Abstract
To compare clinical presentations, haematological and immunological parameters in urban and rural malaria patients. Clinically suspected malaria patients, resident in either rural or urban communities, were selected from seven health facilities in the Greater Accra region of Ghana. For each suspected malaria patient, parasites were detected microscopically and quantified subsequently. In each study site, an equal number of cases and age-matched controls were selected. In both cases and controls, clinical presentations, nutritional status, haematological, and immunological parameters were profiled. A total of 149 malaria patients and 149 nonmalaria controls were selected. Compared to rural dwellers with malaria, parasitaemia was significantly higher in both males and females and in the various age groups in urban dwellers with malaria. Additionally, mean lymphocytes, haemoglobin, haematocrit, mean cell haemoglobin, platelets, and mean platelet volume levels were significantly lower in urban dwellers with malaria. However, TNF-α, IL-6, and IL-12 levels in urban dwellers with malaria were significantly higher, while IL-10, CD4+, CD3+, CD8+ T-cells levels and CD4+/ CD3+ ratio were significantly lower in urban dwellers with malaria. Furthermore, chills, diarrhoea, fever, and pallor were significantly associated with urban dwellers with malaria. This study concluded that urban dwellers are more prone to severe malaria while rural dwellers tend to have more measured immune response against malaria infection, and therefore experienced better controlled inflammatory processes associated with mild form of the disease.
Keywords: T-cell profiling in malaria, Malaria immunology, Comparative haematological profiling in malaria, Rural and urban malaria, Ghana
Introduction
Malaria is intra-erythrocytic parasitic infection caused by Plasmodium falciparum, P. ovale, P. malaria, P. vivax, and P. knowlesi (White 2008). These parasites are responsible for an average of over 400,000 mortalities yearly (World 2019). In Ghana, over 95% of malaria cases are attributable to P. falciparum (Asante et al. 2011).
Haematological alterations that occur during the asexual stage of the life cycle of the malaria parasite reported to characterize malaria may lead to adverse biochemical changes (Costa et al. 2006). Erythrocytes infected with P. falciparum induce increase in secretion of cytokines such as tumor necrosis factor alpha (TNFα), interleukin (IL)-1, IL-10 and interferon gamma (IFNγ). These cytokines and intracellular P. falciparum induce red cells lysis which leads to low red cell count and attendant abnormalities in haematological profile (Ghosh and Shetty 2008).
Malaria transmission and incidence tend to be higher in rural areas compared to urban communities (Tatem et al. 2013). This is due to lack of improved housing, poor drainage facilities, lack of better health care, lack of knowledge of prevention of malaria (Omumbo et al. 2005), and abundance of mosquito vectors in suitable temperatures and humid climate (Grillet 2000). Although the reverse is true of urbanized communities, increasingly high cases of malaria have been reported in urbanized communities (Maghendji-Nzondo et al. 2016; Parnell and Walawege 2011; Yadouléton and Allagbé 2010; Klinkenberg et al. 2010).
In classical P. falciparum malaria, fever, chills, anorexia, epigastric discomfort, nausea, vomiting, and diarrhoea are common (Uzzan et al. 2006). Routine laboratory examinations may show various degrees of anaemia. In most cases, the white blood cell count is usually normal, but leucopenia may be observed. Thrombocytopenia is common as well as elevation of serum transaminases and lactic dehydrogenase. Moderately or markedly elevated bilirubin concentrations may also be observed (Ahiboh et al. 2008; Chiwakata et al. 2001). However, it is not clearly established if malaria impacts differently on urban and rural patients. Therefore, this study aimed at comparing the haematological, immunological (TNF-α, IL-12, IL-10, IL-6, CD4, CD3, and CD8) and clinical profiles (abdominal discomfort, chills, fever, vomiting, diarrhea, nausea and palor) of malaria patients dwelling in urban and rural communities in the Greater Accra region of Ghana. TNF-α, IL-6 and IL-12, and IL-10 were chosen based on their known proinflammatory and anti-inflammatory and immunoregulatory properties, respectively (Jason et al. 2001; Torre et al. 2002).
Materials and methods
Study sites and study design
The study samples were collected from health facilities located in both rural and urban communities in the Greater Accra region of Ghana. In all, five districts were involved in this study. The districts were Ga West, Ga South, Ashaiman, Ada East and Accra Metropolis. The rural sites were Ada (Ada East), Mayera and Oduman (Ga West), and Obom (Ga South) while the urban sites were Amasaman (Ga West), Maamobi (Accra metropolis) and Ashaiman (Ashaiman district). Health facilities used for this study were Ga West Municipal Hospital in Amasaman, Obom Health Centre, Mayera Health Centre, Oduman Health Centre, Ada East Government Hospital, Maamobi General Hospital and Ashaiman Polyclinic. The study participants were selected strictly based on residence in rural and urban communities.
Details of study area
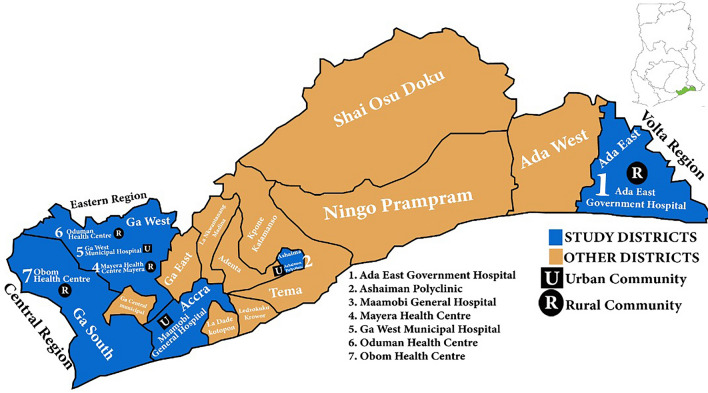
Figure 1 is the map of the Greater Accra region detailing the study region and sites. In Ada East and Ga South districts, rural communities predominate while Ga West is made up of almost equal numbers of rural and urban communities. Ashaiman and Accra metro are entirely urban communities. In Ga West municipality, the municipal hospital, Ga West Municipal Hospital is located in the municipal capital, Amasaman. Mayera health centre is located in Mayera, south-east of Amasaman, a distant of about 15 km from Amasaman. Oduman health centre is also located in Oduman, north-east of Amasaman, a distant of about 20 km from Amasaman. The geographical locations of the study centers were Ga West Municipal Hospital, Amasaman (5.7020708, −0.2992889), Ashaiman Polyclinic (5.6856, −0.0398) and Ada East District Hospital (5.8956754, 0.5340865) and the health centres (latitude, longitude) were Mayera Health Centre (5.720578, −0.2703561), Oduman Health Centre (5.64171, −0.3302) and Obom Health Centre (5.7361, −0.4395).
Fig. 1.
Map of study districts and the sites (map was authors’ own production)
Sample size
This cross-sectional study sampled at least 198 malaria cases from rural and urban communities based on 15.1% prevalence of malaria in the region (Diallo et al. 2017). Sample size was calculated using the Cochrane’s formula, n = z2p(1-p)/d2, where n = sample size, p = prevalence of malaria in Greater Accra Region, z = confidence level at 95% (standard value of 1.96), d = margin of error at 5% (standard value of 0.05). Adjusting for 10% nonresponse rate, a sample size of 218 was obtained. A minimum of 109 suspected malaria cases were recruited from both rural and urban dwellers. For each case recruited age-matched controls were also collected. Cases and controls were collected from March to July 2018.
Sampling and sampling strategy
The suspected malaria cases were sampled randomly by selecting the first four malaria cases each day which consented to be part of the study. On each day, an equal numbers of malaria suspected cases that were negative for malaria were also collected as control cases. To ensure uniformity in the clinical assessment, digital infra-red noncontact thermometers (Kinlee, Guangdong), digital blood pressure meters (Omron, Kyoto-Japan), and digital weighing scales (Omron, Kyoto-Japan) were calibrated and distributed to the study sites.
Blood sample collection
Five mL of whole blood was collected from suspected malaria patients with prior written consent from accompanying parents and guardians. Patients above 18 years consented for themselves. The blood samples were kept on ice packs until they arrive at Ga West Municipal Hospital Laboratory where all analyses were done.
Laboratory procedures
Blood smears for malaria parasite quantification
Thick blood smears were prepared on a new microscope slide for each blood sample. Smears were stained with locally prepared Giemsa stain (pH = 6.8). Parasite densities were estimated by dividing the number of asexual parasites per at least 200 leukocytes and multiplied by the estimated WBC of the patients/μL of blood (Alves-Junior et al. 2014).
Hematological profiling procedure
Peripheral blood’s haematological parameters were determined using Urit 5160 haematological analyzer (Guangzhou, China). The 5-part differential analyzer works on the principle of laser beam multidimensional cell classification, flow cytometry for white cell differentiation, white and red cell estimation. Platelets were counted by optical and electrical impedance principles and haemoglobin concentration measured by cyanide-free colorimetric method. All other parameters were calculated automatically.
Nutritional assessment and determination of body mass index
Body mass index (BMI), protein, and albumin concentrations were used to assess nutritional statuses as previously used (Norgan 1994; Prenner et al. 2014). BMI was calculated as the ratio of weight in kilogram and square of height in meters. The height and body weight were taken by Seca 213 portable stadiometer (New Zealand) and Omron digital weighing scale (Omron, Kyoto-Japan), respectively. The total protein and albumin concentrations (from plasma samples) were measured by PKL-125 Italia fully automated chemistry analyser using Medsource endpoint reagents (Ozone Biomedicals Pvt Ltd, Haryana, India).
Cytokines profiling using enzyme immuno-assay
Plasma levels of interleukin (IL)-12, IL-10, IL-6, and tumor necrosis factor alpha (TNF-α) were determined by using Cusabio Biotech sandwich ELISA kit (Wuhan, China). Strict manufacturer’s protocols were followed. Cytokines in stored plasma (below −20 °C) were analyzed on the same day using Mindray MR-96A ELISA plate reader (Shenzhen, China).
Enumeration of CD4 and CD3 T cells
CD3 and CD4 T cells were enumerated using BD FACS count flow cytometer (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Briefly, the CD4/CD3 reagent tube was brought to room temperature, vortexed for approximately 5 s each, and then opened with the coring station. Subsequently, 50 µL of whole blood was added to a reagent tube, vortexed upright, and incubated in the workstation for one hour. After incubation, 50 µL of formalin was added, vortexed, and incubated until the samples were analyzed. The samples were then vortexed and immediately run on BD FACS count flow cytometer. The absolute values of CD4, CD3 T cells, and the CD4/CD3 ratio were then automatically printed out by an inbuilt thermal printer.
Interpretation of results
The Ghanaian normal reference range for CD4+ T lymphocytes is 660–1493 cell/µL, CD3+ T lymphocytes is 1140–2685 cell/µL and CD4/CD3 ratio is 0.51–0.65. A person is said to be immuno-suppressed if the CD4+ T lymphocytes, CD3+ T lymphocytes, and CD4/CD3 ratio are less than 660 cells/µL 1140 cells/µL and 0.50 respectively. CD8+ cells (normal ranges 187–1139 cells/μL) were calculated by subtracting CD4 cells from CD3 cells (Ampofo et al. 2006).
Statistical analyses
The statistical analyses were done by Graphpad Prism version 8.4.3.686. One-way analysis of variance (ANOVA) was used to test the level of differences among urban, rural, and control patients’ samples. Tukey post hoc analysis was used to perform multiple comparisons among the parameters. Additionally, student t-test was used to test the differences in geometric mean of parasite densities in the malaria cases. Furthermore, Chi-square tool was used to test the association of clinical variables to residential status. In all inferential statistics, p-value less than 0.05 was considered statistically significant.
Results
Cases and controls selected for the study
A total of 298 suspected malaria patients participated in this study. Of this total, 166 patients were rural dwellers (n = 83 were infected with malaria parasites and n = 83 were not). From urban-dwelling patients, 132 patients were selected (n = 66 were infected with malaria parasites and n = 66 were not). The distributions of the malaria cases and controls in each health facility, situated in either rural or urban communities, are presented in Table 1.
Table 1.
Samples collection sites and number of samples
| Parameters | Study samples | |
|---|---|---|
| Sample collection sites | ||
| Rural patients | Cases (n = 83) | Controls (n = 83) |
| Ada East hospital | 17 (20.5%) | 17 (20.5%) |
| Mayera health center | 13 (15.7%) | 13 (15.7%) |
| Oduman health center | 23 (27.7%) | 23 (27.7%) |
| Obom health center | 30 (36.1%) | 30 (36.1%) |
| Urban patients | Cases (n = 66) | Controls (n = 66) |
| Ga West hospital | 29 (43.9%) | 29 (43.9%) |
| Maamobi Gen. hospital | 13 (20.0%) | 13 (20.0%) |
| Ashaiman polyclinic | 24 (36.4%) | 24 (36.4%) |
Comparison of cases and controls
Table 2 represents demographic and clinical parameters of the malaria cases and control patients. In both cases and controls, the number in each age range reduces with increasing age. The number of individuals belonging to each age range in both cases and controls were comparable. Similarly, the gender distributions between cases and controls was also comparable. However, mean temperature readings were significantly higher in cases than controls (38.6 °C vs. 37.8 °C, p = 0.041). It was also found that all clinical findings assessed were associated with malaria.
Table 2.
The demographic and clinical findings of the study participants
| Parameters | Rural | Urban | Total | p-value | |||
|---|---|---|---|---|---|---|---|
| Controls (n = 83) | Cases (n = 83) | Controls (n = 66) | Cases (n = 66) | Controls (n = 149) | Cases (n = 149) |
||
| Age range (years) | |||||||
| 0–5 | 37 (44.6%) | 45 (54.2%) | 18 (27.3%) | 18 (27.3%) | 55 (36.9%) | 63 (42.3%) | 0.39 (0.532) |
| 6–10 | 28 (33.7%) | 21 (25.3%) | 25 (37.9%) | 27 (40.9%) | 53 (35.6%) | 48 (32.2%) | 0.13 (0.667) |
| 11–15 | 7 (8.4%) | 8 (9.6%) | 17 (25.8%) | 14 (21.2%) | 24 (16.1%) | 22 (14.7%) | 0.07 (0.784) |
| 16–20 | 11 (13.3%) | 9 (10.8%) | 6 (9.1%) | 7 (10.6%) | 17 (11.4%) | 16 (10.7%) | 0.03 (0.869) |
| Gender | |||||||
| Males | 39 (47.0%) | 30 (36.1%) | 19 (28.8%) | 20 (30.3%) | 58 (38.9%) | 50 (33.6%) | 0.43 (0.509) |
| Females | 44 (53.0%) | 53 (63.9%) | 47 (71.2%) | 46 (69.7%) | 91 (61.1%) | 99 (66.4%) | 0.21 (0.650) |
| Clinical findings | |||||||
| Mean temperature (°C) | 37.7 ± 0.3 | 38.1 ± 0.3 | 37.8 ± 0.4 | 39.0 ± 0.7 | 37.8 ± 0.4g | 38.6 ± 0.6g | 2.9 (0.041)* |
| Abdominal discomfort | 33 (39.8%) | 52 (62.7%) | 36 (54.5%) | 51 (77.3%) | 69 (46.3%) | 103 (69.1%) | 4.2 (0.038) |
| Chills | 29 (24.9%) | 61 (73.4%) | 36 (54.5%) | 62 (94.0%) | 65 (43.6%) | 123 (82.6%) | 11.1 (0.008) |
| Diarrhoea | 11 (13.2%) | 37 (44.6%) | 17 (25.8%) | 48 (72.7%) | 28 (18.8%) | 85 (57.0%) | 21.3 (< 0.001) |
| Fever | 42 (50.6%) | 63 (75.9%) | 39 (59.1%) | 62 (94.0%) | 81 (54.4%) | 125 (83.9%) | 5.6 (0.017) |
| Nausea | 23 (27.7%) | 67 (80.7%) | 30 (45.5%) | 55 (83.3%) | 53 (35.6%) | 122 (81.9%) | 17.5 (< 0.001) |
| Pallor | 17 (20.5%) | 39 (47.0%) | 38 (57.6%) | 45 (68.2%) | 55 (36.9%) | 84 (56.4%) | 4.1 (0.041) |
| Vomiting | 9 (10.8%) | 48 (57.8%) | 16 (19.3%) | 41 (62.1%) | 25 (16.8%) | 89 (59.7%) | 26.6 (< 0.001) |
*Test statistic and p-value determined by T-test. The other statistics are presented at Chi statistic x2 (p-value)
Parasitological characteristics of the study patients
Parasitological analysis revealed that the geometric means of parasitaemia among males were higher in urban dwellers than rural dwellers (15,169 vs. 11,031 parasites/µL of blood). Additionally, parasitaemia was significantly higher in each age group in urban dwellers than rural dwellers. Comparing parasitaemia among each age group, level in 0–5 year age group, in both urban and rural dwellers, were higher than the level seen in the other age groups. Similarly, higher mean parasite densities were seen in 6–10 year group compared to 11–15 and 16–20 year groups. However, while mean parasitaemia in 11–15 year group in rural dwellers did not differ from density seen in the 16–20 year group (10,478 vs. 8901 parasites/µL, p > 0.05), in urban dwellers, mean parasitaemia density was significantly higher in 11–15 year age group compared to 16–20 year group (15,489 vs. 11,254 parasites/µL, p < 0.05). On the other hand, a similar trend was seen in the females. Parasitaemia was higher in female urban dwellers (18,365 vs. 14,008 parasites/µL, p = 0.001). Among the female age groups, significant higher densities were seen in each age group. In female rural dwellers, parasitaemia was higher in the 0–5 year age group compared to the other age groups. Likewise, parasitaemia in the 6–10 year age group was higher than densities seen in 11–15 year and 16–20 year groups. However, in urban dwellers, parasite density was only significantly higher in the 0–5 year group. Densities in the other age groups did not differ from each other (Table 3).
Table 3.
Age-gender segregation of malaria parasitaemia
| Gender | Number of patients (n = 149) | GM of parasite densities (parasites/µL) | p-value | |
|---|---|---|---|---|
| Rural dwellers n (parasite count) |
Urban dwellers n (parasite count) |
|||
| Males | 30 (11,031)* | 20 (15,169)* | 0.032 | |
| 0–5 years | 19 (38.0%) | 14 (19,578)a | 5 (28,015)a | 0.011 |
| 6–10 years | 17 (34.0%) | 10 (13,201)a, b | 7 (19,090)a, b | 0.008 |
| 11–15 years | 9 (18.0%) | 3 (10,478)a, b | 6 (15,489)a, b, c | 0.021 |
| 16–20 years | 5 (10.0%) | 3 (8901)a, b | 2 (11,254)a, b, c | 0.013 |
| Females | 53 (14,008)* | 46 (18,365)* | 0.001 | |
| 0–5 years | 44 (44.4%) | 31 (17,336)a | 13 (21,505)a | 0.038 |
| 6–10 years | 31 (31.3%) | 11 (13,407)a, b | 20 (16,888)a | 0.027 |
| 11–15 years | 13 (13.1%) | 5 (12,114)a, b | 8 (15,509)a | 0.009 |
| 16–20 years | 11 (11.1%) | 6 (11,456)a, b | 5 (16,358)a | 0.016 |
GM – geometric mean, n – number of patients in category, *Significant difference in parasitaemia between males and females, a, b, c parasitaemia differed significantly in respective groups
Haematological parameters in malaria cases
The levels of peripheral blood neutrophils, eosinophils, and monocytes in infected cases did not significantly differ between controls and cases (Table 4). However, whereas leukocytes count, lymphocyte count, basophil count and mean cell haemoglobin (MCH) were significantly lower in controls compared to cases, red blood cells (RBC) count, haemoglobin, mean cell volume (MCV), haematocrit and platelet count were significantly higher in controls compared to cases. Additionally, Tukey post hoc analysis identified total white blood cells, lymphocytes, haemoglobin, haematocrit, mean cell volume, mean cell haemoglobin, platelets and mean platelet volume levels in urban dwellers to be significantly different from rural dwellers. Lymphocytes (2.08 ± 0.16 vs. 2.15 ± 0.05), haemoglobin (10.53 ± 1.75 vs. 12.04 ± 1.45), haematocrit (27.88 ± 3.93 vs. 31.71 ± 3.93), mean cell haemoglobin (28.29 ± 3.39 vs. 30.58 ± 1.97), platelets (126.3 ± 62.52 vs. 166.80 ± 75.20) and mean platelet volume (8.60 ± 0.87 vs. 10.02 ± 1.55) levels in urban dwellers were found to be significantly lower than rural dwellers. However, mean cell volume levels were significantly higher in urban dwellers (79.62 ± 10.80) compared to rural dwellers (77.47 ± 6.31).
Table 4.
Haematological parameters among confirmed cases and controls
| Parameters | Mean ± standard deviation | p-value | ||
|---|---|---|---|---|
| Controls (n = 149a) | Rural (n = 83) | Urban cases (n = 66) | ||
| White blood cells (× 109/L) | 4.99 ± 1.12 | 6.43 ± 1.14 | 7.95 ± 1.93 | 0.003 |
| Neutrophils (× 109/L) | 3.0 ± 0.14 | 4.0 ± 0.05 | 5.25 ± 0.15 | 0.113 |
| Lymphocytes (× 109/L) | 1.87 ± 0.14 | 2.15 ± 0.05* | 2.08 ± 0.16* | 0.012 |
| Eosinophil (× 109/L) | 0.16 ± 0.03 | 0.19 ± 0.012 | 0.23 ± 0.02 | 0.503 |
| Monocytes (× 109/L) | 0.12 ± 0.02 | 0.23 ± 0.02 | 0.38 ± 0.03 | 0.068 |
| Basophils (× 109/L) | 0.01 ± 0.002 | 0.03 ± 0.002 | 0.03 ± 0.003 | 0.015 |
| Red blood cells (× 1012/L) | 5.08 ± 0.46 | 4.02 ± 0.39 | 3.62 ± 0.57 | 0.022 |
| Haemoglobin (g/dL) | 12.26 ± 1.39 | 12.04 ± 1.45* | 10.53 ± 1.75* | 0.039 |
| Haematocrit (%) | 36.79 ± 3.89 | 31.71 ± 3.93* | 27.88 ± 3.93* | 0.009 |
| Mean cell volume (fL) | 86.51 ± 8.12 | 77.47 ± 6.31* | 79.62 ± 10.80* | 0.011 |
| Mean cell haemaoglobin (pg) | 24.23 ± 2.95 | 30.58 ± 1.97* | 28.29 ± 3.39* | 0.002 |
| Platelet (× 109/L) | 242.90 ± 65.53 | 166.80 ± 75.20* | 126.3 ± 62.52* | 0.001 |
| Mean platelet volume (fL) | 8.61 ± 0.99 | 10.02 ± 1.55* | 8.60 ± 0.87* | 0.012 |
| Plateletcrit (%) | 0.21 ± 0.06 | 0.16 ± 0.07 | 0.16 ± 0.11 | 0.006 |
| Platelet large cell ratio | 16.76 ± 4.62 | 23.32 ± 5.02 | 22.78 ± 4.01 | 0.031 |
*Significant differences between rural and urban parameters, aThe control values presented in the table were mean values of all the control cases selected from both rural and urban communities
Nutritional status of cases and controls
The body mass index, total protein, and albumin levels of urban dwellers with malaria were statistically not different from control values. However, levels in rural dwellers with malaria were significantly lower than control values and levels observed in urban dwellers with malaria (Table 5).
Table 5.
Anthropometric and nutritional status of the participants
| Parameters | Mean ± standard deviation | p-value | ||
|---|---|---|---|---|
| Controls (n = 149a) | Rural cases (n = 83) | Urban cases (n = 66) | ||
| Body mass index (kg/m2) | 23.1 ± 3.7 | 19.1 ± 2.9* | 24.5 ± 3.4 | 0.011 |
| Total protein (g/L) | 75.7 ± 4.2 | 69 ± 3.6* | 78.3 ± 5.1 | 0.008 |
| Albumin (g/L) | 42.8 ± 4.6 | 34.1 ± 2.8* | 44.1 ± 5.0 | 0.037 |
Underweight (< 18.5), Normal (18.5 to −24.9), Overweight (25.0 to −29.9), Obese (> 30.0) (World Health Organization 2020), *Significantly different from control values, aThe control values presented in the table were mean values of all the control cases selected from both rural and urban communities
Levels of immunological markers in malaria patients
Presented in Table 6 are the immunological parameters of the malaria cases, tested by one-way analysis of variance (ANOVA). Whereas serum IL-10 and CD4 + cells were significantly higher in controls than cases, TNF- α, IL-12, IL-6, CD3 + , and CD8 + cell count were significantly lower in controls compared to cases. Tukey post hoc analysis found that TNF-α (392.6 ± 149.6 vs. 284.9 ± 93, p = 0.02), IL-6 (239.6 ± 80.3 vs. 186.3 ± 35.3, p = 0.0015) and IL-12 (130.2 ± 40.6 vs. 102.8 ± 52.8, p = 0.008) levels in urban residents were significantly higher than their rural counterparts. However, the reverse was observed in IL-10 (110.6 ± 41.3 vs. 158.8 ± 44.2, p < 0.0001). In addition, the levels of CD4+ T-cells, CD3+ T-cells, and CD8+ T-cells in urban-infected residents were significantly lower compared to rural residents.
Table 6.
Levels of immunological parameters among confirmed cases and controls
| Parameters | Mean ± standard deviation | p-value | ||
|---|---|---|---|---|
| Controls (n = 149a) | Rural (n = 83) | Urban cases (n = 66) | ||
| TNF-α (pg/mL) | 83.9 ± 46.51 | 284.9 ± 93* | 392.6 ± 149.6* | < 0.001 |
| IL-12 (pg/mL) | 75.92 ± 17.42 | 102.8 ± 52.8* | 130.2 ± 40.6* | < 0.001 |
| IL-10 (pg/mL) | 190.5 ± 63.2 | 158.8 ± 44.2* | 110.6 ± 41.3* | < 0.001 |
| IL-6 (pg/mL) | 71.7 ± 17.19 | 186.3 ± 35.3* | 239.6 ± 80.3* | < 0.001 |
| CD4+ T-cells (cells/µL) | 944 ± 244 | 703 ± 110* | 511 ± 175* | < 0.001 |
| CD3+ T-cells (cells/µL) | 1533 ± 254 | 1840 ± 431* | 1630 ± 330* | < 0.001 |
| CD8+ T-cells (cells/µL) | 588 ± 273 | 1337 ± 359 | 1149 ± 345 | < 0.001 |
| CD4+/ CD3+ ratio | 0.62 ± 0.69 | 0.38 ± 0.25* | 0.31 ± 0.19* | < 0.001 |
*Significant differences between rural and urban parameters, a The control values presented in the table were mean values of all the control cases selected from both rural and urban communities
Association of clinical findings to dwelling places
Of the seven clinical findings reported by patients, four of them were strongly associated with urban residents (Table 7). The associated clinical findings were chills (94.0%, x2 = 10.7, p = 0.001), diarrhoea (72.7%, x2 = 11.9, p = 0.0006), fever characterized by temperature above 38 oC (94.0%, x2 = 8.9, p = 0.003) and pallor (68.2%, x2 = 6.7, p = 0.001).
Table 7.
Clinical findings of the study participants
| Clinical finding | Rural dwellers (n = 83) | Urban dwellers (n = 66) | x2 (p-value) |
|---|---|---|---|
| Abdominal discomfort | 3.7 (0.055)* | ||
| Yes | 52 (62.7%) | 51 (77.3%) | |
| No | 31 (37.3%) | 15 (22.7%) | |
| Chills | 10.7 (0.001)** | ||
| Yes | 61 (73.4%) | 62 (94.0%) | |
| No | 22 (26.5%) | 4 (6.0%) | |
| Diarrhoea | 11.9 (0.0006)** | ||
| Yes | 37 (44.6%) | 48 (72.7%) | |
| No | 46 (55.4%) | 18 (27.3) | |
| Fever (temp > 38.0 °C) | 8.9 (0.003)** | ||
| Yes | 63 (75.9%) | 62 (94.0%) | |
| No | 20 (24.1%) | 4 (6.0%) | |
| Nausea | 0.7 (0.404) | ||
| Yes | 67 (80.7%) | 55 (83.3%) | |
| No | 16 (19.3%) | 9 (16.7%) | |
| Pallor | |||
| Yes | 39 (47.0%) | 45 (68.2%) | 6.7 (0.01)** |
| No | 44 (53.05) | 21 (31.8%) | |
| Vomiting | 0.3 (0.595) | ||
| Yes | 48 (57.8%) | 41 (62.1%) | |
| No | 35 (42.2%) | 25 (37.9%) | |
*Confirmed by accompanying adults for children under 10 years
**Significant association
Discussion
In Ghana, malaria is prevalent in both urban (Diallo et al. 2017) and rural communities (Babayara and Addo 2018). In this case–control study, we report that Ghanaian urban dwellers were hard hit with malaria compared to rural dwellers, even though the nutritional status assessment was significantly poor in rural dwellers. In both age and gender categories, parasitaemia was significantly higher in urban dwellers than rural dwellers. Although the actual causality of this observation was not explored in the present study, a review of previous studies in this subject area offers some insights as to the plausible causes of the significantly higher parasitaemia in urban dwellers compared to rural dwellers. Low transmission intensity of malaria in urban areas (Padilla et al. 2015) is due partly to factors such as improved housing and limited mosquito breeding sites and abundance (Kokwaro 2009). In Ghana, the predominant malaria causing Anopheles species in urban communities were identified as An. gambiae (s.l.) of which more than 99% are An. coluzzii and An. gambiae (s.s.) (Mattah et al. 2017) while in rural Ghana, An. gambiae s.l. and An. funestus constitute over 94% of malaria causing mosquito population (of which almost 98% of An. gambiae.s.l. were identified as An. gambiae s.s.). Relatively fewer numbers of An. pharoensis and An. rufipes has also been identified in rural communities (Appawu et al. 2004). Entomological inoculating rates (EIR) of female Anopheles mosquitoes have also been reported to be significantly reduced in urbanized communities (Robert et al. 2003). For these reasons, transmission of malaria in urban areas is expected to be low with its attendant lower levels of multiclonality. Multiclonality of malaria parasites is directly related to malaria immune status. In communities with stable malaria transmission, multiclonality tends to increase as host immunity to malaria sharpens (Smith et al. 1999). Additionally, several studies have also shown an inverse relationship between multiclonality and severity of malaria (al-Yaman 1997; Farnert et al. 1999; Muller et al. 2001). Hence, the hyperparasitaemia observed in urban residents in the current study could be as a result of incompetent malaria immunity that characterizes the urban dwellers observed in this study. In such patients, malaria parasites will undergo unchecked multiplication from few parasites to tens of millions of parasites within a short time (Dietz et al. 2006; Simpson et al. 2002). As malaria parasites multiply in such individuals, peripheral blood haematological indices are radically deranged. Not surprisingly, the urban dwellers studied in this study recorded significantly low levels of lymphocytes, haemoglobin, haematocrit, mean cell haemoglobin, and platelets. This trend may be sequalae to the hyper-parasitaemia reported herein, further corroborating the earlier findings. In both rural and urban dwellers with malaria, thrombocytopenia was observed with levels further reduced in urban dwellers. Tumour necrosis factor alpha (TNF-α) which promotes cellular apoptosis, is reported to be inversely related to platelet count (Raza et al. 2013). Urban dwellers with malaria recorded higher levels of TNF-α than rural dwellers, hence the reduction in their platelet count compared to rural dwellers conformed to earlier publications.
Low levels of lymphocytes observed in urban dwellers reflected in significantly reduced T-lymphocyte subpopulations specifically CD4+ T-cells, CD3+ T-cells, and CD8+ T-cells. In urban dwellers with malaria, mean CD4+ T cells and mean CD4+/CD3+ ratios were not only significantly reduced but also fell below the lower reference ranges established for Ghanaians by Ampofo et al. (2006). There is compelling evidence to confirm that CD4+ T-cells play an important role in clearance of pathogens in humans and in animal models (Soghoian and Streeck 2010). Specifically, in humans, CD4+ T-cell subtype, T-helper 2 (Th2) is responsible for controlling parasitic infections such as Plasmodium falciparum mediated by interleukin (IL)-4, IL-5, IL-13 and IL-10 (Zhu and Paul 2008). In this study, mean CD4+ T-cells and mean CD4+/CD3+ ratios were significantly lower; suggesting their immunocompromised states. Therefore, these categories of individuals are highly susceptible to infections as well as complications associated with the infections, hence higher parasitaemia in urban dwellers with malaria. High parasitaemia correlated with high body temperatures (> 38.0 °C) in the study participants of urban origin. Additionally, in urban dwellers with malaria, hyperthermia directly correlated with TNF-α, IL-6 and IL-12. However, high level of TNF-α have been associated with associated with fever and headache in malaria patients (Kwiatkowski and Nowak 1991) and disease severity and complications (Shaffer et al. 1991). In effect, urban dwellers are more likely to develop severe malaria compared to rural dwellers and by inference, urban dwellers may be more prone to malaria complications than rural dwellers. Based on the foregoing, urban dwellers with malaria must be treated as an emergency case, with all the attention to reduce malaria parasitaemia within the shorted possible time with the ultimate aim to subside inflammatory response and tissue damage.
The protective effect of IL-10 was observed in this study which corroborates earlier reports that high level of IL-10 is beneficial to the host by modulating the P. falciparum parasite-induced inflammatory response (Perera et al. 2013; Day et al. 1999). IL-10 has been shown in a previous study to be inversely related to TNF-α and other proinflammatory cytokines (Ho et al. 1998), a phenomenon that was observed in this study. More importantly, IL-10 has been shown to be directly produced by CD4+ T cells. It exerts its activity by suppressing inflammation via the inhibition of T cell functions and the upstream activities of antigen-presenting cells (Kumar et al. 2019). The findings from this study indicated a corresponding reduction in both CD4+ T cells and IL-10 in urban residents. This observation confirms a reduced control of inflammatory responses and the attendant heightening of host tissue damage in urban dwellers (Ado and Langhorne 2012). It has been demonstrated experimentally in mice that reduced IL-10 levels exacerbates disease pathology and enhanced proinflammatory cytokine (IFN-γ and TNF-α) activity (Li et al. 1999).
Taken together, elevation of TNF-α, IL-6, IL-12, and reduction in IL-10 could be responsible for the simultaneous presentations of chills, diarrhoea, fever, and pallor as observed in urban dwellers with malaria. These presentations are usually associated with severe and prolonged inflammation as a result of sustained higher levels of proinflammatory cytokines.
Conclusion
In summary, urban dwellers exhibited severer forms of malaria compared to rural dwellers with malaria. This could be as a result of reduced or no malaria immunity which occurs due to irregular exposure of urban dwellers to infected mosquito bites. This situation predisposes infected individuals to severe outcomes of malaria Additionally, combinations of symptoms such as abdominal discomfort, chills, diarrhoea, fever, nausea, pallor, and vomiting observed in the urban dwellers could be due to cytokine storm as a result of prolong inflammation.
Abbreviations
- ANOVA
Analysis of variance
- BMI
Body mass index
- CD
Cluster of differentiation
- fL
Femtoliter (equivalent to 10–15 L)
- GM
Geometric mean
- IL
Interleukin
- MCH
Mean cell haemoglobin
- MCV
Mean cell volume
- RBC
Red blood cells
- TNFα
Tumor necrosis factor alpha
Author contributions
Conceptualization: [DOA, EA], Methodology: [DOA, PA, PA, KOD, EA], Formal analysis and investigation: [DOA, PA, KOD, EA], Writing–original draft preparation: [EA, PA]; Writing–review and editing: [DOA, PA, KOD], Resources: [DOA, PA, PA, KOD, EA], Supervision: [EA].
Funding
This study was funded from the authors own resources.
Data availability
The study data have been deposited at Harvard dataverse (https://doi.org/10.7910/DVN/DMUDQ3).
Declarations
Conflicts of interest
The authors have none to declare.
Consent to participate
Written consent to participate was sought from each participant or relatives or guardians of children below 18 years.
Ethics approval
Ethical approval for the study was obtained from Ghana Health Service Ethical Review Committee (study number: GHS-REC002/03/18).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ado RPF, Langhorne J. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. Int J Parasitol. 2012;42:549–555. doi: 10.1016/j.ijpara.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Ahiboh H, Oga AS, Yapi HF, Kouakou G, Boua KD, Edjeme N, et al. Anaemia, iron index status and acute phase proteins in malaria (Abidjan, Cote d'Ivoire) Bull Soc Pathol Exot. 2008;101:25–28. [PubMed] [Google Scholar]
- Al-Yaman F, Genton B, Reeder JC, Anders RF, Smith T, Alpers MP. Reduced risk of clinical malaria in children infected with multiple clones of Plasmodium falciparum in a highly endemic area: a prospective community study. Trans R Soc Trop Med Hyg. 1997;91:602–605. doi: 10.1016/S0035-9203(97)90046-8. [DOI] [PubMed] [Google Scholar]
- Alves-Junior ER, Gomes LT, Ribatski-Silva D, Mendes CRJ, Leal-Santos FA, Simões LR, et al. Assumed white blood cell count of 8,000 Cells/μL overestimates malaria parasite density in the brazilian amazon. PLoS ONE. 2014;9(4):e94193. doi: 10.1371/journal.pone.0094193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampofo W, Torpey K, Mukadi Y, Koram K, Nolan K, Amenyah R, Kaitoo E, Antwi P, Ofori-adjei D, Lamptey P. Normal CD4T lymphocyte levels in HIV seronegative individuals in the Manya/Yilo Krobo communities in the eastern region of Ghana. Viral Immunol. 2006;19:260–266. doi: 10.1089/vim.2006.19.260. [DOI] [PubMed] [Google Scholar]
- Appawu M, Owusu-Agyei S, Dadzie S, Asoala V, Anto F, Koram K, Rogers W, Nkrumah F, Hoffman SL, Fryauff DJ. Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop Med Int Health. 2004;9(1):164–170. doi: 10.1046/j.1365-3156.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- Asante KP, Zandoh C, Dery DB, Brown C, Adjei G, Antwi-Dadzie Y, Adjuik M, Tchum K, Dosoo D, Amenga-Etego S, Mensah C, Owusu-Sekyere KB, Anderson C, Krieger G, Owusu-Agyei S. Malaria epidemiology in the Ahafo area of Ghana. Malar J. 2011;10:211. doi: 10.1186/1475-2875-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayara MNK, Addo B. Presumptive treatment of malaria in Ghana: was it ever useful? evidence from the Kassena-Nankana District of Northern Ghana. Malar Res Treat. 2018 doi: 10.1155/2018/3408089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiwakata CB, Manegold C, Bonicke L, Waase I, Julch C, Dietrich M. Procalcitonin as a parameter of disease severity and risk of mortality in patients with Plasmodium falciparum malaria. J Infect Dis. 2001;183:1161–1164. doi: 10.1086/319283. [DOI] [PubMed] [Google Scholar]
- Costa FTM, Ml Avri, Nogueira PA, Gysin J. Cytoadhesion of Plasmodium falciparum-infected erythrocytes and the infected placenta: a two-way pathway. Brazilian J Med Biol Res. 2006;39(12):1525–1536. doi: 10.1590/S0100-879X2006001200003. [DOI] [PubMed] [Google Scholar]
- Day PJ, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, et al. The prognostic and pathophysiologic role of pro- and anti-inflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–1297. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- Diallo N, Akweongo P, Maya E, Aikins M, Sarfo B. Burden of malaria in mobile populations in the greater accra region, Ghana: a cross-sectional study. Malar J. 2017;16:109. doi: 10.1186/s12936-017-1751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K, Raddatz G, Molineaux L. Mathematical model of the first wave of Plasmodium falciparum asexual parasitaemia in non-immune and vaccinated individuals. Am J Trop Med Hyg. 2006;75(2 Supplement):46–55. doi: 10.4269/ajtmh.2006.75.46. [DOI] [PubMed] [Google Scholar]
- Farnert A, Rooth I, Svensson Å, Snounou G, Bjorkman A. Complexity of Plasmodium falciparum infections is consistent over time and protects against clinical disease in Tanzanian children. J Infect Dis. 1999;179:989–995. doi: 10.1086/314652. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Shetty S. Blood coagulation in falciparum malaria—a review. Parasitol Res. 2008;102:571–576. doi: 10.1007/s00436-007-0832-0. [DOI] [PubMed] [Google Scholar]
- Grillet ME. Factors associated with distribution of anopheles aquasalis and anopheles oswaldoi (Diptera: Culicidae) in a malarious area, northeastern Venezuela. J Med Entomol. 2000;37:231–238. doi: 10.1603/0022-2585-37.2.231. [DOI] [PubMed] [Google Scholar]
- Ho M, Schollaardt T, Snape S, Looareesuwan S, Suntharasamai P, White NJ. Endogenous interleukin-10 modulates pro-inflammatory response in Plasmodium falciparum malaria. J Infect Dis. 1998;178:520–525. doi: 10.1086/515640. [DOI] [PubMed] [Google Scholar]
- Jason J, Archibald LK, Nwanyanwu OC, Bell M, Buchanan I, Larned J, Kazembe PN, Dobbie H, Parekh B, Byrd MG, Eick A, Han A, Jarvis WR. Cytokines and malaria parasitemia. Clin Immunol. 2001;100(2):208–218. doi: 10.1006/clim.2001.5057. [DOI] [PubMed] [Google Scholar]
- Klinkenberg E, Onwona-Agyeman KA, McCall PJ, et al. Cohort trial reveals community impact of insecticide-treated nets on malariometric indices in urban Ghana. Trans R Soc Trop Med Hyg. 2010;104:496–503. doi: 10.1016/j.trstmh.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Kokwaro G. Ongoing challenges in the management of malaria. Malar J. 2009;8:S2. doi: 10.1186/1475-2875-8-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Ng S, Engwerda C. The role of IL-10 in malaria: a double edged sword. Front Immunol. 2019;10:229. doi: 10.3389/fimmu.2019.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D, Nowak M. Periodic and chaotic host-parasite interactions in human malaria. Proc Natl Acad Sci USA. 1991;88:5111–5113. doi: 10.1073/pnas.88.12.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in PLASMODIUM chabaudi chabaudi infection in mice. Infect Immun. 1999;67:4435–4442. doi: 10.1128/IAI.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghendji-Nzondo S, Kouna L, Mourembou G, et al. Malaria in urban, semi-urban and rural areas of southern of Gabon: comparison of the Pfmdr 1 and Pfcrt genotypes from symptomatic children. Malar J. 2016;15:420. doi: 10.1186/s12936-016-1469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattah PAD, Futagbi G, Amekudzi LK, Mattah MM, de Souza DK, Kartey-Attipoe WD, Bimi L, Wilson MD. Diversity in breeding sites and distribution of Anopheles mosquitoes in selected urban areas of southern Ghana. Parasites Vectors. 2017;10:25. doi: 10.1186/s13071-016-1941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DA, Charlwood JD, Felger I, Ferreira C, do Rosario V, Smith T. Prospective risk of morbidity in relation to multiplicity of infection with Plasmodium falciparum in Sao Tome. Acta Trop. 2001;78:155–162. doi: 10.1016/S0001-706X(01)00067-5. [DOI] [PubMed] [Google Scholar]
- Norgan NG. Population differences in body composition in relation to the BMI. Eur J Clin Nutr. 1994;48(Supplement 3):S1O–S27. [PubMed] [Google Scholar]
- Omumbo JA, Guerra CA, Hay SI, Snow RW. The influence of urbanisation on measures of Plasmodium falciparum infection prevalence in east Africa. Acta Trop. 2005;93:11–21. doi: 10.1016/j.actatropica.2004.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla JC, Chaparro PE, Molina K, et al. Is there malaria transmission in urban settings in Colombia? Malar J. 2015;14:453. doi: 10.1186/s12936-015-0956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell S, Walawege R. Sub-Saharan African urbanization and global environmental change. Glob Environ Chang. 2011;21:12–20. doi: 10.1016/j.gloenvcha.2011.09.014. [DOI] [Google Scholar]
- Perera MK, Herath NP, Pathirana SL, Phone-Kyaw M, Alles HK, Mendis KN, Premawansa S, Handunnetti SM. Association of high plasma TNF-alpha levels and TNF-alpha/IL-10 ratios with TNF2 allele in severe P. falciparum malaria patients in Sri Lanka. Pathog Global Health. 2013;107(1):21–29. doi: 10.1179/2047773212Y.0000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner G, Wasler A, Fahrleinter-Pammer A, et al. The role of serum albumin in the prediction of malnutrition in patients at least five yr after heart transplantation. Clin Transplant. 2014;28:737–742. doi: 10.1111/ctr.12370. [DOI] [PubMed] [Google Scholar]
- Raza A, Ghanchi NK, Sarwar Zubairi AB, et al. Tumor necrosis factor -α, interleukin-10, intercellular and vascular adhesion molecules are possible biomarkers of disease severity in complicated Plasmodium vivax isolates from Pakistan. PLoS ONE. 2013;8:e81363. doi: 10.1371/journal.pone.0081363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–176. doi: 10.4269/ajtmh.2003.68.169. [DOI] [PubMed] [Google Scholar]
- Shaffer N, Grau GE, Hedberg K, Davachi F, Lyamba B, Hightower AW, Breman JG, Phuc ND. Tumor necrosis factor and severe malaria. J Infect Dis. 1991;163:96–101. doi: 10.1093/infdis/163.1.96. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Aarons L, Collins WE, Jeffery GM, White NJ. Population dynamics of untreated Plasmodium falciparum malaria within the adult human host during the expansion phase of the infection. Parasitology. 2002;124:247–263. doi: 10.1017/S0031182001001202. [DOI] [PubMed] [Google Scholar]
- Smith T, Felger I, Tanner M, Beck HP. Premunition in Plasmodium falciparum infection: insights from the epidemiology of multiple infections. Trans R Soc Trop Med Hyg. 1999;93:59–64. doi: 10.1016/S0035-9203(99)90329-2. [DOI] [PubMed] [Google Scholar]
- Soghoian DZ, Streeck H. Cytolytic CD4+ T cells in viral immunity. Expert Rev Vaccines. 2010;9(12):1453–1463. doi: 10.1586/erv.10.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ, Gething PW, Smith DL, Hay SI. Urbanization and the global malaria recession. Malar J. 2013;12:1–10. doi: 10.1186/1475-2875-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre D, Speranza F, Giola M, Matteelli A, Tambini R, Biondi G. Role of Th1 and Th2 cytokines in immune response to uncomplicated Plasmodium falciparum malaria. Clin Diagn Lab Immunol. 2002;9:348–351. doi: 10.1128/CDLI.9.2.348-351.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzan B, Izri A, Durand R, Deniau M, Bouchaud O, Perret GY. Serum procalcitonin in uncomplicated falciparum malaria: a preliminary study. Travel Med Infect Dis. 2006;4:77–80. doi: 10.1016/j.tmaid.2005.04.003. [DOI] [PubMed] [Google Scholar]
- WHO. World malaria report 2019. Geneva: World Health Organization; 2019; https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019
- White NJ. Plasmodium knowlesi: the fifth human malaria parasite. Clin Infect Dis. 2008;46(2):172–173. doi: 10.1086/524889. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Body mass index–BMI. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Assessed Jul 27, 2020
- Yadouléton RN, Allagbé H, et al. The impact of the expansion of urban vegetable farming on malaria transmission in major cities of Benin. Parasites and Vectors. 2010;3:118. doi: 10.1186/1756-3305-3-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data have been deposited at Harvard dataverse (https://doi.org/10.7910/DVN/DMUDQ3).