Key Points
Question
How are differences in national treatment strategies for multiple sclerosis associated with clinical outcomes?
Findings
In this cohort study comparing patients in the Danish and Swedish multiple sclerosis registries, the use of highly effective disease-modifying treatment was far more frequent in the Swedish cohort and was associated with significant reductions in the rate of confirmed disability worsening and relapse outcomes.
Meaning
This study suggests that escalation of treatment was inferior to using a more effective disease-modifying treatment as initial treatment for multiple sclerosis.
Abstract
Importance
Treatment strategies for relapsing-remitting multiple sclerosis (RRMS) vary markedly between Denmark and Sweden. The difference in the association of these national strategies with clinical outcomes is unknown.
Objective
To investigate the association of national differences in disease-modifying treatment (DMT) strategies for RRMS with disability outcomes.
Design, Setting, and Participants
This cohort study used data on 4861 patients from the Danish and Swedish national multiple sclerosis (MS) registries from the date of index DMT initiation (between January 1, 2013, and December 31, 2016) until the last recorded visit at time of data extraction (October 2, 2019).
Exposures
All MS-specific DMTs initiated during the observation period were included in the analysis.
Main Outcomes and Measures
The primary study outcome was time to 24-week confirmed disability worsening. Secondary outcomes were 24-week confirmed disability improvement, milestone Expanded Disability Status Scale scores of 3 and 4, annualized relapse rate, time to first relapse, and treatment switching. Data were analyzed using inverse probability of treatment weighting–based models using a propensity score to weight and correct the comparison for the imbalance of confounders observed at baseline between the 2 countries.
Results
A total of 2700 patients from the Swedish MS registry (1867 women [69.2%]; mean [SD] age, 36.1 [9.5] years) and 2161 patients from the Danish MS registry (1472 women [68.1%]; mean [SD] age, 37.3 [9.4 years]) started a first DMT between 2013 and 2016, were included in the analysis, and were observed for a mean (SD) of 4.1 (1.5) years. A total of 1994 Danish patients (92.3%) initiated a low to moderately effective DMT (teriflunomide, 907 [42.0%]) and 165 (7.6%) initiated a highly effective DMT, whereas a total of 1769 Swedish patients (65.5%) initiated a low to moderately effective DMT (teriflunomide, 64 [2.4%]) and 931 (34.5%) initiated a highly effective DMT. The Swedish treatment strategy was associated with a 29% reduction in the rate of postbaseline 24-week confirmed disability worsening relative to the Danish treatment strategy (hazard ratio, 0.71; 95% CI, 0.57-0.90; P = .004). The Swedish treatment strategy was also associated with a 24% reduction in the rate of reaching an expanded disability status scale score of 3 (hazard ratio, 0.76; 95% CI, 0.60-0.97; P = .03) and a 25% reduction in the rate of reaching an expanded disability status scale score of 4 (hazard ratio, 0.75; 95% CI, 0.61-0.96; P = .01) relative to Danish patients.
Conclusions and Relevance
The findings of this study suggest that there is an association between differences in treatment strategies for RRMS and disability outcomes at a national level. Escalation of treatment efficacy was inferior to using more efficacious DMT as initial treatment.
This cohort study uses data from Danish and Swedish national multiple sclerosis (MS) registries to investigate the association of national differences in disease-modifying treatment strategies for relapsing-remitting MS with disability outcomes.
Introduction
The clinical development of new disease-modifying treatments (DMTs) of relapsing-remitting multiple sclerosis (RRMS) has, in recent years, given rise to an increasing number of treatment alternatives. The possibility of achieving personalized treatment decisions has thereby increased, and there are a growing number of guidelines and treatment algorithms to support the clinician and patient in their choice of DMT.1,2,3,4,5 The treatment strategy used most often is to start with a first-line agent and, when there are signs of breakthrough disease activity, to switch to a second-line agent that is considered more effective, often at the expense of safety and higher costs. This escalating strategy aims to find the optimal effective treatment while taking as little risk as possible.6,7,8 However, in cases with clinical or neuroradiologic signs of high disease activity from the onset, a more effective DMT is often suggested to reduce the risk of nerve tissue damage, which may occur if the inhibition of inflammation is delayed. Other than the effectiveness of DMTs, there are several reasons for switching therapy, such as safety, adverse events, planning of pregnancy, tolerability, adherence, and costs.9,10,11 Ideally, all these aspects are included in the shared decision-making to select the most suitable DMT at the individual level.
There are several lines of evidence supporting early treatment in multiple sclerosis (MS), including trials of clinically isolated syndromes in delaying the conversion to MS, observational studies, and postmarketing studies.2,12 However, although evidence suggests that delaying initiation of DMTs is associated with future clinical outcomes, surveys of treatment practices among physicians show that DMTs are usually not started during clinically isolated syndromes (after the onset of symptoms but before the diagnosis of MS).13,14
The first approved DMTs have been evaluated in randomized clinical trials (RCTs), whereas later RCTs used an approved DMT as a comparator (often a first-line agent). There are only a few head-to-head RCTs that have compared the efficacy and safety of approved DMTs of similar treatment category. Three trials showed similar effects with interferon beta and glatiramer acetate,14,15,16 whereas, to our knowledge, no RCT has compared highly effective DMTs, such as natalizumab, fingolimod, alemtuzumab, ocrelizumab, rituximab, or cladribine, in a head-to-head manner. Thus, the evidence base regarding treatment algorithms for the management of RRMS, considering all available treatment alternatives, must be considered weak. However, recent real-world evidence indicates that the commencement of highly effective treatment within 2 years from disease onset compared with delayed commencement improved long-term disability outcomes, indicating that timing to switch therapy is of importance.17
Denmark and Sweden have developed nationwide MS registries with coverage of more than 90% and 80% of patients, respectively.18,19 From September 2013 until 2019, teriflunomide became the recommended first-line treatment in Denmark of patients with RRMS with mild to moderate disease activity, except for women with pregnancy plans in the near future. In Sweden, teriflunomide treatment was initially approved only for patients with RRMS whose treatment with interferon beta failed, and it did not receive a full indication for RRMS until 2016. Another major difference in the choice of DMT is the increasing use of rituximab as off-label treatment for MS in Sweden. It has become the most frequently used DMT, initially as second- or third-line treatment, but during recent years, it has been used increasingly as a first-line option. Thus, while both countries have similar socioeconomic standards and health care systems, the choice of DMT and the treatment strategy for RRMS demonstrate significant differences. The objective of this retrospective cohort study, based on data obtained from the Swedish and Danish MS registries, was to investigate whether national treatment recommendations and clinical practice are associated with disability outcomes after 3 to 7 years of follow-up.
Methods
Data Sources
The Swedish MS registry was established in 2000 to capture and collate demographic, treatment, disease, and clinical outcomes data on patients with MS.18 The Danish Multiple Sclerosis Registry is a nationwide population-based registry established in 1956.19,20 Consent in the Swedish MS registry begins at the point of first inclusion in the registry. This consent extends to any study that uses data sourced from the Swedish MS registry, and no additional consent procedure is required. In Denmark, there is no requirement for informed consent from the patient for inclusion in the Danish Multiple Sclerosis Registry, on the explicit condition that the data can never be used for any other purpose than pure science and statistics. This study was approved by the Swedish Ethical Review Authority and the Danish Data Protection Authority.
Inclusion Criteria
Adult patients aged 18 to 55 years at baseline with clinically isolated syndrome or RRMS who initiated DMT for the first time between January 1, 2013, and December 31, 2016, were included in the analysis. Patients with primary progressive MS or secondary progressive MS at baseline were excluded from the analysis. To limit confounding from comorbid conditions21 and the relative unresponsiveness to DMT associated with increasing age, we did not include patients older than 55 years.22,23 Data were extracted from the registries on October 2, 2019 (ie, the maximum follow-up period was 7 years).
Definitions and Outcomes
Baseline was defined as the date that DMT was first started. The primary analysis compared time to 24-week confirmed disability worsening (CDW) between the Danish and Swedish cohorts. Confirmed disability worsening was defined as an increase in Expanded Disability Status Scale (EDSS) score with at least 1 point from baseline sustained between 2 follow-up visits separated by no less than 6 months (1.5 points if the EDSS score at baseline was 0 and 0.5 points if the baseline EDSS score was ≥5.5). Time to milestone EDSS scores of 3 and 4 and the annual relapse rate were also studied as secondary outcomes. Expanded Disability Status Scale scores recorded within 90 days of relapses were excluded from the analysis of all outcomes.
Statistical Analysis
Categorical variables were summarized using frequency and percentage. Continuous variables were summarized using mean (SD) values or median values and interquartile ranges as appropriate. Baseline demographic characteristics, disease activity, treatment, and examination factors were compared between the Danish and Swedish cohorts using standardized differences. Time to the primary 24-week CDW outcome, time to the secondary 24-week confirmed disability improvement, and time to the milestone EDSS score of 3 and 4 end points were analyzed using inverse probability of treatment weighting–based Cox proportional hazards regression models with robust SEs. The inverse probability of treatment weighting model used a propensity score to weight and correct the comparison for the imbalance of confounders observed at baseline between the 2 countries. As a sensitivity analysis, we reran the comparisons of clinical outcomes, limiting the Swedish cohort to only patients treated with the highly effective DMT (fingolimod, natalizumab, rituximab, alemtuzumab, and ocrelizumab) and compared these with the entire Danish cohort. Hazard proportionality for each model was assessed via analysis of scaled Schoenfeld residuals. Kaplan-Meier survival and failure curves were used to visualize time-to-event outcome. All analyses were conducted in R, version 3.6.3 (R Group for Statistical Computing).24 Additional details on the data sources, outcome definitions, and statistical analysis can be found in the eMethods in the Supplement.
Results
Baseline Demographic and Clinical Characteristics
A total of 2700 patients from the Swedish registry (1867 women [69.2%]; mean [SD] age, 36.1 [9.5] years) and 2161 patients from the Danish MS registry (1472 women [68.1%]; mean [SD] age, 37.3 [9.4 years]) met the inclusion criteria and started a DMT between 2013 and 2016 were included in the analysis. The weighted standardized comparison between the study cohorts revealed significant differences only in sex and in the 12- and 24-month relapse rates prior to baseline (Table 1). Although a higher relapse rate was recorded among Swedish patients, this prebaseline relapse activity was considered comparable between the 2 countries (standardized difference <10%). A total of 1966 Danish patients (92.4%) initiated a low moderately effective DMT as the first treatment. Teriflunomide was the most frequent first-line treatment for Danish patients (907 [42.0%]), followed by interferon beta-1a (643 [29.8%]). A total of 1769 Swedish patients (65.5%) initiated a low to moderately effective DMT as the first-line treatment. The pattern was more mixed in the Swedish cohort, with both dimethyl fumarate (615 [22.8%]) and interferon beta-1a (616 [22.8%]) accounting for the most frequently used first-line DMT. However, 931 patients (34.5%) in the Swedish cohort (rituximab, 484 [17.9%]; natalizumab, 299 [11.1%]; fingolimod, 148 [5.5%]) started the first-line treatment with a second-line or moderately to highly effective DMT compared with 165 of the Danish patients (7.6%). Overall, the mean (SD) follow-up was 4.1 (1.5) years, with the Danish cohort having a slightly longer mean (SD) follow-up (4.4 [1.5] years) compared with the Swedish cohort (3.7 [1.4] years).
Table 1. Comparison of Baseline Characteristics by Country.
| Baseline characteristic | Patients, No. (%) | Standardized difference | Weighted standardized difference | |
|---|---|---|---|---|
| Denmark (n = 2161)a | Sweden (n = 2700)b | |||
| Age, mean (SD), y | 37.3 (9.4) | 36.1 (9.5) | −0.128 | −0.079 |
| Sex | ||||
| Female | 1472 (68.1) | 1867 (69.2) | 0.022 | −0.006 |
| Male | 689 (31.9) | 831 (30.8) | ||
| Not reported | 0 | 2 (0.1) | ||
| Disease duration, y | ||||
| Mean (SD) | 3.8 (5.4) | 3.3 (5.4) | −0.107 | −0.073 |
| Median (IQR) | 1.5 (0.5-4.9) | 0.9 (0.3-3.6) | ||
| Total relapses, mean (SD), No. | ||||
| With 12 mo before baseline | 0.5 (0.7) | 0.5 (0.7) | 0.081 | 0.005 |
| With 24 mo before baseline | 0.6 (0.8) | 0.6 (0.8) | 0.079 | 0.002 |
| EDSS score, median (IQR)c | 2 (1-2.5) | 1.5 (1-2.5) | −0.256 | −0.094 |
| MRI, count of T2 lesions at DMT startd | ||||
| 0 | 17 (0.8) | 14 (0.5) | −0.168 | −0.098 |
| 1-9 | 313 (14.5) | 535 (19.8) | ||
| ≥10 | 995 (46.0) | 841 (31.2) | ||
| MRI conducted, T2 lesions not reported | 573 (26.5) | 548 (20.3) | ||
| No baseline MRI | 263 (12.2) | 762 (28.2) | ||
| First treatmente | ||||
| Interferon beta-1a | 643 (29.8) | 616 (22.8) | −1.01 | −0.327 |
| Interferon beta-1b | 6 (0.3) | 176 (6.5) | ||
| Glatiramer acetate | 128 (5.9) | 128 (4.7) | ||
| Dimethyl fumarate | 280 (13.0) | 615 (22.8) | ||
| Rituximab | 3 (0.1) | 484 (17.9) | ||
| Natalizumab | 104 (4.8) | 299 (11.1) | ||
| Fingolimod | 58 (2.7) | 148 (5.5) | ||
| Pegylated interferon beta-1a | 30 (1.4) | 86 (3.2) | ||
| Teriflunomide | 907 (42.0) | 64 (2.4) | ||
| Other | 2 (0.1) | 84 (3.1) | ||
| Follow-up time, mean (SD), y | 4.4 (1.5) | 3.7 (1.4) | −0.487 | −0.151 |
Abbreviations: DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale; IQR, interquartile range; MRI, magnetic resonance imaging.
Primary treatment escalation strategy.
Primary immediate highly effective DMT strategy.
Defined as the EDSS score recorded nearest to baseline date within 6 months.
Defined as the MRI recorded nearest to baseline date within 6 months.
Not included in derivation of the propensity score.
24-Week Confirmed Disability Progression
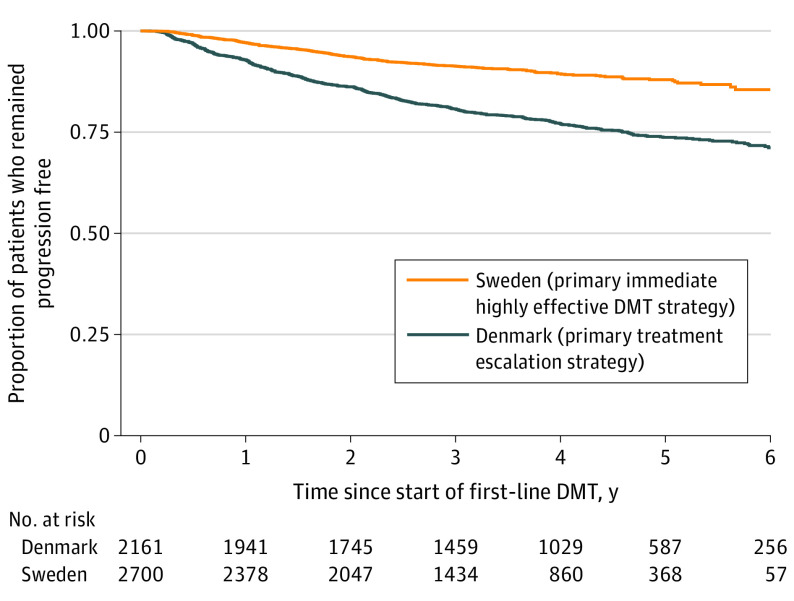
The Swedish treatment strategy was associated with a 29% reduction in the rate of postbaseline 24-week CDW relative to the Danish treatment strategy (inverse probability of treatment weighting hazard ratio [HR], 0.71; 95% CI, 0.57-0.90; P = .004) (Figure 1). This difference was maximized among Swedish patients initiating therapy in either 2015 or 2016 (HR, 0.69; 95% CI, 0.57-0.84; P < .001) compared with Danish patients, although there remained a clear reduction favoring Sweden in the earlier 2013-2014 subgroup (HR, 0.78; 95% CI, 0.64-0.94; P = .008). These differences were preserved when the analysis was limited to the index (first) DMT follow-up only, with the Swedish treatment strategy again associated with a similarly significant reduction in the rate of CDW with the first treatment relative to Danish patients (HR, 0.80; 95% CI, 0.67-0.98; P = .001). Although the earlier 2013-2014 subgroup (HR, 0.83; 95% CI, 0.70-1.02; P = .08) did not demonstrate a significant difference in the rate of CDW, the later 2015-2016 cohort (HR, 0.78; 95% CI, 0.64-0.95; P = .006) demonstrated a significant reduction in CDW favoring the Swedish patients.
Figure 1. Time to Confirmed Disability Progression by Treatment Strategy Cohort.
DMT indicates disease-modifying treatment.
24-Week Confirmed EDSS Score Improvement
Although the Swedish treatment strategy was associated with a decreased rate of EDSS improvement events in unadjusted modeling, there was no statistically significant difference in the rate of 24-week confirmed improvement events between the 2 groups on the propensity score–weighted model (HR, 0.97; 95% CI, 0.84-1.14; P = .73). Similar nonsignificant results were observed in both the 2013-2014 subgroup (HR, 1.05; 95% CI, 0.87-1.27; P = .82) and the later 2015-2016 subgroup (HR, 0.83; 95% CI, 0.65-1.08; P = .19). The results were also similar when a longer minimum 12-month confirmation period was used (HR, 1.11; 95% CI, 0.90-1.35; P = .57).
Time to Milestone EDSS Score of 3 and 4
The Swedish treatment strategy was associated with a 24% reduction in the rate of reaching an EDSS score of 3 relative to the Danish treatment strategy (HR, 0.76; 95% CI, 0.60-0.97; P = .03). However, this reduction was not observed in the earlier treatment cohort (HR, 0.87; 95% CI, 0.68-1.13; P = .25) but only in the later treatment group (HR, 0.73; 95% CI, 0.62-0.92; P = .001). For the subset of patients in each cohort reaching an EDSS score of 3 at any time during follow-up, the mean (SD) time to reach an EDSS score of 3 in the Swedish cohort was 2.2 (1.5) years compared with 1.7 (1.2) in the Danish cohort. Similarly, the Swedish treatment strategy was associated with a 25% decrease in the rate of reaching an EDSS score of 4 relative to the Danish treatment strategy (HR, 0.75; 95% CI, 0.61-0.96; P = .01). The mean (SD) time to reach an EDSS score of 4 in the Swedish cohort was 3.9 (1.4) years compared with 2.6 (1.6) years in the Danish cohort.
Treatment Switching
The Danish treatment strategy was associated with significantly higher proportions of both index DMT discontinuations and subsequent switching to an alternate DMT relative to the Swedish cohort (Table 2). The Swedish treatment strategy was associated with a 22% reduction in the rate of discontinuation (HR, 0.78; 95% CI, 0.71-0.68; P < .001) and a 12% reduction in treatment switching (HR, 0.88; 95% CI, 0.78-0.98; P = .02) relative to the Danish treatment strategy. Lack of effectiveness was the most commonly reported reason for discontinuation of the index treatment in the Danish cohort (563 of the 1504 patients who discontinued treatment [37.4%]), followed by adverse effects (508 of the 1504 patients who discontinued treatment [33.8%]) (eTable 1 in the Supplement). By comparison, adverse effects were the most frequently cited reason for discontinuation in the Swedish cohort (587 of the 1702 patients who discontinued treatment [34.5%]). Discontinuation due to lack of effectiveness was less frequent in the Swedish cohort (523 of the 1702 patients who discontinued treatment [30.7%]).
Table 2. Index Treatment Discontinuations and Treatment Switching by Cohort.
| Outcome | Denmark (n = 2161) | Sweden (n = 2700) | P value |
|---|---|---|---|
| Discontinuation of index DMT | 1504 (69.6) | 1702 (63.0) | <.001 |
| Switched to alternate DMT | |||
| Any switch gap | 1365 (63.2) | 1547 (57.3) | <.001 |
| Maximum 6-mo switch gap | 1190 (55.1) | 1399 (51.8) | .02 |
Abbreviation: DMT, disease-modifying treatment.
Among patients switching from the index treatment to an alternative DMT, a second-line treatment was chosen by 534 patients in the Danish cohort (35.5%), and fingolimod was the most common choice (290 of 1504 discontinuations [19.3%]) (eTable 2 in the Supplement). In the Swedish cohort, 859 patients (50.5%) switched to a second-line DMT, and rituximab was the most common choice (529 of 1702 discontinuations [31.1%]). Of the 1994 of 2161 Danish patients (92.3%) who started therapy with a first-line DMT (interferon beta-1a, interferon beta-1b, glatiramer acetate, dimethyl fumarate, or teriflunomide), 554 (27.8%) switched to a highly effective-efficacy DMT. By comparison, 1685 of 2700 patients (62.4%) in the Swedish cohort started treatment with a first-line DMT; of these, 585 (34.7%) switched to a highly effective DMT. Subgroup and sensitivity analyses are described in Table 3, Figure 2, and the eResults in the Supplement.
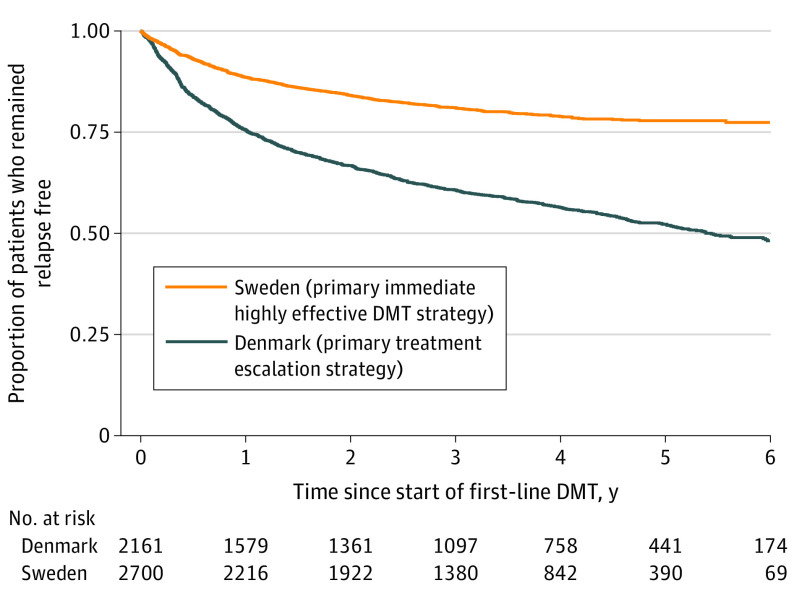
Table 3. Annualized Relapse Rate by Country.
| Observation period | Relapse count | Follow-up years | Annualized relapse rate (95% CI) | P value |
|---|---|---|---|---|
| All | ||||
| Denmark | 1828 | 9605.4 | 0.190 (0.182-0.199) | <.001 |
| Sweden | 739 | 9521.6 | 0.078 (0.072-0.083) | |
| Early (2013-2014 DMT initiated) | ||||
| Denmark | 1099 | 5878.2 | 0.187 (0.176-0.198) | <.001 |
| Sweden | 481 | 5865.3 | 0.082 (0.075-0.090) | |
| Late (2015-2016 DMT initiated) | ||||
| Denmark | 729 | 3727.2 | 0.196 (0.182-0.210) | <.001 |
| Sweden | 258 | 3656.3 | 0.071 (0.062-0.080) | |
Abbreviation: DMT, disease-modifying treatment.
Figure 2. Time to First Relapse by Treatment Strategy Cohort.
DMT indicates disease-modifying treatment.
Discussion
We used the Danish and Swedish nationwide MS registries to investigate whether there is an association between differences in treatment strategies and disability outcomes. Swedish patients had a significantly lower rate of disability progression than Danish patients during an observational time of 3 to 7 years, and fewer Swedish patients reached thresholds of clinically evident disability compared with Danish patients. Our data suggest that there was an association between differences in the choice of first-line DMT and of treatment strategy and disability outcomes. Danish patients almost exclusively started treatment with a conventional first-line DMT, primarily teriflunomide, whereas one-third of Swedish patients initiated treatment with a highly effective DMT, mostly rituximab and natalizumab. This difference may explain why more Danish patients discontinued and subsequently switched from the index DMT to another DMT and why breakthrough disease was more often recorded as the reason for the DMT switch for Danish than Swedish patients. In addition, Swedish patients switched to a highly effective DMT more often than Danish patients did, which might have been associated with the more favorable disability outcomes among Swedish patients.
In both the Danish and Swedish guidelines of MS care, the escalation treatment strategy is recommended.24,25,26 However, there have been discrepancies in MS treatment and in the evolution of treatment strategies between the countries. In Denmark, teriflunomide became the first-line DMT of choice between September 2013 and 2019, whereas in Sweden, teriflunomide was initially approved for patients with RRMS whose treatment with interferon beta failed and who did not receive a full indication for RRMS until 2016. These circumstances may explain to some extent why teriflunomide was the first-line treatment in 42.0% of Danish patients and in only 2.4% of Swedish patients. Although injectable DMT with interferon beta and glatiramer acetate constituted approximately 37% of baseline treatments in both countries, 22.8% of patients commenced treatment with dimethyl fumarate in Sweden compared with 13.0% of patients in Denmark. There may be an association between this difference in the selection of oral treatment and disability outcomes. Teriflunomide and dimethyl fumarate are both considered first-line DMTs, and phase 3 RCTs have shown significant reductions in the annual relapse rate and in lesion formations as detected on magnetic resonance imaging (MRI) scans.27,28,29,30 Although the pivotal trials of teriflunomide also showed significant reductions in 12-week confirmed disability progression, this end point was only reached in one of the dimethyl fumarate trials (ie, DEFINE [Determination of the Efficacy and Safety of Oral Fumarate in Relapsing–Remitting MS]).28 To our knowledge, no head-to-head trials have been conducted between oral medications. However, analyses of retrospective real-world data from MS registries using propensity score weighting suggest that dimethyl fumarate was just as effective as fingolimod31,32 and superior to teriflunomide32,33 with regard to the annual relapse rate, but no difference in disability progression was shown. Similar results were recently reported in a nationwide cohort study from Denmark34 supporting the use of dimethyl fumarate rather than teriflunomide to inhibit disease activity.
Although escalation therapy is the most accepted treatment strategy for RRMS, it is not evidence based. There is no consensus on how to define the threshold for breakthrough disease activity or when a patient should be considered to have highly active or aggressive RRMS.35,36 Nevertheless, there are clinical, MRI, and cerebrospinal fluid findings that are associated with a severe clinical course and early development of disability.35 According to Danish recommendations, highly effective DMT is advised for patients with high disease activity who have not been previously treated and for patients who have disease activity while receiving first-line treatment. In the Swedish guidelines, patients with more than 1 relapse in the last year, or more than 2 gadolinium contrast–enhancing lesions detected on MRI scans, and the presence of negative prognostic markers (high T2 lesion volume detected on MRI scans or onset of relapse with motor symptoms and/or with incomplete remission) are considered eligible for highly effective DMT.24 Thus, the recommendations to initiate DMT and when to switch the DMT in case of breakthrough disease activity are similar in Danish and Swedish MS care. However, in clinical practice in Sweden, there has been a shift to begin highly effective DMT also in patients with less disease activity or fewer neurologic adverse effects. At baseline, the first treatment was a highly effective DMT for 34.5% of Swedish patients (rituximab, 17.9%; natalizumab 11.1%; fingolimod 5.5%) compared with 7.6% of Danish patients. Thus, early intervention with a more effective DMT was more prevalent in the Swedish cohort and may have been the main factor associated with the better disability outcome that we observed among Swedish patients. In a previous study, highly effective DMT (alemtuzumab, rituximab, ocrelizumab, natalizumab, or mitoxantrone) started within 2 years of the onset of clinical disease was associated with less disability after 6 to 10 years of follow-up compared with highly effective DMT started later in the disease course.17 Our results suggest that intervention with a highly effective DMT as the first DMT reduces disability progression after a mean follow-up of 4 years. The larger difference in annualized relapse rates and disability outcomes during the second period probably reflects the increasing use of rituximab among Swedish patients with RRMS as both the first-line DMT and as rescue DMT when switching therapy. In our study, escalation remained a common event across both cohorts. Of the 1685 of 2700 Swedish patients who initiated therapy using a first-line DMT, 585 (34.7%) switched to the highly effective DMT; in comparison, 27.8% of Danish patients who initiated first-line treatment switched to a highly effective DMT. However, our sensitivity analysis limiting the Swedish cohort to only patients who started highly effective therapy as their first DMT suggested that the association of this treatment strategy with clinical outcomes may be larger than escalating therapy.
More patients in the Danish cohort than in the Swedish cohort discontinued and switched their index DMT. The most common reason was the occurrence of breakthrough disease. This was expected because conventional first-line DMT was the most prevalent first treatment in Denmark, whereas Swedish patients more often started treatment with a highly effective DMT. We also observed that significantly more Swedish patients switched their index treatment to a highly effective second-line DMT compared with patients in the Danish cohort, with almost one-third switching to rituximab. Fingolimod was the most-used therapy in the switch to a second-line DMT in the Danish cohort. In previous observational studies, rituximab was considered significantly more effective than fingolimod.37,38,39 Thus, in Sweden, highly effective DMT was more common both as the first-line DMT and when switching treatment, compared with the Danish cohort. A somewhat different pattern of treatment interruption was observed when the analysis was limited to first-line DMT only (interferon beta-1a, interferon beta-1b, glatiramer acetate, dimethyl fumarate, and teriflunomide). In this subgroup analysis, Swedish patients receiving first-line DMT switched therapy more frequently than the Danish subgroup, opposite to the trend observed across the entire cohort. This observation is consistent with the phenomenon of Swedish patients who initiate therapy with a non–first-line DMT being far more persistent with therapy and far less likely to switch than either Danish patients or the subgroup of Swedish patients who initiated first-line therapy.
Limitations
This study has some limitations; the main limitations are the retrospective and observational nature of the data. We selected 24-week confirmed disability progression as the primary outcome and 24-week disability improvement, time to reach an EDSS score of 3 and 4, annualized relapse rate, and time to first relapse as secondary outcomes. Disability progression is the essential outcome in MS and is probably more reliable than relapse rate for evaluation of MS treatment. However, Danish patients had significantly more EDSS score assessments than Swedish patients, which may be associated with the disability outcome. To limit this possible confounder, the EDSS score–based outcome models were further adjusted to correct for differences in assessment frequency. Although EDSS score assessment has several limitations concerning its interrater and intrarater reliability and sensitivity, it is internationally the most accepted instrument for quantifying disability in MS.40 The 24-week confirmation of EDSS score progression or improvement should minimize the effect from relapses, and the EDSS scores of 3 and 4 are meaningful thresholds for disability. We found a more than 50% lower annualized relapse rate among Swedish patients with RRMS compared with Danish patients with RRMS. We cannot rule out that this difference was due to fewer assessments of patients by Swedish than Danish physicians. However, the higher prestudy relapse rate observed among Swedish patients does not support such an assumption. Moreover, a higher proportion of patients received DMT as infusions in Sweden, which entails intensified monitoring, including monitoring of relapses. The coverage of the Swedish and Danish registry is more than 80% and 90%, respectively. The data density including scoring of the EDSS has been validated and was recently improved in the Swedish MS registry.41 According to the 2017-2018 annual quality report, a yearly EDSS score was registered for 80% of the Danish patients receiving DMT.42 Further details around additional limitations can be found in the eDiscussion in the Supplement.43,44
Conclusions
This study shows that, for the first time to our knowledge, differences in national treatment recommendations and strategies had a significant association with disability outcomes after a few years of follow-up. Starting with a more effective therapy and switching to a more effective DMT at treatment discontinuation irrespective of reason seemed to be superior to commencing a conventional first-line DMT and escalation.
eTable 1. Reason for Discontinuation of Index DMT by Cohort
eTable 2. Treatment Switch Product by Cohort
eMethods. Materials and Methods – Additional Details
eResults. Relapse, Subgroup and Sensitivity Analyses
eDiscussion. Additional Study Limitations
References
- 1.Col N, Alvarez E, Springmann V, et al. A novel tool to improve shared decision making and adherence in multiple sclerosis: development and preliminary testing. MDM Policy Pract. 2019;4(2):2381468319879134. doi: 10.1177/2381468319879134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comi G, Radaelli M, Soelberg Sørensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet. 2017;389(10076):1347-1356. doi: 10.1016/S0140-6736(16)32388-1 [DOI] [PubMed] [Google Scholar]
- 3.Gafson A, Craner MJ, Matthews PM. Personalised medicine for multiple sclerosis care. Mult Scler. 2017;23(3):362-369. doi: 10.1177/1352458516672017 [DOI] [PubMed] [Google Scholar]
- 4.Ingwersen J, Aktas O, Hartung HP. Advances in and algorithms for the treatment of relapsing-remitting multiple sclerosis. Neurotherapeutics. 2016;13(1):47-57. doi: 10.1007/s13311-015-0412-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trojano M, Tintore M, Montalban X, et al. Treatment decisions in multiple sclerosis—insights from real-world observational studies. Nat Rev Neurol. 2017;13(2):105-118. doi: 10.1038/nrneurol.2016.188 [DOI] [PubMed] [Google Scholar]
- 6.Cree BAC, Mares J, Hartung HP. Current therapeutic landscape in multiple sclerosis: an evolving treatment paradigm. Curr Opin Neurol. 2019;32(3):365-377. doi: 10.1097/WCO.0000000000000700 [DOI] [PubMed] [Google Scholar]
- 7.Río J, Comabella M, Montalban X. Multiple sclerosis: current treatment algorithms. Curr Opin Neurol. 2011;24(3):230-237. doi: 10.1097/WCO.0b013e328346bf66 [DOI] [PubMed] [Google Scholar]
- 8.Ziemssen T, Derfuss T, de Stefano N, et al. Optimizing treatment success in multiple sclerosis. J Neurol. 2016;263(6):1053-1065. doi: 10.1007/s00415-015-7986-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mäurer M, Tiel-Wilck K, Oehm E, et al. Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419892077. doi: 10.1177/1756286419892077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller AE. Switching or discontinuing disease-modifying therapies for multiple sclerosis. Continuum (Minneap Minn). 2016;22(3):851-863. doi: 10.1212/CON.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 11.Saccà F, Lanzillo R, Signori A, et al. Determinants of therapy switch in multiple sclerosis treatment–naïve patients: a real-life study. Mult Scler. 2019;25(9):1263-1272. doi: 10.1177/1352458518790390 [DOI] [PubMed] [Google Scholar]
- 12.Chalmer TA, Baggesen LM, Nørgaard M, Koch-Henriksen N, Magyari M, Sorensen PS; Danish Multiple Sclerosis Group . Early versus later treatment start in multiple sclerosis: a register-based cohort study. Eur J Neurol. 2018;25(10):1262-e110. doi: 10.1111/ene.13692 [DOI] [PubMed] [Google Scholar]
- 13.Fernández O, Delvecchio M, Edan G, et al. Survey of diagnostic and treatment practices for multiple sclerosis in Europe. Eur J Neurol. 2017;24(3):516-522. doi: 10.1111/ene.13236 [DOI] [PubMed] [Google Scholar]
- 14.Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983. doi: 10.1212/01.wnl.0000345970.73354.17 [DOI] [PubMed] [Google Scholar]
- 15.Mikol DD, Barkhof F, Chang P, et al. ; REGARD study group . Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903-914. doi: 10.1016/S1474-4422(08)70200-X [DOI] [PubMed] [Google Scholar]
- 16.O’Connor P, Filippi M, Arnason B, et al. ; BEYOND Study Group . 250 μg or 500 μg Interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8(10):889-897. doi: 10.1016/S1474-4422(09)70226-1 [DOI] [PubMed] [Google Scholar]
- 17.He A, Merkel B, Brown JWL, et al. ; MSBase study group . Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307-316. doi: 10.1016/S1474-4422(20)30067-3 [DOI] [PubMed] [Google Scholar]
- 18.Hillert J, Stawiarz L. The Swedish MS registry—clinical support tool and scientific resource. Acta Neurol Scand. 2015;132(199):11-19. doi: 10.1111/ane.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magyari M, Joensen H, Laursen B, Koch-Henriksen N. The Danish Multiple Sclerosis Registry. Brain Behav. 2021;11(1):e01921. doi: 10.1002/brb3.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magyari M, Koch-Henriksen N, Sørensen PS. The Danish Multiple Sclerosis Treatment Register. Clin Epidemiol. 2016;8:549-552. doi: 10.2147/CLEP.S99500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murtonen A, Kurki S, Hänninen K, Soilu-Hänninen M, Sumelahti ML. Common comorbidities and survival in MS: risk for stroke, type 1 diabetes and infections. Mult Scler Relat Disord. 2018;19:109-114. doi: 10.1016/j.msard.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 22.Devonshire V, Havrdova E, Radue EW, et al. ; FREEDOMS study group . Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol. 2012;11(5):420-428. doi: 10.1016/S1474-4422(12)70056-X [DOI] [PubMed] [Google Scholar]
- 23.Shirani A, Zhao Y, Petkau J, et al. Multiple sclerosis in older adults: the clinical profile and impact of interferon beta treatment. Biomed Res Int. 2015;2015:451912. doi: 10.1155/2015/451912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swedish Medical Products Agency. Läkemedel vid multipel skleros (MS)—behandlingsrekommendation. Accessed October 2, 2019. https://www.lakemedelsverket.se/sv/behandling-och-forskrivning/behandlingsrekommendationer/sok-behandlingsrekommendationer/lakemedel-vid-mulitpel-skleros-ms%20-%20hmainbody1
- 25.The National Board of Health and Welfare . National Guideline for Healthcare of Multiple Sclerosis (MS) and Parkinson Disease (PD). National Board of Health and Welfare; 2016. [Google Scholar]
- 26.The Swedish MS Society. News. Accessed October 2, 2019. https://www.mssallskapet.se
- 27.Fox RJ, Miller DH, Phillips JT, et al. ; CONFIRM Study Investigators . Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087-1097. doi: 10.1056/NEJMoa1206328 [DOI] [PubMed] [Google Scholar]
- 28.Gold R, Kappos L, Arnold DL, et al. ; DEFINE Study Investigators . Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098-1107. doi: 10.1056/NEJMoa1114287 [DOI] [PubMed] [Google Scholar]
- 29.Confavreux C, O’Connor P, Comi G, et al. ; TOWER Trial Group . Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(3):247-256. doi: 10.1016/S1474-4422(13)70308-9 [DOI] [PubMed] [Google Scholar]
- 30.O’Connor P, Wolinsky JS, Confavreux C, et al. ; TEMSO Trial Group . Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293-1303. doi: 10.1056/NEJMoa1014656 [DOI] [PubMed] [Google Scholar]
- 31.Hersh CM, Love TE, Cohn S, et al. Comparative efficacy and discontinuation of dimethyl fumarate and fingolimod in clinical practice at 12-month follow-up. Mult Scler Relat Disord. 2016;10:44-52. doi: 10.1016/j.msard.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 32.Boster A, Nicholas J, Wu N, et al. Comparative effectiveness research of disease-modifying therapies for the management of multiple sclerosis: analysis of a large health insurance claims database. Neurol Ther. 2017;6(1):91-102. doi: 10.1007/s40120-017-0064-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson M, Fox RJ, Havrdova E, et al. Efficacy and safety of BG-12 (dimethyl fumarate) and other disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis: a systematic review and mixed treatment comparison. Curr Med Res Opin. 2014;30(4):613-627. doi: 10.1185/03007995.2013.863755 [DOI] [PubMed] [Google Scholar]
- 34.Buron MD, Chalmer TA, Sellebjerg F, et al. Comparative effectiveness of teriflunomide and dimethyl fumarate: a nationwide cohort study. Neurology. 2019;92(16):e1811-e1820. doi: 10.1212/WNL.0000000000007314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iacobaeus E, Arrambide G, Amato MP, et al. ; 2018 ECTRIMS Focused Workshop Group . Aggressive multiple sclerosis (1): towards a definition of the phenotype. Mult Scler. 2020;1352458520925369. doi: 10.1177/1352458520925369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arrambide G, Iacobaeus E, Amato MP, et al. ; 2018 ECTRIMS Focused Workshop Group . Aggressive multiple sclerosis (2): treatment. Mult Scler. 2020;1352458520924595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950-958. doi: 10.1002/ana.24651 [DOI] [PubMed] [Google Scholar]
- 38.Boremalm M, Juto A, Axelsson M, et al. Natalizumab, rituximab and fingolimod as escalation therapy in multiple sclerosis. Eur J Neurol. 2019;26(8):1060-1067. doi: 10.1111/ene.13936 [DOI] [PubMed] [Google Scholar]
- 39.Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75(3):320-327. doi: 10.1001/jamaneurol.2017.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58. doi: 10.1186/1471-2377-14-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group . Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233. doi: 10.1097/EDE.0000000000000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heydarpour P, Manouchehrinia A, Beiki O, et al. Smoking and worsening disability in multiple sclerosis: a meta-analysis. Acta Neurol Scand. 2018;138(1):62-69. doi: 10.1111/ane.12916 [DOI] [PubMed] [Google Scholar]
- 43.Magyari M, Sorensen PS. Comorbidity in multiple sclerosis. Front Neurol. 2020;11:851. doi: 10.3389/fneur.2020.00851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Östergren O, Martikainen P, Tarkiainen L, Elstad JI, Brønnum-Hansen H. Contribution of smoking and alcohol consumption to income differences in life expectancy: evidence using Danish, Finnish, Norwegian and Swedish register data. J Epidemiol Community Health. 2019;73(4):334-339. doi: 10.1136/jech-2018-211640 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Reason for Discontinuation of Index DMT by Cohort
eTable 2. Treatment Switch Product by Cohort
eMethods. Materials and Methods – Additional Details
eResults. Relapse, Subgroup and Sensitivity Analyses
eDiscussion. Additional Study Limitations