Abstract
Introduction
Segmental arterial mediolysis (SAM) is a rare non-atherosclerotic, non-inflammatory vasculopathy causing arterial wall necrosis that leads to strictures, dissections, and aneurysms, particularly in medium-sized abdominal arteries. Awareness of SAM is important because, unlike vasculitides, immunosuppressive treatment may worsen the disease process.
Case
A 58-year-old male with multiple medical comorbidities presented with acute epigastric pain and a right incarcerated inguinal hernia that was interpreted as showing bowel strangulation on CT. The hernia was unable to be reduced in the emergency department, so the patient was taken for open reduction by the surgical service. Intraoperatively, he was noted to have a ruptured superior mesenteric artery aneurysm. Conventional angiography demonstrated a bead-like appearance of several jejunal branches of the superior mesenteric artery, raising concern for a vasculitis. His hospital course included rheumatologic consultation, and initial recommendations were to start immunosuppressive therapy for treatment of polyarteritis nodosa. Further testing demonstrated normal ANA, ANCA, and complement levels. Due to a lack of systemic symptoms or signs and otherwise unremarkable laboratory evaluation, the patient was ultimately diagnosed with SAM and immunosuppressive therapy was halted.
Discussion
Unexplained medium arterial stenosis, dissection, aneurysm, and hemorrhage should raise suspicion for possible SAM. The initial management approach should focus on treatment of the acute hemorrhage, usually involving endovascular stenting or coil embolization. Unlike vasculitides, SAM does not benefit from, and may actually be harmed by, immunosuppressive therapy.
Conclusion
Clinicians involved in the longitudinal care of emergency department patients should be aware of this rare clinical entity in order to initiate appropriate treatment.
Keywords: Arterial aneurysm, arterial stenosis, vasculitis, acute abdomen
INTRODUCTION
Segmental arterial mediolysis (SAM) is a rare vasculopathy causing degeneration of the medial layer of arteries, particularly medium-sized abdominal vessels. First described in 1976, this non-inflammatory, non-atherosclerotic disease of unknown origin has been reported to have mortality rates as high as 50%.1,2 While SAM may present at any age, it is most frequently reported in the middle age and elderly and is generally ntoed to have an equal distribution between sexes, though one case series noted a slight male predominance.2-6 The most common acute presentations involve hemorrhage, dissection, or occlusion of the celiac artery (50-60%) and superior mesenteric artery (30%) with less common manifestations in the intracranial, carotid, iliac, coronary, and pulmonary arteries.7 Perhaps due to the difficulty of diagnosis, stenoses secondary to SAM are a rarely diagnosed cause of chronic ischemic abdominal pain.8 In this case report, we describe the presentation of a patient ultimately diagnosed with SAM, followed by a more in depth discussion of the diagnosis and management of this rare entity.
CASE REPORT
A 58-year-old morbidly obese male presented to the emergency department with acute onset of severe, paroxysmal periumbilical pain that radiated bilaterally. His past medical history was significant for paroxysmal atrial fibrillation, venous thromboembolism with IVC filter placement, chronic kidney disease, umbilical hernia with a mesh repair, and a right inguinal hernia. The pain was variably described as sharp or tearing and was accompanied by nausea, vomiting, and a swollen and tender right hemiscrotum. These symptoms occurred mid-morning while the patient was beer-tasting following a breakfast at McDonald’s. On physical examination, his abdomen was soft, but diffusely tender to palpation. Additionally, his right hemiscrotum was enlarged and tender, consistent with an inguinal hernia.
Due to concern for aortic dissection, the patient underwent CT angiogram of his abdomen/pelvis, which was read as showing a right inguinal hernia with inflammatory changes consistent with a closed obstruction, though no abnormalities of the abdominal vasculature were seen, specifically no evidence of aortic dissection. Routine laboratory testing included sodium 140 mmol/L, potassium 4.4 mmol/L, chloride 110 mmol/L, carbon dioxide 24 mmol/L, anion gap 6 mmol/L, blood urea nitrogen 33 mg/dL, creatinine 2.6 mg/dL, glucose 116 mg/dL, albumin 3.4 g/dL, calcium 8.7 mg/dL, total protein 7.9 g/dL, troponin I <0.02 ng/mL, alkaline phosphatase 145 U/L, alanine aminotransferase 16 U/L, aspartate aminotransferase 8 U/L, total bilirubin 0.3 mg/dL, lipase 147 U/L, C reactive protein 3 mg/dL, white cell count 9,700/uL, hemoglobin 14.2 g/dL, hematocrit 43%, and platelet count 277,000/uL.
Attempts to reduce the hernia were unsuccessful and manipulation markedly increased the patient’s pain. The emergency surgery team therefore decided to proceed with operative reduction of the incarcerated right inguinal hernia. Intraoperatively, the patient was noted to have hemoperitoneum with a “massive small bowel mesenteric hematoma at the root of the superior mesenteric artery… with a… palpable thrill.” There was “a rough cobblestoned appearance… of the… entire small bowel mesentery”. The jejunal branch of the superior mesenteric artery was ligated intraoperatively. The patient then proceeded to interventional radiology for endovascular intervention. During that procedure, a superior mesenteric arteriogram showed no evidence of active extravasation, however multiple small jejunal branches were noted to have a beaded, irregular appearance, consistent with polyarteritis nodosa (PAN). One of these branches that was near the known mesenteric hematoma was coil embolized due to concern that it may have been the culprit vessel causing the hematoma.
Over an extended hospitalization, the patient had surgical correction of his hernia and a repeat laparotomy for evaluation of possible abdominal compartment syndrome due to hemodynamic instability. During this subsequent operation, the previously identified hematoma was noted to be significantly decompressed and there was no evidence of active mesenteric bleeding nor signs of mesenteric ischemia. Additional laboratory testing during his hospital stay included a negative antinuclear antibody titer, negative anti-neutrophil cytoplasmic antibody, total C3 complement level 105 mg/dL, and total C4 complement level 19 mg/dL. Due to the beaded appearance of the superior mesenteric artery during conventional angiography and the elevated C reactive protein level, the consulting rheumatologist recommended that the patient start methylprednisolone for treatment of medium-vessel vasculitis, specifically PAN. Additional diagnoses entertained at the time included systemic lupus erythematosus, Behcet’s Disease, fibromuscular dysplasia, Ehlers Danlos Syndrome, and SAM. Lack of constitutional symptoms, however, argued against a systemic vasculitis like Behcet’s and systemic lupus erythematosus. The “string of beads” appearance of the angiogram was noted to be most consistent with either PAN or SAM. Biopsy of either a kidney or the mesentery, while potentially diagnostic for PAN, was not pursued because the consulting rheumatologist felt that a negative result would not be sufficient to rule out the disorder. Empiric steroids and cyclophosphamide were therefore recommended for treatment of PAN on initial consultation.
However, on further consideration, just prior to discharge, the consulting rheumatologist began to consider SAM as the most likely diagnosis. Reasons for this change in diagnosis included the fact that the patient exhibited no other system involvement, particularly no skin, pulmonary, central nervous system, or peripheral nervous system signs. Additionally, the conventional angiogram demonstrated abnormalities in only one medium-sized vessel instead of several, which favored the diagnosis of SAM over PAN. Further, the presenting symptom of mesenteric artery rupture was viewed as more consistent with the case reports of SAM available in the literature. Though the patient was continued on prednisone and azathioprine at the time of discharge, he was advised to taper off all immunosuppressive medicines at his one month follow-up visit due to the rheumatologist’s final determination that the patient’s disease process was most consistent with SAM and not PAN. Review of the initial CT noted a misinterpretation of an extensive hematoma as small bowel inflammatory changes (Figure). Further imaging evaluation of the patient’s abdominal vasculature has not been available.
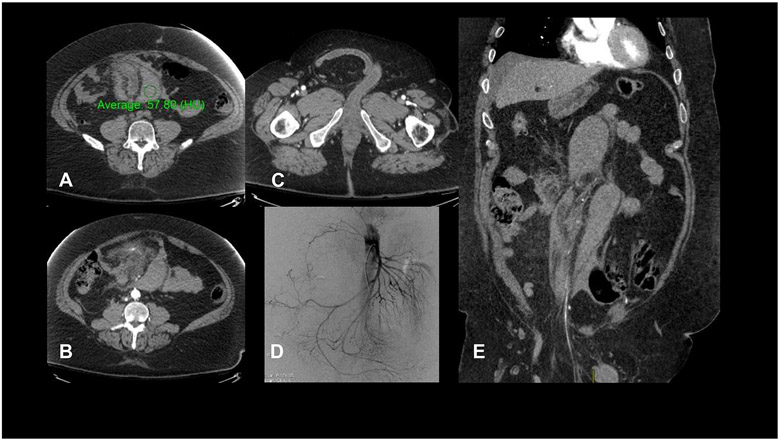
Figure:
CT imaging for the presented patient at the time of his ED visit. (A) High attenuation (57 HU on non-contrast imaging) crescentic mesenteric collection, consistent with mesenteric hematoma; (B) blush from a branch of the superior mesenteric artery, indicating the site of active bleeding; (C) right inguinal hernia with unremarkable small bowel; (D) conventional angiography demonstrating subtle beading of small superior mesenteric artery branches; (E) coronal depiction of two large hematomas and part of the inguinal hernia.
DISCUSSION
Though considered a unique disease entity, the etiology of SAM is not well understood. What is known is that mediolysis, characterized by vacuolization and lysis of outer smooth muscles cells in the media on histopathology, is characteristic of SAM. Tears can then develop, separating the outer medial muscle from the adventitia, leading to areas of weakness between the arterial lumen and adventitial layer and ultimately formation of intramural hematomas and dissecting aneurysms.9 Minimal to no inflammatory changes are present and atherosclerotic plaques are absent. These dissections may thrombose, dissect further, or rupture. Stable lesions are filled with granulation tissue; subsequent resolution ranges from radiographically normal vessels to vessel stenosis.7,10
The presentation of SAM has classically been described as nonspecific abdominal or flank pain affecting middle-aged and older patients, perhaps with a slight male predominance.11 More profound presentations due to aneurysm rupture occur in up to one-third of patients and is associated with a 50% mortality.11,12 Cases have been reported to resolve over days to weeks while others resolve over years.1,13,14 Most case reports document a single clinically significant presentation without repeat exacerbations. Though this occurs in 50-80% of cases, up to 40% of patients in literature have disease recurrence.15,16
Angiographic findings of SAM include single or multiple areas of medium visceral artery dissection (the hallmark image finding of SAM), dilation, or occlusion and do not have a predilection for bifurcations as is seen with mycotic aneurysms.9,13 Large vessel involvement would argue against SAM as a diagnosis, rather pointing toward a collagen vascular disease like Ehlers-Danlos or Marfan’s Syndrome. Though multiple aneurysms are noted in one-third of cases, isolated arterial dissection of a visceral artery is more characteristic of SAM.1,17 Intramural hematomas along the course of the affected artery cause a beaded appearance, which can be also be seen in PAN and fibromuscular dysplasia.5 Notably, while conventional angiography assists in the treatment of SAM, CT or MR angiography are sufficient for the diagnosis and follow-up of patients with SAM.13,18 Due to the significant overlap between the image findings of SAM and other vasculopathies, correlation with physical examination and laboratory findings are often required when histopathology is not pursued.
The differential diagnosis of SAM, as discussed in the case report, includes several rheumatologic conditions. Acute phase reactants (C reactive protein and erythrocyte sedimentation rate) as well as rheumatologic tests like anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, and complement levels can corroborate or argue against systemic diseases like systemic lupus erythematosus, Behcet’s Disease, and PAN. Physical examination is also key to evaluating for non-SAM diagnoses. Joint hypermobility, lens subluxation, and skin laxity, for instance, would point toward a collagen vascular disease instead of SAM. Neurologic findings, caused by involvement of the carotid arteries, are more common in PAN and fibromuscular dysplasia.19 Further, fibromuscular dysplasia typically affects the renal arteries of young females, causing stenosis and subsequent premature hypertension, while SAM is typically observed at an older age, affects primarily the celiac and superior mesenteric arteries, and causes arterial dissections and hemorrhage.8 Though histopathology remains the reference standard for the diagnosis of SAM, guidelines for its diagnosis without histopathology have been described and incorporate the findings noted above.17 In particular, patients should have no evidence of atherosclerosis on imaging, normal inflammatory markers, and no findings suggestive of collagen vascular diseases. One case series of 85 patients reported that nearly one-third of patients are diagnosed with only image findings.11
Initial management for SAM presenting with intra-abdominal hemorrhage is focused on surgical or endovascular repair of structural complications with stenting, coiling, and resection being described.16,20 Due to a 10% intra-operative mortality for patients undergoing surgical management, an endovascular approach has become preferred and is successful in nearly 90% of cases.11 Subsequent medical management of SAM, as well as management of patients without intra-abdominal hemorrhage, is unclear; immunosuppression has been described as either potentially worsening the natural course or, at best, being ineffective.7,19 Use of anti-hypertensive medicines is indicated for those with existing aneurysms.21 Antiplatelet and anticoagulation medicines have no definite role in the treatment of SAM. Mortality of the acute phase is estimated in the 40-50% range, however this is likely an overestimate given greater scrutiny applied to catastrophic presentations of SAM.1 Of those who survive the acute phase, most case reports found patients to generally be asymptomatic, with follow-up imaging showing complete resolution or no change in the angiographic findings.11
The aim of this case report is to describe an unexpected presentation of SAM, which coincided with signs of a strangulated hernia that had been thought to be the cause of the patient’s symptoms. We also highlight the importance of not treating this disease with immunosuppressive medications, as would otherwise be standard treatment for other vasculitides.
CONCLUSIONS
Segmental arterial mediolysis represents a rare cause of medium-vessel acute arterial hemorrhage as well as acute and subacute ischemia. Its presenting symptoms overlap considerably with other acute abdominal conditions commonly seen in the emergency department. Although it would appear that a high degree of clinical curiosity would be needed to evaluate for SAM, a standard CT abdomen/pelvis protocol using intravenous contrast is adequate for many presentations. Care should be taken, however, to ensure that the interpreting radiologist is aware of the potential vascular pathology questioned: CT angiography can definitively evaluate for this disease process.13 Initiation of a vasculitis workup early in the treatment of these patients expedites their long-term care. Due to its apparently unique pathophysiology, empiric immunosuppression may worsen both SAM and wound healing from surgical repair.
REFERENCES
- 1.Sakano T, Morita K, Imaki M, Ueno H. Segmental arterial mediolysis studied by repeated angiography. Br J Radiol. 1997;70(834):656–658. doi: 10.1259/bjr.70.834.9227264. [DOI] [PubMed] [Google Scholar]
- 2.Slavin RE, Gonzalez-Vitale JC. Segmental mediolytic arteritis: a clinical pathologic study. Lab Investig J Tech Methods Pathol. 1976;35(1):23–29. [PubMed] [Google Scholar]
- 3.Gruenwald P Necrosis in the coronary arteries of newborn infants. Am Heart J. 1949;38(6):889–97, illust. [DOI] [PubMed] [Google Scholar]
- 4.Slavin RE, Cafferty L, Cartwright J. Segmental mediolytic arteritis. A clinicopathologic and ultrastructural study of two cases. Am J Surg Pathol. 1989;13(7):558–568. [PubMed] [Google Scholar]
- 5.Slavin RE, Saeki K, Bhagavan B, Maas AE. Segmental arterial mediolysis: a precursor to fibromuscular dysplasia? Mod Pathol Off J U S Can Acad Pathol Inc. 1995;8(3):287–294. [PubMed] [Google Scholar]
- 6.Slavin RE, Inada K. Segmental arterial mediolysis with accompanying venous angiopathy: a clinical pathologic review, report of 3 new cases, and comments on the role of endothelin-1 in its pathogenesis. Int J Surg Pathol. 2007;15(2):121–134. doi: 10.1177/1066896906297684. [DOI] [PubMed] [Google Scholar]
- 7.Chao CP. Segmental arterial mediolysis. Semin Interv Radiol. 2009;26(3):224–232. doi: 10.1055/s-0029-1225666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker-LePain JC, Stone DH, Mattis AN, Nakamura MC, Fye KH. Clinical diagnosis of segmental arterial mediolysis: differentiation from vasculitis and other mimics. Arthritis Care Res. 2010;62(11):1655–1660. doi: 10.1002/acr.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slavin RE. Segmental arterial mediolysis: course, sequelae, prognosis, and pathologic-radiologic correlation. Cardiovasc Pathol Off J Soc Cardiovasc Pathol. 2009;18(6):352–360. doi: 10.1016/j.carpath.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Horsley-Silva JL, Ngamruengphong S, Frey GT, Paz-Fumagalli R, Lewis MD. Segmental arterial mediolysis: a case of mistaken hemorrhagic pancreatitis and review of the literature. JOP J Pancreas. 2014;15(1):72–77. [DOI] [PubMed] [Google Scholar]
- 11.Shenouda M, Riga C, Naji Y, Renton S. Segmental arterial mediolysis: a systematic review of 85 cases. Ann Vasc Surg. 2014;28(1):269–277. doi: 10.1016/j.avsg.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Tameo MN, Dougherty MJ, Calligaro KD. Spontaneous dissection with rupture of the superior mesenteric artery from segmental arterial mediolysis. J Vasc Surg. 2011;53(4):1107–1112. doi: 10.1016/j.jvs.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Michael M, Widmer U, Wildermuth S, Barghorn A, Duewell S, Pfammatter T. Segmental arterial mediolysis: CTA findings at presentation and follow-up. AJR Am J Roentgenol. 2006;187(6):1463–1469. doi: 10.2214/AJR.05.0281. [DOI] [PubMed] [Google Scholar]
- 14.Ryan JM, Suhocki P V, Smith TP. Coil embolization of segmental arterial mediolysis of the hepatic artery. J Vasc Interv Radiol JVIR. 2000;11(7):865–868. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto T, Deguchi J, Endo H, Miyata T. Successful treatment tailored to each splanchnic arterial lesion due to segmental arterial mediolysis (SAM): report of a case. J Vasc Surg. 2008;48(5):1338–1341. doi: 10.1016/j.jvs.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 16.Shimohira M, Ogino H, Sasaki S, et al. Transcatheter arterial embolization for segmental arterial mediolysis. J Endovasc Ther Off J Int Soc Endovasc Spec. 2008;15(4):493–497. doi: 10.1583/08-2384.1. [DOI] [PubMed] [Google Scholar]
- 17.Kalva SP, Somarouthu B, Jaff MR, Wicky S. Segmental arterial mediolysis: clinical and imaging features at presentation and during follow-up. J Vasc Interv Radiol JVIR. 2011;22(10):1380–1387. doi: 10.1016/j.jvir.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Pillai AK, Iqbal SI, Liu RW, Rachamreddy N, Kalva SP. Segmental arterial mediolysis. Cardiovasc Intervent Radiol. 2014;37(3):604–612. doi: 10.1007/s00270-014-0859-4. [DOI] [PubMed] [Google Scholar]
- 19.Lie JT. Segmental mediolytic arteritis. Not an arteritis but a variant of arterial fibromuscular dysplasia. Arch Pathol Lab Med. 1992;116(3):238–241. [PubMed] [Google Scholar]
- 20.Davran R, Cinar C, Parildar M, Oran I. Radiological findings and endovascular management of three cases with segmental arterial mediolysis. Cardiovasc Intervent Radiol. 2010;33(3):601–606. doi: 10.1007/s00270-009-9651-2. [DOI] [PubMed] [Google Scholar]
- 21.Soulen MC, Cohen DL, Itkin M, Townsend RR, Roberts DA. Segmental arterial mediolysis: angioplasty of bilateral renal artery stenoses with 2-year imaging follow-up. J Vasc Interv Radiol JVIR. 2004;15(7):763–767. [DOI] [PubMed] [Google Scholar]