Abstract
In the present study, eggs and copepodid stages of Argulus japonicus were treated with ethanol and methanol extract of Azadirachta indica (neem) leaf and its antiparasitic efficacy (AE %) was determined. The experiments were performed in triplicate along with the positive (2% DMSO) and negative (without DMSO and extract) control groups. The reduced cumulative hatching percentage of eggs by 13% (in ethanolic) and 17% (in methanolic) extract of neem leaf at 1.5 g L−1 was obtained during 15-day exposure compared to the control group showing 70–85% eggs hatching. The AE of 100% for ethanolic and 91.66% for methanolic extract against the copepodid stage was found at 1.25 and 1.5 g L−1 respectively in 6 h. The histological analysis of the eggs showed the undifferentiated decaying mass of cells with extensively damaged eggs when treated with ethanolic extract of neem leaf. Further, severe degeneration in the branchial region, digestive tract and eye cells was observed in the copepodids treated with ethanol extract than the methanol extract. The terpenoids a potential antiparasitic compound of ethanolic extract produced more AE than the methanolic extract. Thus, the ethanolic extract of neem leaf can be potentially utilized as a natural parasiticide to disrupt the egg and other life phases of A. japonicus.
Electronic supplementary material
The online version of this article (10.1007/s12639-021-01355-4) contains supplementary material, which is available to authorized users.
Keywords: Azadirachta indica, Ethanol extract, Antiparasitic efficacy, Argulus japonicus, Eggs, Copepodid
Introduction
Modern aquaculture practices rely on high stocking densities and feeding rates, which creates stressful environmental conditions, leading to many infectious diseases (Mishra et al. 2017). Argulosis is one such disease caused by a crustacean parasite Argulus which contributes 20% of the total parasitic infection reported by the National Surveillance Programme for Aquatic Animal Diseases (NSPAAD) during 2014–2018 in aquaculture system (Sahoo et al. 2020). The Argulus sp. is a prolific breeder with a direct life cycle with several stages: eggs, metanauplius, copepodid, juvenile, and adult stage (Shimura 1983; Shafir and Van 1986; Rushton-Mellor 1994). The eggs hatch into metanauplii and subsequently into copepodid that immediately parasitizes the fish (Woo 1995). Argulus attaches with the host through suckers and feeds on blood and mucus that irritates the fish, cause dermal lesions, and immunosuppression (Yildiz and Kumantas 2002; Walker et al. 2004; Saurabh et al. 2011). It has been recognized as a major constraint in aquatic animal health since it causes a significant economic loss through morbidity and mortality of both wild and farmed fish (Noga 2010; Sahoo et al. 2013a, b; Muchlisin et al. 2014; Kar et al. 2017). Argulosis alters the general morphology of the infected fish-like scale loss, discolor, pathological changes, affecting the marketability of contaminated fish. Besides, the parasite acts as a vector for carp spring viremia (SVCV) (Ahne et al. 2002). Argulus japonicus is an opportunistic pathogen, and infections lead to catastrophic fish kills (Taylor et al. 2006) in a short period. Therefore, the management of argulosis must be the top priority to save the aquaculture industry from this massive loss.
Various management strategies against argulosis in aquaculture have been discussed in a recent review article by Kumari et al. 2020. Chemotherapeutics are most common in use to control parasitic disease in aquaculture. Still, they are highly toxic to humans and fishes with less degradability in the environment (Rath et al. 2016) and, most critically, the parasitic resistance development (Hemaprasanth et al. 2012). Further, the Integrated Pest Management (IPM) and good husbandry practices result in a significant elimination of Argulus parasite from the aquaculture system (Walker et al. 2004; Bandilla et al. 2005; Hakalahti et al. 2008). The current approaches in developing vaccines against Argulus siamensis could only partially protect the fish (Kar et al. 2017). Therefore, it is the need of the hour to find a sound management option to control hazardous argulosis, and recently phytotherapeutics offering such prospects need to be looked into thoroughly.
Azadirachta indica (neem) is a member of the family Meliaceae, regarded as a "village pharmacy" by the people of India (Raman 2017). Neem is one of the most promising medicinal plants with broad-spectrum antibacterial, antiviral, and antiparasitic activities in the aquaculture industry (Biswas et al. 2002; Kumar et al. 2012a, b; Devi et al. 2019; Kumari et al. 2019). This plant is one of the abundant sources of secondary metabolite especially, the terpenoids which is known for its broad pharmaceutical activities (Pandreka et al. 2015). The bioactive compounds have potent effects on Argulus parasite as reported for azadirachtin (Kumar et al. 2012b), piperine (Kumar et al. 2012a), rotenone and nicotine (Banerjee and Saha 2013). The crude plant extracts do exhibit antiparasitic activity against Argulus revealed by earlier workers like neem extract (Mamadou et al. 2013), juice of papaya seeds (Kismiyati et al. 2015) aqueous extract of neem leaf (Kumari et al. 2019), Moringa oleifera leaf extract (Idris and Mahasri 2020). Neem extract also demonstrated an effective chemotherapeutic on crustaceans with a minimal damaging effect on non‐target aquatic organisms (Goktepe et al. 2004).
A very few comprehensive approaches have been made towards the treatment of different larval stages of the Argulus parasite. Since Argulus spawns continuously and lay thousands of eggs in one stretch, targeting its various life stages could make possible inputs for formulating a control measure or complete eradication of argulosis from the aquaculture system (Banerjee and Saha 2013). In this context, the present study aimed to evaluate the antiparasitic efficacy (AE) of ethanolic and methanolic extracts of A. indica leaf on eggs hatchability and mortality of copepodid stage of A. japonicus under in vitro condition.
Material and methods
Collection and preparation of neem leaf extract
The fresh leaves of A. indica were collected from the old campus of ICAR-CIFE, Mumbai, in March 2019. The leaves were washed thoroughly under running tap water, followed by soaking the leaves in a solution of KMnO4 of 2 ppm for 5 min to avoid any fungal infection. Finally, the leaves were washed in distilled water to elude toxicity may cause due to KMnO4. The washed leaf was then dried at room temperature, under shade for 8 days, and crushed to a fine powder in an electric blender. The coarse leaf powder was lyophilized at −54 °C to confirm complete moisture removal and was stored in air-tight plastic containers for further use. Ten grams of dried leaf powder was dissolved in each 100 mL of methanol (absolute) and ethanol (absolute) in a 250 ml conical flask (1: 10 (w/v)). Extraction was allowed for 48 h on a laboratory shaker (ROTEK LSV, India) at 108 rpm, subsequently filtered using Whatman no.1 filter paper. The filtrate was then concentrated under reduced pressure using a vacuum rotary evaporator until solvents were completely evaporated to get the semisolid crude extract. The concentrated extracts were stored in air-tight amber glass bottles at 4 °C for further use.
Preparation of working test solution
The stock solution was prepared by dissolving each pre-weighed concentrated ethanol and methanol extract of neem leaf in 2% dimethyl sulphoxide (DMSO) solution (Ling et al. 2012). The strength of each extract was calculated, and working test solutions were prepared as per the experimental requirement.
Phytochemical analysis
The phytochemical screening of the prepared extracts of neem leaf was determined by following the standard methods (Hashemi and Hossain 2016; Kumar et al. 2018).
Maintenance of various larval stages of Argulus japonicus under laboratory condition
The required population of A. japonicus was maintained in a glass aquaria tank in ICAR-CIFE Central Wet Lab along with the host Carassius auratus. The goldfishes were fed with commercial pellet feed and the water quality parameters like pH, temperature, and dissolved oxygen were checked intermittently to assess their influence on the escape of the parasite from the system. Once the desired number of adult Argulus was gained, the infested goldfish was used to transmit the infection to other naïve fish maintained in separate FRP tanks of forty-liter capacity to establish a stock of parasites of all its life stages. The egg clutches of the Argulus parasite were recovered from the sidewalls and base of the FRP tanks, aeration tube, air stones, and the substratum (rock and marble piece) provided in the tank. The eggs collected were incubated in a glass beaker of 1 L capacity, containing filtered tank water, provided with proper aeration and daylight exposure. The beaker was periodically refilled with filtered tank water to maintain the level and avoid drying the eggs and subsequent mortality. The eggs clutches were observed under a microscope at 10 X magnification for their gradual development at 24 h intervals, the photographs were captured and morphological changes were recorded. On day 9th to 12th, the nauplius hatches to metanauplii subsequently develop to copepodid, juvenile, and adult stage with succeeding molting. The present study was mainly focused on the treatment of eggs and the copepodid stage of A. japonicus.
Comparative effects on hatching percentage of eggs of A. japonicus treated with ethanol and methanol extracts of neem leaf
Treatments were given when most of the eggs of Argulus were discovered for eyespots. A total of 2000 eggs with eyespots were immersed in a solution of ethanolic and methanolic extract of neem leaves of concentrations 1.0, 1.25, and 1.5 g L−1 for T1, T2, and T3 respectively. Each treatment group was stocked with 100 eggs in the separate beaker of 250 mL capacity containing 100 mL solutions of each extract. The experiment was conducted in triplicate for fifteen days and the positive control containing 2% DMSO without extract and negative control without DMSO and extract was also maintained throughout the experimentation. The experimental setup was maintained in natural photoperiod (12:12 h) of daylight and darkness for the development and hatching of eggs. The developmental stages of eggs were observed under the microscope by taking the eggs clutches in a cavity slide along with the solution of the respective treatment groups. Care was taken to avoid the breakage of eggs clutches and eggs throughout the experimental period. An untreated positive and negative control set was maintained under similar physicochemical conditions. The respective extracts of a similar concentration in each treatment group were refilled regularly. The hatched metanauplii were removed and counted daily from hatching to day 15th of the exposure. The experimental setup was extended from 12—15 days, depending on the hatchability of eggs under the control group compared with the experimental group. The egg viability in terms of cumulative hatching percentage was determined by counting empty eggshells with longitudinal hatching furrows. Further, the treated unhatched eggs were fixed in Davidson’s fixative for histological analysis.
Comparative in vitro antiparasitic efficacy of ethanolic and methanolic extracts of neem leaf against copepodid stage of A. japonicus.
After 3–5 days of hatching, the suckers appeared in the copepodid stage of Argulus that could parasitize the fish when exposed to C. auratus. This developed stage was used for conducting the in vitro experiment. The test was performed in a 96 well microtitre plate in triplicate along with positive (2% DMSO and without extract) and negative (without DMSO and extracts) control. Two hundreds of actively swimming copepodids were collected from a glass beaker with the help of a dropper. Ten numbers of active copepodids were released in each well of the microtitre plate containing ethanolic and methanolic extracts of neem leaf of concentrations viz. T1 (1.0), T2 (1.25), and T3 (1.5) g L−1, respectively. The treated copepodids were observed periodically for mortality at 30 min intervals for 6 h under the dissection microscope by taking the larvae in a Petri-plate along with the extract. Death of copepodid was assured as the complete motionless condition and settling in the bottom of the plate. Finally, the AE % of neem leaf for copepodid stage of A. japonicus was determined by following the equation (Wang et al. 2009):
where AE = antiparasitic efficacy, B = mean number of surviving parasites in the positive control and T = mean number of surviving parasites in treatment.
Histological analysis of treated eggs and copepodid stage of A. japonicus
This would probably be the novel attempt on the histological study on eggs and copepodid stage of A. japonicus treated with herbal-based solutions reported so far. The eggs and copepodid stages of A. japonicus from control and treated with ethanolic and methanol extract of neem leaf were collected and fixed in Davidson’s fixative for 24 h. Specimens were washed in tap water thoroughly and passed in serial grades of alcohol for dehydration. Cleared in xylene and embedded in paraffin wax in the histo-embedder machine (LEICA-EG1140C). Paraffin wax tissue blocks were prepared for sectioning at 4 microns thickness using a rotary microtome (LEICA-RM2125RT). The obtained tissue sections were collected on glass slides, deparaffinized, differentially stained by hematoxylin and eosin, and examined using light microscopy (Roberts et al. 2001).
Statistical analysis
The data obtained were statistically analyzed by mathematical package SPSS version 16, and the graphs were illustrated using Microsoft excel 2010 graph sheet. The cumulative percentage hatching (% CH), antiparasitic efficacy (AE), and median effective concentration (EC50) of in vitro tests were estimated at the 95% confidence interval (Finney 1971; Wang et al. 2009) by probit analysis.
Results and discussion
Argulus parasites were collected from ornamental fish species Carasius auratus (Mousavi et al. 2011; Kumari et al. 2018) found most prevalent during the summer season (Saha and Bandyopadhyay 2015; Gonzalez et al. 2018). The water quality parameters like pH of 6.9 ± 0.5; temperature 26 ± 2 °C and dissolved oxygen of 4.9 ± 0.5 were obtained in the Argulus infected goldfish tank. The workers revealed that the pH and DO do not have significant impact on the prevalence of Argulus infection but directly correlated with the temperatures (Yunikasari and Mahasri 2020). The phytochemicals, like alkaloids, flavonoids, terpenoids, and saponin, except for tannin, were obtained in the neem leaf ethanol extract. Other than terpenoids, the alkaloids, flavonoids, saponin, and tannin were found in the methanolic extract of neem leaf (Table 1). Nearly comparable results were obtained in the study of Kumari et al. (2019) for ethanolic and methanolic extract of neem leaf except for tannin that might be due to the seasonal variations in the phytoconstituents proportions. Similarly, Kavitha et al. (2017); Hashemi and Hossain (2016) also reported for the neem leaf extracts. In contrast, Kumar et al. (2018) reported the absence of terpenoids in the methanolic extract of neem leaf. These fluctuations might be due to the variations in geographical location, plant age, and extraction process, directly influencing the phytoconstituents type and content (Vazquez-Leon et al. 2017). The female adult Argulus lays eggs coated with gelatin make them sticky, which helps the eggs attach firmly to the substratum (Walker et al. 2004; Sahoo et al. 2013a, b). The growing eggs were characterized by eyespots appearance on 5–7 days of eggs laid followed by the development of organs attached to the thorax from day 7th to 9th. And the third stage was the embryo movement observed from day 9th to 11th, 24–48 h before the eggs hatched to metanauplii (Kismiyati et al. 2015; Idris and Mahasri 2020), shown in (Fig. 1). A significant reduction in the cumulative hatching percentage of eggs of A. japonicus by 13% (ethanol) and 17% (methanol) extracts at a concentration of 1.5 g L−1 than the control groups showed 70–85% hatching on day 15th (Fig. 2). Recently, Idris and Mahasri (2020) found 100% failure in the hatching of eggs of A. japonicus when treated with aqueous extract of Moringa oleifera leaf at 8% concentration.
Table 1.
Phytochemical screening of ethanol and methanol extracts from neem leaves
| Sl. No | Phytochemical tests | Extracts of A. indica leaf Ethanolic extract Methanolic extract |
|
|---|---|---|---|
| 1 | Test for alkaloids Mayer’s test |
+ ve | + ve |
| 2 |
Test for flavonoids Alkaline reagent (NaOH) |
+ ve | + ve |
| 3 |
Test for saponin Saponin test |
+ ve | + ve |
| 4 |
Test for terpenoids Salkowski's test |
+ ve | - ve |
| 5 |
Test for tannin Ferric chloride test |
+ ve | + ve |
Here, (+ ve): presence; (- ve): absence
Fig. 1.
The larval developmental stages of Argulus japonicus were observed under the microscope at 10X objective. a Eggs clutch laid on the FRP tank (seen through the naked eye). b The beaker containing scraped eggs clutches immersed in water. c One day old eggs with elliptical shape, arranged in rows and columns. d 5–7 day old eggs with distinct eyespots and developing embryo. e 7–9 day old eggs with characteristic embryo developing inside, which shows movements from day 9th to 11 before it hatches. f On day 12th, nauplius hatched into metanaupli with distinct eyes, antenna, and fused abdominal appendages. g On day 14th, the metanauplii undergo molting and structural development with the appearance of chelated legs (a modified form of antennules). h 15-day old copepodid with free abdominal appendages, the appearance of a distinct abdominal region, the chelated legs start converting into sucker with developing branchial chamber. i Day 16th–17th sexual dimorphism (male and female) sex organs develop, distinct chelate leg developing into sucker is observed with a well-developed digestive system and branchial region. j On day 20, copepodid develops to juvenile with well developing distinct organs (suckers, branchial chambers, digestive organs, sexual organs) with the horseshoe-shaped body
Fig. 2.
Cumulative hatching percentage (% CH) of Argulus eggs treated with various concentrations of a ethanolic and b methanolic extract of neem leaf for 15 days
Further, severe damage to the eggs of A. japonicus at a percentage of 100% at 100 g L−1 was observed when treated with the aqueous juice of papaya seeds (Kismiyati et al. 2015). It has been observed that Brachionus species form a symbiotic relationship with the eggs of Argulus to feed on the gelatinous coating of eggs that helps them in the hatching process. The removal of Rotifer philodina by mechanical means results in 100% failure in the hatching of eggs of Argulus (Banerjee et al. 2016). This could be one of the possible reasons for the significantly reduced hatching per cent of A. japonicus as the treated eggs were completely devoid of epicommensals than the control group shown in supplementary Figs. 1 and 2. The treated eggs with ethanol and methanol extracts were turned dark brown to black discoloration with wrinkles on the surfaces of the eggs, probably due to extensive damage and degeneration process, consequently making the embryo undeveloped by altering the hydro-mineral content of the egg. The extensive damage to eggs and subsequent wrinkle formation might be due to the scraping off mucus and egg cell walls caused by plant phytoconstituents (Idris and Mahasri 2020).
The larvae hatched out as metanauplii within 12–14 days, which exhibits antennae structure, hook-like first maxillula, shorter abdomen with indistinct gonad, and sexual dimorphism (Fryer 1982). Metanauplii subsequently developed to copepodid stage upon successive molting, characterized by chelated legs transforms to sucker with a well-developed digestive tract, abdomen, and sexually dimorphic larvae were able to attach with the host (Shafir and Van 1986). The copepodids when treated with ethanolic and methanolic extract of neem leaf it showed antiparasitic effects of 100 and 91.66% with median effective concentration (EC50) of 0.92 and 1.11 g L−1 respectively, in 6 h (Table 2, Fig. 3). The control groups were found with actively swimming Argulus larvae with no mortality during 6 h of incubation. Hakalahti et al. (2004) reported exposure of emamectin benzoate, enabled to cause 100% killing of copepodid of A. coregoni.
Table 2.
Comparative antiparasitic efficacy of ethanolic and methanolic extract of neem leaf against copepodid stage of Argulus japonicus at different concentrations and time periods
| Treatments | Time (hours) AE (in %) = {(B-T) × 100}/B |
|||
|---|---|---|---|---|
| Ethanolic extract of neem leaf | Methanolic extract of neem leaf | |||
| 3 h | 6 h | 3 h | 6 h | |
| T1 | 0 | 16.66 | 0 | 16.66 |
| T2 | 50.00 | 100 | 16.66 | 41.66 |
| T3 | 58.33 | - | 50.00 | 91.66 |
Fig. 3.
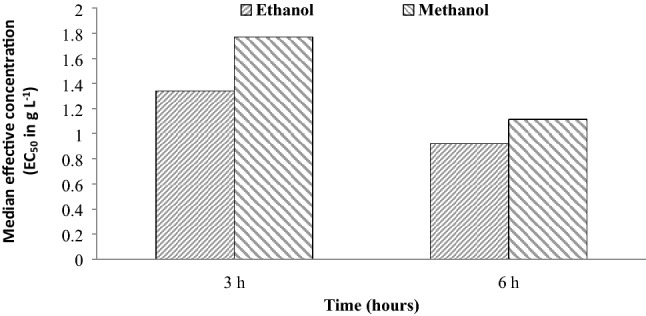
Median effective concentration (EC50) of ethanolic and methanolic extract against copepodid stage of A. japonicus at different concentrations with respect to time periods
Remarkable histological alterations were observed in the treated (1.5 g L−1 ethanolic and methanolic extract of neem leaf) eggs and copepodids. The histological sections of treated eggs showed an undifferentiated mass that probably hampers the embryo developing inside (Fig. 4). Banerjee et al. (2014) revealed similar deformities in embryo development of Argulus when treated with aqueous extract of neem leaf. The treated copepodids stages showed severe degenerative changes in the branchial region (affect respiratory function), digestive tract (impairs with nutrients absorption and digestion process), and marked destruction in the eye cells (cause osmotic shock) (Fig. 5) might be the reason for the mortality of the parasite. In the present study, the extent of histological change was found more prominent for eggs and copepodids treated with ethanol extract than the methanolic extract of neem leaf. The control group showed a normal histological section of eggs and copepodid. The probable reason for the ethanol extract of being more effective than methanol extract might be the high proportion of terpenoids. The terpenoids cause the deficiency of dissolved oxygen in water, which may cause the death of eggs, larvae, and adult parasites (Mordue and Nisbet 2000). Not only terpenoids but the other biochemical compounds also exert significant antimicrobial activity. Because of that, the methanolic extract of neem leaf deficient in terpenoids could also exhibit antiparasitic activity. The compound tannins react with protein and interfere with the absorption process on the embryo impair with the Argulus development further. Saponins, triterpenoid, and flavonoids have been reported for their potential to disrupt the egg membrane, permeability that causes cell dehydration; ultimately the parasitic death (Sari 2014; Kismiyati et al. 2015). Alkaloids can disrupt the peptidoglycan constituent of the parasitic cells (Idris and Mahasri 2020).
Fig. 4.

Histological microphotograph of eggs of A. japonicus. a Control group: showing normal histoarchitecture of embryo developing cells (EDC) inside the eggs and b treatment (Methanolic neem extract) where arrows indicating decaying embryonic cells (DEC) with undifferentiated cellular mass (UCM) architecture. c Ethanolic extract group: showing abnormal embryonic development with extensive DEC and completely undistinguishable cellular mass (UCM) inside the incubated eggs, EES: Empty egg shell (H&E 10 x)
Fig. 5.
Microscopic photograph of ventral view of copepodid stage of A. japonicus with a control group showing normal histoarchitecture. b Treated group (ethanolic neem leaf extract) showing damaged branchial region (BR), digestive tract (DT) and cells of compound eye region (EC) (indicated by arrows) H&E, 10x
Further, azadirachtin is the most promising antiparasitic biocompound of neem that alters the neuroendocrine regulation and molting hormone activity and end-point effect as impair the parasite development (Mordue and Nisbet 2000; Kumar et al. 2012a, b). Although antiparasitic properties of many plant extracts and isolated natural products have been tested in vivo and in vitro effectively explained in the recent article by (Kumari et al. 2020); however, there is scanty of information on the herbal treatment for larval of Argulus parasite. Recently, Sahoo et al. 2019 revealed that the neem leaf extracts could significantly upregulate the expression of ion channel genes such as GABA (Gamma-aminobutyric acid), ICA-3 (Ion channel activator protein 1–4) and NTR (Neurotransmitters proteins) of Argulus siamensis than the other herbal and antiparasitic drugs. These outcomes evidence the potential role of A. indica leaf extract in the effective killing of Argulus parasite. Additionally, the findings of this study may pave the way for development of effective, alternative drugs that can target all the life stages of A. japonicus simultaneously.
Conclusions
The findings of the present study reveal the high antiparasitic effect of ethanolic extract for the eggs and copepodid stage of A. japonicus than the methanolic extract of neem leaf. The extent of histoarchitectural alterations with the treated eggs and copepodid was found more conspicuous in the ethanol extract of neem leaf. Thus the ethanolic extract of neem leaf can be utilized as a natural insecticide to disrupt the egg and other life phases of A. japonicus in the aquaculture industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
The authors owe their thankfulness and gratitude to ICAR, The Director and Vice-Chancellor, ICAR-Central Institute of Fisheries Education, Mumbai, India, for providing all the facilities required for the present work.
Data availability
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the use of laboratory animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahne W, Bjorklund HV, Essbauer S, Fijan N, Kurath G, Winton JR. Spring viremia of carp (SVC) Dis Aquat Org. 2002;52:261–272. doi: 10.3354/dao052261. [DOI] [PubMed] [Google Scholar]
- Bandilla M, Hakalahti T, Hudson PJ, Valtonen ET. Aggregation of Argulus coregoni (Crustacea: Branchiura) on rainbow trout (Oncorhynchus mykiss): a consequence of host susceptibility or exposure. Parasitol. 2005;130:169–176. doi: 10.1017/S0031182004006407. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Manna S, Saha SK. Effect of aqueous extract of Azadirachta indica, A. Juss (neem) leaf on oocyte maturation, oviposition, reproductive potentials and embryonic development of a freshwater fish ectoparasite Argulus bengalensis Ramakrishna, 1951 (Crustacea: Branchiura) Parasitol Res. 2014;113:4641–4650. doi: 10.1007/s00436-014-4155-7. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Poddar S, Manna S, Saha SK. Mutualistic association of rotifer Philodina roseola with the branchiuran fish ectoparasite Argulus bengalensis at its embryonic stage. Biol Lett. 2016;12:20151043. doi: 10.1098/rsbl.2015.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Saha SK. Biphasic control of Argulus bengalensis Ramakrishna (1951) (Crustacea: Branchiura) with plant derivatives. Aquaculture. 2013;414:202–209. doi: 10.1016/j.aquaculture.2013.07.044. [DOI] [Google Scholar]
- Biswas K, Chattopadhyay RK, Banerjee U, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica) Curr Sci. 2002;82:1336–1345. [Google Scholar]
- Devi NP, Das SK, Sanjukta RK, Singh SG. A comparative study on antibacterial activity of integumentary extract of selected freshwater fish Species and Neem extracts against gram-positive and Gram-Negative bacteria. J Entomol Zool Stud. 2019;7:1352–1355. [Google Scholar]
- Finney DJ. Probit Analysis. 3. Cambridge: Cambridge University Press; 1971. p. 333. [Google Scholar]
- Fryer G (1982) The parasitic Copepoda and Branchiura of British freshwater fishes. A handbook and key- Freshw Biol Assoc Sci Publ 46–87
- Goktepe I, Portier R, Ahmedna M. Ecological risk assessment of neem-based pesticides. Environ Sci Health. 2004;39:311–320. doi: 10.1081/PFC-120030244. [DOI] [PubMed] [Google Scholar]
- Gonzalez TA, Becker JA, Hutson KS. Parasite dispersal from the ornamental goldfish trade. Adv Parasitol. 2018;100:239–281. doi: 10.1016/bs.apar.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Hakalahti T, Lankinen Y, Valtonen ET. Efficacy of emamectin benzoate in the control of Argulus coregoni (Crustacea: Branchiura) on rainbow trout Oncorhynchus mykiss. Dis Aquat Org. 2004;60:197–204. doi: 10.3354/dao060197. [DOI] [PubMed] [Google Scholar]
- Hakalahti T, Mikheev VN, Valtonen ET. Control of freshwater fish louse Argulus coregoni: a step towards an integrated management strategy. Dis Aquat Org. 2008;82:67–77. doi: 10.3354/dao01971. [DOI] [PubMed] [Google Scholar]
- Hashemi ZSS, Hossain MA. Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pacific Sci Rev A: Nat Sci Eng. 2016;18:128–131. [Google Scholar]
- Hemaprasanth KP, Kar B, Garnayak SK, Mohanty J, Jena JK, Sahoo PK. Efficacy of two avermectins, doramectin and ivermectin against Argulus siamensis infestation in Indian major carp, Labeo rohita. Vet Parasitol. 2012;190:297–304. doi: 10.1016/j.vetpar.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Idris F, Mahasri G. Different concentration influence of Moringa oleifera leaf aqueous extract immersion against Argulus japonicus egg damage. IOP Conf Series: Earth Environ Sci. 2020;441:0121–131. [Google Scholar]
- Kar B, Mohapatra A, Mohanty J, Sahoo PK. Evaluation of ribosomal P0 peptide as a vaccine candidate against Argulus siamensis in Labeo rohita. Open Life Sci. 2017;12:99–108. doi: 10.1515/biol-2017-0011. [DOI] [Google Scholar]
- Kavitha M, Raja M, Kamaraj C, Karthik RR, Balasubramaniam V. In vitro antimicrobial activity of Azadirachta indica (Leaves) against fish pathogenic bacteria isolated from naturally infected Dawkinsia filamentosa (Blackspot barb) Med Aromat Plants. 2017;6:2167–412. [Google Scholar]
- Kismiyati K, Subekti S, Inaya AFN. The influence of Papaya Seed (Carica papaya) toward the damage eggs of Argulus japonicus. J Ilmiah Perikanan dan Kelautan. 2015;7:159–164. [Google Scholar]
- Kumar A, Raman RP, Kumar K, Pandey PK, Kumar V, Mohanty S, Kumar S. Antiparasitic efficacy of piperine against Argulus spp. on Carassius auratus (Linn. 1758): in vitro and in vivo study. Parasitol Res. 2012;111:2071–2076. doi: 10.1007/s00436-012-3054-z. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sharma S, Devi L. Investigation of total phenolic, flavonoid contents and antioxidant activity from extracts of Azadirachta indica of Bundelkhand Region. Int J Life Sci Sci. 2018;2455:1716. [Google Scholar]
- Kumar S, Raman RP, Kumar K, Pandey PK, Kumar N, Mohanty S, Kumar A. In vitro and in vivo antiparasitic activity of Azadirachtin against Argulus spp. in Carassius auratus (Linn. 1758) Parasitol Res. 2012;110:1795–1800. doi: 10.1007/s00436-011-2701-0. [DOI] [PubMed] [Google Scholar]
- Kumari P, Kumar S, Paul T, Raman RP, Rajendran KV. Potential Management strategies against argulosis in aquaculture. Aquacult Mag. 2020;6:30–36. [Google Scholar]
- Kumari P, Kumar S, Ramesh M, Shameena S, Deo AD, Rajendran KV, Raman RP. Antiparasitic effect of aqueous and organic solvent extracts of Azadirachta indica leaf against Argulus japonicus in Carassius auratus. Aquaculture. 2019;511:634175. doi: 10.1016/j.aquaculture.2019.05.060. [DOI] [Google Scholar]
- Kumari P, Ramesh M, Shameena SS, Raman RP, Kumar S (2018). Argulosis: threat for ornamental fish industry. Aquacult Times 7–11.
- Ling F, Wang JG, Lu C, Wang GX, Lui YH, Gong XN. Effects of aqueous extract of Capsicum frutescens (Solanaceae) against the fish ectoparasite Ichthyophthirius multifiliis. Parasitol Res. 2012;111:841–848. doi: 10.1007/s00436-012-2907-9. [DOI] [PubMed] [Google Scholar]
- Mamadou K, Moussa C, Kouame F, Elena SR, Agathe F, Livui MD (2013). In vivo antiparasitic effects of an African’s traditional plant Ocimum gratissimum (Linn., 1758) on fish louse Argulus spp. infesting the Nile tilapia males Oreochromis niloticus (Linn., 1758) in fish farming. Open Sci. Repository Vet Med N DOI: 10.7392- 23050447.
- Mishra SS, Rakesh D, Dhiman M, Choudhary P, Debbarma J, Sahoo SN, Mishra CK. Present status of fish disease management in freshwater aquaculture in India: state-of-the-art-review. J Aquacult Fish. 2017;1:003. [Google Scholar]
- Mordue AJ, Nisbet AJ. Azadirachtin from the neem tree Azadirachta indica: its actions against insects. Anais Soci Entomol Brasil. 2000;29:615–632. doi: 10.1590/S0301-80592000000400001. [DOI] [Google Scholar]
- Mousavi HE, Behtash F, Rostami-Bashman M, Mirzargar SS, Shayan P, Rahmati-holasoo H. Study of Argulus spp. infestation rate in Goldfish, Carassius auratus (Linnaeus, 1758) in Iran. Humam Vet Med. 2011;3:198–204. [Google Scholar]
- Muchlisin ZA, Munazir AM, Fuady Z, Winaruddin W, Sugianto S, Adlim M, Fadli N, Hendri A. Prevalence of ectoparasites on mahseer fish (Tor tambra Valenciennes, 1842) from aquaculture ponds and wild population of Nagan Raya District, Indonesia. Hum Vet Med. 2014;6:148–152. [Google Scholar]
- Noga EJ (2010) Fish Disease Diagnosis and Treatment. 2nd Edition Mosby-yearbook, Inc. watsworth publishing Co USA 366.
- Pandreka A, Dandekar DS, Haldar S, Uttara V, Vijayshree SG, Mulani FA, Aarthy T, Thulasiram HV. Triterpenoid profiling and functional characterization of the initial genes involved in isoprenoid biosynthesis in neem (Azadirachta indica) Plant Biol. 2015;15:201–214. doi: 10.1186/s12870-015-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman RP. Applicability, feasibility and efficacy of phytotherapy in aquatic animal health management. Am J Plant Sci. 2017;8:257. doi: 10.4236/ajps.2017.82019. [DOI] [Google Scholar]
- Rath S, Pereira LA, Dal Bosco SM, Maniero MG, Fostier AH, Guimaraes JR. Fate of ivermectin in the terrestrial and aquatic environment: mobility, degradation, and toxicity towards Daphnia similis. Environ Sci Pollut Res. 2016;23:5654–5666. doi: 10.1007/s11356-015-5787-6. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Hardy RW, Sugiura SH. Screamer disease in Atlantic salmon, Salmo salar in Chile. J Fish Dis. 2001;24:543–549. doi: 10.1046/j.1365-2761.2001.00328.x. [DOI] [Google Scholar]
- Rushton-Mellor SK. The genus Argulus (Crustacea: Branchiura) in Africa: identification keys. Syst Parasitol. 1994;28:51–63. doi: 10.1007/BF00006909. [DOI] [Google Scholar]
- Saha M, Bandyopadhyay PK. First report of three species of Argulus (Crustacea: Branchiura) infesting on redcap Oranda gold fish (Carassius auratus) in India. Biolife. 2015;3:813–819. [Google Scholar]
- Sahoo PK, Mohanty J, Garnayak SK, Mohanty BR, Kar B, Prasanth H, Jena JK. Estimation of loss due to argulosis in carp culture ponds in India. Indian J Fish. 2013;60:99–102. [Google Scholar]
- Sahoo PK, Mohanty J, Hemaprasanth KB, Mohanty BR, Garnayak SK, Jena JK. Egg laying strategies and effect of temperature on egg development of Argulus siamensis. J Parasit Dis. 2013;37:158–162. doi: 10.1007/s12639-012-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo PK, Parida S, Mohapatra A, Mohanty J. Selection of candidate reference genes for RT-qPCR analysis in Argulus siamensis and their validation through screening of drugs and drug targets. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-54881-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo PK, Paul A, Sahoo MK, Pattanayak S, Kumar PR, Das BK. Incidences of Infectious Diseases In Freshwater Aquaculture Farms Of Eastern India: a passive surveillance based Study from 2014–2018. J Aquacult Res Dev. 2020;11:579. [Google Scholar]
- Sari NDK (2014) Control of Argulus japonicus eggs by means of drying, Doctoral dissertation, Airlangga University. 113–124
- Saurabh S, Mohanty BR, Sahoo PK. Expression of immune–related genes in rohu Labeo rohita (Hamilton) by experimental freshwater lice Argulus siamensis (Wilson) infection. Vet Parasitol. 2011;175:119–128. doi: 10.1016/j.vetpar.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Shafir A, Van JG. Laying, development and hatching of eggs of the fish ectoparasite Argulus japonicus (Crustacea: Branchiura) J Zool. 1986;210:401–413. doi: 10.1111/j.1469-7998.1986.tb03645.x. [DOI] [Google Scholar]
- Shimura S (1983) Seasonal occurrence, sex ratio and site preference of Argulus coregoni Thorell (Crustacea: Branchiura) parasitic on cultured freshwater salmonids in Japan Parasitol 86: 537–552.
- Taylor NGH, Sommerville C, Wootten R. The epidemiology of Argulus spp. (Crustacea: Branchiura) infections in stillwater trout fisheries. J Fish Dis. 2006;29:193–200. doi: 10.1111/j.1365-2761.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Leon LA, Paramo-Calderon DE, Robles-Olvera VJ, Valdes-Rodriguez OA, Perez-Vazquez A, Garcia-Alvarado MA, Rodriguez-Jimenes GC. Variation in bioactive compounds and antiradical activity of Moringa oleifera leaves: influence of climatic factors, tree age, and soil parameters. European Food Res Technol. 2017;243:1593–1608. doi: 10.1007/s00217-017-2868-4. [DOI] [Google Scholar]
- Walker PD, Flik G, Bonga SEW. The biology of parasites from the genus Argulus and a review of the interactions with its host. Symp Soc Exp Biol. 2004;55:107–128. [PubMed] [Google Scholar]
- Wang GX, Han J, Cheng C, Feng TT, Fu-yuan L, Zhu B. Bioassay-guided isolation and identification of active compounds from Fructus cnidii against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus) Parasitol Res. 2009;106:247–255. doi: 10.1007/s00436-009-1659-7. [DOI] [PubMed] [Google Scholar]
- Woo PTK. Fish Diseases and Disorders: Protozoan and Metazoan Infections. CAB Int. 1995;1:542–550. [Google Scholar]
- Yıldız KKA, Kumantas A. Argulus foliaceus infection in a goldfish (Carassius auratus) Israel J Vet Med. 2002;57:118–120. [Google Scholar]
- Yunikasari RD, Mahasri G. Correlation between water quality and prevalence on Koi (Cyprinus carpio) which infested by Argulus in Mungkid Subdistrict and Muntilan Subdistrict, Magelang Regency. Central Java E&ES. 2020;441:012150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author on reasonable request.





