Abstract
Background and Aim:
Hepatocellular carcinoma (HCC) is the third most common reason for cancer-related death worldwide. Many countries either lack appropriate clinical practice guidelines for the diagnosis and treatment of HCC or the quality of their guidelines has never been evaluated. The main objective of our work was to identify published HCC guidelines and assess their quality with the Appraisal of Guidelines for Research and Evaluation instrument (AGREE) and their suitability regarding adaptation for future guidelines.
Methods:
We performed a systematic literature search on HCC clinical practice guidelines of MEDLINE, National Guidelines Clearinghouse and the Guidelines International Network. Methodological quality of selected guidelines was assessed by the AGREE instrument, Version 2001.
Results:
A total of 286 citations were screened and 32 relevant guidelines were identified. Overall, the guidelines performed well in the clarity and presentation domain with a mean score of 67%, followed by scope and purpose (55%) and rigor of development (50%). In contrast, poor scores were given for the remaining domains: stakeholder involvement (23%), applicability (28%) and editorial independence (31%). According to the AGREE instrument, four guidelines can be strongly recommended, 18 with provisos and alterations while the remaining cannot be recommended for adaptation due to poor methodological quality.
Conclusion:
Although existing HCC guidelines may accurately reflect agreed clinical practice, many guidelines lack proper methodological quality. Future guidelines should place more emphasis on these methodological shortcomings.
Keywords: adaptation, Appraisal of Guidelines for Research and Evaluation (AGREE) instrument, evaluation, guidelines, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) accounts for 80–90% of primary liver cancers and represents a major health burden, being the sixth most common cancer and the third most common cause of cancer-related death worldwide.1 The general prognosis is still poor with overall survival rates of 3–5%. In a recently published study, the annual direct as well as indirect costs associated with HCC in the USA were estimated by using a Surveillance, Epidemiology and End Results (SEER)-Medicare database.2 The study estimated that caring for a patient with HCC cost an average of $32 907 in 2005. With the calculated prevalence of approximately 14 000 patients with HCC in 2005, the total economic burden of HCC was estimated to be $454.5m. As more patients are being diagnosed at a younger age and with earlier stage tumors, the total cost of HCC will undoubtedly rise in the future.
Clinical practice guidelines (CPG) have been defined as systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.3 They are designed to evaluate and implement the ever-increasing amount of evidence and opinion on best current medical practice and to improve quality, appropriateness and cost-effectiveness of care.4 This implies that CPG need to follow a certain standard of methodology. However, recent studies revealed that the methodological quality of clinical practice guidelines is highly variable.5 Nonetheless, the recent increase in the production of clinical practice guidelines has been accompanied by a growing concern about variations in guideline recommendations and quality.6 Reasons for this variation have been suggested. For example, recommendations are often formed by consensus with research evidence being used to support this.7 Furthermore, the influence on the production of guidelines by public agencies and medical societies results in inconsistencies between guidelines dealing with the same topic.8 Ethical considerations, social influences and practical necessities vary between cultures and different health-care systems reinforcing differences between guidelines.9 The development of CPG also requires considerable time, expertise and resources. Therefore, the adaptation of already existing high-quality guidelines is not only cost-effective, but avoids duplication of effort.
Many countries either lack appropriate treatment guidelines for HCC or their quality has never been evaluated. The aim of this study was to search systematically for all existing HCC guidelines, and assess and compare their quality with the Appraisal of Guidelines for Research and Evaluation (AGREE) instrument.10 This tool evaluates the process of practice guideline development and the quality of reporting. Consequently, already existing guidelines of sufficient quality can be used as a source for adaptation, especially in those countries with limited resources and experience on guideline development.
Methods
Data sources and selection process
Between October 2009 and January 2010 a systematic search was performed to identify evidence-based clinical practice guidelines for HCC. As computerized databases indexing guidelines, MEDLINE, the National Guidelines Clearinghouse (NGC) and the Guidelines International network (GIN) library were used by a search algorithm (Fig. 1). Furthermore, homepages of international medical societies and institutions were screened for current CPG publications. All full-text clinical practice guidelines published between 1999 and November 2009 on diagnosis and treatment of HCC were included in the study. Each guideline was checked for the following topics: prevention, screening and surveillance, diagnosis, clinical staging, curative treatment, trans-arterial therapies and systemic therapies. Excluded were non-evidence-based expert consensus statements, secondary or multiple publications or adaptations of original practice guidelines, editorials, letters to the editor, case histories, hepatitis B and C guidelines and all guidelines not written in English or German.
Figure 1.
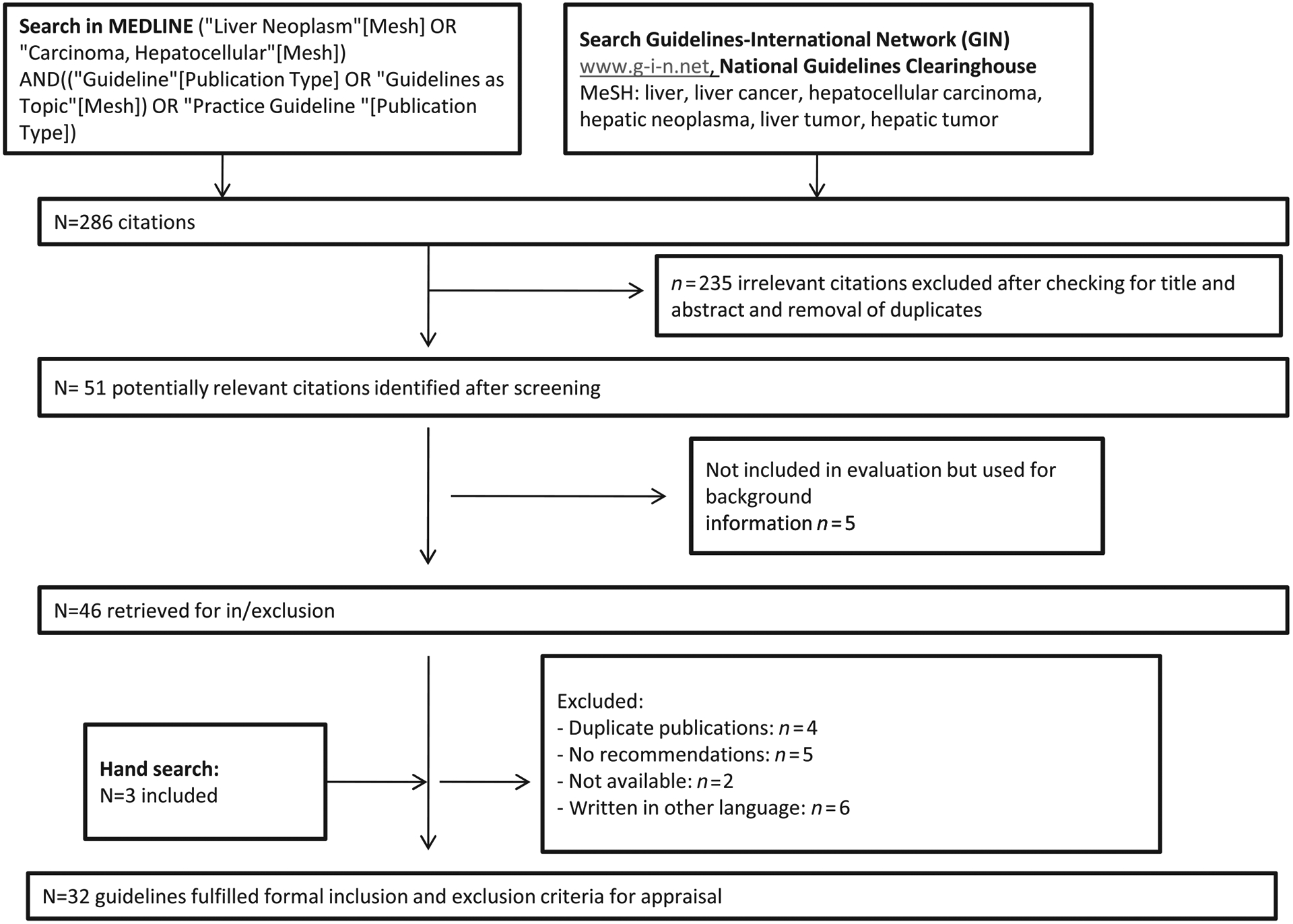
Searching and selecting guidelines flowchart.
Quality appraisal of guidelines
The AGREE instrument was selected as the appraisal tool. Twenty-three criteria of six domains, including scope and purpose, stakeholder involvement, rigor of development, clarity and presentation, applicability and editorial independence, were rated on a four-point Likert scale ranging from 1 (Strongly Disagree) to 4 (Strongly Agree) with two mid-points: 3 (Agree) and 2 (Disagree). Two experts independently conducted an evaluation of the chosen guidelines according to the instructions for using the AGREE instrument. Domain scores were calculated by summing up all the scores of each individual criterion in a domain and by standardizing the total as a percentage of the maximum possible score for this domain. Major discrepancies in the scores (> 1 point) assigned by the two reviewers were identified and resolved through discussion. A mean score was also calculated for each criterion, derived from the final scores assigned by the two reviewers. The final component of the AGREE instrument involves a recommendation regarding the use of a guideline in practice by taking each of the appraisal criteria into consideration. A guideline is “strongly recommended” if the guideline rates high (3 or 4) on the majority of items and most domain scores are above 60%. A guideline is “recommended,” if it rates high (3 or 4) or low (1 or 2) on a similar number of items with domain scores between 30% and 60%. A guideline is “not recommended” if it rates low (1 or 2) on the majority of items and most domain scores are below 30%, indicating that the guideline has a low overall quality and serious shortcomings.
Results
Searching for and selection of source CPG
Our literature search resulted in 286 citations identified through computerized database searches. An additional three citations had been identified through hand-searching in reference lists of papers, and website searches of CPG resources (Fig. 1). We identified 32 CPG for further evaluation and critical appraisal, which are listed in Table 1. For exclusion criteria see Figure 1. All 32 CPG covered either diagnosis and management of HCC (n = 11)11–21 or only specific aspects, such as diagnosis or specific therapies (radiological intervention and others) (n = 21).22–42 Diagnosis of HCC was addressed in three of 21 guidelines, with surgery and transplantation being covered by nine guidelines and locoregional therapies and radioembolization being covered in three and two guidelines, respectively. The role of systemic treatment was evaluated in three guidelines. All guidelines were published between May 2001 and October 2009. The former mainly originated from Europe (5/11), two CPG were published in the USA, three in Asian countries and one originated from Saudi Arabia. The specific guidelines were also predominantly produced in Europe (12/21), eight were from the USA and one was Japanese. Eighty-four percent of the guidelines (27/32) were disseminated national or regional, the rest of them (5/32) had an international scope; with the exception of the “Consensus statement from the Asian Oncology Summit 2009,”21 all guidelines were produced by a professional organization. Finally, 81% (26/32) of the guidelines were evidence-based. Quality scores of the CPG by the AGREE instrument are shown in Table 2 and are described below.
Table 1.
List of all 32 guidelines included in our analysis
| Organization | Origin | Date | Type | Topics covered | Reference |
|---|---|---|---|---|---|
| FNCLCC | France | 2001 | EB | Diagnosis, clinical staging, curative treatment, trans-arterial therapies, systemic therapies | 11 |
| EASL | Europe | 2001 | EB | Prevention, screening, surveillance, diagnosis, clinical staging, curative treatment, trans-arterial therapies, systemic therapies | 12 |
| BSG | Great Britain | 2003 | EB | Screening, surveillance, diagnosis, clinical staging, curative treatment, trans-arterial therapies, systemic therapies | 13 |
| BASL | Belgium | 2004 | EB | Prevention, screening, surveillance, diagnosis, clinical staging, curative treatment, trans-arterial therapies, systemic therapies | 14 |
| AASLD | USA | 2005 | EB | Prevention, screening, surveillance, diagnosis, clinical staging, curative treatment, trans-arterial therapies, systemic therapies | 15 |
| SGA | Saudi Arabia | 2006 | EB | Prevention, screening, surveillance, diagnosis, clinical staging, curative treatment, trans-arterial therapies, systemic therapies | 16 |
| Japanese Ministry of Health, Labour and Welfare | Japan | 2006 | EB | Prevention, screening, surveillance, diagnosis, curative treatment, trans-arterial therapies, systemic therapies | 17 |
| Japan Society of Hepatology | Japan | 2007 | CB | Prevention, screening, surveillance, diagnosis, clinical staging, curative treatment, trans-arterial therapies, systemic therapies | 18 |
| ESMO | Europe | 2008 | EB | Prevention, screening, surveillance, diagnosis, clinical staging, curative treatment, trans-arterial therapies, systemic therapies including sorafenib | 19 |
| NCCN | USA | 2009 | EB | Screening, surveillance, diagnosis, clinical staging, curative treatment, trans-arterial therapies, radioembolization, systemic therapies including sorafenib | 20 |
| Asian Oncology Summit | ASIA | 2009 | EB | Prevention, screening, surveillance, diagnosis, curative treatment, trans-arterial therapies, systemic therapies | 21 |
| Haute Autorité de santé | France | 2005 | CB | Indications for liver transplantation | 22 |
| REBOC | USA | 2007 | CB | Radioembolization | 23 |
| EFSUMB | Europe | 2008 | EB | Contrast-enhanced ultrasound for diagnosis of liver tumors | 24 |
| SIR | USA | 2009 | EB | Transhepatic arterial chemoembolization | 25 |
| Department of Digestive Surgery, Nihon University School of Medicine, Tokyo | Japan | 2008 | EB | Surgical treatment | 26 |
| AFEF | France | 2008 | EB | Use of sorafenib | 27 |
| ACR | USA | 2007 | CB | Curative treatment, trans-arterial therapies, systemic therapies | 28 |
| ACR | USA | 2006 | CB | Diagnosis | 29 |
| ACR | USA | 2008 | CB | Radioembolization | 30 |
| CCO | Canada | 2008 | EB | Use of sorafenib | 31 |
| CCO | Canada | 2006 | EB | Surgical treatment | 32 |
| NICE | Great Britain | 2007 | EB | Microwave ablation | 33 |
| NICE | Great Britain | 2003 | EB | Radiofrequency ablation | 34 |
| NICE | Great Britain | 2005 | EB | Laparoscopic liver resection | 35 |
| NICE | Great Britain | 2006 | EB | Living-donor liver transplantation | 36 |
| NICE | Great Britain | 2009 | EB | Surgical treatment | 37 |
| NICE | Great Britain | 2007 | EB | Surgical treatment | 38 |
| CIRSE | Europe | EB | Radiofrequency ablation | 39 | |
| SAGES | USA | 2007 | EB | Diagnostic laparoscopy | 40 |
| Royal COLLEGE of Surgeons in Ireland | Ireland | 2005 | EB | Diagnostic laparoscopy | 41 |
| SNLG | Italy | 2009 | EB | Diagnosis | 42 |
ACR, American College of Radiology; AFEF, L’Association Française pour l’Etude du Foie; AASLD, American Association for the Study of the Liver; BASL, Belgian Association for the Study of the Liver; BSG, British Society of Gastroenterology; CB, consensus-based; CCO, Cancer Care Ontario CIRSE, Cardiovascular and Interventional Radiological Society of Europe; EASL, European Association for the Study of the Liver; EB, evidence-based; EFSUMB, European Federation of Societies for Ultrasound in Medicine and Biology; ESMO, European Society for Medical Oncology; FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer; NCCN, The National Comprehensive Cancer Network ; NICE, National Institute for Health and Clinical Excellence; REBOC, The Radioembolization Brachytherapy Oncology Consortium; SAGES, Society of American Gastrointestinal and Endoscopic Surgeons; SGA, Saudi Gastroenterology Association; SNLG, Sistema Nazionale Linee Guida; SIR, Society of Interventional Radiology.
Table 2.
Assessment of HCC guidelines with AGREE instrument (Domain scores in %)
| Reference | Organization | Origin | Scope & purpose | Stakeholder involvement | Rigor of development | Clarity & presentation | Applicability | Editorial independence | Overall recommendation |
|---|---|---|---|---|---|---|---|---|---|
| 11 | FNCLCC | France | 28 | 0 | 19 | 50 | 0 | 0 | Not recommended |
| 12 | EASL | Europe | 11 | 8 | 16 | 46 | 5 | 33 | Not recommended |
| 13 | BSG | Britain | 61 | 8 | 62 | 75 | 16 | 0 | Recommended |
| 14 | BASL | Belgium | 55 | 16 | 29 | 66 | 33 | 0 | Not recommended |
| 15 | AASLD | USA | 61 | 12.5 | 45 | 87.5 | 61 | 33 | Strongly recommended |
| 16 | SGA | Arabia | 44 | 25 | 50 | 71 | 27 | 0 | Recommended |
| 17 | Japanese Ministry of Health, Labour and Welfare | Japan | 55 | 5 | 95 | 75 | 33 | 16 | Recommended |
| 18 | Japan Society of Hepatology | Japan | 66 | 25 | 26 | 75 | 33 | 16 | Not recommended |
| 19 | ESMO | Europe | 33 | 8 | 24 | 58 | 0 | 33 | Not recommended |
| 20 | NCCN | USA | 44 | 42 | 52 | 75 | 16 | 83 | Recommended |
| 21 | Asian Oncology Summit | Asia | 77 | 21 | 57 | 66 | 33 | 50 | Recommended |
| 22 | Haute Autorité de santé | France | 66 | 25 | 38 | 66 | 22 | 33 | Recommended |
| 23 | REBOC | USA | 88 | 33 | 50 | 58 | 11 | 50 | Recommended |
| 24 | EFSUMB | Europe | 44 | 25 | 29 | 50 | 11 | 17 | Not recommended |
| 25 | SIR | USA | 55 | 33 | 71 | 50 | 33 | 33 | Recommended |
| 26 | Japan | 55 | 25 | 48 | 58 | 22 | 33 | Recommended | |
| 27 | AFEF | France | 88 | 25 | 10 | 42 | 22 | 0 | Not recommended |
| 28 | ACR | USA | 22 | 16 | 57 | 50 | 0 | 17 | Not recommended |
| 29 | ACR | USA | 22 | 16 | 57 | 50 | 0 | 17 | Not recommended |
| 30 | ACR | USA | 44 | 25 | 38 | 50 | 11 | 17 | Recommended |
| 31 | CCO | Canada | 100 | 50 | 77 | 83 | 55 | 50 | Strongly recommended |
| 32 | CCO | Canada | 100 | 33 | 62 | 83 | 44 | 66 | Strongly recommended |
| 34 | NICE | Britain | 55 | 50 | 52 | 92 | 33 | 50 | Recommended |
| 34 | NICE | Britain | 55 | 50 | 38 | 66 | 33 | 50 | Recommended |
| 35 | NICE | Britain | 55 | 50 | 48 | 83 | 33 | 50 | Recommended |
| 36 | NICE | Britain | 55 | 50 | 64 | 79 | 50 | 50 | Recommended |
| 37 | NICE | Britain | 55 | 50 | 64 | 79 | 50 | 50 | Recommended |
| 38 | NICE | Britain | 55 | 50 | 64 | 79 | 50 | 50 | Recommended |
| 39 | CIRSE | Europe | 55 | 33 | 71 | 50 | 33 | 33 | Recommended |
| 40 | SAGES | USA | 55 | 16 | 66 | 83 | 44 | 0 | Recommended |
| 41 | Royal College of Surgeons in Ireland | Ireland | 33 | 8 | 33 | 66 | 0 | 0 | Not recommended |
| 42 | SNLG | Italy | 77 | 45 | 81 | 83 | 83 | 55 | Strongly recommended |
| Mean | 55.3 | 22.9 | 49.8 | 67 | 28 | 30.8 | |||
| Range | 11–100 | 0–50 | 10–95 | 42–92 | 0–83 | 0–83 |
ACR, American College of Radiology; AFEF, L’Association Française pour l’Etude du Foie; AASLD, American Association for the Study of the Liver; BASL, Belgian Association for the Study of the Liver; BSG, British Society of Gastroenterology; CCO, Cancer Care Ontario CIRSE, Cardiovascular and Interventional Radiological Society of Europe; EASL, European Association for the Study of the Liver; EFSUMB, European Federation of Societies for Ultrasound in Medicine and Biology; ESMO, European Society for Medical Oncology; FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer; NCCN, The National Comprehensive Cancer Network ; NICE, National Institute for Health and Clinical Excellence; REBOC, The Radioembolization Brachytherapy Oncology Consortium; SAGES, Society of American Gastrointestinal and Endoscopic Surgeons; SGA, Saudi Gastroenterology Association; SNLG, Sistema Nazionale Linee Guida; SIR, Society of Interventional Radiology.
Scope and purpose
This domain covers the overall aim of a guideline, the specific clinical questions/problems and the target patient population. Overall, the mean score was 55% (ranging from 11% to 100%) indicating that the criteria of scope and purpose were met by most of the guidelines. Of the 32 guidelines, only 11 scored more than 60% and four less than 40%. A closer look revealed that the target population in particular (criterion 3) was not specifically described in 22 guidelines, in contrast to criteria 1 and 2, where most of the guidelines performed well. The overall objective of the guideline was well defined in 19 guidelines (59%) and the clinical questions addressed in 27 guidelines (84%), respectively.
Stakeholder involvement
This domain evaluates the degree to which the guideline represents the views of its intended users. Included are questions regarding the composition of the guideline development group, whether patients were involved, whether the target users of the guideline were well defined, and whether the guideline was piloted among end-users. Overall, the mean score for this domain was 23% (ranging from 0% to 50%). Fifteen guidelines had been developed by a multi-disciplinary team (47%) and a total of 28 guidelines were produced by health organizations. Only seven guidelines addressed patients’ views and preferences during the development process. None of the guideline development groups described a process of piloting among target group members and just 13 guidelines provided some information about their target users (41%).
Rigor of development
This domain assesses if systematic methods and specific criteria were used for searching and selecting the evidence and for formulating recommendations, whether the recommendations and the supporting evidence were explicitly linked, whether health benefits, side-effects and risks have been considered, whether the guidelines were externally reviewed and whether a procedure for updating was provided. The mean score for this domain was 50% (ranging from 10% to 95%) with 21 guidelines scoring < 60%. Merely 12 guidelines reported details of the strategy used to search for evidence and 18 reported the criteria for selecting the evidence. Moreover, we observed that just over two thirds of the guidelines provided information about the methods used for formulating the recommendations. The recommendations were explicit in 19/32 of the guidelines regarding health benefits, side-effects and risks. In 14 guidelines there was an explicit link between supporting evidence and recommendations. A minority of 12 guidelines was reviewed before publication and most of the guidelines (31/32) did not provide any information about updating.
Clarity and presentation
This domain describes whether the recommendations were specific and unambiguous, whether the different management options were clearly presented, whether key recommendations were easily identifiable, and if the guidelines were supported with tools for application. Overall, the mean score for this domain was 67% (range, 42–92%), indicating that, on average, 67% of the criteria for clarity and presentation were met. Most guidelines performed well in this domain with only 12 guidelines scoring < 60% of which only two achieved less than 50%. The recommendations were specific in all of the guidelines and easily identifiable in 25 guidelines. Thirty of them described different disease management options, but only slightly more than one third of the guidelines (n = 11) included tools for application.
Applicability
This domain evaluates issues that are pertinent to guideline implementation, such as organizational barriers, cost implications, and monitoring criteria. The mean score in this domain was the lowest of all with a mean of 28% (range, 0–83%) and only two CPG scored > 60%. A total of 15 CPG discussed more or less potential organizational barriers and seven provided some kind of indicator for monitoring and audit purposes. Only two CPG discussed cost implications.
Editorial independence
This domain addresses conflict of interest, specifically whether the guideline was editorially independent from the funding body and whether potential conflicts of interest were reported for the members of the guideline development group. The score in this domain was also poor, with a mean score of 31% (range, 0–83%). Two guidelines scored > 60%. Potential conflicts of interests of CPG developers were stated in just two CPG and 13 guidelines (40%) were editorially independent from the funding body.
Overall recommendations
After completing the AGREE instrument, the authors made an overall recommendation for each guideline. The authors recommended for adaptation those guidelines that demonstrated acceptable quality, depending on their AGREE score. Four guidelines can be strongly recommended, the majority of the domains scoring above 60% showing a good overall quality of the guidelines. Eighteen can be recommended with provisos and alterations, the majority of the domains scoring between 30% and 60%. These guidelines revealed flaws in certain domains indicating their average quality. If in future CPG development more attention is paid to these shortcomings, the overall quality of them could be improved significantly. The remaining 10 CPG cannot be recommended (Table 2) due to their poor scoring in the majority of the domains.
Discussion
Over the last years, we have witnessed an increasing number of clinical practice guidelines in HCC produced by different bodies, not only with regard to diagnosis and treatment but also in several other clinical areas. This proliferation of guidelines has produced a need for standardized international criteria for their proper establishment. The AGREE instrument was developed in response to this need. It is a validated instrument for the evaluation of guidelines and for defining the steps in producing high-quality guidelines endorsed by the leading producers, raters, and compilers of international CPGs43 and it is considered the best current tool for assessing guideline quality.44 The AGREE instrument assesses both the quality of the reporting, and the quality of some aspects of the recommendations. Like other appraisal tools, the AGREE instrument does not differentiate between a guideline failing to meet a criterion due to poor methodology or lack of reporting, and which can result in distrust in and/or misuse of recommendations.45 Furthermore, it has to be considered that the quality of the recommendations are beyond the scope of AGREE.
Overall, the assessed guidelines demonstrated considerable flaws. Almost all the guidelines scored poorly with respect to stakeholder involvement, applicability and editorial independence. Not a single guideline included the views of all relevant professional groups and/or addressed patients’ views and preferences. Furthermore, evidence of pilot testing was missing. Although few studies have assessed the impact of guideline development on patient outcome, it has been demonstrated that guidelines can improve clinical practice.46
According to the results of studies relating to other clinical topics,47 the assessed guidelines received the lowest scores on the applicability domain. These findings underline the fact that guideline producers should be more attentive both to potential barriers to guideline implementation as well as to monitoring criteria, which assess the guideline’s impact.
HCC guidelines also consistently failed to perform well in the domain of editorial independence. It should be borne in mind that potential conflicts of interest—for example, between a committee member and pharmaceutical industry—can have an impact on guideline drafting. Therefore, conflict of interests need to be clearly stated. A few studies recently underlined that authors of CPG were influenced by pharmaceutical industries and it is important to know to what extent these interactions might have influenced the recommendations.48
One of the key factors regarding the adequacy of a set of guidelines pertains to the rigor of their development. Although most of the guidelines included references to published literature, many did not clearly describe the literature review methodology employed or the mechanism by which recommendations were formulated. This step is crucial in determining whether the recommendations are truly based on the best available evidence and also in understanding how the evidence is synthesized. For example, a recent study illustrated that less than one-third of cardiovascular risk management recommendations in national guidelines were based on high-quality evidence.49 However, very often it is not clear how final recommendations have been arrived at50 and these recommendations can vary as a result of local bias, differences in data interpretation or be a manifestation of available resources. Analyses of guidelines on other medical topics, such as the methodology of current psoriasis guidelines by Nast et al., have revealed similar results with a high score for scope, purpose and clarity, but low scores for stakeholder involvement, applicability and editorial independence.5 Since most guidelines on HCC scored low in these domains, special attention should be paid to these shortcomings in future guideline development.
As a consequence of our appraisal, we were able to strongly recommend four guidelines as source guidelines for future CPG. These included two guidelines with most domain scores above 60% developed by Cancer Care Ontario (CCO)31 and an Italian guideline by Sistema Nazionale Linee Guida (SNLG),42 indicating that these guidelines had a high overall quality. Two other guidelines, published by the American Association for the Study of the Liver (AASLD)15 and CCO32 scored only in three domains above 60%. However, these domains best reflect the adherence to the currently best available evidence and the transferability to local settings. Therefore, the authors considered, that they can be “strongly recommended.”
Eighteen other guidelines can be recommended with provisos and alterations. Most domains scored between 30% and 60% in these guidelines, while six guidelines could not be recommended because they rated low on the majority of items with a majority of domain scores below 30%, indicating a low overall quality and serious shortcomings. More than three domains had more than 30% in the other four guidelines,14,18,19,41 but a closer look revealed that some of the domains were rated concisely about 30% and they especially failed to convince at the rigor of development and applicability domains. Therefore these guidelines cannot be recommended. However, it must be emphasized that the validity of overall assessment is limited due to the subjective nature of our appraisal, as there were no clear rules on how to weight the different domains in making a final recommendation.
Several high-ranking international guidelines have already been developed. Adaptation of already existing high-quality guidelines represents one possibility to save costs and avoid duplication of efforts when new guidelines need to be developed. However, until today there has been no validated process for adapting guidelines which have been produced in one cultural setting for use in another.
In conclusion, our analysis of current CPG for the diagnosis and treatment of HCC revealed several methodological flaws, although these may accurately reflect agreed clinical practice. Even if methodological standards for the development of GCP guidelines are published adherence to these remains unsatisfactory.
Acknowledgment
Our work is funded by the German Guideline Program in Oncology of the Association of the Scientific Medical Societies, the German Cancer Society, and the German Cancer Aid. We would like to thank Dr Austin Duffy for critical reading of the manuscript and helpful comments.
Footnotes
Conflict of interest statement: The authors indicated no potential conflicts of interest
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010; 127: 2893–917. [DOI] [PubMed] [Google Scholar]
- 2.Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J. Hepatol 2009; 50: 89–99. [DOI] [PubMed] [Google Scholar]
- 3.Field MJ, Lohr KN, eds. Clinical Practice Guidelines: Directions for A New Program. Washington DC: Institute of Medicine, National Academy Press, 1990. [PubMed] [Google Scholar]
- 4.Smith TJ, Hillner BE. Ensuring quality cancer care by the use of clinical practice guidelines and critical pathways. J. Clin. Oncol 2001; 19: 2886–97. [DOI] [PubMed] [Google Scholar]
- 5.Nast A, Spuls PH, Ormerod AD et al. A critical appraisal of evidence-based guidelines for the treatment of psoriasis vulgaris: “AGREE-ing” on a common base for European evidence-based psoriasis treatment guidelines. J. Eur. Acad. Dermatol. Venereol 2009; 23: 782–7. [DOI] [PubMed] [Google Scholar]
- 6.Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA 1999; 281: 1900–5. [DOI] [PubMed] [Google Scholar]
- 7.Burgers JS, Bailey JV, Klazinga NS et al. Inside guidelines: comparative analysis of recommendations and evidence in diabetes guidelines from 13 countries. Diabetes Care 2002; 25: 1933–9. [DOI] [PubMed] [Google Scholar]
- 8.Georg G, Colombet I, Durieux P, Ménard J, Meneton P. A comparative analysis of four clinical guidelines for hypertension management. J. Hum. Hypertens 2008; 22: 829–37. [DOI] [PubMed] [Google Scholar]
- 9.Christiaens T, De Backer D, Burgers J, Baerheim A. Guidelines, evidence, and cultural factors. Scand. J. Prim. Health Care 2004; 22: 141–5. [DOI] [PubMed] [Google Scholar]
- 10.Cluzeau FA, Burgers JS, Brouwers M et al. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual. Saf. Health Care 2003; 12: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giovannini M, Elias D, Monges G, Raoul JL, Rougier P; French National Federation of Cancer (FNCLCC). Hepatocellular carcinoma. Br. J. Cancer 2001; 84 (Suppl.2): 74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, Llovet JM et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol 2001; 35: 421–30. [DOI] [PubMed] [Google Scholar]
- 13.Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 2003; 52 (Suppl.3): iii1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Vlierberghe H, Borbath I, Delwaide J et al. BASL guidelines for the surveillance, diagnosis and treatment of hepatocellular carcinoma. Acta Gastroenterol. Belg 2004; 67: 14–25. [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–36. [DOI] [PubMed] [Google Scholar]
- 16.Abdo AA, Al Abdul Karim H, Al Fuhaid T et al. Saudi Gastroenterology Association guidelines for the diagnosis and management of hepatocellular carcinoma: summary of recommendations. Ann. Saudi Med 2006; 26: 261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makuuchi M, Kokudo N. Clinical practice guidelines for hepatocellular carcinoma: the first evidence based guidelines from Japan. World J. Gastroenterol 2006; 12: 828–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Okanoue T. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology 2007; 72 (Suppl.1): 2–15. [DOI] [PubMed] [Google Scholar]
- 19.Parikh P, Malhotra H, Jelic S. Hepatocellular carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol 2008; 19 (Suppl.2): ii27–8. [DOI] [PubMed] [Google Scholar]
- 20.Benson AB 3rd, Abrams TA, Ben-Josef E et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J. Natl. Compr. Canc. Netw 2009; 7: 350–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poon D, Anderson BO, Chen LT et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009; 10: 1111–18. [DOI] [PubMed] [Google Scholar]
- 22.Haute Autorité de Santé. [Indications for hepatic transplantation—January 19 and 20, 2005, Lyon (Palais des congres)]. J. Chir. (Paris) 2005; 142: 177–83. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy A, Nag S, Salem R et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int. J. Radiat. Oncol. Biol. Phys 2007; 68: 13–23. [DOI] [PubMed] [Google Scholar]
- 24.Claudon M, Cosgrove D, Albrecht T et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—update 2008. Ultraschall Med. 2008; 29: 28–44. [DOI] [PubMed] [Google Scholar]
- 25.Brown DB, Cardella JF, Sacks D et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J. Vasc. Interv. Radiol 2009; 20 (Suppl. 7): S219–S226. S226 e1–10. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki S, Takayama T. Surgical treatment of hepatocellular carcinoma: evidence-based outcomes. World J. Gastroenterol 2008; 14: 685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boige V, Barbare JC, Rosmorduc O. [Use of sorafenib (Nexavar) in the treatment of hepatocellular carcinoma: PRODIGE AFEF recommendations]. Gastroenterol. Clin. Biol 2008; 32 (1 Pt 1): 3–7. [DOI] [PubMed] [Google Scholar]
- 28.Brown DB, Bakal CW, Weintraub JL et al. Expert Panel on Interventional Radiology, Hepatic malignancy. American College of Radiology (ACR), January2007. [Google Scholar]
- 29.Foley WD, Bree RL, Gay SB et al. Expert Panel on Gastrointestinal Imaging, Liver lesion characterization. American College of Radiology (ACR)2006. [Google Scholar]
- 30.Radiology ACO. ACR Practice Guideline for Radioembolization with microsphere brachytherapy device (RMBD) for treatment of liver malignancies. 2008.
- 31.Knox J, Cosby R, Chan K, Sherman M. Sorafenib for Advanced Hepatocellular Carcinoma: Recommendations and Evidentiary Base. Cancer Care Ontario CED-SOS Advice Report #7. 2008. [Google Scholar]
- 32.Marcaccio M, Langer B, Rumble B, Hunter A et al. Hepatic, Pancreatic, and Biliary Tract (HPB) Surgical Oncology Standards. Cancer Care Ontario. Surgery Standards and Evidence-based Series (EBS). 17–2Standards Special Report; 2006. [Google Scholar]
- 33.National Institute for Health and Clinical Excellence. NICE GUIDANCE IPG214 Microwave ablation of hepatocellular carcinoma. 2007.
- 34.National Institute for Health and Clinical Excellence. NICE GUIDANCE IPG2 Radiofrequency ablation of hepatocellular carcinoma. 2003.
- 35.National Institute for Health and Clinical Excellence. NICE GUIDANCE IPG 135 Laparoscopic liver resection. 2005.
- 36.National Institute for Health and Clinical Excellence. NICE GUIDANCE IPG 194 Living-donor-liver transplantation. 2006.
- 37.National Institute for Health and Clinical Excellence. NICE GUIDANCE IPG 298 Ex-vivo hepatic resection and reimplantation for liver cancer. 2009.
- 38.National Institute for Health and Clinical Excellence. NICE GUIDANCE IPG 211 Radiofrequency-assisted liver resection. 2007.
- 39.Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc. Intervent. Radiol 2010; 33: 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Guidelines for Diagnostic Laparoscopy. 2007.
- 41.Redmond HP, Andrews E, Hill ADK. Royal College of Surgeons in Ireland. Diagnostic laparoscopy. 2005. [Google Scholar]
- 42.Sistema Nazionale Linee Guida (SNLG). Diagnostic imaging of focal hepatic lesions. 2009.
- 43.Burgers JS, Cluzeau FA, Hanna SE, Hunt C, Grol R. Characteristics of high-quality guidelines: evaluation of 86 clinical guidelines developed in ten European countries and Canada. Int. J. Technol. Assess. Health Care 2003; 19: 148–57. [DOI] [PubMed] [Google Scholar]
- 44.Vlayen J, Aertgeerts B, Hannes K, Sermeus W, Ramaekers D. A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int. J. Qual. Health Care 2005; 17: 235–42. [DOI] [PubMed] [Google Scholar]
- 45.Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ 1999; 318: 527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nast A, Erdmann R, Hofelich V et al. Do guidelines change the way we treat? Studying prescription behaviour among private practitioners before and after the publication of the German Psoriasis Guidelines. Arch. Dermatol. Res 2009; 301: 553–9. [DOI] [PubMed] [Google Scholar]
- 47.Fervers B, Burgers JS, Haugh MC et al. Predictors of high quality clinical practice guidelines: examples in oncology. Int. J. Qual. Health Care 2005; 17: 123–32. [DOI] [PubMed] [Google Scholar]
- 48.Choudhry NK, Stelfox HT, Detsky AS. Relationships between authors of clinical practice guidelines and the pharmaceutical industry. JAMA 2002; 287: 612–17. [DOI] [PubMed] [Google Scholar]
- 49.McAlister FA, van Diepen S,Padwal RS, Johnson JA, Majumdar SR. How evidence-based are the recommendations in evidence-based guidelines? PLoS Med. 2007; 4: e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van der Wees PJ, Hendriks EJ, Custers JW, Burgers JS, Dekker J, de Bie RA. Comparison of international guideline programs to evaluate and update the Dutch program for clinical guideline development in physical therapy. BMC Health Serv. Res 2007; 7: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]


