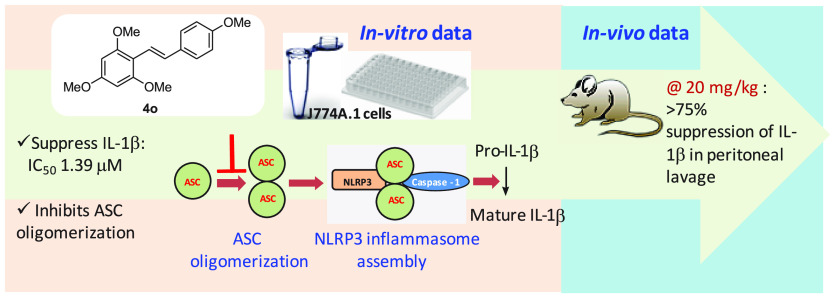
Abstract
Nucleotide-binding domain leucine-rich repeat family pyrin domain containing 3 (NLRP3) inflammasome complex regulates the caspase-1 activity and subsequent processing of interleukin-1β (IL-1β). Various inflammatory diseases involve the activation of inflammasome complexes; thus, the intervention in complex formation via small molecules offers a new therapeutic opportunity. The structure-guided design and synthesis of a series of methoxystilbenes and methoxy-2-phenylnaphthalenes identified new inhibitors of NLRP3 inflammasome complex. The tetramethoxystilbene 4o and trimethoxy 2-phenylnaphthalene 1t inhibit the release of a mature form of IL-1β in J774A.1 cells with IC50 values of 1.39 and 2.07 μM, respectively. Mechanistic investigation revealed that tetramethoxystilbene 4o blocks the oligomerization of apoptosis-associated speck-like protein (ASC), which is the vital step in the formation of NLRP3 inflammasome assembly, thus preventing the activation of caspase-1 and the IL-1β release. Treatment of LPS+ATP challenged mice with 20 mg/kg of 4o significantly suppressed the levels of IL-1β. The data presented herein warrant further investigation of methoxystilbenes in disease-specific models of inflammatory diseases.
Keywords: NLRP3 inflammasome, methoxystilbenes, 2-phenylnaphthalenes, IL-1β, ASC oligomerization
Inflammasomes are the high molecular protein complexes that play a central role in the innate immune system.1,2 They are activated in response to the pathogen-associated molecular pattern (PAMP) or damage-associated molecular pattern (DAMP) released during tissue injury or stress.1,2 Four inflammasomes have been characterized including NLRP1, nucleotide-binding domain leucine-rich repeat family pyrin domain containing 3 (NLRP3), nucleotide-binding domain leucine-rich repeat caspase recruitment domain-containing receptor 4 (NLRC4), and absent in melanoma 2 (AIM2).2,3 Among them, NLRP3 inflammasome is well-studied and accused of the pathogenesis for various diseases such as Alzheimer’s disease (AD),4 gout,5 type II diabetes,6 acute myocardial infarction,7 traumatic brain injury,8 multiple sclerosis,9 COVID-19,10,11 breast cancer,12 and also in cryopyrin-associated periodic syndrome.13 NLRP3 inflammasome complex comprises three important proteins viz. NLRP3 protein, an apoptosis-associated speck-like protein containing caspase recruitment domain (ASC) and procaspase-1.14,15 The NLRP3 protein further consists of three domains, each playing a specific role in the activation of inflammasome pathway. ASC recruits procaspase-1 thereby forming a NLRP3 inflammasome complex and the release of active caspase-1, which maturate IL-1β cytokine.16,17 The matured IL-1β is implicated in the pathogenesis of aforementioned diseases.18 Therefore, the NLRP3 inflammasome has been considered as a potential therapeutic target to impede the activation of IL-1β and ultimately stop the progression of various inflammatory diseases.19 During past few years, many small molecules were discovered that block the NLRP3 inflammasome pathway, including MCC950,20 oridonin,21 tranilast,22 CP424174,23 BAY11-7082,24 INF39,25 FC11A-2,26 TAK242,27 MNS,28 glyburide,29 JC124,30 YQ128,31 quinazolinone,32 IIIM-1266, and IIIM-1270 (Figure 1).33
Figure 1.
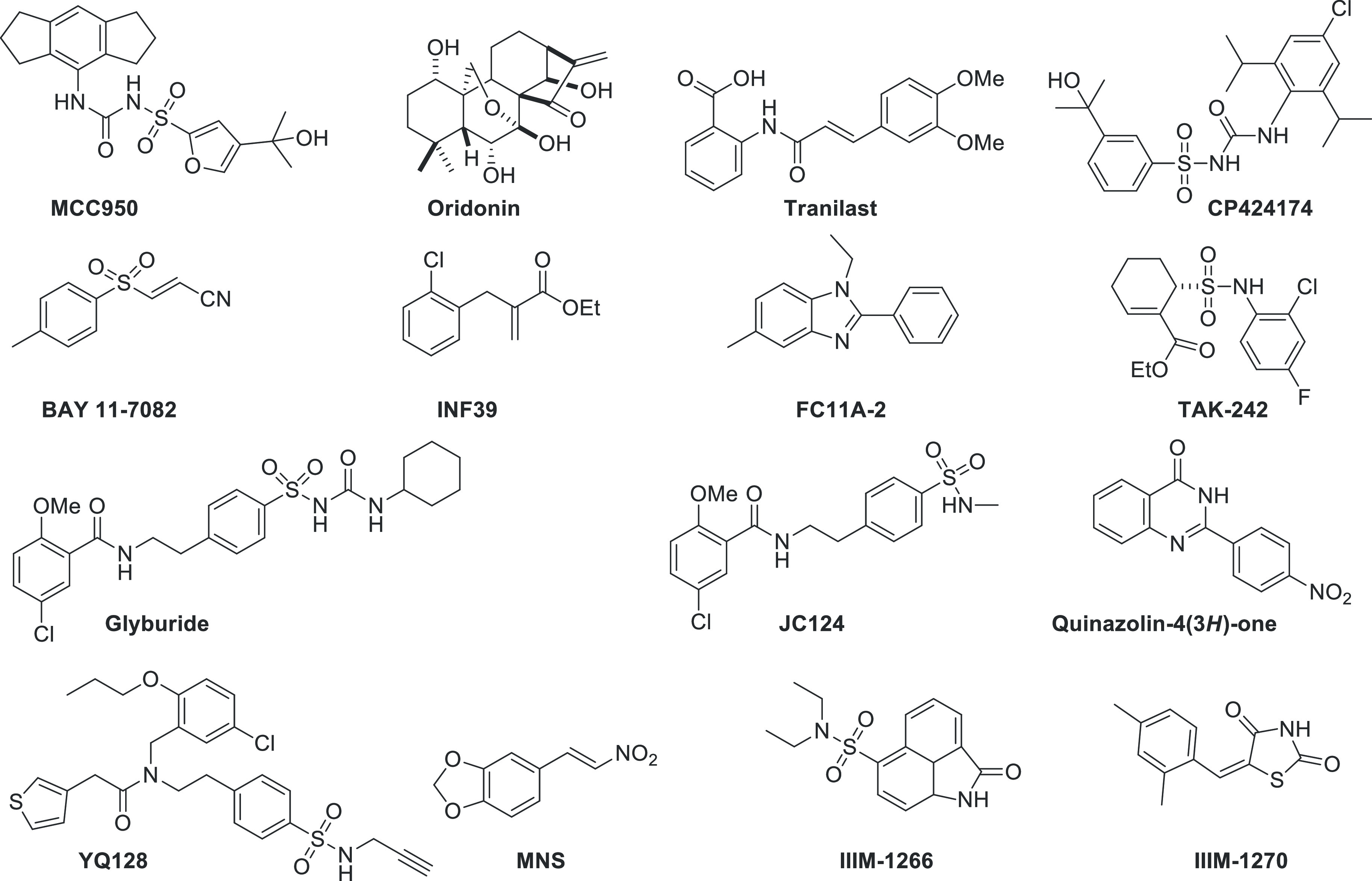
Chemical structures of some of the reported inhibitors of NLRP3 inflammasome complex.
As a part of our efforts in this area, recently, we reported a quinazolin-4(3H)-one and benzo[cd]indol-2-ones as NLRP3 inflammasome inhibitors.32,33 In continuation to these efforts, we were further interested in finding out new inhibitors of the NLRP3 inflammasome complex. The screening of our internal collection of small molecules in IL-1β release assay in J774.1 cells has provided us an interesting active compound IIIM-983 (1), a 2-(4′-hydroxy-phenyl)-7-hydroxynaphthalene. The structure-directed drug design followed by the synthesis of IIIM-983 analogs yielded methoxy-substituted stilbenes and 2-phenylnaphthalenes as NLRP3 inflammasome inhibitors. Herein, we report the detailed mechanistic investigation and in vivo validation of one of the most active compounds 4o.
Results and Discussion
Synthesis and Biological Evaluation of IIIM-983 (1)
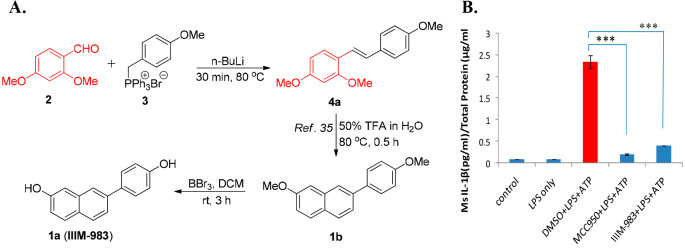
The 2-phenylnaphthalene 1 (IIIM-983), a known ER-β agonist,34 was previously synthesized in our laboratory35 using the scheme presented in Figure 2A. In this scheme, instead of using a conventional Suzuki coupling approach to generate 2-phenylnaphthalenes, we employed stilbene as a key precursor for trifluoroacetic acid (TFA)-mediated C–C bond cleavage followed by Diels–Alder cycloaddition chemistry.35 The desired stilbene intermediate 4a was constructed via Wittig reaction between dimethoxybenzaldehyde 2 and the Wittig salt 3. The treatment of trimethoxystilbene intermediate 4a with aqueous TFA produced 2-phenylnaphthalene 1b via TFA-catalyzed C–C bond cleavage followed by intermolecular [4+2]-Diels–Alder cycloaddition reaction between in situ formed diene and a dienophile.35 The demethylation of methoxy-substituted 2-phenylnaphthalene 1b yielded IIIM-983 (1a) (Figure 2A).
Figure 2.
Synthetic scheme for IIIM-983 (1a) (A) and its effect on IL-1β suppression in J774A.1 cells (B). The J774A.1 cells were first treated with compounds (10 μM) for 1 h and then with LPS (1 μg/mL) for 5.5 h, followed by ATP (3 mM, 30 min). Supernatants were analyzed for IL-1β by ELISA. The statistical comparisons were made, as shown in the figure by using one way ANOVA and post-hoc Bonferroni test.*p < 0.05; **p < 0.01; ***p < 0.005).
Meshaw et al.34 reported 1a as an potent and selective agonist of ER-β (EC50 44 nM). Based on the established inverse relation between ER-β and NLRP3 inflammasome,36 we envisaged the potential of 2-phenylnapthalene 1a and its analogs for inhibition of this complex. As a part of our internal library screening efforts, the compound 1a along with other available diverse sets of small molecules within the group were screened in a J774A.1 cell-based IL-1β secretion assay. MCC950 was used as a positive control in this assay. The J774A.1 mouse macrophage cell line was used to assess the release of IL-1β after activation of NLRP3 inflammasome upon stimulation with lipopolysaccharide (LPS) and adenosine triphosphate (ATP). The levels of secreted IL-1β were monitored by an enzyme-linked immunosorbent assay (ELISA). This screen has identified IIIM-983 (1a) as an inhibitor of IL-1β secretion (82% suppression) at 10 μM (Figure 2B).
Synthesis of Structural Analogs of IIIM-983
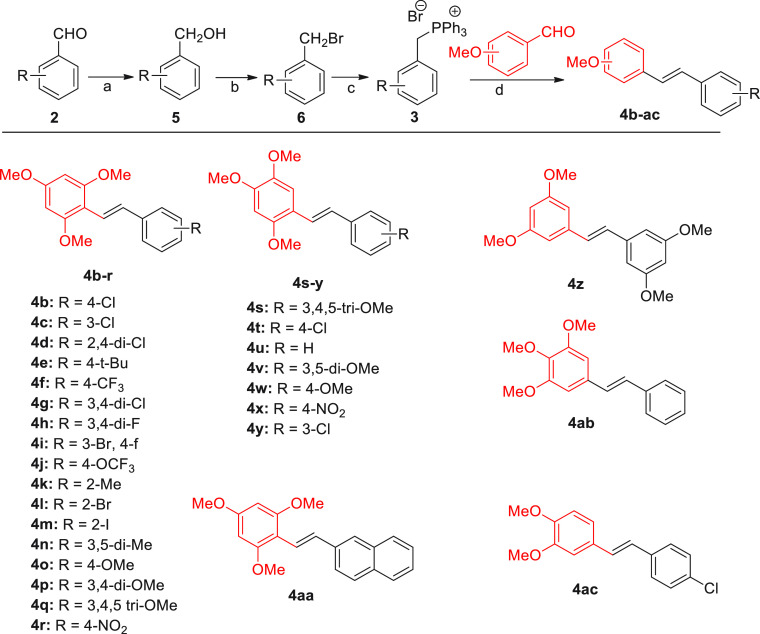
The structural diversification was planned via targeting two series of compounds viz. substituted phenylnaphthalenes and its open form (flexible) compounds, that is, stilbenes. The scheme for the synthesis of a series of stilbenes is shown in Figure 3. The aryl benzaldehydes 2 were reduced to benzyl alcohols 5 using sodium borohydride, which were further transformed to benzyl bromides 6 by reacting with N-bromosuccinamide. The Wittig salt 3 was then prepared by treating benzyl bromides 6 with triphenylphosphine. The Wittig reaction of triphenylphosphine salt 3 with methoxy-substituted benzaldehydes provided stilbenes 4b–4ac in >80% yield (Figure 3).
Figure 3.
Synthesis of methoxy-substituted stilbenes 4b–4ac. Reagents and conditions: (a) NaBH4 (1.5 equiv), MeOH, 0 °C, 30 min, >97% yield; (b) NBS, PPh3, DCM, 60 °C, 30 min; (c) PPh3, 80 °C, THF, 1 h, >97%; and (d) t-BuOK, dry THF, 80 °C, 1 h, >80% yield.
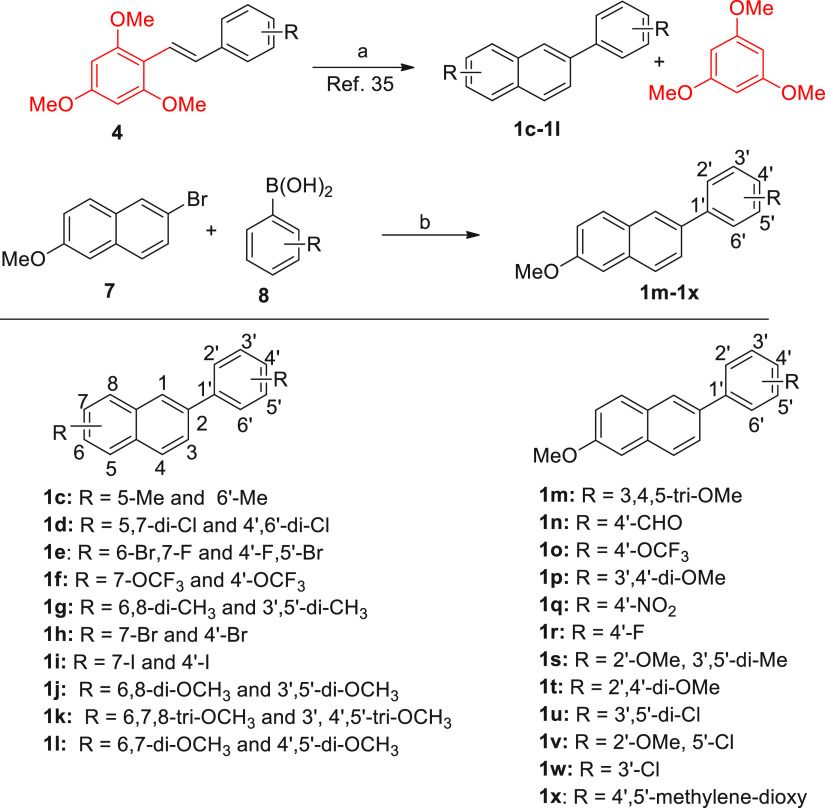
Next, we synthesized a series of 2-phenylnaphthalenes using two approaches. The first approach involved the use of stilbenes as a precursor as per Figure 1A, which produced 2-phenylnaphthalenes 1c–1l. In the second approach, the Suzuki coupling of 2-bromo-substituted naphthalene 7 with aryl boronic acids 8 provided 2-phenylnaphthalenes 1m–1x (Figure 4).
Figure 4.
Synthesis of 2-phenylnaphthalenes 1c–1x. Reagents and conditions: (a) TFA, water, 80 °C, 0.5 h, >90%; and (b) Pd(PPh3)4, CaCO3, dioxane, 80 °C, 6 h, >85%.
Biological Evaluation of a Series of Stilbenes and 2-Phenylnaphthalenes
The assay protocol employed in our initial investigations (Figure 2B) is the indirect assay for inflammasome inhibition, where cells were pretreated with test compounds and then with LPS. In contrast, the direct assay protocol, which we later utilized, consisted of first treating the J774A.1 cells with LPS to up-regulate the expression of NLRP3, and ultimately pro-IL-1β. Then, the cells were treated with test compounds. This change in assay protocol was desirable to make sure that the observed impact on IL-1β readout is primarily because of the intervention at one of the steps of the NLRP3 inflammasome pathway.
In the assay, LPS priming of the cells is required to upregulate the TLR4/NF-κB pathway. NF-κB is a transcription factor that enhances the expression of NLRP3 and pro-IL-1β, which are usually present at low concentrations in the resting macrophages. However, cleavage of pro-IL-1β takes place only after the assembly of NLRP3 inflammasome takes place in response to varied signals including ATP, nigericin, uric acid, single standard DNA, etc. One of the key changes that happen after exposure to these signals is the efflux of K+ ions, which triggers the assembly of NLRP3 inflammasome proteins to form a complex (NLRP3, ASC, and pro-caspase-1), which cleaves pro-caspase-1 to its active caspase-1 form. ATP is also reported to induce NLRP3 inflammasome assembly by causing efflux of potassium ions. It requires only few minutes to cause this change and hence cause cleavage of pro-IL-1β by caspase-1, thus triggering its release from the cells.37,38 Both of the steps, that is addition of LPS for 5.5 h and addition of ATP for 30 min prior to termination of the experiment, are necessary for this assay in macrophages like J774A.1.
IIIM-983 (1a) strongly suppressed the release of IL-1β when cells were treated first with a test compound, followed by the addition of LPS to cells. However, when it was screened in the direct assay of NLRP3 inflammasome (Figure 5B and Table 1), to our surprise, it was inactive. Rather, the levels of IL-1β were higher in 1a (+LPS+ATP)-treated cells compared with the cells treated only with LPS+ATP (Figure 5B). In this assay, the compound behaved like ATP; however, the detailed reason is unexplainable only based on these data. The repetition of the experiment multiple times provided a similar observation. This possibly indicates that the mechanism of suppression of IL-1β by IIIM-983 (1a) is not related to the NLRP3 inflammasome pathway. Next, we wanted to investigate two newly prepared analogs 4c (stilbene) and 1j (2-phenylnaphthalene) in both sets of assays to know whether they also behave like IIIM-983. The results presented in Figure 5 indicate that unlike IIIM-983 (1a), both new compounds suppressed IL-1β in both sets of assays. This has confirmed that new compounds follow the NLRP3 inflammasome pathway for suppressing the IL-1β secretion. Thus, for screening the rest of the series of compounds, the direct assay was employed.
Figure 5.
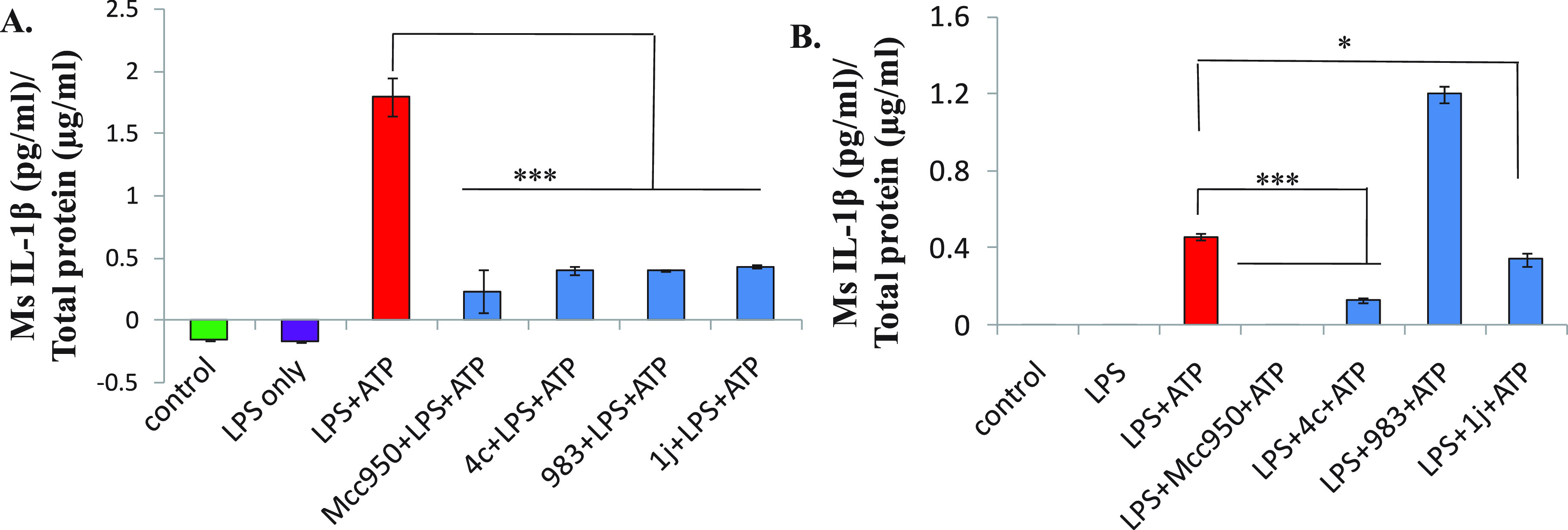
Screening of IIIM-983 (1a) and analogs 4c and 1j in indirect and direct assays of NLRP3 inflammasome activation. (A). Indirect assay: J774A.1 cells were first treated with compounds (10 μM) for 1 h and then with LPS (1 μg/mL) for 5.5 h, followed by ATP (3 mM, 30 min). Supernatants were analyzed for IL-1β by ELISA. (B) Direct assay: J774A.1 cells were treated with LPS (1 μg/mL) for 5.5 h. Cells were then treated with compounds (10 μM) for 1 h and then with ATP (30 min). Supernatants were analyzed for IL-1β by ELISA. Data shown here are an average of three independent experiments. The statistical comparisons were made as shown in the figure by using one-way ANOVA and post-hoc Bonferroni test. *p < 0.05; **p < 0.01; ***p < 0.005).
Table 1. In Vitro Suppression of IL-1β Release in J774A.1 Cells by Stilbenes 4b–4aca.
| entry | % suppression of IL-1β release at 10 μM [mean ± SD] | entry | % suppression of IL-1β release at 10 μM [mean ± SD] |
|---|---|---|---|
| 1a | –8.24 ± 1.02e | 4p | 29.07 ± 8.07b |
| 4b | 75.9 ± 0.01d | 4q | 62.8 ± 9.8d |
| 4c | 40.9 ± 0.71d | 4r | 14.3 ± 4.64e |
| 4d | 57.79 ± 1.35d | 4s | 32.2 ± 5.7c |
| 4e | 51.3 ± 3.75d | 4t | 2.9 ± 1.88e |
| 4f | 62.4 ± 1.57d | 4u | 5.5 ± 6.0e |
| 4g | 19.7 ± 0.9c | 4v | –64.6 ± 3.08d |
| 4h | 34.7 ± 0.73d | 4w | –29.9 ± 0.03b |
| 4i | 60.99 ± 6.25d | 4x | 7.9 ± 1.57e |
| 4j | 63.5 ± 10.08d | 4y | –22.84 ± 2.7e |
| 4k | 37.31 ± 1.28d | 4z | –4.5 ± 2.16e |
| 4l | 76.8 ± 6.54d | 4aa | 13.3 ± 3.69e |
| 4m | 35.9 ± 4.18d | 4ab | –62 ± 15.7d |
| 4n | 35.16 ± 1.99d | 4ac | 23.8 ± 6.07e |
| 4o | 76.25 ± 0.22d | MCC950 | 98 ± 0.13d |
J774A.1 cells were treated with LPS (1 μg/mL) for 5.5 h. Cells were then treated with compounds (10 μM) for 1 h and then with ATP (3 mM, 30 min). Supernatants were analyzed for IL-1β by ELISA. Data shown here are an average of three independent experiments. The statistical comparisons were made as shown by using one way ANOVA and post-hoc Bonferroni test.
p < 0.05.
p < 0.01.
p < 0.005.
Not significant.
All synthesized 51 analogs (28 stilbenes and 23 phenylnaphthalenes) were tested in a direct assay at 10 μM. MCC950 was used as a positive control in this assay. Among 28 stilbenes tested, the analogs bearing 2,4,6-trimethoxy substitution on one of the rings were found to be most active compared with any other substitution pattern of −OMe groups. Further, among the 2,4,6-trimethoxy-substituted stilbenes, those bearing 4-Cl (4b), 2,4-di-Cl (4d), 4-CF3 (4f), 3-Br, 4-F (4i), 4-OCF3 (4i), 2-Br (4l), 4-OMe (4o), and 3,4,5-tri-OMe (4q) functionalities on another ring were superior, showing >60% suppression of IL-1β release at 10 μM. The stilbene 4aa bearing 2,4,6-tri-OMe functionality on one ring but no substitution on another ring was only marginally active (13% suppression at 10 μM). The rest of the stilbenes were devoid of the 2,4,6-tri-OMe pattern and were found to be less active or inactive (Table 1).
From the 2-phenylnaphthalene series, four compounds were most active. The 2-phenylnaphthalene bearing halogen substitutions 1e and 1i (58% suppression) and those carrying 6-OMe group on naphthalene along with 4-NO2 or 4-OMe group on phenyl ring (1q, 1t) were the most active (58, and 76% suppression). Overall, the 6,2′,4′-trimethoxy-substituted 2-phenylnaphthalene 1t was the most active analog, displaying 76% suppression of IL-1β secretion (Table 2).
Table 2. In Vitro Suppression of IL-1β Release in J774A.1 Cells by 2-Phenylnaphthalenes 1c–1xa.
| entry | % suppression of IL-1β release at 10 μM [mean ± SD] | entry | % suppression of IL-1β release at 10 μM [mean ± SD] |
|---|---|---|---|
| 1a | –8.24 ± 1.02e | 1n | 20.6 ± 4.51c |
| 1c | –10.37 ± 2.09e | 1o | 2.18 ± 0.38e |
| 1d | 46.94 ± 2.07d | 1p | –97.44 ± 0.003d |
| 1e | 57.91 ± 4.64d | 1q | 58.19 ± 5.65d |
| 1f | 47.3 ± 3.13d | 1r | 30.18 ± 12.33d |
| 1g | 25.87 ± 9.76c | 1s | 0.79 ± 5.25e |
| 1h | 27.09 ± 1.41c | 1t | 75.98 ± 1.13d |
| 1i | 50.83 ± 9.57d | 1u | 22.46 ± 5.05c |
| 1j | 27.85 ± 3.6c | 1v | 14.33 ± 1.33e |
| 1k | –15.45 ± 7.24e | 1w | 5.69 ± 4.8e |
| 1l | –3.4 ± 6.47e | 1x | 26.41 ± 6.07d |
| 1m | –47.37 ± 9.03d | MCC950 | 98 ± 0.13d |
J774A.1 cells were treated with LPS (1 μg/mL) for 5.5 h. Cells were then treated with compounds (10 μM) for 1 h and then with ATP (3 mM, 30 min). Supernatants were analyzed for IL-1β by ELISA. Data shown here are the average from three independent experiments. Samples were compared statistically by using one way ANOVA and post-hoc Bonferroni testing.
p < 0.05.
p < 0.01.
p < 0.005.
Not significant.
The IC50 determination using the concentration range of 0.156–40 μM has shown that the two most active compounds, the stilbene 4o and 2-phenylnaphthalene 1t, suppressed the levels of IL-1β in J774A.1 cells with IC50 values of 1.39 and 2.0 μM, respectively. The cytotoxicity assessment in J774A.1 cells indicated that compound 4o is nontoxic up to 40 μM (Figure 6).
Figure 6.
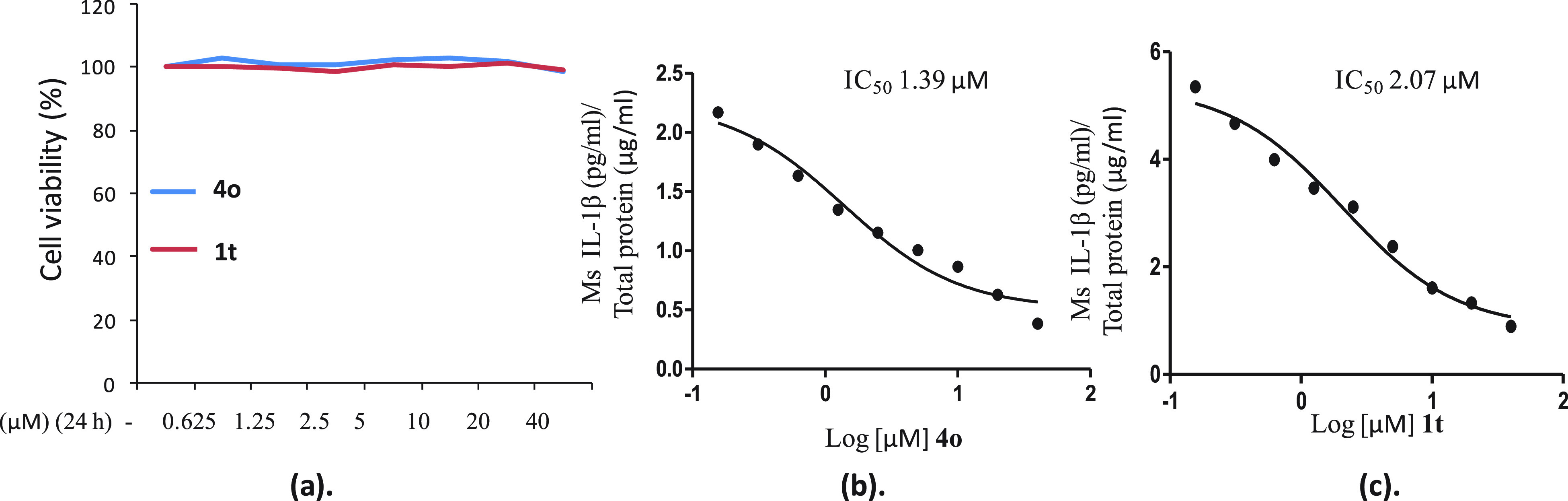
Cytotoxicity assessment and IC50 determination of 4o and 1t. (a) Cytotoxicity of 4o and 1t in J774A.1 cells. (b) Dose–response curve for 4o for suppression of IL-1β release. (c) Dose–response curve for 1t for suppression of IL-1β release.
The stilbene 4o and 2-phenylnaphthalene 1t were then investigated for their effect on the expression of various proteins associated with the NLRP3 inflammasome pathway. The proteins included in the western-blot study were pro-IL-1β, IL-1β, procaspase-1, caspase-1, and ASC. The Western blot results are shown in Figure 7. Both compounds were able to suppress the levels of cleaved forms of IL-1β and caspase-1 that are present in the cell supernatant. However, they were ineffective against procaspase-1, pro-IL-1β, ASC, and NLRP3 like MCC950. This possibly indicates that these compounds are not interacting with individual components of the NLRP3 inflammasome complex. Instead, they must be interacting at the stage of oligomerization of the complex or inhibiting the caspase-1, the downstream protein to the trimeric complex.
Figure 7.
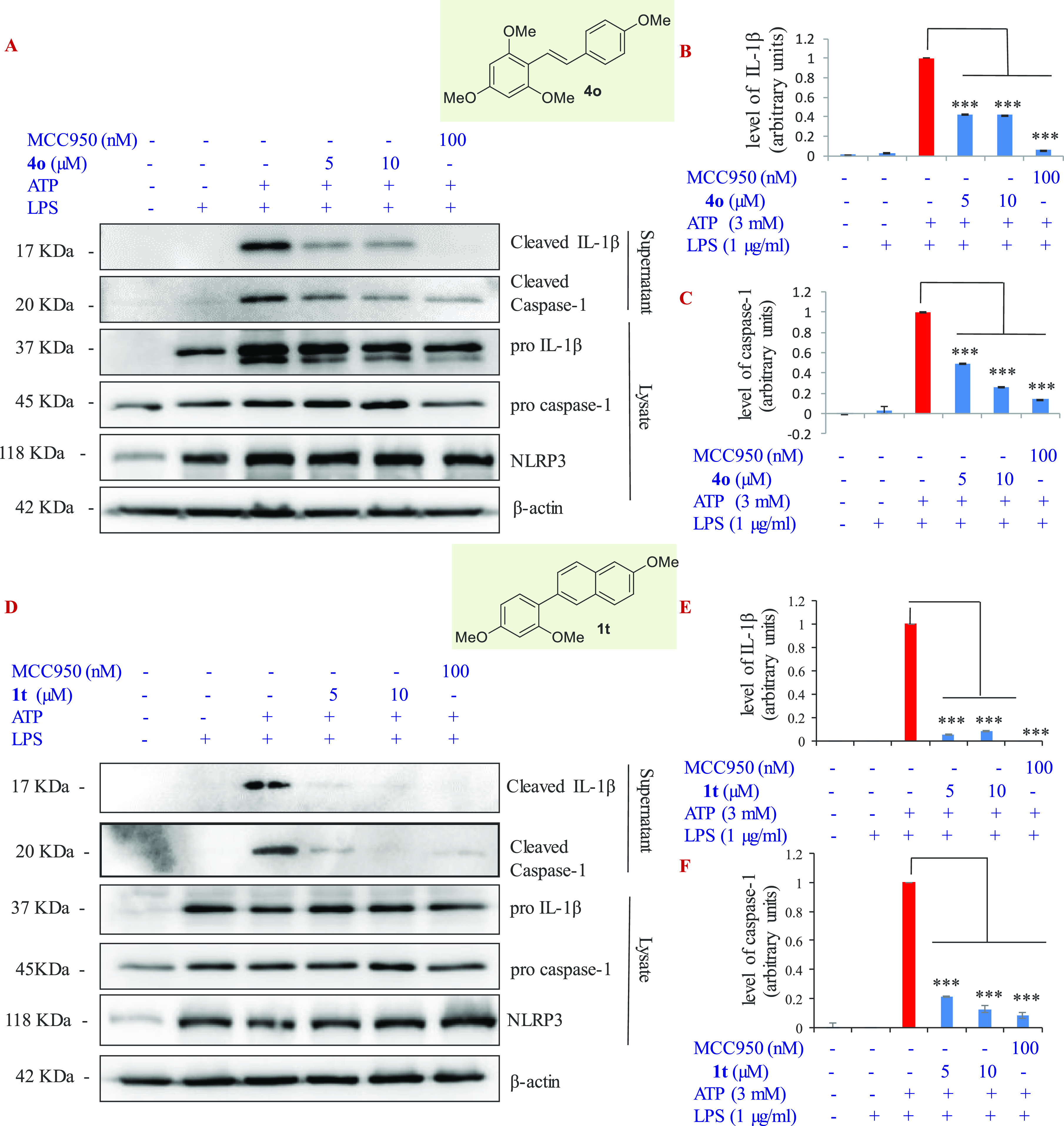
Western blot analysis of stilbene 4o and 2-phenylnaphthalene 1t to study their effect on the expression of IL-1β, pro-IL-1β, caspase-1 (in supernatants), pro-caspase-1, and NLRP3 (in cell lysates) from J774A.1 cells. The cells were stimulated with LPS (1 μg/mL) for 5.5 h, followed by treatment with test compounds (5 and 10 μM) for 1 h before ATP (3 mM, 30 min). (A) Western blot analysis results of stilbene 4o. (B) Quantitative data for levels of cleaved IL-1β upon treatment with stilbene 4o. (C) Quantitative data for levels of cleaved caspase-1 upon treatment with stilbene 4o. (D) Western blot analysis results of phenylnaphthalene 1t. (E) Quantitative data for levels of cleaved IL-1β upon treatment with phenylnaphthalene 1t. (F) Quantitative data for levels of cleaved caspase-1 upon treatment with phenylnaphthalene 1t. Quantification of Western blots was done by using ImageJ software, and statistical comparisons were made by using one way ANOVA and post-hoc Bonferroni test. *p < 0.05; **p < 0.01; ***p < 0.005.
The ASC oligomerization is a vital step in the formation of the NLRP3 inflammasome complex and further activation of the pathway; therefore, blocking the oligomerization step is an ideal approach toward suppressing the production of IL-1β cytokine. Coll et al.20 have shown that MCC950 inhibits the NLRP3 inflammasome via blocking the ASC oligomerization. Recently Dai et al.39 have also shown that MCC950 and tetrahydroquinoline derivative dose dependently inhibits ASC oligomerization. We, therefore, tested the effect of methoxystilbene 4o on the formation of ASC dimers and oligomers and used MCC950 as the positive control. J774A.1 cells were treated with compound 4o after LPS priming and before ATP stimulation. The formation of ASC dimers and oligomers was observed by Western blotting. The data showed that compound 4o had displayed concentration-dependent suppression of monomers, dimers, as well as oligomers. As shown in Figure 8, at 5 μM, 40–60% inhibition of all three forms was seen, whereas the treatment with 10 μM concentration resulted in complete inhibition of all three forms of ASC. The results thus indicate that 4o arrests the NLRP3 inflammasome activation by interfering with ASC oligomerization that results in the reduced levels of caspase-1 and subsequently the suppression of IL-1β release.
Figure 8.
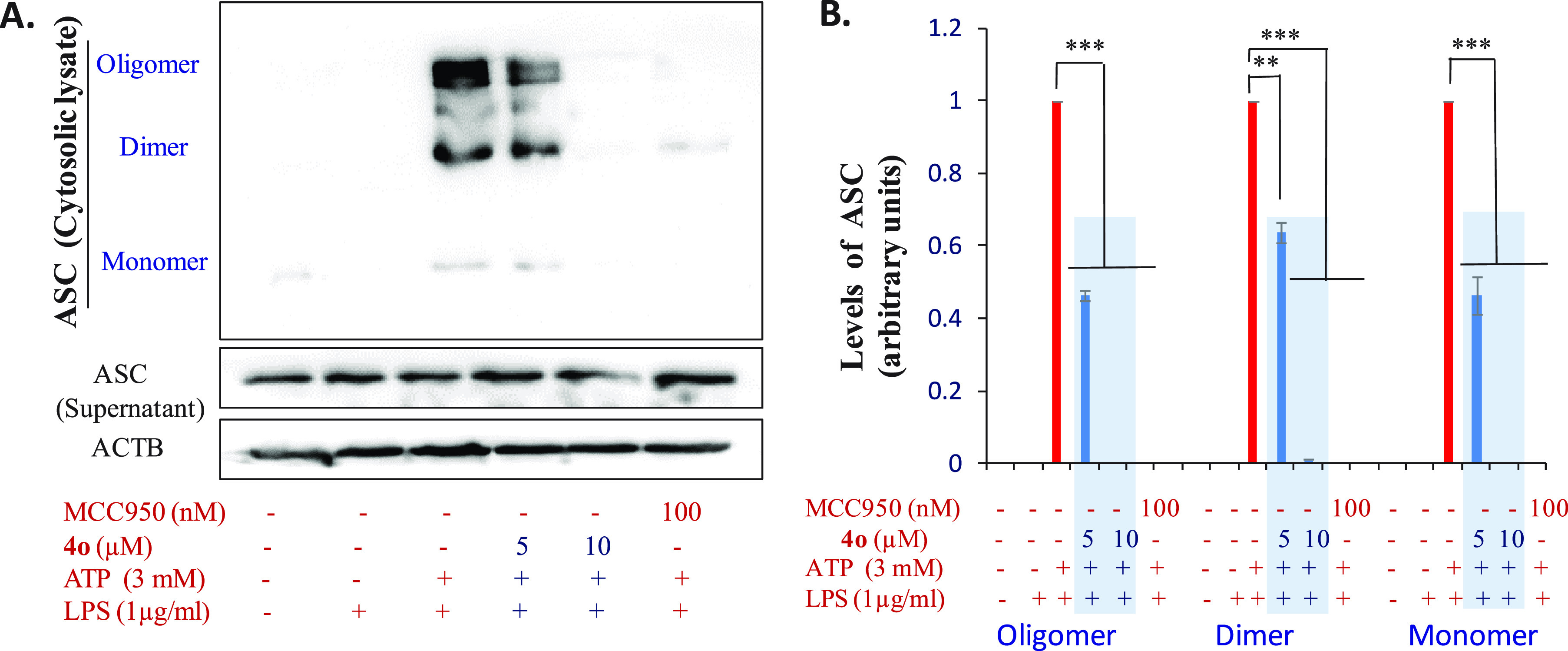
Western blot studies of 4o to study its effect on ASC oligomerization in LPS/ATP challenged J774A.1 cells. (A) Western blot analysis results of 4o for ASC oligomerization. (B) Quantitative data for the effect of compound 4o on various forms of ASC. Quantification of Western blots was done by using ImageJ software, and statistical comparisons were made by using one way ANOVA and post-hoc Bonferroni test; *p < 0.05; **p < 0.01; ***p < 0.005.
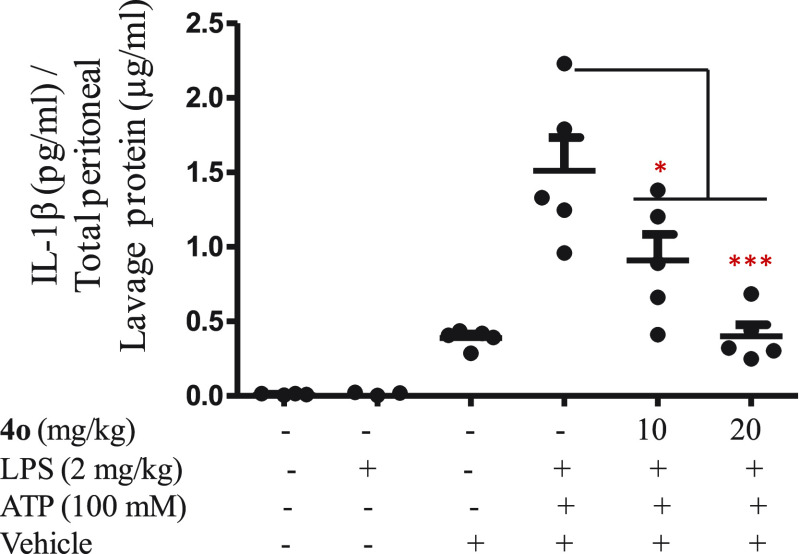
The effect of compound 4o was also studied in the peritoneal inflammation mice model. The BALB/c mice were injected with LPS and ATP to induce the NLRP3 inflammasome activation. The mice were injected with different doses of compound 4o (10 and 20 mg/kg), 30 min before the treatment of LPS for 4 h. The peritoneal lavage was collected, and the level of IL-1β was analyzed by ELISA. The IL-1β level was found to be significantly reduced in the mice group treated with 4o (10 mg and 20 mg/kg). The results suggest that the 4o significantly inhibits the NLRP3 inflammasome in the peritoneal inflammation (Figure 9). These data provide the in vivo proof of concept that the tested compound reduces IL-1β secretion in vivo, similar to the in vitro studies. However, results of this animal model do not indicate translational potential of the compound.
Figure 9.
Effect of 4o treatment on IL-1β levels in peritoneal lavage of LPS/ATP challenged BALB/c mice. Statistical comparisons were made using one way ANOVA and post-hoc Bonferroni test; *p < 0.05; **p < 0.01; ***p < 0.005.
Conclusion
In this study, we have reported methoxystilbenes and 2-phenylnaphthalenes as a new class of NLRP3 inflammasome inhibitors. A total of 51 analogs were synthesized and tested for suppression of IL-1β, which provided two most active compounds 4o and 1t. Both compounds have decreased levels of cleaved forms of IL-1β and caspase-1 in the western-blot analysis. Further, the compound 4o was found to block the formation of ASC dimers and oligomers, preventing the formation of NLRP3 inflammasome assembly and thereby affecting the release of IL-1β. Treatment of LPS/ATP challenged BALB/c mice with 4o has resulted in decreased levels of IL-1β, thus, indicating its potential for further investigation in disease-specific preclinical models.
Experimental Section
General
All chemicals were obtained from Sigma-Aldrich, TCI Chemicals, and Fischer Scientific Company and used as received. The spectral analysis of all compounds was done using Bruker-Avance FT-NMR (500 MHz), Agilent 1100 LC-Q-TOF mass spectrophotometer, and PerkinElmer IR spectrophotometer.
General Procedure for Preparation of Benzyl Alcohols (5)
The sodium borohydride was added slowly to the solution of aromatic aldehydes in methanol at 0 °C with continuous stirring. The resulting mixture was stirred for 30 min in an ice bath. The mixture was concentrated on a rotary evaporator and then partitioned between ethyl acetate and water. The ethyl acetate fraction was evaporated to dryness and used as such for the next step.
General Procedure for Preparation of Benzyl Bromides (6)
Triphenylphosphine was added slowly to the solution of N-bromosuccinimide in methylene chloride at 0 °C with continuous stirring and was then refluxed at 60 °C for 15 min. The reaction mixture was cooled to room temperature. The benzyl alcohol was added to the cooled reaction mixture, and it was then refluxed again at 60 °C for 30 min. The reaction mixture was concentrated to dryness and used as such for the next step.
General Procedure for Preparation of Benzyl Triphenylphosphinebromide Salts 3
The triphenylphosphine (1.69 g, 6.46 mmol) was added in the solution of benzyl bromide (1 g, 5.88 mmol) in 20 mL of THF. Then, reaction mixture was refluxed at 85 °C for 2 h. The white solid was formed, indicating the formation of Wittig’s salt. The reaction mixture was allowed to cool and then filtered. The solid residue was washed with ethyl acetate or THF (15 mL × 3) and then dried under vacuum and used for the next step.
General Procedure for Preparation of (E/Z)-4-Styrylpyridine 4a–4ac
Potassium tert-butoxide (2.5 mmol) was added to the solution of triphenylphosphine halide salts (2 mmol) in dry THF at room temperature, and the resulting mixture was refluxed for 30 min. The color of reaction mixture turned orange which was then cooled at room temperature. The pyridine-4-carboxaldehyde (2 mmol) was added, and the reaction mixture was refluxed at 80 °C for 30 min. THF was evaporated in vacuo in a rotavapor, and the resultant solid mass was partitioned between ethyl acetate and water. The ethyl acetate layer was dried on anhydrous sodium sulfate, which was further evaporated on a rotary evaporator to obtain the crude product. The purification was done using routine silica gel (mesh 100–200) column chromatography using ethyl acetate-hexane as a mobile phase to get styrylmethoxybenzenes 4a–4ac in 78–95% of yield. The analytical data of 4b–4o, 4q, and 4u are available in our earlier publication.40 The spectral data for newly prepared stilbenes are provided below.
(E/Z)-1,2,3-Trimethoxy-5-(2,4,6-trimethoxystyryl)benzene (4p)
Mixture of cis/trans isomers (33:68 ratio, determined based on HPLC. Yield: 88%; white solid; IR (νmax): 3000, 2936, 1957, 1603, 1580, 1574, 1463, 1454, 952, cm–1; 1H NMR (400 MHz, CDCl3) of trans isomer δ 7.38 (d, J = 16.5 Hz, 1H), 7.26 (d, J = 16.4 Hz, 1H), 6.72 (s, 2H), 6.16 (s, 2H), 3.90 (s, 6H), 3.87 (s, 6H), 3.63 (s, 6H); 13C NMR (101 MHz, CDCl3) of trans isomer δ 160.25, 159.45, 153.26, 135.57, 129.97, 119.46, 103.23, 90.80, 60.96, 56.13, 55.80, 55.75, 55.33; ESI-MS m/z: 369.0 [M + H]+.
(E)-1,3,5-Trimethoxy-2-(4-nitrostyryl)benzene (4r)
Trans isomer (98.2% determined based on HPLC). Yield: 87%; yellow solid; mp 199–201 °C; IR (νmax): 3584, 3396, 2913, 1684, 1600, 1604, 1513, 1461, 1324, 1019 cm–1; 1H NMR (400 MHz, CDCl3) δ 8.17 (d, J = 8.8 Hz, 2H), 7.62–7.57 (m, 3H), 7.50 (d, J = 16.6 Hz, 1H), 6.17 (s,2H), 3.91 (s, 6H), 3.86 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 161.40, 160.10, 146.74, 145.83, 127.01, 126.28, 124.76, 124.03, 107.16, 90.62, 55.82, 55.41; ESI-MS m/z: 316.0 [M + H]+.
(E/Z)-1,2,3-Trimethoxy-5-(2,4,5-trimethoxystyryl)benzene (4s)
Mixture of cis/trans isomers (14:85 ratio, determined based on HPLC). Yield: 89%; white solid; IR (νmax): 3416, 2998, 2932, 1603, 1576, 1506, 1463, 1455, 953 cm–1; 1H NMR (400 MHz, CDCl3) of trans isomer δ 7.37 (d, J = 16.5 Hz, 1H), 7.26 (d, J = 16 Hz, 1H), 6.72 (s, 2H), 6.18 (s, 2H), 3.91 (s, 6H), 3.89 (s, 6H), 3.85 (s, 3H), 3.85 (s, 3H); 13C NMR (101 MHz, CDCl3) of trans isomer δ 160.26, 159.45, 153.27, 135.57, 129.98, 119.47, 103.26, 90.83, 60.96, 56.14, 55.82, 55.34; ESI-MS m/z: 369.0 [M + H]+.
(E/Z)-1-(4-Chlorostyryl)-2,4,5-trimethoxybenzene (4t)
Mixture of cis/trans isomers (59:40 ratio, determined based on HPLC). Yield: 91%; white solid; IR (νmax): 3051, 2998, 1695, 1607, 1582, 1488, 1455, 1437 cm–1; 1H NMR (400 MHz, CDCl3) of cis isomer δ 7.42 (d, J = 12 Hz, 1H), 7.23 (d, J = 12 Hz, 1H), 7.18 (d, J = 4.4 Hz, 2H), 6.69 (s, 1H), 6.66 (s, 1H), 6.49 (d, J = 3.6 Hz, 2H), 3.88 (s, 3H), 3.79 (s, 3H), 3.51 (s, 3H); 13C NMR (126 MHz, CDCl3) of mixture of cis/trans isomers δ 151.88, 151.81, 149.91, 149.46, 143.48, 136.69, 132.49, 130.21, 128.72, 127.45, 125.51, 123.67, 117.90, 109.51, 97.65, 97.36, 56.66,56.59, 56.14, 56.02; ESI-MS m/z: 305.0 [M + H]+.
(E)-1-(3,5-dimethoxystyryl)-2,4,5-trimethoxybenzene (4v)
Trans isomer (97% determined based on HPLC). Yield: 85%; white solid; mp 82–84 °C; IR (νmax): 3453, 3000, 2954, 1845, 1731, 1666, 1590, 1514 cm–1; 1H NMR (400 MHz, CDCl3) δ 7.39 (d, J = 16.4 Hz, 1H), 6.90 (d, J = 16.4 Hz, 1H), 6.79 (s, 1H), 6.66(s, 1H), 6.46 (s, 2H), 6.29 (s, 1H), 3.88 (s, 3H), 3.82 (s, 3H), 3.66 (s, 6H), 3.52 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 160.49, 151.76, 149.17, 142.38, 139.54, 128.75, 125.31, 117.18, 113.36, 106.71, 104.37, 99.47, 97.10, 56.57, 56.10, 56.00, 55.26; ESI-MS m/z: 331.0 [M + H]+.
(E/Z)-1,2,4-Trimethoxy-5-(4-methoxystyryl)benzene (4w)
Mixture of cis/trans isomers (10:89 ratio, determined based on HPLC). Yield: 85%; brown oily; IR (νmax): 3444, 2934, 2866, 1719,1605, 1510 cm–1; 1H NMR (400 MHz, CDCl3) of trans isomer δ 7.29 (d, J = 16 Hz, 1H), 7.23(s,2H), 7.21(s,2H), 6.92 (d, J = 16 Hz, 1H), 6.51 (s, 2H), 3.89 (s, 3H), 3.81 (s, 3H), 3.76 (s, 3H), 3.52 (s, 3H); 13C NMR (126 MHz, CDCl3) of mixture of cis/trans δ 158.90, 158.47, 151.68, 151.46, 149.26, 148.94, 143.42, 142.46, 130.95, 130.18, 130.04, 128.52, 127.49, 126.45, 123.50, 120.90, 118.60, 117.70, 114.04, 113.44, 113.17, 109.17, 97.75, 97.31,56.59, 56.08, 55.98, 55.21; ESI-MS m/z: 301.0 [M + H]+.
(E/Z)-1,2,4-Trimethoxy-5-(4-nitrostyryl)benzene (4x)
Mixture of cis/trans isomers (32:67 ratio, determined based on HPLC). Yield: 90%; white solid; IR (νmax): 3098, 3002, 2954, 1653, 1590, 1513, cm–1; 1H NMR (400 MHz, CDCl3) of mixture of cis and trans isomers δ 8.18 (d, J = 8.8 Hz, 1H), 8.07 (d, J = 8.8 Hz, 2H), 7.62–7.58(m, 3H), 7.42 (d, J = 8.7 Hz, 1H), 7.12 (s, 1H), 7.01 (d, J = 16.4 Hz, 1H), 6.86 (d, J = 12.1 Hz, 2H), 6.62 (s, 1H), 6.54 (d, J = 3 Hz, 1H), 6.52 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H), 3.92 (s, 3H), 3.90 (s, 3H), 3.80 (s, 3H), 3.51 (s, 3H); 13C NMR (126 MHz, CDCl3) of mixture cis and trans isomers δ 152.56, 151.99, 150.85, 150.12, 146.18, 144.92, 144.81, 143.43, 142.65, 129.52, 129.31, 127.82, 126.54, 126.49, 124.14, 123.40, 116.85, 116.05, 113.11, 109.48, 97.14, 97.09, 56.58, 56.50, 56.31, 56.26, 56.10, 56.04; ESI-MS m/z: 316.0 [M + H]+.
(E/Z)-1-(3-Chlorostyryl)-2,4,5-trimethoxybenzene (4y)
Mixture of cis/trans isomers (51:48 ratio, determined based on HPLC). Yield: 93%; white solid; IR (νmax): 3472, 3062, 2999, 1627, 1608, 1562, 1514 cm–1; 1H NMR (400 MHz, CDCl3) of cis and trans isomers δ 7.50 (s, 1H), 7.42 (d, J = 16.4 Hz, 1H), 7.36 (d, J = 7.7 Hz, 1H), 7.29 (s, 4H), 7.24 (d, J = 7.8 Hz, 1H), 7.19–7.11 (m, 4H), 7.10 (s, 1H), 6.90 (d, J = 16.4 Hz, 1H), 6.73 (s, 1H), 6.53–6.54 (m, 3H), 3.91 (d, J = 3.3 Hz, 3H), 3.90 (s, 3H), 3.89 (s, 3H), 3.87 (s, 3H), 3.81 (s, 3H), 3.51 (s, 3H); 13C NMR (126 MHz, CDCl3) of mixture of cis and trans isomers δ 151.97, 151.87, 150.00, 149.51, 143.39, 142.48, 140.09, 139.59, 134.52, 133.91, 129.79, 129.34, 128.76, 127.15, 127.07, 126.87, 126.84, 126.32, 126.03, 125.27, 124.56, 124.46, 117.59, 116.61, 113.11, 109.38, 97.45, 97.15, 56.62, 56.59, 56.55, 56.48, 56.31, 56.14, 56.09, 56.05, 55.99, 55.23; ESI-MS m/z: 305.0 [M + H]+.
(E/Z)-1,2-Bis(3,5-dimethoxyphenyl)ethane (4z)
Yield: 93%; white solid; mp 63–65 °C; IR (νmax): 3078, 2999, 2960, 1592, 1462 cm–1; 1H NMR (400 MHz, CDCl3) δ 6.96 (s, 1H), 6.61 (d, J = 2.2 Hz, 2H), 6.47 (s, 1H), 6.40 (d, J = 2.2 Hz, 2H), 6.35 (t, J = 2.0 Hz, 1H), 6.27 (t, J = 2.2 Hz, 1H), 3.75 (s, 6H), 3.60 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 160.97, 160.49, 139.14, 139.00, 130.59, 129.18, 106.75, 104.60, 100.11, 99.90, 55.40, 55.26; ESI-MS m/z: 301.0 [M + H]+.
(E)-1,2,3-Trimethoxy-5-styrylbenzene (4ab)
Cis isomer (99% determined based on HPLC). Yield: 83%; brown solid; mp 199–201 °C; IR (νmax): 3080, 3057, 3022, 2937, 1695, 1582, 1504 cm–1; 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 8.4 Hz, 2H), 7.38–7.34 (m, 2H), 7.27 (d, J = 12.4 Hz, 1H), 7.03 (d, J = 2.6 Hz, 2H), 6.74 (brs, 2H), 3.94 (s, 6H), 3.89 (s, 3H).13C NMR (126 MHz, CDCl3) δ 153.41, 137.88, 137.21, 133.12, 128.75, 128.64, 128.20, 127.65, 126.46, 103.50, 61.02, 56.13; ESI-MS m/z: 271.0 [M + H]+.
(E)-4-(4-Chlorostyryl)-1,2-dimethoxybenzene (4ac)
Trans isomer (96%, determined based on HPLC). Yield: 87%; white semisolid; IR (νmax): 3584, 3396, 2913, 1684, 1600, 1604, 1513, 1461 cm–1;1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 8.2 Hz, 2H), 7.30 (d, J = 8.2 Hz, 2H), 7.05–6.99 (m, 3H), 6.92 (s, 1H), 6.89–6.83 (m, 1H), 3.94 (s, 3H), 3.89 (s, 3H);13C NMR (126 MHz, CDCl3) δ 149.13, 136.05, 132.78, 130.07, 129.09, 128.83, 127.43, 125.46, 120.05, 111.17, 108.64, 55.95, 55.88; ESI-MS m/z: 275.0 [M + H]+.
Synthesis of 2-Phenylnaphthalenes 1a–1x
The 2-phenylnaphthalenes 1a–1l were prepared using the reported method from our group.35 For the synthesis of phenylnaphthalenes 1m–1x, the Suzuki coupling approach was followed. In a round-bottom flask, 2 M K2CO3 in dioxane was taken and purged with nitrogen for 15 min at room temperature. Then, the 2-bromo-6-methoxynaphthalene (7, 1 mmol) and aryl boronic acids (8, 1.2 mmol) were added to the reaction mixture. The mixture was again purged with nitrogen for 15 min. Pd(PPh3)4 (0.05 mmol) was added, and the resultant mixture was refluxed at 85 °C for 10 h. After completion of the reaction, the product was extracted with ethyl acetate. The organic layer was dried over sodium sulfate and concentrated. The product was purified using silica gel column chromatography using ethyl acetate-hexane as the eluent. The analytical data of 2-phenylnaphthalenes 1a–1i, 1l are already published in our previous work.35 The spectral data of newly synthesized 2-phenylnaphthalenes are provided below.
7-(3,5-Dimethoxyphenyl)-1,3-dimethoxynaphthalene (1j)
Yield: 91%; purity = 97%; white solid; mp 199–201 °C; IR (νmax): 3584, 3396, 2913, 1684, 1600, 1604, 1513, 1461 cm–1; 1H NMR (400 MHz, CDCl3) δ 8.38 (s, 1H), 7.73(d, J = 8.5 Hz, 1H), 7.69(d, J = 10.3 Hz, 1H), 6.86 (d, J = 2.2 Hz, 2H), 6.75 (s, 1H), 6.52 (s, 1H), 6.47 (s, 1H), 4.02 (s, 3H), 3.96 (s, 3H), 3.91 (s, 6H);13C NMR (101 MHz, CDCl3) δ 161.11, 158.46, 156.89, 143.78, 135.66, 134.51, 126.92, 126.56, 121.86, 120.19, 105.61, 99.06, 98.01, 97.78, 55.62, 55.49, 55.40; ESI-MS m/z: 325.0 [M + H]+.
1,2,3-Trimethoxy-7-(3,4,5-trimethoxyphenyl)naphthalene (1k)
Yield: 89%; purity = 99%; white solid; mp 168–170 °C; IR (νmax): 3004, 2935, 2836, 1626, 1585, 1515, 1495 cm–1; 1H NMR (400 MHz, CDCl3) δ 8.16 (s, 1H), 7.72 (d, J = 8.4 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H), 6.95 (s, 1H), 6.86 (s, 2H), 4.05 (s, 3H), 3.97 (s, 6H), 3.93 (s, 6H), 3.88 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 153.55, 153.14, 148.10, 141.32, 137.59, 136.77, 130.02, 127.04, 125.47, 124.53, 119.44, 104.76, 102.24, 61.54, 61.22, 61.01, 56.33, 55.92; ESI-MS m/z: 385.0 [M + H]+.
2-Methoxy-6-(3,4,5-trimethoxyphenyl)naphthalene (1m)41
Yield: 87%; purity = 99%; white solid; mp 114–116 °C; IR (νmax): 33098, 3072, 3002, 2954, 1653, 1623, 1608, 1590, 1464 cm–1; 1H NMR (400 MHz, CDCl3) δ 7.92 (s, 1H), 7.79 (d, J = 8.4 Hz, 2H), 7.67 (d, J = 8.5 Hz, 1H), 7.19–7.16 (m, 2H), 6.88 (s, 2H), 3.96 (s, 6H), 3.94 (s, 3H), 3.91 (s, 3H); ESI-MS m/z: 325.0 [M + H]+.
4-(6-Methoxynaphthalen-2-yl)benzaldehyde (1n)
Yield: 92%; purity = 98%; white solid; mp 199–201 °C; IR (νmax): 3584, 3396, 2913, 1684, 1600, 1604, 1513, 1461, 1324, 1265, 1173, 1134, 1019 cm–1; 1H NMR (400 MHz, CDCl3) δ 10.07 (s, 1H), 8.04 (s, 1H), 7.98 (d, J = 8.2 Hz, 2H), 7.87 (d, J = 8.3 Hz, 2H), 7.82 (d, J = 9.2 Hz, 2H), 7.74 (d, J = 10.2 Hz, 1H), 7.22–7.18 (m, 2H), 3.95 (s, 3H);13C NMR (101 MHz, CDCl3) δ 193.26, 159.78, 148.64, 136.47, 136.18, 135.88, 131.74, 131.35, 130.49, 129.05, 127.82, 127.00, 120.96, 107.11, 56.79.
2-Methoxy-6-(4-(trifluoromethoxy)phenyl)naphthalene (1o)
Yield: 90%; purity = 98%; white solid; mp 152–154 °C; IR (νmax): 3385, 3019, 2957, 2869, 2848, 2351, 2342, 1918, 1729, 1695, 1658, 1632, 1607, 1516, 1501, 1082 cm–1; 1H NMR (400 MHz, DMSO) δ 7.93 (s, 1H), 7.80 (t, J = 8.7 Hz, 2H), 7.68 (dd, J = 14.3, 8.5 Hz, 3H), 7.31 (d, J = 8.3 Hz, 2H), 7.18 (dd, J = 11.6, 2.5 Hz, 2H), 3.94 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 157.96, 148.49, 140.00, 134.97, 133.93, 129.74, 129.08, 128.50, 127.47, 125.77, 121.35, 119.42, 105.54, 55.38; ESI-MS m/z: 319.0 [M + H]+.
2-(3,4-Dimethoxyphenyl)-6-methoxynaphthalene (1p)
Yield: 90%; purity = 99%; white solid; mp 127–129 °C; IR (νmax): 3390, 2952, 2846, 1684, 1669, 1629, 1595, 1554, 1517 cm–1; 1H NMR (400 MHz, CDCl3) δ 7.91 (s, 1H), 7.80–7.74 (m, 2H), 7.67 (dd, J = 8.5, 1.7 Hz, 1H), 7.23 (d, J = 2.0 Hz, 1H), 7.22 (d, J = 1.9 Hz, 1H), 7.18 (d, J = 2.5 Hz, 1H), 7.15 (s, 1H), 6.97 (d, J = 8.2 Hz, 1H), 3.98 (s, 3H), 3.93 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 159.13, 150.83, 150.12, 137.71, 135.80, 135.02, 130.96, 130.70, 128.62, 127.37, 126.45, 120.99, 120.51, 113.24, 112.17, 107.18, 57.49, 56.73; ESI-MS m/z: 295.0 [M + H]+.
2-Methoxy-6-(4-nitrophenyl)naphthalene (1q)42
Yield: 87%; purity = 95%; white solid; mp 146–148 °C; IR (νmax): 3419, 3037, 2958, 1695, 1653, 1627, 1606, 1591, 1512, 1386 cm–1; 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 8.9 Hz, 1H), 8.03 (s, 1H), 7.87–7.83 (m, 4H), 7.82 (s, 1H), 7.72 (dd, J = 8.5, 1.9 Hz, 1H), 7.22 (dd, J = 8.9, 2.5 Hz, 1H), 7.18 (d, J = 2.4 Hz, 1H), 3.96 (s, 3H); ESI-MS m/z: 280.0 [M + H]+.
2-(4-Fluorophenyl)-6-methoxynaphthalene (1r)43
Yield: 90%; purity = 99%; white solid; mp 145–147 °C; IR (νmax): 33164, 2921, 2848, 2356, 1895, 1844, 1792, 1695, 1662, 1653, 1603, 1558, 1521, 1501, 1457, 1451, 1399, 1269, 1252, 1207, 1167, 1032, 891, 858, 829,820, 771, 733, 667, 585,514, 506 cm–1; 1H NMR (400 MHz, CDCl3) δ 7.91 (s, 1H), 7.78 (t, J = 7.9 Hz, 2H), 7.68–7.61 (m, 3H), 7.20–7.11 (m, 4H), 3.94 (s, 3H) ; ESI-MS m/z: 253.0 [M + H]+.
2-Methoxy-6-(2-methoxy-3,5-dimethylphenyl)naphthalene (1s)
Yield: 91%; purity = 99%; white solid; mp 110–112 °C; IR (νmax): 3418, 3056, 2953, 2925, 2351, 1903, 1734, 1632, 1607, 1492 cm–1;1H NMR (400 MHz, DMSO) δ 8.08 (s, 1H), 7.90–7.86 (m, 2H), 7.76 (dd, J = 8.6, 1.9 Hz, 1H), 7.45 (s, 2H), 7.33 (d, J = 2.5 Hz, 1H), 7.18 (dd, J = 9.0, 2.5 Hz, 1H), 3.89 (s, 3H), 3.70 (s, 3H), 2.32 (s, 6H);13C NMR (126 MHz, CDCl3) δ 157.70, 156.56, 136.82, 136.23, 133.66, 131.27, 129.73, 129.30, 127.73, 127.23, 126.14, 125.33, 119.16, 105.65, 77.46, 77.21, 76.95, 59.87, 55.35, 16.42; ESI-MS m/z: 293.0 [M + H]+.
2-(2,4-Dimethoxyphenyl)-6-methoxynaphthalene (1t)
Yield: 91%; purity = 99%; white solid; mp 117–119 °C; IR (νmax): 3059, 2999, 1631, 1580, 1512, 1499 cm–1; 1H NMR (400 MHz, CDCl3) δ 7.84 (s, 1H), 7.75 (d, J = 3.3 Hz, 1H), 7.72 (s, 1H), 7.62 (d, J = 9.9 Hz, 1H), 7.33 (d, J = 9.0 Hz, 1H), 7.14 (s, 1H), 7.12 (d, J = 2.4 Hz, 1H), 6.61 (d, J = 2.3 Hz, 1H), 6.59 (d, J = 2.2 Hz, 1H), 3.93 (s, 3H), 3.86 (s, 3H), 3.81 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 160.36, 157.73, 157.62, 133.89, 133.41, 131.49, 129.60, 129.09, 128.72, 127.71, 126.12, 123.79, 118.67, 105.72, 104.86, 99.22, 55.67, 55.49, 55.36; ESI-MS m/z: 295.0 [M + H]+.
2-(3,5-Dichlorophenyl)-6-methoxynaphthalene (1u)
Yield: 91%; purity = 99%; white solid; mp 134–136 °C; IR (νmax): 2954, 2922, 2870, 1663, 1635, 1559 cm–1; 1H NMR (400 MHz, CDCl3) δ 7.93 (s, 1H), 7.82 (s, 1H), 7.79 (d, J = 8.7 Hz, 2H), 7.63 (d, J = 10.3 Hz, 1H), 7.52 (s, 2H), 7.19 (dd, J = 8.9, 2.4 Hz, 1H), 7.16 (s, 1H), 3.95 (s, 3H);13C NMR (126 MHz, CDCl3) δ 158.14, 141.26, 134.17, 133.83, 132.88, 131.14, 130.72, 129.78, 129.02, 128.96, 127.61, 126.40, 125.76, 125.36, 119.56, 105.57, 55.39; ESI-MS m/z: 303.0 [M + H]+.
2-(5-Chloro-2-methoxyphenyl)-6-methoxynaphthalene (1v)
Yield: 90%; purity = 99%; white solid; mp 124–126 °C; IR (νmax): 3453, 3060, 3000, 2956, 1729, 1653, 1607, 1592 cm–1;1H NMR (400 MHz, DMSO) δ 7.95 (s, 1H), 7.87 (d, J = 9.0 Hz, 1H), 7.84 (d, J = 8.6 Hz, 1H), 7.61 (d, J = 10.2 Hz, 1H), 7.42 (s, 1H), 7.40 (d, J = 2.7 Hz, 1H), 7.35 (d, J = 2.4 Hz, 1H), 7.20–7.16 (m, 2H), 3.90 (s, 3H), 3.80 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 157.98, 155.39, 133.90, 132.66, 132.38, 130.75, 129.81, 128.90, 128.21, 128.14, 128.06, 126.40, 125.81, 119.02, 112.60, 105.65, 55.95, 55.37; ESI-MS m/z: 299.0 [M + H]+.
2-(3-Chlorophenyl)-6-methoxynaphthalene (1w)
Yield: 93%; purity = 99%; white solid; mp 169–171 °C; IR (νmax): 3417, 3063, 3018, 2959, 1629, 1603, 1590, 1559, 1540, 1534, 1495, cm–1; 1H NMR (400 MHz, CDCl3) δ 7.92 (s, 1H), 7.78 (t, J = 8.0 Hz, 2H), 7.64 (d, J = 10.0 Hz, 1H), 7.60 (d, J = 8.4 Hz, 2H), 7.42 (d, J = 8.4 Hz, 2H), 7.19–7.15 (m, 2H), 3.91 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 157.93, 139.67, 135.11, 133.91, 133.14, 129.72, 129.68, 129.64, 129.11, 128.97, 128.52, 128.44, 127.43, 125.68, 125.57, 119.80, 119.37, 105.76, 105.57, 55.36 ; ESI-MS m/z: 269.0 [M + H]+.
5-(6-Methoxynaphthalen-2-yl)benzo[d][1,3]dioxole (1x)
Yield: 89%; purity = 100%; white solid; mp 106–108 °C; IR (νmax): 3346, 2955, 2924, 1606, 1576, 1559, 1550,1540, 1533, 1516, 1251 cm–1; 1H NMR (400 MHz, CDCl3) δ 7.88 (s, 1H), 7.78 (d, J = 2.8 Hz, 1H), 7.76 (d, J = 3.0 Hz, 1H), 7.63 (d, J = 10.0 Hz, 1H), 7.18 (brs, 2H), 7.15 (brs, 2H), 6.91 (d, J = 7.9 Hz, 1H), 6.01 (s, 2H), 3.93 (s, 3H);13C NMR (126 MHz, CDCl3) δ 157.65, 148.19, 146.94, 136.13, 135.63, 133.55, 129.61, 129.15, 127.23, 125.97, 125.18, 120.70, 119.20, 108.67, 107.76, 101.17, 55.36; ESI-MS m/z: 279.0 [M + H]+.
In vitro NLRP3 Inflammasome Inhibition Assay
The biological evaluation of test compounds for NLRP3 inflammasome inhibition was performed using the mouse macrophage J774A.1 cell line in 24-well plates as per the protocol described in our earlier publication.32 Two sets of protocols were adopted for treating cells with test compound: The first one (indirect assay) where the cells were first treated with compounds (10 μM) for 1 h and then with LPS (1 μg/mL) for 5.5 h, followed by ATP (30 min). However, in the direct assay, the cells were treated with LPS (1 μg/mL) for 5.5 h. Then, cells were then treated with compounds (10 μM) for 1 h and then with ATP (3 mM) for 30 min. In both cases, supernatants were analyzed for IL-1β by ELISA using the protocol as described earlier. The Western blot analysis for pro-IL-1β, IL-1β, procaspase-1, and caspase-1 was carried out using the protocol described in our earlier publication.32
Cross-Linking and Oligomerization of ASC
J774A.1 cells after various treatments were washed with cold PBS and incubated briefly in 300 μL of cold buffer containing 20 mM HEPES-KOH (pH 7.5), 1% NP-40, 150 mM KCl, 0.1 mM PMSF, 1 mM sodium orthovanadate, and protease inhibitor cocktail (1×).The cells were centrifuged at 6000 rpm for 10 min at 4 °C. From the supernatant here, 50 μL was collected and used for Western blot as input control for ASC. To chemically induce the cross-linking of ASC, the pellets were further incubated in freshly prepared suberic acid (2 mM) in PBS for 30 min at 37 °C followed by centrifugation at 6000 rpm for 10 min. The Supernatants were discarded, and 25 μL of 2× Laemmili buffer was added to the cross-linked pellets. The samples were boiled at 95 °C for 5 min and subjected to separation by SDS-PAGE for analysis of polymeric forms of ASC.
Animals and Ethical Statement
In this study, BALB/c female mice weighing 20–25 g were used. The animals were acclimatization at 25 °C on 12 h dark and light cycles for 5–7 days. CO2 inhalation was used to sacrifice the animals at the end of the experiment. All animal experiments were done in compliance with the guidelines of Institutional Animal Ethics Committee (IAEC), CSIR-IIIM, Jammu, India. The protocols for all the animal experiments were approved by IAEC. The data presented here represent study number IAEC/1689/75/8/19.
In Vivo Assessment for Effects on IL-1β Release
Female BALB/c mice were separated into different groups. The groups include control (group 1), LPS (group 2), LPS+ATP (group 3), 4o 10 mg/kg+LPS+ATP (group 4), 4o 20 mg/kg+LPS+ATP (group 5), and the vehicle (group 6). For peritoneal injection, compound 4o was dissolved in (5% DMSO, 30% PEG400, 20% PEG200, and 45% distilled water), while LPS and ATP were dissolved in PBS. The mice were given an intraperitoneal injection of compound 4o (10 mg/kg and 20 mg/kg) 30 min prior to the administration of LPS (2 mg/kg) for 4 h. ATP (100 mM) was administrated intraperitoneally for 15 min. Incomplete DMEM (3 mL) was injected, the peritoneal lavage was collected, and the level of IL-β was analyzed by ELISA.
Statistical Analysis
Data presented in the paper are means of three independent experiments, and the error bars show the standard deviation (SD) between the experiments. Instat-3 software was used for statistical analysis and one way ANOVA, and post-hoc Bonferroni test was applied to calculate statistical significance, where a p value <0.05 was considered to be significant with ***p < 0.001, **p < 0.01, and *p < 0.05.
Acknowledgments
M. Abdullaha and M. Ali thank UGC for the research fellowship. The financial support from the CSIR YSA grant (P90807) and CSIR major laboratory project (MLP-5005) is gratefully acknowledged.
Glossary
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid-β
- ATP
adenosine triphosphate
- AIM2
absent in melanoma 2
- ASC
apoptosis-associated speck-like protein
- ELISA
enzyme-linked immunosorbent assay
- IL-1β
interleukine-1β
- LPS
lipopolysaccharide
- NLRP3
nucleotide-binding domain leucine-rich repeat family pyrin domain containing 3
- NLRC4
nucleotide-binding domain leucine-rich repeat caspase recruitment domain-containing receptor 4
- TFA
trifluoroacetic acid
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00126.
NMR spectra scans for the new compounds (PDF)
Author Contributions
M. Abdullaha and R.M. performed the synthesis of compounds. M. Ali, D.K., and P.K. performed all cellular assays for NLRP3 inflammasome activity. V.K.N. performed blood–brain barrier studies. A.K. designed and coordinated all biological experiments. S.B.B. designed and monitored the synthetic studies. S.B.B. designed and coordinated all this work. This is IIIM Publication number. CSIR-IIIM/IPR/00205.
Author Contributions
§ These authors contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- de Zoete M. R.; Palm N. W.; Zhu S.; Flavell R. A. (2014) Inflammasomes. Cold Spring Harbor Perspect. Biol. 6, a016287 10.1101/cshperspect.a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K.; Tschopp J. (2010) The inflammasomes. Cell 140, 821–832. 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Minkiewicz J.; de Rivero Vaccari J. P.; Keane R. W. (2013) Human astrocytes express a novel NLRP2 inflammasome. Glia 61, 1113–1121. 10.1002/glia.22499. [DOI] [PubMed] [Google Scholar]
- Rivera-Escalera F.; Pinney J. J.; Owlett L.; Ahmed H.; Thakar J.; Olschowka J. A.; Elliott M. R.; O’Banion M. K. (2019) IL-1β-driven amyloid plaque clearance is associated with an expansion of transcriptionally reprogrammed microglia. J. Neuroinflammation 16, 261. 10.1186/s12974-019-1645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury S. R.; Conaghan P. G.; McDermott M. F. (2011) The role of the NLRP3 inflammasome in gout. J. Inflammation Res. 4, 39–49. 10.2147/JIR.S11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. M.; Kim J. J.; Kim H. J.; Shong M.; Ku B. J.; Jo E. K. (2013) Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62, 194–204. 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toldo S.; Abbate A. (2018) The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 15, 203–214. 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel C.; Thornton E.; Vink R. (2007) Traumatic brain injury and Alzheimer’s disease: a review. Prog. Brain Res. 161, 303–316. 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- Guo C.; Fulp J. W.; Jiang Y.; Li X.; Chojnacki J. E.; Wu J.; Wang X. Y.; Zhang S. (2017) Development and characterization of a hydroxyl-sulfonamide analogue, 5-chloro-n-[2-(4-hydroxysulfamoyl-phenyl)-ethyl]-2-methoxy-benzamide, as a novel NLRP3 inflammasome inhibitor for potential treatment of multiple sclerosis. ACS Chem. Neurosci. 8, 2194–2201. 10.1021/acschemneuro.7b00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J. W.; Fox T. A.; Halsey R.; Carpenter B.; Kottaridis P. D. (2020) Interleukin-1 blockade with anakinra in acute leukaemia patients with severe COVID-19 pneumonia appears safe and may result in clinical improvement. Br. J. Haematol. 190, e80–e83. 10.1111/bjh.16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P.; Ronconi G.; Caraffa A.; Gallenga C. E.; Ross R.; Frydas I.; Kritas S. K. (2020) Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 34, 327–331. 10.23812/conti-e. [DOI] [PubMed] [Google Scholar]
- Tulotta C.; Ottewell P. (2018) The role of IL-1B in breast cancer bone metastasis. Endocr.-Relat. Cancer 25, R421–r434. 10.1530/ERC-17-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. R.; Leslie K. S. (2011) Cryopyrin-associated periodic syndrome: an update on diagnosis and treatment response. Curr. Allergy Asthma Rep. 11, 12–20. 10.1007/s11882-010-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M.; Shinohara M. L. (2013) The role of interferon-β in the treatment of multiple sclerosis and experimental autoimmune encephalomyelitis - in the perspective of inflammasomes. Immunology 139, 11–18. 10.1111/imm.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M.; Shichita T.; Okada M.; Komine R.; Noguchi Y.; Yoshimura A.; Morita R. (2015) Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 6, 7360. 10.1038/ncomms8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A.; Hornung V.; Petzold G. C.; Stewart C. R.; Monks B. G.; Reinheckel T.; Fitzgerald K. A.; Latz E.; Moore K. J.; Golenbock D. T. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9, 857–865. 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. A.; Bergstralh D. T.; Wang Y.; Willingham S. B.; Ye Z.; Zimmermann A. G.; Ting J. P. (2007) Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. U. S. A. 104, 8041–8046. 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732. 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Wang H.; Kouadir M.; Song H.; Shi F. (2019) Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 10, 128. 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll R. C.; Robertson A. A.; Chae J. J.; Higgins S. C.; Muñoz-Planillo R.; Inserra M. C.; Vetter I.; Dungan L. S.; Monks B. G.; Stutz A.; Croker D. E.; Butler M. S.; Haneklaus M.; Sutton C. E.; Núñez G.; Latz E.; Kastner D. L.; Mills K. H.; Masters S. L.; Schroder K.; Cooper M. A.; O’Neill L. A. (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255. 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H.; Jiang H.; Chen Y.; Ye J.; Wang A.; Wang C.; Liu Q.; Liang G.; Deng X.; Jiang W.; Zhou R. (2018) Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 9, 2550. 10.1038/s41467-018-04947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Jiang H.; Chen Y.; Wang X.; Yang Y.; Tao J.; Deng X.; Liang G.; Zhang H.; Jiang W.; Zhou R. (2018) Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 10, e8689. 10.15252/emmm.201708689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perregaux D. G.; McNiff P.; Laliberte R.; Hawryluk N.; Peurano H.; Stam E.; Eggler J.; Griffiths R.; Dombroski M. A.; Gabel C. A. (2001) Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 299, 187–197. [PubMed] [Google Scholar]
- Juliana C.; Fernandes-Alnemri T.; Wu J.; Datta P.; Solorzano L.; Yu J. W.; Meng R.; Quong A. A.; Latz E.; Scott C. P.; Alnemri E. S. (2010) Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285, 9792–9802. 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco M.; Pellegrini C.; Martínez-Banaclocha H.; Giorgis M.; Marini E.; Costale A.; Miglio G.; Fornai M.; Antonioli L.; López-Castejón G.; Tapia-Abellán A.; Angosto D.; Hafner-Bratkovič I.; Regazzoni L.; Blandizzi C.; Pelegrín P.; Bertinaria M. (2017) Development of an acrylate derivative targeting the NLRP3 inflammasome for the treatment of inflammatory bowel disease. J. Med. Chem. 60, 3656–3671. 10.1021/acs.jmedchem.6b01624. [DOI] [PubMed] [Google Scholar]
- Pan L.; Hang N.; Zhang C.; Chen Y.; Li S.; Sun Y.; Li Z.; Meng X. (2017) Synthesis and biological evaluation of novel benzimidazole derivatives and analogs targeting the NLRP3 inflammasome. Molecules 22, 213. 10.3390/molecules22020213. [DOI] [Google Scholar]
- Ii M.; Matsunaga N.; Hazeki K.; Nakamura K.; Takashima K.; Seya T.; Hazeki O.; Kitazaki T.; Iizawa Y. (2006) A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol. Pharmacol. 69, 1288–1295. 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- He Y.; Varadarajan S.; Muñoz-Planillo R.; Burberry A.; Nakamura Y.; Núñez G. (2014) 3,4-Methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 289, 1142–1150. 10.1074/jbc.M113.515080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters S. L.; Dunne A.; Subramanian S. L.; Hull R. L.; Tannahill G. M.; Sharp F. A.; Becker C.; Franchi L.; Yoshihara E.; Chen Z.; Mullooly N.; Mielke L. A.; Harris J.; Coll R. C.; Mills K. H. G.; Mok K. H.; Newsholme P.; Nuñez G.; Yodoi J.; Kahn S. E.; Lavelle E. C.; O’Neill L. A. J. (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904. 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulp J.; He L.; Toldo S.; Jiang Y.; Boice A.; Guo C.; Li X.; Rolfe A.; Sun D.; Abbate A.; Wang X.-Y.; Zhang S. (2018) Structural insights of benzenesulfonamide analogues as nlrp3 inflammasome inhibitors: Design, synthesis, and biological characterization. J. Med. Chem. 61, 5412–5423. 10.1021/acs.jmedchem.8b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; He L.; Green J.; Blevins H.; Guo C.; Patel S. H.; Halquist M. S.; McRae M.; Venitz J.; Wang X. Y.; Zhang S. (2019) Discovery of Second-Generation NLRP3 Inflammasome Inhibitors: Design, Synthesis, and Biological Characterization. J. Med. Chem. 62, 9718–9731. 10.1021/acs.jmedchem.9b01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullaha M.; Mohammed S.; Ali M.; Kumar A.; Vishwakarma R. A.; Bharate S. B. (2019) Discovery of Quinazolin-4(3 H)-ones as NLRP3 Inflammasome Inhibitors: Computational Design, Metal-Free Synthesis, and in Vitro Biological Evaluation. J. Org. Chem. 84, 5129–5140. 10.1021/acs.joc.9b00138. [DOI] [PubMed] [Google Scholar]
- Abdullaha M.; Ali M.; Kour D.; Kumar A.; Bharate S. B. (2020) Discovery of benzo[cd]indol-2-one and benzylidene-thiazolidine-2,4-dione as new classes of NLRP3 inflammasome inhibitors via ER-β structure based virtual screening. Bioorg. Chem. 95, 103500. 10.1016/j.bioorg.2019.103500. [DOI] [PubMed] [Google Scholar]
- Mewshaw R. E.; Edsall R. J.; Yang C.; Manas E. S.; Xu Z. B.; Henderson R. A.; Keith J. C.; Harris H. A. (2005) ERβ ligands. 3. Exploiting two binding orientations of the 2-phenylnaphthalene scaffold to achieve ERβ selectivity. J. Med. Chem. 48, 3953–3979. 10.1021/jm058173s. [DOI] [PubMed] [Google Scholar]
- Mudududdla R.; Sharma R.; Abbat S.; Bharatam P. V.; Vishwakarma R. A.; Bharate S. B. (2014) Synthesis of 2-phenylnaphthalenes from styryl-2-methoxybenzenes. Chem. Commun. 50, 12076–12079. 10.1039/C4CC05151C. [DOI] [PubMed] [Google Scholar]
- Thakkar R.; Wang R.; Wang J.; Vadlamudi R. K.; Brann D. W. (2018) 17beta-Estradiol Regulates Microglia Activation and Polarization in the Hippocampus Following Global Cerebral Ischemia. Oxid. Med. Cell. Longevity 2018, 4248526. 10.1155/2018/4248526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J.; Shan Y.; Fan Y.; Fan C.; Chen S.; Sun J.; Zhu L.; Qin L.; Yu M.; Lin Z. (2016) NF-κB inhibition attenuates LPS-induced TLR4 activation in monocyte cells. Mol. Med. Rep. 14, 4505–4510. 10.3892/mmr.2016.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thawkar B. S.; Kaur G. (2019) Inhibitors of NF-κB and P2 × 7/NLRP3/caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 326, 62–74. 10.1016/j.jneuroim.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Dai Z.; Chen X. Y.; An L. Y.; Li C. C.; Zhao N.; Yang F.; You S. T.; Hou C. Z.; Li K.; Jiang C.; You Q. D.; Di B.; Xu L. L. (2021) Development of Novel Tetrahydroquinoline Inhibitors of NLRP3 Inflammasome for Potential Treatment of DSS-Induced Mouse Colitis. J. Med. Chem. 64, 871–889. 10.1021/acs.jmedchem.0c01924. [DOI] [PubMed] [Google Scholar]
- Derf A.; Mudududdla R.; Akintade D.; Williams I. S.; Abdullaha M.; Chaudhuri B.; Bharate S. B. (2018) Nonantioxidant tetramethoxystilbene abrogates α-synuclein-induced yeast cell death but not that triggered by the bax or βA4 peptide. ACS Omega 3, 9513–9532. 10.1021/acsomega.8b01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.; Lee H.; Jin Y.; Ha Y. M.; Bae S.; Chung H. Y.; Suh H. (2007) Syntheses of hydroxy substituted 2-phenyl-naphthalenes as inhibitors of tyrosinase. Bioorg. Med. Chem. Lett. 17, 461–464. 10.1016/j.bmcl.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Hachiya S.; Asai K.; Konishi G.-i. (2013) Unique solvent-dependent fluorescence of nitro-group-containing naphthalene derivatives with weak donor–strong acceptor system. Tetrahedron Lett. 54, 1839–1841. 10.1016/j.tetlet.2013.01.096. [DOI] [Google Scholar]
- Denmark S. E.; Smith R. C.; Chang W.-T. T.; Muhuhi J. M. (2009) Cross-Coupling Reactions of Aromatic and Heteroaromatic Silanolates with Aromatic and Heteroaromatic Halides. J. Am. Chem. Soc. 131, 3104–3118. 10.1021/ja8091449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.