Abstract
The ongoing coronavirus disease-2019 (COVID-19) pandemic has necessitated special considerations in the management of diseases. The way presence of pre-existing diseases or treatment for it predisposes to, alters course of, and changes the management of COVID-19, is of relevance and is being extensively studied. Autoimmune hepatitis (AIH) is unique in that it is an autoimmune disease mandating treatment with immunosuppressive drugs, as well as a liver disease with potential for varying degrees of underlying fibrosis. The use of immunosuppressive drugs could alter the risk of acquiring COVID-19, the clinical course and severity of COVID-19 and the degree of underlying liver fibrosis could alter the clinical outcomes of patients with COVID-19. In this review, we try to summarize key areas relevant in understanding and improving the clinical care of patients with AIH in the current pandemic. Special considerations required in the management of patients with AIH in COVID-19 hotspots have been outlined based on the current evidence.
Keywords: Hepatitis, Autoimmune, Liver cirrhosis, COVID-19, SARS-CoV-2 infection, Immunosuppressive agents
Introduction
First noticed as a cluster of viral pneumonia among people known to have visited a market in the Wuhan City of Hubei province in China,1 and later on investigated by the China Center for Disease Control and Prevention and found to be due to infection with a new beta coronavirus,2 the disease was later named Coronavirus disease-2019 (COVID-19),3 and the virus causing it was christened severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2).4 The disease spread throughout the world over a period of a few months, to be declared as a pandemic by the World Health Organization (WHO) on the 11th of March 2020.5 By the 30th of December 2020, it had infected over 80 million people and resulted in the death of over 1.7 million people.6 While predominantly a respiratory pathogen, SARS-CoV-2 has also been shown to cause significant neurologic, cardiac, gastrointestinal, hepatic, renal, hematologic, obstetric, gynecologic, and rheumatologic abnormalities as well.7
The focus of this review is on special considerations for the management of autoimmune hepatitis (AIH) in areas with widespread community transmission of COVID-19. Factors that need to be considered include the risk of acquiring COVID-19 and the risk of poor outcomes with COVID-19. Outcomes in patients with COVID-19 could be altered due to AIH itself, because of the immunosuppressive medicines used to treat AIH, or by virtue of liver impairment that AIH has caused. We will be discussing aspects that are of specific relevance to a practitioner caring for AIH in COVID-19 hotspots. Relevant aspects of COVID-19-induced liver injury, aspects of COVID-19 prevention (including vaccination), and special considerations required in the management of AIH have been discussed herein. We have also discussed possible approaches that a clinician can adopt in various case-scenarios that may be encountered.
Liver injury in COVID-19
Liver abnormalities noted to be present in patients having COVID-19 include transaminitis, hyperbilirubinemia and hypoalbuminemia.8–11 These abnormalities are thought to occur by one or more of the following several mechanisms (Fig. 1): direct cytopathy;12 immune-mediated;13 3. hypoxia-related;14 drug-induced;14 and, microvascular thrombosis.15,16 Patients with COVID-19 and liver injury,17 as well as those with prior hepatic comorbidities, have been shown to have poor outcomes with COVID-19.18 Patients with cirrhosis are thought to be at moderate risk, whereas patients with decompensated cirrhosis are at high risk of poor outcomes with COVID-19.
Fig. 1. Pathogenesis of liver injury in COVID-19.
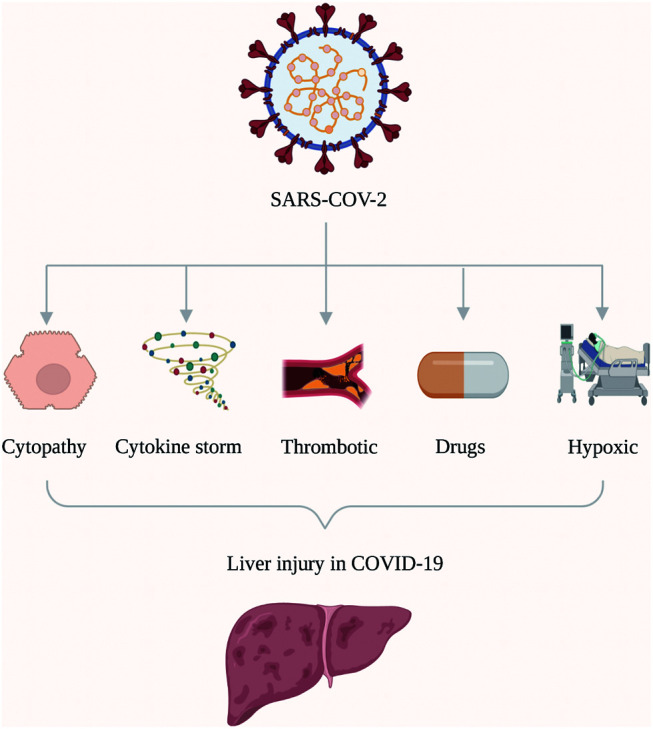
Direct cytopathy
ACE2 receptors, which the SARS-CoV-2 virus utilizes for entering cells have been shown to be expressed in cholangiocytes19 and probably hepatocytes20 as well. It has also been shown that SARS-CoV-2 invades liver cells and causes cytopathy.12 This may, at least partially, be responsible for the hepatic dysfunction seen in COVID-19 patients.12
Immune-mediated
SARS-CoV-2 infection results in a disordered inflammatory response, i.e. the cytokine storm,21 with increase in pro-inflammatory cytokines. This has been shown to be responsible for severe pulmonary and extrapulmonary dysfunction, including liver injury. Liver dysfunction has been shown to be particularly more in patients with increased levels of inflammatory markers, such as CRP, TNF and IL-6.13
Hypoxic/ischemic
In severe COVID-19, multiorgan dysfunction can lead to hypoxia-related to acute-respiratory distress syndrome,22 hypotension,23 or congestive cardiac failure. All of these can result in liver dysfunction.24
Drug-induced liver injury (DILI)
In addition to supportive therapy, antivirals, immunomodulators and antithrombotic drugs are used in the management of COVID-19. Several of these drugs, including antivirals such as lopinavir-ritonavir, remdesivir, favipiravir etc., and immunomodulators such as tocilizumab, baricitinib, etc. may cause liver dysfunction.25,26
Microvascular thrombosis
Endothelial dysfunction along with inflammation in patients with COVID-19 produces vascular thrombosis in multiple organs.27 Elevated D-dimer levels were found to be independently associated with liver dysfunction in one study,15 which could point to an association of thrombosis with liver dysfunction. Studies of liver biopsies from patients with COVID-19 and liver dysfunction have shown significant microvascular thrombosis which could lead to liver dysfunction.16 This could point to a contribution by microvascular thrombosis to the liver dysfunction seen in patients with COVID-19.
Pre-existing liver diseases and COVID-19
Patients with AIH have varying degrees of underlying fibrosis and as much as 40% of these patients develop cirrhosis.28 The degree of underlying fibrosis in patients with AIH has the potential to have an effect on the risk of acquiring COVID-19, as well as on clinical outcomes in patients with COVID-19.
It can be assumed that, despite a reduction in the immunity of patients with cirrhosis, the risk of acquiring COVID-19 does not seem to be higher in patients with cirrhosis, as evidenced by the results of a meta-analysis which demonstrated that the prevalence of cirrhosis in patients with COVID-19 is similar to that in the COVID-19-negative population.18
There seems to be an upregulation of ACE2 receptors in the liver, which probably makes patients with cirrhosis more vulnerable to COVID-19-related liver injury.29 Patients with pre-existing liver disease have been shown to have increased mortality and morbidity, with COVID-19.18 Multiple studies have found deterioration of liver functions and decompensation in cirrhotic patients with COVID-19.30–32 Patients were found to have significantly higher risk of mortality with worsening Child-Pugh status.31,32 Cirrhosis was also found to be an independent predictor of severe COVID-19, in patients with AIH in recent multicenter studies.33,34 With such poor outcomes, it becomes pertinent that patients with cirrhosis be considered high risk.
Immunosuppressants and COVID-19
Patients on immunosuppressants have a complex interplay of factors in favor of and against SARS-CoV-2. On one hand, immunomodulators such as mycophenolate mofetil (MMF)35,36 and calcineurin inhibitors (CNIs) like tacrolimus37 and cyclosporine38,39 have been demonstrated to have antiviral activity against coronaviruses, and glucocorticoids administered for COVID-19 have been shown to prevent the disordered immune response that is responsible for poor outcomes in COVID-19.40 On the other hand, the immunosuppression attributable to these drugs may cause increased susceptibility to SARS-CoV-2 infection,41 secondary bacterial or fungal infections, and prolongation of viral clearance.42 There have been studies that demonstrated increased risk41 as well as others that demonstrated average risk43 of acquiring SARS-CoV-2 infection for patients on immunosuppressants, and the question largely remains unresolved to date. Retrospective studies have demonstrated a risk of bacterial superinfection44 or increased use of antibiotics45 in patients managed with steroids for COVID-19, whereas randomized controlled trials have negated this46–48 as an adverse event of steroids. The data on the effect of steroids on viral shedding is mixed; although, the wealth of evidence suggests that low-dose steroids are not associated with any increase in viral shedding.42 Studies have also been performed to assess how these seemingly opposing actions translate to clinical outcomes in COVID-19. The risk of acquiring SARS-CoV-2 infection seems to be higher in patients with autoimmune diseases on steroids49 and clinical outcomes seem to be worse in patients on steroids as well as in patients on immunomodulators, in patients with autoimmune diseases.49 Early data available specifically in the context of AIH seem to show that continued immunosuppression does not lead to poor outcomes with COVID-19.33,34
AIH and COVID-19
Two large multicenter studies have addressed the impact of COVID-19 in patients with AIH.33,34 In both the studies, the outcomes of patients with AIH were compared with a cohort of non-AIH patients with chronic liver disease. The consistent findings across both the studies was that there is no increase in severity of COVID-19 infection across patients with AIH compared to other etiologies of chronic liver disease.33,34 Presence of cirrhosis, particularly Child-C disease, was the most significant factor of poor outcomes in these patients.34 New-onset liver injury was seen in one-third of the patients with AIH after COVID-19 in one study.33 However, the use of immunosuppressants was not associated with poor outcomes in patients with AIH and COVID-19.33 In fact, one study showed that continuation of immunosuppression was associated with lower risk of new-onset liver injury.33 This suggests that immunosuppression needs to be continued in patients with AIH and COVID-19. Apart from these two studies, however, data on AIH and COVID-19 are limited to a few small case series (Table 1).50–53
Table 1. Published data of AIH patients with COVID.
| Study | Region | Number of COVID-19-positive AIH | COVID-19 requiring hospitalization | Survived |
|---|---|---|---|---|
| Verhelst 202150 | Flanders, Belgium | 1 | 100% (1/1) | 100% (1/1) |
| Rigamonti 202051 | Northern Italy | 4 | 50% (2/4) | 100% (4/4) |
| Di Giorgio 202052 | Northern Italy | 4 | 50% (2/4) | 75% (3/4) |
| Gerussi 202053 | Italy | 10 | 60% (6/10) | 90% (9/10) |
| Marjot 202134 | Multinational | 70 | 76% (53/70) | 77% (54/70) |
| Efe 202133 | Multinational | 110 | 46% (51/110) | 90% (99/110) |
Diagnostic approach
The diagnostic approach for AIH in COVID-19 hotspots can be the same as elsewhere, broadly speaking. Selected patients who are asymptomatic and being evaluated for abnormal transaminases or only mildly symptomatic for AIH can be, in the initial part of the diagnostic workup, evaluated by a telemedicine-based approach. Liver biopsy is necessary for a diagnosis of AIH to be made, as per American Association for the Study of Liver Diseases (AASLD) guidelines54 and can be performed in a COVID-minimal pathway, safely, for COVID-19-negative patients. Liver biopsy in COVID-19-positive patients need to be decided on a case-by-case basis, depending on the urgency to treat AIH, the severity of COVID-19, and other factors like the presence of coagulopathy, sepsis, logistics, chance of cross-infection, etc. The Asia Pacific Association for Study of Liver (APASL) recommends liver biopsy in COVID-19-negative patients when autoimmune flare is suspected and advises against liver biopsy in COVID-19-positive patients.55
Prevention of COVID-19
General measures
Owing to the high risk of poor outcomes that can be expected with the degree of pre-existing liver dysfunction and immunosuppression, prevention of SARS-CoV-2 infection is extremely important in patients with AIH. The preventive strategy against COVID-19 should comprise general measures as well as vaccination. General measures should involve measures to be adopted by the patient,56 hospital-designed infrastructure measures,57 and hospital operational measures,57 which include a COVID-minimal pathway,58 as well as measures to be adopted by health-care personnel.59 Telemedicine becomes particularly relevant during the current COVID-19 epidemic as it has the potential to reduce the need for hospital visits, which in turn reduces the chance of crowding in hospital outpatient clinics, thereby reducing the risk of spreading SARS-CoV-2.60 Telemedicine has been evaluated specifically in the context of management of AIH during the COVID-19 pandemic and has been shown to improve patient adherence to therapy thereby minimizing the chances of relapse, as compared to the standard care group.60
Vaccination
As on December 23rd, 2020, there have been at least seven COVID-19 vaccines61 licensed in different parts of the world and over 200 vaccines in different stages of development.62 In the context of patients with AIH, four specific aspects need to be addressed; these include: 1) safety of the vaccine in patients on immunosuppressants; 2) safety of the vaccine in patients with liver diseases; 3) efficacy of the vaccine in patients on immunosuppressants; and, 4) efficacy of the vaccine in patients with liver diseases.
As far as the licensed vaccines are concerned, patients on immunosuppression were excluded from vaccine licensing trials. While some trials included a small number of patients with pre-existing liver diseases, patients with advanced liver diseases were still excluded and no subgroup analysis was performed to assess outcomes or adverse events specifically in patients with liver diseases.63 Thus, it is not clear at this moment how safe and effective these vaccines would be, in standard doses, for patients with AIH, especially while on immunosuppressive medications. Extrapolating from the experience with vaccination for other diseases in patients with liver diseases,64–66 in patients on immunosuppression,67 and specifically in AIH,68 the likely efficacy of the COVID-19 vaccine in this subgroup of patients is likely to be lower compared to the normal healthy adult.
Considering the high risk of poor outcomes due to COVID-19 infection in patients with liver diseases18 and patients with immunosuppression,69 the benefits of vaccination may outweigh the risks. COVID-19 vaccination is being suggested in patients with chronic liver disease and solid organ transplant recipients on immunosuppression by various international societies, including AASLD63 and European Association for Study of Liver (EASL).70 Till further data on safety and efficacy are available, COVID-19 vaccines need to be administered at standard doses, unless other contraindications are present. Due to the high risk of adverse events, live vaccines71 and replicating viral vector vaccines72 are best avoided in patients on immunosuppressive medications. Further, household members and care providers of these patients should also receive vaccination while continuing appropriate use of masks, sanitizers and social distancing — the keystone of protection against COVID-19. EASL and APASL recommend patients with AIH to be also vaccinated against influenza and Streptococcus pneumoniae.55,73 The formation of neutralizing antibodies in liver transplant recipients (especially those receiving immunosuppressants) have been suboptimal, as shown by a recent study.74 The clinical impact of this suboptimal response remains to be seen. However, this should not deter any clinician from prescribing the vaccine in these patients.
Treatment in COVID-19 negative patients
Principles of treatment
It is clear that the presence of pre-existing liver disease has a significant bearing on the outcomes of patients with COVID-19,18 and given the fact that the present pandemic has been ongoing for the past several months and will continue to do so for some time, it seems prudent that the patients not having active COVID-19 but requiring induction or maintenance therapy for AIH be given immunosuppression as required because withdrawing, delaying or denying it may result in worsening fibrosis or cirrhosis.75–77 Recommendations by APASL55 and the EASL73 seem to support the view that immunosuppression needs to be continued. Strategies for treatment and follow-up should incorporate aspects of prevention as elaborated, including general measures and vaccination. We have discussed special considerations required in the treatment of COVID-19-negative patients with AIH below, and summarized them in Table 2. Decisions for patients with active COVID-19 requiring immunosuppression for AIH induction or maintenance need to be considered on an individualized, case-by-case basis after assessing risks and benefits.
Table 2. Special considerations in the management of AIH in COVID-19 hotspots: A suggested approach.
| COVID-19 status | AIH status | Special considerations |
|---|---|---|
| COVID-19-negative | Diagnosis of AIH | Diagnostic algorithm same as otherwise. Liver biopsy to be planned in COVID–minimal pathway |
| Newly diagnosed patients with activity | Steroids and azathioprine can be given as indicated otherwise. Budesonide to be preferred over prednisolone in appropriate situations, in noncirrhotic patients, and in patients without acute severe AIH | |
| Patients in remission | Continue immunosuppressant at lowest recommended dose required to maintain remission. Decision to stop immunosuppression to be made in patients who have had long-term remission, as per latest guidelines for AIH. Telemedicine-based follow-up in appropriate cases | |
| Patients who require start of second-line agent | CNIs (tacrolimus) may be preferred over mycophenolate in patients with no other contraindicationsa | |
| Patients who require start of third-line agent | Infliximab may be preferred over rituximab in patients with no other contraindicationsa | |
| Decompensated cirrhosis | Treatment algorithm same as otherwise. Living donor liver transplant to be considered for urgent/emergency indications only | |
| Acute severe AIH | Diagnostic and treatment algorithm same as otherwise | |
| ALF due to AIH | Diagnostic and treatment algorithm same as otherwise. May require urgent liver transplantation | |
| COVID-19-positive | Diagnosis of AIH | Evaluation by serology, imaging same as otherwise. Decisions regarding liver biopsy to be taken on case-by-case basis |
| Newly diagnosed patients with activity/patients in remission/patients who require start of second-line agents/patients who require start of third-line agents/patients with decompensated cirrhosis | Decisions regarding management to be taken on an individualized, case-by-case basis. Patients with AIH in remission may continue immunosuppressants as before, unless other contraindications or considerations are present. Treatment decisions in patients requiring induction or escalation of therapy for AIH needs to be taken on a multidisciplinary, case-by-case basis | |
| Acute severe AIH | Need for aggressive immunosuppression likely to override all other considerations, final decision to be taken on a multidisciplinary, case-by-case basis | |
| ALF due to AIH | Decision to be taken on a multidisciplinary, case-by-case basis |
aWeak suggestion, based on data extrapolated from other conditions.
First-line agents
Patients on systemic steroids have been found to have poor COVID-19-related outcomes.49 Prednisolone/prednisone or budesonide in combination with azathioprine is used for first-line management of AIH.54 Owing to high first pass metabolism of budesonide, it is known to have less systemic toxicity and less chance of infections.78 It seems reasonable that patients with new diagnosis of AIH, no cirrhosis and no acute severe AIH be considered for budesonide over predniso(lo)ne especially, as it has been proven to have a higher efficacy,78 and it is biologically plausible that patients on budesonide may fare better than patients on predniso(lo)ne, if infected with SARS-CoV-2.
Data from inflammatory bowel diseases (IBD)79 show that thiopurine monotherapy is associated with poor COVID-19 outcomes. Data regarding the safety of azathioprine specifically in AIH in the context of COVID-19, however, is not available, even though two studies had shown that continuation of immunosuppressive medicines in AIH is not associated with poor outcomes with COVID-19.33,34 Azathioprine still remains the first-line agent of choice as an immunomodulator, till conclusive evidence regarding an alternate first-line agent with better overall outcomes is available.
Second-line agents
Second-line agents for the management of AIH include MMF as well as CNIs, such as tacrolimus and cyclosporine.54 As far as AIH-related outcomes are concerned, MMF and tacrolimus are equivalent.54 Although specific data on AIH patients treated with these medicines and COVID-19-related outcomes are not available, data of safety of these same drugs used in other diseases, in terms of COVID-19-related outcomes, may be cautiously extrapolated to AIH, till specific data are available. In solid organ transplant patients, treatment with mycophenolate has been shown to be risky in a dose-dependent manner,80 but that with CNIs appears to not be.80,81 It would therefore seem appropriate that tacrolimus should be preferred over MMF when no other contraindications are present.
Third-line/salvage agents
Salvage options or third-line agents described by the AASLD guideline for AIH include infliximab and rituximab.54 Being an anti-TNF agent, infliximab has a potential to mitigate the cytokine storm, which is a crucial part of the pathogenesis of COVID-19.82 Studies in IBD83 and in rheumatology84 have shown that anti-TNF drugs are not associated with worse outcomes in COVID-19. Concerns have been expressed regarding risk of poor outcomes for patients on rituximab,85 and early evidence suggests this as well.86 Till conclusive evidence that supports safety in this regard is available, it is better to consider other third-line/salvage agents, such as infliximab, over rituximab, whenever possible.
Overlap syndromes
In addition to the immunosuppressants described above, patients with overlap syndromes may also require ursodeoxycholic acid.54 There is no reason to have concerns regarding the use of this agent in the context of the current COVID-19 pandemic. In fact, ursodeoxycholic acid is an agent with potential to have benefits in the treatment of COVID-19 as well.87 Till evidence to the contrary is available, treatment with this agent can be initiated or continued as indicated.
Liver transplant
Liver transplant is indicated in patients with AIH who present with acute liver failure (commonly referred to as ALF) or as salvage in acute severe AIH or in cirrhosis with decompensation.54 In the presence of widespread community transmission of COVID-19, considerations for liver transplant should consider appropriate local guidelines which specifically discuss this aspect. In view of the risk to donors, living donor liver transplantation is best restricted to urgent indications.88
Management of AIH in COVID-19 patients
The management of AIH in COVID-19-positive patients would require decisions to be taken on a multidisciplinary, case-by-case basis. Decisions should be based on a multitude of factors, such as urgency to treat AIH, severity of COVID-19, presence of co-existing sepsis, requirement of drugs for COVID-19 that may have interactions with drugs given for AIH, etc. APASL recommends continuing immunosuppression in patients with mild COVID-19.55 Data from two multinational studies are available which show no benefit of withdrawing immunosuppression in patients with AIH and active COVID-19.33,34 One study even showed that continuation of immunosuppression lowered the risk of new-onset liver injury.33 The exact reasons for stopping immunosuppression in these studies are not known. The numbers of patients on high-dose steroids and MMF were low in these studies, for meaningful conclusions to be made. However, these studies do support the continuation of immunosuppression in patients with AIH and COVID-19. Some special considerations required while managing patients with AIH who also have COVID-19 are summarized in Table 2.
Drug interactions are particularly important while managing patients with COVID-19 and AIH. Management of COVID-19 is constantly being revised with a variety of drugs being tried for its treatment, with varying efficacy. New data are emerging every day and with the introduction of new drugs, one needs to be aware of the side effect profile of the drugs being used and their potential interaction with the concomitant medications being used for the management of comorbid conditions. Interactions between drugs used for immunosuppression, such as CNIs, may have significant drug interactions, which, if not paid attention to, can be deleterious.89 We have listed the interactions between the drugs used for the management of COVID-19 and the drugs used in AIH in the table below (Table 3). As to the optimal treatment and drugs for COVID-19, the data are still evolving; however, it is suggested to check the side effect profile and drug interactions of the drug being used. One such useful updated resource to check for drug interactions is https://www.covid19-druginteractions.org/checker.
Table 3. Drug interactions between immunosuppressants used for management of AIH and drugs used for management of COVID-19.
| Drug type | Azathioprine | MMF | Tacrolimus | Cyclosporine |
|---|---|---|---|---|
| Interferons | ||||
| Interferon-alpha | Potential additive hematological toxicity | No interaction | No interaction | No interaction |
| Interferon0beta | Potential additive hematological toxicity | No interaction | No interaction | No interaction |
| Antivirals | ||||
| Favipiravir | No interaction | No interaction | No interaction | No interaction |
| Lopinavir-ritonavir | No interaction | Potential altered drug levels of mycophenolate; Drug level monitoring recommended | Increased levels of tacrolimus; Risk of QT prolongation | Increased plasma levels of cyclosporine; Drug monitoring recommended |
| Remdesivir | No interaction | No interaction | No interaction | No interaction |
| Ribavirin | Potential additive hematological toxicity | No interaction | No interaction | No interaction |
| Nitazoxanide | No interaction | No interaction | No interaction | No interaction |
| Antimalarials | ||||
| Chloroquine | Potential additive hematological toxicity | No interaction | Increased levels of tacrolimus; Risk of QT prolongation | Increased plasma levels of cyclosporine; Drug monitoring recommended |
| Hydroxychloroquine | Potential additive hematological toxicity | No interaction | Increased levels of tacrolimus; Risk of QT prolongation | Increased plasma levels of cyclosporine; Drug monitoring recommended |
| Monoclonal antibody | ||||
| Tocilizumab | Potential additive hematological toxicity | No interaction | Monitoring of tacrolimus drug levels recommended | Monitoring of cyclosporine drug levels recommended |
| Bamlanivimab | No interaction | No interaction | No interaction | No interaction |
| Canakinumab | Potential additive hematological toxicity | No interaction | Monitoring of tacrolimus drug levels recommended | Monitoring of cyclosporine drug levels recommended |
| Sarilumab | Potential additive hematological toxicity | No interaction | Monitoring of tacrolimus drug levels recommended | Monitoring of cyclosporine drug levels recommended |
Conclusions
Management of auto-immune hepatitis in areas with widespread community transmission of COVID-19 needs special considerations in the diagnostic approach, preventive aspects and treatment. The management of immunosuppression is particularly complex in this set of patients and specific data in the context of AIH in COVID-19 are lacking. Management of immunosuppression in patients with AIH in COVID-19 hotspots requires a tailored approach based on data from relatively small observational studies and from data extrapolated from other auto-immune diseases till better evidence is available. The management of AIH in patients diagnosed with COVID-19 requires a multidisciplinary approach with decisions considered on a case-by-case basis.
Acknowledgments
We would like to acknowledge the contribution of Madhu Mathew Vennikandam, Advanced Hepatology Fellow, Columbia University Irving Medical Center, New York, USA, in the initial process of the literature search. Figure 1 was created with BioRender.com.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ACLF
acute on chronic liver failure
- AD
acute decompensation
- AIH
autoimmune hepatitis
- ALF
acute liver failure
- APASL
Asia Pacific Association for Study of Liver
- CNIs
calcineurin inhibitors
- COVID-19
coronavirus disease-2019
- DILI
drug-induced liver injury
- EASL
European Association for Study of Liver
- IBD
inflammatory bowel diseases
- MMF
mycophenolate mofetil
- SARS-CoV-2
severe acute respiratory syndrome-coronavirus-2
- WHO
World Health Organization
References
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- 4.The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. WHO Coronavirus disease (COVID-19) dashboard. Available from: https://covid19.who.int/
- 7.Kordzadeh-Kermani E, Khalili H, Karimzadeh I. Pathogenesis, clinical manifestations and complications of coronavirus disease 2019 (COVID-19) Future Microbiol. 2020;15:1287–1305. doi: 10.2217/fmb-2020-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdulla S, Hussain A, Azim D, Abduallah EH, Elawamy H, Nasim S, et al. COVID-19-induced hepatic injury: A systematic review and meta-analysis. Cureus. 2020;12(10):e10923. doi: 10.7759/cureus.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovalic AJ, Huang G, Thuluvath PJ, Satapathy SK. Elevated liver biochemistries in hospitalized chinese patients with severe COVID-19: Systematic review and meta-analysis. Hepatology. 2021;73(4):1521–1530. doi: 10.1002/hep.31472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shokri Afra H, Amiri-Dashatan N, Ghorbani F, Maleki I, Rezaei-Tavirani M. Positive association between severity of COVID-19 infection and liver damage: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2020;13(4):292–304. [PMC free article] [PubMed] [Google Scholar]
- 11.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52(4):584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73(4):807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amiri-Dashatan N, Koushki M, Ghorbani F, Naderi N. Increased inflammatory markers correlate with liver damage and predict severe COVID-19: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2020;13(4):282–291. [PMC free article] [PubMed] [Google Scholar]
- 14.Saviano A, Wrensch F, Ghany MG, Baumert TF. Liver disease and COVID-19: from Pathogenesis to Clinical Care. Hepatology. 2020 doi: 10.1002/hep.31684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsutsumi T, Saito M, Nagai H, Yamamoto S, Ikeuchi K, Lim LA, et al. Association of coagulopathy with liver dysfunction in patients with COVID-19. Hepatol Res. 2021;51(2):227–232. doi: 10.1111/hepr.13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao CL, Rapkiewicz A, Maghsoodi-Deerwester M, Gupta M, Cao W, Palaia T, et al. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19) Hum Pathol. 2021;109:59–68. doi: 10.1016/j.humpath.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar-M P, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, et al. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14(5):711–722. doi: 10.1007/s12072-020-10071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14(5):612–620. doi: 10.1007/s12072-020-10078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 21.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213(2):54–56.e1. doi: 10.5694/mja2.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanidziar D, Bittner EA. Hypotension, systemic inflammatory response syndrome, and COVID-19: A clinical conundrum. Anesth Analg. 2020;131(3):e175–e176. doi: 10.1213/ANE.0000000000005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, et al. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5(1):256. doi: 10.1038/s41392-020-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and research information on drug-induced liver injury. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548196/ [PubMed]
- 26.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-induced liver injury and COVID-19 infection: The rules remain the same. Drug Saf. 2020;43(7):615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali MAM, Spinler SA. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc Med. 2021;31(3):143–160. doi: 10.1016/j.tcm.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czaja AJ. Review article: The prevention and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment Pharmacol Ther. 2014;39(4):385–406. doi: 10.1111/apt.12592. [DOI] [PubMed] [Google Scholar]
- 29.Grace JA, Casey S, Burrell LM, Angus PW. Proposed mechanism for increased COVID-19 mortality in patients with decompensated cirrhosis. Hepatol Int. 2020;14(5):884–885. doi: 10.1007/s12072-020-10084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shalimar, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, et al. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39(3):285–291. doi: 10.1007/s12664-020-01074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14(5):690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74(3):567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Efe C, Dhanasekaran R, Lammert C, Ebi B, Higuera-de la Tijera F, Aloman C, et al. Outcome of COVID-19 in patients with autoimmune hepatitis: an international multi-centre study. Hepatology. 2021 doi: 10.1002/hep.31797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS4th, Armstrong MJ, et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoot TS, Kerckhoffs APM, Hilbrands LB, van Marum RJ. Immunosuppressive drugs and COVID-19: A review. Front Pharmacol. 2020;11:1333. doi: 10.3389/fphar.2020.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai Q, Spoletini G, Bianco G, Graceffa D, Agnes S, Rossi M, et al. SARS-CoV2 and immunosuppression: A double-edged sword. Transpl Infect Dis. 2020;22(6):e13404. doi: 10.1111/tid.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carbajo-Lozoya J, Müller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165(1):112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5(5):1250–1260. doi: 10.3390/v5051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfefferle S, Schöpf J, Kögl M, Friedel CC, Müller MA, Carbajo-Lozoya J, et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011;7(10):e1002331. doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pablos JL, Abasolo L, Alvaro-Gracia JM, Blanco FJ, Blanco R, Castrejón I, et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis. 2020;79(9):1170–1173. doi: 10.1136/annrheumdis-2020-217763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24(1):696. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovvuru S, Nalleballe K, Onteddu SR, Sharma R, Jasti M, Kapoor N, et al. Immunosuppression in chronic autoimmune neurological disorders during the COVID-19 pandemic. J Neurol Sci. 2021;420:117230. doi: 10.1016/j.jns.2020.117230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giacobbe DR, Battaglini D, Ball L, Brunetti I, Bruzzone B, Codda G, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10):e13319. doi: 10.1111/eci.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Y, Zeng H, Zhan Z, Lu H, Zeng Z, He C, et al. Corticosteroid use in the treatment of COVID-19: A multicenter retrospective study in Hunan, China. Front Pharmacol. 2020;11:1198. doi: 10.3389/fphar.2020.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial. JAMA. 2020;324(13):1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): A randomized, double-blind, phase iib, placebo-controlled trial. Clin Infect Dis. 2021;72(9):e373–e381. doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akiyama M, Sakatsume K, Sasaki K, Kawatsu S, Yoshioka I, Takahashi G, et al. The incidence, risk factors, and outcomes of gastrointestinal bleeding in patients with a left ventricular assist device: a Japanese single-center cohort study. J Artif Organs. 2020;23(1):27–35. doi: 10.1007/s10047-019-01138-y. [DOI] [PubMed] [Google Scholar]
- 50.Verhelst X, Somers N, Geerts A, Degroote H, Van Vlierberghe H. Health status of patients with autoimmune hepatitis is not affected by the SARS-CoV-2 outbreak in Flanders, Belgium. J Hepatol. 2021;74(1):240–241. doi: 10.1016/j.jhep.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rigamonti C, Cittone MG, De Benedittis C, Rizzi E, Casciaro GF, Bellan M, et al. Rates of symptomatic SARS-CoV-2 infection in patients with autoimmune liver diseases in northern Italy: A telemedicine study. Clin Gastroenterol Hepatol. 2020;18(10):2369–2371.e1. doi: 10.1016/j.cgh.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, et al. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73(3):702–705. doi: 10.1016/j.jhep.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerussi A, Rigamonti C, Elia C, Cazzagon N, Floreani A, Pozzi R, et al. Coronavirus Disease 2019 (COVID-19) in autoimmune hepatitis: a lesson from immunosuppressed patients. Hepatol Commun. 2020;4(9):1257–1262. doi: 10.1002/hep4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2020;72(2):671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 55.Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14(4):415–428. doi: 10.1007/s12072-020-10054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Güner R, Hasanoğlu I, Aktaş F. COVID-19: Prevention and control measures in community. Turk J Med Sci. 2020;50(SI-1):571–577. doi: 10.3906/sag-2004-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Capolongo S, Gola M, Brambilla A, Morganti A, Mosca EI, Barach P. COVID-19 and healthcare facilities: a decalogue of design strategies for resilient hospitals. Acta Biomed. 2020;91(9-S):50–60. doi: 10.23750/abm.v91i9-S.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boffa DJ, Judson BL, Billingsley KG, Del Rossi E, Hindinger K, Walters S, et al. Results of COVID-minimal surgical pathway during surge-phase of COVID-19 pandemic. Ann Surg. 2020;272(6):e316–e320. doi: 10.1097/SLA.0000000000004455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gan WH, Lim JW, Koh D. Preventing intra-hospital infection and transmission of coronavirus disease 2019 in health-care workers. Saf Health Work. 2020;11(2):241–243. doi: 10.1016/j.shaw.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Efe C, Simşek C, Batıbay E, Calışkan AR, Wahlin S. Feasibility of telehealth in the management of autoimmune hepatitis before and during the COVID-19 pandemic. Expert Rev Gastroenterol Hepatol. 2020;14(12):1215–1219. doi: 10.1080/17474124.2020.1822734. [DOI] [PubMed] [Google Scholar]
- 61.Craven J. COVID-19 vaccine tracker. Available from: https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker.
- 62. World Health Organization. Draft landscape of COVID-19 candidate vaccines. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 63.Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, et al. AASLD expert panel consensus statement: Vaccines to prevent COVID-19 infection in patients with liver disease. Hepatology. 2021 doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gutierrez Domingo I, Pascasio Acevedo JM, Alcalde Vargas A, Ramos Cuadra A, Ferrer Ríos MT, Sousa Martin JM, et al. Response to vaccination against hepatitis B virus with a schedule of four 40-µg doses in cirrhotic patients evaluated for liver transplantation: factors associated with a response. Transplant Proc. 2012;44(6):1499–1501. doi: 10.1016/j.transproceed.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 65.Gaeta GB, Stornaiuolo G, Precone DF, Amendola A, Zanetti AR. Immunogenicity and safety of an adjuvanted influenza vaccine in patients with decompensated cirrhosis. Vaccine. 2002;20(Suppl 5):B33–B35. doi: 10.1016/s0264-410x(02)00510-8. [DOI] [PubMed] [Google Scholar]
- 66.Arguedas MR, Johnson A, Eloubeidi MA, Fallon MB. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology. 2001;34(1):28–31. doi: 10.1053/jhep.2001.25883. [DOI] [PubMed] [Google Scholar]
- 67.Papp KA, Haraoui B, Kumar D, Marshall JK, Bissonnette R, Bitton A, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23(1):50–74. doi: 10.1177/1203475418811335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wörns MA, Teufel A, Kanzler S, Shrestha A, Victor A, Otto G, et al. Incidence of HAV and HBV infections and vaccination rates in patients with autoimmune liver diseases. Am J Gastroenterol. 2008;103(1):138–146. doi: 10.1111/j.1572-0241.2007.01609.x. [DOI] [PubMed] [Google Scholar]
- 69.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021;80(3):384–391. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 70.Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74(4):944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Croce E, Hatz C, Jonker EF, Visser LG, Jaeger VK, Bühler S. Safety of live vaccinations on immunosuppressive therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation or after bone-marrow transplantation - A systematic review of randomized trials, observational studies and case reports. Vaccine. 2017;35(9):1216–1226. doi: 10.1016/j.vaccine.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 72.Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18(6):546–556. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, et al. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2(5):100169. doi: 10.1016/j.jhepr.2020.100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnićk GL, Elveback IR, et al. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63(5):820–833. [PubMed] [Google Scholar]
- 76.Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1(7806):735–737. doi: 10.1016/s0140-6736(73)92125-9. [DOI] [PubMed] [Google Scholar]
- 77.van Gerven NM, Verwer BJ, Witte BI, van Hoek B, Coenraad MJ, van Erpecum KJ, et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58(1):141–147. doi: 10.1016/j.jhep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 78.Manns MP, Woynarowski M, Kreisel W, Lurie Y, Rust C, Zuckerman E, et al. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139(4):1198–206. doi: 10.1053/j.gastro.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 79.Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70(4):725–732. doi: 10.1136/gutjnl-2020-322539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74(1):148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cavagna L, Seminari E, Zanframundo G, Gregorini M, Di Matteo A, Rampino T, et al. Calcineurin inhibitor-based immunosuppression and COVID-19: Results from a multidisciplinary cohort of patients in northern Italy. Microorganisms. 2020;8(7):977. doi: 10.3390/microorganisms8070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson PC, Richards D, Tanner HL, Feldmann M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020;2(11):e653–e655. doi: 10.1016/S2665-9913(20)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry. Gastroenterology. 2020;159(2):481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehta P, Porter JC, Chambers RC, Isenberg DA, Reddy V. B-cell depletion with rituximab in the COVID-19 pandemic: where do we stand? Lancet Rheumatol. 2020;2(10):e589–e590. doi: 10.1016/S2665-9913(20)30270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loarce-Martos J, García-Fernández A, López-Gutiérrez F, García-García V, Calvo-Sanz L, Del Bosque-Granero I, et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int. 2020;40(12):2015–2021. doi: 10.1007/s00296-020-04699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdulrab S, Al-Maweri S, Halboub E. Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID-19-associated cytokine storm. Med Hypotheses. 2020;143:109897. doi: 10.1016/j.mehy.2020.109897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saigal S, Gupta S, Sudhindran S, Goyal N, Rastogi A, Jacob M, et al. Liver transplantation and COVID-19 (Coronavirus) infection: guidelines of the liver transplant Society of India (LTSI) Hepatol Int. 2020;14(4):429–431. doi: 10.1007/s12072-020-10041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zijp TR, Toren-Wielema ML, Nannan Panday PV, Kosterink JGW, Berger SP, Touw DJ. Important interactions of immunosuppressants with experimental therapies for novel coronavirus disease (COVID-19): How to act. Ther Drug Monit. 2020;42(4):652–653. doi: 10.1097/FTD.0000000000000766. [DOI] [PubMed] [Google Scholar]


