Abstract
Cryptosporidium is an emerging opportunistic zoonotic pathogen that causes diarrheal illness in a wide range of hosts including livestock and humans. This study set out to establish the prevalence of Cryptosporidium as well as the circulating genotypes in order to elucidate the potential role of cattle in the spread of human cryptosporidiosis. Rectal coprological samples from 363 cattle in 11 households in Kiruhura district, Southwestern Uganda were collected and screened for the presence of Cryptosporidium oocysts using the phenol auramine staining method followed by fluorescent microscopy. DNA was extracted from the microscopy positive samples and the COWP gene amplified using PCR. PCR products were sequenced and subjected to phylogenetic analysis. Additionally a multiplex realtime PCR was used to identify the Cryptosporidium spp. Multivariable mixed effect logistic regression models were used to identify potential risk factors for Cryptosporidium infection. The overall prevalence of Cryptosporidium was 7.7% (95% CI 5.1–10.9), and herd level prevalence was 33.3% (95% CI 18.5–52.2). We found a statistically significant difference (OR = 30.78, 95% CI 4.31–219.95, p = 0.001) between infection in bulls as compared to cows. There was no significant difference in the prevalence among the different cattle breeds sampled. All the sequenced COWP gene DNA amplicons were confirmed to be C. hominis, with 93%-100% identity to sequences in the GenBank. The amplification of the small subunit rRNA by multiplex realtime PCR further established that the isolates in this study are C. hominis. This study represents the first time naturally occurring C. hominis has been detected from cattle in Uganda.
Keywords: Cryptosporidium, Genotyping, C. hominis, Uganda
Background
Cryptosporidium is an emerging zoonotic enteric pathogen that causes diarrheal illness in both humans and animals, known as cryptosporidiosis (Guerrant 1997). Cryptosporidiosis infections in cattle are more prevalent in calves as compared to the adult animals. Clinical signs occur 3–5 days after infection and include profuse watery diarrhea, gastrointestinal discomfort, nausea as well as fever (De Graaf et al. 1999; Fiuza et al. 2011). These episodes normally result in weight loss and occasionally death (Ryan et al. 2005; Rajendran et al. 2011). In addition to farmers incurring production losses, farmers may acquire infections from their animals.
Thirty-eight species of Cryptosporidium are currently documented (Kissinger 2019) and they infect a wide range of animal species. The important Cryptosporidium species which infect cattle are C. parvum, C. bovis, and C. andersoni (Fayer et al. 2007). However, other Cryptosporidium species and genotypes have sporadically been reported in cattle but these lack epidemiological significance (Ralston 2009; Fiuza et al. 2011). Cattle are the biological reservoir for C. parvum, a zoonotic species commonly implicated in outbreaks of human cryptosporidiosis (Millard et al. 1994; Slifko et al. 2000; Blackburn et al. 2006). C. hominis the main cause of disease in humans is considered host-specific, however, recent studies have reported the isolation of C. hominis in livestock (Smith et al. 2005; Xiao and Fayer 2008; Rajendran et al. 2011). The anthroponotic transmission of C. hominis (environmental loading of wastes) is a public health concern especially in Sub Saharan Africa because almost a quarter of the people lack access to safe drinking water and basic sanitation which are risk factors. The poor sanitation and lack of safe drinking water coupled with the HIV burden have resulted in an enhanced burden of human cryptosporidiosis in Africa (Aldeyarbi et al. 2016).
The global burden of human cryptosporidiosis is unknown, nonetheless, Cryptosporidium is ranked as the fifth most important food-borne parasite globally and the second leading cause of moderate-to-severe diarrhea in children (Kotloff et al. 2013; World Health Organization 2014). Diarrheal episodes in Sub Saharan Africa are responsible for 14% hospital outpatient visits, 16% of hospital admissions and an average of 35 days of illness per year in children (Greenwood et al. 1987) and an estimated 1.8 million deaths (Wardlaw et al. 2010). In non-fatal cases of diarrhea, particularly chronic infections have been strongly correlated with growth retardation and yet good health is a precondition for society to develop. (Tumwine et al. 2003; Prado et al. 2005; Thompson 2008).
Several studies continue to elucidate the role played by livestock in the transmission of Cryptosporidium to humans (Giles et al. 2009; Rajendran et al. 2011; Gormley et al. 2011; Kang’ethe et al. 2012; Samra 2013). These studies provide an in-depth understanding of the host range of Cryptosporidium which is crucial in the development of strategies that prevent both the anthroponotic and the zoonotic transmission of the disease (Giles et al. 2009; Rajendran et al. 2011). Cryptosporidium is transmitted via the fecal–oral route through the ingestion of water or food contaminated with oocysts. Oocysts may also be ingested through direct contact with fecal material from individuals (Slifko et al. 2000; Blackburn et al. 2006; Ponka et al. 2009). Cryptosporidium has a low infective dose with as few as 9 oocysts capable of causing disease (Okhuysen et al. 1999).
In this study, we report the occurrence of C. hominis in cattle from southwestern Uganda. These are communities where the water sources are shared amongst livestock and humans. This information will contribute towards understanding the epidemiology of cryptosporidiosis and contribute to the formulation of control strategies to protect high-risk populations from disease resulting from either human or animal hosts.
Methods
Study area
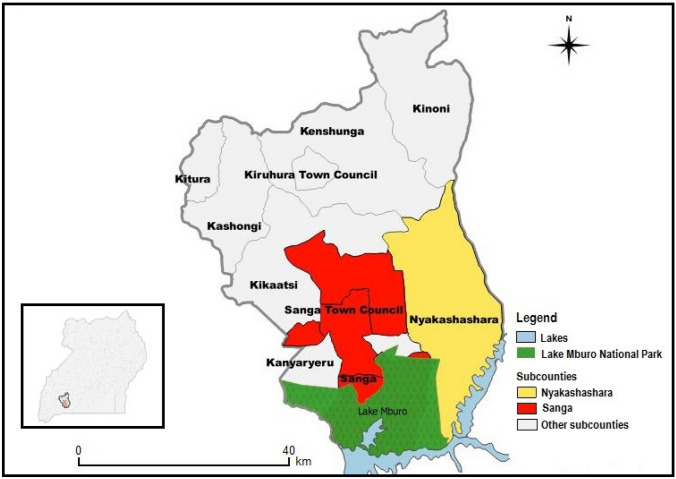
Nyakashashara and Sanga sub-counties are found in Kiruhura district located in the Western Region of Uganda bordering Lake Mburo National Park (LMNP) (See Fig. 1). Kiruhura is a water-stressed area characterised by drought conditions with scarce potential for groundwater. It has a human population of 280,200 and 75% of households use open water sources (“Water and Sanitation | Kiruhura District,” 2015). Kiruhura is a farming district with a cattle population of 342,315 (Ministry Of Agriculture and Uganda Bureau of Statistics 2015). Livestock forms the backbone of economic activity in the district.
Fig. 1.
Map of Uganda showing location of the study area (sub-counties of Kiruhura district). Source this study
Study design
This was a cross-sectional study done in February to March 2014. Cattle were sampled from 11 farms in Nyakashashara and Sanga Sub-counties in Kiruhura district, Western Uganda. Farms with unprotected water sources, shared by both humans and livestock were selected. Within the selected farms, simple random sampling was used to select cattle to be sampled.
Sample size determination
The sample size was determined by the Kish and Leslie formula for cross-sectional studies. A prevalence of 38% for Cryptosporidium (Nizeyi et al. 2002) was used to calculate the sample size
where N is the sample size, Z2 is the abscissa of the normal curve that cuts off an area at 1.96 (1—equals the desired confidence level, e.g., 95%), d is the desired level of precision of 0.05, P is the estimated proportion of an attribute that is present in the population of 0.38 for Cryptosporidium. Therefore; N = (1.962*0.38) (1–0.38)/0.052.
N = 362. However, 363 fecal samples were collected and examined.
Sample collection
Rectal fecal specimens from 363 cattle, each weighing approximately 10 g were collected from eleven (11) farms located in Nyakashashara and Sanga sub-counties. Each specimen was placed into a sterile container and sealed. Details of location, age, and sex of animals were recorded and the specimens were transported in a cool box at 4 °C to the Molecular Biology Laboratory, Makerere University for analysis.
Formalin diethyl ether concentration
Approximately 3 g of the fecal samples were individually weighed and homogenized with 3 ml of phosphate-buffered saline (1Χ PBS) pH 7.4 (Nizeyi et al. 2002). The homogenate was sieved with cotton gauze and transferred to 15 ml falcon tube. After sieving the homogenate, 7 ml of 10% formalin and 3 ml of diethyl ether were added, hand shaken and the mixture was centrifuged at 2000 rpm for 3 min. The diethyl ether layer, the particulate plug, and the formalin below it were discarded and the sediment was retained for examination (Alexander 2014).
Auramine-phenol staining and microscopic analysis
The sediment was washed in 10 ml of 1Χ PBS) pH 7.4 and span at 5000 g for 10 min and the supernatant discarded. This process was repeated three times. The sediment was re-suspended in 200 µl of 1Χ PBS pH 7.4 and 50 µl of the mixture was used to prepare smears on slides. The slides were air dried and fixed with absolute methanol for 3 min before staining.
The slides were stained using the auramine phenol technique according to the Alexander (2014). The slides were immersed in auramine phenol stain for 10 min. The stain was then rinsed off in tap water and the smears decolorized with 3% acid alcohol for 5 min. The smears were counterstained in 0.1% potassium permanganate for 30 s and rinsed in water to remove the excess stain. The smears were air dried at room temperature and examined for the presence of oocysts, using a fluorescent microscope (Leica DM IL LED, Germany) equipped with FITC filters, by scanning the slide under the ×40 objective lens and confirming for the presence of oocysts under the ×100 objective lens (See Fig. 2).
Fig. 2.
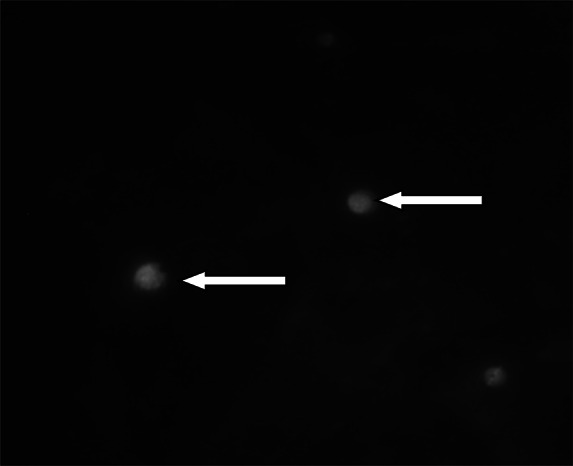
Cryptosporidium spp. oocysts stained with the fluorescent stain auramine-phenol under a Χ100 objective
DNA extraction
DNA was extracted from 150 µl of each re-suspended fecal sediment, using the QIAamp Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany). After extraction, we used agarose gel electrophoresis to confirm the presence of DNA in the samples. The extracted DNA was then stored at − 20 °C for Polymerase Chain Reaction (PCR).
Multiplex realtime PCR
The multiplex realtime PCR was carried out in a Biorad Cycler CFX96 Dx (Biorad Laboratories, USA) targeting the small subunit rRNA and 60 KDa glycoprotein (gp60). The Primer and probe sets used in this study to differentiate C. parvum and C. hominis, were those previously described by Hadfield et al. (2011). For the identification of C. meleagridis a primer probe set designed by Mor et al. (2018) was used. The gp60 gene and the SSU rRNA gene are widely used in the typing and subtyping of Cryptosporidium (Chalmers et al. 2005; Plutzer and Karanis 2009; Ryan et al. 2014; Lombardelli et al. 2019).
The PCR was performed in 30 µl reactions which contained 12.5 µl of powermix (biorad), 0.3 µl for each forward and reverse primer, 0.1 µl (each) hybridisation probe and 6.0 µl of DNA template. Each PCR mixture was then subjected to an initial denaturation at 95 °C for 3 min then 50 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 10 s, and extension at 72 °C for 15 s. Detection of the fluorescent signal was made after each cycle’s annealing phase. A sample was considered positive if the quantification cycle (Cq) was before the 40th cycle. If no amplification was observed before the 40th cycle the sample was reported as negative. FAM (6-carboxyfluorescein) labeled probes were specific for C. parvum and the amplification curves appeared green, HEX (Hexachloro-fluorescein) labeled probes for C. hominis gave blue amplification curves and Texas red probe was for C. meleagridis with the amplification curves appearing red. Each diagnostic run contained one negative and one positive control (Table 1).
Table 1.
Primer sets for Cryptosporidium species identification by Realtime PCR
| Cryptosporidium spp | Primers | Probe | Target gene |
|---|---|---|---|
| C. parvum | FAM (6-carboxyfluorescein) | ||
| Forward primer | 5′TCCTTGAAATGAATATTTGTGACTCG3’ | Small subunit rRNA | |
| Reverse primer | 5′TTAATGTGGCCGTAGTTACGGTTGAAC3’ | ||
| C. hominis | HEX (Hexachloro-fluorescein) | ||
| Forward primer | 5′TCCTTGAAATGAATATTTGTGACTCG3’ | Small subunit rRNA | |
| Reverse primer | 5′AAATGTGGTAGTTGCGGTTGAAA3’ | ||
| C. meleagridis | Texas red | ||
| Forward primer | 5′GAGCTCAGCACTCTCTCTACTA3’ | 60 KDa glycoprotein | |
| Reverse primer | 5′GCGTCTGTGAGTGATCTTCTT3’ |
PCR amplification of Cryptosporidium COWP gene
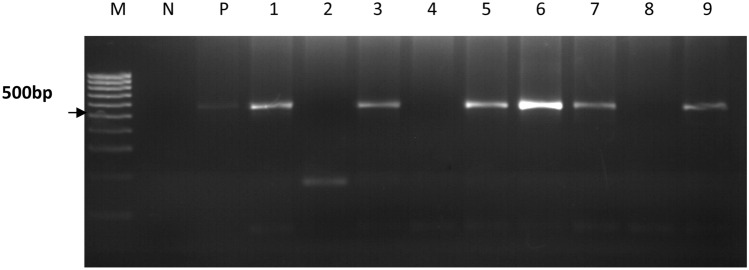
DNA extracted from the oocysts was used to amplify a 553 bp fragment of the COWP gene using a nested PCR (Spano et al. 1997) (Table 2). PCR amplification was performed in 25 µl volumes with 2Χ Ready-mix (Bioline, UK) (volume 12.5 µl, final concentration 1Χ), forward primer (CWPF), 10 pmol (volume 2.5 µl, final concentration 1.0 µm), reverse primer (CWPR), 10 pmol (volume 2.5 µl, final concentration 1.0 µm), DNA template 2.5 µl, nuclease free water to 25 µl. A PCR mastermix without template DNA was used as a negative control and included in each experiment. A positive control was also included. The following cycling conditions were used in a PTC-200 thermocycler (MJ Research, USA); initial denaturation for 5 min at 94 °C, followed by 50 cycles of denaturation at 94 °C for 30 s, annealing 55 °C for 1 min and extension 72 °C for 45 s with a final extension of 72 °C for 7 min. and a 12 °C hold. A second run was performed on the samples with the second set of primers (Cry9 and Cry15). The PCR conditions were identical to the one in the primary run except for the annealing temperature which was reduced to 52 °C for 1 min. PCR products were separated on a 1% agarose gel stained with ethidium bromide and visualized using the UV pro gel documentation system (See Fig. 3). A 1 kb molecular weight marker (Invitrogen®) was used during the agarose electrophoresis as a standard. All the nested PCR products of COWP genes were purified using a DNA purification kit (QIAGEN, Germany). The quality and quantity of the purified PCR products was checked with the NanoDrop 1000 spectrophotometer (Thermo Fisher, USA) and then sent for Sanger sequencing at Inqaba biotech in South Africa.
Table 2.
COWP primer sequences
| Primer | Sequence | Fragment size | Melting temperature | Source |
|---|---|---|---|---|
| CWPF | 5′-ACC GCT TCT CAA CAACCA TCT TGT CCT C-3' | 769 bp | 66.6 °C | (Spano et al. 1997) |
| CWPR | 5′-CGC ACC TGT TCC CAC TCA ATG TAA ACC C-3 | 68.0 °C | ||
| Cry9 | 5′-GGA CTG AAA TAC AGG CAT TAT CTT G-3' | 553 bp | 59.7 °C | (Spano et al. 1997) |
| Cry15 | 5′-GTA GAT AAT GGA AGA GAT TGT G-3' | 54.7 °C |
Fig. 3.
A representative 2% agarose gel showing the amplification of the 553 bp fragment of the COWP gene. Lane M is a 1 kb molecular weight marker, lane N is the negative control and lane P is the positive control. Lanes 1, 3, 5, 6, 7 and 9 are positive samples with a 553 bp band size. Lanes 2, 4 and 8 are negative samples
DNA sequencing
DNA Sequencing was done by a commercial company (Inqaba biotech, South Africa), using the Sanger sequencing method.
Analysis of COWP gene sequences
To determine the taxonomic positions of newly generated COWP sequences relative to published sequences, phylogenetic trees were constructed using the Maximum Likelihood method based on the Tamura 3-parameter model in the computer program MEGA6. The robustness of groupings was assessed using 1000 bootstrap replicates of the data (Tamura et al. 2017). All sequences generated during this study were deposited in GenBank and assigned accession numbers KY586953-KY586963.
Data management and statistical analysis
In preparation for statistical analysis, data were entered into Microsoft Excel version 2010 spreadsheets and coded. Prevalence was calculated by dividing the number of microscopy positive cattle by the total number of cattle tested while herd prevalence was determined by dividing positive herds to total number of herds. Likely risk factors were tested for by multivariable mixed effect logistic regression analysis, using farm ID (herd) as a random effect to account for clustering at herd level. In all the analyses performed, confidence levels were calculated at 95%, and a P value < 0.05 was used to determine the level of statistical significance. Data analysis was done using STATA 2010, version 16 software.
Results
Prevalence of Cryptosporidium as quantified by Microscopy
The overall prevalence of Cryptosporidium infections in the cattle quantified by microscopy using phenol auramine staining method was 7.7% (28/ 363). Farm 2 had the highest infection rate (33.3%), followed by farm 7 (25%), 8 (18.5%), 11 (7.1%), 1 (6.9%), 6 (4.3%) and lastly 4 (3.6%) (Table 3).
Table 3.
Prevalence of Cryptosporidium species in cattle in the study sites
| Sub county | Farm | Number of animals sampled | Positives | % Prevalence | 95% CI |
|---|---|---|---|---|---|
| Sanga | 1 | 29 | 2 | 6.9 | 0.84–22.76 |
| Nyakashashara | 2 | 27 | 9 | 33.3 | 16.51–53.96 |
| Nyakashashara | 3 | 27 | 0 | 0 | 0–12.77 |
| Nyakashashara | 4 | 28 | 1 | 3.6 | 0.09–18.34 |
| Nyakashashara | 5 | 25 | 0 | 0 | 0–13.71 |
| Nyakashashara | 6 | 46 | 2 | 4.3 | 0.05–14.83 |
| Nyakashashara | 7 | 20 | 5 | 25 | 8.65–49.10 |
| Nyakashashara | 8 | 27 | 5 | 18.5 | 6.30–38.01 |
| Sanga | 9 | 39 | 0 | 0 | 0–9.02 |
| Sanga | 10 | 39 | 0 | 0 | 0–9.02 |
| Nyakashashara | 11 | 56 | 4 | 7.1 | 1.98–17.29 |
| Total | 363 | 28 | 7.7 | 5.18–10.95 |
A difference in the prevalence by breed was observed (Crosses 9.2%, Ankole 5.7%, Friesian 7.1%, Boran 2.8%), (Table 4). However, the difference observed was found not to be statistically significant (Table 5).
Table 4.
Prevalence of Cryptosporidium by breed
| Number of positive | Number sampled | % Prevalence | (95% CI) | |
|---|---|---|---|---|
| Boran | 1 | 36 | 2.8 | 0.07–14.52 |
| Ankole | 3 | 53 | 5.7 | 1.18–15.66 |
| Crosses | 20 | 218 | 9.2 | 5.69–13.81 |
| Friesian | 4 | 56 | 7.1 | 1.98–17.29 |
| Total | 28 | 363 | 7.7 | 5.18–10.95 |
Table 5.
Risk factors for Cryptosporidium infection
| Risk factors | Coefficient | Standard error | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|---|---|
| Sex | |||||
| Female | Ref | 1 | |||
| Male | 3.42 | 30.88 | 30.78 | 4.31–219.95 | 0.001† |
| Age | |||||
| Calf | Ref | 1 | |||
| Adult | 0.64 | 1.07 | 1.55 | 0.41–5.98 | 0.523 |
| Breed | |||||
| Boran | Ref | 1 | |||
| Crossbreed | 0.92 | 5.52 | 3.80 | 0.22–65.34 | 0.358 |
| Friesian | 0.51 | 3.36 | 2.19 | 0.11–43.92 | 0.607 |
| Ankole | 1.06 | 9.37 | 5.71 | 0.23–142.73 | 0.289 |
| Subcounty | |||||
| Sanga | Ref | 1 | |||
| Nyakashashara | 1.85 | 9.55 | 8.34 | 0.88–78.76 | 0.064 |
†Significant p value
Risk factors for infection with Cryptosporidium
Risk factor analysis of sex, age, breed and subcounty presented sex as a risk factor for Cryptosporidium positivity. Males (Bulls) are 30 times (OR = 30.78, 95% CI 4.31–219.95) more likely to have Cryptosporidium spp infection than the Females (Cows). However putative risk factors age, breed and subcounty where the farm is located, were found not to be statistically significant factors for positivity to Cryptosporidium as shown in Table 5.
PCR amplification of the COWP gene
Genomic DNA was extracted from the 28 microscopy positives samples. Of the 28 positive samples, the 553 bp COWP gene product was successfully amplified in 20 samples. Failure to amplify the COWP gene product in the 8 samples could be due to fecal constituents such as bilirubin, bile salts, and complex polysaccharides which inhibit PCR even when present at low concentrations (Morgan et al. 1998; Thornton and Passen 2004).
Sequence analysis of Cryptosporidium COWP gene
The PCR products of the 553 bp COWP gene amplification from 11 of the 20 samples were successfully sequenced by Sanger method. All the sequences were identified as C. hominis by BLAST search and had 93–100% identity to sequences in the GenBank as shown in Table 6.
Table 6.
Cryptosporidium species detected by PCR and sequencing of the COWP gene in faecal samples collected from in Kiruhura District, Uganda
| Accession number from this study | Cryptosporidium spp | Sequence identity % from other studies (Accession number) | Reference for accession number |
|---|---|---|---|
| KY586953 | C. hominis | 100 (GU904388.1) | (Bouzid et al. 2010) |
| KY586954 | C. hominis | 93 (DQ388389.1) | (Wielinga et al. 2008) |
| KY586955 | C. hominis | 99 (GU904388.1) | (Bouzid et al. 2010) |
| KY586956 | C. hominis | 99 (KP314261.1) | (Liu et al. 2015) |
| KY586957 | C. hominis | 99 (GU904388.1) | (Bouzid et al. 2010) |
| KY586958 | C. hominis | 100 (KP314261.1) | (Liu et al. 2015) |
| KY586959k | C. hominis | 98 (GU904388.1) | (Bouzid et al. 2010) |
| KY586960 | C. hominis | 96 (DQ388389.1) | (Wielinga et al. 2008) |
| KY586961 | C. hominis | 99 (GU904388.1) | (Bouzid et al. 2010) |
| KY586962 | C. hominis | 98 (GU904388.1) | (Wielinga et al. 2008) |
| KY586963 | C. hominis | 99 (GU904388.1) | (Bouzid et al. 2010) |
Phylogenetic analysis of the COWP gene
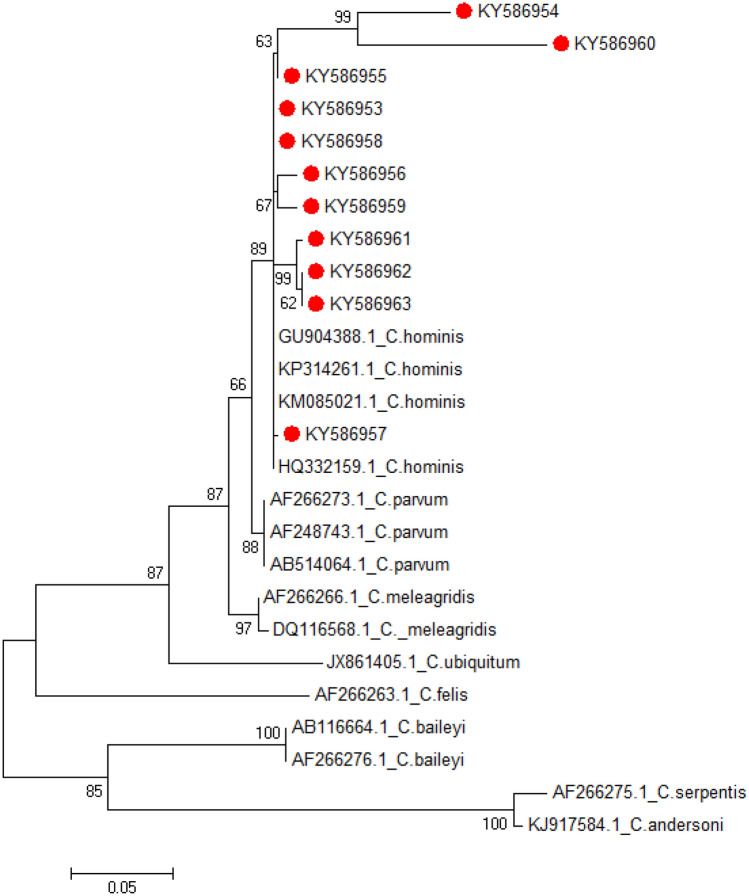
The nucleotide sequences of the COWP gene fragment were aligned using ClustalW and showed that the sequences from this study were highly identical to C. hominis sequences from the GenBank. Phylogenetic analysis of the newly generated COWP gene sequences and representative published sequences yielded a tree where all Cryptosporidium sequences from this study (KY586953-KY586963) clustered within a clade containing known C. hominis sequences with a bootstrap value of 89, therefore indicating that this clustering is highly supported (Fig. 4).
Fig. 4.
Dendogram of Cryptosporidium sequences isolated from cattle in Kiruhura district, south western, Uganda. The tree with the highest log likelihood (− 2058.7706) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches and is estimated from 1000 re-samplings of the sequence data. Reference sequences are shown with GenBank accession numbers and species name. The scale bar indicates nucleotide substitutions per site
Multiplex Real time PCR results
The samples were identified by multiplex realtime PCR to be C. hominis (Table 7).
Table 7.
Multiplex RT PCR results
| Sample ID | C.hominis (Cq Value) | C.meleagridis | C. parvum |
|---|---|---|---|
| CP1 (KY586953) | 29.99 (+) | No amplification(–) | No amplification(–) |
| CP2 (KY586954) | 30.54 (+) | – | – |
| CP3 (KY586955) | 34.88 (+) | – | – |
| CP4 (KY586956) | 28.88 (+) | – | – |
| CP5 (KY586957) | 33.42 (+) | – | – |
| CP6 (KY586958) | 31.10 (+) | – | – |
| CP7 (KY586959) | 13.24 (+) | – | – |
| CP8 (KY586960) | 29.30 (+) | – | – |
| CP9 (KY586961) | 22.70 (+) | – | – |
| CP10( KY586962) | 30.53 (+) | – | – |
| CP11 (KY586963) | 30.42 (+) | – | – |
Discussion
The aim of this study was first to determine the prevalence of Cryptosporidium in cattle bordering the LMNP using microscopy. The study also aimed to genotype the isolated Cryptosporidium species in order to determine if the cattle posed a zoonotic threat to the local human population.
The overall Cryptosporidium prevalence in this study was 7.7% which is comparable to the prevalence of 7.7% and 7.8% obtained in Kenya and Ethiopia respectively (Kang’ethe et al. 2012; Wegayehu et al. 2013). However, the prevalence obtained in this study is higher than 2.2% previously reported in western Uganda (Salyer et al. 2012). The possible explanation for this difference could be due to the variation in sampling techniques as well as seasonality. The samples in this study were collected during the dry season when higher pressure is exerted on the scarce water sources which result in poor sanitation practices that facilitate transmission (Kang’ethe et al. 2012). Furthermore, the prevalence reported in this study was much lower than a previous report of 38% in calves (Nizeyi et al. 2002). This difference in prevalence could be due to age-related susceptibility with calves at a higher risk of Cryptosporidium infection than adult cattle because of their naive immunological status (Maddox-Hyttel et al. 2006; Brook et al. 2008; Maikai et al. 2011; Smith et al. 2014).
In this study, we found sex to be a significant risk factor for Cryptosporidium infection, with male cattle being 30 times (OR = 30.78, 95% CI 4.31–219.95) more likely to be microscopy positive when compared to female cattle. These findings are different from findings by Maurya et al. (2013) who reported female cattle as a significant risk factor for Cryptosporidium infection. The very high odds ratio reported here should be however interpreted with precaution due to the small number of male cattle sampled in this study. We additionally found breed associated differences in the distribution of Cryptosporidium infection. The infection rate of Cryptosporidium in crosses (9.2%) was higher than that of the Friesian breed (7.1%), Ankole (5.7%) and Boran (2.8%). This variation could be due to native breeds being more resistant to diseases than the exotic breeds and crosses (Mwai et al. 2015). However this difference in infection was found to not be statistically significant. Age was not a risk factor in the prevalence of Cryptosporidium infections in this study but this differed from reports in previous studies where calves were at a significantly higher risk of infection as compared to adult cattle (Fayer et al. 2007).
The COWP gene was chosen for molecular analysis because the COWP gene is a sensitive and specific gene target that can be used to diagnose and identify Cryptosporidium species (Kato et al. 2003). The 553 bp COWP gene product was successfully amplified in 20 of the 28 microscopy positive samples. The unsuccessful amplification of expected DNA fragment in the rest of microscopy positive samples may be explained by the low oocyst concentration in the faecal samples analysed. Furthermore, the unsuccessful amplification could have been due to fecal constituents such as bilirubin, bile salts, and complex polysaccharides that inhibit PCR (Morgan et al. 1998; Thornton and Passen 2004). In addition, the COWP gene primers in general only amplify DNA of C. hominis, C. meleagridis C. parvum and species or genotypes closely related to C. parvum. This narrow specificity may also have led to the failure to successfully amplify the 8 isolates (Xiao 2010).
BLAST search comparison of the sequenced COWP gene fragments indicated that the all sequences generated in this study (KY586953-KY586963) are C. hominis. These C. hominis sequences also showed great similarity with C. parvum and this is because there is only a 3–5% sequence divergence between C. hominis and C. parvum (Xu et al. 2004). Phylogenetic analysis of gene sequences from this study showed that all the sequences clustered into a single clade, with known C. hominis sequences from the GenBank, with a bootstrap value of 89%. This bootstrap value indicates that this clustering is highly supported and further emphasizes that the sequences generated from this study were isolated from C. hominis. The eleven sequences (KY586953-KY586963) were further confirmed by multiplex realtime PCR to be C. hominis.
The findings of the present study indicate that Cryptosporidium spp. infections are prevalent in cattle in Kiruhura district. This is the first report documenting the isolation of C. hominis from cattle in Uganda. It is generally accepted that C. hominis primarily infects humans with no animal reservoir; however, there is growing evidence indicating that C. hominis infects livestock (Guk et al. 2004; Smith et al. 2005; Giles et al. 2009; Rajendran et al. 2011; Kang’ethe et al. 2012; Zahedi et al. 2016; Zhang et al. 2018). The public health implications of this study are significant because 75% of the population in Kiruhura use open water sources and yet unsafe water sources are a major transmission route (“Water and Sanitation | Kiruhura District,” 2015). In addition, HIV is endemic in Uganda and when infected, people with congenital immunodeficiency are at a higher risk of severe, life-threatening cryptosporidiosis (Aldeyarbi et al. 2016; “United Nations Programme on HIV/AIDS (UNAIDS),” 2018). Whereas the role animals play in the epidemiology of human cryptosporidiosis is controversial, particularly as potential zoonotic reservoirs of infection. This study reports a natural completed life cycle of C. hominis in a bovine host and indicates that animals may play an important role in the epidemiology of human cryptosporidiosis. This, therefore, highlights the need to understand the host range and the transmission dynamics of C. hominis.
Supplementary materials
Multiple sequence alignment of the COWP gene by BioEdit programme with sequences generated from this study and sequences obtained from the Genbank.
Acknowledgements
Special thanks go to Phillip Kimuda Magambo, Kiseka Henry, Jonan Tubenawe, Monica Nambi and Mary Frances Nakamya for numerous helpful suggestions during the study.
Author contributions
SGW contributed to the conception of the idea, design, sample collection and data analysis and interpretation. SGW also contributed to preparation of the manuscript. AK contributed to conception of the idea, study design and interpretation of results and manuscript preparation. GA contributed to sample analysis and drafting of the manuscript. CK contributed to developing the study design, interpretation of results and preparation of the manuscript. CMM contributed to the designing of the molecular methods and preparation of the manuscript. SO contributed to the study design, data analysis and contributed to the development of the manuscript.
Funding
This research was partly funded by CIMTRADZ but they had no role in the design and execution of the study as well as the decision to publish this manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on request.
Code availability
All software used to analyse data in this study is available online.
Compliance with ethical standards
Conflicts of interest
The authors of this paper do not have any financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper. The authors therefore, declare that they have no competing interests in the publication of this paper.
Consent for publication
All authors read and approved the manuscript.
Ethics approval and consent to participate
This study was reviewed and approved by the Research Ethics Committee, College of Health Sciences, Makerere University under the reference number REC/2011/195. Written and verbal consent was obtained from the farmers before the sampling of animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sarah Gift Witto, Email: sarahgiftwito@gmail.com, Email: sarah.witto@kiu.ac.ug.
Clovice Kankya, Email: clokankya@gmail.com.
Gloria Akurut, Email: akurutgloria@gmail.com.
Claire Mack Mugasa, Email: claire1mack@covab.mak.ac.ug.
Anne Kazibwe, Email: annejkazibwe@yahoo.com.
Sylvester Ochwo, Email: sochwo@covab.mak.ac.ug.
References
- Aldeyarbi HM, Abu El-Ezz NMT, Karanis P. Cryptosporidium and cryptosporidiosis: the African perspective. Environ Sci Pollut Res. 2016;23:13811–13821. doi: 10.1007/s11356-016-6746-6. [DOI] [PubMed] [Google Scholar]
- Alexander D (2014) Manual of diagnostic tests and vaccines for terrestrial animals. Off Int Des Epizoot Paris, Fr Chapter 2
- Blackburn BG, Mazurek JM, Hlavsa M, et al. Cryptosporidiosis associated with ozonated apple cider. Emerg Infect Dis. 2006;12:684–686. doi: 10.3201/eid1204.050796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzid M, Tyler KM, Christen R, et al. Multi-locus analysis of human infective Cryptosporidium species and subtypes using ten novel genetic loci. BMC Microbiol. 2010;10:213. doi: 10.1186/1471-2180-10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook E, Hart CA, French N, Christley R. Prevalence and risk factors for Cryptosporidium spp. infection in young calves. Vet Parasitol. 2008;152:46–52. doi: 10.1016/j.vetpar.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Chalmers RM, Ferguson C, Cacciò S, et al. Direct comparison of selected methods for genetic categorisation of Cryptosporidium parvum and Cryptosporidium hominis species. Int J Parasitol. 2005;35:397–410. doi: 10.1016/j.ijpara.2005.01.001. [DOI] [PubMed] [Google Scholar]
- De Graaf DC, Vanopdenbosch E, Ortega-Mora LM, et al. A review of the importance of cryptosporidiosis in farm animals. Int J Parasitol. 1999;29:1269–1287. doi: 10.1016/S0020-7519(99)00076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R, Santin M, Trout JM. Prevalence of Cryptosporidium species and genotypes in mature dairy cattle on farms in eastern United States compared with younger cattle from the same locations. Vet Parasitol. 2007;145:260–266. doi: 10.1016/j.vetpar.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Fiuza VRS, Almeida AJ, Frazão-Teixeira E, et al. Occurrence of Cryptosporidium andersoni in Brazilian cattle. J Parasitol. 2011;97:952–953. doi: 10.1645/GE-2726.1. [DOI] [PubMed] [Google Scholar]
- Giles M, Chalmers R, Pritchard G, et al. Cryptosporidium hominis in a goat and a sheep in the UK. Vet Rec. 2009;164:24–25. doi: 10.1136/vr.164.1.24. [DOI] [PubMed] [Google Scholar]
- Gormley FJ, Little CL, Chalmers RM, et al. Zoonotic cryptosporidiosis from petting farms, England and Wales, 1992–2009. Emerg Infect Dis. 2011;17:151–152. doi: 10.3201/eid1701.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BM, Bradley AK, Greenwood AM, et al. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81:478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- Guerrant RL. Cryptosporidiosis: an emerging, highly infectious threat. Emerg Infect Dis. 1997;3:51–57. doi: 10.3201/eid0301.970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guk S, Yong T, Park S, et al. Genotype and animal infectivity of a human isolate of Cryptosporidium parvum in the Republic of Korea. Korean J Parasitol. 2004;42:85–90. doi: 10.3347/kjp.2004.42.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield SJ, Robinson G, Elwin K, Chalmers RM. Detection and differentiation of Cryptosporidium spp. in human clinical samples by use of real-time PCR. J Clin Microbiol. 2011;49(3):918–924. doi: 10.1128/JCM.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang’ethe EK, Mulinge EK, Skilton RA, et al. Cryptosporidium species detected in calves and cattle in Dagoretti, Nairobi, Kenya. Trop Anim Health Prod. 2012;44:25–31. doi: 10.1007/s11250-012-0202-5. [DOI] [PubMed] [Google Scholar]
- Kato S, Lindergard G, Mohammed HO. Utility of the Cryptosporidium oocyst wall protein (COWP) gene in a nested PCR approach for detection infection in cattle. Vet Parasitol. 2003;111:153–159. doi: 10.1016/S0304-4017(02)00353-9. [DOI] [PubMed] [Google Scholar]
- Kissinger JC. Evolution of Cryptosporidium. Nat Microbiol. 2019;4:730–731. doi: 10.1038/s41564-019-0438-1. [DOI] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Liu X, Xie N, Li W, et al. Emergence of Cryptosporidium hominis monkey genotype II and novel subtype family Ik in the squirrel monkey (Saimiri sciureus) in China. PLoS ONE. 2015;10:e0141450. doi: 10.1371/journal.pone.0141450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardelli JA, Tomazic ML, Schnittger L, Tiranti KI. Prevalence of Cryptosporidium parvum in dairy calves and GP60 subtyping of diarrheic calves in central Argentina. Parasitol Res. 2019;118:2079–2086. doi: 10.1007/s00436-019-06366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox-Hyttel C, Langkjær RB, Enemark HL, Vigre H. Cryptosporidium and Giardia in different age groups of Danish cattle and pigs—Occurrence and management associated risk factors. Vet Parasitol. 2006;141:48–59. doi: 10.1016/j.vetpar.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Maikai BV, Umoh JU, Kwaga JKP, et al. Molecular characterization of Cryptosporidium spp. in native breeds of cattle in Kaduna State. Nigeria Vet Parasitol. 2011;178:241–245. doi: 10.1016/j.vetpar.2010.12.048. [DOI] [PubMed] [Google Scholar]
- Maurya PS, Rakesh RL, Pradeep B, et al. Prevalence and risk factors associated with Cryptosporidium spp. infection in young domestic livestock in India. Trop Anim Health Prod. 2013;45:941–946. doi: 10.1007/s11250-012-0311-1. [DOI] [PubMed] [Google Scholar]
- Millard PS, Gensheimer KF, Addiss DG, et al. An outbreak of cryptosporidiosis from fresh-pressed apple cider. JAMA J Am Med Assoc. 1994;272:1592–1596. doi: 10.1001/jama.1994.03520200048034. [DOI] [PubMed] [Google Scholar]
- Ministry Of Agriculture AI and F, Uganda Bureau of Statistics (2015) The National Livestock Census a Summary Report of the National Livestock Census
- Mor SM, Ascolillo LR, Nakato R, Ndeezi G, Tumwine JK, Okwera A, Sponseller JK, Tzipori S, Griffiths JK. Expectoration of Cryptosporidium parasites in sputum of human immunodeficiency virus-positive and -negative adults. Am J Trop Med Hyg. 2018;98(4):1086–1090. doi: 10.4269/ajtmh.17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan UM, Pallant L, Dwyer BW, et al. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: clinical trial. J Clin Microbiol. 1998;36:995–998. doi: 10.1128/jcm.36.4.995-998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwai O, Hanotte O, Kwon Y, Cho S. Invited Review—African indigenous cattle: unique genetic resources in a rapidly changing world. Asian-Australas J Anim Sci. 2015;28:911–921. doi: 10.5713/ajas.15.0002R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizeyi J, Cranfield M, Graczyk T. Cattle near the Bwindi Impenetrable National Park, Uganda, as a reservoir of Cryptosporidium parvum and Giardia duodenalis for local community and free-ranging gorillas. Parasitol Res. 2002;88:380–385. doi: 10.1007/s00436-001-0543-x. [DOI] [PubMed] [Google Scholar]
- Okhuysen P, Chappell C, Crabb J. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- Plutzer J, Karanis P. Genetic polymorphism in Cryptosporidium species: an update. Vet Parasitol. 2009;165:187–199. doi: 10.1016/j.vetpar.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Ponka A, Kotilainen P, Rimhanen-Finne R, et al. A foodborne outbreak due to Cryptosporidium parvum in Helsinki, November 2008. Euro Surveill. 2009;14:14–16. doi: 10.2807/ese.14.28.19269-en. [DOI] [PubMed] [Google Scholar]
- Prado MS, Cairncross S, Strina A, et al. Asymptomatic giardiasis and growth in young children; a longitudinal study in Salvador, Brazil. Parasitology. 2005;131:51–56. doi: 10.1017/S0031182005007353. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Ajjampur SSR, Chidambaram D, et al. Short report: Investigation of potential zoonotic transmission of cryptosporidiosis in Southern India. Am J Trop Med Hyg. 2011;85:657–659. doi: 10.4269/ajtmh.2011.10-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston B (2009) A Comparative study of Giardia and Cryptosporidium infections in feedlot cattle in Western Australia and Alberta, Canada. Dissertation, University of Calgary
- Ryan UM, Bath C, Robertson I, et al. Sheep may not be an important zoonotic reservoir for Cryptosporidium and Giardia parasites. Appl Environ Microbiol. 2005;71:4992–4997. doi: 10.1128/AEM.71.9.4992-4997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U, Fayer R, Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014;141(13):1667–1685. doi: 10.1017/S0031182014001085. [DOI] [PubMed] [Google Scholar]
- Salyer SJ, Gillespie TR, Rwego IB, et al. Epidemiology and molecular relationships of Cryptosporidium spp. in people, primates, and livestock from Western Uganda. PLoS Negl Trop Dis. 2012;6:2–7. doi: 10.1371/journal.pntd.0001597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samra NA (2013) An epidemiological study of cryptosporidiosis at the wildlife/livestock/human interface in Mpumalanga Province, South Africa. Dissertation,University of Pretoria
- Slifko TR, Smith HV, Rose JB. Emerging parasite zoonoses associated with water and food. Int J Parasitol. 2000;30:1379–1393. doi: 10.1016/S0020-7519(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Smith RP, Cheney T, Giles M. Prevalence and molecular typing of Cryptosporidium in dairy cattle in England and Wales and examination of potential on-farm transmission routes. Vet Parasitol. 2014;204:111–119. doi: 10.1016/j.vetpar.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HV, Nichols RAB, Mallon M, et al. Natural Cryptosporidium hominis infections in Scottish cattle. Vet Rec. 2005;156:710–711. doi: 10.1136/vr.156.22.710. [DOI] [PubMed] [Google Scholar]
- Spano F, Putignani L, McLauchlin J, et al. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150:209–217. doi: 10.1016/S0378-1097(97)00115-8. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2017;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RCA. Giardiasis: modern concepts in control and management. Ann Nestlé (English ed) 2008;66:23–29. doi: 10.1159/000113306. [DOI] [Google Scholar]
- Thornton C, Passen S. Inhibition of PCR amplification by phytic acid, and treatment of bovine fecal specimens with phytase to reduce inhibition. J Microbiol Methods. 2004;59:43–52. doi: 10.1016/j.mimet.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Tumwine JK, Kekitiinwa A, Nabukeera N, et al. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am J Trop Med Hyg. 2003;68:710–715. doi: 10.4269/ajtmh.2003.68.710. [DOI] [PubMed] [Google Scholar]
- Uganda | UNAIDS. https://www.unaids.org/en/regionscountries/countries/uganda. Accessed 6 Aug 2020
- Wardlaw T, Salama P, Brocklehurst C, et al. Diarrhoea: why children are still dying and what can be done. Lancet. 2010;375:870–872. doi: 10.1016/S0140-6736(09)61798-0. [DOI] [PubMed] [Google Scholar]
- Water and Sanitation | Kiruhura District. (2015). https://www.kiruhura.go.ug/dept/water-and-sanitation. Accessed 16 Sep 2020
- Wegayehu T, Adamu H, Petros B. Prevalence of Giardia duodenalis and Cryptosporidium species infections among children and cattle in North Shewa Zone. Ethiopia BMC Infect Dis. 2013;13:419. doi: 10.1186/1471-2334-13-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielinga PR, de Vries A, van der Goot TH, et al. Molecular epidemiology of Cryptosporidium in humans and cattle in The Netherlands. Int J Parasitol. 2008;38:809–817. doi: 10.1016/j.ijpara.2007.10.014. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2014) Multicriteria-based ranking for risk management of food-borne parasites. Microbiol Risk Assess Ser xvii
- Xiao L. Molecular epidemiology of cryptosporidiosis: An update. Exp Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Xiao L, Fayer R. Molecular characterisation of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int J Parasitol. 2008;38:1239–1255. doi: 10.1016/j.ijpara.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Xu P, Widmer G, Wang Y, et al. The genome of Cryptosporidium hominis. Nature. 2004 doi: 10.1038/nature02990. [DOI] [PubMed] [Google Scholar]
- Zahedi A, Monis P, Aucote S, et al. Zoonotic Cryptosporidium species in animals inhabiting sydney water catchments. PLoS ONE. 2016 doi: 10.1371/journal.pone.0168169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jian Y, Li X, et al. Molecular detection and prevalence of Cryptosporidium spp. infections in two types of domestic farm animals in the Qinghai-Tibetan Plateau Area (QTPA) in China. Parasitol Res. 2018;117:233–239. doi: 10.1007/s00436-017-5697-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on request.
Code availability
All software used to analyse data in this study is available online.