Summary
In the central nervous system, developmental and pathophysiologic conditions cause a large-scale reorganization of functional connectivity of neural circuits. Here, by using a mouse model for peripheral sensory nerve injury, we present a protocol for combined electrophysiological and anatomical techniques to identify neural basis of synaptic remodeling in the mouse whisker thalamus. Our protocol provides comprehensive approaches to analyze both structural and functional components of synaptic remodeling.
For complete details on the use and execution of this protocol, please refer to Ueta and Miyata, (2021).
Subject areas: cell biology, microscopy, model organisms, neuroscience
Graphical abstract
Highlights
-
•
The infraorbital nerve cut for preparing a peripheral nerve injury mouse model
-
•
Pressure or iontophoretic drug application via stereotaxic injection
-
•
Assessing functional synaptic remodeling by whole-cell patch-clamp in acute slices
-
•
Immunohistochemical identification of structural synaptic remodeling
In the central nervous system, developmental and pathophysiologic conditions cause a large-scale reorganization of functional connectivity of neural circuits. Here, by using a mouse model for peripheral sensory nerve injury, we present a protocol for combined electrophysiological and anatomical techniques to identify neural basis of synaptic remodeling in the mouse whisker thalamus. Our protocol provides comprehensive approaches to analyze both structural and functional components of synaptic remodeling.
Before you begin
Structures and functions of neural circuitry in the central nervous system are dynamically refined during development and in disease. Because neural and glial mechanisms both involve in the induction and maintenance of synaptic remodeling, investigating how neural or glial mechanisms change physiological and morphological properties of synapses and characterizing interactions between neurons and glial cells, such as astrocytes or microglia, are of particular importance to understand neural mechanisms underlying developmental maturation or etiology.
We have recently reported the microglia-dependent mechanism of structural and functional synaptic remodeling in the mouse whisker thalamus (Ueta and Miyata, 2021), which is triggered by peripheral sensory nerve injury (Nagumo et al., 2020; Takeuchi et al., 2012; 2017). In that study, we have established region-specific depletion or activation of microglia using stereotaxic injections of microglial inhibitor or activator.
Here, we describe step-by-step procedures for development of mouse model for peripheral nerve injury, stereotaxic injection, and electrophysiological and histological assessment of synaptic remodeling in the whisker thalamus. These approaches include fundamental techniques for mouse surgery, whole-cell patch-clamp using acute brain slices, and immunohistochemistry combined with mouse genetics, and will be useful for studies investigating neural and glial contribution to synaptic remodeling in diverse regions under pathophysiological conditions, and also during development.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat polyclonal anti-Iba1 antibody (1:500–1,000 dilution) | Abcam | Cat#ab5076; PRID: AB_2224402 |
| Rabbit Polyclonal Anti-Iba1 Antibody (1:500–1,000 dilution) | FUJIFILM Wako Pure Chemical Corporation | Cat#019-19741; PRID: AB_839504 |
| Rabbit polyclonal anti-NeuN antibody (1:1,000–5,000 dilution) | Merck (Sigma-Aldrich) | Cat#ABN78; PRID: AB_10807945 |
| Mouse monoclonal anti-NeuN antibody (1:1,000–2,000 dilution) | Merck (Sigma-Aldrich) | Cat#MAB377; PRID: AB_2298772 |
| Rabbit polyclonal anti-TMEM119 antibody (1:500–1,000 dilution) | Abcam | Cat#ab209064; PRID: AB_2800343 |
| Guinea Pig Polyclonal Anti-VGluT2 Antibody (1:500–1,000 dilution) | Nittobo Medical Co., Ltd. (transferred from Frontier Institute Co., Ltd.) | Cat#MSFR106290; PRID: AB_2341096 |
| Guinea Pig Polyclonal Anti-VGluT2 Antibody (1:1,000–5,000 dilution) | Merck (Sigma-Aldrich) | Cat#AB2251-I; PRID: AB_2665454 |
| Rabbit Polyclonal Anti-VGluT2 Antibody (1:500–1,000 dilution) | Nittobo Medical Co., Ltd. (transferred from Frontier Institute Co., Ltd.) | Cat#MSFR106310; PRID: AB_2619683 |
| Donkey anti-Guinea Pig IgG Secondary Antibody, Alexa Fluor 488 Conjugate (1:500 dilution) | Jackson ImmunoResearch Laboratories | Cat#706-545-148; PRID: AB_2340472 |
| Donkey Anti-Guinea Pig IgG Secondary Antibody, Alexa Fluor 647 Conjugate (1:500 dilution) | Jackson ImmunoResearch Laboratories | Cat#706-605-148; PRID: AB_2340476 |
| Donkey anti-Rabbit IgG Secondary Antibody, Alexa Fluor 488 conjugate (1:500 dilution) | Thermo Fisher Scientific | Cat#A21206; PRID: AB_2535792 |
| Chemicals, peptides, and recombinant proteins | ||
| Agar | FUJIFILM Wako Pure Chemical Corporation | Cat#010-15815; CAS: 9002-18-0 |
| Agarose S | Nippon Gene Co., Ltd. | Cat#312-01193; CAS: 9012-36-6 |
| Atipamezole (Antisedan) | Nippon Zenyaku Kogyo Co., Ltd. | CAS: 104075-48-1 |
| Bicuculline methochloride | Tocris | Cat#0131; CAS: 53552-05-9 |
| Biocytin | Toronto Research Chemicals Inc. or Sigma | Cat#B388100 or #B4261, CAS: 576-19-2 |
| Butorphanol (Vetorphale) | Meiji Seika Pharma Co., Ltd. | CAS: 42408-82-2 |
| CaCl2·2H2O | Kanto Chemical Co., Inc. | Cat#07058-00; CAS: 10035-04-8 |
| CGP55845 | Tocris | Cat#1248; CAS: 149184-22-5 |
| Cholera toxin subunit B, Alexa Fluor 555 conjugate | Thermo Fisher Scientific | Cat#C34776 |
| Cholera toxin subunit B, Alexa Fluor 647 conjugate | Thermo Fisher Scientific | Cat#C34778 |
| Chromium (III) potassium sulfate·12H2O | Kanto Chemical Co., Inc. | Cat#07360-00; CAS: 7788-99-0 |
| Clodronate liposomes | Katayama Chemical | Cat#160-0430-1 |
| CsMeSO3 | Merck (Sigma-Aldrich) | Cat#C1426; CAS: 2550-61-0 |
| CsOH 50 wt. % solution in water | Merck (Sigma-Aldrich) | Cat#232041; CAS: 21351-79-1 |
| D-(+)-glucose | FUJIFILM Wako Pure Chemical Corp. | Cat#049-31165; CAS: 50-99-7 |
| EDTA·2Na·2H2O | Nacalai Tesque | Cat#15111-45; CAS: 6381-92-6 |
| EGTA | Nacalai Tesque | Cat#15214-92; CAS: 67-42-5 |
| Ethylene glycol | Merck (Sigma-Aldrich) | Cat#09-1540-5; CAS: 107-21-1 |
| Fluoromount-G (refractive index, ~1.4) | SouthernBiotech | Cat#0100-01 |
| Glycerol | Nacalai Tesque | Cat#17017-35; CAS: 56-81-5 |
| HCl (1 M) | FUJIFILM Wako Pure Chemical Corporation | Cat#083-01095; CAS: 7647-01-0 |
| Heparin sodium salt from porcine intestinal mucosa | Merck (Sigma-Aldrich) | Cat#H3393; CAS: 9041-08-1 |
| HEPES | Merck (Sigma-Aldrich) | Cat#H3375; CAS: 7365-45-9 |
| Isoflurane | Viatris (Mylan N.V.) | CAS: 26675-46-7 |
| KCl | FUJIFILM Wako Pure Chemical Corporation | Cat#163-03545; CAS: 7447-40-7 |
| K-gluconate | Merck (Sigma-Aldrich) | Cat#G4500; CAS: 299-27-4 |
| KOH (1 M) | FUJIFILM Wako Pure Chemical Corporation | Cat#169-03885; CAS: 1310-58-3 |
| Lactic acid | Nacalai Tesque | Cat#20006-62; CAS: 50-21-5 |
| Lipopolysaccharides from E.Coli O26 | FUJIFILM Wako Pure Chemical Corporation | Cat#120-05131; CAS: 93572-42-0 |
| Medetomidine (Domitor) | Nippon Zenyaku Kogyo Co., Ltd. | CAS: 86347-15-1 |
| MgCl2·6H2O | FUJIFILM Wako Pure Chemical Corporation | Cat#135-00165; CAS: 7791-18-6 |
| Midazolam | Sandoz K.K. | CAS: 59467-70-8 |
| myo-Inositol | FUJIFILM Wako Pure Chemical Corporation | Cat#094-00281; CAS: 87-89-8 |
| Na2-ATP | Merck (Sigma-Aldrich) | Cat#A7699; CAS: 34369-07-8 |
| Na2HPO4·12H2O | FUJIFILM Wako Pure Chemical Corporation | Cat#196-02835; CAS: 10039-32-4 |
| NaCl | FUJIFILM Wako Pure Chemical Corporation | Cat#191-01665; CAS: 7647-14-5 |
| Na-GTP | Merck (Sigma-Aldrich) | Cat#G8877; CAS: 36051-31-7 |
| NaH2PO4·2H2O | FUJIFILM Wako Pure Chemical Corporation | Cat#192-02815; CAS: 13472-35-0 |
| NaHCO3 | FUJIFILM Wako Pure Chemical Corporation | Cat#191-01305; CAS: 144-55-8 |
| NaOH (1 M) | FUJIFILM Wako Pure Chemical Corporation | Cat#192-02175; CAS: 1310-73-2 |
| Neurobiotin | Vector Laboratories | Cat#SP-1120; CAS: 111822-45-8 |
| NeuroTrace™ 435/455 Blue Fluorescent Nissl Stain (1:100–200 dilution) | Thermo Fisher Scientific | Cat#N21479 |
| Normal donkey serum | ImmunoBioScience Corp. | Cat#IHR-8135 |
| Ofloxacin gel (Tarivid opthalamic ointment 0.3%) | Santen Pharmaceutical Co., Ltd. | CAS: 82419-36-1 |
| Paraformaldehyde | TAAB laboratories Equipment Ltd. | Cat#P001; CAS: 30525-89-4 |
| Picric acid | Nacalai Tesque | Cat#27926-02; CAS: 88-89-1 |
| PLX3397 (Pexidartinib) | Selleck Chemicals | Cat#S7818; CAS: 1029044-16-3 |
| ProLong™ Gold Antifade Mountant (refractive index, 1.47) | Thermo Fisher Scientific | Cat#P36934 |
| QX314 (lidocaine N-ethyl bromide) | Merck (Sigma-Aldrich) | Cat#L5783, CAS: 21306-56-9 |
| Sevoflurane | Viatris (Mylan N.V.) | CAS: 28523-86-6 |
| SlowFade™ Diamond Antifade Mountant (refractive index, 1.47) | Thermo Fisher Scientific | Cat#S36972 |
| Sodium ascorbate ((+)-sodium L-ascorbate) | Merck (Sigma-Aldrich) | Cat#A7631; CAS: 134-03-2 |
| Sodium pyruvate (pyruvic acid sodium salt) | Nacalai Tesque | Cat#29806-12; CAS: 113-24-6 |
| Spongel | LTL Pharma | N/A |
| Streptavidin, Alexa Fluor 488 conjugate (1:500 dilution) | Thermo Fisher Scientific | Cat#S11223 |
| Sucrose | FUJIFILM Wako Pure Chemical Corporation | Cat#196-00015; CAS: 57-50-1 |
| Thiourea | FUJIFILM Wako Pure Chemical Corporation | Cat#204-01202; CAS: 62-56-6 |
| Tris base (Trizma base) | Merck (Sigma-Aldrich) | Cat#T6066; CAS: 77-86-1 |
| Tris-HCl | Nacalai Tesque | Cat#35433-15; CAS: 1185-53-1 |
| Trisodium citrate dihydrate | FUJIFILM Wako Pure Chemical Corporation | Cat#204-16675; CAS: 6132-04-3 |
| Triton X-100 | Nacalai Tesque | Cat#12967-32; CAS: 9002-93-1 |
| Xylocaine jelly 2% (lidocaine hydrochloride) | Aspen Japan K.K. | CAS: 73-78-9 |
| Xylocaine with 1:80,000 adrenaline | Dentsply Sirona K.K. | CAS: 73-78-9 (for lidocaine hydrochloride), 51-43-4 (for adrenaline) |
| Experimental models: organisms/strains | ||
| Mouse: C57BL/6JJmsSlc | Sankyo Labo Service Corporation Inc. | MGI: 5488963; PRID: MGI:5488963 |
| Mouse: Egr2tm2(cre)Pch/J (Krox20-Cre) | The Jackson Laboratory | JAX: 025744; PRID: IMSR_JAX:025744 |
| Mouse: B6;129S-Gt(ROSA) 26Sortm34.1(CAG-Syp/tdTomato)Hze/J (Ai34D) |
The Jackson Laboratory | JAX: 012570; RRID: IMSR_JAX:012570 |
| Software and algorithms | ||
| Adobe Photoshop/Illustrator CC | Adobe Inc. | https://www.adobe.com/ |
| Igor Pro 6 | WaveMetrics | https://www.wavemetrics.com/ |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| NeuroMatic | Rothman and Silver, 2018 | http://www.neuromatic.thinkrandom.com/ |
| pClamp 8 | Molecular Devices | https://www.moleculardevices.com/ |
| Zen | Carl Zeiss | https://www.zeiss.de/corporate/home.html |
| Other | ||
| 1 or 20 mL syringe | Terumo Corporation | Cat#SS-01T or SS-20ESZ |
| 21-, 26-, or 27-gauge syringe needle | Terumo Corporation | Cat#NN-2138R, NN-2613S, or NN-2719S |
| 35G beveled needle | World Precision Instruments | Cat#NF35BV-2 |
| Anesthetic apparatus with anesthesia induction chamber | Shinano MFG Co. Ltd. | Cat#SN-487-0T |
| Aron Alpha (cyanoacrylate) | Toagosei Co. Ltd. | Cat#31204 |
| Concentric stimulating electrode | Inter Medical Co. Ltd. | Cat#IMB-160820 |
| Confocal laser scanning microscope | Carl Zeiss | Cat#LSM710 |
| Cooled CCD camera (for histology) | Quantum Scientific Imaging, Inc. | Cat#QSI RS 6.1 |
| Coverslip (24 × 32, 40, 50, and 60 mm) | Matsunami Glass Ind. Ltd. | Cat#C024321, C024401, C024501, and C024601 |
| DC temperature controller (for surgery) | FHC | Cat#40-90-8D |
| Digitizer | Molecular Devices | Cat#Digidata 1322A |
| Electrical tape | Yamato Co. Ltd. | Cat#NO200-19 |
| Electronic stimulator | Nihon Kohden | Cat#SEN-7203 |
| Epifluorescence microscope (for electrophysiology) | Olympus Corporation | Cat#BX51WI |
| Epifluorescence microscope (for histology) | Carl Zeiss | Cat#Axio Scope.A1 |
| Flexible needle | World Precision Instruments | Cat#MF28G-5 |
| Flow tube for peristaltic pump (for electrophysiology; I.D., 1.29 mm) | M&S Instruments Inc. | F117940 |
| Flow tube for peristaltic pump (for paraformaldehyde fixation; I.D., 2.28 mm) | M&S Instruments Inc. | F117946 |
| Fluorescence microscope | Keyence | Cat#BZ-X810 |
| Glass capillary (O.D., 1.0 and I.D., 0.6 mm) | Narishige | Cat#G-1 |
| Glass capillary (O.D., 1.5 and I.D., 0.9 mm) | Narishige | Cat#G-1.5 |
| Glass capillary (O.D., 1.5 and I.D., 0.86 mm) | Sutter Instrument | Cat#B150-86-10 |
| Glass capillary (O.D., 1.5 and I.D., 1.17 mm) | Harvard Apparatus | Cat#30-0062 |
| Glass microscope slides | Matsunami Glass Ind. Ltd. | Cat#S2441 |
| Hamilton syringe (10 μL) | World Precision Instruments | Cat#Nanofil |
| Heat pad | Natsume Seisakusho Co. Ltd. | Cat#KN-475-2-40 |
| Injector blade (for preparing thin sections of the fixed brain) | Schick Japan K. K. | Cat#SII-10 |
| Iontophoresis pump | Kation Scientific | Cat#BAB-600 |
| Magnetic hot plate stirrer | IKA | Cat#RH B2 S004 |
| Microelectrode amplifier | Molecular Devices LLC. | Cat#Multiclamp 700A |
| Micromanipulator (for electrophysiology) | Sutter Instrument | Cat#MP-225 |
| Micromanipulator (for stereotaxic injection) | Narishige | Cat#SM-15R/SM-15M-2 |
| Micromotor system | Minitor Co. Ltd. | Cat#Minimo one series ver.2 |
| Micropipette puller | Sutter Instrument | Cat#P-1000 or P-97 |
| Micro-Sample Osmometer | Fiske Associates Inc. | Cat#Model 210 |
| Microsyringe pump controller | World Precision Instruments | Cat#Micro 4 |
| Microtome blade (for trimming the fixed brain) | Feather Safety Razor Co. Ltd. | Cat#A35 |
| Near-infrared camera | Hamamatsu Photonics | Cat#C3077-79 |
| Near-infrared camera controller | Hamamatsu Photonics | Cat#C2741-62 |
| Paraffin oven (laboratory incubator) | Hirasawa Works | Cat#SC-4d-CP |
| Peristaltic pump | Gilson Inc. | Cat#Minipuls 3 |
| pH indicator paper (pH 5.0–10.0) | Merck | Cat#109533 |
| pH meter | Horiba Ltd. | Cat#B-712 |
| Razor blade (for preparing acute brain slices) | Feather Safety Razor Co. Ltd. | Cat#81-S |
| Rodent brain matrix | ASI Instruments Inc. | Cat#RBM-2000C |
| Shaker | TAITEC Corporation | Cat#Mix-VR and NR-80 |
| Steel bur (Midwest carbide bur) | Dentsply Sirona K.K. | Cat#386301 |
| Stereo microscope (for surgery) | Leica Microsystems Inc. | Cat#M60 |
| Stereotaxic apparatus | Narishige | Cat#SR-5M-HT |
| Stimulus isolator | A.M.P.I. | Cat#Iso-Flex |
| Surgical blade (for trimming the brain) | Feather Safety Razor Co. Ltd | Cat#21 |
| Syringe filter (0.2 μm, non-sterile) | Millipore (Merck) | Cat#SLLGH04NL |
| Temperature controller (line heater) | Warner Instruments | Cat#TC-324B |
| Vibratome | Leica Microsystems Inc. | Cat#VT-1000S and VT-1200S |
| Vinyl repair patch | Otsuka Corporation | Cat#TGK-280 |
| Water bath system | AS ONE Corporation | Cat#TR1 |
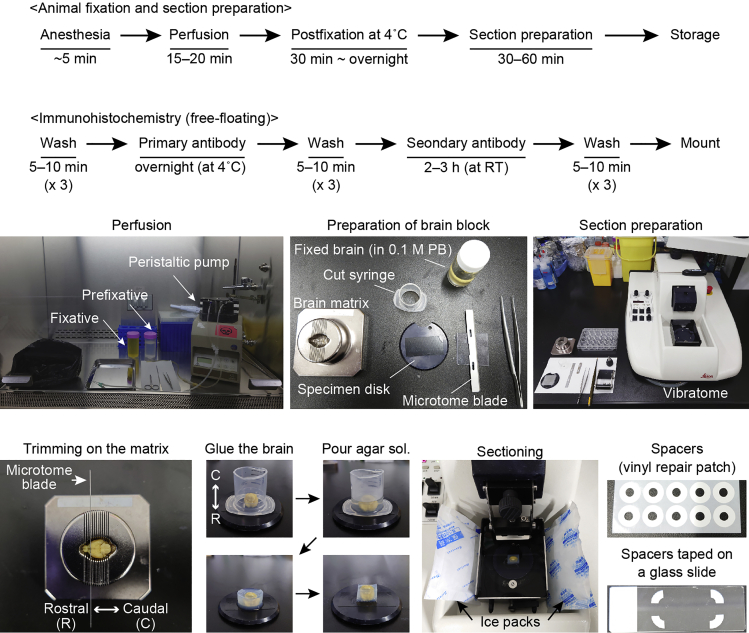
Materials and equipment
10× artificial cerebrospinal fluid (aCSF) stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 1.25 M | 73.05 g |
| NaHCO3 | 260 mM | 21.84 g |
| NaH2PO4·2H2O | 12.5 mM | 1.95 g |
| KCl | 25 mM | 1.86 g |
| ddH2O | N/A | Fill up to 1,000 mL |
| Total | N/A | 1,000 mL |
The solution can be stored at 4°C for 1 month.
aCSF solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 10× aCSF | 1× | 100 mL |
| 1 M CaCl2 solution | 2 mM | 2 mL |
| 1 M MgCl2 solution | 1 mM | 1 mL |
| D-(+)-Glucose | 20 mM | 3.6 g |
| ddH2O | N/A | Fill up to 1,000 mL |
| Total | N/A | 1,000 mL |
Add 1 M CaCl2 solution and 1 M MgCl2 solution after diluting 10× aCSF solution with ddH2O and bubbled with a mixture of 95% O2 and 5% CO2 for 10–30 min at 20°C–25°C.
Although the solution may be stored at 4°C for one week, we recommend to prepare the fresh solution on the day of use.
Osmolarity of the solution is 310 ± 5 mOsm.
pH of the solution falls to 7.2–7.3 after bubbling with a mixture of 95% O2 and 5% CO2 for 10 min at 20°C–25°C .
1 M CaCl2 solution
| Reagent | Final concentration | Amount |
|---|---|---|
| CaCl2·2H2O | 1 M | 14.7 g |
| ddH2O | N/A | Fill up to 100 mL |
| Total | N/A | 100 mL |
The solution can be stored at 4°C for at least 3 months.
1 M MgCl2 solution
| Reagent | Final concentration | Amount |
|---|---|---|
| MgCl2·6H2O | 1 M | 20.3 g |
| ddH2O | N/A | Fill up to 100 mL |
| Total | N/A | 100 mL |
The solution can be stored at 4°C for at least 3 months.
Cutting solution (sucrose-based solution)
| Reagent | Final concentration | Amount |
|---|---|---|
| Sucrose | 234 mM | 80.1 g |
| NaHCO3 | 25 mM | 2.1 g |
| NaH2PO4·2H2O | 1.25 mM | 0.2 g |
| KCl | 2.5 mM | 0.19 g |
| 1 M CaCl2 solution | 0.5 mM | 0.5 mL |
| 1 M MgCl2 solution | 10 mM | 10 mL |
| myo-Inositol | 0.5 mM | 0.09 g |
| D-(+)-Glucose | 20 mM | 3.6 g |
| ddH2O | N/A | Fill up to 1,000 mL |
| Total | N/A | 1,000 mL |
The composition is originally based on the previous study (Gentet and Ulrich, 2003).
The solution can be stored at 4°C for 1 month.
Osmolarity of the solution is 360 ± 10 mOsm.
pH of the solution falls to 7.1–7.3 after bubbling with a mixture of 95% O2 and 5% CO2 for 10 min on ice.
Alternatives: Cold aCSF solution can be used as the cutting solution.
1.25× Cs+-based pipette internal solution for voltage-clamp recording
| Reagent | Final concentration | Amount |
|---|---|---|
| CsMeSO3 | 120 mM | 547 mg |
| NaCl | 20 mM | 23 mg |
| QX314 | 5 mM | 34 mg |
| Na2-ATP | 2 mM | 22 mg |
| Na-GTP | 0.5 mM | 5 mg |
| 1 M CaCl2 solution | 0.1 mM | 2 μL |
| 1 M MgCl2 solution | 2 mM | 40 μL |
| HEPES | 10 mM | 48 mg |
| EGTA | 1 mM | 8 mg |
| ddH2O | N/A | Fill up to 16 mL |
| Total | N/A | 16 mL |
Add 15 mL of ddH2O first and mix.
After pH adjustment to 7.2–7.3 with CsOH, fill up to 16 mL by ddH2O.
A 0.8-mL aliquot in the 1.5 mL or 2 mL microtube can be stored at −30°C for at least 3 months.
Thaw an aliquot on the day of use, and adjust osmolarity to 290 mOsm by adding ddH2O.
Filter the solution (nearly 1 mL of total amount) using non-sterile 0.20 μm mini-syringe filter before use.
If necessary, add 0.5% (w/v) biocytin and vortex the solution before adjusting osmolarity. Sonication is effective to prepare the biocytin solution.
Biocytin can also be dissolved in the initial step before aliquot.
Aliquots can be stored at −30°C for at least 3 months as described above.
Once prepared, the solution may be stored at 4°C for a week.
Alternatives: Neurobiotin can be used instead of biocytin for visualizing cell morphology.
1.25× K+-based pipette internal solution for current-clamp recording
| Reagent | Final concentration | Amount |
|---|---|---|
| K-gluconate | 130 mM | 609 mg |
| Na2-ATP | 2 mM | 22 mg |
| Na-GTP | 0.3 mM | 3 mg |
| 1 M MgCl2 solution | 2 mM | 40 μL |
| HEPES | 10 mM | 48 mg |
| EGTA | 0.2 mM | 1.6 mg |
| Biocytin | 0.5% (w/v) | 100 mg |
| ddH2O | N/A | Fill up to 16 mL |
| Total | N/A | 16 mL |
Prepare the solution similar to the Cs+-based internal solution described above, but adjust pH using KOH.
Biocytin is always included in the K+-based internal solution in our experiments.
A 0.8-mL aliquot in the 1.5 mL or 2 mL microtube can be stored at −30°C for at least 3 months.
Thaw a 0.8-mL aliquot on the day of use, and adjust osmolarity to 290 mOsm by adding ddH2O.
Total amount of the solution becomes nearly 1 mL.
Filter the solution before use as described above.
Once prepared, the solution may be stored at 4°C for a week.
CRITICAL: As it is difficult to measure the small amount of reagent, you can prepare larger amount of internal solutions or concentrated solution, especially for Na2-ATP, Na-GTP, and EGTA. We adjust osmolarity just before the experiment, because osmolarity may be slightly changed during the storage. For more convenience, you can fill up to 20 mL after pH adjustment and adjust osmolarity to 290 mOsm. In that case, a 1-mL aliquot can be stored, but we recommend to measure osmolarity of thawed solution just before the experiment.
0.1 M phosphate buffer (PB) (pH 7.4)
| Reagent | Final concentration | Amount |
|---|---|---|
| Na2HPO4·12H2O | 80 mM | 29 g |
| NaH2PO4·2H2O | 20 mM | 2.95 g |
| ddH2O | N/A | Fill up to 1,000 mL |
| Total | N/A | 1,000 mL |
If pH is lower than 7.4, add few drops of 1 M NaOH and adjust pH to 7.4.
Add 9 g NaCl for preparing phosphate buffered saline (PBS). The solution can be stored at 4°C for 1 year.
0.05 M Tris buffer (TB) (pH 7.6)
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris-HCl | 500 mM | 6.06 g |
| Tris base | 11.5 mM | 1.39 g |
| ddH2O | N/A | Fill up to 1,000 mL |
| Total | N/A | 1,000 mL |
Add 0.9 g NaCl for preparing tris buffered saline (TBS). The solution can be stored at 4°C for 1 year.
Prefixative solution (sucrose-based solution)
| Reagent | Final concentration | Amount |
|---|---|---|
| Sucrose | 250 mM | 8.5 g |
| 1 M MgCl2 solution | 5 mM | 0.5 mL |
| 0.1 M PB | 20 mM | 20 mL |
| ddH2O | N/A | Fill up to 100 mL |
| Total | N/A | 100 mL |
The solution can be stored at 4°C for 1 month.
Alternatives: Saline (0.9% NaCl) can be used as the prefixative solution. To prevent blood coagulation, heparin can be included in the prefixative solution (10–20 units/mL).
Fixative solution (4% paraformaldehyde and 0.2% picric acid)
| Reagent | Final concentration | Amount |
|---|---|---|
| Paraformaldehyde | 4% (w/v) | 40 g |
| Picric acid | 0.2% (w/v) | 2 g |
| 0.2 M PB | 0.1 M | 500 mL |
| ddH2O | N/A | Fill up to 1,000 mL |
| Total | N/A | 1,000 mL |
Add 500 mL 0.2 M PB and 300 mL ddH2O in a 1000-mL conical flask.
Add 40 g paraformaldehyde powder.
Heat the solution at 60°C and stir the solution until paraformaldehyde powder is completely solubilized when the cloudy solution becomes clear. It will require 1–2 h. Add 7–9 drops of 1 M NaOH using a 3-mL disposable transfer pipette if necessary. pH of the solution is adjusted to around 7.5 using a pH indicator paper.
Cool the solution to 4°C by placing the flask on ice.
Add 2 g picric acid powder and stir until the powder is completely solubilized. It will finish within 10 min.
After filtering the solution using a filter paper, fill up to 1000 mL with ddH2O.
The solution can be stored at 4°C for at least 3 months.
50- or 100-mL aliquots can be stored at −30°C for a long time.
Alternatives: 4% paraformaldehyde solution without picric acid can be used as the fixative solution. To avoid oxidation of the paraformaldehyde without picric acid, we recommend the solution to be used within a week (storing at 4°C until use). The solution can be stored at −30°C for a long time.
CRITICAL: Preparation of the fixative solution should be performed under the fume hood. To avoid scattering of paraformaldehyde powder, we recommend to use a weighing dish rather than a weighing paper for measuring the powder. Picric acid is not only toxic but also explosive when dried. Do not keep the lid open especially under the fume hood.
3% agar solution (for embedding a fixed brain)
| Reagent | Final concentration | Amount |
|---|---|---|
| Agar | 3% (w/v) | 6 g |
| Agarose S | 0.3% (w/v) | 0.6 g |
| 0.1 M PB | N/A | Fill up to 200 mL |
| Total | N/A | 200 mL |
Dissolve agar and agarose S by boiling the solution using microwave radiation.
Aliquot the solution into 50-mL conical tubes and keep until the liquid becomes solid.
Solid agar can be stored at 4°C for at least 3 months.
Citrate-based antigen retrieval solution (pH 6.0)
| Reagent | Final concentration | Amount |
|---|---|---|
| Trisodium citrate | 10 mM | 2.94 g |
| ddH2O | N/A | Fill up to 1,000 mL |
| Total | N/A | 1,000 mL |
Adjust pH to 6.0 by adding 1 M HCl (nearly 9 mL for the 1,000-mL solution).
The solution can be stored at 20°C–25°C for 1 year.
For antigen retrieval, boil the solution using microwave irradiation.
Soak sections in it and keep at 90°C for 10–30 min in the laboratory incubator.
After that, return it to 20°C–25°C and carry out performing immunohistochemistry.
Alternatives: As another option, the Tris-EDTA solution can be used for antigen retrieval. Add 1.21 g Tris base and 0.372 g EDTA in 1,000 mL ddH2O and adjust pH to 9.0 by adding 1 M NaOH (nearly 0.3 mL). The solution can be stored at 20°C–25°C for 1 year.
10% normal donkey serum with 0.3% Triton X-100
| Reagent | Final concentration | Amount |
|---|---|---|
| Normal donkey serum | 10% (v/v) | 1 mL |
| 20% Triton X-100 | 0.3% (w/v) | 150 μL |
| 0.1 M PB | N/A | 9 mL |
| Total | N/A | 10 mL |
The solution can be stored at 4°C for 1 month.
10% sucrose solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Sucrose | 10% (w/v) | 10 g |
| 0.1 M PB | N/A | Fill up to 100 mL |
| Total | N/A | 100 mL |
The solution can be stored at 4°C for 1 month.
30% sucrose solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Sucrose | 10% (w/v) | 30 g |
| 0.1 M PB | N/A | Fill up to 100 mL |
| Total | N/A | 100 mL |
The solution can be stored at 4°C for 1 month.
Antifreeze solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Glycerol | 30% (w/v) | 150 g |
| Ethylene glycol | 30% (v/v) | 150 mL |
| NaCl | 0.85% (w/v) | 4.25 g |
| 0.4 M TB∗ | 0.04 | 50 mL |
| ddH2O | N/A | Fill up to 500 mL |
| Total | N/A | 500 mL |
The solution can be stored at −30°C for a long time.
For preparation of 0.4 M TB, dissolve 4.85 g Tris-HCl and 1.11 g Tris base in 100-mL ddH2O.
Gelatin solution for preparing gelatin-coated glass slides
| Reagent | Final concentration | Amount |
|---|---|---|
| Gelatin | 1% (w/v) | 2 g |
| Chromium (III) Potassium Sulfate·12H2O | 0.005% (w/v) | 10 μg |
| ddH2O | N/A | 200 mL |
| Total | N/A | 200 mL |
Add chromium (III) potassium sulfate after melting gelatin by heating the solution at 60°C and filter the solution. We recommend to prepare the solution on the day of use.
After washing glass microscope slides with 70% ethanol and then with ddH2O, dip them into the warm gelatin solution keeping at 60°C.
Dry gelatin-coated glass slides in the laboratory incubator at 40°C for 12–16 h (at least for 4–6 h).
Step-by-step method details
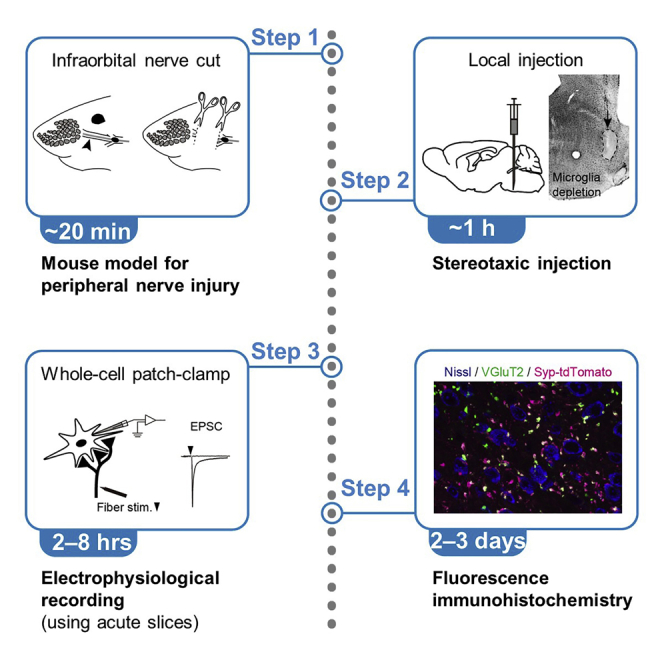
Development of mouse model for the infraorbital nerve injury
Timing: 15–20 min
In the mouse whisker somatosensory pathway, thalamic neurons undergo synaptic remodeling not only during development but also after peripheral nerve injury. To examine structural and functional changes in synapses after peripheral nerve injury, we perform transection of the infraorbital nerve, which is the second branch of the trigeminal nerve and conveys tactile information from the whiskers.
-
1.Mouse anesthesia
-
a.Anesthetize a mouse (older than 3 weeks) by 2–3% isoflurane in an anesthesia induction chamber for 5 min (Figure 1A).
-
b.Anesthesia is maintained by intraperitoneal injection of a mixture of medetomidine (0.3 mg/kg), midazolam (4 mg/kg), and butorphanol (5 mg/kg) in saline (0.9% NaCl).
-
a.
Note: We use C57 BL/6 wild type mice or transgenic mice produced from C57 BL/6 genetic background of both sexes for this study. Synaptic remodeling in the thalamus is observed both in male and female mice. Mice in the age range of 3–8 weeks old, which corresponds to the period after the developmental synapse remodeling and maturation in the whisker thalamus (Takeuchi et al., 2014), are used.
-
2.Unilateral digestion of the infraorbital nerve
- a.
-
b.Expose the infraorbital nerve under a microscope using two forceps (Figure 1B). The nerve should be freed from the connective tissue without bleeding. Troubleshooting 1
-
c.Locally anesthetize the nerve by applying 2% xylocaine with 1:80,000 adrenaline on the surface of the nerve using a 1-mL syringe with a 26- or 27-gauge needle.
-
d.Lift the nerve up and cut the nerve at a central site first and then a peripheral site at a distance of 1–2 mm from the central site to prevent axonal regeneration (Figure 1C).
-
3.Postoperative care
-
a.After suturing the skin, inject atipamezole (0.1 mg/kg) intraperitoneally to reverse the sedative effect of medetomidine.
-
b.Return the mouse to the home cage and house until the electrophysiological or histological experiment is performed.
-
a.
Note: Synaptic remodeling in the thalamus is induced 5–6 days from the nerve cut (Takeuchi et al., 2012).
CRITICAL: Operation should be terminated within 30 min because sedative effect of medetomidine usually continues only 30 min from the intraperitoneal injection. We recommend continuous administration of 0.5–1% isoflurane (or sevoflurane) in addition to medetomidine injection.
Figure 1.
Development of mouse model for the infraorbital nerve (ION) cut
(A) A setup for mouse surgery.
(B) Exposed ION. Scale bar, 5 mm.
(C) No running ION after ION cut (IONC). Scale bar, 5 mm.
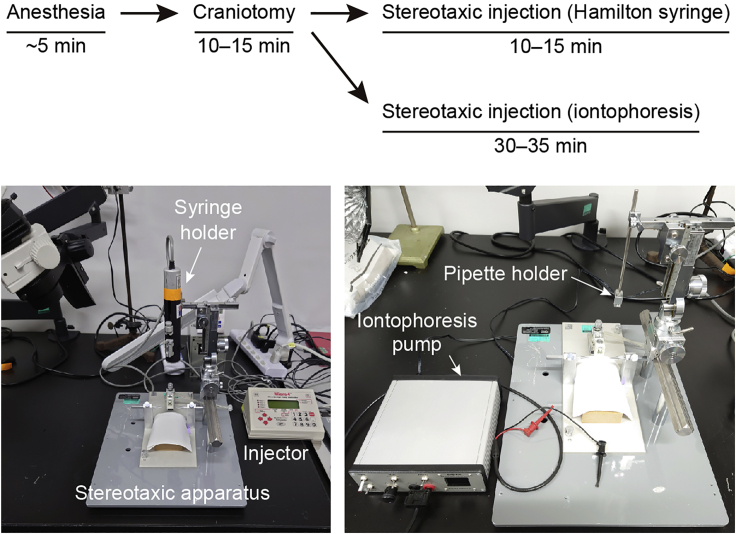
Stereotaxic injection
Timing: 25–60 min
Refer to ‘‘materials and equipment’’ for information about reagents used. The timing and workflow are depicted in Figure 2.
-
4.Mouse anesthesia
-
a.Anesthetize a mouse by 2–3% isoflurane in an anesthesia induction chamber for 5–10 min (Figure 1A).
-
b.Anesthesia is maintained by intraperitoneal injection of a mixture of medetomidine (0.3 mg/kg), midazolam (4 mg/kg), and butorphanol (5 mg/kg) in saline (0.9% NaCl).
-
a.
Note: When the operation is expected to be prolonged (more than 25 min), apply 0.5–1% isoflurane continuously to maintain anesthesia. Body temperature should be monitored and maintained during the operation.
-
5.Craniotomy
-
a.Place the anesthetized mouse in a stereotaxic apparatus using an auxiliary ear bar (Figure 2A). Apply 2% xylocaine jelly to the outer ear for local anesthesia using a swab before the auxiliary ear bar is attached.
-
b.Apply 0.3% ofloxacin gel to both eyes using a swab to protect eyes during the operation.
-
c.Make a skin incision to expose a skull after local anesthesia with 2% xylocaine jelly application.
-
d.Adjust the nose position using an incisor bar until z coordinates of bregma and lambda become the same.
-
e.Drill the skull above the marked sited using a micromotor system with a steel bur. To reduce heat damage of the brain, the drilling should be paused by cooling with saline of 20°C–25°C. Troubleshooting 2
-
a.
Note: Diameter of the drilled hole is typically around 0.5 mm.
-
6.Injection using a Hamilton syringe (Figure 2)
-
a.Attach a 10 μL Hamilton syringe with a stainless-steel needle to a syringe holder connected with a microprocessor-controlled injector.
 CRITICAL: We recommend the use of a 35-gauge beveled needle to reduce brain damage. After the injection, the Hamilton syringe with a needle should be washed 2–3 times with water and then with 70% ethanol.
CRITICAL: We recommend the use of a 35-gauge beveled needle to reduce brain damage. After the injection, the Hamilton syringe with a needle should be washed 2–3 times with water and then with 70% ethanol. -
b.Fill the injection solution into the needle from the tip.
-
c.Move the syringe to the surface of the drilled site.
-
d.Insert the needle into the target depth of the brain and inject the solution continuously.
-
e.Remove the needle.Note: After the injection, we recommend the needle remained in place for at least 5 min before being withdrawn. Volume of the injection solution typically ranges between 100 and 500 nL. Injection is completed within 5 min. The speed of the injection is adjusted to 40–100 nL/min.
-
f.Fill the drilled hole with hemostatic gelatin sponge (e.g., Spongel, see ‘‘key resources table’’) and close the scalp wound.
-
g.Inject atipamezole (0.1 mg/kg) intraperitoneally to reverse the sedative effect of medetomidine.
-
h.Return the mouse to the home cage and house until the electrophysiological or histological experiment is performed.Note: Injection by air pressure can be performed using Picospritzer as described in the recent protocol (Okamoto et al., 2020). When you need to inject into more restricted site or you need to reduce neuronal damages, iontophoretic delivery is available as described below (step 7).
-
a.
-
7.Iontophoretic delivery using a glass pipette (Figure 2)
-
a.Pull borosilicate glass capillaries (1 or 1.5 mm O.D.) using a single pull with a micropipette puller (P1000 or P97, Sutter Instrument).Note: We use a single cycle for pulling an injection pipette using the micropipette puller (P-1000 or P-97, Sutter Instrument) to obtain pipettes with long tapers (e.g., step 1: heat, ramp; pull, 50; velocity, 200; delay, 250; pressure, 500).
-
b.Cut off the tip of a pulled-pipette using scissors (tip diameter, 20–50 μm).
-
c.Draw the injection solution into the pipette tip by capillary action and backfill saline slowly using the 1-mL syringe with a flexible needle. Troubleshooting 3
-
d.Insert an Ag/AgCl wire into the pipette and attach the pipette to the pipette holder.
-
e.Move the pipette to the surface of the drilled site.
-
f.Insert the pipette into the target depth of the brain and apply current with 7-s on/off cycles for 20–30 min using an iontophoresis pump. Troubleshooting 4
 CRITICAL: Current polarity (positive or negative) depends on the reagent and the solvent used (e.g. ddH2O, saline, or 0.1 M PBS). Current intensity depends on the tip diameter or the target region and usually ranges between 0.3–5 μA. To make focal injection, lower current intensity is suitable for injection into the neocortex (Ueta et al., 2014), while larger one is necessary for injection into dense structures such as the thalamus and brainstem (Ueta and Miyata, 2021).
CRITICAL: Current polarity (positive or negative) depends on the reagent and the solvent used (e.g. ddH2O, saline, or 0.1 M PBS). Current intensity depends on the tip diameter or the target region and usually ranges between 0.3–5 μA. To make focal injection, lower current intensity is suitable for injection into the neocortex (Ueta et al., 2014), while larger one is necessary for injection into dense structures such as the thalamus and brainstem (Ueta and Miyata, 2021). -
g.Remove the pipette. Leaving the pipette before withdrawn is not necessary when the diameter of the pipette is 20 μm, because the injection solution does not leak out from such narrow tip (Figure 3).
-
h.Carry out postoperative care as described above.Note: For local depletion of microglia, PLX3397, which inhibits microglial proliferation, or clodronate liposomes, which induces microglial apoptosis, is available (Figure 4). For local activation of microglia, lipopolysaccharides is available (refer to ‘‘key resources table’’) (Ueta and Miyata, 2021). Genetic regulation of local microglia by such as the diphtheria toxin receptor expression system using transgenic mouse lines (Willis and Vukovic, 2020), or virally-introduced DREADD system are also useful (Grace et al., 2018). These transgenic mouse lines or DREADD expressing viral vectors under microglial promoter are commercially available from the Jackson Laboratory or Addgene.
-
a.
Figure 2.
The timing and workflow of stereotaxic injection
Figure 3.
Focal injection using iontophoresis
(A) A retrograde tracer, Alexa Fluor 647-conjugated cholera toxin subunit B (CTB647, 0.2% in ddH2O), is iontophoretically delivered to the dorsal part of the thalamic ventral posteromedial nucleus (VPM). VPM is identified by VGLUT2 immunohistochemistry using a guinea pig anti-VGluT2 antibody and an Alexa Fluor 488-conjugated secondary antibody. Gray-scale images are acquired using an epifluorescence microscope (Axio Scope.A1, Carl Zeiss) equipped with a cooled CCD camera (QSI RS 6.1, Quantum Scientific Imaging) and pseudocolored using Adobe Photoshop software. D, dorsal; L, lateral; VPL, ventral posterolateral nucleus. Scale bar, 0.5 mm.
(B) Retrogradely labeled cells in the ventral part of the principal trigeminal nucleus of the brainstem (Pr5) in the contralateral hemisphere. M, medial. Scale bar, 0.5 mm.
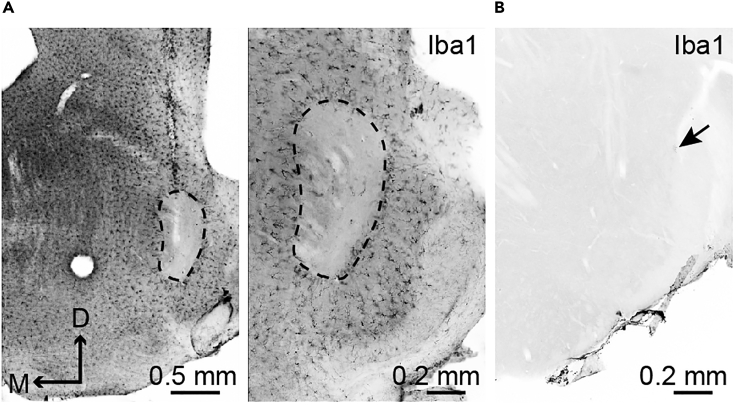
Figure 4.
Local and brain-wide depletion of microglia
(A) Clodronate liposomes, which induce apoptosis of microglia, are locally injected to the brainstem using a Hamilton syringe. No immunoreactivity against a myeloid cell marker Iba1 is found in the injection site. Immunohistochemistry is performed using a rabbit anti-Iba1 antibody and an Alexa Fluor 488-conjugated secondary antibody. Gray-scale images are acquired using the epifluorescence microscope same as in Figure 3 and inverted using Adobe Photoshop software. Scale bar, 0.5 mm (left) or 0.2 mm (right).
(B) Brain-wide microglia are depleted by systemic administration of PLX3397, which inhibits microglial proliferation. An arrow indicates a remaining Iba1-positive cell in the brainstem. Scale bar, 0.2 mm.
Focal injection using iontophoresis is also useful for neuronal tracing from a restricted area (Ueta et al., 2014). For example, we can successfully obtain retrogradely labeled cells in the whisker region in the brainstem, which corresponds to the ventral part of the principal trigeminal nucleus (Pr5), by a small injection (100–200 μm of the diameter) restricted in the whisker thalamus, which corresponds to the dorsal part of the ventral posteromedial nucleus (VPM) (Figure 3). In this protocol, Alexa Fluor 647-conjugated cholera toxin subunit B is used as a retrograde tracer (0.2%, dissolved in 0.1 or 0.05 M PBS) (Figure 3). We have previously utilized Alexa Fluor 555-conjugated cholera toxin subunit B (0.2%) and obtained successful retrograde labeling from the restricted cortical layers (Ueta et al., 2014).
Whole-cell patch-clamp recording using acute brain slices
Timing: ≥2 h
The timing and workflow are depicted in Figure 5.
-
8.Preparation of solutions (refer to ‘‘materials and equipment’’)
-
a.10× aCSF stock solution
-
b.1M CaCl2 solution
-
c.1M MgCl2 solution
-
d.Cutting solution
-
e.Intracellular pipette solution for voltage-clamp or current-clamp recording
-
a.
-
9.Preparation of acute brain slices
-
a.Dilute the 10× aCSF stock solution to 1 L with ddH2O, add 3.6 g glucose (20 mM), and bubble with a mixture of 95% O2 and 5% CO2.
-
b.Add 2 mL of 1M CaCl2 solution (2 mM) and 1 mL of 1M MgCl2 solution (1 mM).
-
c.Transfer 100–150 mL of aCSF to a glass beaker and place it in the water bath adjusted to 32°C. Bubble this aCSF solution continuously.
-
d.Bubble the cutting solution on ice.Note: To improve recovery of neurons, add Na-ascorbate (5 mM), Na-pyruvate (3 mM), and thiourea (2 mM) to both aCSF and the cutting solution. All of Na-ascorbate, Na-pyruvate, and thiourea should be removed from aCSF during recording. Alternatively, add lactic acid (4 mM) instead of Na-ascorbate, Na-pyruvate, and thiourea (Ueta et al., 2019). Lactic acid is also removed from aCSF during recording.
-
e.After a deep anesthesia of a mouse with isoflurane, perfuse 5 mL of the cooled cutting solution transcardially using a 10 mL syringe with a 21-gause syringe needle within 1 min.Note: More details about transcardial perfusion are described in the step 13.
-
f.Decapitate, remove the brain, and soak it into the cutting solution on ice for 1–3 min.
 CRITICAL: Removing the brain and soaking it in the air-bubbled cutting solution after decapitation should be done quickly to avoid neuronal damages or death.
CRITICAL: Removing the brain and soaking it in the air-bubbled cutting solution after decapitation should be done quickly to avoid neuronal damages or death. -
g.Trim the brain on a filter paper using a razor blade or a surgical blade.
-
h.Glue the brain block onto the electrical-taped specimen disk using cyanoacrylate (Aron alpha). Glue a block of gelatin (2–3%) behind the brain block. Place the disk onto the buffer tray with the cutting solution continuously bubbled with 95% O2 and 5% CO2.
-
i.Cut sagittal slices of 250- or 300-μm thickness including the whisker thalamus (VPM) using a vibratome with a razor blade and collect on the holding chamber in the water bath. Cutting speed is basically 0.04 mm/s (VT1200S, Leica Microsystems). Frequency amplitude normally ranges between 0.4 and 0.5 mm (VT1200S). Troubleshooting 5Note: A double edge razor blade is cut in half using tinner’s snips and is attached to blade holder of the vibratome. We can cool the buffer tray with ice or icepacks and apply the sherbet-like chilled cutting solution in the buffer tray. Temperature during slice cutting may affect various physiological properties probably depending on the cell type or the age of animals (e.g., Eguchi et al., 2020). It is important to select appropriate methods depending on the application or the purpose of experiments.
-
j.After cutting, incubate brain slices in the aCSF solution for 30 min at 32°C using a water bath and then for at least 30 min at 20°C–25°C until use.Note: Electrophysiological recording is normally finished within 6–8 h from the slice cutting. To obtain synaptic responses, we recommend to finish recordings within 4–6 h from the decapitation.
-
a.
-
10.Assessment of number of innervated axons by recording of excitatory postsynaptic currents (EPSCs) in individual neurons of the whisker thalamus (Figure 6)
-
a.To block inhibitory postsynaptic currents, add GABAA receptor antagonist bicuculline methochloride (10 μM) and GABAB receptor antagonist CGP55845 hydrochloride (1 μM) to the perfusing aCSF continuously bubbled with 95% O2 and 5% CO2. Temperature of aCSF is maintained at 31°C–32°C using a line heater (Figure 6A).
-
b.Establish a whole-cell patch on a thalamic neuron which is identified using a near-infrared camera (C3077-79, Hamamatsu Photonics)-equipped microscope (BX51WI, Olympus Corp.) with a 60× water-immersion objective (numerical aperture = 0.9, Olympus Corp.).Note: VPM is visually identifiable in the bright-field images (Takeuchi et al., 2012; 2014). VPM in mice consists of glutamatergic thalamocortical neurons and is mostly devoid of local interneurons.
-
c.EPSC is evoked by an electrical stimulation using a concentric electrode placed on the lemniscal fiber bundle.
-
d.The number of EPSC steps corresponds to the number of axons innervating a recorded neuron. For example, a single neuron which receives three axonal inputs expresses three EPSC steps when a stimulus intensity is gradually increased, but a neuron receiving a single axonal input expresses a single EPSC step in an all-or-none fashion (Figure 6B).Note: We use 5 or 6 cycles for pulling a whole-cell patch pipette (4–8 MΩ) using the micropipette puller to obtain pipettes with short tapers (e.g., step 1: heat, ramp; pull, 0; velocity, 19, delay, 1; pressure, 500; step 2: ramp – 5; pull, 0; velocity, 19; delay, 1; steps 3 and 4: heat, ramp – 15; pull, 0; velocity, 19; delay, 1; steps 5 and 6: ramp – 25; pull, 0; velocity, 20; delay, 1). We usually increase or decrease velocity in the last step to correct the tip diameter. The internal solution is kept on ice during recording. A recent detailed protocol for patch-clamp techniques would help your experiments (Manz et al., 2021).
 CRITICAL: Neurons in the mouse whisker thalamus receive glutamatergic excitatory inputs from lemniscal fibers, which originate from the brainstem, and/or from corticothalamic axons. EPSCs evoked by these two different pathways have different EPSC kinetics and short-term plasticity: lemniscal fiber-evoked EPSCs, fast EPSC decay and paired-pulse depression; corticothalamic EPSCs, slower decay of EPSCs and paired-pulse facilitation (Miyata and Imoto, 2006).
CRITICAL: Neurons in the mouse whisker thalamus receive glutamatergic excitatory inputs from lemniscal fibers, which originate from the brainstem, and/or from corticothalamic axons. EPSCs evoked by these two different pathways have different EPSC kinetics and short-term plasticity: lemniscal fiber-evoked EPSCs, fast EPSC decay and paired-pulse depression; corticothalamic EPSCs, slower decay of EPSCs and paired-pulse facilitation (Miyata and Imoto, 2006).
-
a.
-
11.Post hoc visualization of dendritic and axonal morphologies of a recorded neuron.
-
a.After recording, the slices of 250- or 300-μm thickness are collected in a glass vial or a 24-well plate, soaked into the fixative solution, and stored for 12–16 h at 4°C. The fixative solution of 2 mL is enough for a single slice.Note: The slices can be stored in the fixative solution at 4°C for few weeks. It can be possible to store for longer time (e.g., 2–3 months).
-
b.Wash the slices three times with 0.1 M PBS for 10–20 min on a shaker.
-
c.Incubate the slices with fluorescent streptavidin (e.g., Alexa Fluor 488-conjugated streptavidin, 1:500) in 0.1 M PBS with 0.05% Triton X-100 for 2–3 h at 20°C–25°C or 12–16 h at 4°C.
-
d.Wash the slices two times with 0.1 M PBS for 5–10 min and then with 0.1 M PB for 5–10 min to remove NaCl.
-
e.Mount the slices on a gelatin-coated glass slide (refer to ‘‘materials and equipment’’).Note: Use spacers (two sheets of electrical tape, 200 μm thickness for one sheet) when sections are coverslipped not to flatten the slices.
-
f.Seal the sections with antifade mountant and apply a coverslip.Note: After fixation, the sections can be resectioned at a thickness of 40 or 50 μm. Such thinner sections are useful for simultaneous immunohistochemical staining (Nagumo et al., 2020) (Figure 7).
-
a.
Figure 5.
The timing and workflow of preparation of acute brain slices
Figure 6.
A setup for whole-cell patch clamp recording and example experiments
(A) A setup for patch-clamp experiments.
(B) Estimation of innervated axon numbers by excitatory postsynaptic current (EPSC) recording from a thalamic VPM neuron under voltage-clamp.
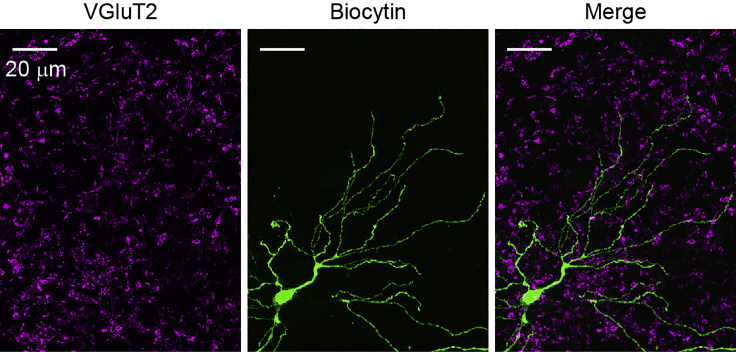
Figure 7.
Post hoc visualization of cell morphology after electrophysiological recording
A VPM neuron is visualized with Alexa Fluor 488-conjugated streptavidin. Immunohistochemistry against vesicular glutamate transporter type 2 (VGluT2) and Alexa Fluor 647-conjugated secondary antibody is simultaneously performed. Gray-scale images are acquired using a fluorescent microscope (BZ-X810, Keyence) and pseudocolored. The slice is resectioned at 40 μm. Scale bar, 20 μm.
Immunohistochemistry of fixed brain tissue
Timing: 2–3 days
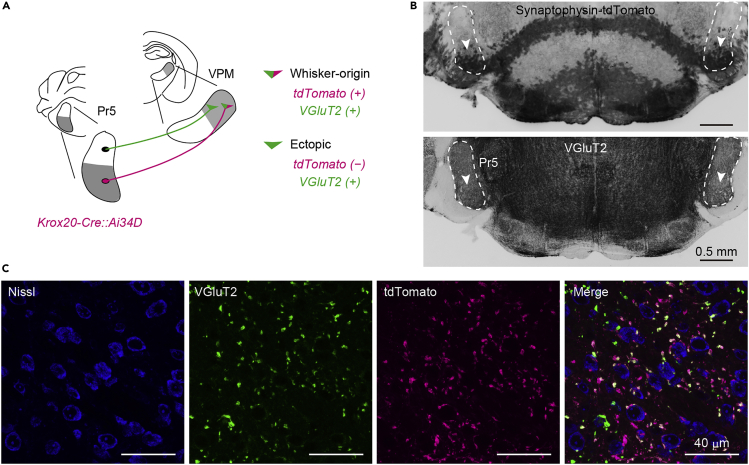
To identify whisker-originated axon terminals in the whisker thalamus, we have crossed a Krox20-Cre mouse and an Ai34D reporter mouse, which expresses synaptophysin-tdTomato under Cre inducing conditions (Figure 8). In the brainstem trigeminal nucleus of Krox20-Cre mice, Cre is expressed in thalamus-projecting principal neurons in the whisker region but not in neurons in non-whisker regions (Bechara et al., 2015; Takeuchi et al., 2014; Voiculescu et al., 2000).
Figure 8.
Schematic illustrating the identification of pathway-specific axon terminals in the thalamus and example sections
(A) Identification of whisker-origin and ectopic (non-whisker-origin) axon terminals in the thalamic VPM using Krox20-Cre mice crossed with Ai34D mice, which express synaptophysin-tdTomato under Cre.
(B) Cre recombinase is expressed in principal neurons in the ventral whisker region of Pr5. Scale bar, 0.5 mm.
(C) Axon terminals in VPM. Gray-scale images are acquired using a confocal laser scanning microscope (LSM710, Carl Zeiss) and pseudocolored. Scale bar, 40 μm.
The timing and workflow are depicted in Figure 9.
-
12.Preparation of solutions (refer to ‘‘materials and equipment’’)
-
a.0.1 M PB and PBS
-
b.0.05 M Tris buffer (TB) and Tris-buffered saline (TBS)
-
c.Prefixative solution
-
d.Paraformaldehyde-based fixative solution
-
e.Agar solution for embedding a brain block
-
f.Antigen retrieval solutions
-
g.10% normal donkey serum solution with 0.3% Triton X-100
-
h.10% or 30% Sucrose solution
-
i.Antifreeze solution
-
a.
-
13.Preparation of fixed brain tissue
-
a.Deeply anesthetize the Krox20-Cre : Ai34D mouse with isoflurane and place it on the shallow tray.
-
b.Make an abdominal incision on the liver and find the xiphoid process.
-
c.Lift up the xiphoid process, expose the pleural cavity by making a lateral incision in the diaphragm, and cut along the lateral wall of the rib cage on both sides until both the heart and the thymus, which is in front of and above the heart, are exposed.Note: To avoid injuring the lung, carefully displace it during cutting the rib cage.
-
d.Make a small incision in the right atrial ear and quickly insert a 21-gauge needle, which connects to the peristaltic pump system (Figure 9), to the left ventricle of the heart.Note: You can insert the needle to the left ventricle first and cut the right atrial ear next.
-
e.Perfuse the fixative solution followed by the 5 mL of the prefixative solution at 20°C–25°C for 10–15 min using a peristaltic pump. Troubleshooting 6Note: The volume of the fixative solution is twice the body weight.
-
f.Brains are usually post-fixed in the fresh fixative for 30 min or 12–16 h at 4°C. After post-fixation, the fixative solution is replaced by 0.1 M PB.
 Pause point: Although the fixed brain can be stored in 0.1 M PB for few weeks at 4°C, we recommend to prepare thin sections within few days from the perfusion.
Pause point: Although the fixed brain can be stored in 0.1 M PB for few weeks at 4°C, we recommend to prepare thin sections within few days from the perfusion.
-
a.
-
14.Preparation of thin sections using a vibratome
-
a.Trim the fixed brain on the brain slicer matrix using a microtome blade.
-
b.Glue the brain block onto the specimen disk using Aron Alpha. Mount the block with agarose gel solution using a mold (20 mL cut syringe). Allow the disk to cool to set the gel in the refrigerator.
-
c.Remove the mounted brain block from the disk after trimming gels and glue onto the specimen disk again.Note: By this step, the brain block embedded in agarose gel is tightly glued onto the disk.
-
d.Set the disk onto the buffer tray with cooled 0.1 M PB.
-
e.Cool the buffer tray with ice or ice packs during cutting using a vibratome with an injector blade. Cutting speed ranges between 0.05–0.2 mm/s (scale 1–4.5 for Leica VT1000S). Frequency is normally set at 40–50 Hz (scale 4–5).
-
f.Collect sections and submerge them into a 24-well plate with 0.1 M PB.Note: To observe both the brainstem and the thalamus, we usually use coronal sections for histological experiments. Section thickness is usually set to 40 or 50 μm for handling and antibody penetration. We use sections of 8–20 μm thickness for analyzing colocalization of different markers or labeling in individual cell somata using single-focus images under epifluorescence microscopy.
 Pause point: Sections may be stored at 4°C for a couple of weeks, but we recommend that sections are immersed in an antifreeze solution after a sucrose-substitution procedure and kept at −30°C for long-term storage. Use a cryostat to obtain thinner sections after a sucrose-substitution procedure (10% sucrose for 4–6 hours to 12–16 h and 30% sucrose for one day or few days at 4°C).
Pause point: Sections may be stored at 4°C for a couple of weeks, but we recommend that sections are immersed in an antifreeze solution after a sucrose-substitution procedure and kept at −30°C for long-term storage. Use a cryostat to obtain thinner sections after a sucrose-substitution procedure (10% sucrose for 4–6 hours to 12–16 h and 30% sucrose for one day or few days at 4°C).
-
a.
-
15.Fluorescent immunohistochemistry on free-floating sections
-
a.Wash the sections three times with 0.1 M PBS for 5–10 min in a 12-well plate on a shaker.
-
b.Incubate the sections with primary antibody (guinea pig polyclonal antibody against vesicular glutamate transporter type2 (VGluT2), 1:500 or 1,000) in 10% normal donkey serum solution with 0.3% Triton X-100 for 12–16 h at 4°C or 2–3 h at 20°C–25°C on an orbital shaker.Note: Use 0.4–0.5 mL of the antibody solutions for a single well in a 12-well plate (less than 6 sections per well). Incubation with primary antibodies is usually carried out for 12–16 h at 4°C.Alternatives: For VGluT2 immunohistochemistry, rabbit polyclonal antibody is also available and working well (refer to “key resources table”).
-
c.Wash the sections three times with 0.1 M PBS for 5–10 min.
-
d.Incubate the sections with secondary antibody (Alexa Fluor 488-conjugated antibody against guinea pig IgG, 1:500) and Fluorescent Nissl (NeuroTrace 435/455, 1:100) in 10% normal donkey serum solution with 0.3% Triton X-100 for 12–16 h at 4°C or 2–3 h at 20°C–25°C on an orbital shaker.Note: Incubation with secondary antibodies is usually carried out in a dark place for 2–3 h at 20°C–25°C.Alternatives: NeuN immunohistochemistry is also useful for visualizing neurons (refer to “key resources table”).
-
e.Wash the sections two times with 0.1 M PBS for 5–10 min and then with 0.1 M PB for 5–10 min.
-
f.Mount the sections on a gelatin-coated glass slide.
-
g.Seal the sections with antifade mountant and apply a coverslip. Troubleshooting 7
-
h.Acquire images using an epifluorescent microscope or by confocal microscopy. Troubleshooting 8Note: Use spacers (Vinyl repair patch, 70 μm thickness) when sections are coverslipped for confocal microscopic observation.
-
a.
Figure 9.
The timing and workflow of immunohistochemistry using fixed brain sections
When we need to visualize myeloid cells including microglia, we use anti-Iba1 antibody for immunohistochemistry (refer to “key resources table”) (Figure 4). Because anti-Iba1 antibody detects diverse type of myeloid cells, we recommend to use anti-TMEM119 antibody for detecting microglia specifically (refer to “key resources table”) (Ueta and Miyata, 2021).
Alternatives: When we only need to acquire lower magnification images (e.g., 2×, 4×, or 5×), we use an aqueous mountant without antifade reagents (e.g., Fluoromount-G).
Expected outcomes
Infraorbital nerve cut (IONC) induces microglial accumulation in the whisker region of the brainstem but not in the thalamus (Ueta and Miyata, 2021). Using stereotaxic injections, we can manipulate local microglial activity as well as neuronal activity (for genetic approaches, refer Willis and Vukovic, 2020). Systemic microglial depletion using oral administration of microglial inhibitor is also commercially available and successful (Elmore et al., 2014; Ueta and Miyata, 2021). Combined these local and systemic regulation of microglia, the understanding of the role of microglia in various developmental and pathophysiological conditions will be progressed.
To understand the mechanisms for synaptic remodeling, it is important to know both structural and physiological characteristics of synapses. A whole-cell patch-clamp recording from an identified single neuron in acute brain slices has some limitations described below, but is still useful for detailed analyses of synaptic properties including kinetics and structure; for example, we can estimate the number of innervated axons by counting EPSC steps. In addition, dendritic and axonal morphologies can be reconstructed by biocytin or neurobiotin filling during recording and post hoc visualization (Ueta et al., 2019; Ueta and Miyata, 2021). Combined electrophysiological and anatomical techniques, together with utilization of region-specific Cre mice, are advantageous strategy to label and manipulate specific circuits or projection pathways.
Limitations
Depletion or activation effect of microglia by using a single focal injection will not continue for a long-time period. We have observed that microglia are still depleted at the injection site after 5–6 days from a single injection of clodronate liposomes (Ueta and Miyata, 2021). Genetic approaches using microglia-targeted recombinant viral infection probably overcome this limitation. In addition, genetic approaches enable targeting different microglial population with activation of state-specific gene expression (Friedman et al., 2018; Hammond et al., 2019). Repopulation of microglia is another important issue when microglia are systemically or locally depleted, because repopulated microglia have both neurotoxic and neuroprotective effects (Rubino et al., 2018; Willis et al., 2020), and also because the rate of microglial repopulation depends on the extent of depletion (Najafi et al., 2018). Long-term effects of microglial manipulation will be highlighted in future studies.
It is difficult to know whether individual axonal input originates from the whisker region or non-whisker regions in electrophysiological recording. However, using Cre-off viral injection into Krox20-Cre mice combined with direct recording from an identified presynaptic terminal, we have recently enabled the pathway-specific measurement of electrophysiological properties in acute slices (Midorikawa and Miyata, 2021).
Troubleshooting
Problem 1
Bleeding occurs intraoperatively (step 2).
Potential solution
Add one drop of saline or 2% xylocaine with 1:80,000 adrenaline using a 1-mL syringe with a 26- or 27-gauge needle to stop bleeding.
Problem 2
Bleeding occurs during drilling (step 5).
Potential solution
Place an absorbent cotton piece wet with warmed saline for a few minutes to stop bleeding. Rapid vibrations of the hand motor will cause bleeding from the skull. To minimize such vibrations, the hand motor can be controlled by a micromanipulator with a drill holder (e.g., Cat#SD-102, Narishige).
Problem 3
No capillary action (step 7).
Potential solution
The tip diameter is too narrow (probably smaller than 20 μm). Cut off the tip using scissors or gently break the tip using forceps.
Problem 4
No current flows (step 7).
Potential solution
It is probably due to an air bubble in the pipette. Tap the pipette gently not to mix the injection solution and backfilled saline to avoid reducing concentration of the injection solution. In our experiments, injection solution is filled within the tapering part of the glass capillary. Normally, an Ag/AgCl wire is incapable of inserting into the thin tapering part. So, backfilled saline is needed as the electric carrier. Alternatively, the injection solution can be backfilled. In that case, collect the solution after finishing the injection and use it in the next experiments.
Problem 5
Vibratome fails to accurately and repeatedly slice sections (step 9).
Potential solution
The brain block is not tightly glued onto specimen disk. We recommend to cut at low cutting speed (around 0.05 mm/s) after the brain block is tightly glued on the disk again. If that doesn’t work, prepare thicker sections (e.g., 100 μm).
Problem 6
Uneven whole-brain fixation due to insufficient perfusion (step 13).
Potential solution
When the solution is released from the nose and/or the mouth during perfusion, it does not flow to the brain. This is probably due to clogged or ruptured blood vessels. To avoid this, remove air bubbles from a silicone tube connecting to a needle before inserting it into the left ventricle. We recommend to use a blunt-end needle, which can be made from a syringe needle by using diagonal and needle-nose pliers for example.
Problem 7
Undesirable air bubbles are formed under the coverslip (step 15).
Potential solution
After drying sections, place a generous amount (0.2–0.3 mL mountant for one slide) of mountant on the one side of the glass slide. The use of insufficiently small amount of mountant will result in air bubble formation. Hold one edge of a coverslip with fine tweezers (curved one is better) and place the other edge of the coverslip on the droplet. Slowly lower the tweezers together with the coverslip until the tip of the tweezers is close to the surface of the glass slide. Then, slowly pull out the tweezers from under the coverslip. Absorb excess mountant using a twisted paper (e.g., KimWipes). Finally, seal the coverslip with nail polish.
Problem 8
The signal is weak or the background of an acquired image is high (step 15).
Potential solution
There may be several causes. We recommend to perform antigen retrieval before antibody penetration or use TBS as a buffer instead of PBS (refer to ‘‘materials and equipment’’), because PBS is known to inhibit some enzymatic actions. Tyramine-based signal enhancement method will be helpful as described in a recent protocol (Okamoto et al., 2020).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Yoshifumi Ueta (yueta@twmu.ac.jp).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate any unique data sets or code.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 16K18995, 21K06444 (to Y.U.), 16H01344, 17H05752, 19H03343, and 20H05916 (to M.M.). This work was also supported by the Narishige Neuroscience Research Foundation, Japan, the Nakatomi Foundation, Japan, and TWMU Promotion Fund for Basic Research, Japan (to Y.U.). We thank Sachie Sekino for assistance with stereotaxic injections, immunohistochemistry, and maintaining animals; Yumi Tani for assistance with genotyping and maintaining animals; Akiko Tamaki for support; Institute of Laboratory Animals (ILA) of Tokyo Women’s Medical University for help with animal care and management; and Medical Research Institute (MRI) of Tokyo Women’s Medical University for maintaining and managing confocal microscopy, respectively.
Author contributions
Conceptualization, methodology, writing – original draft, writing – review & editing, and funding acquisition, Y.U. and M.M.; investigation, Y.U.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Yoshifumi Ueta, Email: yueta@twmu.ac.jp.
Mariko Miyata, Email: mmiyata@twmu.ac.jp.
References
- Bechara A., Laumonnerie C., Vilain N., Kratochwil C.F., Cankovic V., Maiorano N.A., Kirschmann M.A., Ducret S., Rijli F.M. Hoxa2 selects barrelette neuron identity and connectivity in the mouse somatosensory brainstem. Cell Rep. 2015;13:783–797. doi: 10.1016/j.celrep.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Eguchi K., Velicky P., Hollergschwandtner E., Itakura M., Fukazawa Y., Danzl J.G., Shigemoto R. Advantages of acute brain slices prepared at physiological temperature in the characterization of synaptic functions. Front. Cell. Neurosci. 2020;14:63. doi: 10.3389/fncel.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore M.R., Najafi A.R., Koike M.A., Dagher N.N., Spangenberg E.E., Rice R.A., Kitazawa M., Matusow B., Nguyen H., West B.L. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B.A., Srinivasan K., Ayalon G., Meilandt W.J., Lin H., Huntley M.A., Cao Y., Lee S.H., Haddick P.C.G., Ngu H. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of alzheimer's disease not evident in mouse models. Cell Rep. 2018;22:832–847. doi: 10.1016/j.celrep.2017.12.066. [DOI] [PubMed] [Google Scholar]
- Gentet L.J., Ulrich D. Strong, reliable and precise synaptic connections between thalamic relay cells and neurones of the nucleus reticularis in juvenile rats. J. Physiol. 2003;546:801–811. doi: 10.1113/jphysiol.2002.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace P.M., Wang X., Strand K.A., Baratta M.V., Zhang Y., Galer E.L., Yin H., Maier S.F., Watkins L.R. DREADDed microglia in pain: Implications for spinal inflammatory signaling in male rats. Exp. Neurol. 2018;304:125–131. doi: 10.1016/j.expneurol.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond T.R., Dufort C., Dissing-Olesen L., Giera S., Young A., Wysoker A., Walker A.J., Gergits F., Segel M., Nemesh J. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253–271. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz K.M., Siemann J.K., McMahon D.G., Grueter B.A. Patch-clamp and multi-electrode array electrophysiological analysis in acute mouse brain slices. STAR Protoc. 2021;2:100442. doi: 10.1016/j.xpro.2021.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midorikawa M., Miyata M. Distinct functional developments of surviving and eliminated presynaptic terminals. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2022423118. e2022423118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata M., Imoto K. Different composition of glutamate receptors in corticothalamic and lemniscal synaptic responses and their roles in the firing responses of ventrobasal thalamic neurons in juvenile mice. J. Physiol. 2006;575:161–174. doi: 10.1113/jphysiol.2006.114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagumo Y., Ueta Y., Nakayama H., Osaki H., Takeuchi Y., Uesaka N., Kano M., Miyata M. Tonic GABAergic inhibition is essential for nerve injury-induced afferent remodeling in the somatosensory thalamus and ectopic sensations. Cell Rep. 2020;31:107797. doi: 10.1016/j.celrep.2020.107797. [DOI] [PubMed] [Google Scholar]
- Najafi A.R., Crapser J., Jiang S., Ng W., Mortazavi A., West B.L., Green K.N. A limited capacity for microglial repopulation in the adult brain. Glia. 2018;66:2385–2396. doi: 10.1002/glia.23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Yamauchi K., Sohn J., Takahashi M., Ishida Y., Furuta T., Koike M., Fujiyama F., Hioki H. Exclusive labeling of direct and indirect pathway neurons in the mouse neostriatum by an adeno-associated virus vector with Cre/lox system. STAR Protoc. 2020;2:100230. doi: 10.1016/j.xpro.2020.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.S., Silver R.A. NeuroMatic: an integrated open-source software toolkit for acquisition, analysis and simulation of electrophysiological data. Front. Neuroinform. 2018;12:14. doi: 10.3389/fninf.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino S.J., Mayo L., Wimmer I., Siedler V., Brunner F., Hametner S., Madi A., Lanser A., Moreira T., Donnelly D. Acute microglia ablation induces neurodegeneration in the somatosensory system. Nat. Commun. 2018;9:4578. doi: 10.1038/s41467-018-05929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Asano H., Katayama Y., Muragaki Y., Imoto K., Miyata M. Large-scale somatotopic refinement via functional synapse elimination in the sensory thalamus of developing mice. J. Neurosci. 2014;34:1258–1270. doi: 10.1523/JNEUROSCI.3865-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Osaki H., Yagasaki Y., Katayama Y., Miyata M. Afferent fiber remodeling in the somatosensory thalamus of mice as a neural basis of somatotopic reorganization in the brain and ectopic mechanical hypersensitivity after peripheral sensory nerve injury. eNeuro. 2017;4 doi: 10.1523/ENEURO.0345-16.2017. ENEURO.0345-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Yamasaki M., Nagumo Y., Imoto K., Watanabe M., Miyata M. Rewiring of afferent fibers in the somatosensory thalamus of mice caused by peripheral sensory nerve transection. J. Neurosci. 2012;32:6917–6930. doi: 10.1523/JNEUROSCI.5008-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta Y., Miyata M. Brainstem local microglia induce whisker map plasticity in the thalamus after peripheral nerve injury. Cell Rep. 2021;34:108823. doi: 10.1016/j.celrep.2021.108823. [DOI] [PubMed] [Google Scholar]
- Ueta Y., Otsuka T., Morishima M., Ushimaru M., Kawaguchi Y. Multiple layer 5 pyramidal cell subtypes relay cortical feedback from secondary to primary motor areas in rats. Cereb. Cortex. 2014;24:2362–2376. doi: 10.1093/cercor/bht088. [DOI] [PubMed] [Google Scholar]
- Ueta Y., Sohn J., Agahari F.A., Im S., Hirai Y., Miyata M., Kawaguchi Y. Ipsi- and contralateral corticocortical projection-dependent subcircuits in layer 2 of the rat frontal cortex. J. Neurophysiol. 2019;122:1461–1472. doi: 10.1152/jn.00333.2019. [DOI] [PubMed] [Google Scholar]
- Voiculescu O., Charnay P., Schneider-Maunoury S. Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones, and peripheral nervous system. Genesis. 2000;26:123–126. doi: 10.1002/(sici)1526-968x(200002)26:2<123::aid-gene7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Willis E.F., MacDonald K.P.A., Nguyen Q.H., Garrido A.L., Gillespie E.R., Harley S.B.R., Bartlett P.F., Schroder W.A., Yates A.G., Anthony D.C. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell. 2020;180:833–846. doi: 10.1016/j.cell.2020.02.013. [DOI] [PubMed] [Google Scholar]
- Willis E.F., Vukovic J. Protocol for brain-wide or region-specific microglia depletion and repopulation in adult mice. STAR Protoc. 2020;1:100211. doi: 10.1016/j.xpro.2020.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unique data sets or code.