Abstract
Background:
Transarterial chemoembolization (TACE) is indicated for unresectable hepatocellular carcinoma.
Methods:
This was a retrospective study of 50 hepatocellular carcinoma patients treated with TACE using doxorubicin-loaded LifePearl™ to investigate the safety and efficacy of TACE.
Results:
There was no 30-day mortality, and limited adverse events were reported. At best tumor response, complete response and disease control were 58% and 94%, respectively, with a median of 4.5 months of follow-up. Median overall survival was 33.8 months. Patients with Barcelona Clinic Liver Cancer stage 0 and stage A at best tumor response showed a higher complete response rate (83%) than patients with Barcelona Clinic Liver Cancer stage B (complete response: 50%; p = 0.0414).
Conclusion:
Doxorubicin-loaded LifePearl™ TACE might be an effective treatment, with a good safety profile, for patients with early/intermediate-stage hepatocellular carcinoma. Further prospective data, especially with a small cohort of selected patients, are required to confirm these results.
Keywords: : DEM-TACE, doxorubicin, LifePearl™, unresectable hepatocellular carcinoma
Tweetable abstract
Transarterial chemoembolization with LifePearl™ microspheres loaded with doxorubicin in 50 unresectable hepatocellular carcinoma patients is demonstrated to be an effective treatment with a median survival of 33.8 months and a good safety profile.
Hepatocellular carcinoma (HCC) mortality rates are increasing in many countries, including northern and central Europe and North and Latin America [1]. HCC is estimated to be the third leading cause of cancer death worldwide. The Barcelona Clinic Liver Cancer (BCLC) staging system is used to classify HCC, taking into account the number and size of lesions and the patient's performance status [2]. Most patients are not resectable at diagnosis due to liver impairment or extended disease and hence are indicated for liver-directed therapy. Several liver-directed therapies are used for local disease control (DC), downstaging or palliative care in HCC patients, including radiofrequency and microwave ablation, which are mainly used in early stages of HCC (BCLC stage 0/A) [3,4]. Liver-directed therapies for BCLC stage B are lipiodol-based conventional transarterial chemoembolization (TACE), drug-eluting microsphere TACE and transarterial radioembolization, where microspheres are impregnated with 90Y or 166Ho [5–8].
According to the most updated version of the European Society for Medical Oncology HCC guidelines, TACE is also recommended as a bridge to transplant in earlier HCC stages to help keep patients on the waiting list until transplantation [7]. The guidelines also report the benefit of TACE in increasing overall survival (OS) in selected patients with unresectable BCLC stage 0/A and stage B HCC with small tumor burden and good liver function.
TACE can be used in different HCC stages, as highlighted in a French survey [9]. Moreover, the combination of ablation and TACE for early HCC stages has been evaluated in several studies, providing a new avenue for managing these types of tumors [10–13].
The literature shows that TACE performed with drug-eluting microspheres has similar efficacy and is safer than conventional TACE, providing a more standardized way of performing TACE [7,10,14]. There are several types of drug-eluting microspheres for TACE that have different characteristics [15]. Among these are LifePearl™ microspheres (Terumo Europe, Leuven, Belgium), which are made of polyethylene glycol (PEG), a hydrophilic material that offers good compressibility and elasticity as well as an extended time in suspension [15,16].
LifePearl™ microspheres are approved for loading with doxorubicin, idarubicin and epirubicin and have good tolerability and efficacy in HCC treatment [6,17–24]. Doxorubicin is the most frequently used drug for TACE in HCC treatment [17,23,24] and is used in the current study, which is aimed at assessing the tolerability, treatment efficacy and safety of LifePearl™ in HCC patients.
Methods
Patient population
This was a single-center retrospective cohort study on 50 consecutive patients who were treated with TACE using LifePearl™ loaded with doxorubicin from April 2015 to January 2019. This was a retrospective data collection using an anonymized study file free of personal identification data.
Inclusion criteria were >18 years of age, diagnosis of unresectable BCLC stage 0/A or stage B HCC, TACE treatment with LifePearl™ loaded with doxorubicin and no indication for percutaneous or surgical treatment. Exclusion criteria were hemodynamic instability, hypo- or hypertension, fever, pregnancy, tumor extension to both lobes, total or partial intra- or extrahepatic portal thrombosis, hepatic impairment with a Child–Pugh score B >7 or C, tumor arteriovenous fistula, renal impairment (creatinine >2 mg/dl), active heart disease or altered coagulation tests (prothrombin time <50% and platelets <50 × 109/l).
TACE with LifePearl™
LifePearl™ PEG embolization microspheres with a diameter of 200 or 400 μm were loaded with doxorubicin following the manufacturer's instructions as follows. Doxorubicin powder was reconstituted with sterile water for injection (25 mg/ml) and mixed thoroughly in the pharmacy. The doxorubicin dose was computed according to the tumor size. Specifically, one syringe loaded with 75 mg was used in patients with single or multiple nodules of a small size (<3 cm in diameter) and two syringes loaded with 75 mg each, for a total of 150 mg, were used in patients with moderate-sized multifocal or large masses (≥3 cm in diameter).
With regard to the size of the microspheres chosen for TACE, the 200-μm microspheres were initially used for every patient following the international consensus, and then the 400-μm microspheres were introduced for larger tumors. Preprocedural treatment included analgesic medication and antiemetic prophylaxis. Anatomical eligibility was assessed and identification of tumor feeder arteries was performed prior to TACE with angiography of the hepatic and mesenteric arteries. Selected hepatic arteries were catheterized with a Progreat 2.7F microcatheter (Terumo Europe), a Radifocus M 0.035 180-cm guidewire (Terumo Europe) and a Simmons 15F Radifocus Glidecath (Terumo Europe), and doxorubicin-loaded LifePearl™ microspheres mixed with nonionic contrast agent were slowly injected. Additional TACE procedures were performed when clinically required (i.e., presence of radiological signs of recurrence or tumor persistence on follow-up computed tomography [CT] scan or MRI, such as contrast enhancement compatible with HCC in previously treated or new tumors).
Outcome measures
Efficacy and safety were evaluated during the entire follow-up period (39 months). Tumor response was assessed by CT scan or MRI, and response rate was assessed according to RECIST 1.1 criteria because it was easier to collect from clinical data. OS was calculated from the date of first treatment to the last follow-up or death of the patient.
Liver toxicity was assessed by laboratory evaluation, which included aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT), alkaline phosphatase (ALK) and bilirubin levels. The type and intensity of adverse events (AEs) were recorded according to the Common Terminology Criteria for Adverse Events, version 4.03.
Statistical analysis
Descriptive statistics were applied. Median, mean ± standard deviation and range were used for continuous data, and proportions were expressed using percentages. Statistical tests to assess significance included chi-square and Student's t-test, and a significant difference between samples was intended when p < 0.05. Kaplan–Meier plots were used for OS analysis.
Results
Patient characteristics
The sample included 50 patients who were affected by BCLC stage 0 (4%), stage A (20%) or stage B (76%) HCC (Table 1). Mean age was 69.8 ± 9.2 years, and patients were mostly males (82.0%; 41 of 50). Average lesion diameter was 48.6 ± 25.1 mm and number of lesions at diagnosis was one in 20 patients (40.0%), two in 16 patients (32.0%) and three or more in 14 patients (28.0%).
Table 1. . Baseline patient characteristics.
| Characteristic | Patients (n = 50) |
|---|---|
| Age (years), mean ± SD (n) | 69.8 ± 9.2 (50) |
| Sex, % (n) | |
| Male | 82.0 (41) |
| Child–Pugh score, % (n) | |
| A | 86.0 (43) |
| B | 14.0 (7) |
| ECOG, % (n) | |
| 0 | 74.0 (37) |
| 1 | 24.0 (12) |
| 2 | 2.0 (1) |
| BCLC tumor stage, % (n) | |
| 0 – very early stage | 4.0 (2) |
| A – early stage | 20.0 (10) |
| B – intermediate stage | 76.0 (380) |
| Baseline number of tumors, % (n) | |
| 1 | 40.0 (20) |
| 2 | 32.0 (16) |
| ≥3 | 28.0 (14) |
| Tumor diameter (mm), mean ± SD (n) | 48.6 ± 25.1 (49) |
BCLC: Barcelona Clinic Liver Cancer; ECOG: Eastern Cooperative Oncology Group; SD: Standard deviation.
The median number of TACE procedures per patient was 2.0 (range: 1.00–6.00), and the mean doxorubicin dose per TACE procedure was 79.2 ± 15.9 (Table 2). The size of the LifePearl™ used for each TACE procedure was 200 μm for nine patients (18.0%) and 400 μm for 41 patients (82.0%).
Table 2. . TACE procedures.
| Doxorubicin dose | |
|---|---|
| Successful administration of total dose, % (n) | 88% (44/50) |
| Dose (mg), mean ± SD (n) | 79.2 ± 15.9 (47/50) |
| Cumulative dose across all procedures | |
| Dose (mg), mean ± SD (n) | 174.4 ± 80.2 (49/50) |
| Number of procedures per patient, % (n) | |
| 1 DEM-TACE procedure | 28.0 (14/50) |
| 2 DEM-TACE procedures | 24.0 (12/50) |
| ≥3 DEM-TACE procedures | 48.0 (24/50) |
| Median number of TACE procedures (range) | 2.0 (1.00–6.00) |
| Size of microspheres, % (n) | |
| 200 μm | 18.0 (9/50) |
| 400 μm | 82.0% (41/50) |
DEM-TACE: Drug-eluting microsphere transarterial chemoembolization; TACE: Transarterial chemoembolization.
Toxicity
There was no 30-day mortality. A total of 19 AEs were reported in 30% (15 of 50) of patients, and the most common AEs were postembolization syndrome (52.6%; ten of 19) and fever (26.3%; five of 19). A total of 73.7% of reported AEs were grade 1–2, or mild to moderate intensity, whereas 26.3% of AEs were grade 3 or higher. There was one case of pain of grade 4 intensity, but no AEs were of grade 5 intensity. Heart rate and blood pressure were not affected by treatment, and no significant variations were observed at follow-up visits. With regard to liver toxicity, 8% (four of 50) of patients had an increase in ALT and AST values from baseline.
Serious AEs were reported in 8% (four of 50) of patients, and there were a total of five serious AEs, which included two cases of postembolization syndrome, one case of right femoral pseudoaneurysm, one case of edematous ascitic decompensation and one case of cholecystitis. The patients with postembolization syndrome did well with conservative treatment, the patient with the pseudoaneurysm was treated with an injection of thrombin to the pseudoaneurysm and did well, the patient with edematous ascitic decompensation did well with medical treatment and the patient with cholecystitis was treated with surgery (cholecystectomy). With regard to liver toxicity in the patient who underwent cholecystectomy, although AST, ALT and GGT enzyme levels did not change significantly after the procedure, ALK levels slightly increased (p = 0.0179), though they remained within normal limits (Table 3).
Table 3. . Liver toxicity.
| Baseline | 3 months | 15 months | p-value† | |
|---|---|---|---|---|
| AST (IU/l), mean ± SD (n) | 65.3 ± 43.1 (46) | 64.6 ± 46.1 (46) | 63.8 ± 37.1 (46) | >0.05 |
| AST (IU/l), median (range) | 55.5 (18.40–204.0) | 53.5 (17.0–277.0) | 53.5 (17.0–156.0) | |
| ALT (U/l), mean ± SD (n) | 63.2 ± 50.6 (49) | 57.0 ± 42.9 (49) | 57.5 ± 40.0 (49) | >0.05 |
| ALT (U/l), median (range) | 42.0 (15.0–259.0) | 44.0 (7.0–184.0) | 47.0 (10.0–162.0) | |
| GGT (U/l), mean ± SD (n) | 105.2 ± 69.7 (44) | 143.9 ± 197.6 (44) | 166.2 ± 236.9 (44) | >0.05 |
| GGT (U/l), median (range) | 83.0 (25.0–287.0) | 82.0 (33.0–1277.0) | 96.0 (21.0–1277.0) | |
| ALK U/l), mean ± SD (n)‡ | 117.7 ± 61.4 (37) | 142.0 ± 77.4 (37) | 147.6 ± 78.0 (37) | 0.0179 |
| ALK (U/l), median (range)‡ | 97.0 (51.6–340.0) | 115.0 (69.0–393.0) | 117.0 (69.0–393.0) |
Change from baseline to last follow-up.
Two values <35 U/l were considered outliers and excluded from the analyses.
ALK: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; GGT: Gamma-glutamyl transferase; SD: Standard deviation.
Tumor response
Tumor response was available for 47 patients (94%). A satisfactory complete response (CR) was observed at first and best tumor response (32 and 58%, respectively). DC rate was 90% at first evaluation and 94% at best tumor response, according to RECIST 1.1, with a mean time to follow-up of 4.5 months (Figure 1). Partial response rates at first and best tumor response were 58 and 36%, respectively, whereas progressive disease was observed in 10 and 6% of patients, respectively. Mean time to first tumor response was 86.1 ± 118.2 days, and mean time to best tumor response was 135.3 ± 140.0 days.
Figure 1. . Tumor response.
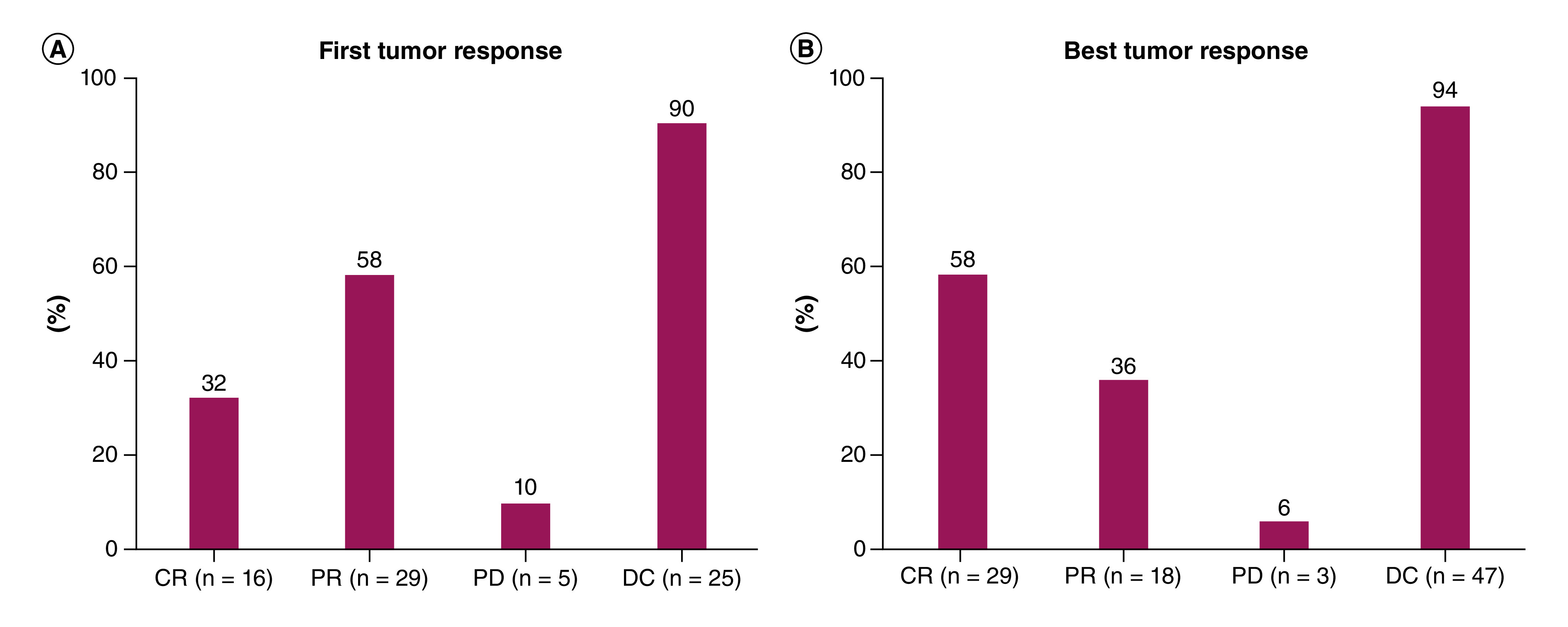
(A) First tumor response. (B) Best tumor response.
CR: Complete response; DC: Disease control; PD: Progressive disease; PR: Partial response.
At first tumor response, patients with stage BCLC stage 0/A HCC showed a high CR (58.3%), whereas patients with BCLC stage B had a CR of 23.7% (Figure 2). Partial response values were 33.3 and 65.8% for BCLC stage 0/A and BCLC stage B, respectively, whereas the corresponding values for progressive disease were 8.3 and 10.5%, respectively. DC was comparable for BCLC stage 0/A (91.6%) and BCLC stage B patients (89.5%).
Figure 2. . Tumor response according to BCLC stage.
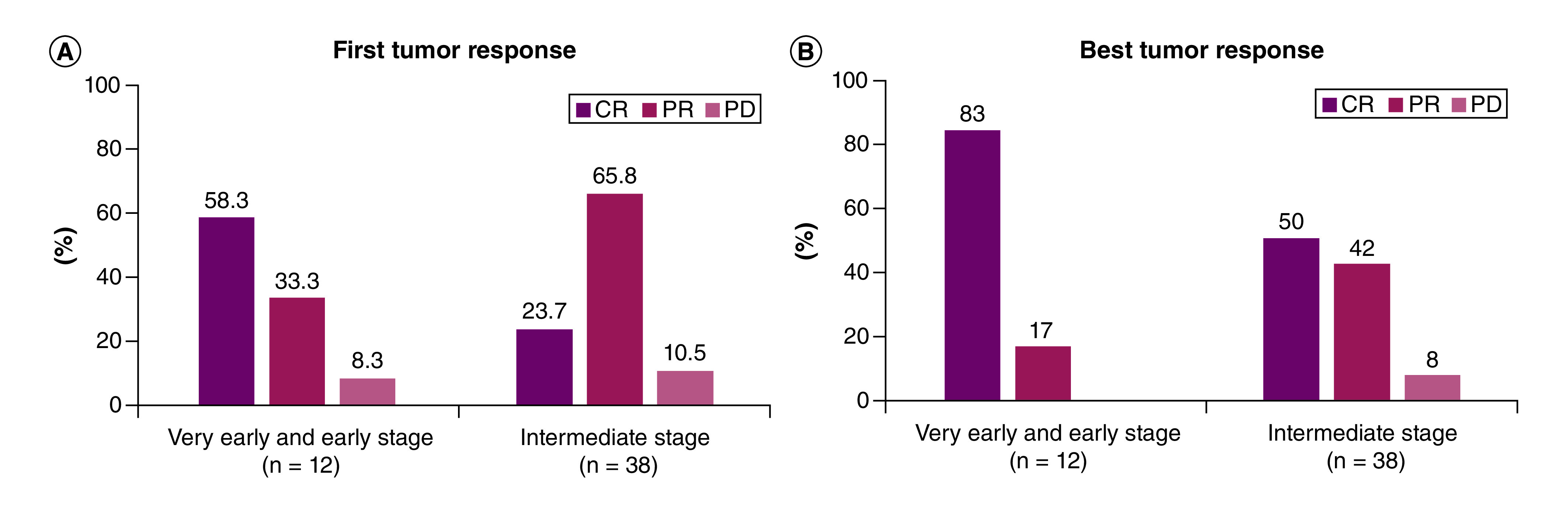
(A) First tumor response according to BCLC stage. (B) Best tumor response according to BCLC stage.
BCLC: Barcelona Clinic Liver Cancer; CR: Complete response; PD: Progressive disease; PR: Partial response.
When considering the best tumor response to TACE, the results showed that 83% of patients with BCLC stage 0/A HCC showed a higher CR after TACE treatment with LifePearl™ microspheres compared with BCLC stage B patients, who achieved a 50% response (p = 0.0414) (Figure 2). Partial response values were 17 and 42% for BCLC stage 0/A and BCLC stage B, respectively, whereas progressive disease values were 0 and 10.5%, respectively. Mean time to first tumor response was 135.3 ± 140.0 days and mean time to best tumor response was 86.1 ± 118.2 days.
Survival
Median OS of the total population was 33.8 months (range: 3–40) (Figure 3). Median OS for very early- and early-stage disease (BCLC stage 0/A) was 35.8 (26.4; n.e.), whereas median OS for intermediate-stage disease (BCLC stage B) was 31.9 months (range: 27.0; n.e.; p = 0.26) (Figure 3).
Figure 3. . Overall survival.

(A) Overall survival of total sample. (B) Overall survival according to BCLC stage.
Discussion
The aim of this study was to investigate the overall performance and safety of TACE with LifePearl™ microspheres loaded with doxorubicin in patients with BCLC stage 0/A or stage B HCC. The main finding of this study was that TACE with doxorubicin-loaded LifePearl™ microspheres is effective for the treatment of patients with different stages of HCC, improving tumor response and survival of properly selected patients as previously reported [7,23].
Treatment with LifePearl™ was well tolerated in the authors' study and agreed with the findings of several previous studies, which demonstrated good procedure safety and AEs of mild intensity (e.g., postembolic syndrome and fever) [6,17–25]. Liver toxicity with TACE with doxorubicin-loaded microspheres was usually reflected by increases in AST, GGT, ALT and bilirubin levels [6,23,24]. The results of this study showed that AST, ALT and GGT levels did not change significantly after TACE compared with baseline, whereas ALK levels significantly increased versus baseline (though never exceeding normal values). These data were in agreement with liver toxicity reports in the literature [17–20].
With regard to general tolerability, in this study, a total of 19 AEs were reported in 30% (15 of 50) of patients and were mostly fever (26.3%; five of 19) and other AEs associated with postembolization syndrome (52.6%; ten of 19). AE intensity was mainly grade 1–2, or mild to moderate (73.7%), with grade 3 or higher AEs observed in 26.3% of cases. This good safety profile was also observed when using 400-μm microspheres, without any 30-day mortality.
The low toxicity using larger microspheres agrees with a study reporting no significant difference in complications related to microspheres smaller or larger than 100 μm (p = 0.98) [26]. This high level of tolerability was also reported in other studies of HCC treatment with TACE and doxorubicin-loaded LifePearl™, which showed fever (33%), postembolization syndrome-related symptoms (6%), transaminase increases (17%), pain (33%) of mild to moderate intensity (grade: 1–2) that resolved without complication and no grade 3 or 4 AEs [17–20].
The results of this study demonstrated that the use of LifePearl™ loaded with doxorubicin was efficacious, with high CR percentages of 32 and 58% at first and best tumor response, respectively. DC was >90%, according to RECIST 1.1, with a mean follow-up time of 4.5 months. The differential analyses of best tumor response according to BCLC stages showed that BCLC stage 0/A patients achieved higher CR (83%) than BCLC stage B patients (50%; p = 0.0414), whereas DC was comparable in the two groups, at 91.6% for BCLC stage 0/A patients and 89.5% for BCLC stage B patients. DC was similar to or higher than that reported by other studies using modified RECIST (79–85.5% at 1 month, 72% at 3 months and 62–64% at 6 months after TACE with LifePearl™) [9–18]. Reported tumor response achieved with LifePearl™ is consistent across all studies.
DC obtained in this study was higher than that reported for conventional TACE (52–65.4%) or TACE performed with other microspheres: DC Bead (Boston Scientific, MA, USA) (63.8%), HepaSphere (Merit Medical, UT, USA) (53.8%) and Embozene Tandem beads (Varian Medical Systems, CA, USA) (81%) [27–31]. This may be due to the physio-mechanical properties of the LifePearl™ core material, which allows higher drug release in comparison with other drug-eluting microspheres, as shown in the in vitro comparative study of LifePearl™, DC Bead, HepaSphere and Embozene Tandem microspheres, which eluted 30 ± 5%, 21 ± 2%, 8 ± 3% and 6 ± 0%, respectively, of the loaded doxorubicin [16].
According to EASL guidelines, TACE is recommended as the standard of care for BCLC stage B patients. TACE is also used in BCLC stage 0/A patients for downstaging or as a bridge to transplantation [9,23] and is recommended by recent European Society for Medical Oncology guidelines [7]. TACE enhances downstaging and bridge to transplant with excellent tumor response [23].
The median OS of the total population in the current study was 33.8 months, whereas BCLC stage 0/A patients had a median OS of 35.8 months and BCLC stage B patients had a median OS of 31.9 months, showing that patients with early HCC stages can also benefit from the use of TACE. These data were in agreement with best reported median OS in previous studies on TACE with other drug-eluting microspheres loaded with doxorubicin, which showed OS of 33.9 months (95% CI: 28.9–38.9 months) and 1- and 2-year survival of 97.1 and 65.7%, respectively [32,33].
The main limitations of this study were the retrospective data collection, absence of a control arm, low number of patients and use of RECIST 1.1, as it may not be comparable with other publications using modified RECIST criteria. Further multicenter prospective studies with a larger number of patients are currently ongoing and are expected to confirm these data.
Conclusion
TACE with LifePearl™ microspheres loaded with doxorubicin is well tolerated when used to treat patients with unresectable HCC, as indicated by the limited number of AEs and minimal changes in enzymes indicative of liver function. TACE with LifePearl™ microspheres loaded with doxorubicin is also effective for the treatment of patients with very early-, early- and intermediate-stage HCC, resulting in satisfactory CR, high DC and good OS.
Future perspective
TACE using doxorubicin-loaded LifePearl™ was used for the treatment of 50 patients with early- and intermediate-stage HCC. Few AEs were reported. CR and DC were 58 and 94%, respectively, at time of best tumor response, with a mean time to follow-up of 4.5 months. Patients with BCLC stage 0/A at best tumor response showed a higher CR rate (83%) than patients with BCLC stage B (CR: 50%; p = 0.0414). Median OS was 33.8 months.
Doxorubicin-loaded LifePearl™ TACE is an effective treatment, with a good safety profile, for unresectable patients with early- and intermediate-stage HCC. TACE has several applications in HCC patients, including tumor downstaging and bridging to transplantation. Moreover, future treatments for HCC include the combination of targeted and immunotherapy and checkpoint inhibitors (anti-PD-1 or anti-CTLA-4) to TACE. The rationale for this combination is that TACE induces tumor necrosis, resulting in antigen release of dying tumor cells that are known to induce antitumor immune responses and synergize T-cell stimulation through checkpoint inhibition. For this reason, the treatment of all stages of HCC, from early to intermediate to advanced, will change considerably in the near future.
Summary points.
Transarterial chemoembolization is indicated for unresectable hepatocellular carcinoma (HCC), increasing overall survival (OS) in selected patients with unresectable Barcelona Clinic Liver Cancer stage 0/A and stage B HCC with small tumor burden and good liver function.
Fifty consecutive HCC patients from a single center, treated with transarterial chemoembolization using doxorubicin-loaded LifePearl™, were enrolled.
Adverse events were reported for 30% of patients, and 26.3% of adverse events were grade 3 or higher, with only one grade 4 event and no grade 5 events.
Complete response (CR) rates were 32 and 58% at first evaluation and best tumor response, respectively.
Disease control rates were 90 and 94% at first evaluation and best tumor response, respectively.
Median OS was 33.8 months. Patients with Barcelona Clinic Liver Cancer stage 0/A at best tumor response showed a higher CR rate (83%) than patients with Barcelona Clinic Liver Cancer stage B (CR: 50%; p = 0.0414), with a trend toward better OS, with a mean of 35.8 and 31.9 months (p = 0.26), respectively.
Transarterial chemoembolization with LifePearl™ microspheres loaded with doxorubicin was well tolerated when used to treat patients with very early-, early- and intermediate-stage HCC and resulted in satisfactory CR, high disease control and good OS.
Footnotes
Financial & competing interests disclosure
This study was funded by a research grant from Terumo Europe NV (Leuven, Belgium). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All procedures performed in this study were conducted in accordance with the ethical standards of the institutional research committee of German Trias I Pujol (Can Ruti) University Hospital and with the 1964 Helsinki Declaration and its later amendments.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Bertuccio P, Turati F, Carioli Get al. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 67(2), 302–309 (2017). [DOI] [PubMed] [Google Scholar]
- 2.de Jesus VHF, Dettino ALA. Update on hepatocellular carcinoma from the 2018 Gastrointestinal Cancer Symposium (ASCO GI). J. Hepatocell. Carcinoma 5, 87–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viveiros P, Riaz A, Lewandowski RJ, Mahalingam D. Current state of liver-directed therapies and combinatory approaches with systemic therapy in hepatocellular carcinoma (HCC). Cancers (Basel) 11(8), 1085 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo W, Zhang Y, He Get al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J. Surg. Oncol. 15(1), 126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallini JR, Gabz A, Salem R, Lewandowski RJ. Transarterial radioembolization with yttrium-90 for the treatment of hepatocellular carcinoma. Adv. Ther. 33, 699–714 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gjoreski A, Popova-Jovanovska R, Eftimovska-Rogac I, Vejseli J. Safety profile and efficacy of chemoembolization with doxorubicin-loaded polyethylene glycol microspheres in patients with hepatocellular carcinoma. Open Access Maced. J. Med. Sci. 7(5), 742–746 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel A, Cervantes A, Chau Iet al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30(5), 871–873 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Radosa CG, Radosa JC, Grosche-Schlee Set al. Holmium-166 radioembolization in hepatocellular carcinoma: feasibility and safety of a new treatment option in clinical practice. Cardiovasc. Intervent. Radio. 42, 405–412 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Fohlen A, Tasu JP, Kobeiter H, Bartoli JM, Pelage JP, Guiu B. Transarterial chemoembolization (TACE) in the management of hepatocellular carcinoma: results of a French national survey on current practices. Diagn. Interv. Imaging 99(9), 527–535 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Kim AR, Park E, Kwon SYet al. Efficacy and safety of combined radiofrequency ablation with transarterial chemoembolization in patients with Barcelona Clinic Liver Cancer stage A hepatocellular carcinoma ineligible for curative treatment. Korean J. Gastroenterol. 73(3), 167–176 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamai T, Oshige A, Tabu Ket al. Utility of percutaneous radiofrequency ablation alone or combined with transarterial chemoembolization for early hepatocellular carcinoma. Oncol. Lett. 14(3), 3199–3206 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornton LM, Cabrera R, Kapp M, Lazarowicz M, Vogel JD, Toskich BB. Radiofrequency vs microwave ablation after neoadjuvant transarterial bland and drug-eluting microsphere chembolization for the treatment of hepatocellular carcinoma. Curr. Probl. Diagn. Radiol. 46(6), 402–409 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan T, Chang L, Rahmathullah MN, Wu L, Yuan YF. Comparative efficacy of interventional therapies for early-stage hepatocellular carcinoma: a PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore) 95(15), e3185 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiu B, Deschamps F, Aho Set al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J. Hepatol. 56(3), 609–17 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Monier A, Guiu B, Duran Ret al. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur. Radiol. 27(4), 1431–1439 (2017). [DOI] [PubMed] [Google Scholar]
- 16.de Baere T, Plotkin S, Yu R, Sutter A, Wu Y, Cruise GM. An in vitro evaluation of four types of drug-eluting microspheres loaded with doxorubicin. J. Vasc. Interv. Radiol. 27(9), 1425–31 (2016). [DOI] [PubMed] [Google Scholar]; •• Evaluated the suspension time, loadability and release of doxorubicin for different types of microspheres, including LifePearl™, DC Bead™, Tandem™ and HepaSphere™.
- 17.Fiorentini G, Sarti D, Carandina Ret al. A review discussing the use of polyethylene glycol microspheres in the treatment of hepatocellular carcinoma. Future Oncol. 15(7), 695–703 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Aliberti C, Carandina R, Sarti Det al. Hepatic arterial infusion of polyethylene glycol drug-eluting beads for primary and metastatic liver cancer therapy. Anticancer Res. 36(7), 3515–21 (2016). [PubMed] [Google Scholar]; • First data on Lifepearl™ for the treatment of hepatocellular carcinoma (HCC) and liver metastases.
- 19.Aliberti C, Carandina R, Sarti Det al. Chemoembolization adopting polyethylene glycol drug-eluting embolics loaded with doxorubicin for the treatment of hepatocellular carcinoma. AJR Am. J. Roentgenol. 209(2), 430–434 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Argirò R, Cirelli C, Corona Met al. Transarterial chemoembolization of hepatocellular carcinoma with 100 ± 25-μm and 200 ± 25-μm LifePearl microspheres, short-term follow-up and safety profile. Cardiovasc. Intervent. Radiol. 40(2), 25–412 (2017). 28828739 [Google Scholar]; • Important study on LifePearl™ safety.
- 21.de Baere T, Verset G, Guiu Bet al. LifePearl anthracyclin registry in selective chemo-embolization of patients with unresectable hepatocellular carcinoma. https://library.cirse.org/cirse2018/crs/lifepearl-r-anthracyclin-registry-in-selective-chemo-embolization-of-patients-with-unresectable-hepatocellular-carcinoma-paris-study-preliminary-results; • Largest prospective registry of LifePearl™ loaded with anthracyclines for the treatment of HCC.
- 22.Malagari K, Burrel M, Reig Met al. Pharmacokinetic study of doxorubicin in the treatment of unresectable HCC by LifePearl™. https://library.cirse.org/cirse2018/crs/pharmacokinetic-analyses-in-patients-with-unresectable-hepatocellular-carcinoma-treated-by-tace-with-doxorubicin-drug-eluting-microspheres; • Pharmacokinetic study of LifePearl™ loaded with doxorubicin for the treatment of HCC.
- 23.Veloso Gomes F, Oliveira JA, Correia MTet al. Chemoembolization of hepatocellular carcinoma with drug-eluting polyethylene glycol embolic agents: single-center retrospective analysis in 302 patients. J. Vasc. Interv. Radiol. 29(6), 841–849 (2018). [DOI] [PubMed] [Google Scholar]; • Study with the largest number of patients treated with LifePearl™ loaded with doxorubicin for the treatment of HCC.
- 24.Lucatelli P, Argirò R, De Rubeis Get al. Polyethylene glycol epirubicin-loaded transcatheter arterial chemoembolization procedures utilizing a combined approach with 100 and 200 μm microspheres: a promising alternative to current standards. J. Vasc. Interv. Radiol. 30(3), 305–313 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Malagari K, Pomoni M, Moschouris Het al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc. Intervent. Radiol. 35, 1119–1128 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Balli H, Aksungur E, Khalatai B, Aikimbaev K. Super-selective transarterial chemoembolization with doxorubicin-loaded drug-eluting beads sized below and above 100 microns in hepatocellular carcinoma: a comparative study. J. Belg. Soc. Radiol. 103(1), 47 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Defreyne L. Interventional radiology for liver diseases. Eur Radiol. 31(4), 2227–2230 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J. Hepatol. 9(18), 808–814 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan F, Wang EQ, Lam MGet al. Superselective chemoembolization of HCC: comparison of short-term safety and efficacy between drug-eluting LC Beads, QuadraSpheres, and conventional ethiodized oil emulsion. Radiology 278(2), 612–21 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Lammer J, Malagari K, Vogl Tet al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc. Intervent. Radiol. 33(1), 41–52 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malagari K, Kiakidis T, Pomoni Met al. Pharmacokinetics, safety, and efficacy of chemoembolization with doxorubicin-loaded tightly calibrated small microspheres in patients with hepatocellular carcinoma. Cardiovasc. Intervent. Radiol. 39(10), 1379–91 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Burrel M, Reig M, Forner Aet al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J. Hepatol. 56(6), 1330–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popovic P, Stabuc B, Jansa R, Garbajs M. Survival of patients with intermediate stage hepatocellular carcinoma treated with superselective transarterial chemoembolization using doxorubicin-loaded DC Bead under cone-beam computed tomography control. Radiol. Oncol. 50(4), 418–426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


