Abstract
Background
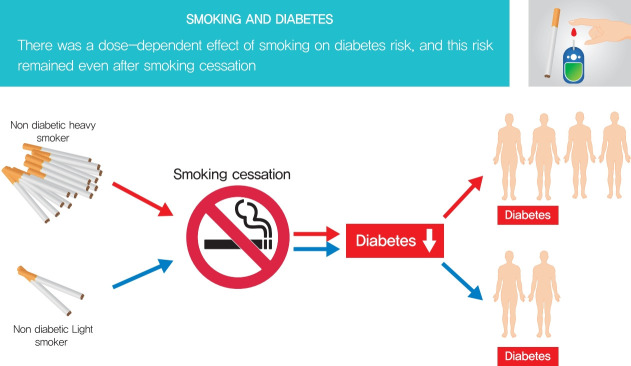
This study aimed to evaluate the dose-dependent effects of smoking on risk of diabetes among those quitting smoking.
Methods
We analyzed clinical data from a total of 5,198,792 individuals age 20 years or older who received health care check-up arranged by the national insurance program of Korea between 2009 and 2016 using the Korean National Health Insurance Service database. Cumulative smoking was estimated by pack-years. Smokers were classified into four categories according to the amount of smoking: light smokers (0.025 to 5 smoking pack-years), medium smokers (5 to 14 smoking pack-years), heavy smokers (14 to 26 smoking pack-years), and extreme smokers (more than 26 smoking pack-years).
Results
During the study period, 164,335 individuals (3.2% of the total population) developed diabetes. Compared to sustained smokers, the risk of diabetes was significantly reduced in both quitters (hazard ratio [HR], 0.858; 95% confidence interval [CI], 0.838 to 0.878) and nonsmokers (HR, 0.616; 95% CI, 0.606 to 0.625) after adjustment for multiple risk factors. The risk of diabetes gradually increased with amount of smoking in both quitters and current smokers. The risk of diabetes in heavy (HR, 1.119; 95% CI, 1.057 to 1.185) and extreme smokers (HR, 1.348; 95% CI, 1.275 to 1.425) among quitters was much higher compared to light smokers among current smokers.
Conclusion
Smoking cessation was effective in reducing the risk of diabetes regardless of weight change. However, there was a potential dose-dependent association between smoking amount and the development of diabetes. Diabetes risk still remained in heavy and extreme smokers even after smoking cessation.
Keywords: Diabetes, Risk factors, Smoking, Smoking cessation, Weight
Graphical abstract
INTRODUCTION
Smoking is well established as a causal risk factor for major chronic diseases and lower life expectancy [1]. Although many environmental and genetic factor are known to be the cause of diabetes [2] active smoking also has been recognized as a risk factor for type 2 diabetes mellitus (T2DM) [3]. The hypothesis that smoking increases the risk for incident T2DM is plausible due to the general inflammation caused by smoking, along with increased insulin resistance and greater abdominal obesity in smokers [4]. Although smoking cessation is known to significantly reduce the risk of chronic disease in both Asian and Western populations, subsequent weight gain has been recognized as one of the minor disadvantages and concerns among smokers who may attempt to quit smoking. T2DM is strongly related to weight gain in adult life and accumulation of excess fat within major organs [5,6].
Recent studies suggest that weight gain after smoking cessation might cause a temporary increase in the risk of T2DM. However, it is not clear whether the severity of weight gain after quitting smoking is similar between Asians and non-Asians [7], and only a few studies have addressed the issue of weight gain following smoking cessation in an Asian population [8,9]. Furthermore, there is not enough evidence to evaluate correlations between the amount of smoking and risk of diabetes after smoking cessation. With longitudinal, repeated assessments of smoking status and body weight in a large cohort study of men and women, the current investigation aimed to evaluate the risk trajectories of diabetes among those who reported quitting smoking, according to body weight changes after smoking cessation. We also evaluated the association between risk of diabetes and cumulative smoking in pack-years as measures of smoking exposure after quitting smoking.
METHODS
Study population and source of data
We evaluated data from the records of 5,198,792 Korean residents aged 20 years or older who had undergone one biennial medical evaluation provided by the National Health Insurance Service (NHIS) between January 1, 2005 and December 31, 2016. Enrollees in the National Health Insurance Corporation are recommended to undergo standardized medical examination every 2 years, so we used 2-year windows to define screening program participation. The Health Insurance Review and Assessment database includes data on approximately 7.0% of the Korean population’s health insurance claims; details of the NHIS database have been described elsewhere [10]. The database is open to any researchers whose study protocols are approved by the official review committee.
The median follow-up period was 4.87 years. Medical examinations in 2009–2010 and 2015–2016 included measurements of height, weight, blood pressure, and laboratory tests such as fasting glucose and cholesterol, serum creatinine, liver enzymes, and urinalysis (Supplementary Fig. 1). Past medical history and health-related behaviors such as life style factors and smoking, alcohol intake, and physical activity were collected using standardized self-reporting questionnaires.
Quality control procedures for laboratory tests were performed in accordance with the Korean Association of Laboratory Quality Control. These databases and the aforementioned nationwide medical records were combined and analyzed to construct a cohort for investigating health problems, after the NHIS approved the use of its database for the research (research number NHIS-2019-1-151). Our study protocol was approved by the official review committee and the Institutional Review Board of the Korea National Institute for Bioethics Policy (P01-201603-21-005) and informed consent was waived because of the anonymous nature of the data. This study was carried out according to the ethical principles of the Declaration of Helsinki.
Definition
The primary outcome was development of diabetes between 1 January 2009 and 31 December 2016 in each participant. We excluded people previously diagnosed diabetes. For diagnosis of diabetes, we additionally used anti-diabetic drug descriptions with codes for insulins, sulfonylureas, metformin, meglitinides, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, and α-glucosidase inhibitors along with the 10th International Statistical Classification of Diseases (ICD-10) codes E11–14 [11]. Dyslipidemia was defined as total cholesterol level ≥240 mg/dL (≥6.21 mmol/L) or presence of one or more claims per year for anti‐hyperlipidemic medications with ICD‐10 code E78. The diagnosis of hypertension was confirmed using laboratory data (systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg) or ICD-10 code (I10–15) with a claim for anti-hypertensive medication.
Data on socioeconomic characteristics such as income level, and lifestyle factors, including smoking, alcohol drinking, and exercise, were obtained using standardized questionnaires. Low income level was defined as being in the lower one‐fifth of the whole population. Heavy alcohol drinking was defined as drinking equal to or more than 30 g/day. Exercise (regular) was defined as mid-term exercise ≥5 days or vigorous exercise ≥3 days in a week. At each health check-up period, the participants responded to a self-reported health survey questionnaire and participated in a physical examination.
Assessment of changes in smoking behavior and weight
Participants in the NHIS biennial health check-up were grouped into current smokers, quitters, and nonsmokers based on responses to the self-reported survey between baseline at 2005–2006 and health check-up from 2009–2010. We identified participants who had reported that they were smokers in 2005–2006 but were not smokers in 2009–2010 as quitters. Current smokers and quitters were further classified by smoking amount. Cumulative smoking was measured using packyears, where 1 pack-year equaled smoking 20 cigarettes smoked per day during 1 year. Smokers were classified according to current smoking habits into four categories: light smokers (0.025 to 5 smoking pack-years); medium smokers (5 to 14 smoking pack-years); heavy smokers (14 to 26 smoking packyears); and extreme smokers (more than 26 smoking packyears) [12].
Weight change was calculated as the difference in body weight between 2009–2010 and 2015–2016, corresponding to the period between the first and last health check-up. Based on the study performed by Corrada et al. [13], we defined the weight stable group as weight change within 5%, and we categorized weight change into three groups by 5% increase or decrease as follows: weight loss ≥5%; weight change <5%; weight gain ≥5%.
Statistical analysis
The general characteristics of subjects are expressed as mean±standard deviation for continuous variables and number (percentage) for categorical variables, according to smoking status. Continuous variables were evaluated using analysis of variance (ANOVA), and categorical variables were evaluated using chisquare tests.
The hazard ratios (HRs) using the Cox proportional hazards model with a 95% confidence interval (CI) for risk of diabetes according to smoking status and smoking amount in packs per day were analyzed by multivariable Cox proportional hazard models, after adjusting for age and sex in model 1; age, sex, alcohol drinking, income, exercise, body mass index (BMI), hypertension, and dyslipidemia in model 2; and age, sex, alcohol drinking, income, exercise, BMI, hypertension, dyslipidemia, and weight change in model 3. Subgroup analyses were performed by sex, age group, hypertension, dyslipidemia, regular exercise, weight change according to smoking status and amount. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA), and P<0.05 for two tailed tests was considered statistically significant.
RESULTS
Baseline characteristics
Participant baseline characteristics are shown in Table 1. The participants were divided based on smoking status as follows: nonsmokers, 3,734,732 (71.8%); quitters, 252,661 (4.9%); and current smokers, 1,211,399 (23.3%). The proportion of men was higher among current smokers and quitters compared with nonsmokers. The risk of diabetes was slightly increased in quitters and current smokers compared with nonsmokers. Quitters and current smokers had a lower risk of hypertension and dyslipidemia, higher waist circumference, higher fasting glucose level, and higher systolic blood pressure compared with nonsmokers. The prevalence of hypertension and dyslipidemia, BMI, waist circumference, and fasting glucose level were all higher among quitters than among current smokers.
Table 1.
Participant baseline characteristics according to smoking status
| Characteristic | Nonsmokers (n=3,734,732) | Quitters (n=252,661) | Current smoker (n=1,211,399) | P value |
|---|---|---|---|---|
| Sex | <0.0001 | |||
| Male | 971,103 (26) | 245,020 (96.98) | 1,179,838 (97.39) | |
| Female | 2,763,629 (74) | 7,641 (3.02) | 31,561 (2.61) | |
| Amount of smoking | <0.0001 | |||
| Mean±SD | - | 17.07±14.72 | 17.19±12.49 | |
| Light smoker (0.025–5 smoking pack-years) | - | 35,314 (13.98) | 100,463 (8.29) | |
| Medium smoker (5–14 smoking paack-years) | - | 90,036 (35.64) | 455,872 (37.63) | |
| Heavy smokers (14–26 smoking pack-years) | - | 76,669 (30.34) | 427,226 (35.27) | |
| Extreme smokers (more than 26 smoking pack-years) | - | 50,642 (20.04) | 227,838 (18.81) | |
| Age group, yr | 50.64±13.38 | 46.32±11.89 | 44.14±11.5 | <0.0001 |
| 20–39 | 856,729 (22.94) | 85,642 (33.9) | 505,637 (41.74) | |
| 40–64 | 2,261,763 (60.56) | 144,964 (57.37) | 626,837 (51.74) | |
| ≥65 | 616,240 (16.5) | 22,055 (8.73) | 78,925 (6.52) | |
| Alcohol drinking (heavy) | 70,792 (1.9) | 35,165 (13.92) | 205,777 (16.99) | <0.0001 |
| Income (low) | 632,423 (16.93) | 27,696 (10.96) | 139,403 (11.51) | <0.0001 |
| Exercise (regular) | 695,790 (18.63) | 63,060 (24.96) | 212,562 (17.55) | <0.0001 |
| Hypertension prevalence | 939,834 (25.16) | 64,417 (25.5) | 246,788 (20.37) | <0.0001 |
| Dyslipidemia prevalence | 723,005 (19.36) | 49,047 (19.41) | 183,332 (15.13) | <0.0001 |
| Weight, kg | 59.72±10.06 | 70.86±10.29 | 69.6±10.91 | <0.0001 |
| BMI, kg/m2 | 23.39±3.08 | 24.44±2.9 | 23.93±3.1 | <0.0001 |
| Waist circumference, cm | 78.02±8.75 | 84.18±7.48 | 82.9±7.77 | <0.0001 |
| Fasting glucose level, mg/dL | 92.24±10.88 | 94.46±11.51 | 93.27±11.72 | <0.0001 |
| Systolic blood pressure, mm Hg | 120.91±15.07 | 124.26±13.47 | 123.32±13.44 | <0.0001 |
| Diastolic blood pressure, mm Hg | 75.16±9.88 | 78.08±9.47 | 77.62±9.44 | <0.0001 |
| Total cholesterol level, mg/dL | 197±36.25 | 199.19±35.92 | 196.8±35.41 | <0.0001 |
Values are presented as number (%) or mean±standard deviation.
SD, standard deviation; BMI, body mass index.
Risk of diabetes according to baseline smoking status and intensity of smoking
After a median follow-up period of 4.87 years, 164,335 individuals (3.2% of the total population) developed diabetes. Diabetes incidence over time according to smoking status is shown in Table 2. Current smokers served as the reference, and the age, sex-adjusted HR (95% CI) showed that the risk of diabetes was lower in quitters and nonsmokers (HR, 0.922; 95% CI, 0.901 to 0.943) (HR, 0.619; 95% CI, 0.61 to 0.628). The multivariate models that considered age, sex, alcohol, income, exercise, BMI, hypertension, dyslipidemia, and weight change also showed a similarly lower risk of diabetes for quitters and nonsmokers (HR, 0.858; 95% CI, 0.838 to 0.878) (HR, 0.616; 95% CI, 0.606 to 0.625). Nonsmokers had the lowest risk of diabetes according to smoking status among all group (Table 2).
Table 2.
Risk of diabetes according to baseline smoking status by sex and age group
| Smoking status | No. of subjects | Diabetes | HR (95% Cl) |
||
|---|---|---|---|---|---|
| Model | Model 2 | Model 3 | |||
| Nonsmokers | 3,734,732 | 114,250 | 0.619 (0.61–0.628) | 0.616 (0.606–0.625) | 0.616 (0.606–0.625) |
| Quitters | 252,661 | 8,826 | 0.922 (0.901–0.943) | 0.828 (0.809–0.847) | 0.858 (0.838–0.878) |
| Current smokers | 1,211,399 | 41,259 | 1 (reference) | 1 (reference) | 1 (reference) |
Model 1: adjusted for age and sex; Model 2: adjusted for age, sex, alcohol drinking (heavy), income (low), exercise (regular), body mass index, hypertension, and dyslipidemia; Model 3: adjusted for age, sex, alcohol drinking (heavy), income (low), exercise (regular), body mass index, hypertension, dyslipidemia, and weight change.
HR, hazard ratio; CI, confidence interval.
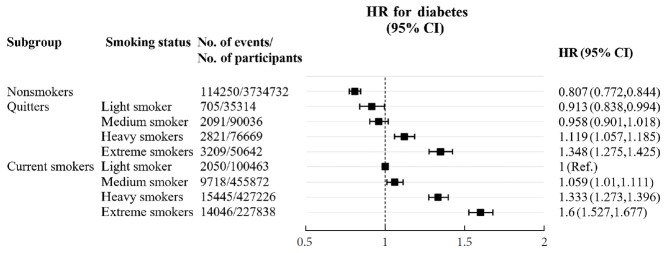
Fig. 1 presents the risk of diabetes based on cumulative smoking by packs per year (Supplementary Table 1). The risk of diabetes was lowest in the nonsmoker group, and gradually increased with amount of smoking in both quitters and current smokers. Quitters who had been heavy and extreme smokers showed a higher risk of diabetes compared with current smokers with light smoking.
Fig. 1.
Risk of diabetes according to intensity of smoking in packs per day. HR, hazard ratio; CI, confidence interval.
In subgroup analyses, the result of smoking with respect to the risk of diabetes tended to be similar across subgroups. Weight gain did not modify the associations between smoking status and risk of T2DM (Fig. 2, Supplementary Table 2). The overall effects of cumulative smoking on risk estimates for diabetes were also consistent across the categories for subgroup analyses (Supplementary Table 3).
Fig. 2.
Smoking status and risk of diabetes by subgroup. HR, hazard ratio; CI, confidence interval.
DISCUSSION
We found that smoking cessation was associated with a reduced risk of diabetes regardless of post-cessation weight changes, based on a nationally representative database linked to clinical records of more than 5,000,000 Koreans. Further, we identified dose-dependent associations between cumulative smoking by pack-years and the risk of diabetes.
An association between smoking cessation and the risk of T2DM has been controversial. Previous studies showed increased risk of diabetes after smoking cessation, suggesting that weight gain after quitting smoking might affect the development of diabetes. Considerable weight gain may occur in quitters after smoking cessation, probably due to increased appetite and reduced energy expenditure [14]. It was reported that adults who quit smoking experienced relatively more adverse changes in their metabolic profile and an increased risk for incident diabetes, peaking within 3 years of quitting, but still observable 6 years after quitting [15]. Another study also observed that the peak of risk of T2DM was observed among quitters with the most weight gain at 5 to 7 years of cessation [16]. However, our study showed that those who sustained smoking cessation over 6 years did not increase the risk of diabetes. Although weight gain is a well-known risk factor for diabetes, our data also showed that subsequent weight gain did not attenuate the apparent benefits of smoking cessation on diabetes within 6 years. Consistent with our data, Jee et al. [17] also found that the risks for diabetes were higher in current smokers than in quitters in Koreans. We observed a beneficial role for smoking cessation in potentially reducing the disease burden of diabetes despite the subsequent weight change. The discrepancies resulting from such studies may be due to different participant characteristics and ethnicities, sample size, or measurement methods.
When smoking exposure was measured in pack-years, both current smokers and quitters carried an increased risk of diabetes based on the amount smoked after adjustment for other risk factors, including weight change. Although quitters still had a lower risk compared to current smokers, the risk of diabetes in heavy and extreme smokers among quitters was much higher compared to light smokers among current smokers. It is of note that the harmful effect of smoking on the development of diabetes seems to be dependent on the amount of smoking and remained in quitters with >14 pack-years even after smoking cessation. Only a few studies have examined the effect of cumulative smoking on the risk of diabetes [15-17]. Previous studies usually focused on a dose-response relationship between smoking amount in current smokers and diabetes risk. Our study expanded on previous findings by showing a dose-dependent effect of smoking on diabetes risk even after smoking cessation.
The exact mechanism by which smoking increases the risk of diabetes and deteriorates glucose homeostasis has not been fully elucidated, but a few biological mechanisms associated with post-cessation changes in health status and diabetes may explain our study findings. Cigarette smoking can lead to insulin resistance [18]. Smoking reduces insulin-mediated glucose uptake by 10% to 40% in men who smoke compared with non-smoking men [19]. Additionally, several studies have shown that current cigarette smokers have lower β-cell function than never smokers, suggesting that smoking may impair measures of β-cell function [20,21].
This study had some limitations. First, we only analyzed one time smoking cessation and could not confirm the exact starting point and duration of smoking cessation. Quitters are likely to relapse [18], and their subsequent disease risk may vary according to the duration of cessation and cumulative smoking. Second, there was a possibility of bias in the confirmation of characteristics through self-questionnaires and diagnosis of outcome through claim of insurance. Also, the smoking amount assessed by self questionnaries, and some smoking amount could have been missed depend on memory. Third, we were not able to examine the exact reason for smoking cessation among quitters. There is a possibility that those who decided to quit smoking had already experienced worsening health conditions due to smoking, which may have affected study outcomes. Fourth, we also recognized the heterogeneity of sex ratio. However, according to other studies, the portion of smoker was higher in men [22]. Thus, our study also reflects this trend. Last, our study only included Asians (Korean) and results may not be generalizable to other countries or races.
Despite limitations, our results provide important evidence of the beneficial role of smoking cessation in potentially reducing the disease burden of diabetes, despite subsequent weight change. Our findings also suggest that there was a dose-dependent effect of smoking on diabetes risk, and this risk remained even after smoking cessation. Smoking should be recognized as a modifiable risk factor in diabetes prevention or screening strategies.
Acknowledgments
We would like to thank the Korean National Health Insurance Corporation and all the participants of the study and health check-up.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: S.E.P., M.H.S., E.J.R., W.Y.L.
Acquisition, analysis, or interpretation of data: K.D.H., J.H.J.
Drafting the work or revising: S.E.P., M.H.S., J.H.C., H.K.,
Y.H.K., Y.G.P., E.J.R., W.Y.L.
Final approval of the manuscript: S.E.P., M.H.S., E.J.R., W.Y.L.
FUNDING
None
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0061.
Risk of diabetes according to intensity of smoking in packs per day
Smoking status and risk of diabetes by subgroup
Adjusted HR for incident diabetes according to quitting smoking and smoking intensity
Study design.
REFERENCES
- 1.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-Century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 2.Kwak SH, Park KS. Pathophysiology of type 2 diabetes in Koreans. Endocrinol Metab (Seoul) 2018;33:9–16. doi: 10.3803/EnM.2018.33.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 4.Berlin I. Smoking-induced metabolic disorders: a review. Diabetes Metab. 2008;34(4 Pt 1):307–14. doi: 10.1016/j.diabet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia. 2008;51:1781–9. doi: 10.1007/s00125-008-1116-7. [DOI] [PubMed] [Google Scholar]
- 6.Rhee EJ. Diabetes in Asians. Endocrinol Metab (Seoul) 2015;30:263–9. doi: 10.3803/EnM.2015.30.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim EH, Lee H, Shin DW, Yun JM, Shin JH, Lim YK, et al. Association between weight changes after smoking cessation and cardiovascular disease among the Korean population. Korean J Fam Med. 2017;38:122–9. doi: 10.4082/kjfm.2017.38.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura U, Tanaka T, Okamura T, Kadowaki T, Yamato H, Tanaka H, et al. Changes in weight, cardiovascular risk factors and estimated risk of coronary heart disease following smoking cessation in Japanese male workers: HIPOP-OHP study. J Atheroscler Thromb. 2010;17:12–20. doi: 10.5551/jat.1800. [DOI] [PubMed] [Google Scholar]
- 10.Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YH, Han K, Ko SH, Ko KS, Lee KU, Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association Data analytic process of a nationwide population-based study using national health information database established by National Health Insurance Service. Diabetes Metab J. 2016;40:79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G, Wang H, Wang K, Wang W, Dong F, Qian Y, et al. The association between smoking and blood pressure in men: a cross-sectional study. BMC Public Health. 2017;17:797. doi: 10.1186/s12889-017-4802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–49. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–9. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 15.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152:10–7. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Zong G, Liu G, Wang M, Rosner B, Pan A, et al. Smoking cessation, weight change, type 2 diabetes, and mortality. N Engl J Med. 2018;379:623–32. doi: 10.1056/NEJMoa1803626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jee SH, Foong AW, Hur NW, Samet JM. Smoking and risk for diabetes incidence and mortality in Korean men and women. Diabetes Care. 2010;33:2567–72. doi: 10.2337/dc10-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong C, Nimmo L, Elatrozy T, Anyaoku V, Hughes C, Robinson S, et al. Smoking is associated with increased hepatic lipase activity, insulin resistance, dyslipidaemia and early atherosclerosis in type 2 diabetes. Atherosclerosis. 2001;156:373–8. doi: 10.1016/s0021-9150(00)00664-x. [DOI] [PubMed] [Google Scholar]
- 19.Chang SA. Smoking and type 2 diabetes mellitus. Diabetes Metab J. 2012;36:399–403. doi: 10.4093/dmj.2012.36.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morimoto A, Tatsumi Y, Deura K, Mizuno S, Ohno Y, Watanabe S. Impact of cigarette smoking on impaired insulin secretion and insulin resistance in Japanese men: The Saku Study. J Diabetes Investig. 2013;4:274–80. doi: 10.1111/jdi.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostgren CJ, Lindblad U, Ranstam J, Melander A, Rastam L, Skaraborg Hypertension and Diabetes Project Associations between smoking and beta-cell function in a non-hypertensive and non-diabetic population. Skaraborg Hypertension and Diabetes Project. Diabet Med. 2000;17:445–50. doi: 10.1046/j.1464-5491.2000.00294.x. [DOI] [PubMed] [Google Scholar]
- 22.Shin DY, Jang YK, Lee JH, Wee JH, Chun DH. Relationship with smoking and dyslipidemia in Korean adults. J Korean Soc Res Nicotine Tob. 2017;8:73–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of diabetes according to intensity of smoking in packs per day
Smoking status and risk of diabetes by subgroup
Adjusted HR for incident diabetes according to quitting smoking and smoking intensity
Study design.