, as shown on both gross anatomy and histology (Figs. 1 and 3B). In contrast to humans, no other species demonstrated an organized fibrocartilaginous labrum either grossly or histologically. Low and high magnification photographs of histological staining with Mallory trichrome or Safranin O/Fast green are shown in Figs. 4 and 5, and Supplemental Figures 1 and 2, respectively.
Fig. 4.
Lower magnification Mallory trichrome-stained sections of the glenocapsular junction at each clockface position across species. Box on human 12 o’clock image (top left) indicates higher magnification region of interest shown in Fig. 5
Fig. 5.
Higher magnification Mallory trichrome-stained sections of the glenocapsular junction at each clockface position across species
Discussion
The most important finding of this study was that none of the included animal species possessed a distinct fibrocartilaginous glenoid labrum discernible by either gross or histological evaluation, as compared to the human glenoid labrum (Fig. 6). The hypothesis that goats would be most similar to humans was partially supported, as the dimensions of the goat glenoid were most similar to that of humans, yet goats did not possess a true labrum and had different attachment sites for the capsulolabral complex.
Fig. 6.
Attachment site comparison between human (left) and goat (right) at 4 o’clock position, exemplifying the presence and absence of a discrete labral structure in humans compared to other animal species, respectively. Articular cartilage (AC), labrum (L), and capsule (C)
The human labrum and capsule have been extensively studied [3, 9, 10, 17, 26, 37, 38], but similar analyses of other species are scarce and inconsistent [1, 5, 21, 27, 34]. While non-human primates ostensibly possess shoulder anatomy closest to humans given our relatively recent evolutionary divergence, the use of non-human primates is expensive and increasingly tempered by ethical considerations. As with most animal models, studies of the structure and function of the non-human primate shoulder have focused extensively on the rotator cuff rather than the capsulolabral complex [28]. Additionally, an investigation of the effect of anterior shoulder dislocation on cadaveric simian shoulders only characterized the capsule and not the labrum [8]. Similarly, in a sheep model of capsular plication, effects on the labrum were not described [19].
Of the large animal models, the capsulolabral complex of the dog has been best characterized. In a study similar to the present, the labrum and capsular ligaments of the dog shoulder were characterized grossly and histologically according to clock face positions [35]. However, a direct comparison to human anatomy was only performed for gross morphology using formalin-fixed dog specimens. While a discrete labrum in dogs was described, fixation can often increase the apparent thickness of a multiple-layered capsule so as to give the appearance of a distinct structure [14]. In this prior study, only dog specimens were prepared for histology and there was inconsistent demonstration of a fibrocartilaginous labrum at each clockface position. These findings, with the aforementioned limitations, contrast the findings of the present study which did not find a discrete fibrocartilaginous labrum at any position, in any specimen, of the dog glenoid.
Approaching greater clinical relevance, albeit with a smaller animal, an anteroinferior labral injury (i.e., Bankart lesion) was simulated in a rabbit model by sharp incision of the capsulolabral complex at the glenoid edge [1]. Biomechanical and histological analyses were then performed at sequential timepoints to assess capsulolabral healing. While neotissue formation at the iatrogenic defect was demonstrated over time, a distinct fibrocartilaginous labrum was not apparent on the histological images. In related studies of a simulated anteroinferior dislocation in a rat model, the capsulolabral complex was incised at the glenoid junction and the humeral head was manually dislocated [25, 27]. As with the rabbit model above [1], histology demonstrated neotissue formation at the capsule-glenoid junction, but no distinct fibrocartilaginous labrum was clearly discernible. As suggested above, the past literature has largely assumed the presence of a distinct fibrocartilaginous labrum in animal models of labral injury and healing, yet review of the results does not conclusively support this position. In contrast, this study thoroughly evaluated the putative glenoid-labral interface of five model animals using morphological and histological analyses and found no distinct fibrocartilaginous labrum as clearly demonstrated in human shoulders. It is also notable that the capsulolabral complex was continuous with the glenoid surface anteriorly in humans, but not in any animal species. This unique feature of human shoulders may in part be due to differences in locomotion (i.e., bipedal vs. quadrupedal) and the increased propensity for anterior dislocation of the human shoulder, while acknowledging that the incidence and injury patterns of shoulder instability in animals is largely unreported.
This study is not without limitations. Similar to the existing literature on the topic, the results presented herein are largely qualitative and therefore subjective. While subjectivity is often inherent in the assessment of gross morphology and histology, the inclusion of a human specimens provided a positive control for defining the presence or absence of a fibrocartilaginous labrum in model animals. Nevertheless, inclusion of additional stains or immunohistochemistry may have further supported distinct structural and biochemical differences in the capsulolabral complex of humans and the included animal species. The five species of animals investigated herein are among the most commonly used for models of shoulder pathology, but similar characterization of additional species, including non-human primates, would be useful. The focus of this study was the characterization of the labrum and glenoid-labrum interface; while inspection of the capsule for glenohumeral ligaments was performed during gross dissection, these data were not formally included herein. Future characterization of the glenohumeral ligaments and other static stabilizers will be important for ongoing development of novel animal models, as these structures likely play a vital role in glenohumeral stability, especially in the absence of a discrete labrum, and in keeping with the conceptualization of the static stabilizers as a periarticular fiber system of the shoulder [15]. Furthermore, as we continue to learn about the synergistic relationships of passive and dynamic stabilizers of the human shoulder, parallel studies in animal models may illuminate how animals, absent a labrum, can have largely stable shoulders.
Conclusion
The species examined in the present study did not possess a labrum by gross or histological evaluation. While models using these animals may be acceptable for examining other shoulder pathologies, they are not adequate to evaluate labral pathology.
Supplementary Information
Additional file 1: Supplemental Figure 1. Lower magnification safranin-O-stained sections of the glenocapsular junction at each clockface position across species. Box on human 12 o’clock image (top left) indicates higher magnification region of interest show in Supplemental Figure 2.
Additional file 2: Supplemental Figure 2. Higher magnification Safranin-O-stained sections of the glenocapsular junction at each clockface position across species.
Acknowledgements
None.
Authors’ contributions
All authors were active participants, including contributing to the conception and design of the study, acquisition of data, and analysis and interpretation of data. All authors contributed by drafting, revising, editing and approving the final manuscript for submission.
Funding
This study was funded by the Albert B. Ferguson Orthopaedic Fund of the Pittsburgh Foundation (Grant #: AD2017–91229).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Approval was obtained from the Committee for Oversight of Research and Clinical Training Involving Decedents (CORID #131) from the University of Pittsburgh.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abe H, Itoi E, Yamamoto N, Minagawa H, Tomioka T, Kijima H, Shimada Y. Healing processes of the glenoid labral lesion in a rabbit model of shoulder dislocation. Tohoku J Exp Med. 2012;228(2):103–108. doi: 10.1620/tjem.228.103. [DOI] [PubMed] [Google Scholar]
- 2.Adam M, Attia AK, Alhammoud A, Aldahamsheh O, Al Ateeq Al Dosari M, Ahmed G. Arthroscopic Bankart repair for the acute anterior shoulder dislocation: systematic review and meta-analysis. Int Orthop. 2018;42(10):2413–2422. doi: 10.1007/s00264-018-4046-0. [DOI] [PubMed] [Google Scholar]
- 3.Alashkham A, Alraddadi A, Felts P, Soames R. Histology, vascularity and innervation of the glenoid labrum. J Orthop Surg. 2018;26:2309499018770900. doi: 10.1177/2309499018770900. [DOI] [PubMed] [Google Scholar]
- 4.Antonio GE, Griffith JF, Yu AB, Yung PSH, Chan KM, Ahuja AT. First-time shoulder dislocation: High prevalence of labral injury and age-related differences revealed by MR arthrography. J Magn Reson Imaging. 2007;26(4):983–991. doi: 10.1002/jmri.21092. [DOI] [PubMed] [Google Scholar]
- 5.Bardet J. Diagnosis of shoulder instability in dogs and cats: a retrospective study. J Am Anim Hosp Assoc. 1998;34(1):42–54. doi: 10.5326/15473317-34-1-42. [DOI] [PubMed] [Google Scholar]
- 6.Boffano M, Mortera S, Piana R. Management of the first episode of traumatic shoulder dislocation. EFORT Open Rev. 2017;2:35–40. doi: 10.1302/2058-5241.2.160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavert P. Glenoid labrum pathology. Orthop Traumatol Surg Res. 2015;101(1):S19–S24. doi: 10.1016/j.otsr.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Cooper ME, Hutchinson MR. The microscopic pathoanatomy of acute anterior shoulder dislocations in a simian model. Arthrosc J Arthrosc Relat Surg. 2002;18(6):618–623. doi: 10.1053/jars.2002.32588. [DOI] [PubMed] [Google Scholar]
- 9.Cordasco FA, Steinmann S, Flatow EL, Bigliani LU. Arthroscopic treatment of glenoid labral tears. Am J Sports Med. 1993;21:425–431. doi: 10.1177/036354659302100317. [DOI] [PubMed] [Google Scholar]
- 10.De C, Sp A, Sj O, Rf W, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74:46–52. doi: 10.2106/00004623-199274010-00007. [DOI] [PubMed] [Google Scholar]
- 11.Derwin KA, Baker AR, Iannotti JP, McCarron JA. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev. 2010;16(1):21–30. doi: 10.1089/ten.teb.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gigis I, Heikenfeld R, Kapinas A, Listringhaus R, Godolias G. Arthroscopic versus conservative treatment of first anterior dislocation of the shoulder in adolescents. J Pediatr Orthop. 2014;34(4):421–425. doi: 10.1097/BPO.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 13.Good CR, MacGillivray JD. Traumatic shoulder dislocation in the adolescent athlete: advances in surgical treatment. Curr Opin Pediatr. 2005;17(1):25–29. doi: 10.1097/01.mop.0000147905.92602.bb. [DOI] [PubMed] [Google Scholar]
- 14.Herbst E, Albers M, Burnham JM, Shaikh HS, Naendrup J-H, Fu FH, Musahl V. The anterolateral complex of the knee: a pictorial essay. Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 2017;25(4):1009–1014. doi: 10.1007/s00167-017-4449-2. [DOI] [PubMed] [Google Scholar]
- 15.Huber WP, Putz RV. Periarticular fiber system of the shoulder joint. Arthrosc J Arthrosc Relat Surg. 1997;13(6):680–691. doi: 10.1016/S0749-8063(97)90001-3. [DOI] [PubMed] [Google Scholar]
- 16.Itoi E, Lee S-B, Berglund LJ, Berge LL, An K-N. The effect of a glenoid defect on Anteroinferior stability of the shoulder after Bankart repair: a cadaveric study*. JBJS. 2000;82(1):35–46. doi: 10.2106/00004623-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Itoigawa Y, Itoi E. Anatomy of the capsulolabral complex and rotator interval related to glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):343–349. doi: 10.1007/s00167-015-3892-1. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsen BW, Johannsen HV, Suder P, Søjbjerg JO. Primary repair versus conservative treatment of first-time traumatic anterior dislocation of the shoulder: a randomized study with 10-year follow-up. Arthrosc J Arthrosc Relat Surg. 2007;23(2):118–123. doi: 10.1016/j.arthro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Kelly B, Turner A, Bansal M, Terry M, Wolf B, Warren R, Altchek D, Allen A. In vivo healing after capsular plication in an ovine shoulder model. Iowa Orthop J. 2005;25:95–101. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S-H, Ha K-I, Kim S-H. Bankart repair in traumatic anterior shoulder instability. Arthrosc J Arthrosc Relat Surg. 2002;18(7):755–763. doi: 10.1053/jars.2002.31701. [DOI] [PubMed] [Google Scholar]
- 21.Kovacevic D, Baker AR, Staugaitis SM, Kim M-S, Ricchetti ET, Derwin KA. Development of an arthroscopic joint capsule injury model in the canine shoulder. PLoS One. 2016;11(1):e0147949. doi: 10.1371/journal.pone.0147949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo UG, Loppini M, Rizzello G, Ciuffreda M, Maffulli N, Denaro V. Management of Primary Acute Anterior Shoulder Dislocation: systematic review and quantitative synthesis of the literature. Arthrosc J Arthrosc Relat Surg. 2014;30(4):506–522. doi: 10.1016/j.arthro.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Mohtadi NGH, Chan DS, Hollinshead RM, Boorman RS, Hiemstra LA, Lo IKY, Hannaford HN, Fredine J, Sasyniuk TM, Paolucci EO. A randomized clinical trial comparing open and arthroscopic stabilization for recurrent traumatic anterior shoulder instability: two-year follow-up with disease-specific quality-of-life outcomes. JBJS. 2014;96(5):353–360. doi: 10.2106/JBJS.L.01656. [DOI] [PubMed] [Google Scholar]
- 24.Monk AP, Garfjeld Roberts P, Logishetty K, Price AJ, Kulkarni R, Rangan A, Rees JL. Evidence in managing traumatic anterior shoulder instability: a scoping review. Br J Sports Med. 2015;49(5):307–311. doi: 10.1136/bjsports-2013-092296. [DOI] [PubMed] [Google Scholar]
- 25.Mulcahey MK, Marshall M, Gallacher SE, Kaback LA, Blaine TA. Factors expressed in an animal model of Anteroinferior Glenohumeral instability. Orthop J Sports Med. 2015;3:2325967115599733. doi: 10.1177/2325967115599733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ovesen J, Nielsen S. Stability of the shoulder joint: cadaver study of stabilizing structures. Acta Orthop Scand. 1985;56(2):149–151. doi: 10.3109/17453678508994342. [DOI] [PubMed] [Google Scholar]
- 27.Packer JD, Varthi AG, Zhu DS, Javier FG, Young JD, Garver JV, Henry H, Tommasini SM, Blaine TA. Ibuprofen impairs capsulolabral healing in a rat model of anterior glenohumeral instability. J Shoulder Elb Surg. 2018;27(2):315–324. doi: 10.1016/j.jse.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Plate JF, Bates CM, Mannava S, Smith TL, Jorgensen MJ, Register TC, Stehle JR, High KP, Shively CA, Kaplan JR, Saul KR, Tuohy CJ. Age-related degenerative functional, radiographic, and histological changes of the shoulder in nonhuman primates. J Shoulder Elb Surg. 2013;22(8):1019–1029. doi: 10.1016/j.jse.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polyzois I, Dattani R, Gupta R, Levy O, Narvani AA. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4(2):104–108. [PMC free article] [PubMed] [Google Scholar]
- 30.Porcellini G, Campi F, Pegreffi F, Castagna A, Paladini P. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. JBJS. 2009;91(11):2537–2542. doi: 10.2106/JBJS.H.01126. [DOI] [PubMed] [Google Scholar]
- 31.Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19(4):493–499. doi: 10.1016/j.knee.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothrauff BB, Sasaki H, Kihara S, Overholt KJ, Gottardi R, Lin H, Fu FH, Tuan RS, Alexander PG. Point-of-care procedure for enhancement of meniscal healing in a goat model utilizing infrapatellar fat pad–derived stromal vascular fraction cells seeded in Photocrosslinkable hydrogel. Am J Sports Med. 2019;47:3396–3405. doi: 10.1177/0363546519880468. [DOI] [PubMed] [Google Scholar]
- 33.Rothrauff BB, Smith CA, Ferrer GA, Novaretti JV, Pauyo T, Chao T, Hirsch D, Beaudry MF, Herbst E, Tuan RS, Debski RE, Musahl V. The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats. J Shoulder Elb Surg. 2019;28(4):654–664. doi: 10.1016/j.jse.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 34.Sager M, Herten M, Dreiner L, Engelhardt E, Assheuer J, Kramer M, Jäger M. Histological variations of the glenoid labrum in dogs. Anat Histol Embryol. 2013;42(6):438–447. doi: 10.1111/ahe.12035. [DOI] [PubMed] [Google Scholar]
- 35.Sager M, Herten M, Ruchay S, Assheuer J, Kramer M, Jäger M. The anatomy of the glenoid labrum: a comparison between human and dog. Comp Med. 2009;59(5):465–475. [PMC free article] [PubMed] [Google Scholar]
- 36.Stubbs AJ, Howse EA, Mannava S. Tissue engineering and the future of hip cartilage, labrum and ligamentum teres. J Hip Preserv Surg. 2016;3(1):23–29. doi: 10.1093/jhps/hnv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tokish LCJM, McBratney MCM, Solomon CDJ, LeClere LL, Dewing LCB, Provencher CMT. Arthroscopic repair of circumferential lesions of the glenoid labrum. JBJS. 2009;91(12):2795–2802. doi: 10.2106/JBJS.H.01241. [DOI] [PubMed] [Google Scholar]
- 38.Wright-Fitzgerald AS, Balceniuk MD, Burrows AM. Shouldering the burdens of locomotion and posture: Glenohumeral joint structure in prosimians. Anat Rec. 2010;293(4):680–691. doi: 10.1002/ar.21127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Figure 1. Lower magnification safranin-O-stained sections of the glenocapsular junction at each clockface position across species. Box on human 12 o’clock image (top left) indicates higher magnification region of interest show in Supplemental Figure 2.
Additional file 2: Supplemental Figure 2. Higher magnification Safranin-O-stained sections of the glenocapsular junction at each clockface position across species.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.