Abstract
Background:
Children with sickle cell disease (SCD) are at increased risk for bacterial infections including osteomyelitis (OM). Fever and bone pain, key presenting symptoms of OM, are common in SCD, thus complicating diagnosis. We reviewed presentation, imaging features, and microbiologic etiologies of children with SCD treated for OM.
Methods:
The comprehensive SCD clinical database of children and adolescents with SCD followed at a single, large tertiary pediatric center was searched to identify all diagnostic coding for potential cases of osteomyelitis in children ages 6 months to 21 years from 2010 – 2019. Medical charts were reviewed to determine OM diagnostic probability based on radiographic and microbiologic findings and the duration of prescribed antibiotic treatment for OM.
Results:
Review of 3,553 patients (18,039 person-years) identified 20 episodes of probable OM in 19 children. Magnetic resonance imaging (MRI) findings to support OM were definitive in 4/19 (21%), probable in 10/19 (53%), suspected in 5/19 (26%), based on blinded radiologist review. Blood and/or operative cultures from bone and tissue debridement isolated Salmonella species in 7 (35%) cases and Methicillin-susceptible Staphylococcus aureus (MSSA) in 2 (10%). Six patients received antibiotic treatment prior to obtainment of cultures. Of culture-positive cases, MRI findings for OM were definitive or probable in 6/9 (67%), suspected in 3/9 (33%).
Conclusions:
Distinction between OM and sickle-related bone infarct or vaso-occlusion is difficult based on imaging findings alone. Early attainment of blood and operative cultures increases the likelihood of identifying and adequately treating OM.
Keywords: Sickle cell disease, osteomyelitis, Salmonella, Staphylococcus aureus
BACKGROUND
Children with sickle cell disease (SCD) are particularly susceptible to osteoarticular complications including vaso-occlusive crisis (VOC), avascular necrosis, septic arthritis and osteomyelitis1–3. The increased susceptibility to osteoarticular infections is thought to result from impaired splenic function, complement activity, and intermittent episodes of vaso-occlusion, infarction and necrosis of the bone providing an environment conducive for bacteria to thrive2. Osteomyelitis (OM) is an acute or chronic inflammatory process in the bone caused by bacterial infection. The cumulative occurrence of OM in children with SCD has been reported to range broadly from 1.4 and 12% in different series4–6.
Differentiating OM from VOC may pose a diagnostic challenge, as several clinical, radiological and laboratory features overlap. However, early recognition and treatment of OM is necessary to prevent long-term morbidity such as chronic OM or recurrent relapses2,4,7. While there are no standard diagnostic criteria for osteomyelitis, isolation of a bacterial pathogen from the suspected site of infection or from blood in a patient with clinical and radiographic findings consistent with osteomyelitis is highly suggestive8. Staphylococcus aureus is the most common cause of osteomyelitis in children, while Salmonella species are a unique cause in children with SCD9–12. Less commonly, other enteric pathogens have been described as the cause of osteoarticular infections in children with SCD, including Serratia, Enterobacter cloacae, Enterococcus faecium, and Pseudomonas aeruginosa5.
The epidemiology of invasive bacterial infections and the treatment of osteomyelitis in general pediatrics have evolved over the past few decades. Recent data indicate that the proportion of pediatric S. aureus infections secondary to community-acquired methicillin resistant S. aureus (MRSA) may be decreasing13,14. The purpose of this retrospective study was to conduct an updated review of the radiologic and microbiological etiologies of OM in children with SCD as well as medical and surgical treatments.
METHODS
We conducted a retrospective review of children 6 months to 21 years of age with SCD diagnosed with OM at Children’s Healthcare of Atlanta (CHOA) from January 1, 2010 to December 31, 2019. Eligible subjects were identified from our center’s comprehensive SCD Clinical Database which captures all patients with a confirmed SCD diagnosis who have had at least one inpatient or outpatient medical encounter at CHOA in a given calendar year. International Classification of Diseases (ICD) discharge codes were reviewed to identify potential cases of OM (ICD-9 code 730.X; ICD-10 codes M86.X and H05.022). Subjects were excluded from study if they had: primary bone abnormality syndromes, bacteremia without evidence of bone involvement, lack of supporting evidence for OM by either microbiology culture or radiographic findings, lack of at least 4 weeks of antibiotic therapy prescribed for treatment of OM. This study was approved by the Institutional Review Board of CHOA.
Electronic medical records were reviewed to determine presenting symptoms, anatomic location(s) of the bone involvement, presence of concomitant septic arthritis, blood and biopsy culture results, date(s) of surgical debridement, laboratory parameters at admission and during the course of OM therapy, bacterial antibiotic susceptibility patterns when available, radiographic findings, prescribed intravenous (IV) and oral antibiotic therapy and treatment duration, animal or travel exposures within the family, and whether there were subsequent episodes of osteomyelitis. A blinded, pediatric radiologist reviewed all imaging results and to provide an assessment of the strength of evidence for OM based on imaging alone. Magnetic resonance imaging (MRI) at our institution for the indication of OM are performed following a protocol that includes initial large field of view images of the area of concern followed by focused smaller field of view images of the region of interest. Exams are usually performed with and without contrast but may be performed without contrast alone; exams were perfomed on either a 1.5 or 3.0 Tesla system. Radiographic evidence for the diagnosis of OM were categorized as definitive (findings most consistent with bone infection), probable (findings favored infection but could not entirely exclude VOC), or suspected (findings could support either infection or VOC).
Blood or tissue samples for cultures were processed in automated, continuous monitor systems. Isolates were identified by phenotypic tests or matrix assisted laser desorption ionization mass spectrometry time of flight (MALDI-TOF). Descriptive statistics were reported by total numbers, percentages, means, medians, and interquartile ranges (IQR) as appropriate.
RESULTS
In the study timeframe, there were 3,553 eligible children and adolescents with SCD in the clinical database, with 18,039 person-years captured. From this group, we identified 38 patient encounters with ICD codes for OM. Following chart reviews, 18 were excluded due to absence of either microbiologic or radiographic findings consistent with osteomyelitis. There were a remaining 20 episodes of probable OM in 19 patients included in the study (Table 1). One patient had two episodes of OM that occurred >2 years apart in different bone locations (Cases 10 and 14). The median age of OM diagnosis was 6.5 years (IQR 2.8 – 9.8 years). Two patients (including the individual with recurrent OM) had a history of splenectomy. Presenting symptoms included pain at the area of presumed OM in 19 (94%), fever in 11 (55%), limited weight-bearing or movement of the affected area in 8 (40%), and swelling of the affected area in 7 (35%). Two children with culture-confirmed Salmonella osteomyelitis had household members with occupational or personal chicken handling (Cases 8 and 9).
Table 1.
Microbiology culture and magnetic resonance imaging findings of children with SCD and osteomyelitis
| Case | Age (Years) | SCD Geno-type | Culture Findings | Radiographic Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic Pre-treated | Blood | Operative | Radiographic Evidence of OM | Multi-focal | Area of bone | Abscess/ fluid | Myositis / muscle edema | Effusion/ synovitis | Osteo-necrosis | |||
| 1 | 1.6 | SC | No | Salmonella species | N/A | No Imaging | Elbow, forearm | |||||
| 2 | 1.6 | SS | No | No growth | N/A | Definitive | No | Radius | No | Yes | No | No |
| 3 | 1.8 | Sβ0 thal | Yes | No growth | MSSA | Definitive | No | Ischium | Yes | Yes | Yes | Yes |
| 4 | 2.3 | SS | No | No growth | No growth | Probable | No | Humerus | No | Yes | Yes | No |
| 5 | 2.8 | SC | No | Salmonella species | N/A | Suspected | No | Sternum | Yes | No | No | No |
| 6 | 3.5 | SS | No | No growth | N/A | Probable | Yes | Humerus, radius, ulna | Yes | Yes | Yes | Yes |
| 7 | 5.5 | SS | Yes | No growth | No growth | Probable | No | Femur | Yes | Yes | Yes | Yes |
| 8 | 5.9 | SS | No | Salmonella species | Salmonella enterica | Definitive | Yes | Humerus, tibia | Yes | Yes | Yes | No |
| 9 | 6.8 | SS | No | No growth | Salmonella species | Probable | Yes | 1st metatarsals bilateral | Yes | No | No | Yes |
| 10 | 6.9 | SS | Yes | No growth | Contaminant* | Probable | Yes | Distal femur, proximal tibia, tarsals, metatarsals | Yes | No | Yes | No |
| 11 | 7.6 | SS | No | Salmonella species | N/A | Probable | Yes | Superior pubic ramus, femur | No | Yes | Yes | No |
| 12 | 8.3 | Sβ0 thal | Yes | No growth | No growth | Probable | Yes | Humerus, radius, ulna | Yes | Yes | Yes | Yes |
| 13 | 9.1 | SS | No | No growth | N/A | Suspected | Yes | Ilia bilateral | No | Yes | No | Yes |
| 14 | 9.1 | SS | No | No growth | No growth | Probable | No | Humerus | Yes | Yes | Yes | No |
| 15 | 10.0 | SS | No | MSSA | N/A | Suspected | No | Femur | No | Yes | No | Yes |
| 16 | 12.5 | Sβ0 thal | Yes | No growth | N/A | Suspected | Yes | Acetebula bilateral, femur | No | Yes | No | Yes |
| 17 | 13.1 | SS | No | N/A | N/A | Probable | No | Femur | Yes | Yes | No | No |
| 18 | 16.6 | SS | No | Salmonella species | Salmonella species | Probable | Yes | Humerus, radius, ulna | No | Yes | Yes | No |
| 19 | 17.8 | SS | Yes | No growth | N/A | Probable | Yes | Parietal bones bilateral | Yes | No | Yes | Yes |
| 20 | 18.9 | SS | No | No growth | Salmonella species | Definitive | No | Femur | Yes | Yes | Yes | No |
Micrococcus species and coagulase-negative Staphylococcus cultured from 1 of 4 purulent debridement samples, in broth only, samples obtained after systemic antibiotics administered. MSSA = methicillin-sensitive Staphylococcus aureus; thal = thalassemia
Initial laboratory findings and maximum values during the hospitalization are shown in Figure 1, demonstrating leukocytosis (white blood cell count mean of 15.6 ×103/μL at initial, and 19.1×103/μL at maximum) and significant elevations in erythrocyte sedimentation rate (ESR) (mean 58 mm/hr initial, 77.8 mm/hr at maximum) and C-reactive protein (CRP) (mean 10.6 mg/dL initial, 12.2 mg/dL at maximum).
Figure 1. Laboratory findings at initial presentation and maximum values during episodes of OM in children with SCD.
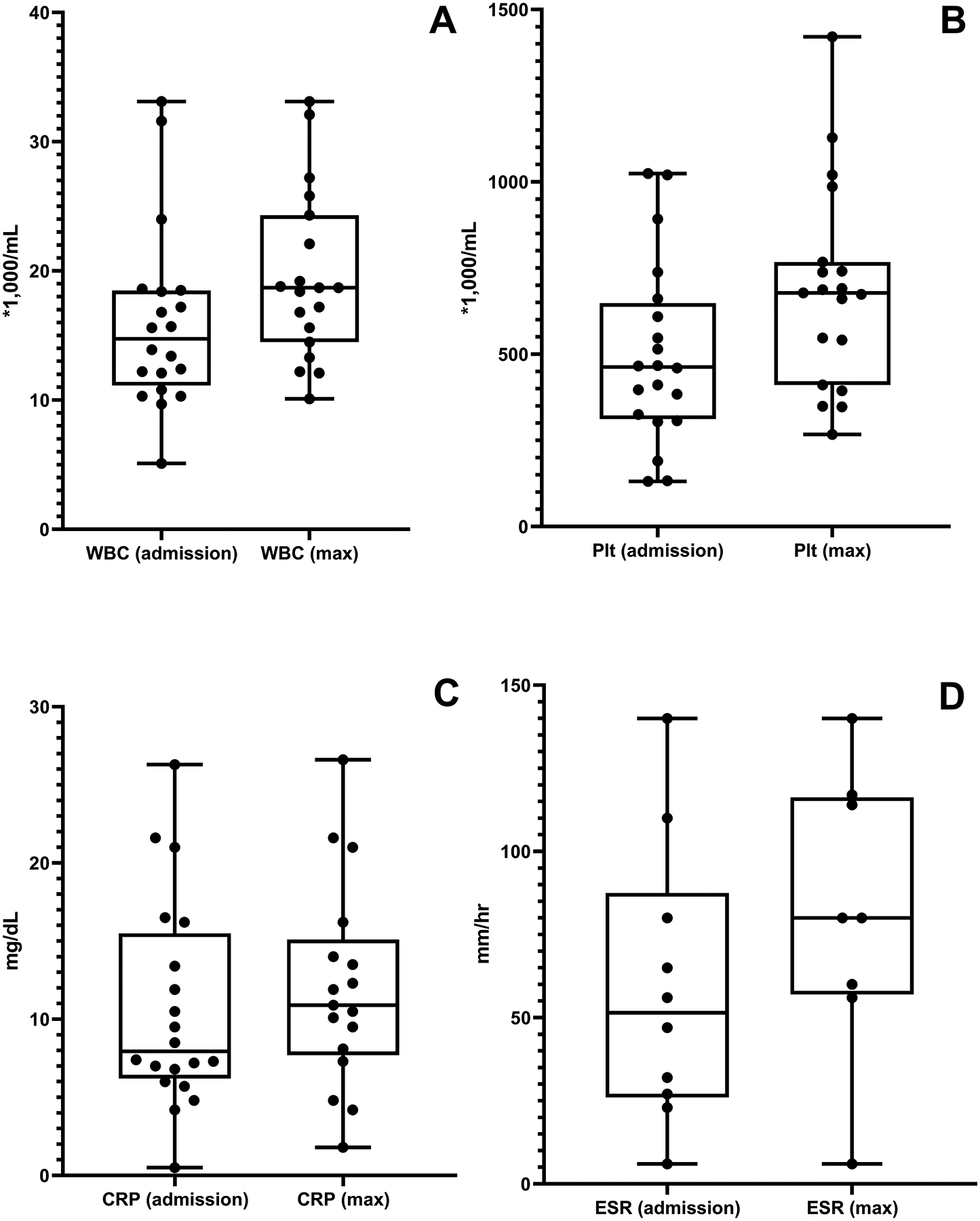
Box and whisker plots demonstrate median values, IQR, and 1.5 times IQR (whisker) for: A) White blood cell count (WBC), B) Platelet count (Plt), C) C-reactive protein (CRP), D) Erythrocyte sedimentation rate (ESR).
Imaging was obtained in 19 cases: 18 had magnetic resonance imaging (MRI) and 1 had computerized tomography (CT) in order to evaluate both lungs and bone (case 5). Evidence for OM by imaging findings alone was categorized as definitive in 4/19 (21%), probable in 10/19 (53%), and suspected in 5/19 (26%). Abscess or fluid collection was noted in 11/19 (58%), adjacent myositis or muscle edema in 15/19 (79%), joint effusion in 12/19 (63%), and osteonecrosis in 9/19 (47%). Among the cases of culture-confirmed infection, MRI findings for osteomyelitis were definitive in only 2 (22%), probable in 4 (44%), and suspected in 3 patients (33%).
Blood cultures were obtained in all but 1 case, and surgical incision & drainage with tissue cultures was undertaken in 9 (45%). A microbiologic etiology was confirmed in 9 cases: 7 with Salmonella species, 2 with Methicillin-susceptible staphylococcus aureus. Six patients received antibiotic pre-treatment prior to blood or operative cultures; of these, 1 patient had a positive operative culture (Case 3) despite antibiotic treatment. Antibiotic pre-treatment occurred in 5/9 (55%) culture-negative OM as compared to 1/11 (9%) culture-positive OM.
Among the 7 cases of Salmonella, 6/7 isolates were susceptible to all antibiotics tested, while one isolate (Case 11) was resistant to ampicillin, but susceptible to ceftriaxone, ciprofloxacin and trimethoprim/sulfamethoxazole.
One patient with antibiotic pre-treatment (Case 10) had growth of presumed bacterial contaminants from operative cultures, with Micrococcus that grew in broth only in 1 of 4 abscess cultures and coagulase-negative Staphylococcus that grew from a deep bone culture. Given the nature of these 2 organisms, they were presumed to be culture contaminants at the time of treatment; however the patient’s clinical presentation greatly favored the diagnosis of OM over VOC, including initial MRI findings showing OM with posterior compartment abscess and intraoperative findings of purulent fluid at the interosseous membrane and within the bone.
By study inclusion criteria, all patients received at least 4 weeks of antibiotics (median 5 weeks, IQR 4 – weeks). The median duration of IV antibiotics was 9 days (IQR 6 – 17 days). The median duration of total antibiotics in culture-positive vs culture-negative cases was 6 weeks (IQR 4 – 12 weeks) vs 4 weeks (IQR 4 – 6 weeks). One outlier (Case 8) was a 6-year-old patient who presented with diarrhea, septic shock with hepatic insufficiency and disseminated intravascular coagulation, with initial blood cultures that grew Salmonella enterica. After this presentation she developed left shoulder pain, and MRI showed OM of the humeral epiphysis and metaphysis as well as extensive surrounding myositis. The first surgical debridement occurred 14 days after initial presentation and antibiotic initiation, with growth of Salmonella from the bone marrow culture although no growth from joint or abscess aspiration. This patient received 40 days of IV antibiotics and 28 weeks of total antibiotic therapy (IV and oral combined).
Recurrence of OM occurred in 2 patients. Cases 10 and 14 represent the same patient who had 2 distinct episodes of OM at different times (>2 years apart) and different bone locations. Case 9 represents an individual originally from Tanzania with chronic OM of the metatarsals of bilateral feet. At the time of initial presentation, this patient had swollen abscesses on the dorsi of both feet, both of which underwent incision & drainage, with isolation of a Salmonella species. MRI showed OM of the first metatarsals, with a possible Brodie’s abscess of the left first metatarsal, suggestive of chronic OM. The patient completed 3 months of antibiotic therapy with resolution of pain and swelling; however, 13 months later presented again with a raised lesion on the dorsum of the right foot and sequential MRI suggestive of OM of the right first metatarsal. The persistence of these radiographic findings, in addition to the initial appearance of the Brodie’s abscess suggest longstanding chronic OM.
DISCUSSION
The diagnosis of osteomyelitis requires a high degree of clinical suspicion, as presenting symptoms and laboratory parameters can overlap between OM and VOC. OM in children with SCD is relatively uncommon compared to VOC; however children with SCD are at increased risk for OM due to their functional or anatomical asplenia, thus clinicians must maintain high vigilance for possible infection in the setting of bone pain and fever. Our findings were in agreement with prior studies that found Salmonella species to be the most common organism identified followed by Staph aureus, without identification of any other definitive causes of infection in our large institutional cohort9–11,15,16.
A recent study provides similar findings to ours, highlighting the diagnostic difficulty of OM in SCD. Weisman et al. identified cases of OM through electronic review of MRI reports for the terms “sickle” and “osteomyelitis,” then reviewed reports to determine if the diagnosis of OM was determined to be consistent, indeterminant, or inconsistent with OM based on the radiographic report. Of 28 cases identified, the majority had no organism identified by bone or operative culture, while 9 (32%) had non-typhi Salmonella identified. Our study provides contrast to this series in several ways. Our group reviewed an entire defined cohort of known SCD patients to seek all potential cases of OM over a 9-year period through search of diagnostic codes followed by review of microbiologic cultures and MRI results. Thus, our series was able to include cases that were diagnosed by findings other than bone imaging. Through our methods, we identified a somewhat higher prevalence of culture-positive OM cases (45% of cases), and we identified S. aureus as well as Salmonella as infectious etiologies of OM.
Our study highlights the limitations of MRI as the only diagnostic tool for identifying OM, particularly in light of the fact that among patients with positive blood and/or tissue cultures, only 20% were found to have MRI findings deemed definitive for osteomyelitis diagnosis. Additionally, through examination of culture results, we confirmed that S. aureus in addition to Salmonella remains an important consideration in the initial, empiric antibiotic therapy for children with SCD with suspected OM. The low prevalence of antibiotic resistance in our cohort, even among children with exposure to daily penicillin prophylaxis, suggests that there may be a range of options for empiric therapy that covers both Salmonella as well as MSSA.
A strength of our study was the large number of children with SCD captured through our center’s clinical database with availability of long-term follow-up data. In addition, we used multiple criteria including microbiologic and radiographic findings to identify cases of OM and exclude cases in which VOC was a more likely diagnosis. Limitations of our study include the retrospective nature and relatively small sample size taken from a single geographic area. Given the lack of a standardized definition of OM, it is also possible that we overestimated the number of cases as we rely on the radiology reader’s interpretation of imaging results. We attempted to minimize this bias by grading the strength of the imaging to suggest OM by a blinded pediatric radiologist.
Our results highlight the unique susceptibility of children with SCD to invasive bacterial infections and the persistence of Salmonella as a common pathogen in cases of OM in SCD. Blood cultures, radiographic imaging, and surgical incision & drainage of areas of suspected OM are all important diagnostic considerations in distinguishing OM from the more common diagnosis of VOC in children with SCD. Obtaining blood cultures prior to antibiotic therapy and operative cultures when possible are potential steps to increasing the likelihood of identification of a definitive diagnosis of OM.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Abraham J. & Phyllis Katz Foundation. Marianne Yee received funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number 1K23HL146901-01A1. Nitya Bakshi received funding from the National Heart, Lung and Blood Institute of the National Institutes of Health under award number 1K23HL140142-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATION KEY
- CRP
C-reactive protein
- ESR
Erythrocyte sedimentation rate
- IQR
Interquartile range
- MRSA
Methicillin-sensitive Staphylococcus aureus
- MSSA
Methicillin-resistant Staphylococcus aureus
- OM
osteomyelitis
- PLT
platelet
- SCD
Sickle cell disease
- WBC
White blood cell
Footnotes
Conflict of Interest: All of the authors have no conflicts of interest to disclose
REFERENCES
- 1.Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol 2005;129:482–90. [DOI] [PubMed] [Google Scholar]
- 2.Bennett OM, Namnyak SS. Bone and joint manifestations of sickle cell anaemia. J Bone Joint Surg Br 1990;72:494–9. [DOI] [PubMed] [Google Scholar]
- 3.Rao SP, Miller S, Solomon N. Acute bone and joint manifestations of sickle cell disease in children. N Y State J Med 1986;86:254–60. [PubMed] [Google Scholar]
- 4.Fontalis A, Hughes K, Nguyen MP, et al. The challenge of differentiating vaso-occlusive crises from osteomyelitis in children with sickle cell disease and bone pain: A 15-year retrospective review. J Child Orthop 2019;13:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gouveia C, Duarte M, Norte S, et al. Osteoarticular infections in paediatric sickle cell disease: in the era of multidrugresistant bacteria. Br J Haematol 2020. [DOI] [PubMed] [Google Scholar]
- 6.Neonato MG, Guilloud-Bataille M, Beauvais P, et al. Acute clinical events in 299 homozygous sickle cell patients living in France. French Study Group on Sickle Cell Disease. Eur J Haematol 2000;65:155–64. [DOI] [PubMed] [Google Scholar]
- 7.Inusa BP, Oyewo A, Brokke F, Santhikumaran G, Jogeesvaran KH. Dilemma in differentiating between acute osteomyelitis and bone infarction in children with sickle cell disease: the role of ultrasound. PLoS One 2013;8:e65001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger E, Saunders N, Wang L, Friedman JN. Sickle cell disease in children: differentiating osteomyelitis from vaso-occlusive crisis. Arch Pediatr Adolesc Med 2009;163:251–5. [DOI] [PubMed] [Google Scholar]
- 9.Burnett MW, Bass JW, Cook BA. Etiology of osteomyelitis complicating sickle cell disease. Pediatrics 1998;101:296–7. [DOI] [PubMed] [Google Scholar]
- 10.Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther 2010;8:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan J, Ikeda S, McNeil JC, Kaplan SL, Vallejo JG. Microbiology of Osteoarticular Infections in Patients with Sickle Hemoglobinopathies at Texas Children’s Hospital, 2000–2018. Pediatr Infect Dis J 2019;38:1251–3. [DOI] [PubMed] [Google Scholar]
- 12.Weisman JK, Nickel RS, Darbari DS, Hanisch BR, Diab YA. Characteristics and outcomes of osteomyelitis in children with sickle cell disease: A 10-year single-center experience. Pediatr Blood Cancer 2020;67:e28225. [DOI] [PubMed] [Google Scholar]
- 13.Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA 2012;308:50–9. [DOI] [PubMed] [Google Scholar]
- 14.Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing Susceptibility of Staphylococcus aureus in a US Pediatric Population. Pediatrics 2016;137. [DOI] [PubMed] [Google Scholar]
- 15.Chambers JB, Forsythe DA, Bertrand SL, Iwinski HJ, Steflik DE. Retrospective review of osteoarticular infections in a pediatric sickle cell age group. J Pediatr Orthop 2000;20:682–5. [DOI] [PubMed] [Google Scholar]
- 16.Syrogiannopoulos GA, McCracken GH Jr., Nelson JD. Osteoarticular infections in children with sickle cell disease. Pediatrics 1986;78:1090–6. [PubMed] [Google Scholar]


