Abstract
Background:
Evidence-based interventions are needed to address the use of stimulants such as methamphetamine as a driver of onward HIV transmission and faster clinical HIV progression among sexual minority men. Prior randomized controlled trials with people living with HIV who use substances indicate that financial incentives provided during contingency management (CM) are effective for achieving short-term reductions in stimulant use and HIV viral load. However, the benefits of CM are often not maintained after financial incentives for behavior change end.
Purpose:
Data from a recently completed randomized controlled trial with 110 sexual minority men living with HIV who use methamphetamine was leveraged to examine mediator of the efficacy of a positive affect intervention for extending the benefits of CM.
Methods:
An autoregressive cross-lagged model was fit to determine if reductions in HIV viral load were mediated by intervention-related increases in positive affect and decreases in stimulant use measured in four waves over 15 months.
Results:
Higher baseline positive affect predicted significantly lower self-reported stimulant use immediately following the 3-month CM intervention period, even after controlling for self-reported stimulant use at baseline. Moreover, decreased stimulant use emerged as an independent predictor of long-term reductions HIV viral load at 15 months, even after adjusting for HIV viral load at baseline and the residual effect of the positive affect intervention.
Conclusions:
Findings underscore the importance of durable reductions in stimulant use as a primary intervention target that is essential for optimizing the clinical and public health benefits of HIV treatment as prevention.
Keywords: contingency management, men who have sex with men, methamphetamine, positive affect, treatment as prevention
1. Introduction
Treatment as Prevention (TasP) is an evidence-based, biomedical approach where achieving viral suppression with antiretroviral therapy (ART) leads to a 96% reduction of onward HIV transmission while optimizing health outcomes (Holmes et al., 2017; Eshleman et al., 2017; Rodger et al., 2016). In the modern ART era, many patients take one pill daily and can achieve viral suppression at lower rates of adherence, which has begun to address problems of resistance, toxicity, and tolerability (Looney et al., 2015; Nance et al., 2018). Along with dramatic increases in viral suppression in the United States, modern ART regimens have led to significant increases in life expectancy, such that a person diagnosed with HIV today is projected to have a life expectancy that approximates someone without HIV (Samji et al., 2013). With the evidence of effectiveness of TasP, the World Health Organization provided new guidelines for a TasP strategy, initiating all those diagnosed with HIV into ART, regardless of their CD4+ T-cell count (World Health Organization, 2012). In 2014, the United Nations Programme on HIV and AIDS (UNAIDS) launched the 90–90-90 targets for 2020 to: 1) diagnose 90% of all HIV positive persons; 2) deliver ART to 90% of those diagnosed; and 3) achieve viral suppression in 90% of those on ART (Levi et al., 2016). Despite the unprecedented effectiveness of TasP, expanded approaches are needed to optimize its clinical and public health benefits with marginalized, underserved populations such as people who use substances who are at highest risk of experiencing difficulties with managing HIV treatment and more likely to engage in transmission risk behavior (Carrico et al., 2016; Mayer et al., 2014; Shoptaw et al., 2013).
There is a preponderance of evidence linking stimulant use with amplified risk for HIV acquisition and onward transmission, especially among sexual minority men who engage in methamphetamine use (Colfax et al., 2010; Mayer et al., 2006). Furthermore, people living with HIV who use stimulants continue to struggle with engagement along the HIV care continuum. Not only do people who use stimulants initiate ART at lower CD4+ cell counts, but they are also less likely to be retained in HIV care (Carrico et al., 2011; Horvath et al., 2013). Difficulties with ART adherence and persistence, in turn, lead to elevated viral load and thereby increasing the risk for HIV clinical progression and onward HIV transmission (Carrico et al., 2014; Carrico et al., 2011; Cook et al., 2008; Mayer et al., 2014).
Despite the accruing evidence linking stimulant use with difficulties managing HIV treatment and amplified risk of onward HIV transmission, evidence-based interventions for sexual minority men who use stimulants are limited (Carrico et al., 2016; Colfax et al., 2010). Many interventions targeting stimulant use have employed contingency management (CM), an evidence-based behavioral approach wherein participants receive incentives as positive reinforcement for biologically confirmed abstinence of substance use (Prendergast et al., 2006). CM has also been shown effective for achieving short-term reductions in HIV viral load, but an important limitation is that reductions in viral load are not maintained after CM incentives are discontinued (Metsch et al., 2016; Petry et al., 2010).
Affect regulation has been recognized to play an important role in co-occurring substance use and HIV. Of particular interest, the Revised Stress and Coping Theory posits that positive affect (e.g., happiness, contentment, gratitude) may bolster coping efforts during experiences of chronic stress, assisting with managing stressful situations such as stimulant withdrawal, increasing responsivity to non-substance-related sources of reward, and improving HIV disease management (Folkman & Moskowitz, 2000). For instance, in one randomized controlled trial (RCT) that enrolled people newly diagnosed with HIV, a positive affect intervention significantly improved psychological adjustment and decreased antidepressant medication use compared to an attention-control condition (Moskowitz et al., 2017). Moreover, several observational studies have found that positive affect is directly or indirectly associated with decreased stimulant use (Carrico et al., 2013), better ART adherence (Carrico et al., 2010), increased odds of linkage to HIV care and lower HIV viral load (Carrico, & Moskowitz, 2014), and decreased risk of onward HIV transmission (Batchelder et al., 2018).
Affect Regulation Treatment to Enhance Methamphetamine Intervention Success (ARTEMIS) is a positive affect intervention designed to boost and extend the benefits of CM in sexual minority men living with HIV who use methamphetamine. Findings from a recently completed RCT documented the efficacy of the ARTEMIS positive affect intervention for improving mindfulness and reducing methamphetamine craving during CM (Carrico et al., 2018b). Furthermore, the ARTEMIS positive affect intervention has demonstrated efficacy for achieving durable and clinically meaningful reductions in HIV viral load that were paralleled by increases in positive affect and decreases in stimulant use (Carrico et al., 2019b). The primary objective of the present longitudinal study was to examine intervention-related mechanisms accounting for long-term reductions in HIV viral load. Specifically, we hypothesized that intervention-related increases in positive affect as well as reductions in stimulant use would mediate the efficacy of the intervention for reducing viral load over 15 months.
2. Methods
2.1. Recruitment and screening.
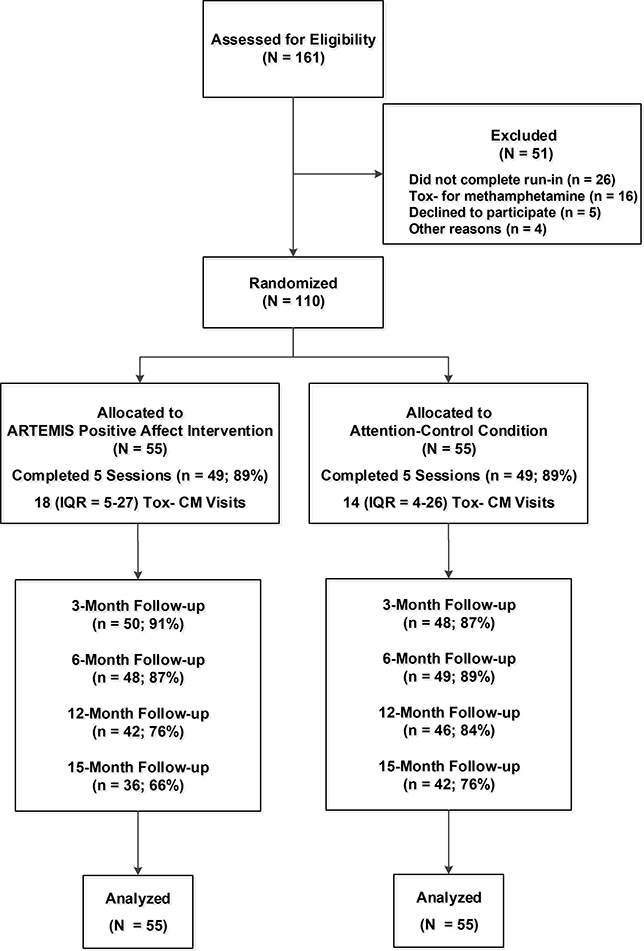
From 2013–2017, a total of 184 individuals were recruited for this RCT from a community-based CM program, with flyers and palm cards distributed in the community, and via an incentivized snowball sampling method where eligible participants received up to $30 for referring other eligible participants. To be eligible for this Phase II RCT, participants were required to meet the following inclusion criteria: 1) 18 years of age or older; 2) report anal sex with a man in the past 12 months; 3) speak English; 4) provide documentation of HIV-positive serostatus (i.e., letter of diagnosis or being on ART medications other than tenofovir disoproxil fumarate/emtricitabine that were matched via photo identification); and 5) provide a urine or hair sample that was reactive for methamphetamine. As shown in Figure 1, of the 161 participants who completed a screening visit 16 (10%) were excluded because they did not test reactive for methamphetamine, five (3%) declined to participate, and four (2%) did not meet other inclusion criteria. Participants could receive up to $400 and an iPod shuffle for completing all research-related visits for this RCT.
Figure 1:
Screening, randomization, and follow-up for ARTEMIS participants
All relevant procedures were approved by the Institutional Review Board for University of California, San Francisco with reliance agreements from the Univesity of Miami and Northwestern University (www.clinicaltrials.gov identifier NCT01926184). All participants completed a signed informed consent and a certificate of confidentiality was provided by the National Institute on Drug Abuse. The University of California, Los Angeles Data Safety and Monitoring Board for Addiction Medicine held annual meetings to review participant-related events and overall progress for this RCT. There were no adverse events or serious adverse events.
2.2. Run-in period and randomization.
Eligible participants completed a waiting period prior to randomization (i.e., run-in) that entailed five separate visits: 1) a baseline assessment with a peripheral venous blood sample; 2) three CM urine screening visits (regardless of the toxicology results); and 3) a separately scheduled randomization visit where the first session of the positive affect intervention or attention-control condition was delivered after randomization. Participants who did not complete the run-in period were not randomized. Of the 136 participants who were eligible and consented to participate in the RCT, 110 (81%) completed the run-in period and were randomized during the first eight weeks of CM. Randomization was accomplished using a computer-generated sequence with randomly permuted block sizes of 2, 4, and 6 to guard against subversion. Only the study data manager had access to the computer- based randomization algorithm.
2.3. Assessments.
Participants completed a baseline assessment during the run-in period that included self-report measures, a urine sample for on-site toxicology screening, and a peripheral venous blood sample. Participants completed follow-up assessments at three, six, 12, and 15 months after the beginning of CM that included computer-based administration of self-report measures and a urine sample for on-site toxicology screening. Peripheral venous blood samples to measure HIV disease markers were collected at baseline, six, 12, and 15 months. In the 110 participants randomized, follow-up rates at three (89%), six (88%), 12 (80%), and 15 (71%) months were acceptable with no significant differences between the experimental conditions. Follow-up assessments were completed in August of 2018.
2.4. Community-based CM program.
This RCT was conducted in partnership with a community-based, 3-month CM program for sexual minority men who use methamphetamine that is operated by the San Francisco AIDS Foundation (Gomez et al., 2017). CM was delivered separately from the individual intervention or attention-control sessions. Urine sample collection is directly observed by CM program staff. The voucher for the initial sample that was non-reactive for methamphetamine and cocaine metabolites was worth $2.00. Vouchers increased in value by 25 cents for each consecutive stimulant-free sample to a maximum of $10.00. Participants earned an $8.50 bonus voucher for every third consecutive stimulant-free sample. Participants who provided a reactive urine toxicology result for stimulants could return to their place in the escalating reinforcement schedule after producing three consecutive urine samples that were non-reactive for methamphetamine and cocaine metabolites. The total possible reinforcement of stimulant abstinence during thrice-weekly urine screening over the three months was $330.
2.5. Positive affect intervention.
ARTEMIS is a multi-component, individually delivered 5-session intervention designed to increase positive affect. The ARTEMIS intervention protocol consists of eight core skills that have been shown to increase positive affect and improve psychological adjustment in prior clinical research (Moskowitz et al., 2017). Informed by prior research examining the efficacy of mindfulness-based relapse prevention (Moskowitz et al., 2017), participants also completed meditation exercises during ARTEMIS intervention sessions to further enhance mindfulness and assist individuals in coping more effectively with methamphetamine withdrawal. Participants received a workbook and an iPod shuffle that was pre-loaded with meditation exercises. Participants received $20 cash for completing each individual session, and 49 (89%) completed all five sessions.
2.6. Attention-control condition.
The attention-control condition consisted of five sessions that included face-to-face administration of psychological measures and neutral writing exercise (Boiler et al., 2013). We chose an attention-control to provide comparable contact time with study staff and identical incentives. Participants were instructed to write as if they were reporting facts without going into thoughts or feelings about the events (e.g., plans for the next 24 hours). Participants received $20 cash for completing each attention-control session and an iPod with three pre-loaded popular songs. A total of 49 (89%) participants completed all five attention-control sessions.
2.7. Fidelity monitoring.
Facilitators with master’s level training in public health or counseling were provided with a detailed manual. Audio recordings of ARTEMIS intervention sessions were reviewed by a clinical supervisor during weekly individual supervision to provide feedback on delivery of intervention content and process-oriented techniques. Monthly group supervision meetings provided opportunities for case presentation and ongoing discussions about optimizing the delivery of both the ARTEMIS intervention skills and attention-control protocol. Audio recordings of intervention sessions were reviewed by an independent fidelity monitor to provide more detailed feedback to facilitators regarding adherence to the ARTEMIS intervention content, interpersonal skills, rapport, and session flow. A total of 71 of the 259 competed ARTEMIS intervention sessions (27%) were coded using fidelity rating checklists with detailed feedback provided to facilitators.
2.8. Measures.
The primary outcome was log10 HIV viral load at 15 months, which was measured using the Abbott RealTime HIV-1 reverse transcription -polymerase chain reaction assay. This assay reliably detects HIV RNA from 40 to 10,000,000 copies/mL. Positive affect was measured using the 26-item Differential Emotions Scale (DES) to assess the frequency of positive and negative affect during the past week, wherein participants rated how frequently they felt a particular emotion during the past week (Cronbach’s alpha = 0.89). Finally, participants self-reported the number of days using powder cocaine, crack-cocaine, or methamphetamine in the past 30 days. The highest number of days participants reported using any of these stimulants was selected.
2.9. Statistical analyses.
All statistical analyses were performed using SAS9.4 (SAS Institute, Cary NC). Consistent with our previously reported findings (Carrico et al., 2019b), intent-to-treat analyses for positive affect, stimulant use, and viral load were initially conducted (Table 2) to determine the mean difference between treatment arms at each time point (i.e., baseline, 3-month, 6-month, 12-month), estimated using generalized linear modeling (PROC GLM in SAS9.4). Then, in order to model the covariance structure, bivariate repeated measurement models with a Kronecker product covariance structure were fit to examine the intra- and inter-variable correlations of the measurement errors (PROC MIXED / REPEATED statement in 9.4). The Kronecker product covariance structure was chosen for its advantages in modeling the correlation pattern separately for each repeated factor with multivariate repeated measures data, which improves accuracy, makes model fitting easier, and allows for the evaluation of the joint evolution of variables (Galecki, 1994; Verbeke, Fieuws, Molenberghs, & Davidian, 2014). Moreover, the approach uses fewer parameters than an unstructured model, with the Kronecker product combining the factor-specific covariance structures into an overall correlation model (Galecki, 1994; Verbeke et al., 2014). In Table 3, the interrelationship among the three variables (i.e., positive affect, stimulant use, and viral load) were noted on the estimates out of the random effects portion, with slope parameters reflecting the average change of the variable over time. For the analyses, we used an unequal time variable coding for assessments at baseline, 3-, 6-, 12- and 15 months. Although there were no differences in results between the coded time and days elapsed from baseline, we obtained a better goodness-of-fit statistic (e.g., the AIC was lower when we used coded time variable). As such, Table 3 presents the results from the models using coded time rather than days elapsed from the baseline visit.
Table 2:
Differences in means for positive affect, stimulant use, and viral load between the treatment and control groups for each time point (i.e., baseline, 3-month, 6-month, and 12 months)
| Variable | Group | Baseline | 3-month | 6-month | 12-month | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||||
| Positive Affect | Treatment | 33.27 | 7.62 | 34.82 | 8.41 | 35.44 | 8.12 | 35.17 | 10.11 |
| Control | 31.25 | 8.76 | 32.27 | 9.47 | 30.96 | 8.84 | 30.74 | 11.19 | |
|
| |||||||||
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||||||
|
| |||||||||
| Mean difference between group by study visit ‡ | 2.02 (1.57) | 2.55 (1.81) | 4.49 (1.73)** | 4.43 (2.28)* | |||||
|
| |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||||
| Days of Stimulant use in past 30 days | Treatment | 10.31 | 9.89 | 5.30 | 7.68 | 4.55 | 7.72 | 5.33 | 8.05 |
| Control | 9.18 | 9.26 | 8.50 | 9.29 | 8.12 | 8.91 | 9.24 | 10.59 | |
|
| |||||||||
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||||||
|
| |||||||||
| Mean difference between group by study visit ‡ | 1.13 (1.83) | −3.20 (1.72) | −3.57 (1.70)* | −3.91 (2.02)* | |||||
|
| |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||||
| Viral Load | Treatment | 1.04 | 1.22 | - | - | 0.69 | 0.75 | 0.93 | 1.21 |
| Control | 1.42 | 1.40 | - | - | 1.82 | 1.61 | 1.52 | 1.51 | |
|
| |||||||||
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||||||
|
| |||||||||
| Mean difference between group by study visit ‡ | −0.39 (0.25) | - | −1.13 (0.27)*** | −0.59 (0.30)* | |||||
p<0.05
p<0.01
p<0.001
estimated using generalized linear modeling
Table 3:
Bivariate mixed models with a Kronecker product covariance (after controlling for treatment group)
| Positive Affect (x) vs. Stimulant Use (y) | Positive Affect (x) vs. Viral Load (z) | Stimulant Use (y) vs. Viral Load (x) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| UN@AR | UN@UN | UN@AR | UN@UN | UN@AR | UN@UN | |
|
| ||||||
| Fixed effect | ||||||
| Slopex | 3.05(0.52)*** | 2.48(0.41)*** | 4.13(0.65)*** | 6.56(0.51)*** | ||
| Slopey | −3.03(0.32)*** | −2.86(2.60)*** | −0.56(0.27)* | −0.34(0.24) | ||
| Slopez | −0.66(0.14)*** | −0.94(0.13)*** | −0.13(0.13) | −0.10(0.12) | ||
|
| ||||||
| Random effect (covariance) | ||||||
|
| ||||||
| σxy | −71.62(8.50)*** | −87.15(12.21)*** | ||||
| σxz | −57.43(10.12)*** | −62.66(15.31)*** | ||||
| σyz | 7.53(2.32)* | 9.86(2.87)*** | ||||
|
| ||||||
| Fit statistics | ||||||
| −2LnL | 5605.5 | 5559.7 | 4368.4 | 4298.7 | 51.93.9 | 5148.9 |
| AIC | 5613.5 | 5593.7 | 4376.4 | 4332.7 | 5201.9 | 5182.9 |
| BIC | 5624.3 | 5639.6 | 4387.2 | 4378.6 | 5212.7 | 5228.8 |
p<0.05
p<0.01
p<0.001
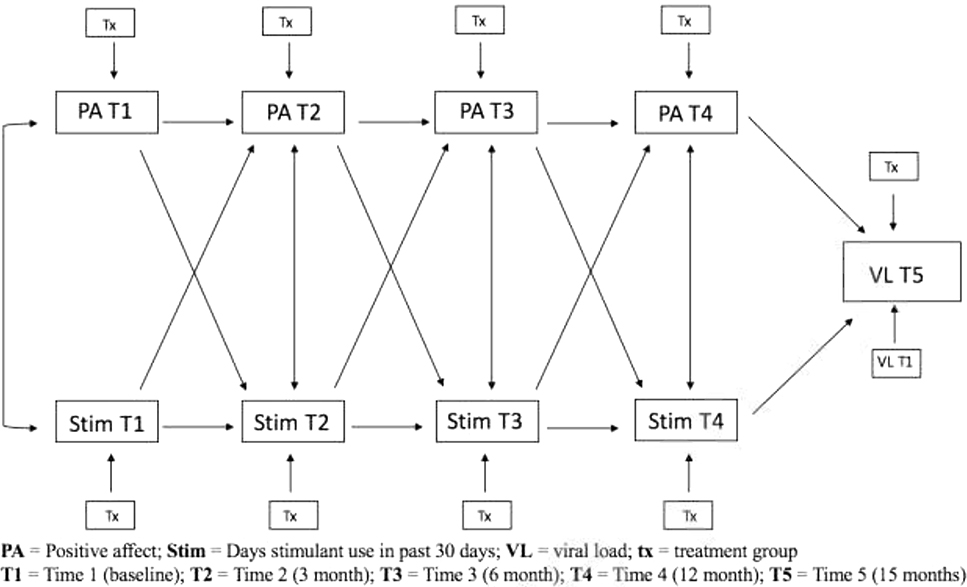
To test our hypothesized model (see Figure 2), an autoregressive cross-lagged model was fit so that measurments of both positive affect and stimulant use at a given time point t were simultaneously regressed on measurements of these two variables at the immediately preceding time point, t-1 (Selig & Little, 2012). The PROC CALIS procedure was used on SAS9.4.The autoregressive cross-lagged model provided information about whether positive affect at time t-1 predicted stimulant use at time t, above and beyond the t-1 observations of stimulant use, and similarly, whether stimulant use at time t-1 predicted positive affect at time t, over and above the measurements of positive affect at time t-1. In addition, our model included treatment group as a exogenous predictor for each time point measurement. Ultimately, the autoregressive cross-lagged model was used to investigate whether the cross-lagged paths significantly improved the overall fit of the model. Any significant paths accounted for the correlations among the variables within a wave and controlled for previous scores on the outcome variable and other predictors in the model to allow for the estimation of the unique relationship between study variables.
Figure 2:
Hypothesized autoregressive cross-lagged model
With regards to invariance testing, we assessed whether the pattern of results as invariant across time. Each cross-lag path was constrained to be equal across time and compared to an unconstrained model where paths were left free to vary. A chi-square difference test of relative fit was used to ascertain whether there was a difference in model fit between the constrained and unconstrained models. Non-significance would indicate no difference in fit between the two models and the more parsimonious constrained model would be kept for further hypothesis testing. Model fit was determined using the chi-square value, degrees of freedom, comparative fit index (CFI), and the root mean square error of approximation (RMSEA) indicators of goodness-of-fit (Hu & Bentler, 1999), and we used Hu and Bentler’s recommended cut-off criteria for a well-specified or close-fitting model as CFI/TLI > 0.95 and a RMSEA < 0.06. To handle missing data, we used full information maximum likelihood estimation (FIML).
3. Results
Table 1 presents the study sample characteristics. Overall, participants were 43 years old (SD = 9, range 23 to 59), identified as non-white (57.3%), identified as exclusively gay (76%), had more than a high school education (77%), had an income less than $16,000 (65%), and had been diagnosed with HIV for 13 years (SD = 9, range= .04 to 36.15). Table 2 presents the differences in means of positive affect, stimulant use, and viral load between the treatment and control groups for each time point (i.e., baseline, 3-month, 6-month, and 12 months). At 6 months, significant differences are observed between treatment groups for positive affect (Mean (SE) = 4.49 (1.73),p < 0.01), stimulant use (Mean (SE) = −3.57 (1.70),p < 0.05), and HIV viral load (Mean (SE) = −1.13 (0.27), p < 0.001). The same is observed at 12 months for positive affect (Mean (SE) = 4.43 (2.28),p < 0.05), stimulant use (Mean (SE) = −3.91 (2.02),p < 0.05), and HIV viral load (Mean (SE) = −0.59 (0.30), p < 0.05). Consistent with our previously reported findings (Carrico et al., 2019b), mean differences over time were found significant.
Table 1:
Baseline characteristics of ARTEMIS trial participants
| All (n=110) | ARTEMIS (n=55) | Attention-Control (n=55) | p-value | |
|---|---|---|---|---|
|
| ||||
| M (SD) | M (SD) | M (SD) | ||
|
| ||||
| Age | 43.2 (8.9) | 43.2 (9.2) | 43.2 (8.5) | 0.88 |
| Time since HIV diagnosis (Years) | 12.9 (8.6) | 13.4 (8.9) | 12.5 (8/4) | 0.62 |
| CD4+ T-cell count (cells/mm3) | 620.9 (298.1) | 642.9 (272.8) | 639.9 (313.6) | 0.99 |
|
| ||||
| n (%) | n (%) | n (%) | ||
|
| ||||
| Race/Ethnicity | 0.12 | |||
| Black/African American | 18 (16.4) | 9 (8.2) | 9 (8.2) | |
| White | 47 (42.7) | 18 (32.7) | 29 (52.7) | |
| Hispanic/Latino | 32 (29.1) | 21 (38.2) | 11 (20.0) | |
| Other ethnic minority | 13 (11.8) | 7 (12.7) | 6 (10.9) | |
|
| ||||
| Education | 0.62 | |||
| Less than high school | 8 (7.3) | 5 (9.1) | 3 (5.5) | |
| HS graduate | 17 (15.5) | 8 (14.6) | 9 (16.4) | |
| Some college/trade school | 57 (51.8) | 25 (45.5) | 32 (58.2) | |
| College graduate | 17 (15.5) | 10 (18.2) | 7 (12.7) | |
| Post graduate | 11 (10.0) | 7 (12.7) | 4 (7.3) | |
|
| ||||
| Income | 0.26 | |||
| < $4,999 | 16 (14.5) | 8 (14.8) | 8 (14.6) | |
| $5,000 – $11,999 | 28 (35.5) | 9 (16.7) | 19 (34.6) | |
| $12,000 – $15,999 | 27 (24.5) | 14 (25.9) | 13 (23.6) | |
| $16,000 – $24,999 | 12 (10.9) | 6 (11.1) | 6 (10.9) | |
| $25,000 – $34,999 | 9 (8.2) | 4 (7.4) | 5 (9.1) | |
| $35,000 – $49,999 | 9 (8.2) | 7 (13.0) | 2 (3.6) | |
| >$50,000 | 8 (7.3) | 6 (11.1) | 2 (3.6) | |
|
| ||||
| HIV viral load ≥ 200 copies/mL | 15 (13.6) | 6 (11.1) | 9 (16.7) | 0/14 |
ARTEMIS = affect regulation to enhance methamphetamine intervention success
In order to examine the intra- and inter-marker correlations of the measurement errors, bivariate repeated measurement models with a Kronecker product covariance structure were conducted. Table 3 presents the results of the fitted mixed models, where the slope parameters reflect the average change of the variables over time, and the estimated correlation coefficients of the random effects portion reflect the interrelationships among the three variables. Based on the fit statistics (−2 Res Log Likelihood, AIC, BIC; See Table 3), the models with a continuous-time first-order autoregressive covariance structure (i.e., UN@AR) provide a good fit to the data. The results also indicate a strong significant negative correlation between positive affect and stimulant use (σxy = −71.62, p < 0.001); a strong significant negative correlation between positive affect and viral load (σxz = −57.43,p < 0.001); and a significant positive correlation between stimulant use and viral load (σyz = 7.53, p < 0.05). Although the models with an unstructured covariance structure (UN@UN) provide a better fit to the data, the continuous-time first-order autoregressive covariance structure was chosen for its parsimony in the number of covariance parameters as well as its common utilization in studies with multivariate longitudinal data (Galecki, 1994; Verbeke et al., 2014).
3.1. Final Model
Figure 3 graphically depicts the final parsimonious model outlining the longitudinal relationship between positive affect and stimulant use on the outcome viral load. The model showed acceptable fit to the data (x2(35) = 74.36, p < 0.0001; CFI = 0.87; RMSEA = 0.08). For all time waves, prior positive affect and stimulant use predicted later positive affect (Autocorrelation [AR] = 0.36, [95% CI = 0.29 – 0.44]) and stimulant use (AR = 0.43, [95% CI = 0.34 – 0.51]), respectively. The longitudinal path for the effect of positive affect on stiimulant use (T1 to T2) was significant, suggesting that higher positive affect at baseline predicted lower stimulant use at 3 months, even after controlling for prior stimulant use at T1. All other cross- lagged paths were non-significant
Figure 3:
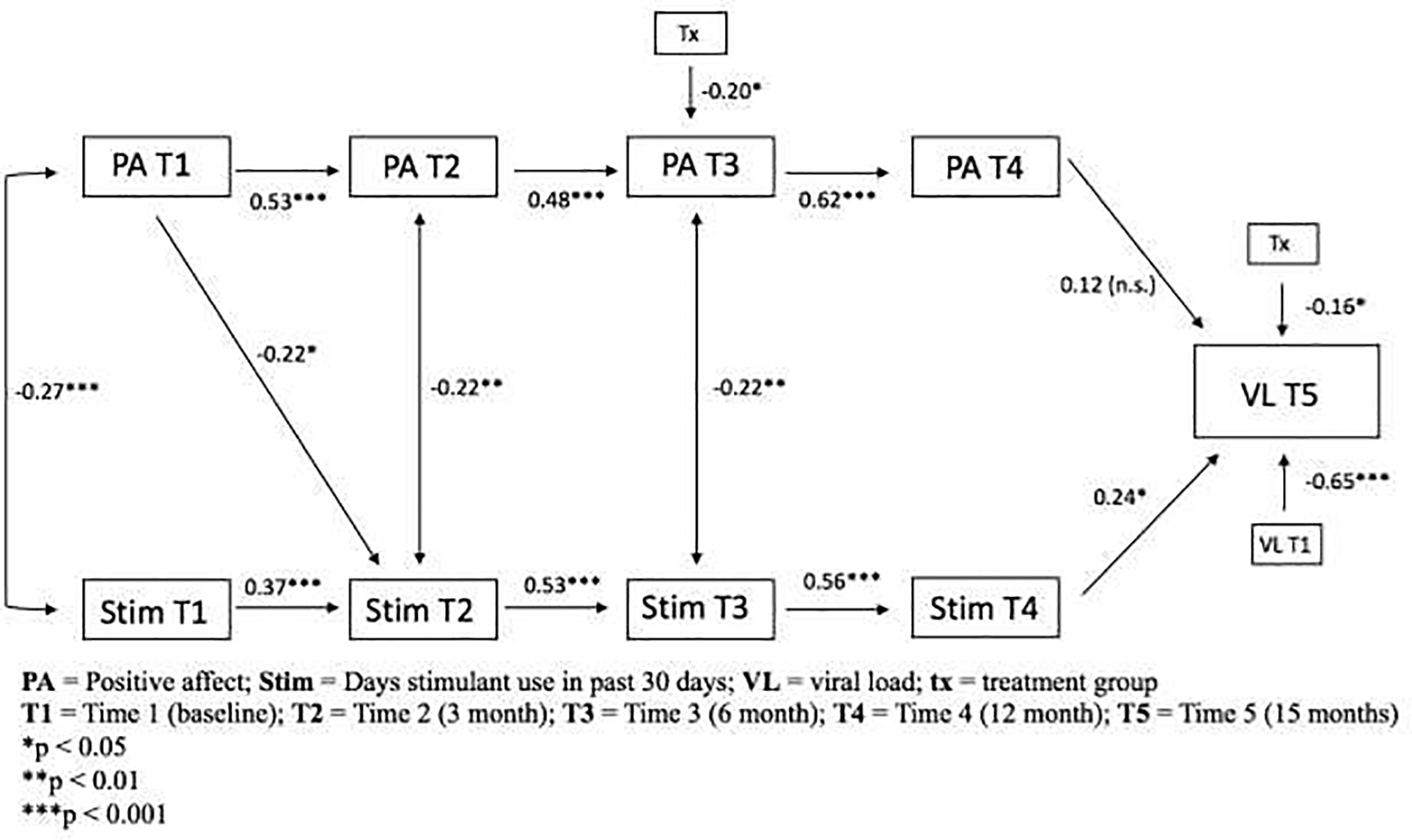
Final parsimonious autoregressive cross-lagged model
4. Discussion
Results from this RCT demonstrate that higher baseline positive affect predicts significantly lower self-reported stimulant use immediately following the 3-month CM intervention period, even after controlling for self-reported stimulant use at baseline. Moreover, decreased stimulant use emerged as an independent predictor of long-term reductions HIV viral load at 15 months, even after adjuring for HIV viral load at baseline and the residual effect of the ARTEMIS positive affect intervention. We have previously demonstrated the efficacy of the ARTEMIS positive affect intervention for achieving durable and clinically meaningful reductions in HIV viral load following CM that were paralleled by significant increases in positive affect and decreases in stimulant use (Carrico et al., 2019b). Interestingly, those randomized to receive the ARTEMIS positive affect intervention continued to display lower HIV viral load at 15 months, even after adjusting for positive affect and stimulant use.
Higher baseline positive affect emerged as an independent predictor of lower self-reported stimulant use at three months. In other words, positive affect was an important prognostic indicator for greater success in reducing stimulant use during CM. It may be that higher positive affect serves as an indicator of positive psychological traits or decreased severity of stimulant use at baseline. Incorporating positive affect assessments into clinical intakes may assist providers in deciding which patients are most likely to benefit from CM. Further clinical research is needed to establish clinically validated cut points for the level at which positive affect is linked to better outcomes following CM and other evidence-based approaches to substance use disorder treatment.
Another important finding from this study was that stimulant use independently predicted higher viral load at 15 months, which underscores the central importance of reducing stimulant use in this population. This lends further empirical support to a large body of research documenting the association of stimulant use with elevated virai load among people living with HIV (Ellis et al., 2003; Carrico et al., 2011), even in the TasP era (Carrico et al., 2019a). Although positive affect was associated with lower self-reported stimulant, there were no direct or indirect effects of positive affect on viral load at 15 months. These findings underscore the importance of achieving durable reductions in stimulant use as a primary intervention target for interventions designed to optimize the clinical and public health benefits of TasP in sexual minority men living with HIV who use methamphetamine. Further clinical research is needed to test novel approaches to optimize the durable efficacy behavioral and biomedical interventions for addressing chronic, relapsing stimulant use disorders in people living with HIV.
Interestingly, those randomized to receive the ARTEMIS positive affect intervention during CM continued to display significantly lower HIV viral load at 15 months, even after adjusting for baseline viral load and self-reported stimulant use. This highlights the possibility that there may be other unmeasured mediational pathways that partially explain the efficacy of the ARTEMIS positive affect intervention for achieving durable and clinically meaningful reductions in HIV viral load following CM. Informed by Revised Stress and Coping Theory,more definitive randomized controlled trials are needed to test other plausible social and psychological mediators of the ARTEMIS positive affect intervention. Elucidating the key theory-based mediators would advance our understanding of the underlying mechanism(s) of the multi-component ARTEMIS positive affect intervention, which would guide efforts to augment its effectiveness.
Findings from this RCT should be interpreted in context of some limitations. Given the modest sample size, tests of mediation did not have sufficient statistical power to test indirect effects. Because this RCT was conducted in San Francisco and focused exclusively on sexual minority men living with HIV who use stimulants, important questions remain regarding the generalizability of these results in other settings as well as with other populations. Clearly, more definitive RCTs of this promising model of delivering an ARTEMIS positive affect intervention during CM for optimizing TasP are needed.
Despite these limitations, this RCT provides important information to guide clinical practice with sexual minority men living with HIV who use methamphetamine. Positive affect at baseline is a key prognostic indicator for greater success during CM. Incorporating measures of positive affect into clinical intake assessments could guide the development of comprehensive care plans to address co-occurring stimulant use and HIV in sexual minority men. Furthermore, stimulant use disorders are chronic, relapsing conditions that often require ongoing treatment to achieve durable and clinically meaningful reductions in stimulant use. Findings from this study underscore the importance of durable reductions in stimulant use as a primary intervention target that is essential for optimizing the clinical and public health benefits of TasP.
Highlights.
Higher positive affect predicts significantly lower stimulant use after CM
Decreased stimulant use independently predicts long term reductions in viral load
Durable reductions in stimulant use should be used as a primary treatment target
5. Acknowledgments
This project was supported by the National Institute on Drug Abuse (R01-DA033854; Carrico, Woods, and Moskowitz, PIs) and the National Institute of Mental Health (K24-MH093225; Moskowitz, PI). Additional support for this project was provided by the University of California, San Francisco Center for AIDS Research’s Virology Core (P30-AI027763; Volberding, PI), the Miami Center for AIDS Research (P30-AI073961; Pahwa, PI), and the Center for HIV Research and Mental Health (P30-MH116867; Safren, PI). This project was investigator initiated without directives from the funding sources.
Role of Funding Source:
This project was supported by the National Institute on Drug Abuse (R01-DA033854; Carrico, Woods, and Moskowitz, PIs). Additional support for this project was provided by the University of California, San Francisco Center for AIDS Research’s Virology Core (P30-AI027763; Volberding, PI), the Miami Center for AIDS Research (P30-AI073961; Pahwa, PI), and the Center for HIV Research and Mental Health (P30-MH116867; Safren, PI). This project was investigator initiated without directives from the funding sources.
Footnotes
Author Disclosures
6. Compliance with Ethical Standards
Disclosure of potential conflicts of interest:
All authors of the present manuscript declare that they have no conflict of interest.
Research involving Human Participants and/or Animals:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent:
Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batchelder AW, Carrico AW, Acree M, Hecht FM, Moskowitz JT, 2018. Positive and negative self-conscious emotion and transmission risk following HIV diagnosis. AIDS Behav. 22, 5, 1496–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolier L, Haverman M, Westerhof GJ, Riper H, Smit F, Bohlmeijer E, 2013. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health, 13, 1, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Gomez W, Jain J, Shoptaw S, Discepola MV, Olem D, … & Evans JL, 2018b. Randomized controlled trial of a positive affect intervention for methamphetamine users. Drug Alcohol Depend. 192, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Hunt PW, Neilands TB, Dilworth S. e., Martin JN, Deeks SG, & Riley ED, 2019a. Stimulant use and viral suppression in the era of universal antiretroviral therapy. J Acquir Immune Defic Syndr. 80, 1, 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Johnson MO, Colfax GN, Moskowitz JT, 2010. Affective correlates of stimulant use and adherence to anti-retroviral therapy among HIV-positive methamphetamine users. AIDS Behav. 14, 4, 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Moskowitz JT, 2014. Positive affect promotes engagement in care after HIV diagnosis. Health Psychol. 33, 7, 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Neilands TB, Dilworth SE, Evans JL, Jain JP, Gomez W, … Moskowitz JT, 2019b. Randomized controlled trial of a positive affect intervention to reduce HIV viral load among sexual minority men who use methamphetamine. J Int AIDS Soc. 22, 12, e25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Riley ED, Johnson MO, Charlebois ED, Neilands TB, Remien RH, … Rotheram-Borus MJ, 2011. Psychiatric risk factors for HIV disease progression: the role of inconsistent patterns of anti-retroviral therapy utilization. JAIDS. 56, 2, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Shoptaw S, Cox C, Stall R, Li X, Ostrow DG, … & Plankey MW (2014). Stimulant use and progression to AIDS or mortality after the initiation of highly active anti-retroviral therapy. J Acquir Immune Defic Syndr (1999), 67(5), 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Woods WJ, Siever MD, Discepola MV, Dilworth SE, Neilands TB, Miller N and Moskowitz JT, 2013. Positive affect and processes of recovery among treatment-seeking methamphetamine users. Drug and Alcohol Dependence, 132(3), 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Zepf R, Meanley S, Batchelder A, Stall R, 2016. When the party is over: A systematic review of behavioral interventions for substance-using men who have sex with men. J Acquir Immune Defic Syndr. 73, 3, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colfax G, Santos GM, Chu P, Vittinghoff E, Pluddemann A, Kumar S, Hart C, 2010. Amphetamine-group substances and HIV. The Lancet. 376, 9739, 458–474. [DOI] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, … Plankey MW, 2008. Crack cocaine, disease progression, and mortality in a multi-center cohort of HIV-1 positive women. AIDS. 22, 11, 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, & HIV Neurobehavioral Research Center Group, 2003. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 188, 12, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Eshleman SH, Wilson EA, Zhang XC, Ou SS, Piwowar-Manning E, Eron JJ, … Kumarasamy N, 2017. Virologic outcomes in early antiretroviral treatment: HPTN 052. HIV Clinical Trials. 18, 3, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT, 2000. Positive affect and the other side of coping. Am Psychol. 55, 6, 647. [DOI] [PubMed] [Google Scholar]
- Galecki AT, 1994. General class of covariance structures for two or more repeated factors in longitudinal data analysis. Communications in Statistics-Theory and Methods. 23, 11, 3105–3119. [Google Scholar]
- Gómez W, Olem D, Andrews R, Discepola MV, Ambrose P, Dilworth SE, Carrico AW, 2018. Optimizing contingency management with methamphetamine-using men who have sex with men. Cogn Behav Pract. 25, 2, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Hallett T, Walensky R, Barnighausen T,pillay Y, Cohen M, 2017Effectiveness and cost-effectiveness of treatment as prevention for HIV. Major Infectious Diseases. 3. [PubMed] [Google Scholar]
- Horvath KJ, Carrico AW, Simoni J, Boyer EW, Amico KR, Petroll AE, 2013. Engagement in HIV medical care and technology use among stimulant-using and nonstimulant-usnig men who have sex with men. AIDS Res Treat. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Bentler PM, 1999. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 6, 1, 1–55. [Google Scholar]
- Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A, 2016. Can the UNAIDS 90–90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Global Health. 1, 2, e000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney D, Ma A, Johns S, 2015. HIV therapy—the state of art. In The Future of HIV-1 Therapeutics (pp. 1–29). Springer, Cham. [Google Scholar]
- Mayer KH, Colfax G, Guzman R, 2006. Club drugs and HIV infection: a review. Clinical Infectious Diseases. 42, 10, 1463–1469 [DOI] [PubMed] [Google Scholar]
- Mayer KH, Skeer MR, O’cleirigh C, Goshe BM, & Safren SA, 2014. Factors associated with amplified HIV transmission behavior among American men who have sex with men engaged in care: implications for clinical providers. Annals of behavioral Medicine. 47, 2, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsch LR, Feaster DJ, Gooden L, Matheson T, Stitzer M, Das M, … Nijhawan AE, 2016. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: a randomized clinical trial. JAMA. 316, 2, 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz JT, Carrico AW, Duncan LG, Cohn MA, Cheung EO, Batchelder A, … Folkman S, 2017. Randomized controlled trial of a positive affect intervention for people newly diagnosed with HIV.J Consult Clin Psychol. 85, 5, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance RM, Delaney JC, Simoni JM, Wilson IB, Mayer KH, Whitney BM, … Christopoulos KA, 2018. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015: a cohort study. Annals of Interal Medicine. 169, 6, 376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Weinstock J, Alessi SM, Lewis MW, Dieckhaus K, 2010. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol. 85, 1, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J, 2006. Contingency management for treatment of substance use disorders: A meta- analysis. Addiction. 101, 11, 1546–1560. [DOI] [PubMed] [Google Scholar]
- Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Van Lunzen J, … Asboe D, 2016. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 316, 2, 171–181 [DOI] [PubMed] [Google Scholar]
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, … Justice A, 2013. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One. 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig JP, Little TD, 2012. Autoregressive and cross-lagged panel analysis for longitudinal data.
- Shoptaw S, Montgomery B, Williams CT, El-Bassel N, Aramrattana A, Metzger DS, … Strathdee SA, 2013. Not just the needle: the state of HIV prevention science among substance users andfuture directions. JAIDS. 63, 2, S174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke G, Fieuws S, Molenberghs G, Davidian M, 2014. The analysis of multivariate longitudinal data: A review. Stat Methods Med Res. 23, 1, 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization., 2012. Programmatic update: antiretroviral treatment as prevention (TASP) of HIV and TB: executive summary (No. WHO/HIV/2012.12). Geneva: World Health Organization. Accessed on January 8, 2020. https://apps.who.int/iris/handle/10665/70904 [Google Scholar]