Abstract
Background. Currently, bevacizumab (BEV), an antiangiogenic agent, is used as an adjunctive therapy to re-irradiation and surgery in patients with recurrent high-grade gliomas (rHGG). BEV has shown to decrease enhancement on MRI, but it is often unclear if these changes are due to tumor response to BEV or treatment-induced changes in the blood brain barrier. Preliminary studies show that amino acid PET can aid in distinguishing these changes on MRI.
Methods. The authors performed a systematic review of PubMed and Embase through July 2020 with the search terms ‘bevacizumab’ or ‘Avastin’ and ‘recurrent glioma’ and ‘PET,’ yielding 38 papers, with 14 meeting inclusion criteria.
Results. Thirteen out of fourteen studies included in this review used static PET and three studies used dynamic PET to evaluate the use of BEV in rHGG. Six studies used the amino acid tracer [18F]FET, four studies used [11C]MET, and four studies used [18F]FDOPA.
Conclusion. [18F]FET, [11C]MET, and [18F]FDOPA PET in combination with MRI have shown promising results for improving accuracy in diagnosing tumor recurrence, detecting early treatment failure, and distinguishing between tumor progression and treatment-induced changes in patients with rHGG treated with BEV.
Keywords: amino acid PET, bevacizumab, pseudoresponse, recurrent glioma
Key Points.
PET can help distinguish between true and pseudoresponse to BEV in recurrent glioma.
PET can determine BEV treatment failure earlier than MRI in recurrent glioma.
Background
MRI is the clinical imaging modality in glioma patients at all stages of disease.1 The standardized MRI-based criteria for response assessment in neuro-oncology (RANO) includes quantification of contrast-enhancement in each of the 4 disease categories: complete or partial response and stable or progressive disease.2 As reported in a multitude of publications, MRI contrast-enhancement is an unreliable marker for tumor size or treatment response.3–5 Rather, MRI contrast-enhancement is a nonspecific result of vascular permeability of contrast agent (eg, gadolinium) that is also influenced by corticosteroids, antiangiogenic agents like bevacizumab (BEV), and immunotherapy agents.1,4 The most recent RANO update stated that the presence of “significant” enlarging areas of nonenhancing tumor on T2-weighted/FLAIR may be observed following radiation, demyelinating disease, ischemic injury, and cerebral edema, but may not be precisely quantified as an increase size of T2/FLAIR.1
Owing to the poor prognosis of recurrent gliomas in general and considering the limitations of MRI in assessment of recurrent glioma, an evidence-based recommendation for the use of PET imaging in gliomas was published by RANO-PET and European Association of Neuro-Oncology (EANO) in 2016.6 Common treatment modalities in the setting of recurrent high-grade glioma (rHGG) often include re-irradiation and consideration of additional chemotherapeutic agents such as BEV.7 BEV is an antiangiogenic therapy added to the treatment regimen to normalize tumor vasculature and reduce vascular permeability of immature blood vessels supplying the tumor.8 BEV has been shown to increase the overall survival (OS) and progression-free survival (PFS) in some studies in recurrent glioblastoma (GBM); however, this is still disputed.9,10 This therapy is known to decrease tumor enhancement on MRI, irrespective of the tumor’s sensitivity to the drug (ie, pseudoresponse).
However, as alluded to above, in some patients, it may be unclear on standard MRI alone if the decreased enhancement and cerebral edema is due to the tumor’s sensitivity to BEV (true response) or rather a treatment-induced change in the blood–brain barrier (BBB) causing a decrease in contrast extravasation (pseudoresponse).3,7,11 Furthermore, as the BEV-associated decrease in contrast-enhancement on MRI may occur along similar timepoints for pseudo- and true responders alike, early differentiation between these two clinical scenarios is of the utmost importance. More recently, physicians have utilized amino acid PET (AA PET) in addition to standard and/or perfusion MRI to help differentiate between treatment response and pseudoresponse at treatment onset.7,11–13
Independent of the integrity of the BBB, AA PET is a form of molecular imaging that relies on relative differences in the intracellular active uptake of radiolabeled amino acid tracers through the L (large) amino acid transporter system (LAT1 and LAT2).13,14 LAT1 has been shown to have increased expression in high-grade gliomas, which suggests that the images produced through AA PET reflect live proliferating cells rather than structural changes.14,15 Methionine, L-DOPA, and L-tyrosine are specific substrates for the LAT1, which make radiolabeled tracers derived from these amino acids, O-(2-18F-fluoroethyl)-L-tyrosine ([18F]FET), 11C-methyl-L-methionine ([11C]MET), and 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine ([18F]FDOPA), ideal choices for PET scans. 15–17 In HGG tissue samples, the uptake of [11C]MET and [18F]FDOPA significantly correlate to increased expression of LAT1.15,16
The utility of each tracer in clinical practice is largely dependent on its unique chemical properties. Because of the short half-life (t½ = 20 min) of methionine’s isotope label, [11C], the rapid decay of the tracer limits the ability to transport it from outside facilities.3 This means that studies utilizing this amino acid tracer require the presence of an onsite cyclotron unit, which is not readily available at all clinical centers with PET imaging capabilities.3 [18F]FET and [18F]FDOPA are both labeled with the isotope [18F], which has a much longer half-life (t½ = 109 min), making these two tracers feasible for implementation in centers housing an offsite nuclear pharmacy.3 However, [18F]FDOPA is regarded as a difficult tracer to synthesize, which may contribute to the increasing use of [18F]FET for HGG in research studies.18 Because of these properties, [11C]MET, [18F]FET, and [18F]FDOPA are currently being used to evaluate tumor response to BEV in the treatment of recurrent gliomas.
In general, treatment response on AA PET is first assessed by measuring the metabolically active tumor defined by the tumor-to-normal brain ratio (TBR) in the pretreatment phase. Once determined and treatment has been initiated, demonstration of overall reduction in metabolically active tumor volume on repeat amino acid PET to a predefined threshold is consistent with treatment response. Multiple recent studies have shown that AA PET may be used to diagnose BEV treatment failure at an earlier timepoint than standard MRI, which may afford earlier patient-specific treatment changes.13,19,20
In this systematic review, we describe the current knowledge comprising AA PET, specific to the tracers [18F]FET, [11C]MET, and [18F]FDOPA, in the setting of rHGG treated with BEV. Our objective is to demonstrate the diagnostic and prognostic advantages of using AA PET in conjunction with MRI to evaluate BEV-induced changes in recurrent glioma.
Literature Search
Search Strategy and Eligibility Criteria
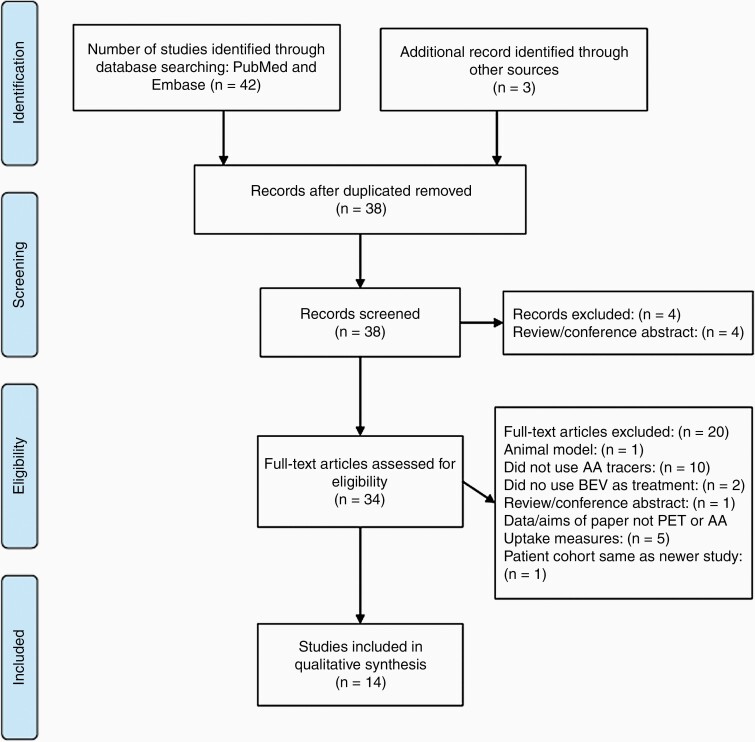
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were utilized to conduct a systematic review of studies utilizing AA PET in patients with recurrent glioma. A search of PubMed and Embase databases was conducted through July 15, 2020 using the following terms: “bevacizumab” OR “Avastin” AND “recurrent glioma” AND “PET.” The following inclusion criteria were utilized: (1) studies must include patients who underwent PET scans with [18F]FET, [11C]MET, or [18F]FDOPA, (2) at least one patient was diagnosed with a recurrent glioma, and (3) patients were treated with BEV. Studies were excluded if the previously mentioned amino acid tracers were not used, if the patients evaluated were newly diagnosed glioma patients, if the paper did not focus on the effect of BEV, or if the study was a narrative or systematic review, meta-analysis, commentary, animal study, letter to the editor, or editorial and did not contribute original research. Studies were also excluded if the PET scans were not compared to MRI. Articles that were not accessible in English or able to be translated were also excluded. See Figure 1 for detailed screening and exclusion of studies.
Figure 1.
PRISMA flow diagram for amino acid PET studies.
Data Extraction and Quality Assessment
The first author, year of publication, number of patients, type of tracers utilized, treatment regimen including frequency of BEV, types of imaging, and measurement outcomes for the included studies were extracted, reviewed, and agreed upon by 2 reviewers. The primary outcomes of interest were amino acid uptake measures (see detailed definitions of uptake measures in Table 1). Secondary outcomes of interest included any measures of diagnostic accuracy of AA PET. Studies were assessed for quality and overall level of evidence using the Oxford Center for Evidence Based Medicine (OCEBM) criteria.21 Studies with appropriate blinding measures, utilization of an MRI-reference standard, and applicability of the treatment group to this systematic review were evaluated as major factors for quality assessment, in addition to study design.
Table 1.
Definitions of Commonly Used Parameters in AA PET Studies
| Commonly Used Definitions | Studies Referenced | |
|---|---|---|
| PFS | Calculated as time from the beginning of BEV therapy to the first scan showing progression of disease. | Beppu et al., 20167 Beppu et al., 201927 Deuschl et al., 201726 Galldiks et al., 201311 George et al., 201823 Harris et al., 201228 Hutterer et al., 201113 Schwarzenberg et al., 201420 Wardak et al., 201430 |
| OS | Time from beginning BEV therapy until date of death or last follow-up. Note: Fleischmann et al., 2017 used post-recurrence survival, defined as time from initiation of radiation therapy with concomitant BEV until last follow-up or death. Hutterer et al., 2011 used a similar definition and termed it recurrent OS (rOS). Another variation reported by Galldiks et al., 2013 is total OS (tOS), defined as the standard OS or time from first diagnosis of tumor to death. |
Bashir et al., 201922 Deuschl et al., 201726 Fleischmann et al., 201725 Galldiks et al., 201311 George et al., 201823 Harris et al., 201228 Hutterer et al., 201113 Schwarzenberg et al., 201420 Wardak et al., 201430 |
| SUV | Calculated by adjusting activity concentration by the dose given and patient’s weight (injected activity/body weight). Note: Fleischmann et al., 2017 used early summation images to find ROIs with increased FET uptake, which aided in extraction of TACs for dynamic analysis. |
Beppu et al., 20167 Beppu et al., 201927 Deuschl et al., 201726 Fleischmann et al., 201725 Galldiks et al., 201311 Garcia et al., 201512 George et al., 201823 Harris et al., 201228 Humbert et al., 201929 Hutterer et al., 201113 Schwarzenberg et al., 201420 Wardak et al., 201430 |
| Normal, or nontumor SUV (SUVnormal) (alternatively known as tumor VOI) |
Calculated by outlining one or multiple ROIs of normal brain parenchyma in the contralateral hemisphere and determining the tracer uptake in this region. Note: George et al., 2018 used VOI to characterize tumor volume, however the definition is synonymous with SUV |
Bashir et al., 201922 Beppu et al., 20167 Beppu et al., 201927 Galldiks et al., 201311 Galldiks et al., 201824 George et al., 201823 Harris et al., 201228 Humbert et al., 201929 Hutterer et al., 201113 Schwarzenberg et al., 201420 |
| TBR or SUV of tumor/Normal- suvt/n | A comparison of the amount of amino acid tracer uptake (therefore metabolic activity) of the tumor (SUVtumor) to the apparently normal brain tissue (SUVnormal). Most studies used the threshold TBR greater than or equal to 1.6. Deuschl et al., 2017 used a threshold ratio of 1.3. Schwarzenberg et al., 2014 used TBRmax of 1.5. Fleischmann et al., 2017 used a TBRmax of 1.8. Harris et al., 2012 used a 95% confidence interval to determine which voxels had significant increases to calculate TBR, which they referred to as PRM. Note: Beppu et al, 2016 and 2019, and George et al., 2018, used the same measurement as above but used the terms SUVT/N and voxel-wise Spearman’s correlation coefficient, respectively. |
Bashir et al., 201922 Beppu et al., 20167 Beppu et al., 201927 Deuschl et al., 201726 Fleischmann et al., 201725 Galldiks et al., 201311 Galldiks et al., 201824 George et al., 201823 Harris et al., 201228 Schwarzenberg et al., 201420 |
| BTV or MTV | Volume of metabolically active tumor greater than the threshold determined by TBR. Most studies used the threshold TBR greater than or equal to 1.6. |
Bashir et al., 201922 Beppu et al., 20167 Beppu et al., 201927 Deuschl et al., 201726 Fleischmann et al., 201725 Galldiks et al., 201311 Galldiks et al., 201824 George et al., 201823 Harris et al., 201228 Humbert et al., 201929 Hutterer et al., 201113 Schwarzenberg et al., 201420 |
| Mean TBR (tbrmean) | The mean TBR is the average tracer uptake above the TBR threshold, therefore, it is the average BTV. | Beppu et al., 20167 Galldiks et al., 201311 Galldiks et al., 201824 Schwarzenberg et al., 201420 |
| Max TBR (TBRmax) | Calculated by the max SUV of the lesion (SUVlesion) divided by the mean SUV of normal brain parenchyma (SUVcontralateral normal brain tissue). Determined by images 5–40 min after injection of the tracer, depending on study methodology. | Beppu et al., 20167 Fleischmann et al., 201725 Galldiks et al., 201311 Galldiks et al., 201824 Schwarzenberg et al., 201420 |
| Tumor reduction | Threshold used to determine if the metabolically active tumor volume had significantly decreased. Most studies qualified this as tumor volume reduction of equal or greater than 45%. | Galldiks et al., 201311 Hutterer et al., 201113 |
| TAC) | Graph of the amount of tracer uptake in a specific tissue in relationship to the time since the injection of the tracer. | Fleischmann et al., 201725 Galldiks et al., 201311 Wardak et al., 201430 |
| TTP | Time between beginning of “dynamic acquisition” to the max SUV of the tumor. Alternatively described as the starting time frame plus half of the frame duration. | Fleischmann et al., 201725 Galldiks et al., 201311 |
BEV = bevacizumab; BTV = biological tumor volume; OS = overall survival; PFS, progression-free survival; MTV = metabolic tumor volume; PRM = parametric response maps; ROI = regions of interest; SUV = standardized uptake value; TAC = time activity curve; TBR = tumor-to-background ratio; TTP = time to peak; VOI = volume of interest.
Review of Studies
The literature search yielded 35 papers, 11 of which met criteria for inclusion in this review (Figure 1). Three studies (Galldiks et al. 2018, Beppu et al. 2019, and Deuschl et al. 2017) were identified through other sources and met the criteria of our review. Of the 14 studies included in this literature review, 4 evaluated the tracer [11C]MET, 6 evaluated the tracer [18F]FET, and 4 evaluated the tracer [18F]FDOPA. The number of patients included in each study ranged from 1 to 57. The majority of the studies (13 studies, 93%) evaluated tumor recurrence with static measurements like standardized uptake values and tumor-to-normal brain ratios (SUV and TBR, respectively), while only 3 studies (21%) used dynamic measurements of amino acid tracer uptake, like time-activity curves, and time-to-peak minimum (TAC and TTPmin, respectively). The results of each study included in our literature review are summarized in Table 2.
Table 2.
Overview of Included AA PET Studies
| Study | Tracer and Study Type | Number of Patients | Chemotherapy Agents Used in Recurrence | OCEBM Level of Evidence | Measurements | Results and Conclusions |
|---|---|---|---|---|---|---|
| Hutterer et al., 201113 | Static FET Retrospective |
11 | BEV + IR | 4 | PFS OS (rOS) SUV Tumor reduction |
FET-PET detected BEV-treatment failure earlier than RANO criteria with MRI alone, with a mean time benefit of 9 weeks - AUC-ROC curve for PFS using tumor volume reduction was 0.875 ± 0.117 with a sensitivity of 87.5% and specificity of 100% - FET responders had longer PFS than RANO responders, but not significantly - FET volumes decreased in long-term survivors (PFS ≥ 6 months) but increased in short-term survivors (PFS< 6 months) - Contrast-enhancement on MRI does not always correspond to areas of metabolically active tumor with FET-PET |
| Harris et al., 201228 | Static F-DOPA Retrospective |
24 | BEV +/- IR | 4 | PFS OS SUV TBR (PRM) |
F-DOPA-PET stratified patients by short-term and long-term survival - Analyzed PRMs corresponding to regions of pretreatment contrast enhancement on MRI - PRMs with increased F-DOPA uptake stratified patients by 3-month PFS and 6-month OS, with increased uptake corresponding to shorter PFS and OS (sensitivity of 75%, specificity of 70%) - When comparing 2 post-BEV treatment PET scans, an increased volume fraction of F-DOPA uptake stratified patients by short- and long-term PFS and OS (sensitivity of 91%, specificity of 83%) |
| Galldiks et al., 201311 | Static and Dynamic FET Prospective |
10 | BEV + IR | 3 | PFSOS (tOS) SUV TBR Tumor reduction TAC TTP |
Static and dynamic FET predicted BEV-treatment failure earlier than what was reported by Hutterer et al., with a median of 4.9 weeks and a mean time benefit of 10.5 weeks - Changes in TBRmax greater than 17% differentiated responders (PFS greater than 6 mos) from nonresponders with a sensitivity of 83% and specificity of 100% - Response on FET had sig longer PFS than nonresponders (9 vs 3 mos) and OS (23 mos vs 3.5 mos) - Mean FET tumor volumes decreased in long term survivors but unchanged in short term survivors Differentiated responders from nonresponders by characterizing TTP and kinetic patterns - TTP at baseline could differentiate responders and nonresponders with an AUC of 1.0 and sensitivity and specificity of 100% - Shorter TTP at baseline and follow-up in nonresponders - Nonresponders more likely to have an early peak of FET uptake than a constant descent (Type 3 kinetic pattern) Proposed BEV-response is suggested if 2 of 3 criteria are met: 1. Increase of TTP ≥ 10 min at follow-up 2. TTP ≥ 25 min at baseline 3. Type 1 (SUV peaks at the end) or 2 kinetics (SUV peaks at least half-way through and plateaus or has slow descent) |
| Wardak et al., 201430 | Dynamic F-DOPA Prospective |
21 | BEV + IR | 3 | PFS OS SUV TAC |
Looking at dynamic parameters can provide predictive information of OS after BEV - The average PFS and OS was 183 days and 13.2 months, respectively - OS and PFS significantly correlated (r = 0.85, P < .0001) - FLT more predictive of OS than F-DOPA (Multiple Linear Regression = R2 = 0.83 for both, R2 = 0.82 for FLT alone) |
| Schwarzenberg et al., 201420 | Static F-DOPA Prospective |
30 | BEV +/- IR | 3 | PFS OS SUV TBR MTV |
F-DOPA PET at 2 weeks was statistically significant of distinguishing treatment response to antiangiogenic therapy with the PET parameter MTV - F-DOPA PET SUV parameters were not predictive of outcomes - MTV was highly prognostic and predictive of treatment response, most significantly at 2 weeks with an OS (P < .0001) and PFS (P = .001) - ROC curve identified 18 mL as the optimal threshold for MTV |
| Garcia et al., 201512 | Static MET Case report |
1 | BEV | NA | SUV | Pseudoprogression evident on MRI after BEV - MRI demonstrated increased lesion size after initiation of BEV-MET-PET demonstrated normalization of metabolic activity |
| Beppu et al., 20167 | Static MET Pilot study |
20 | BEV + TMZ | 3 | PFS SUV |
MET-PET at 8 weeks differentiated pseudoresponders from true responders after BEV and is correlated with PFS - MRI-response at 4 and 8 weeks is associated with longer PFS - 20% of MRI-responders were reclassified as pseudoresponse by MET-PET at 8 weeks - MET-PET alone and in addition to MRI at 8 weeks predicts longer PFS, but not at 4 weeks - Lower SUVT/N at 8 weeks versus baseline was associated with longer PFS than increased SUVT/N |
| Fleischmann et al., 201725 | Static and Dynamic FETRetrospective | 57a | BEV | 4 | OS (PRS) SUV TBR BTV TAC TTP |
TTPmin in dynamic FET-PET may be used for prognosis in rHGG treated with radiation and BEV - Earlier TTPmin is associated with shorter PRS (P = .027) - Time from pretreatment PET to initiation of radiation and BEV significantly affected TTPmin - Suggest rHGG with decreasing TAC may be evaluated using TTPmin for spatial characterization of aggressive tumor regions |
| Deuschl et al., 201726 | Static C-MET Prospective | 11 | BEV +/- LOM | 3 | PFS OS SUVT/N SUVMax MTV |
Suggested a method for determining treatment response to BEV using PET/MRI: if T/N ratio on PET is greater than 1.6, then response is determined by decrease in T/N ratio of 25%. If T/N is less than 1.6, use RANO guidelines - MRI: 0/11 Complete response, 6/11 partial response, 4/11 stable disease, 1/11 progressive disease - PET: 4/6 partial responders on MRI were metabolic responders on C-MET PET; 2/4 of stable disease on MRI were responders on C-MET PET; 4 PET nonresponders including 2 cases of pseudoresponse (MRI and PET discordant) - PFS and OS was predicted by reduction of T/N of greater than 25% on C-MET and the RANO classification of tumor response on MRI |
| Galldiks et al., 201824 | Static F-FET Prospective | 21 | BEV + LOM | 3 | TBR MTV OS |
Both relative change in MTV and absolute MTV on F-FET at follow-up after BEV predicted long term survival >9 months. - Patients were considered responders to BEV/LOM if they survived >9 months - Median OS of responders versus nonresponders was 12 and 6 months, respectively - Response on FET-PET significantly predicted OS greater than 9 months (P < .05), however, MRI results at 10 weeks did not predict an OS greater than 9 months (P = .203) - Reduction of TBRmax of FET-PET by 27% or a reduction of TBR by 17% distinguished responders from nonresponders. - Patients with MTV less than 5 mL survived significantly longer with average OS of 11 months than those who had a larger tumor volume on follow-up—median of 6 months (P < .001). |
| George et al., 201823 | Static F-FET Prospective |
11 | BEV | 3 | PFS OS SUV (VOI) TBR (Voxel-wise Spearman correlation coefficient) |
Concluded that there is moderate correlation between FET PET uptake and MRI contrast enhancement after BEV - PFS and OS after BEV was 111 days and 223 days, respectively - The voxel-wise Spearman correlation coefficient between FET uptake and T2/FLAIR signal intensity was 0.65 before BEV and 0.61 after BEV (P = .256). - Increased correlation between PET uptake and MRI enhancement after BEV was associated with lower PFS (P < .001) and OS (P = .049). - Post-treatment to pretreatment PET uptake ratio greater than 0.7 was associated with lower PFS and OS. |
| Bashir et al., 201922 | Static F-FET Retrospective |
50a | BEV | 4 | OS TBR BTV |
F-FET PET can distinguish post-treatment changes to BEV from tumor recurrence at 6-month follow-up, and predictive of OS - No significant difference found with F-FET uptakes between measurable and nonmeasurable lesions (TBRmax, 3.0 vs 3.2; TBRmean, 2.0 vs 2.0; and BTV 12 cm3 vs 10.5 cm3; P > .05) - ROC curve threshold of 2.0 for TBRmax, 1.8 for TBRmean, and 0.55 cm3 for BTV in differentiating between recurrence or treatment changes with a sensitivity of 99% and specificity of 94% (P < .0001) - F-FET parameters are higher in patients with recurrent glioblastoma versus post-treatment changes (TBRmax, 3.2 vs 1.6; TBR mean, 2.0 vs 1.6; and BTV 14.8 cm3 vs 0.01 cm3; P < .0001) - Increased BTV correlated with lower OS - 99% accuracy to distinguish recurrence from late post-treatment changes |
| Beppu et al., 201927 | Static C-MET Prospective | 24 | BEV + TMZ | 3 | PFS SUV T/N |
SUVT/N obtained with C-MET PET was more predictive of PFS in patients with rHGG treated with BEV at 8 weeks compared to T/N found using average relative cerebral blood flow from arterial spin labeling - Median PFS was 123 days - Changes in T/N ratios and tumor volumes on ASL significantly correlated to changes on C-MET PET after treatment of rHGG with BEV. - Long PFS could be predicted by T/N ratio at 8 weeks on C-MET and the change in T/N from baseline to 4 weeks follow-up on C-MET and ASL |
| Humbert et al., 201929 | Static F-DOPA Clinical trial and Prospective |
12 | BEV | NA | SUV | Demonstrated the application of F-DOPA PET and MRI in tumor board discussions to implement decisions regarding patient treatment plans - 33.3% cases of suspected tumor recurrence had changed diagnosis and treatment plan with inclusion of F-DOPA PET results |
ASL = arterial spin labeling perfusion imaging; AUC = area under the curve; BEV = bevacizumab; BTV = biological tumor volume; C-MET = [11C]MET; F-DOPA = [18F]FDOPA; F-FET = [18F]FET; IR = irinotecan; LOM = lomustine; MLR = multiple linear regression; MTV = metabolic tumor volumes; NA = not applicable; OCEBM = Oxford Center for Evidence Based Medicine; OS = overall survival; PFS = progression-free survival; PRM = parametric response map; PRS = post-recurrence survival; rHGG = recurrent high-grade glioma; ROC = receiver operating characteristic; rOS = recurrent OS; SUV = standardized uptake value; TAC = time activity curve; TBR = tumor-to-background ratio; TMZ = temozolomide; tOS = total OS; TTP = time to peak; VOI = volume of interest.
aNumber of patients indicates number treated with bevacizumab, not necessarily all patients analyzed in study.
[18F]FET
[18F]FET was used in 6 studies that met criteria for inclusion. Hutterer et al. evaluated the use of [18F]FET in 6 patients with rHGG who received biweekly BEV plus irinotecan (IR).13 This study found that response on MRI or [18F]FET-PET (as defined as meeting RANO criteria on MRI or reduction of SUV by 45%) could independently predict longer PFS in patients than nonresponders on either imaging modality.13 However, patients that demonstrated response on [18F]FET-PET had a longer PFS than those who were RANO responders on MRI, although this was not statistically significant.13 In the 4 patients (36%) that were categorized as MRI responders but [18F]FET-PET nonresponders, tumor progression was detected earlier on [18F]FET-PET than MRI, with a mean time benefit of 9 weeks.13 For the rHGG patient, detecting recurrence 9 weeks earlier is likely to be impactful for some patients. Additionally, long-term survivors (those surviving longer than 6 months) had a statistically significant decrease in tumor volumes relative to short-term survivors (patients who survived less than 6 months) as detected on [18F]FET -PET.13 Hutterer et al. concluded that [18F]FET-PET was superior in determining treatment response to BEV and detecting treatment failure earlier than MRI, suggesting [18F]FET -PET may be beneficial in monitoring BEV therapy.13
Bashir et al. retrospectively evaluated 146 patients who had undergone [18F]FET-PET for comparison to histopathology interpretations, of which 50 were treated with BEV as a second-line treatment.22 [18F]FET uptake measures were higher in patients with recurrent glioblastoma versus post-treatment changes, with a TBRmax of 3.2 versus 1.6, a TBRmean of 2.0 versus 1.6, and a metabolically active tumor volume of 14.8 cm3 versus 0.01 cm3 (P <.0001).22 The authors demonstrated that [18F]FET uptake could accurately differentiate tumor recurrence from late post-treatment changes using TBRmax with a sensitivity of 99% and specificity of 94%, resulting in an overall diagnostic accuracy of 99%.22 If reproducible, this would greatly improve the diagnostic accuracy beyond MRI alone in this setting.
George et al. conducted a prospective study of the use of static [18F]FET-PET compared to MRI in 11 patients with rHGG treated with BEV.23 Participants underwent MRI and [18F]FET-PET scans before treatment and approximately 4–5 weeks after initiation of BEV.23 On MRI, the enhancing tumor volume was significantly decreased after treatment, although total tumor volume was not significantly decreased.23 However, with [18F]FET-PET, the mean tracer uptake was significantly decreased for both the enhancing tumor and the entire tumor.23 This study found that patients with post-to-pretreatment mean [18F]FET-PET SUV ratio greater than 0.7 (90th percentile) had a significantly shorter OS and PFS.23 In other words, increased mean SUV following BEV was suggestive of treatment failure. Additionally, patients had a lower PFS and OS if the correlation between enhancement on MRI and [18F]FET uptake increased after treatment.23
Galldiks et al. 2018 determined the parameters of [18F]FET-PET and MRI that distinguished patients treated with BEV plus lomustine (LOM) who survived more than 9 months after recurrence of HGG (responders) than those who did not (nonresponders).24 This study used the cutoff of OS ≥ 9 months as the definition for response to BEV/LOM therapy because it was the same criteria that the BELOB trial used, which found that rHGG patients that were treated concurrently with BEV and LOM had an increased OS than those treated either agent alone.24,9 After segregating and analyzing MRI and [18F]FET-PET data from responders versus nonresponders, they found that patients in the “responders” group had approximately 27% decrease in [18F]FET uptake at follow-up compared to baseline, and smaller absolute MTV on follow-up compared to the “non-responders” group; Both of these statistics—MTV reduction and absolute MTV—significantly differentiated responders from nonresponders, with P = .036 and P = .001, respectively.24 Interestingly, RANO categorization of disease progression and response on MRI did not significantly differentiate responders from nonresponders.24 One notable difference between Galldiks et al. 2018 and many other 18F]FET-PET studies in this review is that Galldiks et al. 2018 defined “response to BEV” as a discrete time (9 months), whereas most other studies categorized BEV response as a reduction of tracer uptake. Additionally, the goals of Galldiks et al. 2018’s study focused on determining different [18F]FET-PET parameters that predicted long term overall survival, whereas the primary goal of many other studies was to differentiate true BEV response from pseudoprogression. Because of this difference in study design, Galldiks et al. 2018 only used the quantitative measurement of OS rather than OS and PFS; the authors argued that PFS is less reliable measurement than OS because of the limited ability to definitively distinguish true treatment response to BEV from pseudoresponse.24
Galldiks et al. 2013 expanded upon Hutterer’s findings in a prospective study evaluating [18F]FET-PET in 10 rHGG patients prior to and after 5 weeks of biweekly BEV/IR treatment compared to MRI alone.11 Treatment response was determined by a reduction > 45% of metabolically active tumor volume. The active tumor component was defined as TBR of greater than or equal to 1.6. Among the 10 patients in this cohort, MRI did not detect tumor progression. [18F]FET-PET revealed 6 metabolic responders and 4 nonresponders. Initial response on [18F]FET-PET was predictive of longer PFS (median PFS, 9 vs 3 months; P = .001) and OS (median OS 23.0 months vs 3.5 months; P = .001).11 Among the 4 patients whose MRI did not meet RANO criteria for tumor progression, [18F]FET-PET was able to detect treatment failure earlier than MRI alone with a median time benefit of 10.5 weeks, similar to the time benefit of 9 weeks reported by Hutterer et al.13 Although TBRmax as a single point was not predictive of survival, a decrease in TBRmax greater than 17% differentiated long-term survivors from short-term survivors.11 Additionally, when comparing tumor volumes of long-term survivors with short-term survivors, Galldiks et al. 2013 found that long-term survivors had decreased mean [18F]FET-PET tumor volumes (as seen in Hutterer et al.). The authors also evaluated tumor kinetics with TAC and TTP, respectively on dynamic [18F]FET-PET. In order to identify responders on dynamic [18F]FET-PET, Galldiks et al. 2013 utilized the following criteria: (1) an increase in TTP from baseline to follow up greater than or equal to 10 min, (2) a baseline TTP greater than 25 min, and (3) a kinetic pattern of either a SUV peak at the end of the study or a peak in the middle of the study followed by a plateau or slow descent afterward.11 A kinetic pattern characterized by an early peak of [18F]FET followed by a constant and progressive descent was more commonly observed in nonresponders (P = .018). Responders in this study met at least 2 of the 3 criteria and Galldiks et al. 2013 calculated that these criteria had a sensitivity of 100% and specificity of 75%.11
Fleischmann et al. analyzed the results of pre-irradiation dynamic [18F]FET-PET imaging in a larger retrospective study of 72 rHGG patients.25 Seventy-nine percent of these patients were concurrently treated with BEV therapy, however, follow-up [18F]FET-PET scans were only obtained in patients demonstrating MRI changes after radiation.25 Unlike Hutterer et al. and Galldiks et al. 2013, Fleischmann et al. did not compare pretreatment [18F]FET-PET scans to post-treatment scans, and did not specifically compare the presence of BEV treatment with results of the PET scan.25 The authors also found that static measurements of [18F]FET uptake on the pretreatment [18F]FET-PET scan could not predict length of post-recurrence survival; specifically, TBRmax at pretreatment was not correlated with survival length.25 This is consistent with Galldiks et al. 2013’s conclusion that TBRmax alone could not predict OS or PFS.11,25 When evaluating the dynamic measurements, tumors with an early TTPmin (<12.5 min) had a statistically significant decreased survival than those who had a later TTPmin (P = .027) and suggested that this difference in tumor kinetics could be used to determine the most aggressive areas within the tumor.25 As others have demonstrated earlier detection of recurrence on [18F]FET-PET than MRI alone, this study suggests that [18F]FET-PET may provide still valuable diagnostic information even when obtained at suspected recurrence.
[11C]MET
With regard to BEV response assessment, [11C]MET-PET has been reported in 4 studies in patients with rHGG.7,12 Deuschl et al. explored how [11C]MET-PET and MRI could be used to evaluate the response to BEV (with or without LOM) treatment of rHGG. Like many other studies, response to therapy on MRI was defined by RANO criteria whereas response on [11C]MET-PET was monitored using T/N ratios. Of 11 patients, 6 were categorized as partial response on MRI and of these 6 patients, 4 were considered [11C]MET-PET responders. The 2 patients partial response on MRI that did not show response on [11C]MET-PET were categorized as pseudoresponders.26 Four patients had stable disease and out of these patients, 2 were [11C]MET-PET responders; the other 4 patients were nonresponders on [11C]MET-PET. PFS and OS was predicted by reduction of T/N of greater than 25% on C-MET and the RANO classification of tumor response on MRI.26 Deuschl et al. proposed that the rHGG should be evaluated via RANO criteria on MRI as well as determining the T/N ratio with PET; if the T/N ratio is greater than 1.6, treatment response should be determined by the results of the PET scan, while if T/N is less than 1.6, treatment response should be determined based on RANO criteria.26
As seen with [18F]FET-PET, the ability to differentiate pseudoprogression from BEV true response is also a major focus with the AA PET tracer [11C]MET-PET. In a case study of a 60-year-old female with recurrent multifocal GBM receiving BEV treatment, MRI was suggestive of increasing tumor volume; however, a [11C]MET-PET scan showed that the metabolic activity was normalizing, therefore responding to the BEV therapy.12 From this, Garcia et al. concluded that the progression on MRI was most likely pseudoprogression due to the changes in the BBB after treatment.12
This was expanded on in a pilot study of [11C]MET-PET compared to MRI alone in patients with rHGG treated with BEV. Beppu et al. 2016 found that when evaluating the response of BEV therapy on MRI, patients who responded on MRI at 4 weeks or at 8 weeks had a longer PFS than those who did not respond on MRI.7 When evaluating the results of [11C]MET-PET alone, patients who had a lower TBR at 8 weeks than at baseline had longer PFS than those patients who had a higher TBR at 8 weeks compared to baseline.7 Additionally, patients who responded on [11C]MET-PET at 8 weeks had a significantly longer PFS than those who did not respond on [11C]MET-PET at 8 weeks.7 When comparing MRI alone to MRI with [11C]MET-PET, Beppu et al. 2016 found that patients who responded on both modalities at 8 weeks (true responders) had a longer PFS than patients who only demonstrated MRI response (pseudo-responders).7
Beppu et al. 2019 investigating the treatment response to BEV in rHGG using Arterial Spin Labeling perfusion imaging (ASL) and [11C]MET-PET to see if the different modalities of imaging were comparable. If the patient was above the median length of PFS (127 days), he or she was considered a long PFS patient.27 On [11C]MET-PET, Beppu et al. 2019 observed that at 4 weeks, the AA PET tracer may have a decrease in uptake followed by an increase in tracer uptake at 8 weeks.27The regions of the tumor with the most blood flow correlated to the area of highest tracer uptake on the [11C]MET-PET scan.27 Additionally, values of T/N ratio, changes in T/N ratio and tumor volume on ASL and [11C]MET-PET both correlated, however, the most accurate predictor of long PFS was the T/N ratio at 8 weeks follow-up.27
[18F]FDOPA
[18F]FDOPA was utilized in 4 studies, two of which additionally utilized the PET tracer [18F]-3’-deoxy-3’-fluoro-L-thymidine ([18F]FLT), a nucleoside analog that indicates DNA synthesis.20,28–30 The two studies that discussed [18F]FDOPA alone were both prospective and nonrandomized studies.20,29 Humbert et al. used [18F]FDOPA-PET to assess rHGG in 12 patients and residual disease response versus progression in 53 patients with primary low-grade glioma.29 Among glioma patients, the STUPP 31 protocol was utilized as first-line treatment, though BEV was used in 18 patients.29 The objective of this study was to evaluate the diagnostic accuracy of [18F]FDOPA-PET in these patient populations by comparing the post-BEV [18F]FDOPA-PET diagnosis determined by case discussion during tumor board with the final diagnosis as determined by either pathology or clinical follow-up at 3 months post-PET.29 A third of patients with suspected rHGG (4/12) had a change in the care plan after the tumor board reviewed the patient’s [18F]FDOPA-PET results, with most of the changes in treatment course involving switching to or adding a new therapy (3/4).29 This study also appraised the impact of [18F]FDOPA-PET on patient treatment plans by comparing the diagnosis as determined by tumor board discussions of MRI findings to the diagnosis as determined by conclusions drawn from pre- and post-treatment [18F]FDOPA-PET in addition to MRI.29 Notably, the diagnostic accuracy was most improved in those with suspected rHGG, as conclusions drawn from MRI alone resulted in 1 false-positive and 3 false-negative diagnoses.29 In summary, incorporation of [18F]FDOPA-PET findings into tumor board discussion of patients with suspected rHGG resulted in a change in diagnosis and treatment plan in 33.3%.29
Schwarzenberg et al. studied the impact of [18F]FDOPA-PET with and without MRI on PFS and OS in 30 patients with rHGG after BEV therapy.20 In this study, [18F]FDOPA-PET was performed prior to BEV treatment and again at 2- and 6-week intervals following BEV.20 The authors found that reduction in absolute metabolic tumor volume (MTV) on [18F]FDOPA-PET at the 2-week timepoint predicted increased OS (P < .0001) and PFS (P = .001).20 Patients with treatment response as assessed by absolute MTV by [18F]FDOPA-PET at 2 weeks also had a median OS that was 3.5 times longer than those who failed to demonstrate a response (P < .001).20 Treatment response was assessed by MRI at 6 weeks by change in tumor volume, which significantly predicted OS (P = .01) and PFS (P < .001), with MRI responders having a median OS 1.5 times that for MRI nonresponders (P = .03).20 Although both MRI and [18F]FDOPA-PET were able to significantly predict treatment response, it is important to note that treatment response was discordant for 8 patients in this study.20 Six patients demonstrated either a partial response or stable disease on MRI without a corresponding response demonstrated on [18F]FDOPA-PET.20 The median OS for this group was 3.4 months, suggesting that the lack of response on [18F]FDOPA-PET was predictive of treatment failure in as early as 2 weeks after beginning treatment despite reassuring MRI findings.20 Two patients demonstrated [18F]FDOPA-PET response but suggested disease progression on MRI, with survival of 9.5 and 12 months in each patient.20 This is consistent with the notion that the metabolic activity provided by [18F]FDOPA-PET imaging is more consistent with the current disease state than MRI alone.
Two studies utilized [18F]FDOPA in conjunction with [18F]FLT-PET. Although [18F]FLT-PET is not an amino acid tracer and therefore beyond the scope of this review, both studies met the inclusion criteria for this review due to their use of [18F]FDOPA to evaluate patients with rHGG treated with BEV. Because of this, the authors have reported the significant findings related to the [18F]FDOPA data and limited the mention of [18F]FLT -PET to only the most crucial points to accurately represent the study. Harris et al. evaluated the prognostic value of [18F]FDOPA and [18F]FLT-PET in predicting BEV response in HGG by creating parametric response maps (PRM), which demonstrated SUV changes in both amino acid tracers in a voxel-wise manner.28 Receiver operating characteristic (ROC) curve analysis for [18F]FDOPA volume change between pre- and post-treatment PRMs predicted PFS at 3 months with a sensitivity of 75% and specificity of 70% (P = .0110).28 [18F]FDOPA also significantly predicted 6-month OS by comparing volume fraction of increasing tracer uptake between the 1–2 week and 5–7 week post-treatment time points, with a sensitivity of 91% and specificity of 83% (P = .0271).28
Wardak et al. evaluated dynamic [18F]FDOPA-and [18F]FLT-PET 1 week prior to and at 2 and 6 weeks after initiating biweekly BEV/IR to predict OS using multiple linear regression (MLR) analysis in patients with rHGG.30 Changes in kinetic parameters when combined with kinetic parameter values as determined by the MLR model were more predictive of OS than the kinetic parameter values or their changes alone.30 The MLR model was also more predictive of OS when using [18F]FLT-PET versus [18F]FDOPA (R2 = 0.82 and R2 = 0.41, respectively), but was most predictive of OS when it utilized parameters from both [18F]FLT and [18F]FDOPA (R2 = 0.83).30
Summary
Summary of AA PET Use in BEV-Treated High-Grade Glioma
MacDonald et al. first outlined response criteria specific to GBM in 1990, establishing the modern basis for classifying complete versus partial response and stable versus progressive disease.32 Imaging was a fundamental component of this criteria, providing more objective data than a patient’s clinical appearance alone.32 Today, the RANO criteria continually build upon the criteria MacDonald originally outlined to make the guidelines more objective and standardized.4 However, the balance between standardized guidelines and flexible diagnostic and treatment protocols can be elusive, especially in complex disease processes such as GBM. The RANO criteria relies heavily on MRI findings such as contrast enhancement, T2-weighted and FLAIR imaging changes, and further characterized measurable versus nonmeasurable disease.2,4 Despite these advancements, limitations of MRI, such as pseudoprogression and pseudoresponse, are inherent to the RANO criteria.4 Antiangiogenic chemotherapeutic agents, such as BEV, are prone to affect contrast enhancement on imaging with variable actual tumor treatment response regardless of MRI changes.
The literature suggests that amino acid PET can provide additional valuable information about treatment response and prognosis that MRI cannot provide. Specifically, AA PET is helpful in monitoring treatment response to BEV in patients with rHGG. Multiple studies showed that response to treatment detected on [18F]FET-PET were predictive of increased OS and PFS in patients with rHGG treated with BEV.11,13 When there were discordant responses between MRI and PET, [18F]FET-PET was able to determine BEV treatment failure at an earlier timepoint than MRI (9–10.5 weeks prior to MRI changes), which is of upmost importance when an early change in a failed treatment may be beneficial to patient survival and prognosis.11,13
AA PET can also aid in diagnosis and treatment plans for patients with suspected rHGG. One study showed that when a tumor board was shown AA PET in conjunction with MRI rather than MRI alone, they had a change in diagnosis or treatment plan in one third of the patients.28 Furthermore, a study in Germany evaluated the effectiveness of using [18F]FET-PET with MRI in rHGG patients treated with BEV/IR and found that adding [18F]FET-PET increased the rate of correct diagnoses of rHGG by 41%.33 This is advantageous because it may help avoid unnecessary treatments, procedures, or adverse drug reactions.
On a molecular level, AA PET can be advantageous for monitoring tumor progression regardless of changes in the BBB, including postirradiation changes. This is because PET relies on the metabolic activity of tissue rather than changes in BBB permeability, as detected on MRI.5,34 This is reflective of increased expression of L (large) amino acid transporters (LAT1 and LAT2).35 For example, in the setting of temozolomide, AA PET can be useful in differentiating tumor growth from pseudoprogression on MRI which is observed in 20%–30% of cases.36 Pseudoprogression may be difficult to diagnose on MRI alone which may result in overtreatment in some patients.37 In addition, differences in [18F]FET-PET uptake were shown to accurately distinguish between tumor recurrence and late post-treatment changes.25
There is early evidence that changes in temporal assessment of dynamic AA PET, such as late TTP, may be able differentiate between short-term and long-term survivors.11,13,38 However, the widespread application of this imaging modality has not reached the United States. Current implementation of AA PET into the neuro-oncology clinical algorithm in the United States has failed to become mainstream as the FDA requires the treating institution to submit an Investigational New Drug Application (IND).18,39 While there is a wealth of literature supporting AA PET’s role in glioma imaging assessment as a complement to contrast-enhanced MRI, including multiple international guidelines supporting its utility, the assessment of treatment response with BEV is less established.1,4,6,38 As this is likely related to the added difficulty of obtaining an IND from the FDA prior to implementation, significant research efforts will be necessary to fully evaluate the potential of amino acid PET as a monitor for treatment response to BEV.
Limitations
Our review was limited by the small number of studies and limited number of patients who were evaluated with AA PET and MRI after receiving BEV for treatment of recurrent GBM. Additionally, because BEV is an adjuvant treatment in HGG, some studies did not stratify patient outcomes by those receiving second-line therapy, which often included but was not limited to BEV. Only one study (Galldiks et al. 2013) explicitly stated that their study excluded patients if the patient had a previous exposure to BEV. Because BEV is a second line treatment for GBM, it is unlikely that patients in the other 13 studies had a previous exposure to BEV during the initial treatment of the HGG; however, if the patients had been previously exposed to BEV before the time of recurrence, it could introduce a selection bias for those who have already responded to BEV. Finally, because the literature about BEV in rHGG is in its infancy, variations in study protocols and measured outcomes make conducting a meta-analysis implausible.
Conclusions
Despite the limited number of studies of AA PET in rHGG, preliminary clinical studies have shown promising results in the ability of AA PET to detect treatment failure with BEV earlier than on MRI. This suggests that AA PET has the potential to lower morbidity from overtreatment of benign imaging changes on MRI and provide early diagnostic assessment of BEV treatment response in rHGG compared to MRI alone. Using AA PET in conjunction to MRI gives physicians additional information about tumor characteristics to make informed decisions regarding patient care during critical periods of potential tumor growth or treatment response.
Acknowledgments
We thank Tressie M. Stephens for her administrative assistance with coordinating the research and submission of this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement. The authors declare that they have no conflict of interest.
Authorship Statement. Data collection and interpretation: K.L.H., C.M.O., B.J.A., A.M.W., and C.A.G.; Idea conception and design: K.L.H., C.M.O., B.J.A., J.D.B., and C.A.G.; Drafting of original manuscript: K.L.H., C.M.O., B.J.A., and C.A.G.; Edits: K.L.H., C.M.O., A.M.W., and C.A.G.
References
- 1.Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quant EC, Wen PY. Response assessment in neuro-oncology. Curr Oncol Rep. 2011;13(1):50–56. [DOI] [PubMed] [Google Scholar]
- 3.Galldiks N, Langen KJ. Amino acid PET in neuro-oncology: applications in the clinic. Expert Rev Anticancer Ther. 2017;17(5):395–397. [DOI] [PubMed] [Google Scholar]
- 4.Yang D. Standardized MRI assessment of high-grade glioma response: a review of the essential elements and pitfalls of the RANO criteria. Neurooncol Pract. 2016;3(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zikou A, Sioka C, Alexiou GA, Fotopoulos A, Voulgaris S, Argyropoulou MI. Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Media Mol Imaging. 2018;2018:6828396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beppu T, Terasaki K, Sasaki T, et al. MRI and 11C-methyl-L-methionine PET differentiate bevacizumab true responders after initiating therapy for recurrent glioblastoma. Clin Nucl Med. 2016;41(11):852–857. [DOI] [PubMed] [Google Scholar]
- 8.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9): 987–989. [DOI] [PubMed] [Google Scholar]
- 9.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 10.Diaz RJ, Ali S, Qadir MG, De La Fuente MI, Ivan ME, Komotar RJ. The role of bevacizumab in the treatment of glioblastoma. J Neurooncol. 2017;133(3):455–467. [DOI] [PubMed] [Google Scholar]
- 11.Galldiks N, Rapp M, Stoffels G, et al. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40(1):22–33. [DOI] [PubMed] [Google Scholar]
- 12.García JR, Baquero M, Bassa P, Soler M, Moragas M, Riera E. Diagnosed recurrent glioma and antiangiogenic treatment response by 11C-Methionine PET. Rev Esp Med Nucl Imagen Mol. 2015;34(6):398–399. [DOI] [PubMed] [Google Scholar]
- 13.Hutterer M, Nowosielski M, Putzer D, et al. O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52(6):856–864. [DOI] [PubMed] [Google Scholar]
- 14.Verhoeven J, Hulpia F, Kersemans K, et al. New fluoroethyl phenylalanine analogues as potential LAT1-targeting PET tracers for glioblastoma. Sci Rep. 2019;9(1):2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J Neurooncol. 2010;99(2):217–225. [DOI] [PubMed] [Google Scholar]
- 16.Youland RS, Kitange GJ, Peterson TE, et al. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol. 2013;111(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habermeier A, Graf J, Sandhöfer BF, Boissel JP, Roesch F, Closs EI. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids. 2015;47(2):335–344. [DOI] [PubMed] [Google Scholar]
- 18.Lapa C, Linsenmann T, Monoranu CM, et al. Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med. 2014;55(10):1611–1616. [DOI] [PubMed] [Google Scholar]
- 19.Galldiks N, Law I, Pope WB, Arbizu J, Langen KJ. The use of amino acid PET and conventional MRI for monitoring of brain tumor therapy. Neuroimage Clin. 2017;13:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzenberg J, Czernin J, Cloughesy TF, et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20(13):3550–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Group OLoEW. The Oxford 2011 Levels of Evidence. Oxford, UK: Oxford Centre for Evidence-Based Medicine; 2011. [Google Scholar]
- 22.Bashir A, Mathilde Jacobsen S, Mølby Henriksen O, et al. Recurrent glioblastoma versus late posttreatment changes: diagnostic accuracy of O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography (18F-FET PET). Neuro Oncol. 2019;21(12):1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George E, Kijewski MF, Dubey S, et al. Voxel-wise analysis of fluoroethyltyrosine PET and MRI in the assessment of recurrent glioblastoma during antiangiogenic therapy. AJR Am J Roentgenol. 2018;211(6):1342–1347. [DOI] [PubMed] [Google Scholar]
- 24.Galldiks N, Dunkl V, Ceccon G, et al. Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur J Nucl Med Mol Imaging. 2018;45(13):2377–2386. [DOI] [PubMed] [Google Scholar]
- 25.Fleischmann DF, Unterrainer M, Bartenstein P, Belka C, Albert NL, Niyazi M. 18F-FET PET prior to recurrent high-grade glioma re-irradiation-additional prognostic value of dynamic time-to-peak analysis and early static summation images? J Neurooncol. 2017;132(2):277–286. [DOI] [PubMed] [Google Scholar]
- 26.Deuschl C, Moenninghoff C, Goericke S, et al. Response assessment of bevacizumab therapy in GBM with integrated 11C-MET-PET/MRI: a feasibility study. Eur J Nucl Med Mol Imaging. 2017;44(8):1285–1295. [DOI] [PubMed] [Google Scholar]
- 27.Beppu T, Sato Y, Sasaki T, et al. Comparisons between PET with 11C-Methyl-L-methionine and arterial spin labeling perfusion imaging in recurrent glioblastomas treated with bevacizumab. Clin Nucl Med. 2019;44(3):186–193. [DOI] [PubMed] [Google Scholar]
- 28.Harris RJ, Cloughesy TF, Pope WB, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14(8):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humbert O, Bourg V, Mondot L, et al. 18F-DOPA PET/CT in brain tumors: impact on multidisciplinary brain tumor board decisions. Eur J Nucl Med Mol Imaging. 2019;46(3):558–568. [DOI] [PubMed] [Google Scholar]
- 30.Wardak M, Schiepers C, Cloughesy TF, Dahlbom M, Phelps ME, Huang SC. ¹ 8F-FLT and ¹ 8F-FDOPA PET kinetics in recurrent brain tumors. Eur J Nucl Med Mol Imaging. 2014;41(6):1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 32.Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 33.Heinzel A, Müller D, Langen KJ, et al. The use of O-(2-18F-fluoroethyl)-L-tyrosine PET for treatment management of bevacizumab and irinotecan in patients with recurrent high-grade glioma: a cost-effectiveness analysis. J Nucl Med. 2013;54(8):1217–1222. [DOI] [PubMed] [Google Scholar]
- 34.Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(Pt 3):678–687. [DOI] [PubMed] [Google Scholar]
- 35.Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med. 2001;42(3):432–445. [PubMed] [Google Scholar]
- 36.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11): 1963–1972. [DOI] [PubMed] [Google Scholar]
- 37.Galldiks N, Langen KJ, Holy R, et al. Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53(7):1048–1057. [DOI] [PubMed] [Google Scholar]
- 38.Reardon DA, Galanis E, DeGroot JF, et al. Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol. 2011;13(3): 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Products DoMI. Investigational new drug applications for positron emission tomography (PET) drugs: guidance. In: Center for Drug Evaluation and Research, ed. Maryland: Food and Drug Administration; 2012. [Google Scholar]