Abstract
Several PEGylated therapeutic proteins are approved drugs, and more are under development. However, the synthesis and characterization of these bioconjugates, especially heterogeneous mixtures of PEGylated proteins, are challenging. The present study focuses on the development of PEG linkers that can be installed through biocatalytic route and render much simpler and insightful analytical characterization of PEG–protein conjugates. This linker enables traditional peptide mapping assay to determine protein sequence coverage, natural PTMs, and PEG attachment sites. Novel PEG linkers are cleavable during traditional sample preparation, leaving behind reporter amino acids to allow the determination of PEG attachment sites by peptide mapping. Products of transglutaminase-catalyzed bioconjugation of 5K PEG to Interferon α-2b were analyzed, and K31, K134, and K164 were identified as the PEGylation sites; the former two being newly determined sites demonstrates the sensitivity of the approach. In another instance, conjugation sites on Interleukin-2-PEG conjugation were found to be K31, K47, K48, and K75.
Keywords: PEGylated proteins, bioconjugation, PEG linker, Interferon α-2b, peptide mapping
Polyethylene glycols (PEGs) are water-soluble polymers which are often attached to a drug molecule (peptide, protein, nucleotides) to improve a drug’s pharmacological properties.1,2 PEGs have been approved by Food and Drug Administration (FDA) for intravenous, topical, and oral formulations.1,3 Several PEGylated proteins have been developed and commercialized since the 1990s. Some of the prominent marketed PEGylated proteins include Neulasta, a mono-PEGylated granulocyte colony-stimulating factor (20K PEG); and PEG-Intron- Interferon α2b-12K PEG, which has been proven to be more effective than their non-PEGylated counterparts. While great progress has been made toward the development of PEGylated bioconjugates, their characterization continues to pose challenges because of the polydispersity of the PEG chains. The issue is further exacerbated because of the heterogeneous mixture of PEGylated protein conjugates where the therapeutic protein is conjugated to a variable number of PEG chains. The site-selective conjugation methods, both enzymatic and nonenzymatic, have been developed recently to provide homogeneous PEGylated protein conjugates.4−7 For instance, transglutaminase is found to be very effective for site-selective conjugation.8−12 While advances have been made to address synthetic challenges, analytical hurdles remain and pose risk to advance these complex molecules through the drug development pipeline. In most cases, orthogonal approaches are needed to characterize the PEG-protein conjugate and ensure quality control, which is cumbersome and often challenging for routine implementation.13 Among all analytical challenges, determination of PEG attachment site has been most challenging, especially, when analyzing a mixture composed of products with varying degree of PEGylation. Unambiguous determination of PEG attachment site is a regulatory requirement for PEG-protein conjugate characterization.14 PEG attachment site is also important in the discovery space during structure–activity-relationship (SAR) studies and often possess a bottleneck in developing these types of molecules.
The present study addresses the analytical problem by leveraging designer PEG linkers which are cleavable during traditional peptide mapping sample preparation steps. As a result, traditional workflows for PTM analysis can be utilized to determine PEG attachment sites. The linker strategy described herein holds significant potential to expedite the discovery and development of new PEGylated therapeutic proteins. In the discovery phase, thousands of variants are screened for SAR studies, reaction optimization, and so on. Often, determination of the PEG attachment site is critical, but the level of expertise and manual data analysis that are needed with existing methodologies have hampered the progress of these programs. Adopting this linker can enable automated data analysis and high-throughput screening. These cleavable linkers allow independent analysis of protein and PEG units, thereby reducing the analytical challenges.
Matrix-assisted laser desorption/ionization (MALDI) has been traditionally used to estimate the number of PEGs attached to a protein. Polydispersity of PEG and overlapping protein charge states make intact mass analysis of PEG-protein conjugates complicated by ESI. Limited charge reduction coupled with collision-induced dissociation is an emerging LC-MS-based technique that has shown promise in the characterization of PEG-protein conjugates.15 Methods used to determine PEG attachment sites include a traditional peptide mapping strategy combined with in-source fragmentation or decay to detach the PEG chain from peptides or a top-down approach where multiple fragmentation steps are utilized (MSE approach).16−18 In-source decay or the MSE approach requires considerable method development and expertise. In some cases, multiple fragmentation steps are carried out by using a combination collision induced dissociation (CID) and electron capture dissociation (ECD) to locate the PEG attachment site.18,19 Data analyses in these studies are often manual and very time-consuming, especially because the cleavage point on PEG can vary depending on the energy as well as the type of fragmentation (CID or ECD). Manual data analysis can be a significant pain point given the huge number of samples generated during preclinical and clinical development. To avoid the above complexities in MS data interpretation, the loss of the peptide peak in LC-MS data has been used as an indirect way to determine PEG attachment sites.8,9,20 In this case, the loss of the peptide peak is only an in-direct method with no direct evidence to support conclusion. The above description highlights the need for better strategy to develop these types of molecules and can be tackled by better design of linkers as presented in this work.
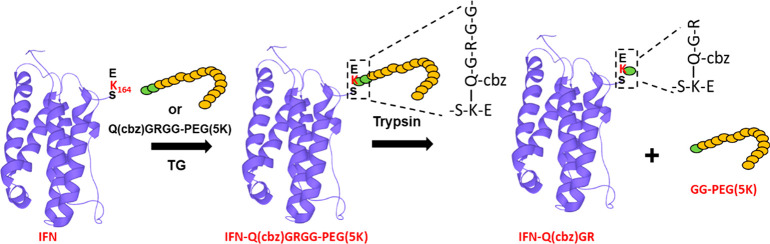
The cleavable linkers, cbzQGRGG-PEG and cbzQGEGG-PEG (Figure 1b–e), can be cleaved at arginine or glutamine residue during trypsin or GluC digestion step, leaving a short reporter amino acid chain-cbzQGR/cbzQGE attached to the protein on the PEG attachment site (as depicted in Figure 1a). This reporter amino acid chain-cbzQGR/cbzQGE can be searched in peptide mapping data (using commercial peptide mapping software) to determine PEG attachment sites along with common post-translational modifications (PTMs) like deamidation and oxidation. Previous studies have explored similar cleavable linker strategies by designing new PEGs that leave reporter residues on a protein, but they required additional steps to cleave off PEG. The reagents used for these cleavage processes could also cause side reactions on the protein methionine residues and further complicate the analysis.21 Additionally, some of these reporter residues were bonded to PEG by labile bonds that are prone to degradation in vivo. The PEG linkers designed in this study comprise natural amino acids and may provide a robust and stable alternative.
Figure 1.
(a) Schematic showing cleavage of our designer PEG linker which leaves behind the reporter amino acid chain Q(cbz)GR for determination of PEG attachment site by peptide mapping. (b–e) Chemical structures of designer PEG linkers. IFN structure was downloaded from UniProt
In this study, we utilized a well-established chemistry published by Fontana and co-workers to test our linkers—transglutaminase-mediated conjugation of Interferon α-2b (IFN).12
Materials and Methods
Materials
IFN was procured from Sigma-Aldrich, TG was obtained from Ajinomoto (trade name of the enzyme is ACTIVA TI), all the PEG linkers were custom designed in-house and prepared by out-sourcing to GenScript. The PEG linkers synthesized at Genscript are cbzQGRGG{PEG10-methylcarboxylic acid}, cbzQGEGG{PEG10-methylcarboxylester}, cbzQGRGG{cys(PEG(5K))}, and cbzQGEGG{cys(PEG(5K))} where PEG10 refers to the PEG chain length and PEG(5K) refers to the molecular weight of the PEG chain. For the sake of consistency, PEG10 will be referred to as PEG 0.5K as the molecular weight of PEG10 is ∼0.5K. Structures of all the PEG linkers can be found in Figure 1b–d. The molecular weight of the 5K PEG linkers are roughly 5.5 kDa (including the amino acid chain). Reagents for peptide mapping and MALDI such as dithiothreitol, iodoacetamide, formic acid, 2,6-dihydroxyacetophenone were procured from Sigma-Aldrich. Trypsin and GluC were obtained from New England Biolabs.
Transglutaminase (TG)-Mediated Conjugation of IFN
The reaction conditions were adopted from Fontana and co-workers’ report.12 IFN, linker, and TG were mixed in 1:20:1 ratio for the reaction. A 100 mM MOPs buffer at pH 7.5 was used as the reaction buffer. Then 10 μM of protein and TG stock were prepared in MOPs and mixed with 1 mM linker stock in the ratio of 1:20:1 (IFN: linker:TG) and incubated at 37 °C for 6 h, followed by a room-temperature incubation overnight.
Intact Mass Analysis
The crude reaction mixture was injected into an Agilent 6560 Ion-mobility Q-TOF for LC-MS analysis. The mass spectrometer was used in Q-TOF mode only. A BEH C18, 2.1 × 150 mm, 1.7 um column, 0.1% formic acid (mobile phase A) and acetonitrile were used for chromatography. About 5 ug of IFN reaction mixture was injected, the temperature of the column was maintained at 60 °C, and a flow rate of 0.3 mL/min was used for chromatography. The gradient used for chromatography is as follows: a gradient of 0–60% B over 30 min and then to 80% B over 5 min was used. The mass range for a full MS scan was 300–3000 m/z. Agilent Bioconfirm software was used for deconvolution.
Peptide Mapping Analysis
The crude reaction mixture was buffer exchanged into 50 mM ammonium bicarbonate (pH 7.8) by using 10 kDa MWCO filters. The conjugated proteins were then treated with 5 mM dithiothreitol (DTT) for 60 min and then 20 mM iodoacetamide for 30 min (in dark) to reduce and alkylate the disulfide bonds. Subsequently, the denatured proteins were digested using trypsin (6 h at 37 °C) or GluC (18 h at 37 °C). The samples were acidified to pH 2 with 10% formic acid and then lyophilized and resuspended in 0.1% formic acid for LC-MS injection. A BEH C18 peptide, 2.1 × 150 mm, 1.7 um column was used for chromatography. For the procedure, 0.1% formic acid–water mobile phase A, 0.1% formic acid-acetonitrile mobile phase B, flow rate of 0.3 mL/min, and column temperature of 40 °C were used.
About 6 μg of protein was injected for each LC-MS run. A gradient of 0–60% B over 30 min and then to 80% B over 5 min was used (2 min postrun for column equilibration).
A Thermo Q-Exactive Plus was used with data-dependent MS acquisition. Positive ion mode and full MS scan range of 300–1500 m/z were used. The AGC target was set at 3 × 106 for MS1 and 5 × 105 for MS2. A dynamic exclusion of 30 s and an isolation window of 2 m/z were used. Monoisotopic precursor selection was enabled, and peaks with +2 to +5 charge were preferentially chosen for MS2. Stepped collision energies of 25 and 28 were used for CID.
The data was searched using Thermo BioPharma Finder 3.1. IFN and TG sequences were obtained from the supplier. A fasta file was created with IFN and TG sequences and used for peptide searches. Peptide mapping data was searched with default error tolerances, and nonspecific modifications were removed from the search. Variable modification of methionine oxidation and deamidation were included in search parameters. The data was searched for unlimited missed cleavages and FDR of 0.1%. MS2 fragments for peptide ID were searched in Xcalibur software.
Matrix-Assisted Laser Desorption/Ionization
The protein bioconjugate was purified by washing three times with water using 10K MWCO ultracentrifugation filters. One mg/mL of the conjugate mixture was mixed with 10 mg/mL 2,6-dihydroxyacetophenone (DHAP) matrix in 1:2 ratio (v/v). DHAP was prepared in 1:1 ACN-water (0.05% TFA). The MALDI spot was prepared by dried droplet method and spotted on a Bruker Anchorchip 384 BC target. A Bruker Autoflex MALDI-TOF was used for acquisition.
Peptide mapping is typically performed on most biologics to characterize the levels of deamidation and oxidation on the protein. A designer PEG linker can enable the determination of PEG attachment sites on proteins, a critical quality attribute (CQA) of PEGylated therapeutic proteins, using the same peptide mapping assay. This study takes advantage of the recent breakthroughs in PEGylated protein synthesis through transglutaminase chemistry and demonstrates how a small change in PEG-linker design can significantly reduce the complexity of PEG-protein bioconjugate characterization.
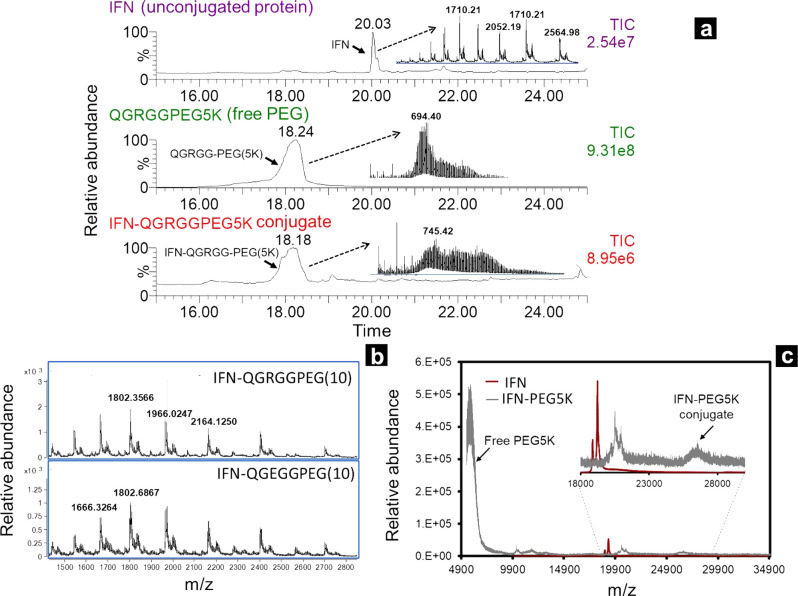
Transglutaminase chemistry enables a bond formation between a lysine and a glutamine residue (Figure S1). Keeping TG chemistry in mind, we designed our linker to constitute a cbz protected glutamine residue followed by a glycine residue and then an arginine or a glutamic acid (cbzQGRGG or cbzQGEGG). The glutamine residue on the linker will conjugate to lysine residues on the protein. We incorporated three glycines (G) in the linker chain (Figure 1b–e) to spatially distance the enzymatic cleavage site from the conjugation site and PEG chain to prevent interference. A systematic study may be required to understand if the glycine residues are necessary and will be a subject of a separate study. IFN served as a good substrate for our study because TG-catalyzed conjugation of PEG at K164 of the IFN is well established.12 In addition, PEGylated IFN is a commercially marketed drug and a perfect case study. PEG chains of two different sizes (0.5K and 5K) were conjugated to IFN. Analysis of the crude reaction mixture by LC-MS revealed the formation of IFN-PEG conjugate as seen from the shift of m/z in Figures 2a,b and Figure S2. Analysis of PEG(0.5K) conjugates were relatively simple but only provided evidence for formation of one IFN-PEG conjugate, that is, IFN conjugated to one PEGylated linker (Figures 2b, S2). Intact mass of IFN-PEG(0.5K) conjugate could be easily deconvoluted (Figure S2) from well-resolved peaks as shown in Figure 2b. Well-resolved peaks for PEG-protein conjugates are achievable from small linkers such as PEG(0.5K), but the product of IFN and 5K PEG conjugation reactions were difficult to analyze by LC-MS (Figure 2a). Hence these conjugation products were subjected to MALDI-MS, which again showed evidence of only one IFN-PEG conjugate (Figure 2c). Of note, free 5K PEG and 5K PEG-protein conjugates were not separated by reverse-phase conditions, making it hard to determine product formation by LC-MS. When subjected to peptide mapping analysis, the conjugate formation was more readily detected with QGRGG-PEG or QGEGG-PEG linkers as discussed below.
Figure 2.
(a) Intact mass analysis of IFN (top), free 5K PEG linker (middle), and IFN-PEG(5K) conjugate (bottom). (b) Intact mass analysis of IFN-PEG(0.5K) conjugate showing clear charge state distribution. Products of PEG(0.5K) conjugation are easy to analyze by intact mass as clear and well-resolved peaks are obtained. (c) MALDI-MS data showing formation of IFN-PEG(5K) conjugate.
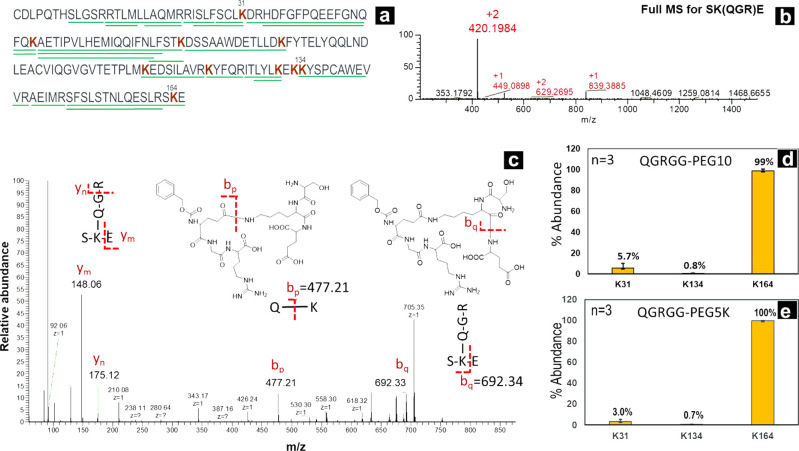
When the reaction mixtures were subjected to trypsin or GluC digestion and peptide mapping, >70% sequence coverage was achieved as shown in Figures 3a and S3. K164 was conjugated with PEG linker very efficiently (>99%) by transglutaminase chemistry (Figure 3d,e). This data was easily attainable by searching for cbz-QGR modification on IFN using standard proteomics data analysis software such as Biopharma Finder. The utilization of commercial proteomics data analysis software tremendously simplified and reduced the time required for data analysis. This type of automated data analysis may not be possible if a noncleavable PEG linker is used because of the polydispersity of 5K PEG. Figure 3b shows the full MS of peptide SKE containing the QGR modification on K164 at m/z 420.19 (+2). Figure 3c shows MS2 data for the 420.19 peak, which proves the formation of the SK(QGR)E peptide post-trypsin digestion. Especially, ion bq proves the formation of the isopeptide bond between lysine of IFN and glutamine of PEG linker.
Figure 3.
(a) Sequence coverage map of IFN-PEG(5K) conjugate showing coverage of peptides (tryptic) conjugated to PEG. This is unlike traditional methods where the loss of the peptide signal is used to determine conjugation sites. Full MS (b) and MS2 signal (c) of peptide SK(QGR)E clearly demonstrating formation of IFN-PEG conjugate and localizing conjugation site to K164. Peptide mapping enabled quantification of conjugation at K31, K134, and K164 of IFN. Relative quantification results from IFN-PEG(0.5K) and IFN-PEG(5K) conjugation are shown in plots (d) and (e), respectively.
The peptide mapping data also showed evidence for PEG modification at K31 and K134 (Figures 3d,e, S4). This forms the first report demonstrating enzymatic K134 modification and the only study showing that K31 and K134 can be modified with a bulky 5K PEG linker by TG catalysis. Detailed mass spectra analysis proving conjugation of K31 and K134 can be found in Supporting Information (Figure S4). These modifications were hard to detect from MALDI or intact mass analysis as we saw no trace of the +2 PEG conjugate of IFN. Given the small amount of conjugation at K31 and K134, and the limited sensitivity of MALDI at higher mass range, the peak for the +2 PEG conjugate of IFN may be hard to detect by traditional techniques. This new analytical strategy has made detection of higher PEG conjugate peaks easier and access to such information may prove critical.
Quantification of >99% conjugation on K164 was based on the relative abundance of peptide SK(QGR)E and SK/SKE. SK is a relatively short peptide and can be hard to retain on the column, making detection and quantification of the peptide tricky. To ensure our quantification data is not skewed by this artifact, we looked at GluC digest of IFN conjugated with QGEGG(PEG(0.5K)) and QGEGG(PEG(5K)). We were able to detect >95% conjugation at K164 from comparing the relative abundance of peptide SLRSK(QGE)E and SLRSKE demonstrating that K164 site is conjugated with high efficiency by TG. Surprisingly, we were not able to detect small amounts of conjugation at K34 and K134 for these reactions with QGEGG(PEG) linkers (Figure 4a). Most likely, modification on K34 was not detected because gluC digest can create a very large peptide fragment containing K34. This large peptide can be very hydrophobic and coelutes with the PEG chain; alternatively, such large chain peptides are hard to fragment by CID and hence not detected by peptide mapping.
Figure 4.
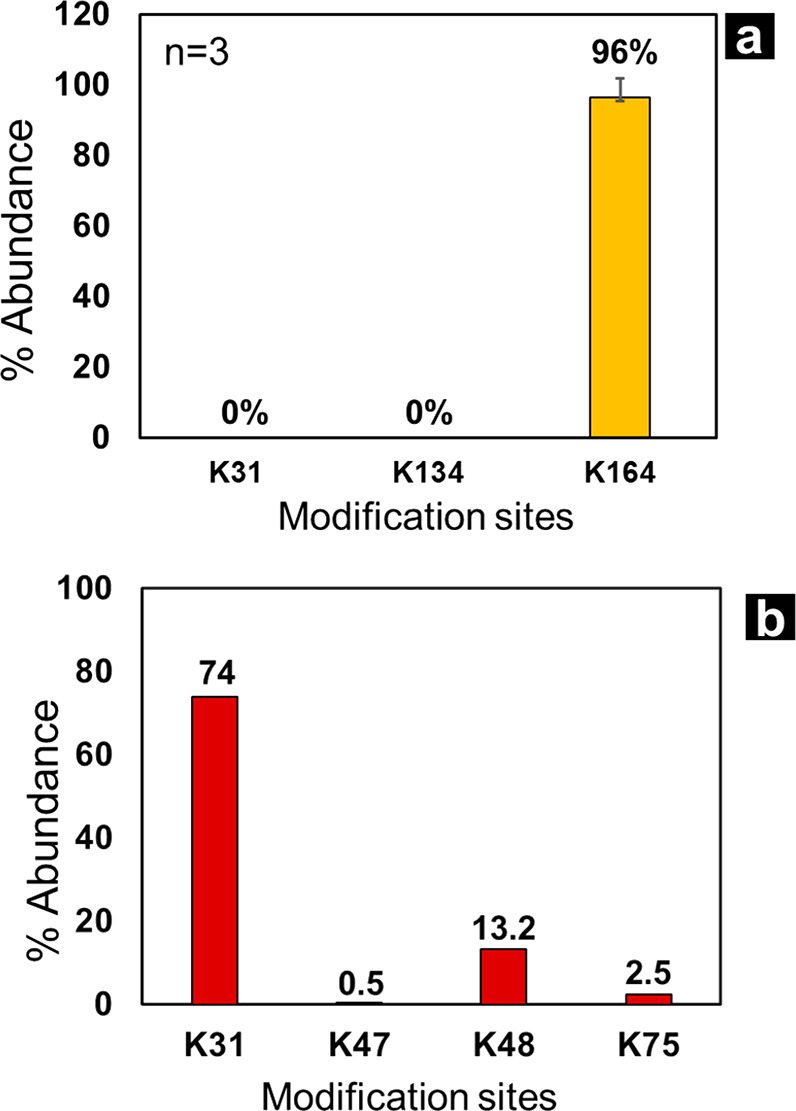
(a) Conjugation results from reacting IFN with QGEGG(PEG(5K)) linker, only K164 was conjugated with high efficiency (96%). (b) Plot showing conjugation efficiency of IL-2 and QGRGG-PEG(0.5K), four conjugation sites were detected, with K31 being the most efficient site (74% conjugation).
After successfully detecting PEG modifications on IFN, we employed the same strategy to determine which sites on Interleukin-2 (IL-2) can be modified by transglutaminase catalysis. We were able to detect four modification sites on IL-2 by using this methodology as shown in Figure 4b. K31, K47, K48, and K75 were the sites on IL-2 that were modified with the wildtype TG and QGRGGPEG(0.5K) proving that this analytical strategy can make detection of PEG modification sites on therapeutic proteins simpler and less time-consuming.
Cleavable PEG linkers were designed and studied that enabled decoupling of analytical complexity of protein and PEGs in a conjugate and enabled detection of PEG attachment sites using traditional peptide mapping workflow along with other CQAs like deamidation and oxidation. Utilization of this linker enables a more thorough characterization of PEG–protein conjugate mixtures. For instance, this study forms the first report of PEG modification on K134 of IFN by TG biocatalysis. Less than 1.0% of K134 is conjugated by TG biocatalysis, which was not identified by traditional methodologies before. This study proved that a bulky 5K PEG can conjugate to K134 and K31, and by careful design of the linker, they can be detected. This strategy allowed the determination of conjugation sites on IFN to be K31, K134, and K164, while IL-2 was conjugated at K31, K47, K48, and K75. Cleavable PEG linkers have been explored extensively in literature to deliver desired in vivo efficacy.22,23 Here, we demonstrate a similar concept that can be used to alleviate analytical challenges.
Acknowledgments
The authors would like to thank Hao Yang, Alycia Shoultz, and Jeffery Moore for discussions on transglutaminase biocatalysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00112.
Additional IFN-PEG conjugate characterization information (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Alconcel S. N. S.; Baas A. S.; Maynard H. D. (2011) FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym. Chem. 2, 1442–1448. 10.1039/c1py00034a. [DOI] [Google Scholar]
- Belén L. H.; Rangel-Yagui C. d. O.; Beltrán Lissabet J. F.; Effer B.; Lee-Estevez M.; Pessoa A.; Castillo R. L.; Farías J. G. (2019) From Synthesis to Characterization of Site-Selective PEGylated Proteins. Front. Pharmacol. 10, 1450. 10.3389/fphar.2019.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R. B. (2001) PEG drugs: an overview. J. Controlled Release 74, 159–171. 10.1016/S0168-3659(01)00331-5. [DOI] [PubMed] [Google Scholar]
- Shadish J. A.; DeForest C. A. (2020) Site-Selective Protein Modification: From Functionalized Proteins to Functional Biomaterials. Matter 2, 50–77. 10.1016/j.matt.2019.11.011. [DOI] [Google Scholar]
- Dozier J. K.; Distefano M. D. (2015) Site-Specific PEGylation of Therapeutic Proteins. Int. J. Mol. Sci. 16, 25831–25864. 10.3390/ijms161025831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaunak S.; Godwin A.; Choi J.-W.; Balan S.; Pedone E.; Vijayarangam D.; Heidelberger S.; Teo I.; Zloh M.; Brocchini S. (2006) Site-specific PEGylation of native disulfide bonds in therapeutic proteins. Nat. Chem. Biol. 2, 312–313. 10.1038/nchembio786. [DOI] [PubMed] [Google Scholar]
- Schumacher F. F.; Nobles M.; Ryan C. P.; Smith M. E. B.; Tinker A.; Caddick S.; Baker J. R. (2011) In Situ Maleimide Bridging of Disulfides and a New Approach to Protein PEGylation. Bioconjugate Chem. 22, 132–136. 10.1021/bc1004685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Freitas D.; Mero A.; Pasut G. (2013) Chemical and Enzymatic Site Specific PEGylation of hGH. Bioconjugate Chem. 24, 456–463. 10.1021/bc300594y. [DOI] [PubMed] [Google Scholar]
- Sato H. (2002) Enzymatic procedure for site-specific pegylation of proteins. Adv. Drug Delivery Rev. 54, 487–504. 10.1016/S0169-409X(02)00024-8. [DOI] [PubMed] [Google Scholar]
- Mero A.; Grigoletto A.; Maso K.; Yoshioka H.; Rosato A.; Pasut G. (2016) Site-selective enzymatic chemistry for polymer conjugation to protein lysine residues: PEGylation of G-CSF at lysine-41. Polym. Chem. 7, 6545–6553. 10.1039/C6PY01616B. [DOI] [Google Scholar]
- Sato H.; Yamamoto K.; Hayashi E.; Takahara Y. (2000) Transglutaminase-Mediated Dual and Site-Specific Incorporation of Poly(ethylene glycol) Derivatives into a Chimeric Interleukin-2. Bioconjugate Chem. 11, 502–509. 10.1021/bc990148b. [DOI] [PubMed] [Google Scholar]
- Spolaore B.; Raboni S.; Satwekar A. A.; Grigoletto A.; Mero A.; Montagner I. M.; Rosato A.; Pasut G.; Fontana A. (2016) Site-Specific Transglutaminase-Mediated Conjugation of Interferon α-2b at Glutamine or Lysine Residues. Bioconjugate Chem. 27, 2695–2706. 10.1021/acs.bioconjchem.6b00468. [DOI] [PubMed] [Google Scholar]
- González-Valdez J.; Rito-Palomares M.; Benavides J. (2012) Advances and trends in the design, analysis, and characterization of polymer–protein conjugates for “PEGylaided” bioprocesses. Anal. Bioanal. Chem. 403, 2225–2235. 10.1007/s00216-012-5845-6. [DOI] [PubMed] [Google Scholar]
- Bossard M. J.PEG–protein conjugates; regulatory requirements for characterization. In Polymer-Protein Conjugates; Pasut G., and Zalipsky S., Eds.; Elsevier: 2020; Chapter 7, pp 141–154. 10.1016/B978-0-444-64081-9.00007-3. [DOI] [Google Scholar]
- Muneeruddin K.; Bobst C. E.; Frenkel R.; Houde D.; Turyan I.; Sosic Z.; Kaltashov I. A. (2017) Characterization of a PEGylated protein therapeutic by ion exchange chromatography with on-line detection by native ESI MS and MS/MS. Analyst 142, 336–344. 10.1039/C6AN02041K. [DOI] [PubMed] [Google Scholar]
- Lu X.; Gough P. C.; DeFelippis M. R.; Huang L. (2010) Elucidation of PEGylation Site with a Combined Approach of In-Source Fragmentation and CID MS/MS. J. Am. Soc. Mass Spectrom. 21, 810–818. 10.1016/j.jasms.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Yoo C.; Suckau D.; Sauerland V.; Ronk M.; Ma M. (2009) Toward top-down determination of PEGylation site using MALDI in-source decay MS analysis. J. Am. Soc. Mass Spectrom. 20, 326–333. 10.1016/j.jasms.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Abzalimov R. R.; Frimpong A.; Kaltashov I. A. (2012) Structural characterization of protein–polymer conjugates. I. Assessing heterogeneity of a small PEGylated protein and mapping conjugation sites using ion exchange chromatography and top-down tandem mass spectrometry. Int. J. Mass Spectrom. 312, 135–143. 10.1016/j.ijms.2011.06.004. [DOI] [Google Scholar]
- Kaltashov I. A.; Bobst C. E.; Abzalimov R. R.; Wang G.; Baykal B.; Wang S. (2012) Advances and challenges in analytical characterization of biotechnology products: Mass spectrometry-based approaches to study properties and behavior of protein therapeutics. Biotechnol. Adv. 30, 210–222. 10.1016/j.biotechadv.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foser S.; Schacher A.; Weyer K. A.; Brugger D.; Dietel E.; Marti S.; Schreitmüller T. (2003) Isolation, structural characterization, and antiviral activity of positional isomers of monopegylated interferon α-2a (PEGASYS). Protein Expression Purif. 30 (1), 78–87. 10.1016/S1046-5928(03)00055-X. [DOI] [PubMed] [Google Scholar]
- Veronese F. M.; Saccà B.; Polverino de Laureto P.; Sergi M.; Caliceti P.; Schiavon O.; Orsolini P. (2001) New PEGs for Peptide and Protein Modification, Suitable for Identification of the PEGylation Site. Bioconjugate Chem. 12, 62–70. 10.1021/bc000061m. [DOI] [PubMed] [Google Scholar]
- Bailon P.; Won C.-Y. (2009) PEG-modified biopharmaceuticals. Expert Opin. Drug Delivery 6, 1–16. 10.1517/17425240802650568. [DOI] [PubMed] [Google Scholar]
- Fang Y.; Xue J.; Gao S.; Lu A.; Yang D.; Jiang H.; He Y.; Shi K. (2017) Cleavable PEGylation: a strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Delivery 24, 22–32. 10.1080/10717544.2017.1388451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.