Abstract
Background
The coronavirus disease-19 (COVID-19) is characterized with intense inflammatory response, cardiac involvement, and coagulopathy. Fibrinogen, as a biomarker for inflammation, cardiovascular disease, and coagulation, has not been fully investigated yet. The aim of this study was to assess the clinical application of fibrinogen in COVID-19 patients.
Methods
We retrospectively analyzed the demographic and laboratory characteristics of 119 COVID-19 patients in the University of Alabama of Birmingham Medical Center. Correlations of fibrinogen on admission with intensive care unit (ICU) admission, disease severity, and laboratory parameters were analyzed.
Results
Among the 119 COVID-19 patients, 77.3% (92/119) had severe disease, and 59.5% (71/119) patients were admitted to the ICU. Elevated fibrinogen was detected in 67.2% (80/119) of the patients. Fibrinogen levels were significantly associated with inflammatory markers and disease severity, but not with cardiac injury biomarker high sensitivity troponin I. Patients with severe disease had increased fibrinogen levels upon admission compared to patients with non-severe disease (P = 0.001). Fibrinogen level at 528.0 mg/dl was the optimal cutoff to predict disease severity, with a sensitivity and specificity of 66.7% and 70.3% (area undty -60er the curve [AUC] 0.72, P = 0.0006).
Conclusions
Fibrinogen is commonly elevated in COVID-19 patients, especially in those with severe disease. Elevated fibrinogen correlates with excessive inflammation, disease severity, and ICU admission in COVID-19 patients.
Keywords: fibrinogen, coronavirus disease 2019 (COVID-19), inflammation, cardiovascular disease, coagulation
Introduction
Coronavirus disease-19 (COVID-19) caused by 2019-nCoV/SARS-CoV-2 has led to a global pandemic (Zhou et al., 2020b). While respiratory illness is the major cause of morbidity and mortality in the COVID-19 patients, coagulopathy (Wang et al., 2020; Zhou et al., 2020a), systemic inflammation (Inciardi et al., 2020) and myocardial injury (Aghagoli et al., 2020) are also involved in the pathogenesis of the disease. Elevated prothrombin (PT), activated partial thromboplastin time (aPTT) and D-dimer have been reported in COVID-19 patients (Chen et al., 2020b; Wang et al., 2020; Zhou et al., 2020a). During the disease progression, activation of the host defense system results in an imbalance between the procoagulant and anticoagulant systems (Liu et al., 2021). This leads to thrombi formation that is termed as immunothrombosis (Key et al., 1990; Engelmann and Massberg, 2013). D-dimer, as a fibrin degradation product, has been widely reported to be elevated in COVID-19 patients (Rostami and Mansouritorghabeh, 2020), though without standard reporting (Favaloro and Thachil, 2020; Thachil et al., 2020). However, fibrinogen as a clotting factor in coagulation and thrombosis, has not been well studied. In addition, fibrinogen acts as a positive acute phase reactants, which is increased during inflammatory response (Engelmann and Massberg, 2013). Furthermore, fibrinogen has been considered as an independent risk factor for cardiovascular disease (Ernst, 1990; Heinrich and Assmann, 1995; Sweetnam et al., 1996; Ahmed et al., 2012). It has been reported that fibrinogen levels are increased in COVID-19 patients (Arachchillage and Laffan, 2020). Additionally, autopsy studies of COVID-19 patients demonstrated the fibrin rich thrombi in small vessels and pulmonary capillaries (Fox et al., 2020). However, the overall profile of fibrinogen in COVID-19 patients has remained unclear. By analyzing the correlation of fibrinogen with disease severity, and laboratory markers, the goal of this study was to investigate the role of fibrinogen in the pathogenesis of COVID-19 and evaluate the role of fibrinogen as a biomarker for disease severity.
Methods
Study Participants and Data Collection
This study included patients with confirmed COVID-19 admitted to the University of Alabama at Birmingham (UAB) Medical Center (Alabama, United States) from March 19, 2020 to June 2, 2020 with fibrinogen tested on initial presentation. The institutional review board at UAB approved this study. The diagnosis was based on World Health Organization guidance and confirmed by 2019-nCoV/SARS-CoV-2 real-time reverse transcription polymerase chain reaction (RT-PCR) assay of nasopharyngeal or oropharyngeal swab samples in the clinical lab of UAB Medical Center (WHO, 2020, May 27). Patients’ demographic, clinical and laboratory data were retrospectively collected from the electronic health records. Clinically, severity of the COVID-19 patients was classified into mild, moderate, severe and critical disease according to the Clinical management of COVID-19 by World Health Organization (WHO, 2020, May 27). Patients were divided into non-severe (mild or moderate) and severe (severe or critical) groups. Severe disease was defined with clinical signs of pneumonia plus one of the following: respiratory rate >30 breaths/min, severe respiratory distress, SpO2 < 90% on room air. Laboratory parameters including complete blood count (CBC) with differentiation, coagulation profile, liver function, renal function, cardiac markers and inflammatory markers were obtained at initial diagnosis. Fibrinogen level was tested using immunoturbidimetric assay with a reference range of 220.0-498.0 mg/dl. Demographic information, clinical characteristics, laboratory parameters and outcomes were compared between patients with elevated and normal fibrinogen to evaluate the role of fibrinogen in COVID-19 patients.
Statistical Analysis
All statistical analyses were performed with SPSS 26 and GraphPad Prism 8. Normally distributed continuous data were expressed as mean ± standard deviation (SD) and Student’s t-test was performed for data analysis. For data that were not normally distributed, values were expressed as median and interquartile range (IQR) and Mann-Whitney U test was performed to determine the differences between two groups. Categorical variables were expressed as number and percentage and compared by Fisher’s exact test. To assess the predictive value of fibrinogen for disease severity and ICU admission, receiver operating characteristic (ROC) analysis was conducted with the area under the ROC curve (AUC), sensitivity and specificity. The P values of < 0.05 and < 0.01 were considered to be statistically significant and highly significant, respectively.
Results
Baseline Clinical Characteristics and Laboratory Findings of the Study Population
During the study period, there were 552 laboratory-confirmed COVID-19 patients in UAB medical center. Among these cases, 119 patients with fibrinogen results on admission were enrolled in our study. The demographic, clinical characteristics, symptoms, and vital sign of the patients are shown in Table 1. The median age of patients was 62 years old (IQR, 50-70 years old). Approximately 56% (67/119) of the patients were male. Most of the patients had at least one comorbidity, with hypertension [70.6% (84/119)] and diabetes mellitus [44.5% (53/119)] were the most frequent ones. The most common presenting symptoms on initial diagnosis were shortness of breath [60.5% (72/119)], fever [37.0% (44/119)], cough (26.1% [31/119]). There were 77.3% (92/119) patients meeting criteria for severe disease and 59.5% (71/119) admitted to ICU.
Table 1.
Comparison of baseline characteristics and outcome between COVID-19 patients with elevated and normal fibrinogen.
| Variable | Total (n=119) | Fibrinogen (≥489.0mg/dl, n=80) | Fibrinogen (<489.0mg/dl, n=39) | P Value |
|---|---|---|---|---|
| Age (IQR), y | 62.0 (50.0-70.0) | 62.0 (53.3-70.0) | 61.0 (42.0-71.0) | 0.301a |
| Male, n (%) | 67 (56.3) | 47 (58.8) | 20 (51.3) | 0.555 |
| Race | ||||
| African American, n (%) | 73 (61.3) | 54 (67.5) | 19 (48.7) | 0.07 |
| White American, n (%) | 38 (31.9) | 20 (25.0) | 18 (46.2) | 0.035 |
| Others, n (%) | 5 (4.2) | 3 (3.8) | 2 (5.1) | 0.662 |
| Unknown, n (%) | 2 (1.7) | 2 (2.5) | 0 (0) | 1 |
| Comorbidities | ||||
| Hypertension, n (%) | 84 (70.6) | 61 (76.3) | 23 (59.0) | 0.058 |
| Diabetes mellitus, n (%) | 53 (44.5) | 42 (52.5) | 11 (28.2) | 0.018 |
| Coronary artery disease, n (%) | 12 (10.1) | 7 (8.8) | 5 (12.8) | 0.525 |
| Chronic renal failure, n (%) | 11 (9.2) | 7 (8.8) | 4 (10.3) | 0.749 |
| Chronic obstructive pulmonary disease, n (%) | 14 (11.8) | 8 (10.0) | 6 (15.4) | 0.383 |
| History of malignancy, n (%) | 18 (15.1) | 14 (17.5) | 4 (10.3) | 0.416 |
| Symptoms | ||||
| Shortness of breath, n (%) | 72 (60.5) | 54 (67.5) | 18 (48.7) | 0.03 |
| Fever, n (%) | 44 (37.0) | 32 (40.0) | 12 (30.8) | 0.419 |
| Cough, n (%) | 31 (26.1) | 23 (28.8) | 8 (20.5) | 0.381 |
| Fatigue, n (%) | 18 (15.2) | 15 (18.8) | 3 (7.7) | 0.172 |
| Nausea, n (%) | 4 (3.3) | 2 (2.5) | 2 (5.1) | 0.596 |
| Diarrhea, n (%) | 9 (7.6) | 8 (10.0) | 1 (2.6) | 0.268 |
| Body ache, n (%) | 4 (3.4) | 2 (2.5) | 2 (5.1) | 0.596 |
| Chest pain, n (%) | 6 (5.0) | 5 (6.3) | 1 (2.6) | 0.662 |
| Lack of appetite, n (%) | 6 (5.0) | 6 (7.5) | 0 (0) | 0.176 |
| Vital signs | ||||
| Temperature (°C) | 37.2 (36.6-38.1) | 37.2 (36.7-38.3) | 36.9 (36.6-38.0) | 0.206a |
| Respiratory rate (breaths/min) | 20.0 (17.0-27.0) | 20.0 (18.0-28.0) | 18.0 (16.0-25.5) | 0.067a |
| Blood pressure (mmHg) | ||||
| Systolic | 128.2 ± 24.7 | 129.2 ± 25.4 | 126.1 ± 23.4 | 0.549b |
| Diastolic | 73.2 ± 15.0 | 72.4 ± 14.4 | 75.0 ± 16.2 | 0.419b |
| Heart rate (beats/min) | 93 (80-107) | 92.0 (80.0-103.0) | 95.5 (77.0-110.3) | 0.347a |
| Oxygen saturation (%) | 96 (93-98.5) | 95.0 (90.0-98.0) | 97.5 (94.8-99.0) | 0.003a |
| Oxygen treatment | ||||
| Room air, n (%) | 22 (18.5) | 10 (12.5) | 12 (30.8) | 0.023 |
| Nasal cannula, n (%) | 31 (26.1) | 19 (23.8) | 12 (30.8) | 0.505 |
| Ventilation, n (%) | 66 (55.5) | 51 (63.8) | 15 (38.5) | 0.011 |
| ICU admission | 71 (59.7) | 55 (68.8) | 16 (41.0) | 0.005 |
| Severe disease, n (%) | 92 (77.3) | 67 (83.8) | 25 (64.1) | 0.021 |
| Outcomes | ||||
| Discharge alive, n (%) | 78 (65.5) | 50 (62.6) | 28 (71.8) | 0.412 |
| Death, n (%) | 27 (22.7) | 20 (25.0) | 7 (17.9) | 0.344 |
| Still hospitalized, n (%) | 14 (11.8) | 10 (12.5) | 4 (10.3) | 1 |
ICU admission: intensive care unit admission. aMann-Whitney U test; bStudent’s t-test; all other analyses were performed using Chi square test.
The bold values indicate P values < 0.05.
Patients’ laboratory findings on initial diagnosis of COVID-19 are shown in Table 2. CBC and differential showed that patients had decreases lymphocyte counts [0.9 (0.6-1.2) ×109/L]. Coagulopathy was developed in patients with prolonged prothrombin time (PT) [14.7 (13.5-16.1) sec], elevated fibrinogen (613.0 ± 223.0 mg/dL) and D-dimer (1053.0 [583.0-2382.0] ng/mL). Patients also showed impaired liver function and renal function with elevated aspartate aminotransferase (AST) [41.0 (29.0-68.0) U/L], lactase dehydrogenase (LDH) [391.5 (292.3-610.3) U/L], blood urea nitrogen (BUN) [23.0 (12.5-31.5) mg/dL], and creatinine [1.4 (0.8-2.2) mg/dL]. Elevated glucose [138.0 (107.3-192.8) mg/dL] was also seen in our study. Cardiac involvement was demonstrated with increased B-type natriuretic peptide (BNP) [122.0(51.0-303.0) pg/mL] and high sensitivity troponin-I (hsTnI) [22.0 (9.0-65.5) ng/L]. While the negative acute phase reactant albumin was decreased (3.1 ± 0.7 mg/dL), the positive acute phase reactants showed increased level with increased C-reactive protein (CRP) (188.3 ± 110.5 mg/L), erythrocyte sedimentation rate (ESR) (55.7 ± 32.0 mm/h), ferritin [454.0 (140.0-1620.0) ng/mL], and procalcitonin (PCT) [0.5 (0.2-1.8) ng/mL].
Table 2.
Comparison of laboratory parameters between COVID-19 patients with elevated and normal fibrinogen.
| Laboratory parameters | Total (n=119) | Fibrinogen (≥489.0mg/dL, n=80) | Fibrinogen (<489.0mg/dL, n=39) | P Value |
|---|---|---|---|---|
| White blood cell (4.0-11.0×109/L) | 7.7 (5.5-11.1) | 8.4 (6.1-12.2) | 7.4 (3.8-9.3) | 0.314 |
| Neutrophil (1.7-7.0×109/L) | 6.2 (4.1-9.6) | 7.0 (4.9-10.7) | 5.7 (2.5-7.4) | 0.134 |
| Lymphocyte (0.9-2.9×109/L) | 0.9 (0.6-1.2) | 0.8 (0.6-1.0) | 1.0 (0.5-1.1) | 0.048 |
| Neutrophil/Lymphocyte | 6.7 (4.2-11.8) | 7.3 (5.7-14.8) | 5.6 (3.1-8.8) | 0.041 |
| Monocyte (0.3-0.9×109/L) | 0.5 (0.3-0.7) | 0.4 (0.3-0.6) | 0.5 (0.3-0.7) | 0.919 |
| Hemoglobin (13.5-17.0g/dL,M, 11.3-15.2, F) | 11.5 (9.9-13.5) | 11.9 (8.9-14.0) | 10.3 (9.5-11.6) | 0.18 |
| Platelet count (150.0-400.0×109/L) | 206.4 (156.7-293.8) | 250.2 (140.3-318.9) | 197.9 (109.1-303.3) | 0.16 |
| PT (12.0-14.5 sec) | 14.7 (13.5-16.1) | 14.5 (13.3-16.5) | 14.9 (14.2-16.4) | 0.221 |
| aPTT (25.0-35.0 sec) | 34.0 (30.0-39.0) | 35.0 (30.0-40.0) | 37.0 (33.8-40.0) | 0.528 |
| Fibrinogen (220.0-498.0 mg/dL) | 613.0 ± 223.0 | 728.8 ± 167.6 | 351.0 ± 116.6 | <0.001a |
| D-dimer (0.0-240.0 ng/mL) | 1053.0 (583.0-2382.0) | 1213.0 (769.0-3741.0) | 610.0 (224.5-1867.3) | 0.107 |
| ALT(7.0-52.0 U/L) | 27.0 (14.5-48.5) | 32.0 (22.0-61.0) | 25.0 (13.8-132.8) | 0.57 |
| AST (12.0-39.0 U/L) | 41.0 (29.0-68.0) | 51.0 (39.0-88.0) | 47.0 (28.5-312.0) | 0.482 |
| LDH (120.0-240.0 U/L) | 391.5 (292.3-610.3) | 458.0 (372.0-690.0) | 270.5 (185.0-1009.5) | 0.001 |
| Total bilirubin (0.3-1.4 mg/dL) | 0.6 (0.4-0.8) | 0.5 (0.4-0.7) | 0.6 (0.5-1.0) | 0.17 |
| Direct bilirubin (0.0-0.3 mg/dL) | 0.2 (0.1-0.3) | 0.2 (0.1-0.3) | 0.2 (0.2-0.4) | 0.84 |
| Indirect bilirubin (0.2-0.8 mg/dL) | 0.4 (0.3-0.5) | 0.3 (0.3-0.5) | 0.4 (0.3-0.4) | 0.19 |
| BUN (5.0-22.0 mg/dL) | 23.0 (12.5-31.5) | 23.0 (11.0-32.0) | 21.5 (18.3-35.8) | 0.446 |
| Creatinine (0.7-1.3 mg/dL,M, 0.4-1.2, F) | 1.4 (0.8-2.2) | 1.3 (0.7-2.2) | 1.6 (0.8-3.7) | 0.331 |
| Glucose (70.0-100.0 mg/dL) | 138.0 (107.3-192.8) | 127.0 (111.0-201.0) | 126.5 (90.0-164.5) | 0.065 |
| BNP (0.0-100.0 pg/mL) | 122.0 (51.0-303.0) | 105.0 (47.0-178.0) | 138.0 (35.0-8909.8) | 0.917 |
| High sensitivity Troponin-I (3.0-20.0 ng/L) | 22.0 (9.0-65.5) | 26.0 (9.0-72.0) | 45.0 (10.5-147.5) | 0.618 |
| Albumin (3.7-5.5 mg/dL) | 3.1 ± 0.7 | 3.1 ± 0.7 | 3.2 ± 0.7 | 0.185a |
| C-reactive protein (0.0-10.9 mg/L) | 188.3 ± 110.5 | 199.3 ± 113.2 | 113.2 ± 85.8 | <0.001a |
| ESR (0.0-20.0 mm/h) | 55.7 ± 32.0 | 65.6 ± 30.6 | 45.3 ± 28.4 | 0.003a |
| Ferritin (11.0-306.8 ng/mL) | 454.0 (140.0-1620.0) | 697.0 (116.5-1936.3) | 676.0 (152.0-1468.0) | 0.016 |
| Procalcitonin (0.00-0.07 ng/mL) | 0.5 (0.2-1.8) | 1.1 (0.1-2.0) | 0.4 (0.2-1.2) | 0.016 |
PT, prothrombin time; aPTT, activated partial thromboplastin time; ALT, alanine transaminase; AST, aspartate aminotransferase; LDH, lactase dehydrogenase; BUN, blood urea nitrogen; BNP, B-type natriuretic peptide; ESR, Erythrocyte sedimentation rate; F, female; M, male; aStudent’s-t test, all other analyses were performed using Mann-Whitney U test.
The bold values indicate P values < 0.05.
Fibrinogen-Associated Factors in COVID-19 Patients
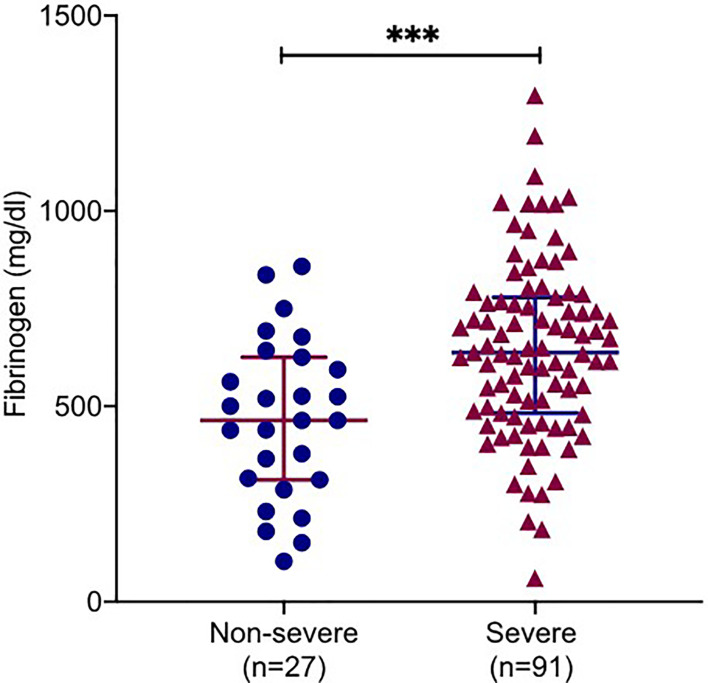
On admission, 67.2% patients (80/119) had increased fibrinogen (normal range 220.0-489.0 mg/dL), and 32.8% patients (39/119) had fibrinogen levels within the normal range. Fibrinogen levels were significantly associated with disease severity of COVID-19 (Figure 1). Patients with severe disease had increased fibrinogen levels than non-severe patients (648.1 mg/dl vs 468.9 mg/dl, P = 0.001). To understand the role of fibrinogen in the pathogenesis of COVID-19, the clinical characteristics and laboratory parameters were compared between normal and elevated fibrinogen group (Tables 1 and 2). When comparing the clinical characteristics, patients with increased fibrinogen levels were more likely to be white (20 vs 18, P =0.035) and had a higher probability of having a comorbidity of diabetes mellitus (42 vs 11, P =0.018), shortness of breath (54 vs 18, P =0.03), lower oxygen saturation (95.0 vs 97.5, P 0.003), mechanical ventilation (51 vs 15, P=0.011), ICU admission (55 vs 16, P=0.005), and severe disease (67 vs 25, P=0.021) (Table 1). When comparing the laboratory parameters, patients with increased fibrinogen levels showed lower lymphocyte count (0.8 [0.6-1.0] vs 1.0[0.5-1.1], P=0.048), higher neutrophil/lymphocyte ratio (7.3 [5.7-14.8] vs 5.6 [3.1-8.8], P=0.041), higher LDH (458.0 [372.0-690.0] vs 270.5 [185.0-1009.5], P=0.001), higher CRP (199.3 ± 113.2 vs 113.2 ± 85.8, P<0.001), higher ESR (65.6 ± 30.6 vs 45.3 ± 28.4, P=0.003), higher ferritin (697.0 [116.5-1936.3] vs 676.0 [152.0-1468.0], P=0.016), and higher PCT (1.1 [0.1-2.0] vs 0.4 [0.2-1.2], P=0.016). Comparison of the inflammatory markers between patients with normal and elevated fibrinogen levels were further demonstrated in Figure 2. However, comparison of other coagulation markers and cardiac markers between patients with normal and elevated fibrinogen levels did not show no significant difference.
Figure 1.
Correlations of fibrinogen levels with clinical severity. Plasma fibrinogen levels in COVID-19 patients with severe and non-severe. All data points are shown and mean ± 95% confidential intervals (CI) are shown by horizontal bars. student t-test was performed to determine the statistical significance. Here, *** indicate a P value < 0.001.
Figure 2.
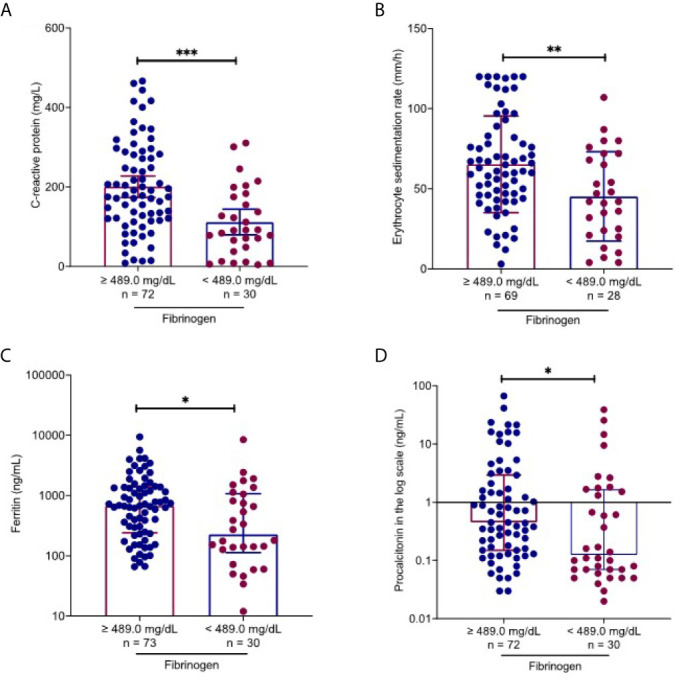
Fibrinogen-associated factors in COVID-19 patients. Using the upper level of the reference laboratory 489.0 mg/dl, inflammatory markers were compared between patients with normal and elevated fibrinogen levels. Patients with elevated fibrinogen levels had higher C-reactive protein (A), erythrocyte sedimentation rate (B), ferritin (C) and procalcitonin (D) levels than those with normal fibrinogen levels. All data points are shown, and medians ± interquartile range are shown by horizontal bars. Mann-Whitney U test was performed to determine the statistical significance. Here, *, ** and *** indicate a P value < 0.05, 0.005 and 0.001, respectively.
Elevated Fibrinogen Levels to Predict Disease Severity and ICU Admission
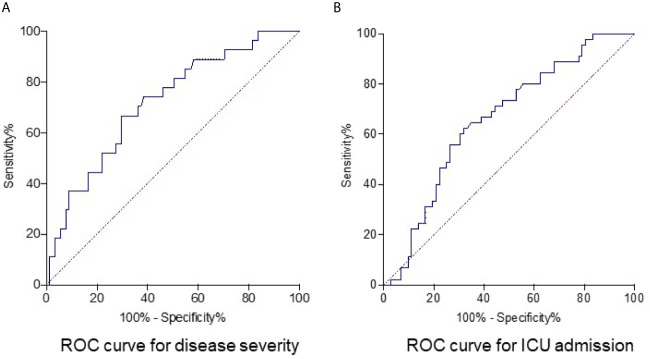
Next, the ROC analysis was performed to evaluate the role of fibrinogen in prediction of disease severity. The results showed that fibrinogen at 528.0 mg/dl on admission as the optimal cutoff level to discriminate severe from non-severe patients (AUC, 0.72; standard error [SE], 0.05; 95% confidence interval [CI], 0.61-0.83; P = 0.0006), with a sensitivity and specificity of 66.7% and 70.3%, respectively (Figure 3A). Seventy seven percent (92/119) patients had a fibrinogen > 528.0 mg/dl in this study cohort. The optimal cutoff for fibrinogen to predict ICU admission in our cohort was 571.0 mg/dl (AUC, 0.66; SE, 0.05; 95% CI, 0.58-0.76; P = 0.0037), with a sensitivity of 62.2% and specificity of 68.1% (Figure 3B).
Figure 3.
Receiver operating characteristics curve for fibrinogen as parameter for predicting disease severity and ICU admission in COVID-19 patients. The optimum cutoff point of fibrinogen to predict severe disease in COVID-19 was 528.0 mg/dl, with 66.7% for sensitivity and 70.3% for specificity. Area under receiver operator characteristic curve (AUC) was 0.72 and P value was 0.0006 (A). The optimum cutoff point of fibrinogen to predict ICU admission of COVID-19 patients was 571.0 mg/dl, with a sensitivity and specificity of 62.2% and 68.1%. The AUC was 0.66 and P value was 0.0037 (B).
Discussion
In this retrospective study, we assessed the distribution of fibrinogen in a cohort of COVID-19 patients. It is worth noting that fibrinogen levels are elevated in COVID-19 patients upon admission, especially in those with severe disease. Fibrinogen levels on admission were associated with elevated inflammatory markers, and elevated fibrinogen levels may be used as an early biomarker to predict disease severity and ICU admission.
COVID-19 is a systemic infection with significant impact on the coagulation system that often manifests in thrombotic complications and coagulopathy (Levi et al., 2020). Previous data have showed that fibrinogen levels are elevated among patients with COVID-19 (Toh and Hoots, 2007; Han et al., 2020) and decreased at later stage of the disease when disseminated intravascular coagulation happens (Tang et al., 2020). However, there is no consensus on the role of fibrinogen in predicting outcomes in COVID-19 patients regarding disease severity, ICU admission and mortality (Gao et al., 2020; Liu et al., 2020; Tang et al., 2020). Tang et al. have reported that fibrinogen levels are elevated in both survived and non-survived group, but the difference between two groups was not statistically significant (Tang et al., 2020). However, studies by other groups reported an association between elevated fibrinogen levels upon admission and poor outcome (Gao et al., 2020; Liu et al., 2020). In this study, we report that elevations in fibrinogen upon admission were significantly associated with disease severity and ICU admission in COVID-19 patients. Consistently, we found that LDH, a biomarker for severe disease or multiple organ failure was significantly associated with the fibrinogen level in our study.
Infection with bacterial, viral, or fungal pathogens initiates the systemic inflammation response. Significant inflammation is present in patients with 2019-nCoV/SARS-CoV-2 infection, based on increased IL-6, CRP and ESR levels upon admission (Chen et al., 2020a). In our study, the decreased negative acute phase reactant albumin and increased positive acute phase reactant CRP, ferritin, ESR and PCT indicated a systemic inflammation response in the patients. In COVID-19 patients with acute respiratory distress syndrome (ARDS), fibrinogen levels were reported to be associated with elevated interleukin-6 values (Ranucci et al., 2020). In the present study, we found that acute inflammatory markers, including CRP, ferritin, ESR and PCT were all significantly associated with elevated fibrinogen. RK Mahat et al. performed a meta-analysis of inflammatory markers in COVID-19, and they found that the inflammatory markers CRP, ferritin, ESR and PCT were associated with disease severity. However, fibrinogen was not analyzed in their study (Mahat et al., 2021).
Myocardial injury has been recognized as a common complication in COVID-19 patients (Boukhris et al., 2020). Myocardial injury is defined as the presence of at least one cardiac troponin value above the 99th percentile upper reference limit (URL) according to the Fourth Universal Definition of Myocardial Infarction (Thygesen et al., 2018). Consistent with many other reports (Lombardi et al., 2020; Garcia De Guadiana-Romualdo et al., 2021; Majure et al., 2021), elevated hsTnI was detected in the COVID-19 patients in our study. The mechanisms for myocardial injury in COVID-19 has not been elucidated. Several possible mechanisms have been proposed such as direct virus attack, inflammation-related injury, coronary plaque rupture, and microvascular thrombosis (Babapoor-Farrokhran et al., 2020; Imazio et al., 2020; Tajbakhsh et al., 2021). Fibrinogen may increase cardiovascular risk due to adverse effects of fibrinogen on plasma viscosity, coagulation, platelet activity, inflammation and atherogenesis (Kakafika et al., 2007). However, the direct myocardial injury biomarker, hsTnI, did not show significant association with fibrinogen level in our study (P=0.087).
The study has several limitations. First, this study was a single-center, retrospective study which needs to be further validated in a prospective study. Second, a proportion of asymptomatic, mild or moderate patients who did not have fibrinogen levels evaluated on admission were excluded from final analysis, which may underestimate the differences between groups. Third, dynamic changes of fibrinogen during the clinical course might also provide more information in the outcome of the patients.
In conclusion, our findings indicated that fibrinogen level on admission were associated with elevated inflammatory markers in COVID-19 patients, and elevated fibrinogen levels may predict severe disease and ICU admission. Monitoring fibrinogen level could be helpful in management of COVID-19 patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Alabama at Birmingham Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JS and LC designed the study, collected the data and drafted the manuscript. DN and SG revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aghagoli G., Gallo Marin B., Soliman L. B., Sellke F. W. (2020). Cardiac Involvement in COVID-19 Patients: Risk Factors, Predictors, and Complications: A Review. J. Card Surg. 35, 1302–1305. 10.1111/jocs.14538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M. S., Jadhav A. B., Hassan A., Meng Q. H. (2012). Acute Phase Reactants as Novel Predictors of Cardiovascular Disease. ISRN Inflammation, 2012 953461. 10.5402/2012/953461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arachchillage D. R. J., Laffan M. (2020). Abnormal Coagulation Parameters are Associated With Poor Prognosis in Patients With Novel Coronavirus Pneumonia. J. Thromb. Haemost. 18, 1233–1234. 10.1111/jth.14820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R. T., Bozorgnia B., Amanullah A. (2020). Myocardial Injury and COVID-19: Possible Mechanisms. Life Sci. 253, 117723. 10.1016/j.lfs.2020.117723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhris M., Hillani A., Moroni F., Annabi M. S., Addad F., Ribeiro M. H., et al. (2020). Cardiovascular Implications of the COVID-19 Pandemic: A Global Perspective. Can. J. Cardiol. 36, 1068–1080. 10.1016/j.cjca.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. (2020. a). Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Invest. 130, 2620–2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. (2020. b). Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 395, 507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann B., Massberg S. (2013). Thrombosis as an Intravascular Effector of Innate Immunity. Nat. Rev. Immunol. 13, 34–45. 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- Ernst E. (1990). Plasma Fibrinogen–an Independent Cardiovascular Risk Factor. J. Intern. Med. 227, 365–372. 10.1111/j.1365-2796.1990.tb00174.x [DOI] [PubMed] [Google Scholar]
- Favaloro E. J., Thachil J. (2020). Reporting of D-Dimer Data in COVID-19: Some Confusion and Potential for Misinformation. Clin. Chem. Lab. Med. 58, 1191–1199. 10.1515/cclm-2020-0573 [DOI] [PubMed] [Google Scholar]
- Fox S. E., Akmatbekov A., Harbert J. L., Li G., Quincy Brown J., Vander Heide R. S. (2020). Pulmonary and Cardiac Pathology in African American Patients With COVID-19: An Autopsy Series From New Orleans. Lancet Respir. Med. 8, 681–686. 10.1016/S2213-2600(20)30243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. (2020). Diagnostic Utility of Clinical Laboratory Data Determinations for Patients With the Severe COVID-19. J. Med. Virol. 92, 791–796. 10.1002/jmv.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia De Guadiana-Romualdo L., Morell-Garcia D., Rodriguez-Fraga O., Morales-Indiano C., Maria Lourdes Padilla Jimenez A., Gutierrez Revilla J. I., et al. (2021). Cardiac Troponin and COVID-19 Severity: Results From BIOCOVID Study. Eur. J. Clin. Invest. 51, e13532. 10.1111/eci.13532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Yang L., Liu R., Liu F., Wu K. L., Li J., et al. (2020). Prominent Changes in Blood Coagulation of Patients With SARS-CoV-2 Infection. Clin. Chem. Lab. Med. 58, 1116–1120. 10.1515/cclm-2020-0188 [DOI] [PubMed] [Google Scholar]
- Heinrich J., Assmann G. (1995). Fibrinogen and Cardiovascular Risk. J. Cardiovasc. Risk 2, 197–205. 10.1097/00043798-199506000-00004 [DOI] [PubMed] [Google Scholar]
- Imazio M., Klingel K., Kindermann I., Brucato A., De Rosa F. G., Adler Y., et al. (2020). COVID-19 Pandemic and Troponin: Indirect Myocardial Injury, Myocardial Inflammation or Myocarditis? Heart 106, 1127–1131. 10.1136/heartjnl-2020-317186 [DOI] [PubMed] [Google Scholar]
- Inciardi R. M., Solomon S. D., Ridker P. M., Metra M. (2020). Coronavirus 2019 Disease (COVID-19), Systemic Inflammation, and Cardiovascular Disease. J. Am. Heart Assoc. 9, e017756. 10.1161/JAHA.120.017756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakafika A. I., Liberopoulos E. N., Mikhailidis D. P. (2007). Fibrinogen: A Predictor of Vascular Disease. Curr. Pharm. Des. 13, 1647–1659. 10.2174/138161207780831310 [DOI] [PubMed] [Google Scholar]
- Key N. S., Vercellotti G. M., Winkelmann J. C., Moldow C. F., Goodman J. L., Esmon N. L., et al. (1990). Infection of Vascular Endothelial Cells With Herpes Simplex Virus Enhances Tissue Factor Activity and Reduces Thrombomodulin Expression. Proc. Natl. Acad. Sci. U.S.A. 87, 7095–7099. 10.1073/pnas.87.18.7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Thachil J., Iba T., Levy J. H. (2020). Coagulation Abnormalities and Thrombosis in Patients With COVID-19. Lancet Haematol 7, e438–e440. 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liao W., Wan L., Xiang T., Zhang W. (2020). Correlation Between Relative Nasopharyngeal Virus RNA Load and Lymphocyte Count Disease Severity in Patients with COVID-19. Viral Immunol. 34, 330–335. 10.1089/vim.2020.0062 [DOI] [PubMed] [Google Scholar]
- Liu Y., Liao W., Wan L., Xiang T., Zhang W. (2021). Correlation Between Relative Nasopharyngeal Virus RNA Load and Lymphocyte Count Disease Severity in Patients With COVID-19. Viral Immunol. 34, 330–335. 10.1089/vim.2020.0062 [DOI] [PubMed] [Google Scholar]
- Lombardi C. M., Carubelli V., Iorio A., Inciardi R. M., Bellasi A., Canale C., et al. (2020). Association of Troponin Levels With Mortality in Italian Patients Hospitalized With Coronavirus Disease 2019: Results of a Multicenter Study. JAMA Cardiol. 5, 1274–1280. 10.1001/jamacardio.2020.3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahat R. K., Panda S., Rathore V., Swain S., Yadav L., Sah S. P. (2021). The Dynamics of Inflammatory Markers in Coronavirus Disease-2019 (COVID-19) Patients: A Systematic Review and Meta-Analysis. Clin. Epidemiol. Glob Health 11, 100727. 10.1016/j.cegh.2021.100727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majure D. T., Gruberg L., Saba S. G., Kvasnovsky C., Hirsch J. S., Jauhar R., et al. (2021). Usefulness of Elevated Troponin to Predict Death in Patients With COVID-19 and Myocardial Injury. Am. J. Cardiol. 138, 100–106. 10.1016/j.amjcard.2020.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. (2020) Clinical Mangement of COVID-19. Available at: https://www.who.int/publications/i/item/clinical-management-of-covid-19.
- Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., et al. (2020). The Procoagulant Pattern of Patients With COVID-19 Acute Respiratory Distress Syndrome. J. Thromb. Haemost. 18, 1747–1751. 10.1111/jth.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami M., Mansouritorghabeh H. (2020). D-Dimer Level in COVID-19 Infection: A Systematic Review. Expert Rev. Hematol. 13, 1265–1275. 10.1080/17474086.2020.1831383 [DOI] [PubMed] [Google Scholar]
- Sweetnam P. M., Thomas H. F., Yarnell J. W., Beswick A. D., Baker I. A., Elwood P. C. (1996). Fibrinogen, Viscosity and the 10-Year Incidence of Ischaemic Heart Disease. Eur. Heart J. 17, 1814–1820. 10.1093/oxfordjournals.eurheartj.a014797 [DOI] [PubMed] [Google Scholar]
- Tajbakhsh A., Gheibi Hayat S. M., Taghizadeh H., Akbari A., Inabadi M., Savardashtaki A., et al. (2021). COVID-19 and Cardiac Injury: Clinical Manifestations, Biomarkers, Mechanisms, Diagnosis, Treatment, and Follow Up. Expert Rev. Anti Infect. Ther. 19, 345–357. 10.1080/14787210.2020.1822737 [DOI] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. (2020). Abnormal Coagulation Parameters are Associated With Poor Prognosis in Patients With Novel Coronavirus Pneumonia. J. Thromb. Haemost. 18, 844–847. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil J., Longstaff C., Favaloro E. J., Lippi G., Urano T., Kim P. Y., et al. (2020). The Need for Accurate D-Dimer Reporting in COVID-19: Communication From the ISTH SSC on Fibrinolysis. J. Thromb. Haemost. 18, 2408–2411. 10.1111/jth.14956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen K., Alpert J. S., Jaffe A. S., Chaitman B. R., Bax J. J., Morrow D. A., et al. (2018). Fourth Universal Definition of Myocardial Infarctio ). Circulation 138, e618–e651. 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- Toh C. H., Hoots W. K. (2007). The Scoring System of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: A 5-Year Overview. J. Thromb. Haemost. 5, 604–606. 10.1111/j.1538-7836.2007.02313.x [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. (2020). Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama 323, 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X. L., Wang X. G., Hu B., Zhang L., Zhang W., et al. (2020. b). A Pneumonia Outbreak Associated With a New Coronavirus of Probable Bat Origin. Nature 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. (2020. a). Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 395, 1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.