Gadolinium-based contrast agents (GBCAs) are MRI contrast agents used clinically to improve the diagnostic accuracy of the MRI scans. The long-term deposition of Gd in normal human tissues, notably the dentate nucleus (DN) and globus pallidus (GP) of the brain, in non-renally impaired patients receiving multiple doses of Gd contrast has been recently reported [2,4]. The implications of Gd deposition are profound given that there are over 30 million contrast-enhanced MRIs performed annually [3]. Although little evidence suggests that this long-term form of Gd deposition results in any clinical manifestations, some GBCAs have been strongly associated with nephrogenic systemic sclerosis (NSF) in patients with renal impairment [1]. In the United States, the Food and Drug Administration now requires warnings on all commercial GBCAs [4].
We report for the first time, the unique histological findings associated with gadolinium deposits on H&E and PAS staining in the DN and GP from a female with NF1 and grade 2 astrocytoma who had a total of 49 GBCA doses with Gadodiamide (Omniscan), and Gadobenate Dimeglumine (MultiHance). These findings may lead to important insights regarding the mechanism of Gd deposition.
This patient was a 16-year old girl with neurofibromatosis type 1 (NF 1), low grade astrocytoma and normal renal function. She was diagnosed at 4 months with NF1. MRI of her brain demonstrated enlargement of the optic tracts and hypothalamus. Biopsy confirmed a grade 2 astrocytoma. She underwent a left pterional craniotomy and tumor resection at age 10, and several courses of chemotherapy. Altogether, she received 21 doses of Omniscan, and 28 doses of MultiHance (0.1mmol/kg) (Supplemental Table 1, online resource). Notably, Omniscan has been strongly associated with the preponderance of NSF cases in renally impaired patients thought to be because of its low stability [1]. She expired at the age of 16.
All imaging was performed on General Electric MRI scanners at both 1.5 and 3T field strength. A control specimen was obtained from a DN of a deceased 18-year old individual with no history of MRI contrast exposure. TEM and Electron microprobe analysis (EMPA) were performed. Gadolinium in the tissue digests were quantified using ICP-MS. Consent was obtained for the use of all imaging and tissue specimens for research purposes.
The last set of MRIs taken 6 weeks prior to death demonstrated stable inherent T1 hyperintensity of the DN, and GP pre-contrast (Figure 1). T1 hyperintensity of the GP was seen on her 9th MRI scan without contrast. H&E stained GP and DN demonstrate large numbers of perivascular dark-staining, spheroid deposits that do not stain for calcium (Von Kossa) or iron (Prussian blue) (Figure 2A–B). These spheroids are also highlighted with PAS stain (Figure 2C). Furthermore, reticulin and trichrome staining did not reveal any organizing connective tissue around the deposits, and GFAP immunohistochemistry and TEM evaluation did not demonstrate glial processes around the deposits.
Figure 1:
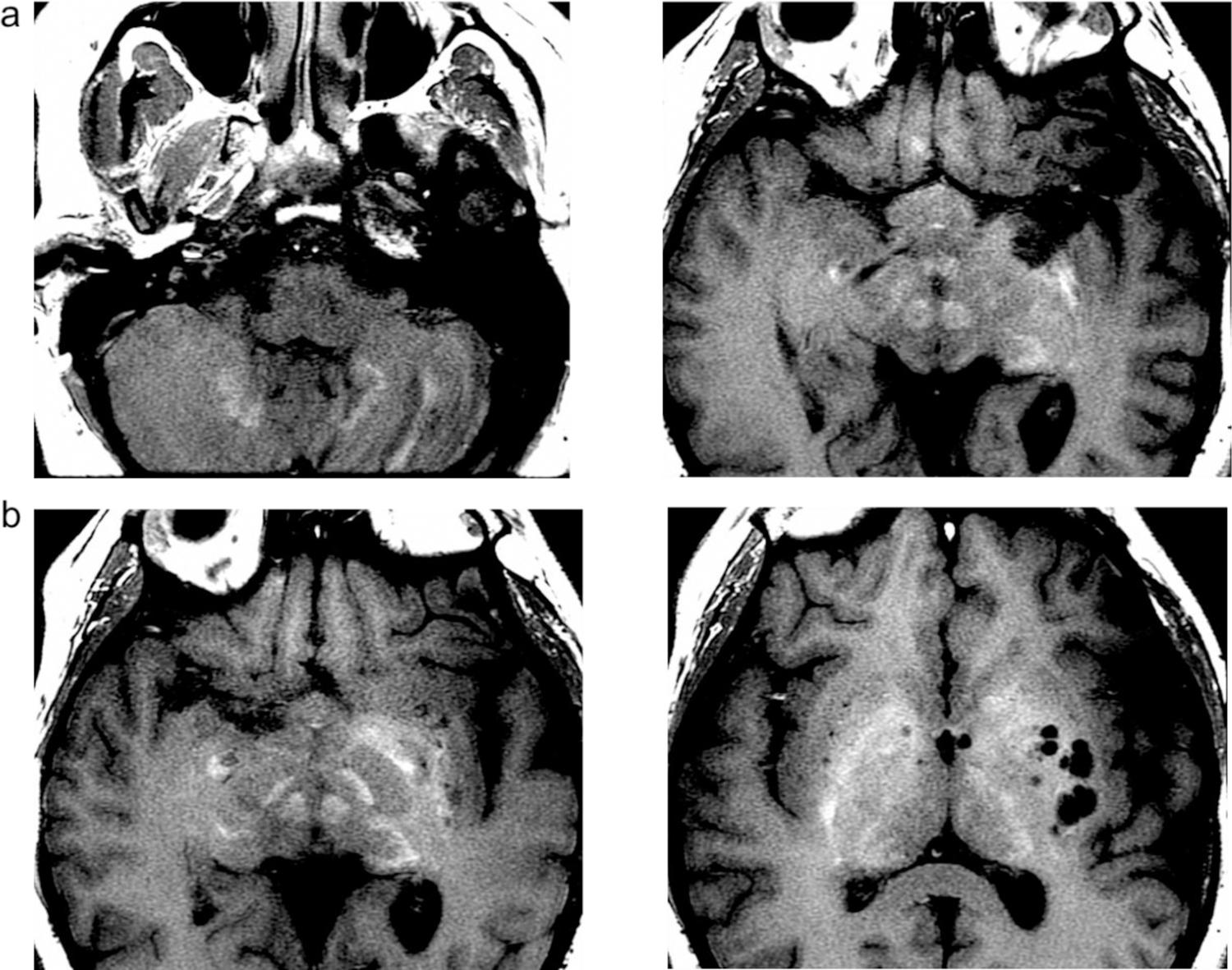
T1-weighted enhancement on MRI imaging of the GP and DN. T1-weighted imaging of the GP (a), and the DN (b) demonstrates enhancement in these two areas without contrast administration.
Figure 2:
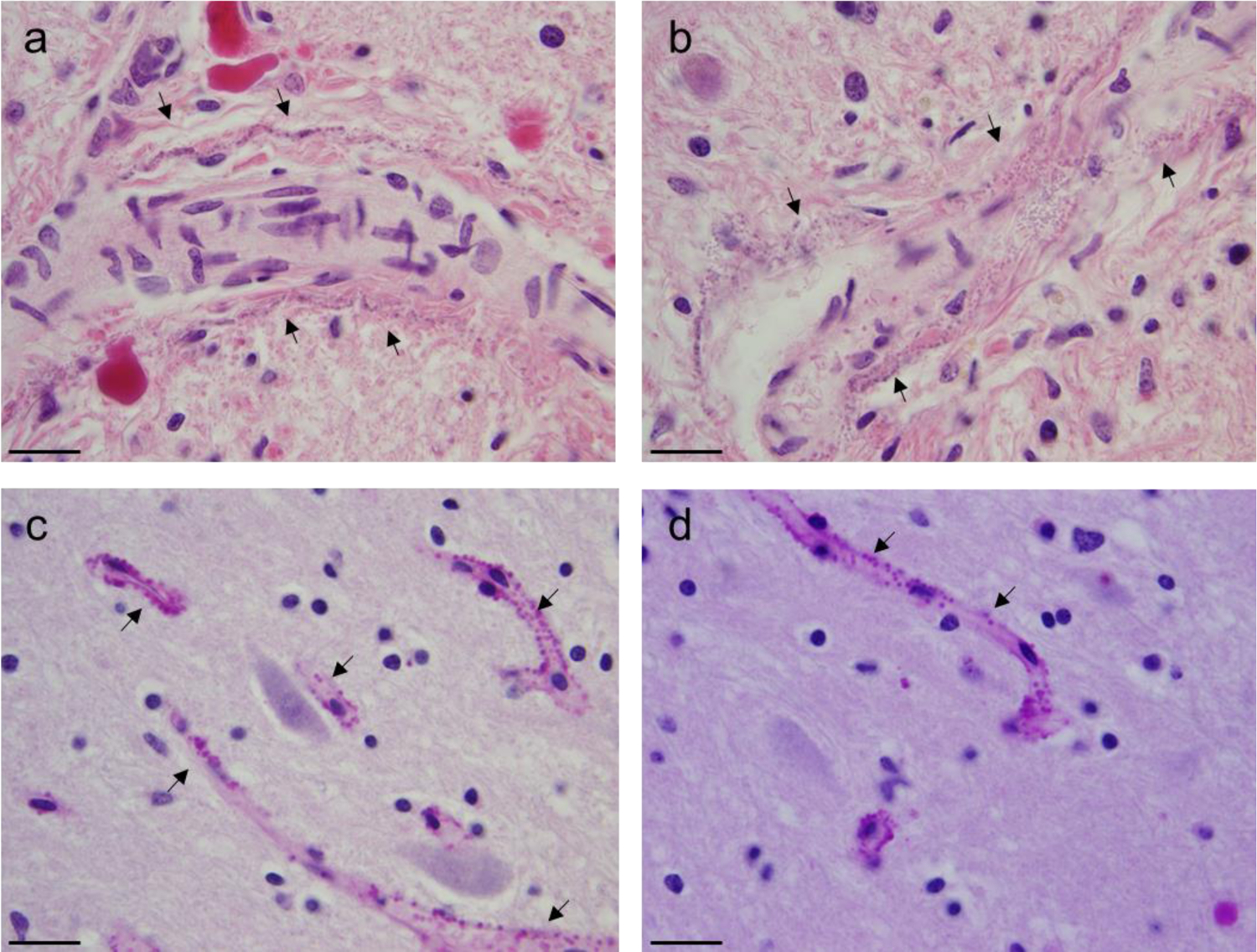
H&E and PAS staining of DN and GP reveals dark perivascular deposits. H&E stain of the GP demonstrates faint amphophilic deposits along the length of the arterioles (scale bars 125µm) (a–b). PAS stain of the dentate nucleus reveals numerous deposits along capillaries (thin arrows, scale bars 50µm) (c–d).
TEM of the DN demonstrates perivascular, round deposits measuring 70–190nm consisting of a more electron-dense center, and a less dense periphery localized in proximity to vascular adventitia (Figure 3A). Higher magnifications reveal that the spheroids are composed of radially-arranged and interwoven filaments of approximately 6nm diameter (Figure 3B–C). Back-scattered imaging located small (~sub-micron) high atomic number contrast regions, with EDS confirming that these deposits corresponded to Gd-rich zones. Wavelength-Dispersive Spectroscopy was then performed, with X-ray maps acquired for Gd La, P Ka, Ca Ka, O Ka and Mn Ka (Supplemental Figure 2, online resource). The Gd, P and Ca maps showed clear concentrations in the bright backscattered electron image zones (Supplemental Figure 1, online resource). The Gd contained in the DN was 2.9 µg/g tissue, and the GP 3.7µg/g of Gd.
Figure 3:
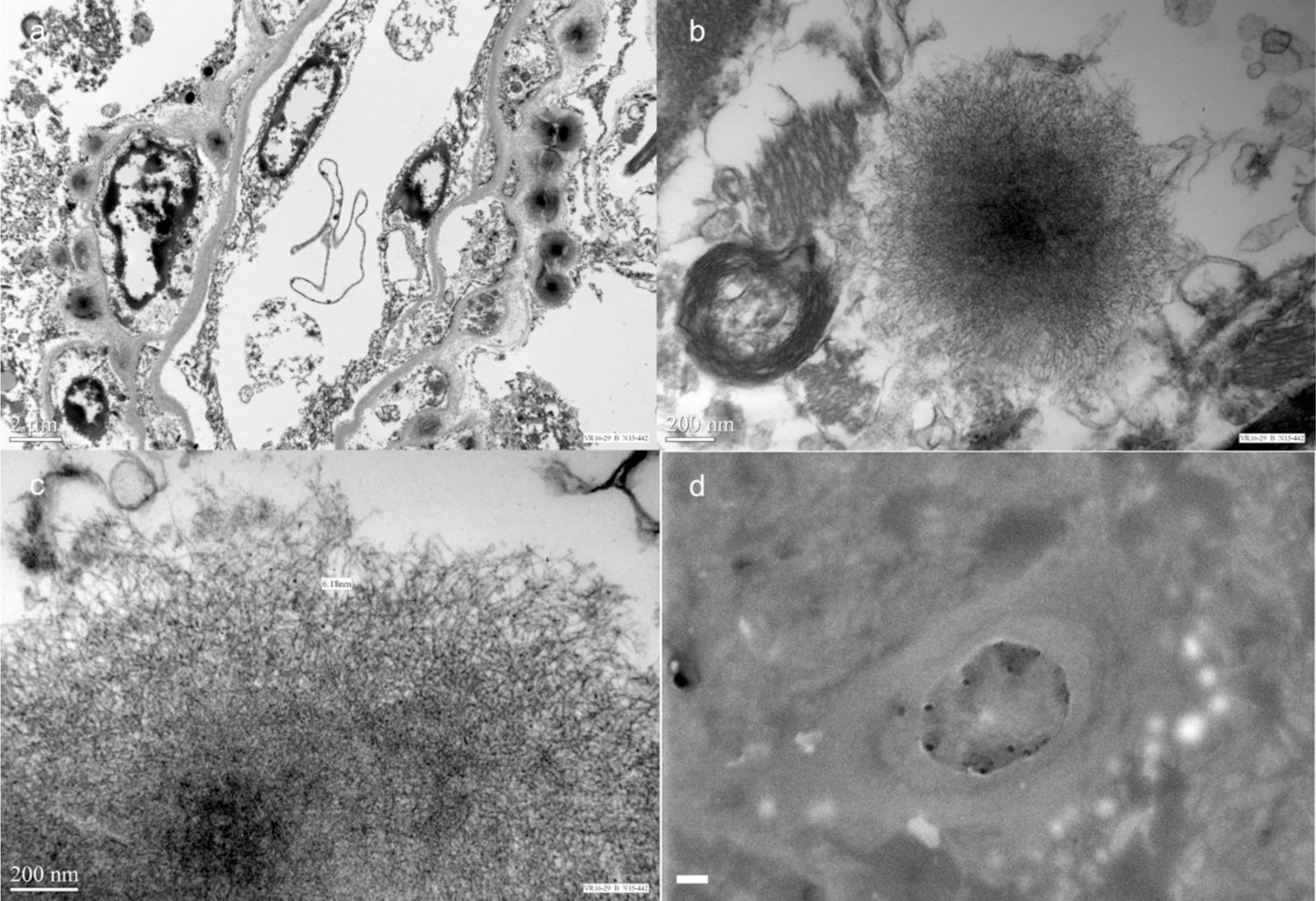
TEM and EMP confirms Gd deposits around perivascular space with intraparenchymal extension. TEM of the DN demonstrates spherical electron-dense deposits surrounding blood vessels (a). Higher magnification TEM reveals that these deposits contain a more electron dense core, and a less dense periphery comprised of filamentous bands measuring approximately 6nm in diameter (b–c). BSE image shows bright globular features around a capillary of the dentate nucleus; these features were confirmed to be from Gd by both EDS and WDS (white scale bar = 1 µm) (d).
Published histological analyses of DN and GP brain specimens containing Gd did not report any significant histological findings associated with Gd deposition [4,2]. We demonstrate for the first time that Gd deposition is associated with perivascular dark-staining deposits around blood vessels in the DN and GP. Confirmation that these deposits are from Gd is accomplished using TEM and EMPA (Figure 3). On TEM, these deposits have a denser electron core, and less electron-dense periphery that are arranged in filaments. This patient’s high cumulative Gd exposure may have allowed for ease of detection of these deposits.
T1-weighted enhancement was seen in the GP on her 9th scan with Omniscan, which has been associated with the preponderance of NSF cases [1] This signal intensity on T1-weighted imaging of her DN and GP increased with subsequent MRI studies, and continued to increase with doses of MultiHance, suggesting that both GBCAs may lead to Gd retention in the brain.
Notably, the clinical implications of Gd deposition is extensive given the 30 million of contrast-enhanced MRIs performed annually [3]. As Gd deposition occurs in patients with normal renal function, any patients receiving GBCAs are at risk for Gd deposition. However, we did not see evidence of any fibrotic or connective tissue processes around these Gd deposits in this patient with high GBCA exposure. Furthermore, inflammatory glial processes were also notably absent. Taken together, our findings suggest that these Gd deposition do not share similar histopathological characteristics as NSF. Further research into the mechanism of deposition and potential long-term health effects are warranted.
Supplementary Material
References
- 1.Broome DR (2008) Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: a summary of the medical literature reporting. Eur J Radiol 66:230–234 [DOI] [PubMed] [Google Scholar]
- 2.Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku Ji, Haruyama T, Kitajima K, Furui S (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232 [DOI] [PubMed] [Google Scholar]
- 3.Lohrke J, Frenzel T, Endrikat J, Alves FC, Grist TM, Law M, Lee JM, Leiner T, Li K-C, Nikolaou K (2016) 25 years of contrast-enhanced MRI: developments, current challenges and future perspectives. Adv Ther 33:1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


