Abstract
Background
Intraoperative parathyroid hormone monitoring (IOPTH) is a widely used adjunct for primary hyperparathyroidism (pHPT). However, the benefit of IOPTH in familial pHPT, such as in multiple endocrine neoplasia type I (MEN1), remains unclear.
Methods
We performed a retrospective analysis of 52 patients with MEN1-associated pHPT undergoing initial parathyroidectomy with IOPTH monitoring at our institution. Parathyroid hormone (PTH) levels were measured before skin incision and 10 min after resection of the last parathyroid gland. Variables analyzed included percent drop of PTH from baseline and the final PTH level compared to the normal reference range (RR).
Results
A total of 52 patients underwent initial subtotal parathyroidectomy with IOPTH. An IOPTH decrease cutoff of ≥75 % from baseline had the highest biochemical cure rate (87 %). In the remaining 13 % who met this cutoff, all had persistent pHPT, with ≥90 % drop of PTH from baseline. The remaining patients, who did not meet the ≥75 % cutoff, were cured. Follow-up was available for three of four patients with final IOPTH levels above the RR: one had persistent pHPT, two had hypoparathyroidism (50 %). When a postresection PTH level was within the RR, 88 % of patients were cured. While considered cured from pHPT, 7 % of patients in this group developed permanent hypoparathyroidism. When the final PTH level dropped below the RR, 28 % developed permanent hypoparathyroidism.
Conclusions
A cutoff in IOPTH decrease of ≥75 % from baseline has the highest biochemically cure rate in patients with pHPT associated with MEN1. However, a 75 % cutoff in IOPTH decrease does not exclude persistent pHPT. The absolute IOPTH value does not accurately predict postoperative hypoparathyroidism.
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is the most common cause of familial primary hyperparathyroidism (pHPT), accounting for 1–5 % of all pHPT cases [1–4]. Other common endocrine tumors associated with MEN1 are anterior pituitary, gastrointestinal, thymic, and pancreatic neuroendocrine tumors. Asymmetrical and sometimes asynchronous, multi-gland parathyroid disease is the hallmark of pHPT associated with MEN1. Therefore, it should be suspected and excluded in all pHPT cases with multi-gland involvement or with recurrent pHPT [5]. Approximately 10 % of patients with multi-gland parathyroid disease in the absence of renal insufficiency have MEN1 [5]. Recurrent and persistent pHPT in patients with MEN1 undergoing parathyroidectomy is high, in part due to missed gland(s), regrowth of parathyroid remnant, or intrathymic foci of parathyroid tissue, which becomes hyperplastic as a result of the inactivated MEN1 tumor suppressor gene.
Intraoperative parathyroid hormone (IOPTH) monitoring is a widely used adjunct for focused or minimally invasive parathyroidectomy in patients with no family history of pHPT or at low risk for having multi-gland parathyroid disease based on preoperative localizing studies. IOPTH may increase the cure rates by detecting additional hyperfunctioning gland(s) in 2–10 % of patients undergoing parathyroidectomy for pHPT [6–8]. IOPTH monitoring can predict cure in cases of sporadic pHPT with an accuracy of 98 % when the Miami criterion is fulfilled [9].
Because patients with MEN1 have pHPT due to multi-gland disease and have lower biochemical cure rates and higher rates of hypoparathyroidism than patients with no family history or sporadic pHPT, IOPTH monitoring may have an important role in guiding the extent of neck exploration and parathyroidectomy. In addition, IOPTH monitoring may be helpful for identifying patients at risk of postoperative hypocalcemia [10].
To our knowledge, there are no data regarding the clinical utility of IOPTH monitoring during initial parathyroidectomy in patients with MEN1-associated pHPT with respect to what is the most accurate criterion for the decrease in PTH level and if it can accurately predict biochemical cure. Thus, the objective of our study was to determine the accuracy of IOPTH for predicting biochemical cure, persistent pHPT, and postoperative hypoparathyroidism.
Material and methods
We performed a retrospective analysis of 52 patients diagnosed with MEN1 undergoing initial parathyroidectomy for pHPT from December 1997 to March 2011 at the National Institutes of Health Clinical Research Center. IOPTH measurement has been available since November 1997 at our institution. The Office of Human Subject Research at the National Institutes of Health approved the study. All patients provided written consent. The diagnosis of MEN1 was based on (1) the presence of hyperparathyroidism combined with an anterior pituitary tumor and/or gastrointestinal and pancreatic neuroendocrine tumor; (2) a diagnosis of pHPT combined with a diagnosis of MEN1 in at least one first-degree relative; or (3) a positive germline mutation in the MEN1 gene. All patients underwent screening and surveillance tests for other manifestations of MEN1 per published guidelines [11]. The diagnosis of pHPT was based on the presence of hypercalcemia (albumin-corrected total calcium and/or ionized calcium) and inappropriately elevated intact PTH in the absence of hypocalciuria. All patients underwent bilateral neck exploration. Subtotal parathyroidectomy and transcervical thymectomy were performed in 45 patients (86.5 %).
Blood sampling and measurement of IOPTH
Blood samples were obtained from peripheral venous or arterial catheters in 47 patients. Blood samples were taken from the internal jugular vein in five patients. The baseline peripheral samples were obtained prior to the skin incision and the final samples 10 min after the last gland was excised. Blood specimens were collected in 2.4 ml of potassium ethylenediaminetetraacetic acid tubes and analyzed immediately for intact PTH by automated immunoassay systems. The assays used during the study period are summarized in Table 1 with their respective normal reference ranges (RRs).
Table 1.
Automated immunoassay systems for IOPTH analysis utilized in this study
| Year | IOPTH system | RR (pg/ml) |
|---|---|---|
| 1997 | DPC Immulite PTHa | 10–65 |
| 1999 | Nichols Advantage PTHb | 10–65 |
| 2002 | Nichols Advantage QuiCk IntraOperative Bio-Intact (1–84) PTH Assayb | 6–40 |
| 2006 | DPC Immulite 2000a | 16–87 |
| 2007 | DPC Imulite 2500a | 16–87 |
IOPTH intraoperative parathyroid hormone, RR reference range
Siemens Medical Solutions, Fernwald, Germany
Nichols Institute Diagnostics, San Clemente, CA, USA
We performed the analysis of IOPTH and the clinical outcome using the final IOPTH value compared to the respective RR as well as the percent drop from the preincision IOPTH. When the final IOPTH absolute value correctly predicted the clinical outcome (cure, hypoparathyroidism, or persistent pHPT), the result was designated true positive (TP). If the final value of IOPTH was predictive of not having the clinical outcome, the result was designated as true negative (TN). False negative (FN) was assigned when the final value of IOPTH failed to predict postoperative hypoparathyroidism or persistent pHPT. The result was designated as false positive (FP) if the final value of IOPTH assay was lower than the RR without postoperative hypoparathyroidism or higher than the RR without persistent pHPT (Table 2). The test performance of IOPTH was also analyzed based on the percent drop of the final IOPTH from the preincision IOPTH using the definitions of test results summarized in Table 2.
Table 2.
Definitions of IOPTH test results in predicting adverse clinical outcome
| Test results predicting adverse outcome | Final IOPTH value | IOPTH percentage | Clinical outcome |
|---|---|---|---|
| True positive | |||
| Hypoparathyroidism | Below lower limits | N/A | Hypoparathyroidism |
| Hyperparathyroidism | Above upper limits | Below cutoff percentage | Persistent hyperparathyroidism |
| True negative | |||
| Hypoparathyroidism | Within or above upper limits | N/A | Eu- or hyperparathyroidism |
| Hyperparathyroidism | Within or below lower limits | Greater or equal to cutoff percentage | Eu- or hypoparathyroidism |
| False positive | |||
| Hypoparathyroidism | Below lower limits | N/A | Eu- or hyperparathyroidism |
| Hyperparathyroidism | Above upper limits | Below cutoff percentage | Eu- or hypoparathyroidism |
| False negative | |||
| Hypoparathyroidism | Within or above upper limits | N/A | Hypoparathyroidism |
| Hyperparathyroidism | Within or below lower limits | Greater or equal to cutoff percentage | Persistent hyperparathyroidism |
Patients were considered biochemically cured if there was no evidence of pHPT for at least 6 months postoperatively. Persistent and recurrent pHPT were defined as pHPT that occurred postoperatively within 6 months and after 6 months, respectively. Permanent hypoparathyroidism was defined as low calcium and PTH in patients who continued to receive calcium and/or vitamin D analogue replacement for more than 6 months postoperatively. Altogether, 35 patients had more than 6 months of follow-up.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 16.0 (SPSS, Chicago, IL, USA). The Mann-Whitney test was used to identify the difference in the IOPTH percent drop by total number of glands removed.
Results
The clinical characteristics of the 52 patients with pHPT due to MEN1 who underwent initial parathyroidectomy with IOPTH are summarized in Table 3. The median IOPTH percent drop when two or fewer parathyroid glands and fewer than three parathyroid glands were removed were 90.7 and 90.6 %, respectively, compared to 91.5 % when three or more glands were removed. There was no significant difference between IOPTH percent drop in patients with fewer than three glands removed compared to those of three or more glands removed (p = 0.739). IOPTH decrease ≥75 % occurred in 49 of 52 patients (94.2 %) with a median of three pathologically confirmed parathyroid glands removed. An IOPTH decrease cutoff of ≥75 % from baseline had the highest biochemically cure rate with a positive predictive value (PPV) of 87 %. In the remaining 13 % who fulfilled this cutoff, all had persistent pHPT with a decrease of ≥90 % from baseline. Of the three patients who did not fulfill ≥75 % cutoff, two were cured and one patient was lost to follow-up. The area under the receiver operating characteristic (ROC) curve of IOPTH was not statistically better than guessing: area under the ROC curve of 0.78 (95 % confidence interval 0.59–0.98; p = 0.07) (Fig. 1a). There was only one patient who had an IOPTH drop of <50 % and not more than ≥75 %. This patient remained euparathyroid postoperatively.
Table 3.
Demographic and clinical data of 52 patients in the study cohort
| Parameter | Data |
|---|---|
| Male sex | 26 (50 %) |
| Age at the time of surgery (years), median and range | 35 (13–78) |
| Preoperative results | |
| Serum creatinine (RR 0.7–1.3 mg/dl) | 0.8 mg/dl (0.5–1.2 mg/dl) |
| Ionized calcium (RR 1.12–1.32 mmol/l) | 1.54 mmol/l (1.27–1.76 mmol/l) |
| Corrected total calcium (RR 8.2–10.0 mg/dl) | 10.8 mg/dl (9.6–12.4 mg/dl) |
| Phosphorus (RR 2.5–4.8 mg/dl) | 2.9 mg/dl (1.8–5.1 mg/dl) |
| Intact PTH (RR 16–87 pg/dl) | 113 pg/ml (39–311 pg/ml) |
| 24-h urine calcium (RR 50–250 mg) | 353 mg (85–658 mg) |
| Postoperative resultsa | |
| Corrected total calcium | 8.8 mg/dl (7.5–11.2 mg/dl) |
| Phosphorus | 3.8 mg/dl (2.4–5.7 mg/dl) |
| Intact PTH | 15 pg/ml (undetectable to 80 pg/ml) |
| Postoperative hypoparathyroidism | |
| Transientb | 13 patients (26.0 %) |
| Permanent | 6 patients (11.5 %) |
| Cryopreserved parathyroid autotransplant | 3 patients (6.0 %) |
| Postoperative pHPT | |
| Persistent | 4 patients (7.7 %) |
| Recurrent | 1 patient (2.0 %) |
| Follow-up time (median and range) | 8.6 months (1 week to 128 months) |
Results are given as the mean and range unless otherwise stated
RR reference range, PTH parathyroid hormone, pHPT primary hyperparathyroidism
Postoperative: within 30 days after parathyroidectomy
Transient postoperative hypoparathyroidism: intact PTH below the reference range
Fig. 1.
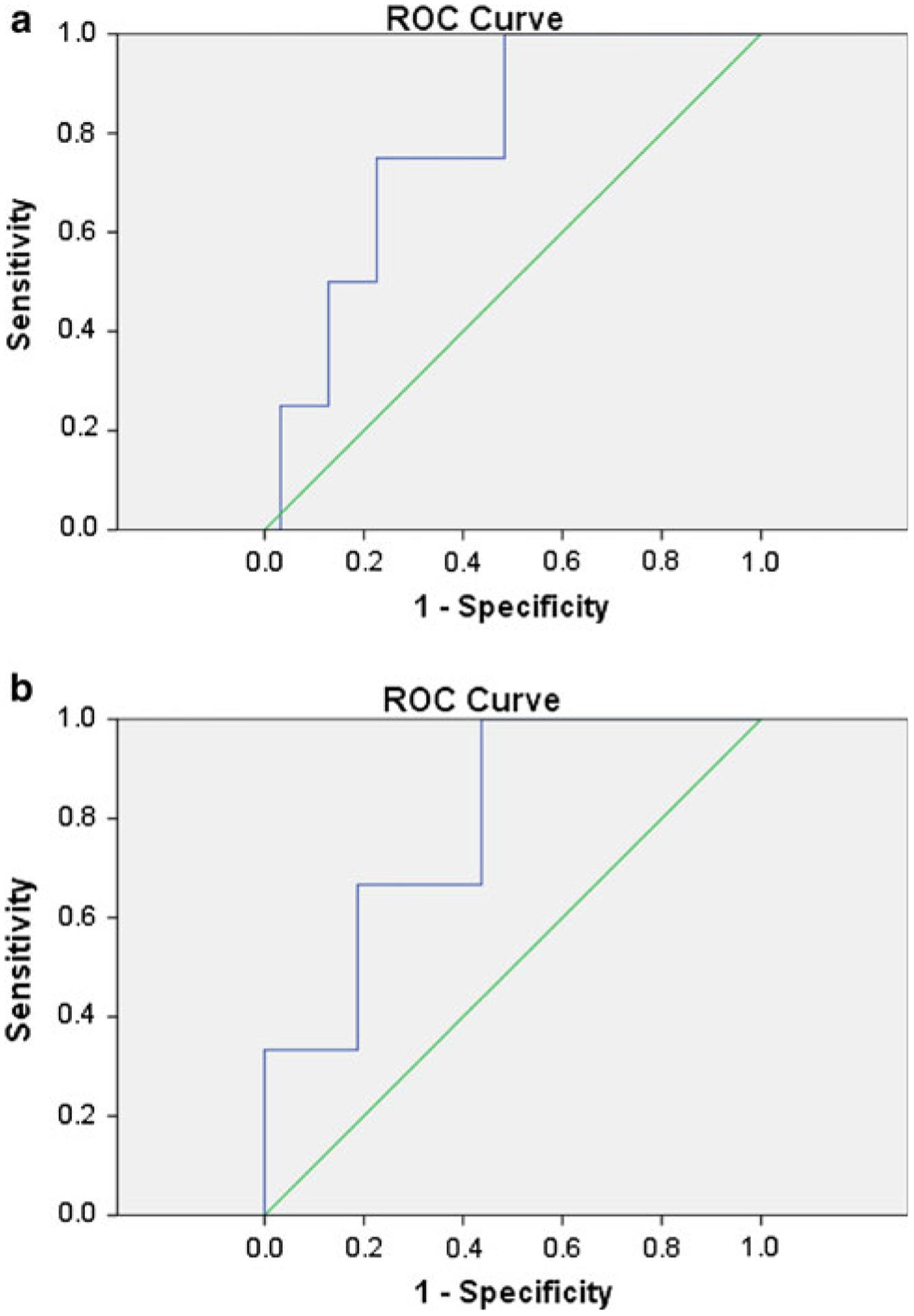
a Receiver operating characteristic (ROC) curve of intraoperative parathyroid hormone (IOPTH) percent drop from baseline for detecting persistent primary hyperparathyroidism in patients with multiple endocrine neoplasia type 1 (MEN1) undergoing initial parathyroidectomy. The area under the ROC curve was 0.78 (95 % confidence interval 0.59–0.98; p = 0.07). b ROC curve of IOPTH percent drop from baseline for detecting persistent primary hyperparathyroidism in patients with MEN1 undergoing initial parathyroidectomy who had fewer than four parathyroid glands identified intraoperatively. The area under the ROC curve was 0.79 (95 % confidence interval 0.54–1.04; p = 0.12)
Three or more parathyroid glands were identified intraoperatively in 46 patients (88.5 %). The biochemical cure rate of patients who had three or more parathyroid glands identified intraoperatively was 87 %.
The final IOPTH level was above normal RR in four patients. One of the four had persistent pHPT, and three were biochemically cured (PPV 25 %). Two patients (50 %) with an elevated final IOPTH developed hypoparathyroidism. When a final PTH level was within the normal RR, 88 % of patients were cured. Postoperative hypoparathyroidism occurred in 39 % of patients in this group and were considered cured from hyperparathyroidism. 7 % of patients with a final PTH level within the normal RR developed permanent hypoparathyroidism. When the final PTH level dropped below the normal RR, 70 % of patients developed hypoparathyroidism (Fig. 2). In all, 28 % of patients with the final PTH levels below the normal RR developed permanent hypoparathyroidism.
Fig. 2.
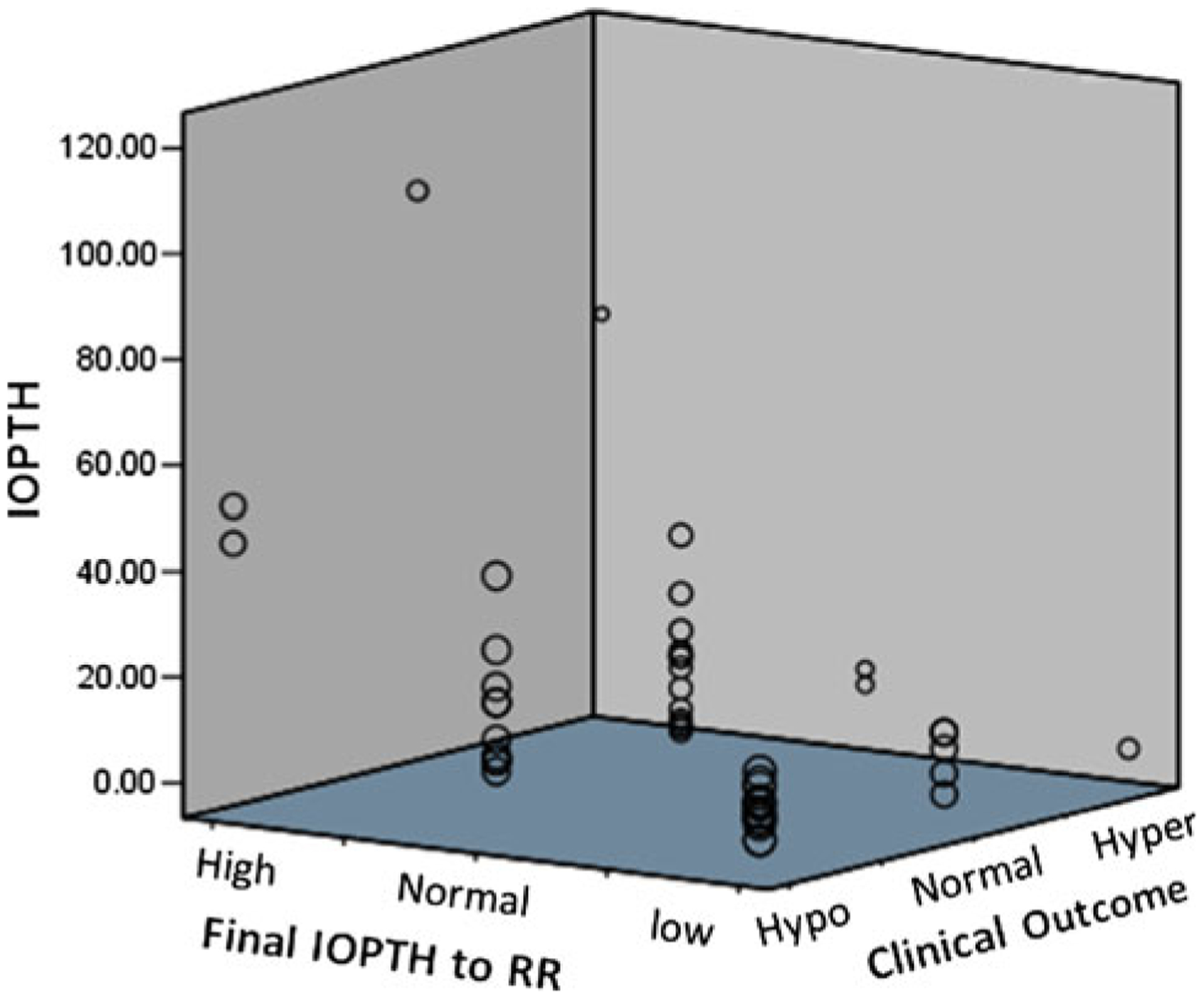
Three-dimensional scatter plots of final intraoperative parathyroid hormone levels (IOPTH) values (Y axis) compared to the normal reference range (X axis), and clinical outcome (Z axis). IOPTH. For Final IOPTH to RR, RR reference range, High final IOPTH ≥upper limits, Normal final IOPTH within normal limits, Low final IOPTH lower limits. For Clinical Outcome, Hypo hypoparathyroidism, Normal eu-parathyroidism, Hyper persistent hyperparathyroidism
Permanent hypoparathyroidism occurred in six patients (11.5 %). The final IOPTH levels were below or within normal RR in four and two patients, respectively.
Four patients had persistent pHPT. Each had three parathyroid glands identified, and all had >90 % drop from the baseline IOPTH. Only one had slightly elevated final IOPTH, at 71 pg/ml (RR 10–65 pg/ml).
The performance of IOPTH for detecting hypoparathyroidism and persistent pHPT is shown in Table 4. We evaluated the role of IOPTH in identifying postoperative persistent pHPT in 27 patients who had fewer than four parathyroid glands identified intraoperatively by surgeons with or without pathology confirmation. Patients in this subgroup with an IOPTH decrease cutoff of ≥75 % from baseline had a biochemically cure rate (PPV) of 76 %. The area under the ROC curve was 0.79 (95 % confidence interval 0.54–1.04; p = 0.12) (Fig. 1b). Three patients in this subgroup had elevated final IOPTH. One of the three had persistent disease, and two were biochemically cured (PPV for persistent pHPT was 33.3 %).
Table 4.
Performance of IOPTH for detecting postoperative hypoparathyroidism and persistent pHPT in MEN1-associated pHPT undergoing initial parathyroidectomy
| Parameter | Final values of IOPTH detecting hypoparathyroidism (%) | Final values of IOPTH detecting persistent pHPT (%) | % IOPTH decrease ≥75 % detecting cure |
|---|---|---|---|
| Sensitivity | 51.9 | 25 | 93.5 |
| Specificity | 72.7 | 90.3 | 0 |
| NPV | 55.2 | 90.3 | 0 |
| PPV | 70 | 25 | 87.8 |
| Likelihood ratio | 1.9 | 2.6 | 93.5 |
MEN1 multiple endocrine neoplasia type 1, pHPT primary hyper-parathyroidism, NPV negative predictive value, PPV positive predictive value
Discussion
The goal of parathyroidectomy for MEN1-associated pHPT is to provide a durable biochemical cure without permanent hypoparathyroidism by removing enough hyperfunctioning parathyroid glands with minimal risk of persistent or recurrent disease. The aim of this study was to evaluate the ability of IOPTH to detect adverse postoperative outcomes, such as hypoparathyroidism or persistent pHPT in patients with MEN1-associated pHPT undergoing initial parathyroidectomy. We analyzed the absolute final IOPTH values compared to the RR as well as the percent drop of IOPTH from baseline.
The results in our study showed that when final IOPTH values were within the RR or had dropped ≥75 % from baseline, 88 and 87 % of patients, respectively, were biochemically cured. As expected, intraoperative identification of three or more parathyroid glands also predicted biochemical cure in 87 % of patients. Hypoparathyroidism occurred in 50, 39, and 70 % of patients when the final IOPTH values were above, within, or below RR, respectively.
Although the role of IOPTH in multi-gland parathyroid disease has been evaluated in several studies, we believe that this study is the first to evaluate the role of IOPTH in MEN1-associated pHPT. When a cutoff of IOPTH decrease of ≥50 % at 10 min after the first enlarged parathyroid gland removed was used in sporadic pHPT patients with multi-gland disease, the FP rate can be as high as 55 % [12–15].
A lower rate of multi-gland disease (5 %) based on PTH secretion [16] during focused parathyroidectomy with IOPTH compared to the rate of multi-gland pHPT (11–26 %) defined by size criteria described in series of patients who had bilateral neck exploration [17–19] suggests the inability of IOPTH to detect enlarged parathyroid glands, which may or may not be hyperfunctioning. Although the long-term outcome of focused parathyroidectomy with IOPTH to confirm biochemical cure remains excellent in patients with sporadic pHPT [20], a missed enlarged parathyroid gland could result in persistent or recurrent pHPT in patients with MEN1 as they have multi-gland disease [21].
A cutoff of 75 % decrease in IOPTH from baseline resulted in a higher PPV in confirming biochemical cure as compared to 50 % decrease [22] in patients with sporadic pHPT. Our results demonstrated that the use of IOPTH decrease ≥75 % or IOPTH within the normal RR can predict biochemical cure in the majority of patients with MEN1-associated pHPT. However, IOPTH failed to identify all four patients who developed persistent pHPT when an IOPTH decrease of ≥75 % cutoff was used. We were unable to accurately analyze the cutoff of <75 % drop in IOPTH because only three patients in this series had <75 % decrease of IOPTH from baseline. On the other hand, only 25 % of patients who had an elevated final IOPTH value and none of the patients with <75 % decrease in IOPTH levels from baseline developed persistent pHPT. Because patients with MEN1-associated pHPT have lower rates of persistent and recurrent pHPT if three or more glands are removed [21, 23], we believe that all four parathyroid glands should be identified and cervical thymectomy performed regardless of IOPTH values, particularly in patients with fewer than four parathyroid glands identified intraoperatively because of the high rate of intrathymic parathyroid tissue [24]. The benefit of the final IOPTH value in a subgroup of patients with fewer than four parathyroid glands identified was limited in this series because of a low PPV (33.3 %) in detecting persistent pHPT.
Our results indicate that the final IOPTH values were inaccurate for predict postoperative hypoparathyroidism because 50 and 40 % of patients with final IOPTH values above and within the normal range developed hypoparathyroidism, respectively, albeit transiently in most of the cases. Therefore, routine calcium and vitamin D analogue supplementation should be considered in patients with MEN1-associated pHPT undergoing subtotal parathyroidectomy if they are not going to be monitored as inpatients. Tonelli et al. [25] proposed that a final PTH level of ≤6 pg/ml by immunochemiluminometric assay is a strong indicator for a successful total parathyroidectomy and recommended immediate forearm autotransplantation if a final PTH is \10 pg/ml. We found that more than 70 % of our patients, who underwent subtotal parathyroidectomy and cervical thymectomy, with the final PTH levels below the RR did not have permanent hypoparathyroidism. Therefore, we do not recommend immediate autotransplantation based on a final PTH value in patients undergoing subtotal thyroidectomy and cervical thymectomy for MEN1-associated pHPT.
Our study does not have information regarding IOPTH dynamics for each resected parathyroid gland. Because bilateral neck exploration and subtotal parathyroidectomy for MEN1-associated HPT requires identification of all parathyroid glands prior to resection with the least abnormal gland partially resected or left in situ. In most operations in our study, IOPTH was not measured after removing each gland. Nonetheless, our previous study showed that removal of fewer than three glands was associated with higher rates of persistent HPT [26], and the current study demonstrated that even with adequate drop of IOPTH four patients had persistent HPT. Therefore, we recommend routine 3.5-gland parathyroidectomy and cervical thymectomy regardless of IOPTH values.
In summary, IOPTH monitoring failed to detect most patients with persistent pHPT. IOPTH provides, in patients with MEN1-associated pHPT, no additional useful information that would alter the surgical approach or management of postoperative hypoparathyroidism.
Conclusions
There is little benefit of using IOPTH in patients with MEN1-associated pHPT undergoing initial parathyroidectomy. Although IOPTH can predict biochemical cure in most patients, it should not alter the operative strategy or the management of postoperative hypoparathyroidism because of its inaccuracy in detecting persistent pHPT and hypoparathyroidism, respectively.
Acknowledgment
The research was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Contributor Information
Robert T. Jensen, Digestive Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA
Steven K. Libutti, Department of Surgery, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA
Stephen Marx, Metabolic Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Electron Kebebew, Endocrine Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 10 Center Drive, MSC1201 Room 3-3940, Bethesda, MD 20892-1201, USA.
References
- 1.Brandi ML, Marx SJ, Aurbach GD et al. (1987) Familial multiple endocrine neoplasia type I: a new look at pathophysiology. Endocr Rev 8:391–405 [DOI] [PubMed] [Google Scholar]
- 2.Sharretts JM, Simonds WF (2010) Clinical and molecular genetics of parathyroid neoplasms. Best Pract Res Clin Endocrinol Metab 24:491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchino S, Noguchi S, Sato M et al. (2000) Screening of the MEN1 gene and discovery of germ-line and somatic mutations in apparently sporadic parathyroid tumors. Cancer Res 60:5553–5557 [PubMed] [Google Scholar]
- 4.Vasen HF, Griffioen G, Lips CJ et al. (1990) Screening of families predisposed to cancer development in The Netherlands. Anti-cancer Res 10:555–563 [PubMed] [Google Scholar]
- 5.Akerstrom G, Stalberg P (2009) Surgical management of MEN-1 and −2: state of the art. Surg Clin North Am 89:1047–1068 [DOI] [PubMed] [Google Scholar]
- 6.Barczynski M, Cichon S, Konturek A et al. (2006) Minimally invasive video-assisted parathyroidectomy versus open minimally invasive parathyroidectomy for a solitary parathyroid adenoma: a prospective, randomized, blinded trial. World J Surg 30:721–731. doi: 10.1007/s00268-005-0312-6 [DOI] [PubMed] [Google Scholar]
- 7.Barczynski M, Konturek A, Cichon S et al. (2007) Intraoperative parathyroid hormone assay improves outcomes of minimally invasive parathyroidectomy mainly in patients with a presumed solitary parathyroid adenoma and missing concordance of preoperative imaging. Clin Endocrinol (Oxf) 66:878–885 [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Pruhs Z, Starling JR et al. (2005) Intraoperative parathyroid hormone testing improves cure rates in patients undergoing minimally invasive parathyroidectomy. Surgery 138:583–587 (discussion 587–590) [DOI] [PubMed] [Google Scholar]
- 9.Carneiro DM, Solorzano CC, Nader MC et al. (2003) Comparison of intraoperative iPTH assay (QPTH) criteria in guiding parathyroidectomy: which criterion is the most accurate? Surgery 134:973–979 discussion 979–981 [DOI] [PubMed] [Google Scholar]
- 10.Barczynski M, Cichon S, Konturek A et al. (2008) Applicability of intraoperative parathyroid hormone assay during total thyroidectomy as a guide for the surgeon to selective parathyroid tissue autotransplantation. World J Surg 32:822–828. doi: 10.1007/s00268-007-9405-8 [DOI] [PubMed] [Google Scholar]
- 11.Brandi ML, Gagel RF, Angeli A et al. (2001) Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86:5658–5671 [DOI] [PubMed] [Google Scholar]
- 12.Clerici T, Brandle M, Lange J et al. (2004) Impact of intraoperative parathyroid hormone monitoring on the prediction of multi-glandular parathyroid disease. World J Surg 28:187–192. doi: 10.1007/s00268-003-7255-6 [DOI] [PubMed] [Google Scholar]
- 13.Gauger PG, Agarwal G, England BG et al. (2001) Intraoperative parathyroid hormone monitoring fails to detect double parathyroid adenomas: a 2-institution experience. Surgery 130: 1005–1010 [DOI] [PubMed] [Google Scholar]
- 14.Gordon LL, Snyder WH III, Wians FJ et al. (1999) The validity of quick intraoperative parathyroid hormone assay: an evaluation in seventy-two patients based on gross morphologic criteria. Surgery 126:1030–1035 [DOI] [PubMed] [Google Scholar]
- 15.Jaskowiak NT, Sugg SL, Helke J et al. (2002) Pitfalls of intraoperative quick parathyroid hormone monitoring and gamma probe localization in surgery for primary hyperparathyroidism. Arch Surg 137:659–668 (discussion 668–659) [DOI] [PubMed] [Google Scholar]
- 16.Molinari AS, Irvin GL III, Deriso GT et al. (1996) Incidence of multi-glandular disease in primary hyperparathyroidism determined by parathyroid hormone secretion. Surgery 120:934–936 (discussion 936–937) [DOI] [PubMed] [Google Scholar]
- 17.Bonjer HJ, Bruining HA, Birkenhager JC et al. (1992) Single and multi-gland disease in primary hyperparathyroidism: clinical follow-up, histopathology, and flow cytometric DNA analysis. World J Surg 16:737–743. doi: 10.1007/BF02067373 (discussion 743–734) [DOI] [PubMed] [Google Scholar]
- 18.Proye C, Quievreux JL, Gontier A et al. (1989) Multi-glandular lesions in primary hyperparathyroidism: late outcome of 86 consecutive patients treated with conservative surgery. Chirurgie 115:723–732 [PubMed] [Google Scholar]
- 19.Van Heerden JA, Grant CS (1991) Surgical treatment of primary hyperparathyroidism: an institutional perspective. World J Surg 15:688–692. doi: 10.1007/BF01665301 [DOI] [PubMed] [Google Scholar]
- 20.Venkat R, Kouniavsky G, Tufano RP et al. (2012) Long-term outcome in patients with primary hyperparathyroidism who underwent minimally invasive parathyroidectomy. World J Surg 36:55–60. doi: 10.1007/s00268-011-1344-8 [DOI] [PubMed] [Google Scholar]
- 21.Elaraj DM, Skarulis MC, Libutti SK et al. (2003) Results of initial operation for hyperparathyroidism in patients with multiple endocrine neoplasia type 1. Surgery 134:858–864 (discussion 864–855) [DOI] [PubMed] [Google Scholar]
- 22.Hughes DT, Miller BS, Doherty GM et al. (2011) Intraoperative parathyroid hormone monitoring in patients with recognized multi-glandular primary hyperparathyroidism. World J Surg 35:336–341. doi: 10.1007/s00268-010-0887-4 [DOI] [PubMed] [Google Scholar]
- 23.Schreinemakers JM, Pieterman CR, Scholten A et al. (2011) The optimal surgical treatment for primary hyperparathyroidism in MEN1 patients: a systematic review. World J Surg 35: 1993–2005. doi: 10.1007/s00268-011-1068-9 [DOI] [PubMed] [Google Scholar]
- 24.Powell AC, Alexander HR, Pingpank JF et al. (2008) The utility of routine transcervical thymectomy for multiple endocrine neoplasia 1-related hyperparathyroidism. Surgery 144:878–883 (discussion 883–874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonelli F, Marcucci T, Fratini G et al. (2007) Is total parathyroidectomy the treatment of choice for hyperparathyroidism in multiple endocrine neoplasia type 1? Ann Surg 246:1075–1082 [DOI] [PubMed] [Google Scholar]
- 26.Norton JA, Venzon DJ, Berna MJ et al. (2008) Prospective study of surgery for primary hyperparathyroidism (HPT) in multiple endocrine neoplasia-type 1 and Zollinger-Ellison syndrome: long-term outcome of a more virulent form of HPT. Ann Surg 247:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]


