Abstract
Purpose of review:
Current biomarkers for chronic kidney disease (CKD) are limited by lack of sensitivity and inability to prognosticate CKD progression. Significant recent research has better characterized novel biomarker candidates that are associated with CKD progression and cardiovascular mortality in CKD. This review discusses the most significant advances within the past year.
Recent findings:
We discuss biomarkers for outcomes in CKD under two categories: (1) Emerging (defined as having been validated in an independent cohort) which include serum Cystatin C, serum β-trace protein, β2-microglobulin, suPAR, soluble Tumor Necrosis Factor Receptors 1/2, urinary monocyte chemotactic protein-1, neutrophil gelatin associated lipocalin, Kidney Injury Molecule-1 and Fibroblast Growth Factor-23 and (2) Novel (which have shown associations in smaller observational studies but have not been validated yet) which include indoxyl sulfate, p-cresyl sulfate, trimethylamine-N-oxide, inteleukin-18, Klotho, markers of endothelial dysfunction, vimentin and procollagen type III N-terminal propeptide. Further, we also discuss future directions for biomarker research including unbiased –omics approaches.
Summary:
There are a number of promising biomarkers that can better prognosticate outcomes in and progression of CKD. Further research is warranted to examine whether these biomarkers validate independently as well and if their incorporation improves clinical practice and/or trial enrollment.
Keywords: Kidney Disease, Renal Injury, Outcomes, Biomarkers, Albuminuria
Introduction:
Chronic kidney disease (CKD) is highly prevalent in the US, leading to progression to end stage renal disease (ESRD) and an increase in cardiovascular mortality. Current biomarkers are limited in their ability to prognosticate CKD progression and mortality.(1) As the need for more sensitive biomarkers has been increasingly recognized over the past decade, the number of biomarker candidates for CKD has dramatically expanded from both animal models and in-vitro human studies.
As a general principle, biomarkers allow clinicians to accurately and reproducibly measure a biologic substance that is an indicator of a biologic process. An effective biomarker for CKD and its progression would have the following attributes: (1) Allow for detection early in the disease process; (2) Effectively risk stratify patients who will progress to ESRD; (3) Prognosticate CKD progression and mortality; (4) Cost-effective; (5) Stable in solution; (6) Widely available testing in clinical labs with timely results; and (7) Be independent of and/or represent different pathophysiological pathways compared to conventional markers. Additionally, since CKD patients are widely underrepresented in clinical trials and clinical trials in nephrology leave much to be desired, newer biomarkers can potentially identify patients that are likely to progress and/or have specific endophenotypes, leading to shorter, more efficient clinical trials.(2,3)
In this review, we will review the most recent developments in biomarkers for CKD progression and outcomes. We will summarize the most current evidence over the past year, and discuss future directions for the field. We will discuss both serum and urine biomarkers. Serum biomarkers are more stable in solution and may have better discriminative performance for AKI, whereas urinary biomarkers are easier to collect noninvasively.(4)
We have categorized biomarkers for CKD progression into “emerging” versus “novel” biomarkers, based on their level of evidence. “Emerging” biomarkers have been examined in multiple studies, and have been validated independently in separate cohorts, whereas “novel” biomarkers may show an association in single or a few observational studies with smaller sample sizes and need further validation.
Weaknesses of Conventional Biomarkers:
The conventional biomarkers for CKD prognostication include estimated glomerular filtration rate (eGFR) by serum creatinine (eGFRcrea) and albuminuria. However, in patients with preserved renal function, eGFRcrea and albuminuria are only modestly useful for risk prediction.(5) In addition, albuminuria has significant limitations including regression either spontaneously or after therapy. Recent epidemiologic studies have also demonstrated CKD progression without albuminuria.(6,7) Thus newer markers are needed, to implement preventive efforts early, improve clinical trials and shed light on therapeutic targets.
Emerging Biomarkers:
We highlight a few biomarkers as “emerging” biomarkers that have been well associated with CKD progression and validated in multiple cohorts and focus on published studies especially in the last year. (Figure 1)
FIGURE 1.
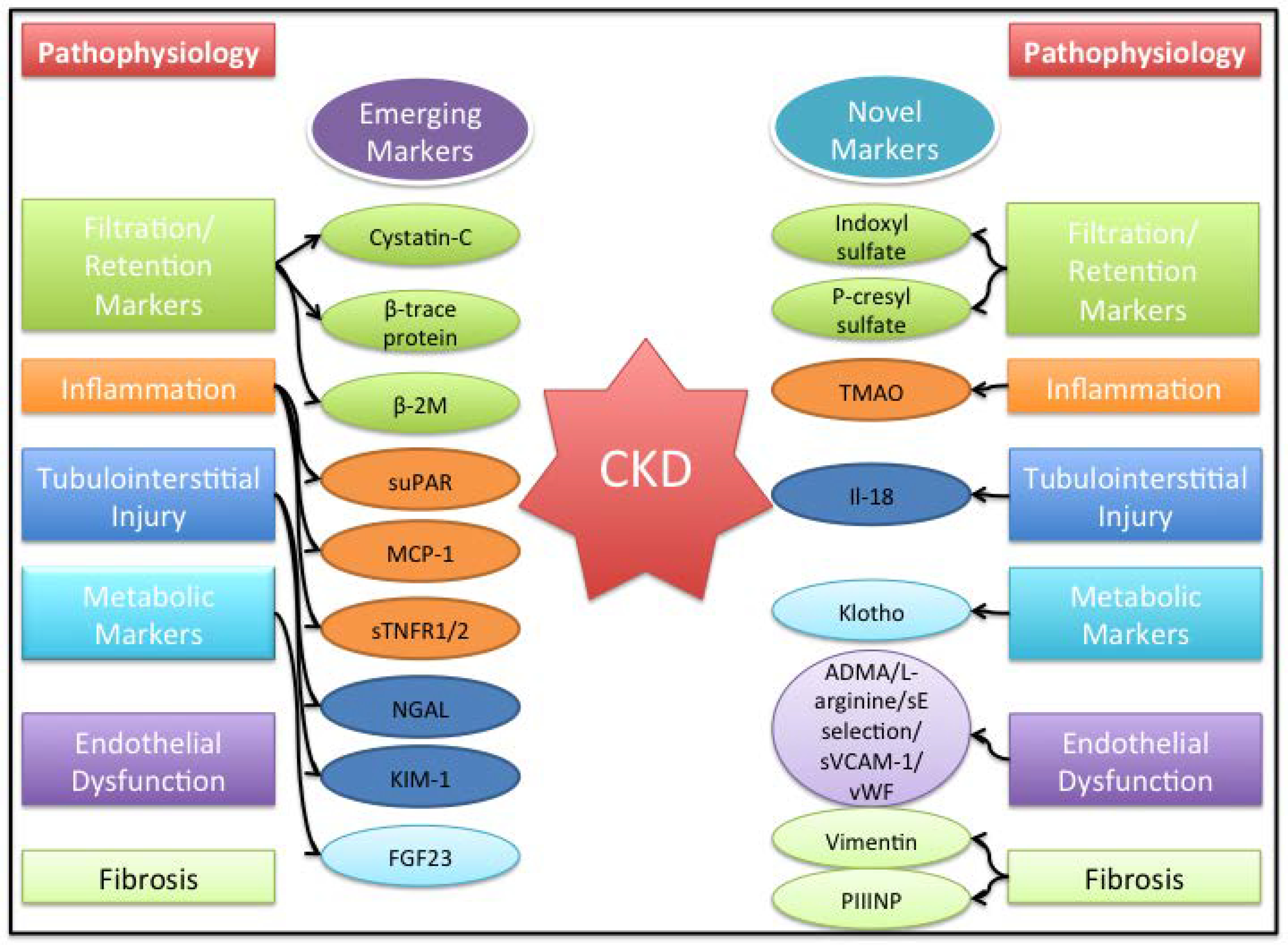
List of emerging and novel biomarker and broad pathophysiologic characteristics. ADMA, asymmetric dimethylarginine; b2M, b2-microglobulin; CKD, chronic kidney disease; FGF23, fibroblast growth factor 23; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemotactic protein-1; NGAL, neutrophil gelatin-associated lipocalin; PIIINP, procollagen type III N-terminal propeptide; sE, soluble E-selectin; sTNFR1/2, soluble tumor necrosis factors receptor 1 and 2; suPAR, soluble urokinase-type plasminogen activator receptor; sVCAM, soluble vascular adhesion molecule-1; TMAO, trimethylamine-N-oxide; vWF, von Willebrand factor.
Serum Cystatin C
Cystatin C is an inhibitor of cysteine proteases, and is a more specific filtration marker of CKD than creatinine, varying less by age, sex, race, or muscle mass. However, levels may be affected in patients with cancer, thyroid dysfunction, HIV, and other conditions. A recent meta-analysis of 19 studies compared the accuracy of cystatin C-based estimates of GFR with GFR measured by nuclear medicine techniques.(8) Cystatin C had a sensitivity and specificity of 0.85 and 0.87 for detecting CKD. Another meta-analysis of 16 studies demonstrated that use of cystatin C improved reclassification of risk for both ERSD and death.(9) In addition, proteoforms of Cystatin C have been recently found to be elevated in diabetic patients with mild CKD and could represent a potential new avenue of investigation.(10) Cystatin C has been well studied and is available inexpensively in some clinical laboratories, and is already being integrated into routine clinical care, especially where serum creatinine is known to be inaccurate.
Serum β-trace protein (BTP) and β2-microglobulin (B2M)
Serum β-trace protein (BTP), a prostaglandin synthase, and β2-microglobulin (B2M), a cause of dialysis-related amyloidosis, are filtration and immunologic markers. In the Chronic Renal Insufficiency Cohort (CRIC) study, BTP and B2M were independent predictors of ESRD and all-cause mortality beyond eGFRcrea.(11) In a recent study, a 4-marker composite score including BTP, B2M, serum creatinine and cystatin C was also an independent predictor of ESRD and mortality, and more strongly associated with these outcomes compared with eGFRcrea. This was seen in a pooled analysis of several cohorts including the MDRD [Modification of Diet in Renal Disease] Study, AASK [African American Study of Kidney Disease and Hypertension], and CRIC [Chronic Renal Insufficiency Cohort] Study). Thus, a multimarker estimation of renal function seems to be robust and although, not ready for clinical use as yet, could represent an advance in estimating kidney function.(12)
suPAR
Soluble urokinase-type plasminogen activator receptor (suPAR) is the circulating form of a membrane protein that is expressed on endothelial cells and podocytes and is involved in cell adhesion. In preclinical and small human studies, suPAR was postulated to have be involved in the pathogenesis of some forms of kidney disease, however its pathogenic role is still controversial.(13) However, higher suPAR levels were associated with CKD progression, in a large cohort study of 3683 patients from the Emory Cardiovascular Biobank and these results were validated in a smaller patient population in the Women’s Interagency HIV Study (WIHS).(14) SuPAR levels showed a graded relationship with longitudinal eGFR decline and incident CKD and these associations were independent of proteinuria. However, suPAR was overall associated with a change in eGFR of only 0.9 percentage points and proteinuria was only measured on a semi-quantitative basis.(15) Despite these study limitations, suPAR could be an important marker for longitudinal renal decline.
Soluble Tumor Necrosis Factors Receptor 1 and 2 (sTNFR1/2)
TNF is a pleotrophic cytokine that is produced predominantly by immune cells that can function in its membrane bound form or can be released as a soluble circulating 17kDa polypeptide upon cleavage by a metalloproteinase.(16–18) TNF may bind to two transmembrane receptors designated TNFR1 (p55 or CD120a) or TNFR2 (p75 or CD120b).(19,20) TNFR1 is present primarily in glomeruli and endothelial cells while TNFR2 is only expressed transcriptionally in renal cells in various kidney diseases.(21,22) Although TNFR2 functions as a ligand presenting receptor to TNFR1, it may have independent functions.(23) Several recent studies have shown that sTNFR1/2 are robust markers for prognostication of renal functional decline in DKD.(24–26) Further studies assessing them as part of multidimensional panels are needed to assess their utility in clinical practice and as part of trial enrichment biomarkers.
Urinary monocyte chemotactic protein-1 (MCP-1)
MCP-1 is a chemokine that promotes recruitment and transformation of monocytes into macrophages. (27) Kidney cells secrete MCP-1 in response to inflammatory stimuli and MCP-1 is upregulated as part of ongoing inflammation. (28) Several studies have shown that urinary MCP-1 is an independent predictor in diabetic kidney disease.(29,30) A recent study demonstrated that urinary MCP-1 to creatinine ratio is predicts sustained eGFR change independent of albuminuria and improved risk discrimination when added to other traditional predictors of kidney disease.(31) Thus, pending further validation, urinary MCP-1 may serve as a useful component assessing inflammation in multidimensional panels for renal functional decline.
Neutrophil gelatin associated lipocalin (NGAL)
NGAL, a ubiquitous lipocalin iron-carrying protein is highly expressed in renal tubular epithelium and released following renal damage. Although it was initially identified as an early biomarker for AKI, and considering that AKI and CKD might be interlinked, there has been great interest in identifying NGAL as a biomarker for CKD.(32,33) However, the evidence for NGAL as a biomarker has been conflicting till date. In the ARIC study, urinary NGAL was associated with higher risk of stage 3 CKD and in Pima Indians with type 2 diabetes NGAL/Cr was associated with increased ESRD risk.(34,35) In contrast, a recent study from the same ARIC cohort demonstrated that NGAL/Cr was not associated with a higher risk of ESRD.(36) These studies differ by the definition of outcome as well as unstandardized biomarker measurements/normalization across studies. Further studies with standardized outcomes and biomarker measurements across heterogenous cohorts are needed to assess the utility of NGAL.
Kidney Injury Molecule-1 (KIM-1)
KIM-1 is a transmembrane glycoprotein that is expressed in the apical membrane of proximal tubular cells in response to injury.(37) Urinary KIM-1 levels rise in renal tubular injury and it is modestly associated with AKI.(38) The literature on urinary KIM-1 and CKD progression is conflicting. A study from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort demonstrated that urinary levels of KIM-1 were associated with incident CKD and rapid renal function decline.(39) Conversely, other studies have not demonstrated any significant associations between urinary KIM-1 and renal outcomes.(31,40) However, a single study has shown that serum KIM-1 at baseline predicted eGFR loss and ESRD during upto 15 years of follow up in patients with type 1 diabetes mellitus. Further study is required in larger, more heterogenous cohorts before assessing whether KIM-1 is an independent prognosticator of CKD.
Fibroblast Growth Factor-23
Fibroblast Growth Factor-23 (FGF-23) is a circulating peptide that mediates phosphate metabolism by decreasing the expression of the sodium-phosphate cotransporter NPT2 in the proximal tubule, thus increasing phosphate excretion. FGF-23 has been previously associated with mortality, ESRD risk and cardiovascular events in large multiethnic cohorts.(41–43)
In addition, recent research suggests that FGF-23 may be a noninvasive indicator of carotid artery intima-media thickness. In a cohort of 91 patients with CKD stage 3 – 4, FGF-23 level was an independent predictor of intima-media thickness.(44) Thus, FGF23 might not just be a marker for incident CKD, but also cardiovascular complications in CKD. Whether FGF23 is a therapeutic target and changes lead to better outcomes will be investigated by the COMBINE study (CKD Optimal Management With BInders and NicotinamidE).(45)
Novel Markers:
Recent research has identified many “novel” biomarkers, which show associations in a smaller number of observational studies and call for further research. (Figure 1)
Indoxyl sulfate (IS) and p-cresyl sulfate (PCS)
A recent meta-analysis from 11 studies found that IS and PCS, two uremic retention toxins that promote inflammation, were independently associated with cardiovascular events and mortality in CKD patients.(46) However, these results may not be clinically significant given small effect sizes.
Trimethylamine-N-oxide (TMAO)
TMAO is a metabolite of gut microbiota, and is linked to inflammation, aging, and CVD. TMAO was found to be independently associated with mortality and inflammatory biomarkers in a cohort of 179 patients with CKD Stage 3–5.(47) Further research is warranted, especially in conjunction with microbiota sequencing studies.
Inteleukin-18 (IL-18)
Urinary IL-18 is a proinflammatory cytokine which is up-regulated in response to ischemia reperfusion injury.(48) While urine IL-18 has been shown to distinguish acute tubular necrosis from prerenal AKI, studies in CKD are lacking. (49,50) Studies in HIV positive patients demonstrate CKD progression with increased urinary IL-18.(51) However, a recent study showed there was no association of urine IL-18/Cr levels and renal function decline in type 2 diabetics with preserved renal function.(31) Thus, it is possible that urine IL-18 offers only prognostic information for AKI but not of progression of CKD.
Klotho
Klotho is a transmembrane protein related to beta-glucouronidases, and Klotho deficiency is associated with aging and accelerated atherosclerosis. Klotho is produced by the kidney and is a co-receptor for FGF23, and may be renal protective through RAS blockade and other mechanisms.(52) Recent work has shown that Klotho-deficient CKD mice had increased cardiac hypertrophy, independent of FGF23 and phosphate.(53) Further studies are needed to disentangle the complicated relationship between FGF23, Klotho and CKD.
Endothelial dysfunction
Markers of endothelial dysfunction have been understudied in CKD. In a recent case-control study with 201 cases and 201 community-based controls without CKD, associations with the following biomarkers were demonstrated: asymmetric dimethylarginine (ADMA), L-arginine, soluble vascular adhesion molecule-1 (sVCAM-1), soluble E-selectin (sE-selectin), and von Willebrand factor (vWF).(54) Whether these markers individually or in combination, improve risk prediction and prognostication for CKD remains to be studied in larger cohorts.
Vimentin
Vimentin is a type III intermediate filament, and novel research indicates that urinary vimentin that may serve as a biomarker of renal fibrosis. Vimentin urinary mRNA levels were found to be 9.99-fold greater in CKD patients vs. controls, and correlated with proteinuria and renal fibrosis scores on biopsy.
Procollagen type III N-terminal propeptide (PIIINP)
PIIINP is cleaved from type 3 collagen during its deposition in the extracellular matrix and then is released into the urine or blood. Biopsy studies demonstrated that urine PIIINP is associated with histopathologic tubulointerstitial fibrosis.(55) A recent analysis from the Cardiovascular Health Study (CHS) cohort demonstrated that higher levels were associated with higher odds of CKD progression in older participants.(56)
Future Directions:
With advances in –omics techniques (genomics, metabolomics, proteomics, and transcriptomics) and improved computational approaches, the horizons for biomarker discovery have been expanded. (Figure 2) However, there are significant barriers to adopting many of these techniques into clinical practice, including cost, feasibility, and accessibility of laboratory testing.
FIGURE 2.

Various forms of –omics relevant to discovery of new biomarkers for chronic kidney disease (CKD).
Because the kidney clears small molecules, metabolite concentrations may be associated with CKD incidence and progression. A recent study used gas and liquid chromatography coupled to mass spectrometry, to analyze 493 small molecules and found that C-mannosyltryptophan, pseudouridine, N-acetylalanine, erythronate, myo-inositol, and N-acetylcarnosine were associated with CKD in the general population.(57) Another study identified certain metabolites including citrulline and choline (renal metabolism markers) and kynurenic acid (secretion marker) which improve prediction of CKD beyond that accorded by baseline eGFR and proteinuria alone.(58)
Computational approaches using high-resolution plasma proteome analysis can simultaneously analyze thousands of plasma proteins, which may yield higher diagnostic accuracy. One plasma proteome analysis identified a CKD association with lysozyme C and leucine-rich alpha-2 glycoprotein, which are involved in neovascularization and heart failure.(59) A proteomic classification score based on 273 different proteins has been recently developed which predicts progression of CKD above and beyond albuminuria and clinical variables which had a significantly better diagnostic accuracy for chronic kidney disease than albuminuria in both diabetic and non-diabetic kidney disease.(60,61)
With growing expertise in transcriptomics, biopsy transcription driven techniques could identify biomarkers that subendophenotype patients for risk of disease progression. Recent research has identified epidermal growth factor (EGF), a tubule-specific protein as an independent predictor of CKD progression.(62)
Finally, biomarkers for disease progression need to be identified in ethnic minorities who are at high genetic risk of progression due to ApolipoproteinL1 (APOL1) risk variants.(63) A combination of genetic markers and biomarkers may be used for risk stratify this vulnerable population for early intervention and disease management.
Conclusions:
Several novel biomarkers for CKD are emerging, while the majority of research remains in early stages. Numerous prospective biomarkers need rigorous validation in larger populations and independent cohorts. -Omics approaches have significant potential for biomarker discovery. Cost and accessibility remain barriers before these biomarkers can be integrated into clinical practice. Analyzing biomarkers in combination is useful, which calls for further research into statistical methodology designing models and alternative approaches such as machine learning.(62,64) While novel independent associations are interesting, biomarkers alone or in combination, should add to risk discrimination or illuminate newer pathogenic pathways.
Key Points:
Recent research has led to discovery and validation of novel biomarkers that improve risk prognostication of renal decline over traditional markers such as serum creatinine and proteinuria
Novel biomarkers have been identified that might outperform traditional markers and elucidate pathogenic pathways but need validation
Advances in –omics approaches may lead to biomarkers that could subendophenotype chronic kidney disease patients at risk of renal progression.
Financial support and sponsorship:
GNN is supported by K23DK107908-01A1 from the National Institute of Diabetes Digestive and Kidney Diseases. SGC is supported by grants from the National Institute of Diabetes Digestive and Kidney Diseases (R01DK096549 and U01DK106962).
Footnotes
Conflicts of interest: None
References:
- 1.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt K-U, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007August; 72(3):247–59. [DOI] [PubMed] [Google Scholar]
- 2. Konstantinidis I, Nadkarni GN, Yacoub R, Saha A, Simoes P, Parikh CR, et al. Representation of Patients With Kidney Disease in Trials of Cardiovascular Interventions: An Updated Systematic Review. JAMA Intern Med. 2016January1;176(1):121–4. ** Demonstrates recent underrepresentation of CKD patients in cardiovascular trials.
- 3. Parikh CR, Moledina DG, Coca SG, Thiessen-Philbrook HR, Garg AX. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int. 2016June;89(6):1372–9. * Demonstrates how biomarkers could be used for clinical trial enrichment
- 4.Schley G, Köberle C, Manuilova E, Rutz S, Forster C, Weyand M, et al. Comparison of Plasma and Urine Biomarker Performance in Acute Kidney Injury. PloS One. 2015;10(12):e0145042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunkler D, Gao P, Lee SF, Heinze G, Clase CM, Tobe S, et al. Risk Prediction for Early CKD in Type 2 Diabetes. Clin J Am Soc Nephrol CJASN. 2015July14; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki S, Haneda M, Sugimoto T, Isono M, Isshiki K, Kashiwagi A, et al. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes. 2005October;54(10):2983–7. [DOI] [PubMed] [Google Scholar]
- 7.Nosadini R, Velussi M, Brocco E, Bruseghin M, Abaterusso C, Saller A, et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes. 2000March;49(3):476–84. [DOI] [PubMed] [Google Scholar]
- 8. Wei L, Ye X, Pei X, Wu J, Zhao W. Diagnostic accuracy of serum cystatin C in chronic kidney disease: a meta-analysis. Clin Nephrol. 2015August;84(2):86–94. * Collaborative meta analysis for risk estimates of cystatin C with kidney disease
- 9.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013September5;369(10):932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yassine HN, Trenchevska O, Dong Z, Bashawri Y, Koska J, Reaven PD, et al. The association of plasma cystatin C proteoforms with diabetic chronic kidney disease. Proteome Sci. 2016; 14:7. * Shows that proteoforms of Cystatin C have been recently found to be elevated in diabetic patients with mild CKD
- 11. Foster MC, Coresh J, Hsu C-Y, Xie D, Levey AS, Nelson RG, et al. Serum β-Trace Protein and β2-Microglobulin as Predictors of ESRD, Mortality, and Cardiovascular Disease in Adults With CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis Off J Natl Kidney Found. 2016July;68(1):68–76. Shows that BTP and β2M are independently associated with ESRD and mortality in CKD patients
- 12. Inker LA, Tighiouart H, Coresh J, Foster MC, Anderson AH, Beck GJ, et al. GFR Estimation Using β-Trace Protein and β2-Microglobulin in CKD. Am J Kidney Dis Off J Natl Kidney Found. 2016January;67(1):40–8. * Shows that eGFR estimation improves with addition of BTP and β2M
- 13. Spinale JM, Mariani LH, Kapoor S, Zhang J, Weyant R, Song PX, et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 2015March;87(3):564–74. * Showed that longitudinal suPAR change was associated with eGFR decline but was not different for patients with FSGS as compared with other diagnoses
- 14. Hayek SS, Sever S, Ko Y-A, Trachtman H, Awad M, Wadhwani S, et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N Engl J Med. 2015November12;373(20):1916–25. ** Study showing that suPAR at baseline is associated with renal decline in two cohorts
- 15.Skorecki KL, Freedman BI. A suPAR Biomarker for Chronic Kidney Disease. N Engl J Med. 2015November12;373(20):1971–2. [DOI] [PubMed] [Google Scholar]
- 16.Vassalli P The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–52. [DOI] [PubMed] [Google Scholar]
- 17.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007April;71(7):619–28. [DOI] [PubMed] [Google Scholar]
- 18.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997February20;385(6618):729–33. [DOI] [PubMed] [Google Scholar]
- 19.Dembic Z, Loetscher H, Gubler U, Pan YC, Lahm HW, Gentz R, et al. Two human TNF receptors have similar extracellular, but distinct intracellular, domain sequences. Cytokine. 1990July;2(4):231–7. [DOI] [PubMed] [Google Scholar]
- 20.Brockhaus M, Schoenfeld HJ, Schlaeger EJ, Hunziker W, Lesslauer W, Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A. 1990April;87(8):3127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int. 2015February;87(2):281–96. * Review article on TNF receptors
- 22.Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J Off Publ Fed Am Soc Exp Biol. 2005October;19(12):1637–45. [DOI] [PubMed] [Google Scholar]
- 23.Grell M Tumor necrosis factor (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J Inflamm. 1995 1996;47(1–2):8–17. [PubMed] [Google Scholar]
- 24.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol JASN. 2012March;23(3):507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS, Niewczas MA. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015April;87(4):812–9. Showed that elevated TNFR 1/2 at baseline predicts ESRD in diabetic American Indians
- 26.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol JASN. 2012March;23(3):516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008April;294(4):F697–701. [DOI] [PubMed] [Google Scholar]
- 28.Rovin BH, Rumancik M, Tan L, Dickerson J. Glomerular expression of monocyte chemoattractant protein-1 in experimental and human glomerulonephritis. Lab Investig J Tech Methods Pathol. 1994October;71(4):536–42. [PubMed] [Google Scholar]
- 29.Verhave JC, Bouchard J, Goupil R, Pichette V, Brachemi S, Madore F, et al. Clinical value of inflammatory urinary biomarkers in overt diabetic nephropathy: a prospective study. Diabetes Res Clin Pract. 2013September;101(3):333–40. [DOI] [PubMed] [Google Scholar]
- 30.Titan SM, Vieira JM, Dominguez WV, Moreira SRS, Pereira AB, Barros RT, et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications. 2012December;26(6):546–53. [DOI] [PubMed] [Google Scholar]
- 31. Nadkarni GN, Rao V, Ismail-Beigi F, Fonseca VA, Shah SV, Simonson MS, et al. Association of Urinary Biomarkers of Inflammation, Injury, and Fibrosis with Renal Function Decline: The ACCORD Trial. Clin J Am Soc Nephrol CJASN. 2016May17; ** Showed that urinary MCP1 is associated with longitudinal renal decline
- 32.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet Lond Engl. 2005April2;365(9466):1231–8. [DOI] [PubMed] [Google Scholar]
- 33.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012September;82(5):516–24. [DOI] [PubMed] [Google Scholar]
- 34.Bhavsar NA, Köttgen A, Coresh J, Astor BC. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis Off J Natl Kidney Found. 2012August;60(2):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Bonventre JV, Sabbisetti V, et al. Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia. 2015January;58(1):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Foster MC, Coresh J, Bonventre JV, Sabbisetti VS, Waikar SS, Mifflin TE, et al. Urinary Biomarkers and Risk of ESRD in the Atherosclerosis Risk in Communities Study. Clin J Am Soc Nephrol. 2015November6;10(11):1956–63. * Tested associations of urinary biomarkers and ESRD in the ARIC cohort
- 37.Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care. 2010December;16(6):556–61. [DOI] [PubMed] [Google Scholar]
- 38.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol CJASN. 2013July;8(7):1079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peralta CA, Katz R, Bonventre JV, Sabbisetti V, Siscovick D, Sarnak M, et al. Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis Off J Natl Kidney Found. 2012December;60(6):904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Bonventre JV, Sabbisetti V, et al. Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia. 2015January;58(1):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011June15;305(23):2432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol JASN. 2014February;25(2):349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol JASN. 2011October;22(10):1913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yilmaz G, Ustundag S, Temizoz O, Sut N, Demir M, Ermis V, et al. Fibroblast Growth Factor-23 and Carotid Artery Intima Media Thickness in Chronic Kidney Disease. Clin Lab. 2015;61(8):1061–70. [DOI] [PubMed] [Google Scholar]
- 45. Isakova T, Ix JH, Sprague SM, Raphael KL, Fried L, Gassman JJ, et al. Rationale and Approaches to Phosphate and Fibroblast Growth Factor 23 Reduction in CKD. J Am Soc Nephrol JASN. 2015October;26(10):2328–39. * Rationale for the COMBINE study
- 46.Lin C-J, Wu V, Wu P-C, Wu C-J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PloS One. 2015;10(7):e0132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, et al. Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PloS One. 2016;11(1):e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faubel S, Edelstein CL. Caspases as drug targets in ischemic organ injury. Curr Drug Targets Immune Endocr Metab Disord. 2005September;5(3):269–87. [DOI] [PubMed] [Google Scholar]
- 49.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis Off J Natl Kidney Found. 2004March;43(3):405–14. [DOI] [PubMed] [Google Scholar]
- 50.Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, et al. Elevated Urinary IL-18 Levels at the Time of ICU Admission Predict Adverse Clinical Outcomes. Clin J Am Soc Nephrol CJASN. 2010August;5(8):1497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, et al. Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr 1999. 2012December15;61(5):565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kalaitzidis RG, Duni A, Siamopoulos KC. Klotho, the Holy Grail of the kidney: from salt sensitivity to chronic kidney disease. Int Urol Nephrol. 2016May23; * Review article on Klotho
- 53. Xie J, Yoon J, An S-W, Kuro-o M, Huang C-L. Soluble Klotho Protects against Uremic Cardiomyopathy Independently of Fibroblast Growth Factor 23 and Phosphate. J Am Soc Nephrol JASN. 2015May;26(5):1150–60. * Shows protective effects of Klotho for uremic cardiomyopathy
- 54.Chen J, Hamm LL, Mohler ER, Hudaihed A, Arora R, Chen C-S, et al. Interrelationship of Multiple Endothelial Dysfunction Biomarkers with Chronic Kidney Disease. PloS One. 2015;10(7):e0132047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghoul BE, Squalli T, Servais A, Elie C, Meas-Yedid V, Trivint C, et al. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin J Am Soc Nephrol CJASN. 2010February;5(2):205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ix JH, Biggs ML, Mukamal K, Djousse L, Siscovick D, Tracy R, et al. Urine Collagen Fragments and CKD Progression-The Cardiovascular Health Study. J Am Soc Nephrol JASN. 2015October;26(10):2494–503. ** Showed association of urine collagen fragments and CKD outcomes
- 57. Sekula P, Goek O-N, Quaye L, Barrios C, Levey AS, Römisch-Margl W, et al. A Metabolome-Wide Association Study of Kidney Function and Disease in the General Population. J Am Soc Nephrol JASN. 2016April;27(4):1175–88. * Association of metabolites and kidney function
- 58. Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, et al. A Combined Epidemiologic and Metabolomic Approach Improves CKD Prediction. J Am Soc Nephrol. 2013August1;24(8):1330–8. * Showed how metabolites might improve CKD prediction
- 59. Glorieux G, Mullen W, Duranton F, Filip S, Gayrard N, Husi H, et al. New insights in molecular mechanisms involved in chronic kidney disease using high-resolution plasma proteome analysis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2015November;30(11):1842–52. * Showed how unbiased proteomics lends insight into CKD mechanisms
- 60.Argilés Á, Siwy J, Duranton F, Gayrard N, Dakna M, Lundin U, et al. CKD273, a new proteomics classifier assessing CKD and its prognosis. PloS One. 2013;8(5):e62837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siwy J, Schanstra JP, Argiles A, Bakker SJL, Beige J, Boucek P, et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2014August;29(8):1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015December2;7(316):316ra193. ** Tissue transcriptome driven identification of EGF as a biomarker
- 63.Parsa A, Kao WHL, Xie D, Astor BC, Li M, Hsu C, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013December5;369(23):2183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swan AL, Stekel DJ, Hodgman C, Allaway D, Alqahtani MH, Mobasheri A, et al. A machine learning heuristic to identify biologically relevant and minimal biomarker panels from omics data. BMC Genomics. 2015January15;16(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]


