Abstract
Background and Aims
Metabolic-associated fatty liver disease (MAFLD) is driven by high caloric intake and sedentary lifestyle. Migration towards high income countries may induce these driving factors; yet, the influence of such on the prevalence of MAFLD is clearly understudied. Here, we investigated the Fatty Liver Index (FLI), a proxy of steatosis in MAFLD, after migration of Ghanaian subjects.
Methods
Cross-sectional data of 5282 rural, urban and migrant participants from the Research on Obesity and Diabetes among African Migrants (also known as RODAM) study were analyzed with logistic regression for geographical differences in FLI and associations with type 2 diabetes mellitus (T2DM), waist-to-hip ratio, and 10-year predicted risk of atherosclerotic cardiovascular disease (ASCVD).
Results
Both FLI and the proportion with an FLI indicative of MAFLD steatosis (FLI ≥60) were higher in migrants compared with non-migrants. Prevalence of elevated FLI (FLI ≥60) in non-migrant males was 4.2% compared to 28.9% in migrants. For females, a similar gradient was observed, from 13.6% to 36.6% respectively. Compared to rural residents, the odds for a FLI ≥60 were higher in migrants living in urban Europe (odds ratio [OR] 9.02, 95% confidence interval [CI]: 5.02–16.20 for men, and 4.00, 95% CI: 3.00–5.34 for women). Compared to controls, the ORs for FLI ≥60 were 2.43 (95% CI: 1.73–3.41) for male T2DM cases and 2.02 (95% CI: 1.52–2.69) for female T2DM cases. One-unit higher FLI was associated with an elevated (≥7.5%) 10-year ASCVD risk (OR: 1.051, 95% CI: 1.041–1.062 for men, and 1.020, 95% CI: 1.015–1.026 for women).
Conclusions
FLI as a proxy for MAFLD increased stepwise in Ghanaians from rural areas, through urban areas, to Europe. Our results clearly warrant awareness for MAFLD in migrant population as well as confirmation with imaging modalities.
Keywords: Fatty liver, Non-invasive test, Migration, African population
Introduction
In recent years, the prevalence of metabolic-associated fatty liver disease (MAFLD) has clearly increased, with an estimated prevalence of 25% worldwide.1 The MAFLD prevalence increases coincide with the global increasing prevalence of type 2 diabetes mellitus (T2DM) and obesity, especially central obesity.2 MAFLD is a spectrum of liver disease ranging from hepatic steatosis through non-alcoholic steatohepatitis (NASH), to fibrosis and cirrhosis.3 MAFLD has a complex pathophysiology, but its root cause is insulin resistance and MAFLD is therefore seen as the hepatic component of the metabolic syndrome.4,5 In turn, MAFLD itself may contribute to the clinical manifestations of the metabolic syndrome and therefore to atherosclerotic cardiovascular disease (ASCVD), potentially by inducing dyslipidemia through increased secretion of triglyceride (TG)-rich lipoproteins, in combination with low-grade inflammation and hypercoagulability.6
Of note, the prevalence of MAFLD varies among ethnic groups. Despite a higher prevalence of obesity among ethnic minority groups, especially in women,7 individuals of African descent are relatively less prone to MAFLD compared to individuals of European, Asian, or Hispanic descent, as assessed in the Multiethnic Cohort in the USA.8 Variance in PNPLA3 has been established to influence susceptibility for MAFLD and may contribute to the observed ethnic difference in MAFLD. The PNPLA3-453I allele, which occurs at a higher frequency in African-ancestry populations (12.5%) compared with other populations (< 1%), is associated with lower hepatic fat content.4,9,10
Migration-related environmental changes and urbanization may facilitate sedentary lifestyle and obesity, potentially driving MAFLD.11 Yet, the influence of migration on the prevalence and severity of MAFLD is understudied. In order to address this relevant question, homogeneity of the studied population is imperative to reduce the effects of differences in genetic background. To achieve this, we used data from the Research on Obesity and Diabetes among African Migrants (RODAM) study, representing a relatively homogenous group of Ghanaians which comprises adults originating from the Ashanti Region in Ghana, living in different environmental contexts.12 Direct measures of hepatic fat content, such as liver biopsy, ultrasound, or Magnetic Resonance Imaging Proton Density Fat Fraction (MRI-PDFF) were unavailable in our study population. Therefore, we used a well-established non-invasive composite proxy to assess the steatotic component of MAFLD: the Fatty Liver Index (FLI).13 We aimed to study the prevalence of MAFLD as assessed by the FLI among Ghanaian residents in rural and urban Ghana and among Ghanaians in three European cities.
Additionally, we assessed the associations of the FLI with T2DM, as well as predicted 10-year risk of ASCVD. Patients with MAFLD are at 1.5- to 2-fold increased risk for atherosclerotic cardiovascular disease (ASCVD), potentially via the mixed hyperlipidemia often observed in MAFLD patients.6 Therefore, we included predicted 10-year risk of ASCVD in our study.
Methods
Study design and population
The RODAM study aims to gain knowledge on the development of obesity and diabetes mellitus among African migrants. Full details of the multicenter RODAM study, initiated in 2012, are published elsewhere.12 In brief, 5,898 Ghanaian men and women aged 25–70 years, were recruited and physically examined from a population residing in rural Ghana, urban Ghana, and Ghanaians residing in three different European cities (Amsterdam, Berlin, and London). As we aim to study the effects of migration on the FLI, Ghanaians living in Europe were categorized as migrants, of which 97% were first-generation migrants. Ethical approval of the study protocols has been received at all sites. All authors had access to the study data and reviewed and approved the final manuscript.
Details on data acquisition, blood sampling and processing procedures can be found in the Supplementary File 1.
FLI
The FLI is validated for people aged 18–75 and is calculated by taking into account body mass index (BMI), waist circumference, TGs, and gamma-glutamyltransferase (γGT) according to the algorithm by Bedogni et al.14 In the RODAM database, TG are measured in mmol/L, whereas the formula for the FLI requires TG expressed as mg/dL. For conversion, the factor 88.57 was used.15 The FLI varies between 0 and 100, a FLI <30 is validated for predicting absence of MAFLD with a sensitivity of 91.5%.13,14 Elevated FLI was defined as a score of ≥60, since a cut-off of 60 predicts presence of hepatic steatosis, with a specificity of 82.3%.13 In our aim to capture MAFLD in the RODAM study, we excluded participants in case of retroviral therapy or treatment for hepatitis C and/or excessive alcohol use, defined as >21 units (168 gram alcohol) per week for men and >14 units (112 gram alcohol) per week for women from the current analysis.
10-year predicted risk of ASCVD
The standardized and clinically oriented American College of Cardiology (ACC)/American Heart Association (AHA) ASCVD risk score was applied to calculate the 10-year risk of clinically manifest ASCVD. This score can be used to calculate total 10-year cardiovascular disease risk percentages.16,17 It accurately predicts ASCVD risk also in non-European populations.18 It is scored as a percentage; a score of ≥7.5% is considered elevated risk of developing ASCVD in the next 10 years based on the prior work by Goff et al.16 More information can be found in the Supplementary File 1. This risk score has been validated for subjects between 40–79 years of age, without prior history of ASCVD. Thus, for the ASCVD analysis, participants below 40 years of age and those with a history of ASCVD were excluded. The RODAM database contained self-reported information on stroke, heart attack, other heart conditions, and peripheral arterial disease. The risk score was calculated with an algorithm that combines age, sex, use of antihypertensive medication, presence of diabetes mellitus, systolic blood pressure, total cholesterol, high-density lipoprotein (i.e. HDL) cholesterol, and smoking status.
Data analysis
Data were analyzed using SPSS Statistical software, version 26 (IBM Corp., Armonk, NY, USA). All analyses were conducted separately for men and women due to a statistically significant interaction between FLI and sex. Normally distributed continuous variables were presented as means and standard deviations. Skewed continuous variables were presented as medians and interquartile ranges (IQRs). Categorical variables were presented as proportions. Differences between rural, urban, and migrant participants were assessed by ANOVA, Kruskal-Wallis tests, and χ2 tests as appropriate. Three models were fitted to adjust for possible confounders. Model 1 was adjusted for age; model 2: model 1 + education; model 3: model 2 + physical activity, alcohol, and T2DM. The results are presented as odds ratios (ORs) and the corresponding 95% confidence intervals (CIs). A p-value of <0.05 was considered statistically significant. In addition, the associations between FLI and elevated 10-year risk of ASCVD were calculated, in which the 10-year risk of ASCVD was the dependent variable. For this ASCVD association, nearly identical models to adjust for potential confounding were used as in the assessment for differences between rural, urban and migrated populations. The only difference between these models in correcting for confounding was the replacement of T2DM by smoking in model 3.
Results
General characteristics
From a total of 5,898 RODAM participants who underwent physical examination, 5,282 were included in the current analysis, based on age and the criteria of assessing FLI. Of these participants, 951 (18.0%) were rural Ghanaians, 1,432 (27.1%) were urban Ghanaians, and 2,899 (54.9%) were migrants. The majority of participants were female (62.7%). Mean age was higher in men than in women in urban participants and migrants. However, in rural participants, mean age was higher in women than in men. The mean duration of living in urban Europe was 18.6 years (standard deviation [SD]: 9.6) for males and 19.0 years (SD: 9.5) for females, calculated from 2,529 out of 2,899 migrants. Migrants had the highest levels of education and rural participants had the lowest levels, in both men and women. The prevalence of any alcohol consumption was higher in rural participants in men (53.1%) compared to urban participants (40.6%) and migrants (41.4%). In women, alcohol consumption was lower in urban participants (26.1%) compared to rural participants (30.9%) and migrants (31.2%). The proportion of current smokers was higher in migrants than in non-migrants (6.3% in migrant men, and 2.1% in migrant women). Compared to the two other groups, rural participants had the highest levels of physical activity, irrespective of sex. Differences in general characteristics are shown in Table 1.
Table 1. General characteristics of rural and urban residing, and migrant participants.
| Variables | Rural (n=951) | Urban (n=1,432) | Migrated (n=2,899) | Total (n=5,282) | p |
|---|---|---|---|---|---|
| Men, n | 356 | 406 | 1,209 | 1,971 | |
| Age, years | 45.8±12.9 | 46.7±11.8 | 47.0±10.4 | 46.7±11.2 | 0.193 |
| Education, n (%) | <0.001 | ||||
| Elementary | 136 (38.2) | 89 (21.9) | 144 (11.9) | 369 (18.7) | |
| Lower secondary | 128 (36.0) | 173 (42.6) | 453 (37.5) | 754 (38.3) | |
| Higher secondary | 47 (13.2) | 85 (20.9) | 279 (23.1) | 411 (20.9) | |
| Tertiary | 21 (5.9) | 37 (9.1) | 250 (20.7) | 308 (15.6) | |
| Alcohol consumption, n (%) | 189 (53.1) | 165 (40.6) | 500 (41.4) | 854 (43.3) | <0.001 |
| Smoking, n (%) | 0.023 | ||||
| Current | 17 (4.8) | 12 (3.0) | 76 (6.3) | 105 (5.3) | |
| Past | 48 (13.5) | 61 (15.0) | 131 (10.8) | 240 (12.2) | |
| Physical activity, n (%) | <0.001 | ||||
| Low levels | 37 (10.4) | 85 (20.9) | 287 (23.7) | 409 (20.8) | |
| Medium levels | 54 (15.2) | 72 (17.7) | 176 (14.6) | 302 (15.3) | |
| High levels | 238 (66.9) | 223 (54.9) | 457 (37.8) | 918 (46.6) | |
| WHR | 0.89±0.06 | 0.90±0.06 | 0.93±0.07 | 0.91±0.07 | <0.001 |
| WHR ≥0.90, n (%) | 126 (35.4) | 222 (54.7) | 785 (64.9) | 1,133 (57.5) | <0.001 |
| T2DM, n (%) | 15 (4.2) | 46 (11.3) | 158 (13.1) | 219 (11.1) | <0.001 |
| BMI, kg/m2 | 21.0±3.0 | 24.1±3.8 | 27.0±3.9 | 25.4±4.4 | <0.001 |
| AST/ALT ratio | 1.99±0.82 | 1.70±0.72 | 1.44±0.56 | 1.60±0.68 | <0.001 |
| Waist circumference, cm | 76.9±8.4 | 84.7±10.3 | 92.6±10.9 | 88.1±12.0 | <0.001 |
| TG, mmol/L, median (IQR) | 0.96 (0.7–1.3) | 1.02 (0.8–1.3) | 0.89 (0.67–1.19) | 0.92 (0.70–1.23) | <0.001 |
| γGT, mmol/L, median (IQR) | 33.1 (24.3–51.6) | 39.5 (27.8–56.6) | 36.7 (27.7–50.1) | 36.5 (27.0–51.5) | 0.003 |
| FLI, median (IQR) | 11.7 (6.6–21.8) | 27.0 (12.3–47.5) | 42.6 (21.8–64.6) | 31.8 (14.3–56.7) | <0.001 |
| FLI, categorized | <0.001 | ||||
| <30, n (%) | 298 (83.7) | 214(52.7) | 426 (35.2) | 938 (47.6) | |
| ≥60, n (%) | 15 (4.2) | 66 (16.3) | 349 (28.9) | 430 (21.8) | |
| Women, n | 595 | 1,026 | 1,690 | 3,311 | |
| Age, years | 46.9±12.6 | 44.7±11.2 | 46.1±9.5 | 45.8±10.7 | <0.001 |
| Education, n (%) | <0.001 | ||||
| Elementary | 367 (61.7) | 520 (50.7) | 427 (25.3) | 1,314 (39.7) | |
| Lower secondary | 154 (25.9) | 367 (35.8) | 571 (33.8) | 1,092 (33.0) | |
| Higher secondary | 18 (3.0) | 87 (8.5) | 362 (21.4) | 467 (14.1) | |
| Tertiary | 11 (1.8) | 28 (2.7) | 186 (11.0) | 225 (6.8) | |
| Alcohol consumption, n (%) | 184 (30.9) | 268 (26.1) | 527 (31.2) | 951 (28.7) | 0.014 |
| Smoking, n (%) | <0.001 | ||||
| Current | 0 (0) | 0 (0) | 21 (2.1) | 21 (0.6) | |
| Past | 5 (0.8) | 21 (2.0) | 67 (4.0) | 93 (2.8) | |
| Physical activity, n (%) | <0.001 | ||||
| Low levels | 130 (21.8) | 406 (39.6) | 406 (24.0) | 942 (28.5) | |
| Medium levels | 126 (21.2) | 158 (15.4) | 289 (17.8) | 582 (17.6) | |
| High levels | 294 (49.4) | 434 (42.3) | 577 (34.1) | 1,305 (39.4) | |
| WHR | 0.89±0.07 | 0.90±0.06 | 0.88±0.08 | 0.89±0.07 | <0.001 |
| WHR ≥0.85, n (%) | 444 (74.6) | 829 (80.8) | 1,153 (68.2) | 2,426 (73.3) | <0.001 |
| T2DM, n (%) | 35 (5.9) | 87 (8.5) | 154 (9.1) | 276 (8.3) | 0.049 |
| BMI, kg/m2 | 23.7±4.5 | 28.0±5.5 | 30.3±5.1 | 28.4±5.6 | <0.001 |
| AST/ALT ratio | 1.96±0.75 | 1.87±0.62 | 1.62±0.49 | 1.76±0.60 | <0.001 |
| Waist circumference, cm | 83.8±11.2 | 90.4±11.9 | 95.7±12.0 | 92.1±12.6 | <0.001 |
| TG, mmol/L, median (IQR) | 0.97 (0.74–1.34) | 1.01 (0.74–1.36) | 0.73 (0.57–0.98) | 0.85 (0.64–1.16) | <0.001 |
| γGT, mmol/L, median (IQR) | 26.6 (20.5–36.7) | 29.5 (22.8–38.5) | 27.3 (21.3–36.9) | 27.9 (21.6–37.3) | <0.001 |
| FLI, median (IQR) | 16.7 (8.3–36.2) | 40.9 (19.3–69.6) | 46.6 (25.4–71.7) | 39.1 (18.5–67.4) | <0.001 |
| FLI, categorized | <0.001 | ||||
| <30, n (%) | 402 (67.6) | 388 (37.8) | 514 (30.4) | 1,304 (39.4) | |
| ≥60, n (%) | 81 (13.6) | 331 (32.3) | 619 (36.6) | 1,031 (31.1) |
Data presented as mean±SD unless stated otherwise. SD, standard deviation; WHR, waist-to-hip ratio; T2DM, type 2 diabetes; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TG, triglycerides; γGT, gamma-glutamyltransferase; FLI, fatty liver index; IQR, interquartile ranges.
FLI and its determinants by location of current residency
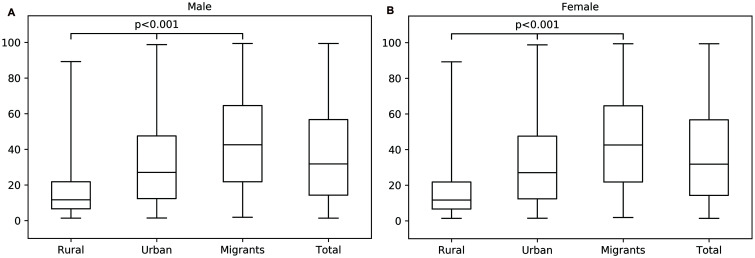
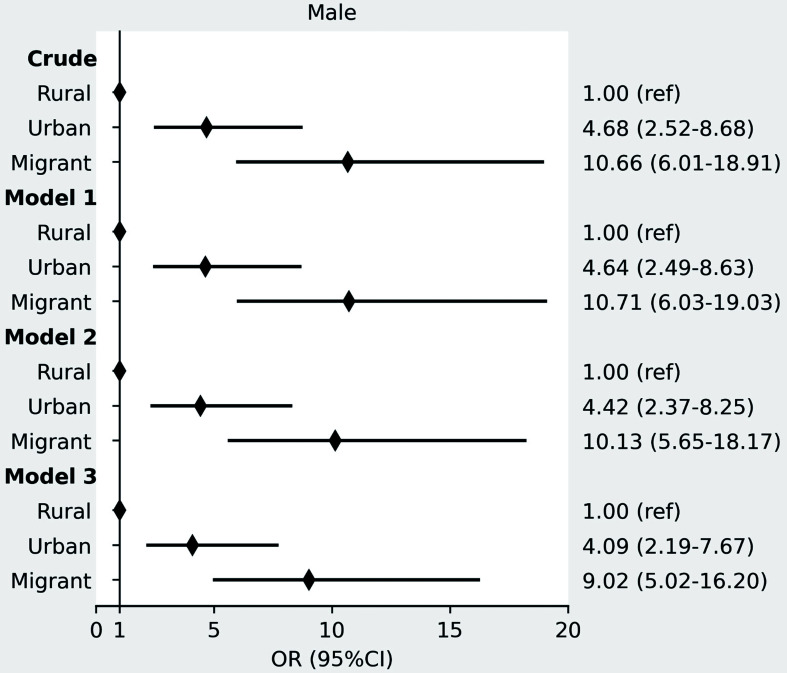
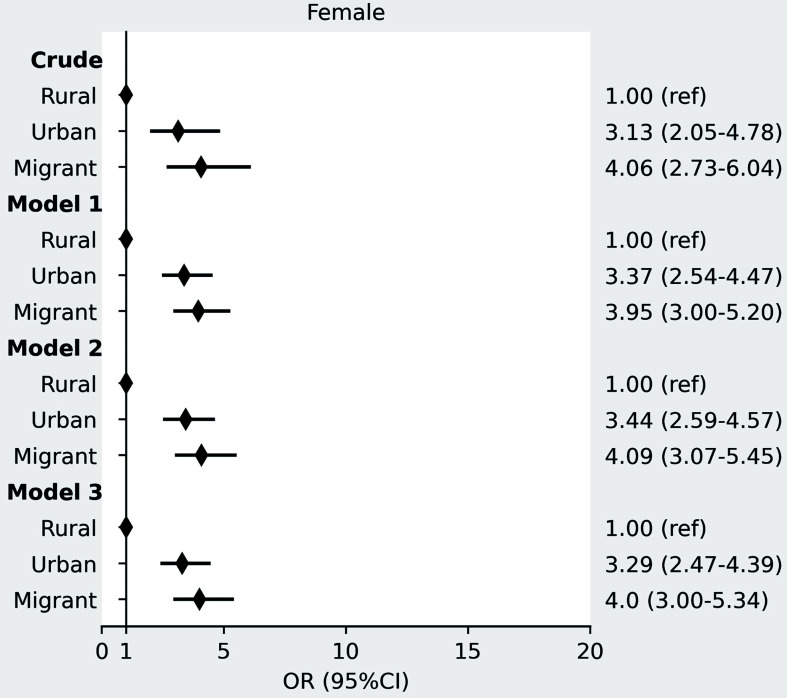
In both men and women, TG levels were highest among urban participants and the lowest among migrants, and γGT was the highest in urban participants and the lowest in rural participants. FLI, BMI, and waist circumference increased stepwise after migration form rural Ghana, through urban Ghana to Europe, whereby the Ghanaian homogenous population living in three distinct environments can be seen as a proxy for migration (as given in Table 1). Median FLI with corresponding IQR per location of residency at inclusion are shown in Figure 1a for men and Figure 1b for women. There was a positive gradient in the prevalence of elevated FLI (FLI ≥60) in males from rural (4.2%) through urban (16.3%) to Europe (28.9%), (p<0.001). A similar positive gradient was observed in females, with 13.6% in rural, 32.3% in urban, and 36.6% in Europe, respectively (p<0.001; Table 1). These differences retained statistical significance even after adjustment for age, education level, physical activity level, alcohol use, and T2DM for urban Ghana (adjusted OR: 4.09, 95% CI: 2.19–7.67 for men and 3.29, 95% CI: 2.47–4.39 for women) and migrants (adjusted OR: 9.02, 95% CI: 5.02–16.20 for men and 4.00, 95% CI: 3.00–5.34 for women), compared to rural Ghana (Fig. 2 for men and Fig. 3 for women).
Fig. 1. Continuous FLI.
(A) FLI in rural Ghana, urban Ghana and Ghanaian migrants in males. (B) FLI in rural Ghana, urban Ghana and Ghanaian migrants in females. FLI, fatty liver index.
Fig. 2. ORs with 95% CIs for elevated FLI (FLI ≥60) in urban Ghana and Ghanaian migrants compared with rural Ghana in men.
Model 1 adjusted for age; model 2: model 1 + education; model 3: model 2 + physical activity, alcohol, and T2DM. OR, odds ratio; CI, confidence interval; FLI, fatty liver index; T2DM, type 2 diabetes.
Fig. 3. ORs with 95% CIs for elevated FLI (FLI ≥60) in urban Ghana and Ghanaian migrants compared with rural Ghana in women.
Model 1 adjusted for age; model 2: model 1 + education; model 3: model 2 + physical activity, alcohol, and T2DM. OR, odds ratio; CI, confidence interval; FLI, fatty liver index; T2DM, type 2 diabetes.
Associations of T2DM and 10-year predicted ASCVD risk with FLI
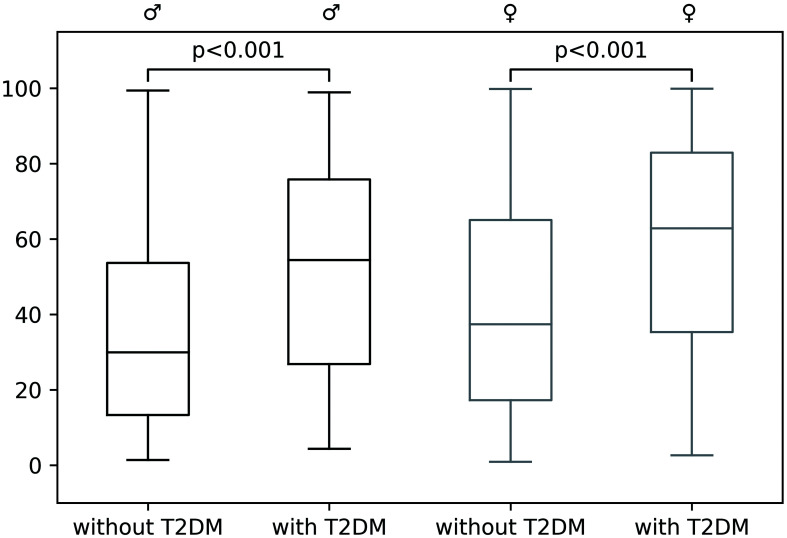
Median FLI in participants with and without T2DM are shown in Figure 4. We found a positive association between T2DM and an elevated FLI (≥60) (adjusted OR: 2.43, 95% CI: 1.73–3.41 for men and 2.02, 95% CI: 1.52–2.69 for women; Supplemental Table 1).
Fig. 4. FLI in male (black) and female (grey) participants with and without T2DM.
FLI, fatty liver index; T2DM, type 2 diabetes.
In total, 2,611 RODAM study participants were included in the analysis for FLI and 10-year ASCVD risk after further exclusion of participants with an age below 40 and those with a history of clinically present CVD (Supplemental Fig. 1). As we hypothesized that MAFLD drives ASCVD, we used the continuous FLI as an independent variable and ASCVD as the dependent variable in this analysis. FLI was positively associated with elevated 10-year ASCVD risk (≥7.5%) among rural participants (adjusted OR: 1.05, 95% CI: 1.01–1.10 for men and 1.03, 95% CI: 1.02–1.05 for women), urban participants (1.08, 95% CI: 1.04–1.11 for men and 1.02, 95% CI: 1.01–1.02 for women), and migrants (men 1.04, 95% CI: 1.03–1.06 and women 1.02, 95% CI: 1.01–1.03). In the total study population, the adjusted OR was 1.05 per 1 unit increase in FLI (95% CI: 1.04–1.06) for men and 1.02 (95% CI: 1.02–1.03) for women (Supplemental Table 2).
Discussion
Key findings
Here, we shed light on the influence of migration on the prevalence of MAFLD by studying the FLI in a homogenous Ghanaian population living in rural Ghana, urban Ghana and Europe. The homogenous Ghanaian population living in three distinct environments is used as a proxy for migration. This study has three important findings. First, the prevalence of an elevated FLI (FLI ≥60) as an indicator of hepatic steatosis increased from rural participants, through urban participants, to European migrants, irrespective of sex. Second, T2DM was positively associated with higher odds for FLI in both Ghanaian men and women. Third, an elevated FLI (FLI ≥60) was associated with an higher odds for 10-year risk of ASCVD (≥7.5%) in both men and women, with a more pronounced effect in men.
Discussion of the key findings
Studies of human migration and features of cardiometabolic disease are scarce. Several studies have reported differences in the prevalence of MAFLD among different ethnicities; however, these were performed in participants residing in a single country.16,19 For instance, multiple studies report a lower prevalence of MAFLD in African Americans compared to Hispanic Americans.19–21 The prevalence of MAFLD in migrants of African descent is often lower than the prevalence of MAFLD in the general population of the host country.20,21 Interestingly, this contrasts with the prevalence of morbidities with a close relation to MAFLD, i.e. obesity, T2DM, and hypertension, which are found to be more prevalent among ethnic minorities in Europe, including African groups.20
Factors driving variation in cardiometabolic health across geographical locations are thought to include changes in nutritional patterns, physical inactivity, and stress, in combination with genetic susceptibility and gene-environment interactions.20,22 As MAFLD is driven by insulin resistance and obesity, one would expect the aforementioned factors to play a similar role in the effects of migration on the prevalence of MAFLD.23 Interestingly, in our models, the effect sizes only slightly changed upon adjustment for lifestyle factors, such as physical activity, alcohol consumption, and T2DM. This may fit with the ‘multiple hit’ hypothesis which postulates that multiple factors act together in inducing MAFLD, and adjustment for a few of these factors would not have a major effect.24 In addition to physical inactivity and genetic susceptibility, gut microbiota have been suggested to play a role. Several studies report the interaction between liver and gut as a critical player in the onset of MAFLD.25–27 Dietary factors may alter the composition of the gut microbiota, which in turn may contribute to the development of MAFLD.28 Evidence for these changed dietary factors in an urban population compared to a rural population was found in a prospective cohort study that was conducted in South Africa. The nutrition intakes of urban-residing men and women were consistently higher than those of their rural counterparts.29 This is often accompanied by a nutrition transition to a Westernized diet, frequently high in fat and sugar.30 This reported change in dietary factors could also play a role in our study population. Taken together, the sizeable disparity in FLI in our study between similar populations living in different environments suggests a more significant role for environmental factors such as dietary changes and alterations in the gut microbiome, in driving the prevalence of MAFLD than for genetic susceptibility.
Of note, we found a strong relation of the FLI with the presence of T2DM. The interplay between T2DM and MAFLD is complex. T2DM is an important risk factor for developing MAFLD, and vice versa, MAFLD may contribute to insulin resistance. Insulin resistance is a central mechanism that leads to lipolysis in peripheral adipose tissue and an increased hepatopetal flux of free fatty acids, driving lipotoxicity in the liver, with subsequent inflammation and hepatocyte injury.4,31 A higher prevalence of MAFLD in patients with T2DM has been found.23,32 In turn, ectopic fat accumulation in MAFLD is thought to affect T2DM. This ectopic fat accumulation is associated with increased gluconeogenesis, decreased glycogen synthesis and inhibition of insulin signalling.32
We observed a significant association of FLI with 10-year risk of ASCVD, bolstering the notion that patients with MAFLD may have increased ASCVD. The relation between MAFLD and ASCVD is supported by studies of subclinical atherosclerosis, such as carotid intima-media thickness (commonly known as carotid IMT) and coronary calcification.33,34 Lee et al.35 conducted a cross-sectional study to investigate the influence of MAFLD on subclinical coronary atherosclerosis as detected by coronary computed tomography angiography (commonly referred to as CCTA). Fatty liver was assessed by ultrasound. In patients with MAFLD, ORs after adjustment for cardiovascular risk factors were higher for atherosclerotic plaques (OR: 1.18). In addition, there was a significant association of FLI ≥30 with non-calcified plaque (OR: 1.37). In addition, meta-analyses of studies with cardiovascular events also support the relation of MAFLD and ASCVD.36 The underlying pathways are likely complex and difficult to decipher since many comorbid factors may co-exist in these patients, such as hypertension, T2DM and obesity. Low grade inflammation38 and hypercoagulable state39 have also been implicated to mediate the relation between NAFLD and asCVD. Yet, evidence from Mendelian randomization studies most strongly supports that the MAFLD may drive ASCVD by mixed hyperlipidemia, through very low-density lipoprotein (i.e. VLDL) hypersecretion.6,37
Strengths and limitations
The FLI is a surrogate marker validated against ultrasonography by Bedogni et al.14 in a Caucasian population and replicated by others.13,40 Potential anthropometric and laboratory data were used in a logistic regression model to obtain a simple and accurate algorithm for the prediction of increased liver fat content, after exclusion of participants with hepatitis B and C. Due to this anthropometric and laboratory data, FLI is not directly based on liver fat content; yet, Bedogni et al.14 reported an area under the receiver operating characteristic curve of 0.85. In the RODAM study, no imaging modalities of hepatic steatosis, such as abdominal ultrasound or MRI-PDFF,41 or liver biopsies, were available to validate our findings with the FLI. When using the applied cut-off value of 60, in order to validate the FLI in a population-based study, the likelihood ratio was 5.10 for the presence of MAFLD. Additionally, a cut-off of ≥60 showed a specificity of 91%.42 Unfortunately, blood platelets were not included in the RODAM study; hence, liver fibrosis proxies, such as Fibrosis-4 and aspartate to aminotransferase (i.e. AST) to platelet ratio (APRI) could not be included in our current analysis. We excluded other liver conditions in our calculations, as much as possible. We were able to exclude participants that used an excessive amount of alcohol and participants that used medication for hepatitis B and retroviral therapy. However, no data on untreated participants was available in the RODAM study. A great strength of the study is the homogeneity of the studied population, which provides a unique opportunity to investigate the metabolic effects of migration.
Conclusion
In conclusion, our study shows that, compared to rural areas, the prevalence of MAFLD as assessed by the FLI in the Ghanaian RODAM population was higher in urban areas and even higher in Europe. In addition, FLI was strongly correlated with T2DM and ASCVD risk. This sheds light on MAFLD in this African population, and highlights the possible influence of migration on the prevalence of MAFLD, providing a clear rationale for future prospective studies with imaging modalities.
Supporting information
Acknowledgments
The authors are very grateful to the advisory board members and Ghanaian volunteers for participating in this project. We thank Jan van Straalen from the Academic Medical Centre for standardization of the lab procedures, and the Academic Medical Centre Biobank for their support in biobank management and high-quality storage of collected samples. We thank Erik Beune, from the department of Public Health at the Amsterdam UMC, the Netherlands, for his input in this project.
Abbreviations
- γGT
gamma-glutamyltransferase
- ACC
American College of Cardiology
- AHA
American Heart Association
- ALT
alanine aminotransferase
- APRI
AST to platelet ratio
- ASCVD
atherosclerotic cardiovascular disease
- AST
aspartate aminotransferase
- BMI
body mass index
- BP
blood pressure
- carotid IMT
carotid intima-media thickness
- CCTA
coronary computed tomography angiography
- CI
confidence interval
- CVD
cardiovascular disease
- FLI
Fatty Liver Index
- GPAQ
Global Physical Activity Questionnaire
- HDL
high-density lipoprotein
- IQR
interquartile ranges
- LDL
low-density lipoprotein
- MAFLD
metabolic-associated fatty liver disease
- MRI-PDFF
Magnetic Resonance Imaging Proton Density Fat Fraction
- NASH
non-alcoholic steatohepatitis
- OR
odds ratio
- PNPLA3
patatin-like phospholipase domain containing 3
- RODAM
Research on Obesity and Diabetes among African Migrants
- SD
standard deviation
- T2DM
type 2 diabetes mellitus
- TG
triglycerides
- VLDL
very low-density lipoprotein
- WHR
waist-to-hip ratio
Data sharing statement
The data that support the findings of this study are available from the corresponding author, AMD, upon reasonable request.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Firneisz G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: the liver disease of our age? World J Gastroenterol. 2014;20(27):9072–9089. doi: 10.3748/wjg.v20.i27.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol Mech Dis. 2016;11:451–496. doi: 10.1146/annurev-pathol-012615-044224. [DOI] [PubMed] [Google Scholar]
- 4.Arab JP, Arrese M, Trauner M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu Rev Pathol Mech Dis. 2018;13:321–350. doi: 10.1146/annurev-pathol-020117-043617. [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM. Non-alcoholic fatty liver disease – a global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Stols-Goncalves D, Hovingh GK, Nieuwdorp M, Holleboom AG. NAFLD and atherosclerosis: two sides of the same dysmetabolic coin? Trends Endocrinol Metab. 2019;30(12):891–902. doi: 10.1016/j.tem.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez DC, Reesor LM, Murillo R. Food insecurity and adult overweight/obesity: gender and race/ethnic disparities. Appetite. 2017;117:373–378. doi: 10.1016/j.appet.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology. 2016;64(6):1969–1977. doi: 10.1002/hep.28677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53(6):1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 11.Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, et al. The changing epidemiology of liver diseases in the Asia-pacific region. Nat Rev Gastroenterol Hepatol. 2019;16(1):57–73. doi: 10.1038/s41575-018-0055-0. [DOI] [PubMed] [Google Scholar]
- 12.Agyemang C, Beune E, Meeks K, Owusu-Dabo E, Agyei-Baffour P, Aikins Ad, et al. Rationale and cross-sectional study design of the research on obesity and type 2 diabetes among African migrants: the RODAM study. BMJ Open. 2014;4(3):e004877. doi: 10.1136/bmjopen-2014-004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11(9):1201–1204. doi: 10.1016/j.cgh.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rugge B, Balshem H, Sehgal R, Relevo R, Gorman P, Helfand M. Screening and treatment of subclinical hypothyroidism or hyperthyroidism. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011. Report No.: 11(12)-EHC033-EF. [PubMed] [Google Scholar]
- 16.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone NJ, Robinson JG, Lichtenstein AH, Goff DC, Jr, Lloyd-Jones DM, Smith SC, Jr, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med. 2014;160(5):339–343. doi: 10.7326/M14-0126. [DOI] [PubMed] [Google Scholar]
- 18.Muntner P, Colantonio LD, Cushman M, Goff DC, Jr, Howard G, Howard VJ, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311(14):1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim U, Monroe KR, Buchthal S, Fan B, Cheng I, Kristal BS, et al. Propensity for intra-abdominal and hepatic adiposity varies among ethnic groups. Gastroenterology. 2019;156(4):966–975.e10. doi: 10.1053/j.gastro.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherif ZA, Saeed A, Ghavimi S, Nouraie SM, Laiyemo AO, Brim H, et al. Global epidemiology of nonalcoholic fatty liver disease and perspectives on US minority populations. Dig Dis Sci. 2016;61(5):1214–1225. doi: 10.1007/s10620-016-4143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113(11):1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agyemang C, van den Born BJ. Non-communicable diseases in migrants: an expert review. J Travel Med. 2019;26(2):tay107. doi: 10.1093/jtm/tay107. [DOI] [PubMed] [Google Scholar]
- 23.Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. doi: 10.1038/nrgastro.2016.147. [DOI] [PubMed] [Google Scholar]
- 24.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 25.De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Candelaresi C, Trozzi L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59(5):1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 26.Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11(2):e9302. doi: 10.15252/emmm.201809302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 28.Kirpich IA, Marsano LS, McClain CJ. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem. 2015;48(13-14):923–930. doi: 10.1016/j.clinbiochem.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wentzel-Viljoen E, Lee S, Laubscher R, Vorster HH. Accelerated nutrition transition in the North West Province of South Africa: results from the Prospective Urban and Rural Epidemiology (PURE-NWP-SA) cohort study, 2005 to 2010. Public Heal Nutr. 2018;21(14):2630–2641. doi: 10.1017/s1368980018001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batal M, Steinhouse L, Delisle H. The nutrition transition and the double burden of malnutrition. Med Sante Trop. 2018;28(4):345–350. doi: 10.1684/mst.2018.0831. [DOI] [PubMed] [Google Scholar]
- 31.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Ampuero J, Gallego-Durán R, Romero-Gómez M. Association of NAFLD with subclinical atherosclerosis and coronary-artery disease: meta-analysis. Rev Esp Enferm Dig. 2015;107(1):10–16. [PubMed] [Google Scholar]
- 34.Zhou YY, Zhou XD, Wu SJ, Fan DH, Van Poucke S, Chen YP, et al. Nonalcoholic fatty liver disease contributes to subclinical atherosclerosis: a systematic review and meta-analysis. Hepatol Commun. 2018;2(4):376–392. doi: 10.1002/hep4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SB, Park GM, Lee JY, Lee BU, Park JH, Kim BG, et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. J Hepatol. 2018;68(5):1018–1024. doi: 10.1016/j.jhep.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646–650. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 37.Brouwers MCGJ, Simons N, Stehouwer CDA, Koek GH, Schaper NC, Isaacs A. Relationship between nonalcoholic fatty liver disease susceptibility genes and coronary artery disease. Hepatol Commun. 2019;3(4):587–596. doi: 10.1002/hep4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med. 2005;22(10):1354–1358. doi: 10.1111/j.1464-5491.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 39.Tripodi A, Fracanzani AL, Primignani M, Chantarangkul V, Clerici M, Mannucci PM, et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;61(1):148–154. doi: 10.1016/j.jhep.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Kwon SY, Lee SW, Lee CH. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int. 2011;31(10):1600–1601. doi: 10.1111/j.1478-3231.2011.02580.x. [DOI] [PubMed] [Google Scholar]
- 41.Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68(1):349–360. doi: 10.1002/hep.29721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee-Moradie F, et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. 2014;171(5):561–569. doi: 10.1530/EJE-14-0112. [DOI] [PubMed] [Google Scholar]
- 43.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Heal. 2009;6(6):790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.