Abstract
Introduction
Medulloblastoma (MDB) often causes signs and symptoms of elevated intracranial pressure (ICP) with imaging findings of mass lesion. Here, we report a case of MDB who initially presented with clinical features imitating idiopathic intracranial hypertension (IIH) and Chiari I malformation (CIM).
Case presentation
A 19-year-old man had clinical symptoms of elevated ICP without mass lesion on imaging. He was initially diagnosed with IIH and CIM, which underwent shunt surgery and posterior fossa decompression. Later on, he had recurrent symptoms, and the new imaging revealed the development of MDB in the right cerebellar hemisphere. After tumor resection, the patient rapidly deteriorated with spinal metastases.
Discussion and conclusion
Management of the coexistence between IIH and CIM in patients with rising ICP is complicated. MDB is one of the aggressive malignant brain tumors showing a wide range of imaging features, including non-enhancing mass. Therefore, recognizing the possibility of brain tumors mimicking IIH or CIM is crucial.
Abbreviations: CIM, Chiari I malformation; CSF, Cerebrospinal fluid; ICP, Intracranial pressure; IIH, Idiopathic intracranial hypertension; MDB, Medulloblastoma; MRI, Magnetic resonance imaging; PFD, Posterior fossa decompression
Keywords: Medulloblastoma, Idiopathic intracranial hypertension, Chiari
Highlights
-
•
The coexistence of idiopathic intracranial hypertension (IIH) and Chiari I malformation (CIM) is complicated.
-
•
Medulloblastoma (MDB) can present with a wide range of imaging features, including non-enhancing mass.
-
•
Exclusion of the secondary cause of rising intracranial pressure is necessary before making the diagnosis of IIH or CIM
-
•
Early diagnosis, including subtype of MDB, and urgent treatment are crucial for better outcome.
1. Introduction
Medulloblastoma (MDB) is one of the primary malignant brain tumors that commonly occur in the cerebellar vermis [1]. The tumor generally arises in the midline and proliferates, compressing surrounding neurovascular structures and occupying the fourth ventricle causing obstructive hydrocephalus. As a result, most patients with MDB have clinical manifestations from mass effect and elevated intracranial pressure (ICP) with a mass on the neuroimaging. Despite the standard treatment protocol, including surgical resection, radiation therapy, and chemotherapy, approximately 30% of patients with MDB will have metastasis disease relapse [2], [3]. In addition, group 3 and 4 MDBS are virulent subtypes, have high rates of metastasis at the diagnosis, and carry poor prognoses.
Here, we presented a case of MDB in evolution, who was initially characterized with the coexisting features of idiopathic intracranial hypertension (IIH) and Chiari I malformation (CIM) [4], complicating diagnosis and treatment. The tumor was not identified at the first presentation, and the patient has received the treatment to control ICP. The tumor was present with rapid spinal metastasis during the follow-up despite tumor resection.
This case report has been prepared according to the SCARE guideline 2020 [5].
2. Case presentation
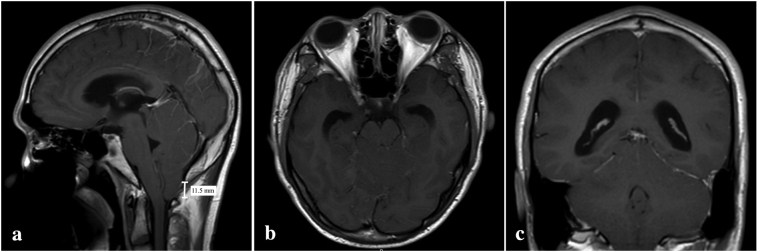
A 19-year-old man presented with a history of progressive suboccipital headache for one year without neurological deficit. He came to the hospital due to worsening of his headache for one month. Neurological examinations were unremarkable except of papilledema. His brain magnetic resonance imaging (MRI) revealed an 11.5 mm descent of cerebellar tonsil to the level of the posterior arch of C1 without mass or other abnormal lesions (Fig. 1a-c). Additional investigation, including computed tomography cerebral venography and cerebrospinal fluid (CSF) study, was performed to exclude secondary cause of intracranial hypertension [6]. CSF profiles were in normal ranges, and there was no malignant cell in the cytological study Therefore, the diagnosis of IIH or CIM was suspected in which endoscopic third ventriculostomy was attempted by RN. During the surgery, ICP was measured at 30 cm H2O. However, the procedure failed to control elevated ICP; therefore, a ventriculoperitoneal shunt was subsequently performed five days later.
Fig. 1.
Post gadolinium T1-weighted magnetic resonance imaging showing the decent of cerebellar tonsil (a) without abnormal mass lesion in axial (b) and coronal views (c).
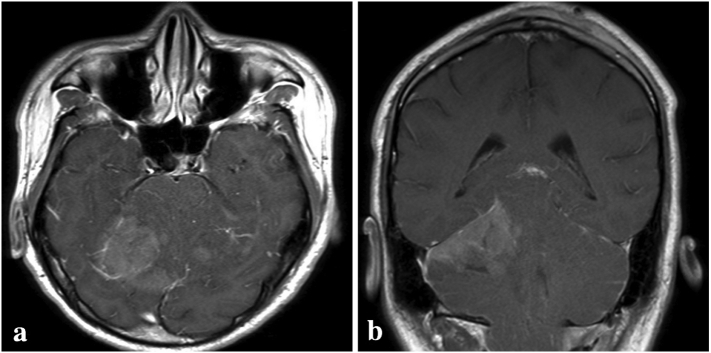
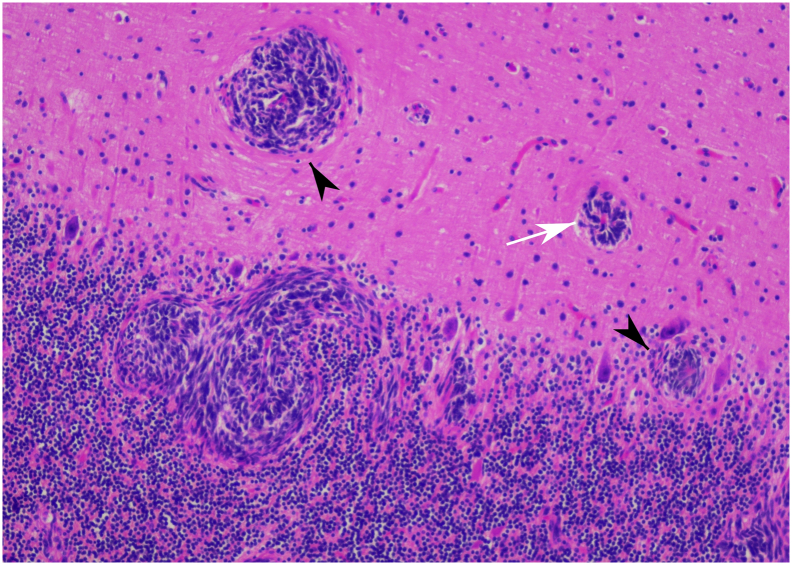
At the two-week follow-up, the headache did not improve. Therefore, we considered the diagnosis of CIM, and posterior fossa decompression (PFD) was then performed. After the surgery, the symptoms were resolved. Two months later, he revisited the hospital due to severe dizziness and blurred vision. The brain MRI revealed leptomeningeal enhancement of the whole cerebellum with ill-defined enhancing mass lesion about 3 × 3 × 2.5 cm at right cerebellar hemisphere causing fourth ventricular and dorsal brainstem compression (Fig. 2a-b). The decision was made to perform the right suboccipital craniectomy with tumor removal. His right cerebellum was markedly swollen during the operation, and a purplish soft intra-axial tumor at the right cerebellar hemisphere, which adhered to the tentorium cerebelli, was identified. The tumor was totally removed and was sent for pathology. According to the pathology report, the tumor was compatible with classic MDB (World Health Organization 2016 grade IV) with high Ki-67 at 80% (Fig. 3) [7]. Besides, the tumor was positive with YAP-1, GAB-1, p75-NGFR, and OTX-2, whereas it was nuclear negative beta-catenin. Therefore, the genetically defined diagnosis based on immunohistochemistry was group 4 MDBs [8].
Fig. 2.
Subsequent axial (a) and coronal views (b) of post gadolinium T1-weighted magnetic resonance imaging showing ill-defined enhancing mass at the superior part of right cerebellar hemisphere.
Fig. 3.
Histologic examination showing small round tumor cells with salt-and-pepper like chromatin and Homer Wright appearance (white arrow) invading cerebellar tissue (arrowhead).
During hospitalization, the patient initially recovered well, and adjuvant therapy was planned. However, he developed sudden quadriparesis two weeks later. The MRI of the whole spine revealed multiple extra- and intramedullary metastases throughout the whole spine (Fig. 4). After discussion with his family, they refused adjuvant treatment and decided to receive palliative care at home.
Fig. 4.
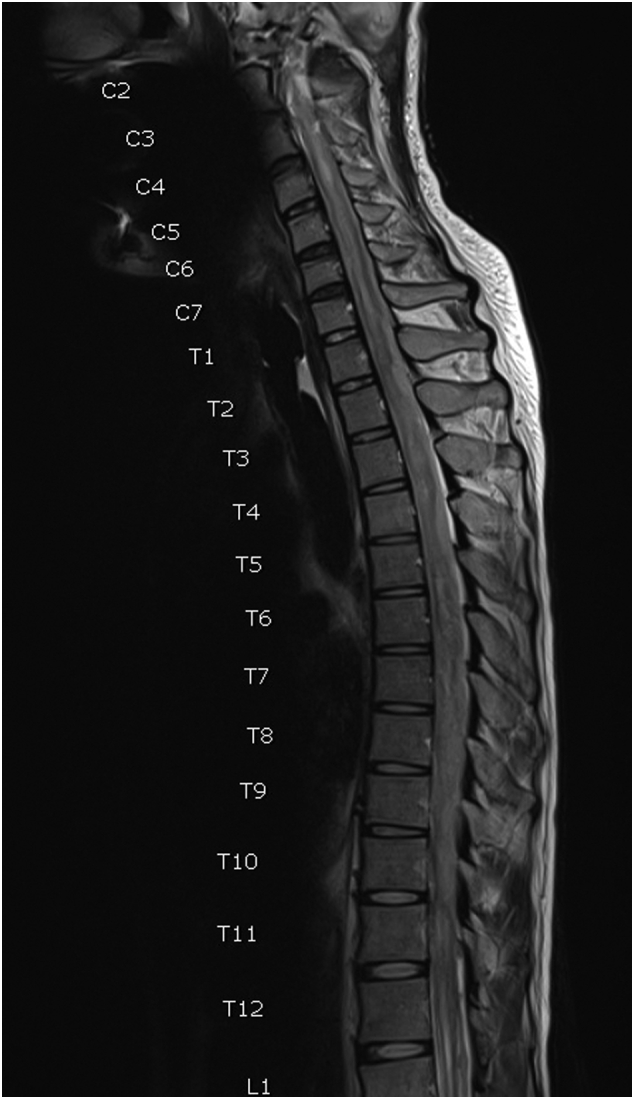
T2-weighted magnetic resonance imaging of the spine showing multiple drop metastases throughout the whole spine.
3. Discussion
IIH is diagnosed with the clinical presentation of elevated ICP without a detectable cause [9]. Several imaging features, including optic disc edema, tortuosity of the optic nerve, empty sella, and cerebellar tonsillar ectopia, may support the diagnosis of IIH [6], [10]. However, in some cases, a clinical diagnosis is uncertain, especially when present with tonsillar ectopia, which is an overlapping feature between IIH and CIM. The coexistence of IIH and CIM has been described in previous studies, but the treatment for a patient in this setting is controversial [11], [12], [13]. Although the surgical options, consisting of shunt surgery and PFD, may improve patients with this coexistence, selection of the first surgical treatment is difficult, and the treatment outcome is unpredictable. Patients often required subsequent surgery after shunt or PFD as an initial treatment because of suboptimal response [4], [14].
Despite the overlapping features of IIH and CIM in our patient, our goal was to overcome the high ICP; therefore, shunt surgery was first performed, followed by PFD. The patient improved temporally and then deteriorated due to newly arising of MDB, which was not identifiable in the first imaging.
MDB is a malignant brain tumor of the posterior fossa, accounting for 18% of all pediatric brain tumors and less than 1% in adults [1]. Theoretically, it originates from the precursors of cerebellar granule neurons forming the cerebellum's external granular layer, which can be found in the roof of the fourth ventricle. Therefore, the tumor is commonly located in the midline. However, some MDBs arise in the cerebellar hemisphere, especially in the adult population [15]. The tumor is highly proliferated, causing mass effect and increased ICP resulting in clinical symptoms including headache, vomiting, visual deterioration, papilledema, and cerebellum or brainstem dysfunction.
Recently, MDB molecular subgroups were proposed, including WNT, SHH, Group 3, and Group 4 MDBs [7]. These subtypes of MDB have distinct characteristics: differences in clinical features, genetic expression, and prognosis. With this molecular classification, the understanding of MDB has been significantly improved, resulting in the development of specific treatments for MDB.
MRI is a mainstay for the diagnosis of medulloblastoma. Typically, the tumor appears as an intra-axial mass with hypo or isointensity on T1-weighted images, whereas it appears hypo, iso, or hyperintensity on T2-weighted images. Most MDBs demonstrate homogeneous or heterogeneous gadolinium enhancement. However, minimal or patchy enhancement pattern can be found in some classic MDBs enhancement. In addition, the molecular subgroup of MDB may be predicted by assessing the location and enhancement pattern of the tumor [16]. WNT and SHH subgroups are likely off-midline enhancing lesions, mostly found in the cerebellopontine angle and cerebellar hemisphere, respectively. In contrast, group 3 and group 4 MDBs are found predominantly in midline location with minimal or no enhancement.
In this patient, the immunohistochemistry was performed and demonstrated subtype of the tumor as Group 4 MDB. This subtype is male predominant and commonly found in young adults. In addition, the majority of Group 4 MDBs demonstrate minimal patchy enhancing lesion on the MRI, exhibit classic histology, and frequently present with metastases at the time of diagnosis; therefore, the Group 4 MDBs carry intermediate to poor prognosis [17].
4. Conclusion
Awareness of the secondary cause of increased ICP is crucial. Despite neuroimaging, MDB is one of the brain tumors showing a wide range of imaging features, including non-enhancing mass; therefore, clinicians should be cautious, especially before establishing the diagnosis of IIH or CIM.
Ethical approval
We declare that our institution does not require ethical approval of clinical case reports and that the study conforms to the ethical regulations of the declaration of Helsinki 1975 (revised, current version).
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
RN: study concept, design, and manuscript editing. NM: data collection, manuscript writing. RN and CT: manuscript revision. All authors: manuscript reviewing and approval.
Guarantor
Dr. Raywat Noiphithak.
Research registration
This study was registered at Thai Clinical Trials Registry. The registration identification number is TCTR20210525004.
Declaration of competing interest
There are no conflicts of interest.
Acknowledgments
Acknowledgement
The authors of this study would like to thank the patient and his family for consenting to the publications. We also thank Dr. Kosakon Uafua, an attending neurosurgeon at Sakaeo Crown Prince Hospital, who is involved in the patient's management.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Millard N.E., De Braganca K.C. Medulloblastoma. J. Child Neurol. 2016;31:1341–1353. doi: 10.1177/0883073815600866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill R.M., Richardson S., Schwalbe E.C., Hicks D., Lindsey J.C., Crosier S., Rafiee G., Grabovska Y., Wharton S.B., Jacques T.S., Michalski A., Joshi A., Pizer B., Williamson D., Bailey S., Clifford S.C. Time, pattern, and outcome of medulloblastoma relapse and their association with tumour biology at diagnosis and therapy: a multicentre cohort study. Lancet Child Adolesc. Health. 2020;4:865–874. doi: 10.1016/S2352-4642(20)30246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer R.J., Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch. Neurol. 2008;65:1419. doi: 10.1001/archneur.65.11.1419. [DOI] [PubMed] [Google Scholar]
- 4.Bejjani G.K. Association of the adult chiari malformation and idiopathic intracranial hypertension: more than a coincidence. Med. Hypotheses. 2003;60:859–863. doi: 10.1016/s0306-9877(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 5.SCARE Group. Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Sivasankar R., Pant R., Indrajit I.K., Negi R.S., Sahu S., Hashim P., D’Souza J. Imaging and interventions in idiopathic intracranial hypertension: a pictorial essay. Indian J. Radiol. Imaging. 2015;25:439–444. doi: 10.4103/0971-3026.169464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 8.Shuangshoti S., Tadadontip P., Techavichit P., Thorner P.S., Shuangshoti S., Teerapakpinyo C. Simplified molecular subtyping of medulloblastoma for reduced cost and improved turnaround time. Appl. Immunohistochem. Mol. Morphol. 2020;28:538–543. doi: 10.1097/PAI.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 9.R. D Idiopathic intracranial hypertension: review of clinical syndrome, imaging findings, and treatment. Curr. Probl. Diagn. Radiol. 2020;49 doi: 10.1067/j.cpradiol.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Batur Caglayan H.Z., Ucar M., Hasanreisoglu M., Nazliel B., Tokgoz N. Magnetic resonance imaging of idiopathic intracranial hypertension: before and after treatment. J. Neuroophthalmol. 2019;39:324–329. doi: 10.1097/WNO.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 11.Aiken A.H., Hoots J.A., Saindane A.M., Hudgins P.A. Incidence of Cerebellar tonsillar ectopia in idiopathic intracranial hypertension: a mimic of the Chiari I malformation. Am. J. Neuroradiol. 2012;33:1901–1906. doi: 10.3174/ajnr.A3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurschel S., Maier R., Gellner V., Eder H.G. Chiari I malformation and intra-cranial hypertension:a case-based review. Childs Nerv. Syst. 2007;23:901–905. doi: 10.1007/s00381-007-0355-0. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair N., Assaad N., Johnston I. Pseudotumour cerebri occurring in association with the Chiari malformation. J. Clin. Neurosci. 2002;9:99–101. doi: 10.1054/jocn.2000.0947. [DOI] [PubMed] [Google Scholar]
- 14.Fagan L.H., Ferguson S., Yassari R., Frim D.M. The Chiari pseudotumor cerebri syndrome: symptom recurrence after decompressive surgery for Chiari malformation type I. Pediatr. Neurosurg. 2006;42:14–19. doi: 10.1159/000089504. [DOI] [PubMed] [Google Scholar]
- 15.Noiphithak R., Yindeedej V., Thamwongskul C. Cerebellopontine angle medulloblastoma with extensive nodularity in a child: case report and review of the literature. Childs Nerv. Syst. 2017;33:839–842. doi: 10.1007/s00381-016-3325-6. [DOI] [PubMed] [Google Scholar]
- 16.Teo W.-Y., Shen J., Su J.M.F., Yu A., Wang J., Chow W.-Y., Li X., Jones J., Dauser R., Whitehead W., Adesina A.M., Chintagumpala M., Man T.-K., Lau C.C. Implications of tumor location on subtypes of medulloblastoma. Pediatr. Blood Cancer. 2013;60:1408–1410. doi: 10.1002/pbc.24511. [DOI] [PubMed] [Google Scholar]
- 17.Taylor M.D., Northcott P.A., Korshunov A., Remke M., Cho Y.-J., Clifford S.C., Eberhart C.G., Parsons D.W., Rutkowski S., Gajjar A., Ellison D.W., Lichter P., Gilbertson R.J., Pomeroy S.L., Kool M., Pfister S.M. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]