Abstract
Cisplatin-based combination treatment is the most effective systemic chemotherapy for bladder cancer; however, resistance to cisplatin remains a significant problem in the treatment of this disease. β-Elemene is a new natural compound that blocks cell-cycle progression and has a broad spectrum of antitumor activity. This study was conducted to explore the potential of β-elemene as a chemosensitizer for enhancing the therapeutic efficacy and potency of cisplatin in bladder cancer and other solid carcinomas. β-Elemene not only markedly inhibited cell growth and proliferation but also substantially increased cisplatin cytotoxicity towards human bladder cancer 5637 and T-24 cells. Similarly, β-elemene also enhanced cisplatin sensitivity and augmented cisplatin cytotoxicity in small-cell lung cancer and carcinomas of the brain, breast, cervix, ovary, and colorectal tract in vitro, with dose-modifying factors ranging from 5 to 124. β-Elemene-enhanced cisplatin cytotoxicity was associated with increased apoptotic cell death, as determined by DNA fragmentation, and increased activities of caspase-3, -7, -8, -9, and -10 in bladder cancer cell lines. Collectively, these results suggest that β-elemene augments the antitumor activity of cisplatin in human bladder cancer by enhancing the induction of cellular apoptosis via a caspase-dependent mechanism. Cisplatin combined with β-elemene as a chemosensitizer warrants further pre-clinical therapeutic studies and may be useful for the treatment of cisplatin-resistant bladder cancer and other types of carcinomas.
Keywords: Apoptosis, bladder cancer, cisplatin resistance, Chinese medicine, β-elemene, caspases
Urinary bladder cancer is the second most common malignant urological tumor, following prostate carcinoma, in the United States (1). During the past 30 years, there has been slow yet steady progress in the development of novel chemotherapeutic strategies for the management of advanced and metastatic bladder cancer (2–4). Nevertheless, current chemotherapy confers only a modest survival benefit on patients with bladder cancer; metastatic disease remains essentially incurable, with only a small number of patients achieving long-term disease control. Typical combination regimens such as cyclophosphamide-doxorubicin-cisplatin and methotrexate-cisplatin produce increased toxicity without a significant improvement in survival compared with single-agent therapy (2, 3, 5, 6). Recently, several novel compounds have been shown to be active against bladder carcinoma and have been tested in combination chemotherapy trials. The combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) is considered the gold standard for treatment of metastatic bladder cancer, with response rates of 40–72% and median survival times of 12–13 months (7–12). However, in a recent long-term study, only 3.7% of patients randomized to MVAC were alive and continuously disease-free for six years (3, 12). The MVAC regimen is also associated with considerable treatment-related toxicity. The development of more effective and less toxic therapeutic regimens for patients with bladder cancer is vital. Combination therapy is gaining attention as an effective approach for enhancing efficacy and minimizing systemic toxicity of chemotherapeutic agents (2, 3, 10, 12).
Elemene (1-methyl-1-vinyl-2,4-di-isopropenyl-cyclohexane) has been extracted from numerous plants and has been identified in more than 50 different medicinal herbs and plants. In China, β-elemene (Figure 1), the main active component of elemene, has been used effectively in the treatment of hyperplastic and proliferative disorders, including prostatic hypertrophy, hysteromyoma, and neoplasms; the State Food and Drug Administration of China has approved one of its formulations (85% β-elemene) for the treatment of primary and secondary brain tumors and other carcinomas (13–17). The major advantages of β-elemene as an anticancer agent are that it has antitumor activity toward a broad spectrum of cancer types, it is associated with a low level of toxicity, and it is well-tolerated by patients with cancer (13–17). Our recent studies have demonstrated that β-elemene exhibits strong inhibitory activity as a single agent and augments the cytotoxic efficacy and potency of cisplatin in ovarian cancer, non-small cell lung cancer, and other tumor cell types (18–28). However, the effect of β-elemene on human bladder cancer and the interaction of β-elemene and cisplatin as a chemotherapeutic regimen for bladder cancer remain to be determined.
Figure 1.
The chemical structure of β-elemene.
This study was conducted to explore the possibility of using β-elemene as an agent to sensitize cancer cells to cisplatin. We found that β-elemene not only inhibited cell proliferation but also enhanced cisplatin-induced cell apoptosis in the human bladder cancer cell lines 5637 and T-24. Moreover, β-elemene enhanced cisplatin sensitivity and increased cisplatin cytotoxicity in carcinomas of the brain, breast, cervix, colorectum, lung, and ovary in vitro. Our results suggest that the novel combination therapy of β-elemene and cisplatin may be beneficial for patients with bladder cancer and other tumor types.
Materials and Methods
Chemicals and reagents.
RPMI-1640 culture medium, fetal bovine serum (FBS), penicillin-streptomycin-glutamine, and 0.25% trypsin-EDTA solution were obtained from Invitrogen Corp., Life Technologies (Carlsbad, CA, USA). (−)-β-Elemene (98% purity) was from Dalian Yuanda Pharmaceutical Co. (Dalian, China). Cisplatin was purchased from Sigma-Aldrich (St. Louis, MO, USA). CellTiter 96 Aqueous ONE Solution Cell Proliferation Assay was from Promega Corp. (Madison, WI, USA), Cell Death Detection ELISAPLUS was from Roche Diagnostics Corp. (Indianapolis, IN, USA), and the Cas-PASE™-9, -3, -7, -10 assay kits were from Geno Technology (St. Louis, MO, USA).
Cells and cell culture.
The two human bladder cancer cell lines 5637 and T-24 were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Two human brain glioblastoma cell lines, one human small cell lung cancer cell line, one human ovarian carcinoma cell line, one human colorectal adenocarcinoma cell line, two human cervical carcinoma cell lines, and two human breast cancer cell lines (all listed in Table I) were also purchased from the ATCC. All human carcinoma cell lines were grown in RPMI-1640 supplemented with 10% FBS, 50 IU/ml penicillin, and 50 μg/ml streptomycin, at 37°C in a humidified atmosphere with 5% CO2.
Table I.
β-Elemene increases cisplatin cytotoxicity and enhances cisplatin sensitivity in human cancer cells, as determined by the MTT assay.
| Cancer cell line | IC50 of cisplatin (μM) | IC50 of cisplatin with β-elemene (μM) | DMF |
|---|---|---|---|
| A-172 brain glioblastoma | 24.0 | 0.25 | 96 |
| U-87MG brain glioblastoma | 10.0 | 1.8 | 5.6 |
| NCI-H69 small cell lung cancer | 8.0 | 1.5 | 5.3 |
| MCAS ovarian carcinoma | 38.0 | 6.5 | 5.8 |
| HeLa cervical adenocarcinoma | 27.5 | 3.0 | 9.2 |
| ME-180 cervical carcinoma | 32.0 | 3.8 | 8.4 |
| COLO 205 colorectal adenocarcinoma | 32.0 | 3.5 | 9.1 |
| MCF-7 breast adenocarcinoma | 28.0 | 0.38 | 73.7 |
| T47D breast carcinoma | 31.0 | 0.25 | 124 |
IC50: Half maximal inhibitory concentration, defined as the concentration of β-elemene or cisplatin needed for 50% inhibition of cell growth and proliferation; DMF: dose-modifying factor, defined as the IC50 for cisplatin without β-elemene divided by the IC50 for cisplatin with β-elemene, i.e. DMF=IC50 (cisplatin) ÷ IC50 (cisplatin + β-elemene); MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Growth inhibition assay.
The antiproliferative effects of β-elemene, cisplatin-alone, and cisplatin plus β-elemene were assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-based assay (Promega Corp.) according to the manufacturer’s instructions. In brief, 5637, T-24, or other carcinoma cells were evenly distributed in 96-well plates (5×103 cells/well), grown overnight, and then treated with different concentrations of β-elemene (0, 20, 40, 60, 80, 100, 120, 140, 160, 180, and 200 μg/ml), or cisplatin (0, 1.0, 2.0, 4.0, 8.0, 16.0, 32.0, 64.0, 128.0, 256.0, and 512.0 μM), or a combination of cisplatin (at the concentrations shown above) plus β-elemene (40 μg/ml) for 24, 48, and 72 h. After incubation, 20 μl of CellTiter 96 Aqueous One Solution reagent were added to each well of the assay plates containing the treated and untreated cells in 100 μl of culture medium, and the plates were incubated at 37°C and 5% CO2 for 1–4 h. The optical density at 590 nm was determined using a 96-well plate reader. Proliferation rates were calculated from the optical densities of the drug-treated cells relative to the optical density of cells with no added drug (control value, 100%).
Cell death detection using enzyme-linked immunosorbent assay (ELISA) for apoptosis.
5637 or T-24 cells were evenly distributed in 96-well plates (1×104 cells/well), grown overnight, and then treated with different concentrations of β-elemene (0, 40, 60, and 80 μg/ml), or cisplatin (0, 4.0, 8.0, and 16.0 μM), or cisplatin (0, 4.0, 8.0, and 16.0 μM) plus β-elemene (40 μg/ml) for 24 and 48 h. The cells were harvested and assayed for apoptosis using a Cell Death Detection ELISA kit (Roche) following the manufacturer’s instructions. This quantitative sandwich enzyme immunoassay uses mouse monoclonal antibodies against DNA and histones for the spectrophotometric quantitation of histone-associated DNA fragments (mono- and oligonucleosomes) in the cytoplasmic fractions of cell lysates.
Caspase activity assay.
The caspase activities were assayed using a CasPASE™ apoptosis assay kit (Geno Technology), according to the manufacturer’s instructions. In brief, T-24 cells (2×107) were treated with β-elemene (0, 40, 60, and 80 μg/ml), or cisplatin (0, 4.0, 8.0, and 16.0 μM), or cisplatin (0, 4.0, 8.0, and 16.0 μM) plus β-elemene (40 μg/ml) for 24 and 48 h. The cells were collected by trypsinization, washed once with phosphate-buffered saline (PBS), collected by centrifugation, suspended in 350 μl of lysis buffer, and lysed by five separate freeze-thaw cycles. The lysates were clarified by centrifugation at 13,000 ×g for 30 min at 4°C, and the caspase-9, caspase-8, and caspase-3/7/10 activities in the supernatants were measured using a CasPASE™ apoptosis assay kit and specific fluorogenic substrates: Asp-Glu-Val-Asp (DEVD) peptide conjugated to 7-amino-4-trifluoromethylcoumarin (AFC) for caspase-3/7/10 activities; Leu-Glu-Thr-Asp (LETD)-AFC for caspase-8 activity; and Leu-Glu-His-Asp (LEHD)-AFC for caspase-9 activity. The absorbance at 490 nm was determined using a microplate reader.
Statistical data analysis.
All quantitative values are presented as means±SD. Data were statistically analyzed using two-way analysis of variance (ANOVA) for comparison among groups. Student’s t-test was used to analyze the statistical significance of differences between untreated controls and drug-treated groups. All p-values were determined using a two-sided test, and p-values <0.05 were considered to indicate significance.
Results
β-Elemene inhibits cell growth and proliferation and promotes cisplatin-induced cytotoxicity in human bladder cancer and other types of cancer cells.
To investigate the effect of β-elemene on bladder cancer cell growth, the dose–response and time course of β-elemene inhibition of the growth of the human bladder cancer cell lines 5637 and T-24 were characterized in vitro using the MTT assay. β-Elemene at concentrations of 20 to 160 μg/ml caused dose-dependent inhibition of the growth and proliferation of both 5637 and T-24 cells at 24, 48, and 72 h (Figure 2A; data at 48 and 72 h not shown). The half-maximal inhibitory concentration (IC50) values of β-elemene at 24, 48, and 72 h were 85, 78, and 72 μg/ml, respectively, for 5637 cells and 76, 72, and 67 μg/ml, respectively, for the T-24 cells (Figure 2A). The values did not differ significantly between the two cell lines (p>0.05), indicating a general antitumor activity of β-elemene towards human bladder cancer cells in vitro.
Figure 2.
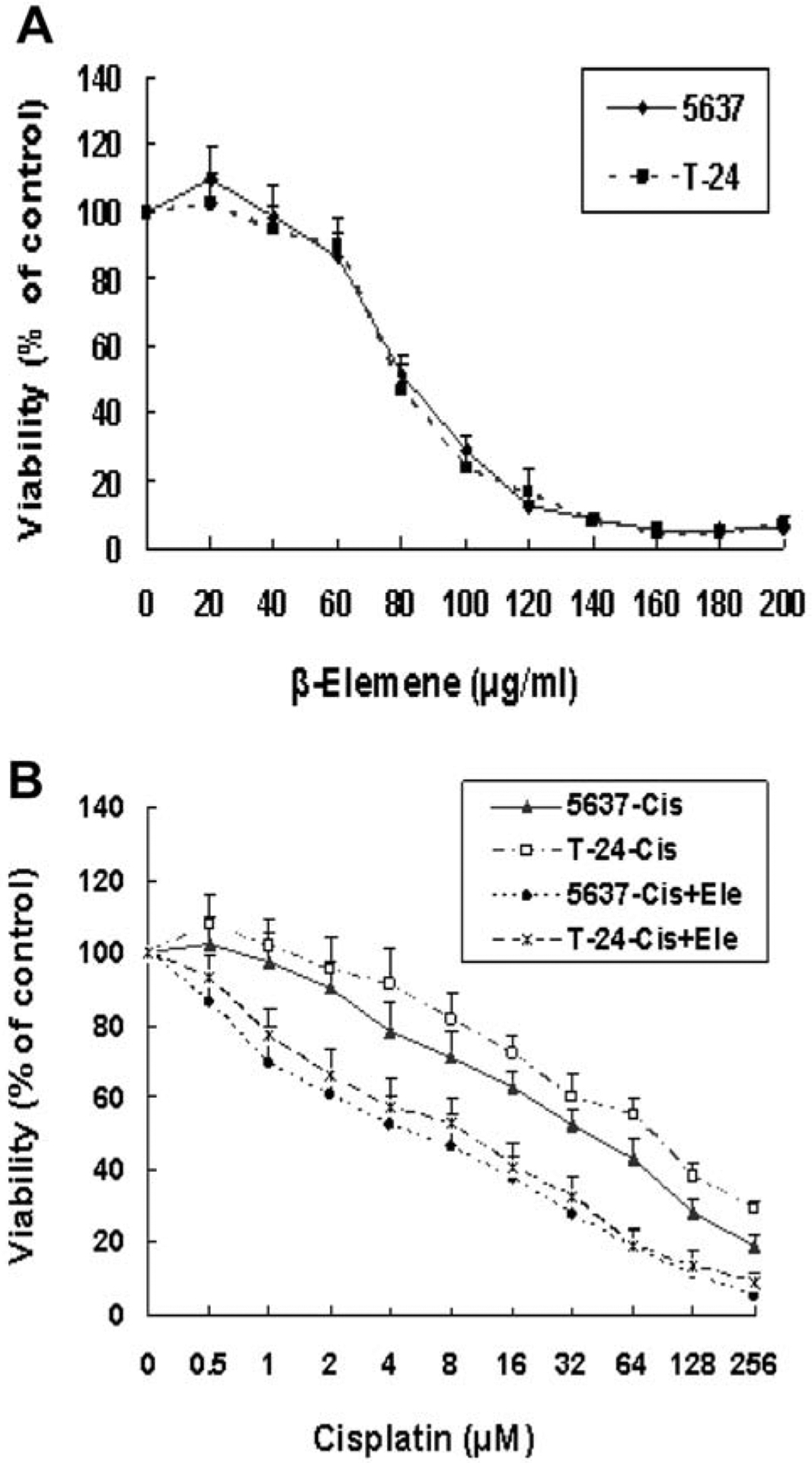
β-Elemene inhibits cell proliferation and promotes cisplatin–induced cytotoxicity in human bladder cancer cells, as determined by MTT assay. 5637 or T-24 cells were evenly distributed in 96-well plates (5×103 cells/well) and treated for 24, 48, and 72 h with β-elemene (Ele)-alone (A), cisplatin (Cis)-alone, or cisplatin at the indicated concentrations plus 40 μg/ml of β-elemene (B). Data at 48 and 72 h are not shown. The inhibition of cell growth was determined by the MTT assay, as described in the Materials and Methods. Cell viability is expressed relative to that of cells in wells with no added drug (untreated control value, 100%). The results represent the means±SD of at least three independent experiments. p<0.05 for the cisplatin group vs. cisplatin + β-elemene group of corresponding cell line. MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Next, we tested the combined effect of β-elemene and cisplatin on bladder cancer cell growth, using the MTT assay. The 5637 and T-24 cells were exposed to different concentrations of cisplatin, either alone or in combination with β-elemene, as indicated for 24, 48, and 72 h, and the inhibition of cell growth was measured in vitro. Cisplatin at concentrations of 0.5 to 256.0 μM caused a dose-dependent inhibition of 5637 and T-24 cell proliferation at all three time points tested (Figure 2B; data at 48 and 72 h not shown). The IC50 values of cisplatin for 5637 cells were 52.0, 36.0, and 20.0 μM at 24, 48, and 72 h, respectively. When cisplatin was combined with β-elemene (40 μg/ml), the IC50 values of cisplatin for the same cell line decreased significantly to 7.0, 4.0, and 2.5 μM at 24, 48, and 72 h, respectively (Figure 2B; p<0.05). Similarly, the IC50 values of cisplatin for T-24 cells were 112.0, 68.0, and 36.0 μM at 24, 48, and 72 h, respectively, and decreased significantly to 12.0, 7.0, and 4.0 μM at 24, 48, and 72 h, respectively, when cisplatin was combined with β-elemene at 40 μg/ml (Figure 2B; p<0.05). These results indicate that β-elemene significantly sensitizes bladder cancer cells to cisplatin-induced growth suppression and proliferation inhibition in our model system.
We have previously shown that β-elemene enhances cisplatin activity in prostate cancer cells and non-small cell lung cancer cells (20, 21, 27). To further explore the spectrum of cancer cells inhibited by the interaction of β-elemene with cisplatin, we used the MTT assay to examine the effect of β-elemene on the in vitro cisplatin cytotoxicity in six other types of human carcinoma cells: two breast carcinoma lines, two brain tumor lines, two cervical carcinoma lines, one colorectal carcinoma line, one ovarian carcinoma line, and one small-cell lung cancer line. As shown in Table I, β-elemene enhanced cisplatin sensitivity and augmented cisplatin antitumor activity in all examined cancer cell lines. The dose-modifying factors (DMFs) ranged from 5.3 to 9.2 in colorectal, cervical, ovarian, and small-cell lung cancer cells, and from 73.7 to 124 in breast and brain carcinoma cells (Table I). These data suggest that β-elemene increases cisplatin antitumor activity across a broad spectrum of solid carcinoma types.
β-Elemene triggers apoptosis and enhances cisplatin-induced apoptotic cell death in human bladder cancer cells.
The ability of cancer chemotherapeutic agents to induce cell-cycle arrest and apoptosis is an important determinant of their therapeutic efficacy. To determine whether the inhibition of bladder cancer cell growth by β-elemene (Figure 2) is attributable to its ability to induce apoptosis, we treated 5637 and T-24 cells with β-elemene at 0, 40, 60, and 80 μg/ml for 24 and 48 h, followed by ELISA-based quantitation of histone-associated DNA fragments, which suggest DNA degradation and apoptosis (29). β-Elemene at 60 and 80 μg/ml markedly enhanced DNA fragmentation, indicating an increased induction of apoptosis in bladder cancer cells at both 24 and 48 h (Figure 3A).
Figure 3.

β-Elemene triggers apoptosis and significantly enhances cisplatin-induced apoptosis of human bladder cancer cells, as assessed by a DNA fragmentation assay. 5637 or T-24 cells were treated with β-elemene (Ele)-alone (A), cisplatin (Cis)-alone, or cisplatin plus β-elemene (40 μg/ml) (B) for 24 and 48 h. Cellular apoptosis was determined based on DNA fragmentation detected with an ELISA-based cell death detection kit, as described in the Materials and Methods. The results are expressed as the means±SD of six independent experiments. (A) β-Elemene-alone treatment. *p<0.05 vs. untreated control of corresponding cell line at the respective time points. (B) Cisplatin-alone and cisplatin + β-elemene treatments. *p<0.05 vs. untreated control of corresponding time point and vs. cisplatin alone at the respective concentrations. ELISA: enzyme-linked immunosorbent assay.
The major goal of cancer chemotherapy is to commit cancer cells to apoptosis following exposure to antitumor agents (30–32). We assessed whether β-elemene promoted cisplatin-induced apoptosis in T-24 cells based on cytoplasmic histone-associated DNA fragments. As shown in Figure 3B, β-elemene and cisplatin, in combination, markedly induced apoptosis compared with cisplatin alone in our model system, suggesting that β-elemene strongly promotes cisplatin-induced apoptotic cell death in human bladder cancer cells.
β-Elemene activates caspases and augments cisplatin-increased caspase activities in human bladder cancer cells.
Caspases are the central executors of the apoptotic process; in particular, caspase-3, caspase-8, and caspase-9 are markers of apoptotic pathways (31–34). To investigate the possible mechanisms by which β-elemene triggers apoptosis and promotes cisplatin-induced apoptosis in human bladder cancer T-24 cells (Figure 3), we examined the effects of β-elemene and cisplatin on the activities of caspase-3/7/10, caspase-8, and caspase-9, using an ELISA-based assay. In T-24 cells at both 24 and 48 h, β-elemene (40 μg/ml) not only significantly induced the activities of caspase-8 (Figure 4A), caspase-9 (Figure 4C), and caspase-3/7/10 (Figure 4E) but also remarkably augmented the cisplatin-induced activities of caspase-8 (Figure 4B), caspase-9 (Figure 4D) and caspase-3/7/10 (Figure 4F), compared with the activities in the presence of cisplatin alone. These results indicate that β-elemene triggers apoptosis and promotes cisplatin-induced apoptotic cell death in human bladder cancer T-24 cells via caspase-dependent apoptotic pathways.
Figure 4.

β-Elemene induces caspase activities and remarkably enhances cisplatin-increased caspase activities in human bladder cancer cells, as measured by an ELISA-based assay. T-24 cells were treated with β-elemene (Ele)-alone, cisplatin (Cis)-alone, or cisplatin plus β-elemene (40 μg/ml) for 24 and 48 h. Caspase-8 (A and B), caspase-9 (C and D), and caspase-3/7/10 activities (E and F) were measured using a CasPASE™ apoptosis assay kit, as described in Materials and Methods. The results are expressed as the means±SD of three independent experiments. A, C and E: β-Elemene alone treatment. *p<0.05 vs. untreated control at the respective time points. B, D and F: Cisplatin alone and cisplatin plus β-elemene treatments. *p<0.05 vs. untreated control at corresponding time point and vs. cisplatin-alone at the respective concentrations. ELISA: enzyme-linked immunosorbent assay.
Discussion
Bladder cancer is the second most common malignant urological tumor, following prostate carcinoma, in the United States (1). Chemotherapy has been used to treat bladder cancer for nearly 50 years. Although several novel compounds have been shown to be active against transitional cell carcinoma and are now being tested in combination chemotherapy trials, cisplatin remains one of the most active ‘standard’ single-agents and the mainstay of typical combination regimens against bladder cancer (2–4, 7–11, 35). However, cisplatin is highly toxic, and its efficacy and effectiveness are limited owing to the development of drug resistance in tumor cells. Thus, the development of new and effective agents to overcome platinum chemoresistance in bladder carcinoma continues to have high priority.
β-Elemene, originally derived from plants, has been recently investigated as a new anticancer agent (13–17). In the present study, we demonstrated that β-elemene inhibited the growth and proliferation of human bladder cancer 5637 and T-24 cells in a concentration-dependent manner. These results are consistent with previous findings that β-elemene is a potent inhibitor of several types of solid tumor, but has only moderate effects on normal and non-cancerous cells (18–28). In addition, we showed that β-elemene enhanced cisplatin sensitivity and increased cisplatin cytotoxicity in bladder cancer and carcinomas of the brain, breast, cervix, colorectum, lung, and ovary in vitro, with dose-modifying factors ranging from 5 to 124. In recent studies, β-elemene augmented cisplatin activity in prostate and ovarian carcinoma and non-small cell lung cancer cells (19, 21, 22, 26, 28). The present study broadens the spectrum of this effect to include bladder, brain, breast, cervical, colorectal, and small-cell lung carcinomas. Most importantly, this study demonstrates that lower doses of cisplatin can be used when administered in combination with β-elemene, and this may result in lower clinical toxicity.
The main goal of chemotherapy for human malignancies is the inhibition of cell proliferation and induction of cell apoptosis. Apoptosis is an important process in tissue homeostasis and a key cytotoxic mechanism of anticancer therapies. It is controlled by many factors that interact in a complex web of regulation. In mammalian cells, two major apoptosis pathways have been proposed: an extrinsic pathway, which involves signal transduction through cell-surface death receptors, and an intrinsic pathway, which is triggered by various stressors such as ultraviolet radiation or chemical agents. It is generally accepted that most human malignancies, including bladder cancer, have aberrations or defects in initiating or executing the apoptotic pathways; therefore, targeting the regulation of apoptosis represents an important pharmacological strategy for the development of chemotherapeutic agents (30–33). In the present study, β-elemene dose-dependently triggered bladder cancer cell apoptosis and remarkably promoted cisplatin-induced DNA fragmentation, consistent with apoptotic cell death, in both bladder cancer cell lines. These observations indicate that the induction of apoptosis by β-elemene and cisplatin may account for their antitumor activity in bladder cancer.
Caspases are cysteine proteases that mediate apoptotic cell death in a variety of cellular systems and are integral to the two major apoptotic pathways (intrinsic and extrinsic). To date, 14 distinct mammalian caspases have been identified and classified as initiator or effector caspases. Various apoptotic signals activate the initiator caspases (caspase-2, -8, -9 and -10), which are cleaved and subsequently activate downstream effector caspases. The effector caspases (caspase-3, -6 and -7) target specific cellular protein substrates in a series of proteolytic events. Thus, caspases play vital roles in the apoptotic process, and caspase-8 and caspase-9 are regarded as markers of different apoptotic pathways (32–34). Increasing evidence demonstrates that caspase cascades, especially via caspse-3, -7, -8 and -9, are involved in the execution of apoptosis in cancer cells in response to diverse stimuli, including therapeutic agents. Therefore, pharmacological manipulation or genetic targeting of caspases is a potential therapeutic strategy (31, 32, 36, 37). The current study showed that β-elemene increased the activities of caspase-8, caspase-9, and caspase-3/7/10, and also promoted cisplatin-induced activation of caspase-8, caspase-9, and caspase-3/7/10 activities in our model system. These results suggest that β-elemene triggers apoptosis and promotes cisplatin-induced apoptotic cell death through caspase-mediated apoptotic pathways in human bladder carcinoma cells.
Altogether, the findings in this study clearly demonstrate that β-elemene not only suppresses the growth and proliferation of human bladder cancer cells, but also augments the antitumor efficacy and potency of cisplatin in carcinomas of the bladder, brain, breast, cervix, colorectum, lung, and ovary in vitro, with dose-modifying factors ranging from 5 to 124. The increased cisplatin cytotoxicity caused by concomitant β-elemene administration was associated with increased apoptotic cell death mediated through caspase-dependent apoptotic pathways in bladder cancer cells. The broad antitumor spectrum of β-elemene makes it an ideal candidate for combination therapy with platinum to treat many different types of cancer. This work provides a rationale for pre-clinical therapeutic studies of cisplatin in combination with β-elemene in in vivo animal models and suggests that combining cisplatin with β-elemene as a chemosensitizer or adjuvant may be potentially useful for the treatment of chemoresistant bladder cancer and other types of carcinomas.
Acknowledgements
This publication was made possible by grants from the Natural Science Foundation of Science & Technology Department of Guangxi Province (No. 0991294) and the Guangxi Scientific Research and Technological Development Program (No. 200901059), and by grants from the National Institutes of Health (Nos. P20RR16440-010003, P20RR16440-020003, P20RR16440-030003, P20RR16440-040003) and a West Virginia University School of Medicine Research Grant (to Q. Q. Li).
References
- 1.Siegel R, Naishadham D and Jemal A: Cancer statistics, 2013. CA Cancer J Clin 63: 11–30, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Bellmunt J and Petrylak DP: New therapeutic challenges in advanced bladder cancer. Semin Oncol 39: 598–607, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF, Dreicer R, George DJ, Milowsky MI, Theodorescu D, Vaughn DJ, Galsky MD, Soloway MS and Quinn DI: ICUD-EAU International Consultation on Bladder Cancer 2012: Chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol 63: 58–66, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Dreicer R, Li H, Stein M, DiPaola R, Eleff M, Roth BJ and Wilding G: Phase 2 trial of sorafenib in patients with advanced urothelial cancer: A trial of the Eastern Cooperative Oncology Group. Cancer 115: 4090–4095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troner M, Birch R, Omura GA and Williams S: Phase III comparison of cisplatin alone versus cisplatin, doxorubicin and cyclophosphamide in the treatment of bladder (urothelial) cancer: A Southeastern Cancer Study Group trial. J Urol 137: 660–662, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Hillcoat BL, Raghavan D, Matthews J, Kefford R, Yuen K, Woods R, Olver I, Bishop J, Pearson B and Coorey G: A randomized trial of cisplatin versus cisplatin plus methotrexate in advanced cancer of the urothelial tract. J Clin Oncol 7: 706–709, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Bamias A, Dafni U, Karadimou A, Timotheadou E, Aravantinos G, Psyrri A, Xanthakis I, Tsiatas M, Koutoulidis V, Constantinidis C, Hatzimouratidis C, Samantas E, Visvikis A, Chrisophos M, Stravodimos K, Deliveliotis C, Eleftheraki A, Pectasides D, Fountzilas G and Dimopoulos MA: Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: A Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol (in press), 2012. [DOI] [PubMed] [Google Scholar]
- 8.Blick C, Hall P, Pwint T, Al-Terkait F, Crew J, Powles T, Macaulay V, Munro N, Douglas D, Kilbey N, Protheroe A and Chester JD: Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (AMVAC) as neoadjuvant chemotherapy for patients with muscle-invasive transitional cell carcinoma of the bladder. Cancer 118: 3920–3927, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Edeline J, Loriot Y, Culine S, Massard C, Albiges L, Blesius A, Escudier B and Fizazi K: Accelerated MVAC chemotherapy in patients with advanced bladder cancer previously treated with a platinum-gemcitabine regimen. Eur J Cancer 48: 1141–1146, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Pliarchopoulou K, Laschos K and Pectasides D: Current chemotherapeutic options for the treatment of advanced bladder cancer: A review. Urol Oncol (in press), 2010. [DOI] [PubMed] [Google Scholar]
- 11.Williams PD, Cheon S, Havaleshko DM, Jeong H, Cheng F, Theodorescu D and Lee JK: Concordant gene expression signatures predict clinical outcomes of cancer patients undergoing systemic therapy. Cancer Res 69: 8302–8309, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts JT, von der Maase H, Sengeløv L, Conte PF, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning M: Long-term survival results of a randomized trial comparing gemcitabine/cisplatin and methotrexate/vinblastine/doxorubicin/cisplatin in patients with locally advanced and metastatic bladder cancer. Ann Oncol 17: 118–122, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Zheng S, Yang H, Zhang S, Wang X, Yu L, Lu J and Li J: Initial study on naturally occurring products from traditional Chinese herbs and vegetables for chemoprevention. J Cell Biochem 27: 106–112, 1997. [PubMed] [Google Scholar]
- 14.Qian J and Qin S: Pharmacological and clinical Studies of elemene, a new anticancer drug. Chin Clin Cancer 26: 1–3, 1999. [Google Scholar]
- 15.Zhou H, Shen J, Hou J, Qiu Y and Luo Q: Experimental study on apoptosis induced by elemene in glioma cells. Ai Zheng 22: 959–963, 2003. [PubMed] [Google Scholar]
- 16.Zou L, Liu W and Yu L: β-Elemene induces apoptosis of K562 leukemia cells. Zhonghua Zhong Liu Za Zhi 23: 196–198, 2001. [PubMed] [Google Scholar]
- 17.Wang B, Guo J, Di J and Shi Q: The experimental study of association between elemene and tumor multidrug-resistance. Chin Clin Cancer 26: 10–13, 1999. [Google Scholar]
- 18.Wang G, Li X, Huang X, Zhao J, Ding H, Cunningham C, Coad J, Flynn D, Reed E and Li QQ: Antitumor effect of β-elemene in non-small cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell Mol Life Sci 62: 881–893, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Wang G, Zhao J, Ding H, Cunningham C, Chen F, Flynn DC, Reed E and Li QQ: Antiproliferative effect of β-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell Mol Life Sci 62: 894–904, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Li QQ, Zou B, Wang G, Li X, Kim JE, Cuff CF, Huang L, Reed E and Gardner K: In vitro combination characterization of the new anticancer plant drug β-elemene with taxanes against human lung carcinoma. Int J Oncol 31: 241–252, 2007. [PubMed] [Google Scholar]
- 21.Li QQ, Wang G, Zhang M, Cuff CF, Huang L and Reed E: β-Elemene, a novel plant-derived antineoplastic agent, increases cisplatin chemosensitivity of lung tumor cells by triggering apoptosis. Oncol Rep 22: 161–170, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Li QQ, Wang G, Reed E, Huang L and Cuff CF: Evaluation of cisplatin in combination with β-elemene as a regimen for prostate cancer chemotherapy. Basic Clin Pharmacol Toxicol 107: 868–876, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Li QQ, Wang G, Huang F, Banda M and Reed E: Antineoplastic effect of β-elemene on prostate cancer cells and other types of solid tumour cells. J Pharm Pharmacol 62: 1018–1027, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Lee RX, Li QQ and Reed E: β-Elemene effectively suppresses the growth and survival of both platinum-sensitive and -resistant ovarian tumor cells. Anticancer Res 32: 3103–3113, 2012. [PMC free article] [PubMed] [Google Scholar]
- 25.Li QQ, Lee RX, Liang H and Zhong Y: Anticancer activity of β-elemene and its synthetic analogs in human malignant brain tumor cells. Anticancer Res 33: 65–76, 2013. [PMC free article] [PubMed] [Google Scholar]
- 26.Li QQ, Lee RX, Liang H, Zhong Y and Reed E: Enhancement of cisplatin-induced apoptosis by β-elemene in resistant human ovarian cancer cells. Med Oncol 30: 424–436, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou B, Li QQ, Zhao J, Li JM, Cuff CF and Reed E: β-Elemene and taxanes synergistically induce cytotoxicity and inhibit proliferation in ovarian cancer and other tumor cells. Anticancer Res 33: 929–940, 2013. [PubMed] [Google Scholar]
- 28.Li QQ, Wang G, Huang F, Li JM, Cuff CF and Reed E: Sensitization of lung cancer cells to cisplatin by β-elemene is mediated through blockade of cell cycle progression: Antitumor efficacies of β-elemene and its synthetic analogs. Med Oncol 30: 488–498, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Kim HS, Ingermann AR, Tsubaki J, Twigg SM, Walker GE and Oh Y: Insulin-like growth factor-binding protein 3 induces caspase-dependent apoptosis through a death receptor-mediated pathway in MCF-7 human breast cancer cells. Cancer Res 64: 2229–2237, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Bellmunt J and Guix M: New agents for bladder cancer. Ann Oncol Suppl 7: 56–58, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, Schlossman R, Ghobrial I, Raje N, Munshi N and Anderson KC: A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene 26: 2374–2380, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Quan T, He T, Franke T, Voorhees J and Fisher G: Epidermal growth factor receptor dependent, NF-κB-independent activation of the phosphatidylinositol 3-kinase/Akt pathway inhibits ultraviolet irradiation-induced caspases-3, -8, and -9 in human keratinocytes. J Biol Chem 278: 45737–45745, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone RW, Ruefli AA and Lowe SW: Apoptosis: A link between cancer genetics and chemotherapy. Cell 108: 153–164, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Thornberry NA and Lazebnik Y: Caspases: Enemies within. Science 281: 1312–1316, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Raghavan D, Shipley WU, Garnick MB, Russell PJ and Richie JP: Biology and management of bladder cancer. N Engl J Med 322: 1129–1138, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Ray S and Almasan A: Apoptosis induction in prostate cancer cells and xenografts by combined treatment with Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand and CPT-11. Cancer Res 63: 4713–4723, 2003. [PubMed] [Google Scholar]
- 37.Fiandalo MV and Kyprianou N: Caspase control: Protagonists of cancer cell apoptosis. Exp Oncol 34: 165–175, 2012. [PMC free article] [PubMed] [Google Scholar]


