Abstract
Acute respiratory distress syndrome is an inflammatory disease with no effective pharmacological treatment. We investigated the therapeutic effect of HY1702, a new small molecule diterpene obtained from the processing and modification of Glaucocalyxin A and may exhibit anti-inflammatory activity. Specifically, we studied the anti-inflammatory effects of HY1702 on lipopolysaccharide-induced inflammatory responses in RAW264.7 and THP-1 cells in vitro and its protective efficacy on lipopolysaccharide-induced mild acute respiratory distress syndrome in mice.
Our results showed that HY1702 significantly decreased lipopolysaccharide-induced inflammatory cytokine expression in RAW264.7 and THP-1 cells and attenuated the secretion of nitric oxide and prostaglandin E2 by down-regulating the expression of inducible nitric oxide synthase and cyclooxygenase 2 in RAW264.7 cells. In mice with lipopolysaccharide-induced mild acute respiratory distress syndrome, HY1702 alleviated histological alterations in the lungs and reduced the alveolar cavity protein leakage and inflammatory cytokine expression in murine bronchial alveolar lavage fluid. HY1702 decreased the myeloperoxidase activity and lung wet to dry weight ratio. In our mechanism studies in lipopolysaccharide-exposed RAW264.7 cells, HY1702 suppressed the inflammation stimulated by lipopolysaccharide through inhibiting phosphorylation of inhibitor of nuclear factor κB kinase subunit α/β (IKKα/β) and inhibitor of nuclear factor κB subunit α (IκBα), further affecting the nuclear transfer of phosphorylated p65. Meanwhile, phosphorylation of p38 mitogen-activated protein (MAP) kinase and extracellular signal-regulated kinase (ERK) was inhibited. These data suggest that HY1702 can reduce inflammation on lipopolysaccharide-stimulated macrophages and attenuate the symptoms of mild acute respiratory distress syndrome in a murine model by regulating the nuclear factor κB and MAP kinase signalling pathways.
Keywords: HY1702, Inflammation, Mild acute respiratory distress syndrome, Nuclear factor κB, MAP kinase
1. Introduction
Acute respiratory distress syndrome (Wheeler and Bernard, 2007), can arise from direct or indirect insults that induce exaggerated pulmonary inflammation, damage the cells of the alveolocapillary membrane, and lead to severe acute respiratory failure. Acute respiratory distress syndrome is characterised by non-cardiogenic pulmonary edema, neutrophil accumulation, inflammatory cytokine overproduction, epithelial integrity disruption, and protein leakage into the alveolar space, severely influencing gas exchange (Bhatia and Moochhala, 2004; Goodman et al., 2003). Despite significant progress in the treatment of acute respiratory distress syndrome, the morbidity and mortality rates remain high (Williams et al., 2004). At present, no clinically effective treatment has been applied for improving the survival of patients with acute respiratory distress syndrome (Bosma et al., 2010). Accordingly, further studies dedicated to identify novel therapeutic approaches to acute respiratory distress syndrome are urgently needed.
Lipopolysaccharide, the major outer membrane component of gram-negative bacteria, induces inflammation (Peng et al., 2004). Acute respiratory distress syndrome induced by intratracheal administration of lipopolysaccharide is well suited to the preliminary study of potential preventive or therapeutic compounds against acute respiratory distress syndrome in humans (Wang et al., 2014). Direct exposure of lipopolysaccharide to lung tissue by tracheal infusion triggers an acute inflammatory response that causes aggravated interstitial and alveolar edema, increased pulmonary capillary permeability, peripheral inflammatory cells infiltration and secretion of inflammatory cytokines (Shen et al., 2015; Zhang et al., 2010). Dexamethasone is the most widely prescribed anti-inflammatory drug. It has been shown to down-regulate pro-inflammatory cytokines and effectively attenuates lipopolysaccharide-induced lung damage (Yu et al., 2009). Therefore, dexamethasone was used as a positive control compound in our in vivo experiments.
Glaucocalyxin A, an ent-kauranoid diterpene from Rabdosia japonica var, is known to possess numerous biological activities, including inhibition of immunosuppressive activity, anti-oxidative and cytotoxic activity (Xiao et al., 2013). However, it has poor pharmacokinetic properties, such as low polarity and poor water solubility, and is not suitable for direct drug administration. Under the premise of retaining the pharmacophore, it has been structurally modified to synthesise the derivate with a better water-solubility. HY1702 is a product obtained by reacting scutellarin and a nitrogen-containing compound as a reaction material under a certain catalyst, solvent and temperature, and acidifying to obtain a diterpenoid compound in the form of its salt. We hypothesised that it has anti-inflammatory activity and thus, in the present study, evaluated the effects of HY1702 on lipopolysaccharide-exposed macrophages and in a murine model of lipopolysaccharide-induced mild acute respiratory distress syndrome, further clarify the potential molecular mechanism.
2. Materials and methods
2.1. Reagents
HY1702 (C22H36ClNO4 ·HCl, purity ≥ 98%) was received from Pharmavan New drug development (China) Co., Ltd. Lipopolysaccharide (Escherichia coli, O111: B4) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse tumor necrosis factor-α, interleukin-6, interleukin-1β and human tumor necrosis factor-α enzyme-linked immunosorbent assay kits were purchased from BOSTER (Wuhan, China). Human interleukin-6 and interleukin-1β enzyme-linked immunosorbent assay kits were purchased from eBioscience (Thermo Fisher Scientific, USA). All the antibodies used for Western blotting and Immunofluorescence were purchased from Cell Signaling Technology. (Beverly, MA, USA).
2.2. Cells culture and treatment
Macrophage RAW264.7 and THP-1 cell lines were derived from the Institutes of Biology and Medical Sciences, Soochow University, and cultured in either Dulbecco's Modified Eagle Medium or Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C with 5% CO2. THP-1 cells were primed with phorbol myristate acetate (10 ng/ml) in serum-free RPMI 1640 medium for 12 h. The medium was discarded and the cells were washed three times with phosphate buffered saline. Complete RPMI 1640 medium was added, and the cells were cultured for subsequent experiments. Phorbol myristate acetate-primed THP-1 and RAW264.7 cells were cultured to 70% to 80% confluence. A range of HY1702 concentrations in complete RPMI 1640 or Dulbecco's Modified Eagle Medium was added to the cells, which were then incubated for 1 h. Then the lipopolysaccharide (100 ng/ml) was added. The cells were cultured for 24 h before we performed total RNA isolation (see Section 2.11) and enzyme-linked immunosorbent assays (see Section 2.7). RAW 264.7 cells were cultured 24 h for protein extraction (see Section 2.13).
2.3. Cell viability assay for RAW 264.7 and THP-1 cells
Cell viability was assessed with Cell Counting Kit-8 assay (DojinDo, JPN) and appropriate concentrations were selected for follow-up tests. Briefly, RAW264.7 and phorbol myristate acetate-primed THP-1 cells were seeded in 96-well plates and incubated at 37 °C with 5% CO2. When cells were cultured to more than 80% confluency, the cells in each group were separately treated with different concentrations of HY1702 for 24 h. Then, 10 μl of Cell Counting Kit-8 solution was added to each well according to the instructions, and the cells were incubated for another 1 h. The absorbance was measured at 450 nm with a microplate reader.
2.4. Nitrite determination and prostaglandin E2 assays in RAW264.7 cells
The nitrite concentration in the culture medium was measured as an indicator of NO production using the Griess reaction (Beyotime Biotechnology, China). Briefly, the 50 μl of cell cultural supernatant and an equivalent amount of Griess reagent were transferred to a new 96-well plate; NO levels were determined by measuring nitrite levels through the absorbance at 540 nm. Nitrite levels in the samples were calculated from a standard curve with known concentrations of sodium nitrite (Babich et al., 1993). The prostaglandin E2 concentration in the cell culture medium was determined using a prostaglandin E2 assay kit (R&D Systems, USA). All procedures were performed according to the manufacturer's instructions.
2.5. Animals experiments
Male C57BL/6 mice (6–8 weeks old) were obtained from Joinn Laboratories (China) Co., Ltd (license No.SCXK (Su) 2018-0006). After a habituation period, 36 mice were divided into six groups: control, lipopolysaccharide, lipopolysaccharide + HY1702 (5, 10 or 30 mg/kg) and lipopolysaccharide + dexamethasone (5 mg/kg). HY1702 or dexamethasone was intragastrically administered 1 h before intratracheal lipopolysaccharide administration; the control and lipopolysaccharide only groups were intragastrically administered normal saline instead of HY1702 or dexamethasone. Then the control group received instilling intratracheal phosphate buffered saline, and mild acute respiratory distress syndrome was induced in the other groups by instilling intratracheal lipopolysaccharide (10 μg of lipopolysaccharide dissolved in 50 μl normal saline). Mice were humanely euthanized at 6 h after lipopolysaccharide administration. Bronchoalveolar lavage fluid and lung tissues were collected for further analysis. Animal experiments procedures were performed following the guidelines of the Detailed Rules and Regulations of Medical Animal Experiments Administration and Implementation, and Ordinance in Experimental Animal Management’ and were approved by the Soochow University Animal Policy and Welfare Committee(approved IACUC number: SUDA20200508A01).
2.6. Histological assessment
The middle lobe of the right lung was collected form each mouse and fixed in 4% paraformaldehyde for 48 h. The tissues were dehydrated, embedded in paraffin, sliced into 5 μm sections and stained with hematoxylin and eosin. The sections were evaluated under a light microscope and then photomicrographs were taken.
2.7. Enzyme-linked immunosorbent assays
The levels of inflammatory factors (tumor necrosis factor-α, interleukin-1β and interleukin-6) in bronchoalveolar lavage fluid and the supernatants of RAW264.7 and THP-1 cell cultures were measured using enzyme-linked immunosorbent assay kits according to the manufacturer's instructions (see Section 2.1).
2.8. Ratio of wet to dry weight for lung tissue
The fresh upper part of the left lung was weighed, dried in an oven at 70 °C for 72 h, then weighed again (once dry) to calculate the lung wet to dry weight ratio.
2.9. Quantifying protein content of bronchoalveolar lavage fluid
Mice were euthanized, an incision was made from the middle of the neck, the neck skin muscles were separated, the trachea was exposed, and the catheter was inserted into the trachea. Phosphate buffered saline (3 × 500 μl) was instilled into the airways and a consistent volume of bronchoalveolar lavage fluid (1400–1450 μl) was recovered from each animal. The lavage was centrifuged at 1000 g for 5 min at 4 °C, and the bronchoalveolar lavage fluid protein content was determined by bicinchoninic acid (BCA) protein assay (Beyotime Biotechnology, China) according to the manufacturer to evaluate the vascular permeability to the airway.
2.10. Myeloperoxidase activity in lung tissue
After a 6 h challenge with lipopolysaccharide, the lung tissues were collected, then homogenised with 10% normal saline buffer and centrifuged at 12,000 g for 10 min at 4 °C. The supernatant of each lung homogenate was collected for the myeloperoxidase assay, which was performed according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.11. RNA isolation and quantitative real-time reverse transcription - polymerase chain reaction
Total RNA was extracted from mice lung samples and RAW264.7 and THP-1 cells using the TRIzol® Universal Reagent (TIANGEN, Beijing, China). The extracted RNA (1000 ng) was reverse-transcribed using a PrimeScript™ RT Master Mix kit (Takara, Dalian, China) according to the manufacturers' instructions. The quantitative real-time polymerase chain reaction was performed using the SYBR Green PCR Mix (Takara, Dalian, China). The cycling reactions were performed using the ABI7900 Sequence Detection System. The polymerase chain reaction thermal cycle was 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 15 s, followed by a 5 min extension at 72 °C and holding at 4 °C. The data were normalised to the geometric mean of housekeeping gene β-actin and calculated using the 2-ΔΔCT method. The sequences of the primers used in the experiments are listed in Table 1 .
Table 1.
Sequences of primers used for qRT-PCR.
| Gene | Forward primers | Reverse primers |
|---|---|---|
| Mouse TNF-α | 5’-ATGGCCTCCCTCTCATCAGT-3’ | 5’-ATAGCAAATCGGCTGACGGT-3’ |
| Mouse IL-6 | 5’- AGCCAGAGTCCTTCAGAGAGAT-3’ | 5’-GGTCTTGGTCCTTAGCCACTC-3’ |
| Mouse IL-1β | 5’-CTGGTGTGTGACGTTCCCAT-3’ | 5’-GTGGGTGTGCCGTCTTTCAT-3’ |
| Mouse actin | 5’-GAACCCTAAGGCCAACCGTGA-3’ | 5’-GATGGCGTGAGGGAGAGCATAG-3’ |
| Human TNF-α | 5’-TAGCCCATGTTGTAGCAAACC-3’ | 5’-ATGAGGTACAGGCCCTCTGAT-3’ |
| Human IL-6 | 5’-GTGTGAAAGCAGCAAAGAGGC-3’ | 5’-TTGGGTCAGGGGTGGTTATT-3’ |
| Human IL-1β | 5’-CTGTCCTGCGTGTTGAAAGATG-3’ | 5’-CTGCTTGAGAGGTGCTGATGT-3’ |
| Human actin | 5’-GGGCATGGAGTCCTGTGGCA-3’ | 5’-GGGTGCCAGGGCAGTGATCTC-3’ |
2.12. Proteomics mass spectrometry
Cultured cells were harvested and lysed in 100 mM Triethylammonium bicarbonate (ThermoFisher Scientific, USA) lysis buffer with 1% SDS (Beyotime Biotechnology, China), followed by sonication at 4 °C for 10 min (15 s on/15 s off). After centrifugation (16,000 g for 10 min, 4 °C), the supernatant was reduced with 10 mmol dithiothreitol Solution (ThermoFisher Scientific, USA) and alkylated with 55 mmol iodoacetamide (Sigma, USA). The solution was added pre-chilled acetone (Tedia, USA) and incubated at −20 °C overnight until a flocculent formed. The precipitation was obtained by centrifugation and resuspend protein pellets with 50 mM Triethylammonium bicarbonate(Thermo, USA). The sample was digested with trypsin (Promega, USA) overnight at 37 °C. Then the samples were desalted using C18 tips (ThermoFisher Scientific, USA) according to the protocol. The peptides eluted were dried and resuspended in 10 μl water with 0.1% formic acid (Sigma, USA). 2 μl of each sample was analyzed by an EASY nano-flow liquid chromatography (ThermoFisher Scientific, USA) coupled to an Orbitrap Fusion Lumos Tribrid mass spectrometer (ThermoFisher Scientific, USA). The raw files were analyzed with Proteome Discoverer 2.3. (ThermoFisher Scientific, USA).
2.13. Protein extraction and western blot analysis
RAW264.7 cells were collected and lysed with radioimmunoprecipitation assay buffer to extract the total, nuclear and cytoplasmic proteins according to the instructions (Beyotime Biotechnology, China). Protein concentrations were determined by the bicinchoninic acid assay kit. Subsequently, the proteins were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% bovine serum albumin at room temperature for 1 h. The membranes were then blocked and probed with the primary antibodies, followed by the respective HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies. Immunoreactive bands were visualized with Super Signal West Dura extended kits (Thermo Scientific). The ImageJ program (http://rsbweb.nih.gov/ij/download.html) was used for densitometric analyses of Western blots.
2.14. Immunofluorescence analysis
RAW264.7 cells were pretreated with HY1702 (2 μM) for 1 h, and lipopolysaccharide (100 ng/ml) was added for another 6 h. Cells were washed three times with phosphate buffered saline, fixed in 4% paraformaldehyde for 15 min, washed three times with phosphate buffered saline and permeabilised with 0.1% Triton X-100 for 15 min, followed by another wash in phosphate buffered saline and blocking in 5% bovine serum albumin for 1 h at room temperature. Samples were incubated with a primary antibody against the nuclear factor κB phosphorylated-p65 subunit (1:200) overnight at 4 °C. On the next day, the cells were probed with an Alexa Fluor 594-conjugated anti-rabbit antibody (1:1000) at room temperature for 1 h and then washed three times with phosphate buffered saline. Besides, 1 mg/ml of 4′,6-diamidino-2-phenylindole (DAPI) dissolved in phosphate buffered saline was added for 20 min to stain the nuclei. Fluorescence images were taken using a laser scanning confocal microscope (Nikon, Japan).
2.15. Statistical analyses
All experiments were repeated at least 3 times, unless stated otherwise, and data were expressed as mean ± S.E.M. One-way analysis of variance and Student's t-test were used to assess the difference for multiple comparisons and single comparisons by using Prism 6 software (Graph Pad, San Diego, USA). P < 0.05 was considered to be a significant difference.
3. Results
3.1. HY1702 extenuates lipopolysaccharide-induced inflammatory response in vitro
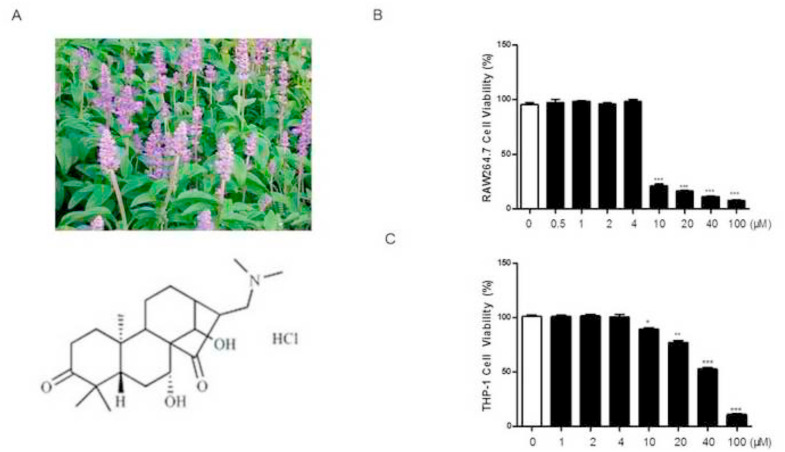
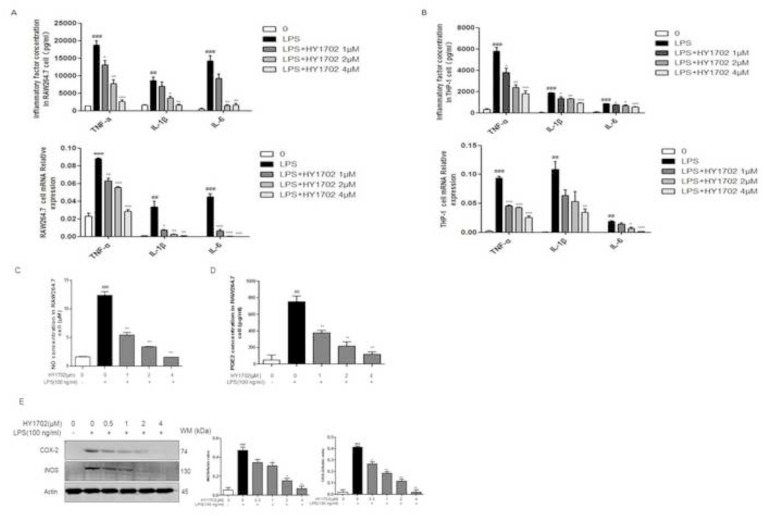
In our cell viability assay, HY1702 (Chemical Structure, Fig. 1 A) displayed no cellular toxicity (inhibition rate < 10%) against RAW264.7 and THP-1 cells at concentrations of at least 4 μM (Fig. 1B and C). Therefore, we chose 4 μM as the highest drug dose for subsequent experiments with HY1702. After lipopolysaccharide exposure, RAW264.7 and THP-1 cells expressed increased levels of tumor necrosis factor-α, interleukin-6 and interleukin-1β compared with the control group (Fig. 2 A and B). Pretreatment with HY1702 significantly inhibited tumor necrosis factor-α, interleukin-6 and interleukin-1β expression. Furthermore, HY1702 down-regulated the secretion of nitric oxide and prostaglandin E2 through inhibiting inducible nitric oxide synthase and cyclooxygenase 2 expression in RAW264.7 cells (Fig 2C to E). These results indicate that HY1702 exerts anti-inflammatory activity in vitro by inhibiting the expression of inflammatory factors and inflammatory mediators.
Fig. 1.
Effect of HY1702 on RAW264.7 and THP-1 cell viabilitys. RAW264.7 and THP-1 cells were treated with different concentrations of HY1702 for 24 h, then 10 μl of Cell Counting Kit-8 solution was added to each well to incubate with the cells for another 1 h. The absorbance was measured at 450 nm. A: The chemical structure of HY1702. B: Effects of HY1702 on the viability of RAW264.7 cells. C: Effects of HY1702 on the viability of THP-1 cells. The values presented are mean ± S.E.M (n = 6) of three independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with control group.
Fig. 2.
Effect of HY1702 on lipopolysaccharide-induced inflammatory response in RAW264.7 and THP-1 cells. RAW264.7 and THP-1 cells were pretreated with HY1702 for 1 h and further treated with lipopolysaccharide for 24 h, then cellular supernatant and protein were collected. Tumor necrosis factor-α, interleukin-1, and interleukin-6 secretion from RAW264.7 (A) and THP-1 (B) cells was detected by using an enzyme-linked immunosorbent assay. Nitric oxide (C) and prostaglandin E2 (D) secretion in RAW264.7 were detected by Griess reagent and enzyme-linked immunosorbent assay. E: Cyclooxygenase 2 and inducible nitric oxide synthase protein level in the lysates of RAW264.7 cells were detected by Western blot. The values presented are mean ± S.E.M (n = 3) of three independent experiments. ###P < 0.001, ##P < 0.01 compared with control; *P < 0.05, **P < 0.01, ***P < 0.001 compared with LPS mode.
3.2. HY1702 alleviates lipopolysaccharide-induced pulmonary histopathological abnormalities
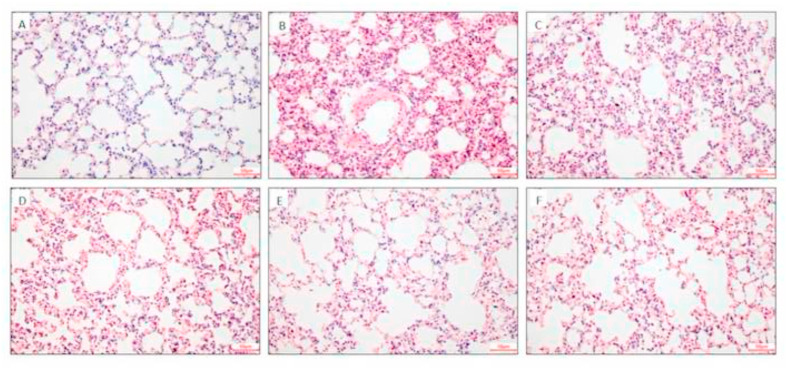
The effects of HY1702 on the histopathological features of lungs from mice with mild acute respiratory distress syndrome were also tested. In the control group, the lung alveolar walls were thin and neatly arranged, with no histological alteration observed in the lung specimens (Fig. 3 A). Compared with the normal structure of those lung tissues, the lipopolysaccharide treatment remarkably increased the alveolar wall thickness, alveolar collapse, and infiltration of pro-inflammatory cells in the lungs (Fig. 3B). In contrast, the groups pretreated with HY1702 or dexamethasone displayed very little histopathological changes (Fig 3C to F). Our results showed that HY1702 improved the pathological changes of lung tissue in mice exposed to lipopolysaccharide and alleviated the development of mild acute respiratory distress syndrome.
Fig. 3.
Effect of HY1702 on histopathology of lung tissues of lipopolysaccharide-induced mild acute respiratory distress syndrome. Mice were treated with HY1702, dexamethasone or vehicle 1 h before lipopolysaccharide challenge. Lung tissues (n = 6) from each experimental group were processed for histological evaluation at 6 h after the lipopolysaccharide challenge. A: Control group. B: Lipopolysaccharide-only group. C: Lipopolysaccharide + HY1702 (5 mg/kg) group. D: Lipopolysaccharide + HY1702 (10 mg/kg) group. E: LPS + HY1702 (30 mg/kg) group. F: Lipopolysaccharide + dexamethasone (5 mg/kg) group. Representative histological lung sections were stained with hematoxylin and eosin at 400 × magnification.
3.3. HY1702 attenuates inflammatory responses in lung tissue of mice with mild acute respiratory distress syndrome
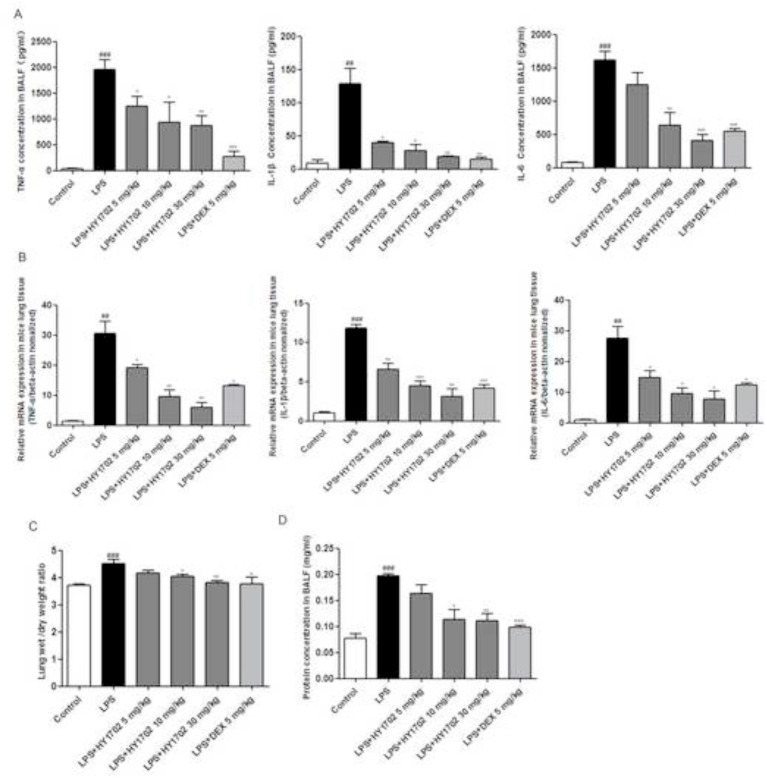
To further evaluate the protective effects of HY1702 against mild acute respiratory distress syndrome, the levels of inflammatory cytokines, lung wet to dry ratios and alveolar cavity protein leakage were measured. Compared with the control group, lipopolysaccharide induction increased the levels of tumor necrosis factor-α, interleukin-1β and interleukin-6 expression in bronchoalveolar lavage fluid (Fig. 4 A and B). Compared with the lipopolysaccharide only group, pretreatment with HY1702 suppressed the expression of tumor necrosis factor-α, interleukin-1β and interleukin-6. The lung wet to dry weight ratio was significantly increased after lipopolysaccharide-stimulation. Pretreatment with HY1702 effectively reduced the lung wet to dry ratio (Fig. 4C). Meanwhile, the protein concentration in the bronchoalveolar lavage fluid was inhibited by pretreatment with HY1702 (Fig. 4D). These data indicated that HY1702 protected against mild acute respiratory distress syndrome by decreasing the expression of inflammatory factors, subsequently reducing pulmonary non-cardiogenic pulmonary edema and destruction of the alveolar wall.
Fig. 4.
HY1702 attenuates mild acute respiratory distress syndrome in mice. Mice were intragastrically administratered with HY1702 (5, 10 or 30 mg/kg), dexamethasone (5 mg/kg) or vehicle 1 h before lipopolysaccharide challenge. 6 h after lipopolysaccharide administration, bronchial alveolar lavage fluid, and lung tissue were collected to quantify the tumor necrosis factor-α, interleukin-1β and interleukin-6 expression by enzyme-linked immunosorbent assay (A) and verse transcription-polymerase chain reaction (B). C: Effects of HY1702 on the lung wet to dry weight ratio in lipopolysaccharide-induced mild acute respiratory distress syndrome. D: Effects of HY1702 on protein content of bronchial alveolar lavage fluid in lipopolysaccharide-induced mild acute respiratory distress syndrome. The values presented are mean ± S.E.M (n = 6) of three independent experiments. ##P < 0.01, ###P < 0.001 compared with control; * P < 0.05, ** P < 0.01, ***P < 0.001 compared with LPS mode.
3.4. HY1702 decreases pulmonary myeloperoxidase activity
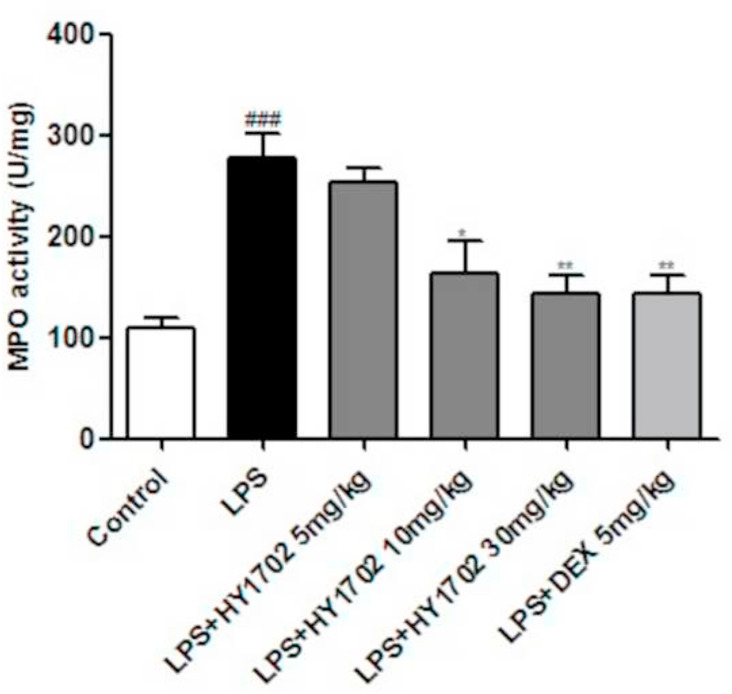
We used lung tissue homogenate to detect myeloperoxidase activity. Compared with control mice, intratracheal instillation of lipopolysaccharide-induced a prominent increase in pulmonary myeloperoxidase activity, which was also decreased by pretreatment with HY1702 (Fig. 5 ). This indicates that HY1702 can inhibit lipopolysaccharide-induced neutrophil infiltration in murine lung tissue, thereby inhibiting further deterioration in mild acute respiratory distress syndrome.
Fig. 5.
Effect of HY1702 on myeloperoxidase activity in lung tissues of lipopolysaccharide-induced mild acute respiratory distress syndrome. Mice were intragastrically administratered with HY1702 (5, 10 or 30 mg/kg), dexamethasone (5 mg/kg) or vehicle 1 h before LPS challenge. 6 h after lipopolysaccharide administration, the myeloperoxidase activity in the lung tissue of mice was detected in lung tissue. The values presented are mean ± S.E.M (n = 6) of three independent experiments. ###P < 0.001 compared with control; * P < 0.05, ** P < 0.01, ***P < 0.001 compared with LPS mode.
3.5. HY1702 extenuates lipopolysaccharide-induced nuclear factor κB and mitogen-activated protein kinase activation in RAW264.7 cells
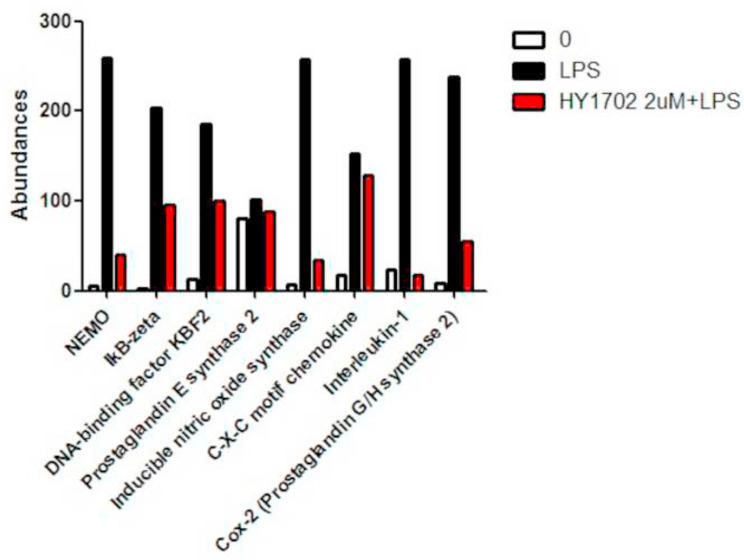
The relative abundance ratio of proteomic data was used to search for the signaling pathways altered by HY1702. The results of our proteomics assay (Fig. 6 ) indicated that HY1702 can effects NEMO (Protein: O88351; Gene: Ikbkg), IkB-zeta (Protein: Q9EST8; Gene: Nfkbiz), DNA-binding factor KBF2 (Protein: Q4U105; Gene: Nfkb2), Prostaglandin E synthase 2 (Protein: Q8BWM0; Gene: Ptges2), Cyclooxygenase-2 (Prostaglandin G/H synthase 2) (Protein: Q05769; Gene: Ptgs2), Nitric oxide synthase (Protein: P29477; Gene: Nos2), C-X-C motif chemokine (Protein: Q3U1J5; Gene: Cxcl2), Interleukin-1 (Protein: Q3U0Y6; Gene: Il1a) in NF-κB signaling pathway in lipopolysaccharide-stimulated RAW264.7 cells after the treatment of HY1702.
Fig. 6.
Application of Proteomics mass spectrometry to detect the alteration of protein expressions in RAW264.7 cells following the treatment with LPS or LPS and HY1702. Pathway analysis involved in inflammation (nuclear factor κB and MAP kinase signaling pathways) following the treatment with LPS or LPS and HY1702 (2 μM) (From http://www.kegg.jp/).
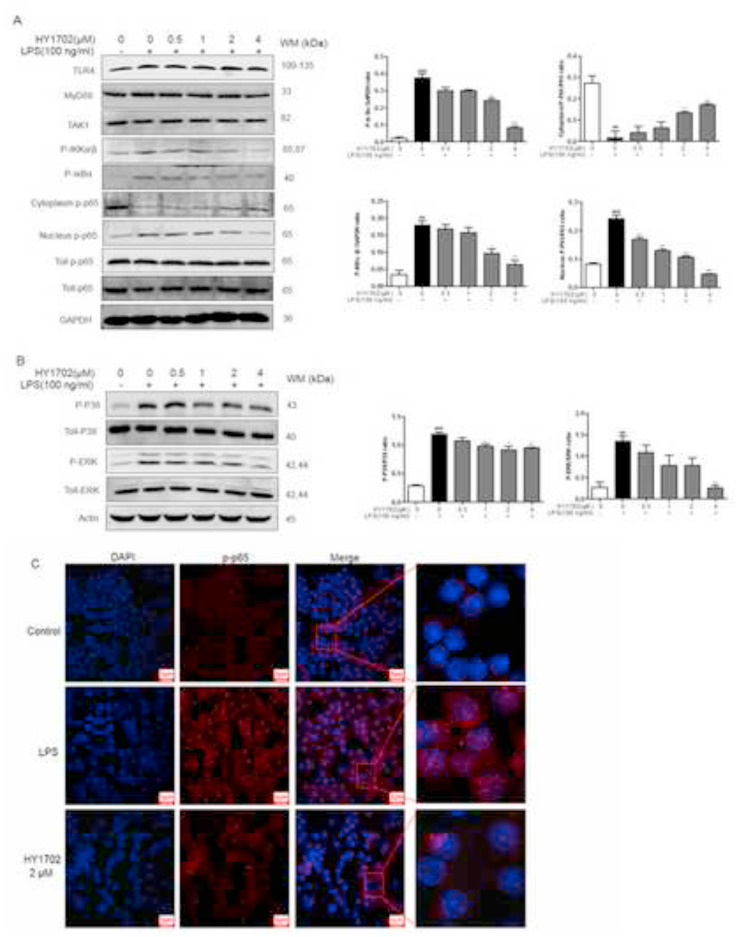
Then we detected the changes of related proteins such as Toll-like receptor 4, MyD88, TAK1, IKKα/β, IκBα and p-p65 by Western Blot experiment. The results showed that HY1702 had no significant effect on the expression of Toll-like Receptor 4, MyD88, TAK-1 and total p-p65 protein, but had a certain effect on the phosphorylation of IKKα/β and IκBα. After lipopolysaccharide administration, the phosphorylation levels of inhibitor of nuclear factor κB kinase subunit α/β (IKKα/β) and inhibitor of nuclear factor κB subunit α (IκBα) were increased. Then we investigated whether HY1702 affected the nuclear translocation of nuclear factor κB (p65 subunit) by Western blot and confocal microscopy analysis of immunofluorescence. We found that pretreatment with HY1702 hindered the transfer of phosphorylated-p65 protein from the cytoplasm to the nucleus (Fig. 7 A and C). Besides, compared with the control group, phosphorylated p38 and phosphorylated extracellular signal regulated kinase (ERK) were increased in the lipopolysaccharide-stimulated group, after HY1702 pretreatment, their expression levels were inhibited (Fig. 7B). These data suggested that HY1702 effectively reduced IKKα/β and IκBα phosphorylation, thereby preventing nuclear factor κB (p65 subunit) nuclear translocation; furthermore, HY1702 decreased p38 and ERK phosphorylation, inhibited the expression of downstream inflammatory factors and protected against lipopolysaccharide-induced mild acute respiratory distress syndrome.
Fig. 7.
HY1702 extenuates the lipopolysaccharide-induced activation of nuclear factor κB and mitogen-activated protein kinase in RAW264.7 macrophages. RAW264.7 cell were pretreated with HY1702 for 1 h and further treated with lipopolysaccharide for 24 h, at 6 h, protein was extracted for experiments. A: Expression of phosphorylated-IKBα/β, phosphorylated-IkBα/β in cytoplasm and nuclear phosphorylated-p65 level. B: The phosphorylated and total extracellular signal–regulated kinase (ERK) and p38 levels were determined by Western blot analysis. Total ERK (ERK), total p38 (p38), or total p65 (p65) protein was used as the loading control. C: The nuclear translocation of nuclear factor κB p65 was visualized by immunofluorescence. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; (blue)). Scale bar = 200 μm. The values presented are mean ± S.E.M of three independent experiments. ###P < 0.001, ##P < 0.01 compared with control; *P < 0.05, **P < 0.01, ***P < 0.001 compared with LPS mode. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Acute respiratory distress syndrome is one of the most common acute critical and high mortality diseases. The molecular mechanisms of acute respiratory distress syndrome are complex and not entirely clear. Inflammation is key players in the progression of acute respiratory distress syndrome, they disrupt alveolar epithelial barriers and injure the microvascular endothelium leading to increased capillary permeability (Bhatia and Moochhala, 2004; Levitt and Matthay, 2012). It is believed that inhibition of the inflammatory response has protective effects against lung injury (Jiang et al., 2016; Nieman et al., 2015). In this study, we observed the anti-inflammation effects of HY1702 on lipopolysaccharide exposed macrophage cells, explored the protective effects against lipopolysaccharide-induced mild acute respiratory distress syndrome, and elucidated the underlying molecular mechanisms.
Reports have demonstrated that pro-inflammatory cytokines play a critical role in the initiation and propagation of inflammatory pathogenesis in lipopolysaccharide-induced acute respiratory distress syndrome (Chen et al., 2015, 2016; Giebelen et al., 2007; Jing et al., 2015). Increased levels of pro-inflammatory cytokines in the bronchoalveolar lavage fluid have been noted in ARDS patients, and the persistent elevation of pro-inflammatory cytokines has been associated with more severe outcomes (Minamino and Komuro, 2006). Inhibition of these cytokines production is beneficial for the treatment of inflammation and inflammatory diseases. Our results showed that HY1702 could suppress the production of tumor necrosis factor-α, interleukin-1β and interleukin-6 in vitro and in vivo, suggesting that HY1702 is useful for novel anti-inflammatory therapies. This indicates that the protective effects of HY1702 on mild acute respiratory distress syndrome may be attributed to the inhibition of inflammatory cytokines and the secretion of inflammatory mediators.
Pulmonary edema is one of the major characteristics of acute respiratory distress syndrome. The wet to dry weight ratio of the lung tissue was used to investigate the degree of pulmonary edema (Deng et al., 2012; Jing et al., 2015). Experiments showed that HY1702 inhibited edema of the lung, as indicated by the significant decrease in lung dry weight ratio. This result provides evidence for the protective effects of HY1702 in mild acute respiratory distress syndrome by reducing the degree of pulmonary edema. Total protein concentration in bronchial alveolar lavage fluid is one of the important indices of pulmonary capillary permeability in lung injury (Chen et al., 2015). Reduction of protein levels by pretreatment with HY1702 suggests that the protective effects of HY1702 on mild acute respiratory distress syndrome may be attributed to the inhibition of increased pulmonary capillary permeability.
Excessive infiltration and accumulation of inflammatory cells can release inflammatory cytokines that may aggravate acute respiratory distress syndrome, particularly neutrophils, in both alveolar and interstitial spaces is one of the pivotal pathological hallmarks of lung injury (Li et al., 2016). Myeloperoxidase is a marker for neutrophils and myeloperoxidase activity that appears in the parenchyma reflects neutrophil accumulation into the lung parenchyma (Abraham, 2003; Haegens et al., 2009; Reumaux et al., 2003). We demonstrate that pretreatment with HY1702 decreases lipopolysaccharide-induced increase in myeloperoxidase activity in the lung tissues, which indicates that HY1702 exerts anti-neutrophil influx effects in lipopolysaccharide-induced mild acute respiratory distress syndrome, suggesting that the inhibition of neutrophil infiltration may be another pathway through which HY1702 attenuates lipopolysaccharide-induced mild acute respiratory distress syndrome. Furthermore, HY1702 significantly alleviated lipopolysaccharide-induced lung histological alterations, showing that HY1702 has a good repair effect on the pathological changes of mild acute respiratory distress syndrome.
Proteomic mass spectrometry analyses allowed us to determine that the mechanism of action of HY1702 is related to nuclear factor κB signaling pathways. Nuclear factor κB and MAP kinase pathways is an important regulatory pathway of inflammation and immune response in the body. Numerous studies have shown that it plays a key role in acute respiratory distress syndrome. Under normal conditions, nuclear factor κB is present in its inactive cytoplasmic form that binds with the nuclear factor κB repressors IκBs (Yamamoto and Gaynor, 2001). Activation of nuclear factor κB induced by lipopolysaccharide involves the phosphorylation of IκBα kinase (IKK), which phosphorylates IκBα, leading to sequent ubiquitination and degradation of IκBα. This contributes to the activation and translocation (cytoplasm to the nucleus) of nuclear factor κB p65 and then induces the transcription of pro-inflammatory cytokines. MAPK families (p38, Jun N-terminal kinase, and ERK 1/2) are involving in lipopolysaccharide-induced acute respiratory distress syndrome (Xu et al., 2015), contribute to the control of the release of cytokines and play critical roles in signal transduction pathways (Tsai et al., 2015). Studies showed that inhibition of nuclear factor κB and MAP kinase attenuates lipopolysaccharide-induced acute respiratory distress syndrome. Our results indicated that HY1702 reduced the phosphorylated ERK, p38, IKKα/β and IκBα, and further suppressed the nuclear translocation of the cytosolic nuclear factor κB (p65) in lipopolysaccharide-treated RAW264.7 cells. Showed that HY1702 may play an anti-inflammatory activity and protective role in lipopolysaccharide-induced mild acute respiratory distress syndrome by inhibiting inflammatory response mediated by nuclear factor κB and MAP kinase activation.
Although our study attests to the pharmacological effects of HY1702 in the mild acute respiratory distress syndrome model and the anti-inflammatory mechanism in RAW264.7 cells, this is a preliminary investigation. Further research for more in-depth mechanisms of HY1702's protective effects on mild acute respiratory distress syndrome and clinical applications is needed.
5. Conclusion
In conclusion, this study shows that HY1702 possesses excellent anti-inflammatory activity and protects against lipopolysaccharide-induced mild acute respiratory distress syndrome by suppressing inflammation. The anti-inflammatory mechanism may be through the inhibition of IKKα/β and IκBα phosphorylation, further inhibiting of nuclear transfer of phosphorylated-p65 and alleviating inflammation. Also, through inhibition of MAP kinase (ERK and p38) and nuclear factor κB signaling, the expression of inducible nitric oxide synthase and cyclooxygenase 2 genes was down-regulated and nitric oxide and prostaglandin E2 production was reduced, this leads to the suppression of inflammatory mediators’ production. Therefore, HY1702 might become a potential drug for the treatment of mild acute respiratory distress syndrome.
Funding
The work was supported by the Natural Science Foundation of Jiangsu Province [BK20160315] and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author contributions
YL, ZC, MW, TZ and LL conceived and designed the experiments. MW and LL performed the experiments. YL, ZC and MW analyzed the data. MW, PW and QX contributed reagents/materials/analysis tools. YL, ZC and MW wrote the manuscript.
CRediT authorship contribution statement
Mengfei Wang: Formal analysis, Data curation, Writing - original draft, conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the manuscript. Tong Zhang: conceived and designed the experiments. Ling Li: conceived and designed the experiments, performed the experiments. Qing Xie: Formal analysis, contributed reagents/materials/analysis tools. Yanping Wang: Formal analysis, Data curation, Writing - original draft, conceived and designed the experiments, analyzed the data, wrote the manuscript. Zijun Chen: Formal analysis, Data curation, Writing - original draft, conceived and designed the experiments, analyzed the data, wrote the manuscript.
Declaration of competing interest
The authors have declared that no competing interest exists.
Acknowledgements
We thank Dr. Elizabeth DeLyria for critical reading of the manuscript and Pharmavan New drug development Capitalized (Soochow, China) assistance with the experiments. The funders had no role in study design, data collection and analysis, or decision to publish.
References
- Abraham E. Neutrophils and acute lung injury. Crit. Care Med. 2003;31:S195–199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- Babich H., Palace M.R., Stern A. Oxidative stress in fish cells: in vitro studies. Arch. Environ. Contam. Toxicol. 1993;24:173–178. doi: 10.1007/BF01141344. [DOI] [PubMed] [Google Scholar]
- Bhatia M., Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- Bosma K.J., Taneja R., Lewis J.F. Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome: current and experimental approaches. Drugs. 2010;70:1255–1282. doi: 10.2165/10898570-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Mou Y., Tan J., Wei L., Qiao Y., Wei T., Xiang P., Peng S., Zhang Y., Huang Z. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharm. 2015;25:55–64. doi: 10.1016/j.intimp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Chen T., Wang R., Jiang W., Wang H., Xu A., Lu G., Ren Y., Xu Y., Song Y., Yong S. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing rho signaling. Inflammation. 2016;39:483–492. doi: 10.1007/s10753-015-0272-4. [DOI] [PubMed] [Google Scholar]
- Deng J., Wang D.X., Deng W., Li C.Y., Tong J., Ma H. Regulation of alveolar fluid clearance and ENaC expression in lung by exogenous angiotensin II. Respir. Physiol. Neurobiol. 2012;181:53–61. doi: 10.1016/j.resp.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Giebelen I.A., van Westerloo D.J., LaRosa G.J., de Vos A.F., van der Poll T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock. 2007;28:700–703. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- Goodman R.B., Pugin J., Lee J.S., Matthay M.A. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523–535. doi: 10.1016/s1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Haegens A., Heeringa P., van Suylen R.J., Steele C., Aratani Y., O'Donoghue R.J., Mutsaers S.E., Mossman B.T., Wouters E.F., Vernooy J.H. Myeloperoxidase deficiency attenuates lipopolysaccharide-induced acute lung inflammation and subsequent cytokine and chemokine production. J. Immunol. 2009;182:7990–7996. doi: 10.4049/jimmunol.0800377. [DOI] [PubMed] [Google Scholar]
- Jiang W., Luo F., Lu Q., Liu J., Li P., Wang X., Fu Y., Hao K., Yan T., Ding X. The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state. Chem. Biol. Interact. 2016;243:127–134. doi: 10.1016/j.cbi.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Jing W., Chunhua M., Shumin W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-kappaB pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015;285:128–135. doi: 10.1016/j.taap.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Levitt J.E., Matthay M.A. Clinical review: early treatment of acute lung injury--paradigm shift toward prevention and treatment prior to respiratory failure. Crit. Care. 2012;16:223. doi: 10.1186/cc11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang J., Foley N.M., Xu Y., Li Y.P., Pan J., Redmond H.P., Wang J.H., Wang J. B7H3 ameliorates LPS-induced acute lung injury via attenuation of neutrophil migration and infiltration. Sci. Rep. 2016;6:31284. doi: 10.1038/srep31284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T., Komuro I. Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. J. Clin. Invest. 2006;116:2316–2319. doi: 10.1172/JCI29637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman G.F., Gatto L.A., Bates J.H.T., Habashi N.M. Mechanical ventilation as a therapeutic tool to reduce ARDS incidence. Chest. 2015;148:1396–1404. doi: 10.1378/chest.15-0990. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Peng X., Hassoun P.M., Sammani S., McVerry B.J., Burne M.J., Rabb H., Pearse D., Tuder R.M., Garcia J.G. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- Reumaux D., de Boer M., Meijer A.B., Duthilleul P., Roos D. Expression of myeloperoxidase (MPO) by neutrophils is necessary for their activation by anti-neutrophil cytoplasm autoantibodies (ANCA) against MPO. J. Leukoc. Biol. 2003;73:841–849. doi: 10.1189/jlb.1102567. [DOI] [PubMed] [Google Scholar]
- Shen Y., Sun Z., Guo X. Citral inhibits lipopolysaccharide-induced acute lung injury by activating PPAR-gamma. Eur. J. Pharmacol. 2015;747:45–51. doi: 10.1016/j.ejphar.2014.09.040. [DOI] [PubMed] [Google Scholar]
- Tsai Y.F., Yu H.P., Chung P.J., Leu Y.L., Kuo L.M., Chen C.Y., Hwang T.L. Osthol attenuates neutrophilic oxidative stress and hemorrhagic shock-induced lung injury via inhibition of phosphodiesterase 4. Free Radic. Biol. Med. 2015;89:387–400. doi: 10.1016/j.freeradbiomed.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Wang J., Liu Y.T., Xiao L., Zhu L., Wang Q., Yan T. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation. 2014;37:2085–2090. doi: 10.1007/s10753-014-9942-x. [DOI] [PubMed] [Google Scholar]
- Wheeler A.P., Bernard G.R. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- Williams M.D., Braun L.A., Cooper L.M., Johnston J., Weiss R.V., Qualy R.L., Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit. Care. 2004;8:R291–298. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Cao W., Jiang X., Zhang W., Zhang Y., Liu B., Cheng J., Huang H., Huo J., Zhang X. Glaucocalyxin A, a negative Akt regulator, specifically induces apoptosis in human brain glioblastoma U87MG cells. Acta Biochim. Biophys. Sin. 2013;45:946–952. doi: 10.1093/abbs/gmt097. [DOI] [PubMed] [Google Scholar]
- Xu C., Chen G., Yang W., Xu Y., Xu Y., Huang X., Liu J., Feng Y., Xu Y., Liu B. Hyaluronan ameliorates LPS-induced acute lung injury in mice via Toll-like receptor (TLR) 4-dependent signaling pathways. Int. Immunopharm. 2015;28:1050–1058. doi: 10.1016/j.intimp.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Gaynor R.B. Role of the NF-kappaB pathway in the pathogenesis of human disease states. Curr. Mol. Med. 2001;1:287–296. doi: 10.2174/1566524013363816. [DOI] [PubMed] [Google Scholar]
- Yu Z., Ouyang J.P., Li Y.P. Dexamethasone attenuated endotoxin-induced acute lung injury through inhibiting expression of inducible nitric oxide synthase. Clin. Hemorheol. Microcirc. 2009;41:117–125. doi: 10.3233/CH-2009-1162. [DOI] [PubMed] [Google Scholar]
- Zhang X., Huang H., Yang T., Ye Y., Shan J., Yin Z., Luo L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury. 2010;41:746–752. doi: 10.1016/j.injury.2010.02.029. [DOI] [PubMed] [Google Scholar]