Abstract
Breast conservation therapy (BCT) consisting of lumpectomy and postoperative radiation has become an accepted alternative to mastectomy (MRM) for the treatment of early stage breast cancer. We currently report the 25 year outcomes of a single institution, prospective, randomized clinical trial at the National Cancer Institute. 237 women with pathologically confirmed invasive breast tumors 5 cm or less were accrued between 1979 and 1987 and randomized to receive either BCT or MRM. Overall survival was the primary endpoint. Patients with node positive disease were included and treated with doxorubicin and cyclophosphamide. Both arms received axillary dissection. BCT patients had radiation to the whole breast followed by a boost. At a median follow-up of 25.7 years, overall survival was 43.8% for the MRM group and 37.9% for BCT (P = 0.38). Although the cumulative incidence of a disease-free survival event was higher in BCT patients (29.0% MRM vs. 56.4% BCT, P = 0.0017), the additional treatment failures were primarily isolated ipsilateral breast tumor recurrences (IBTR’s) requiring salvage mastectomy. 22.3% of BCT patients experienced an IBTR. Distant disease and second cancers were similar in both arms. After 25 years, long term survival between BCT and MRM continues to be similar in patients treated for early stage breast cancer. Patients receiving BCT may be at risk for additional treatment-related morbidity, which may occur as a late event. Further studies are required to delineate patients at higher risk for these events, and prolonged follow up should be encouraged after treatment for all women.
Keywords: Breast conserving therapy, Mastectomy, Radiation, Randomized
Introduction
At the turn of the 20th century, treatment of breast cancer was dominated by techniques of surgical radicalism, believing that complete extirpation of the breast and surrounding tissue was required to arrest centrifugal spread of tumor. The Halsted radical mastectomy, the gold standard of therapy during this time, was the end-result of the progressively larger margins used by surgeons of the era to efface all remnants of contiguous tissue possibly harboring disease [11]. Beginning with Bernard Fisher’s landmark B-04 trial, therapeutic equivalence of increasingly less extensive surgery has been demonstrated in a prospective manner through a number of specific trials [9].
Following the results of B-04, six major phase III trials were initiated in North America and Europe to investigate breast conservation [3, 4, 8, 15, 17, 18]. With up to 20 years of published follow-up data for these trials, breast conserving therapy has been demonstrated to be an accepted alternative to mastectomy with similar survival outcomes. Despite considerable variations in inclusion criteria and treatment details in these trials, pooled analysis of this data has continued to reinforce individual trial findings of noninferiority [5].
We currently present the updated findings of the National Cancer Institute Breast Conservation Trial, which is one of the 6 major original early breast cancer trials [12, 13, 15]. This investigation was originally initiated in 1979, with accrual ending in 1987. Unique to this trial is the use of modern chemotherapy regimens including doxorubicin and cyclophosphamide, the use of CT-simulation for radiotherapy planning, and the length and detail of follow-up. More than half of the surviving patients originally enrolled on this protocol continue to be followed at the Clinical Center at the National Institutes of Health (NIH) to this day. The median follow-up of this trial is now 25.7 years, and among the six major breast conservation trials will be the longest reported follow up. This article updates our previous reports of the trial, as we continue to demonstrate no significant differences in overall survival between the two treatment groups.
Methods
Patient characteristics
This was a single-institution, prospective, randomized clinical trial conducted by NCI at the Clinical Center of the NIH. A detailed description of the study design has previously been reported and this study was approved by the NIH Institutional Review Board [13]. Due to changes in the modern treatment of breast cancer, specific aspects of the study design will be re-emphasized here. Between July 1979 and December 1987, 247 women with pathologically confirmed invasive breast tumors 5 cm or less with clinically negative or positive axillary lymph nodes were enrolled on this trial. Patients were considered ineligible if they had evidence of metastatic disease or previous cancer, were a poor operative risk, or were found to have multi-centric disease. Patients were then stratified by age, clinical nodal status, and the presence of cardiac disease which would preclude them from treatment. Randomization was performed in a 1:1 ratio by the Statistical Data Management Group of the Biometric Research Group of the NCI and the trial was stopped when accrual goals were met. Patients were assigned to treatment either with total mastectomy plus axillary dissection (MRM) or excisional biopsy of the tumor and axillary dissection followed by radiotherapy (BCT).
Intervention
Patients in the MRM group underwent a Patey MRM and level I–III lymph node dissection, as previously described [7]. No postoperative chest wall irradiation was administered and patients were offered breast reconstruction.
Patients in the BCT group initially underwent an excisional biopsy, which was frequently performed at an outside institution (75% of patients). Although microscopically free surgical margins were not required at this time, re-excision was performed if it was felt gross tumor remained. All patients had an axillary dissection at NCI. Following surgery, patients received external beam radiation delivered to 4,500–5,040 cGy to the entire breast with 4–6 MeV photons in 180 cGy fractions 5 days per week. Patients with nodal disease, extracapsular spread, or axillary involvement were treated with a supraclavicular field to 4,500–5,040 cGy. In patients with positive nodes or medial quadrant primaries, tangent fields extended to include internal mammary nodes. CT simulations were performed for all treatment and beginning in 1981, lung inhomogeneity correction factors were applied. A 1,500–2,000 cGy boost to the tumor bed was given using an Iridium-192 implant (1979–1983) or an electron beam (1984–1987).
Chemotherapy with cyclophosphamide and doxorubicin was administered to all patients with positive nodes after surgery. Initially, doxorubicin was given intravenously to 30 mg/m2 on Day 1. On days 3–6, cyclophosphamide was dose escalated orally starting at 150 mg/m2 until hemato-logical toxicity was noted. Twelve cycles were given every 28 days. Interim analysis showed minimal toxicity and multiple amendments were made to the dosing regimen [14]. In 1983, the cyclophosphamide dose was increased to 200 mg/m2 and in 1985 was increased to 40 mg/m2 over only 9 cycles of a shorter period of 21 days. Beginning in 1985 when estrogen testing became more routine, node-positive, postmenopausal patients were also given tamoxifen for 5 years.
Statistical methods
The primary end point was overall survival which was measured from date of enrollment until death from any cause or last known date of survival if alive. It was initially determined that 105 patients were required to be enrolled on each arm to have a 80% power for a 95% confidence limit. Secondary endpoints of disease-free survival and rates of local recurrence were measured from the date of enrollment until recurrence or the last follow-up visit without evidence of disease. Events in the calculation of disease-free survival include any local, regional, or distant recurrence. Contralateral, second cancers or deaths from any cause were not included and were censored at the time of the event. In previous reports of this trial, patients with an isolated ipsilateral breast recurrence (IBTR) successfully treated by mastectomy were considered to be disease-free. [12, 13, 15]. However, emerging evidence suggests these events may be important prognostic factors [2, 20]. The current authors felt it best to include these events as local recurrences and this notion has supported by other trials [17, 18]. Recurrences in the internal mammary, supraclavicular, or ipsilateral axillary nodes were classified as regional occurrences. All other locations were classified as distant recurrences. Distant events following contralateral breast cancers were censored at the time of the event due to the ambiguity of the origin of recurrence.
Overall survival and disease-free survival were compared using the log-rank test and Kaplan–Meier survival estimates were generated. Due to competing causes of death, Cox proportional cause-specific hazard was fit and used to compare the treatment difference in disease-free survival and other cause-specific hazards. Hazard ratio was calculated with MRM as the baseline hazard. Walds test was used to calculate the P value of the treatment effect. Cause-specific cumulative incidence estimated at 25 years was generated. Univariate and multivariate survival analysis was performed and Walds test was used to calculate the P value of each variable. Variables at the 0.05 level were included in the multivariate analysis. Effect of time dependence of IBTR on overall survival was estimated by two approaches; the first used the landmark analysis, with time set at 5 years and the second used Cox proportional hazard model by treating IBTR as a time-dependent covariate (Supplemental Methods) [1].
Results
Patient characteristics
Of 247 patients, 237 participated in the trial because 10 did not wish to be randomized. In total, 116 patients were treated with MRM and 121 patients received BCT (Fig. 1). Patient characteristics were similar on both arms of the trial. The mean age of the patients was 51.6 and 50.0 years on the MRM and BCT arms, respectively. Proportions of side of the breast affected, menopausal status, quadrant of disease, tumor size, and number of lymph nodes positive were also similar (Table 1).
Fig. 1.
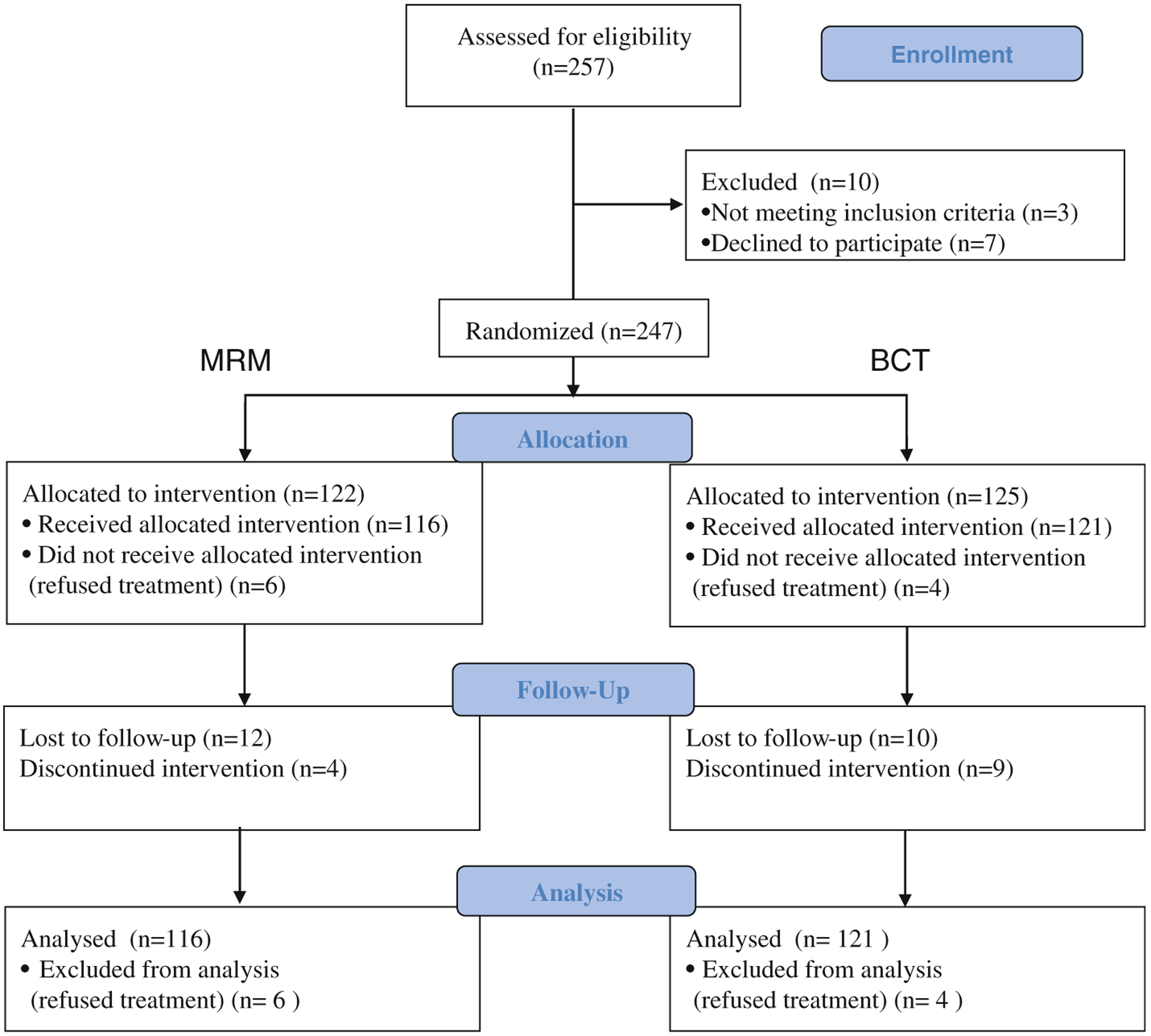
Study flow. MRM indicates modified radical mastectomy and BCT indicates breast conservation therapy
Table 1.
Patient characteristics
| Mastectomy (n = 116), % |
BCT (n = 121), % |
|
|---|---|---|
| Age (median) | ||
| ≤50 | 46 | 47 |
| >50 | 54 | 53 |
| Mass side | ||
| Left | 52 | 58 |
| Right | 48 | 42 |
| Race | ||
| Non-white | 5 | 7 |
| White | 95 | 93 |
| Menopausal status | ||
| Pre | 49 | 50 |
| Post | 51 | 50 |
| Quadrant of disease | ||
| Outer and central | 76 | 74 |
| Inner | 24 | 26 |
| Positive LN’s | ||
| 0 | 58 | 61 |
| 1–3 | 28 | 28 |
| 4–9 | 8 | 4 |
| >10 | 6 | 7 |
| Tumor size (cm) | ||
| 0–2.0 | 48 | 43 |
| 2.1–4.0 | 43 | 50 |
| 4.1–5.0 | 9 | 7 |
| Estrogen receptor | ||
| Negative | 11 | 15 |
| Positive | 46 | 43 |
| Unknown | 43 | 42 |
Locoregional recurrence
The cumulative incidence of a local recurrence at 25.7 years in the BCT arm was 21.9% and 0.9% in MRM arm (HR = 29.9, P < 0.001, Table 2). In the mastectomy group, there was 1 chest wall recurrence, 2 isolated axillary recurrences, and 2 simultaneous local and regional recurrences. In the BCT arm, there were 27 IBTR’s and 85.2% of those recurrences occurred within the same quadrant as the initial tumor. There was only one simultaneous local and regional recurrence in the BCT group. More than 1 out of every 5 patients (22.3%) in the BCT arm experienced an IBTR requiring salvage mastectomy. Median time to IBTR was 5.3 years, although 3 patients experienced late isolated local recurrences after 20 years of follow up. Median survival for patients experiencing an IBTR less than 5 years after diagnosis was 20.2 years, and was 24.1 years if it occurred after 5 years (P = 0.71). When an IBTR was treated as a time-dependent covariate in the Cox proportional hazards model, the hazard ratio of IBTR was 1.72 (95% CI: 0.91–3.25, P = 0.094). The incidence of IBTRs did not seem to correlate with age (Fig. 3).
Table 2.
Event table
| First event | Mastectomy | BCT | Hazard ratio BCT/mastectomy (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| Events, # | Cumulative incidence at 26 years, % (SE) | Events, # | Cumulative incidence at 26 years, % (SE) | |||
| All breast cancer | 50 | 43.4 (4.9) | 70 | 58.5 (4.8) | 1.50 (1.04, 2.16) | 0.028 |
| IBTR | N/A | 0.9 (0.9) | 27 | 21.9 (3.9) | N/A | N/A |
| Breast cancer at logcalregional site | 5 | 3.5 (1.7) | 28 | 23.6 (4.0) | 6.61 (2.55, 17.14) | <0.001 |
| Breast cancer at distant site | 30 | 26.4 (4.2) | 35 | 30.0 (4.3) | 1.10 (0.67, 1.79) | 0.71 |
| Contralateral breast cancer | 15 | 13.6 (3.5) | 11 | 9.1 (2.8) | 0.92 (0.62, 1.36) | 0.66 |
| Non-breast cancer | 13 | 13.3 (3.6) | 12 | 10.6 (3.0) | 1.08 (0.72, 1.62) | 0.71 |
Fig. 3.
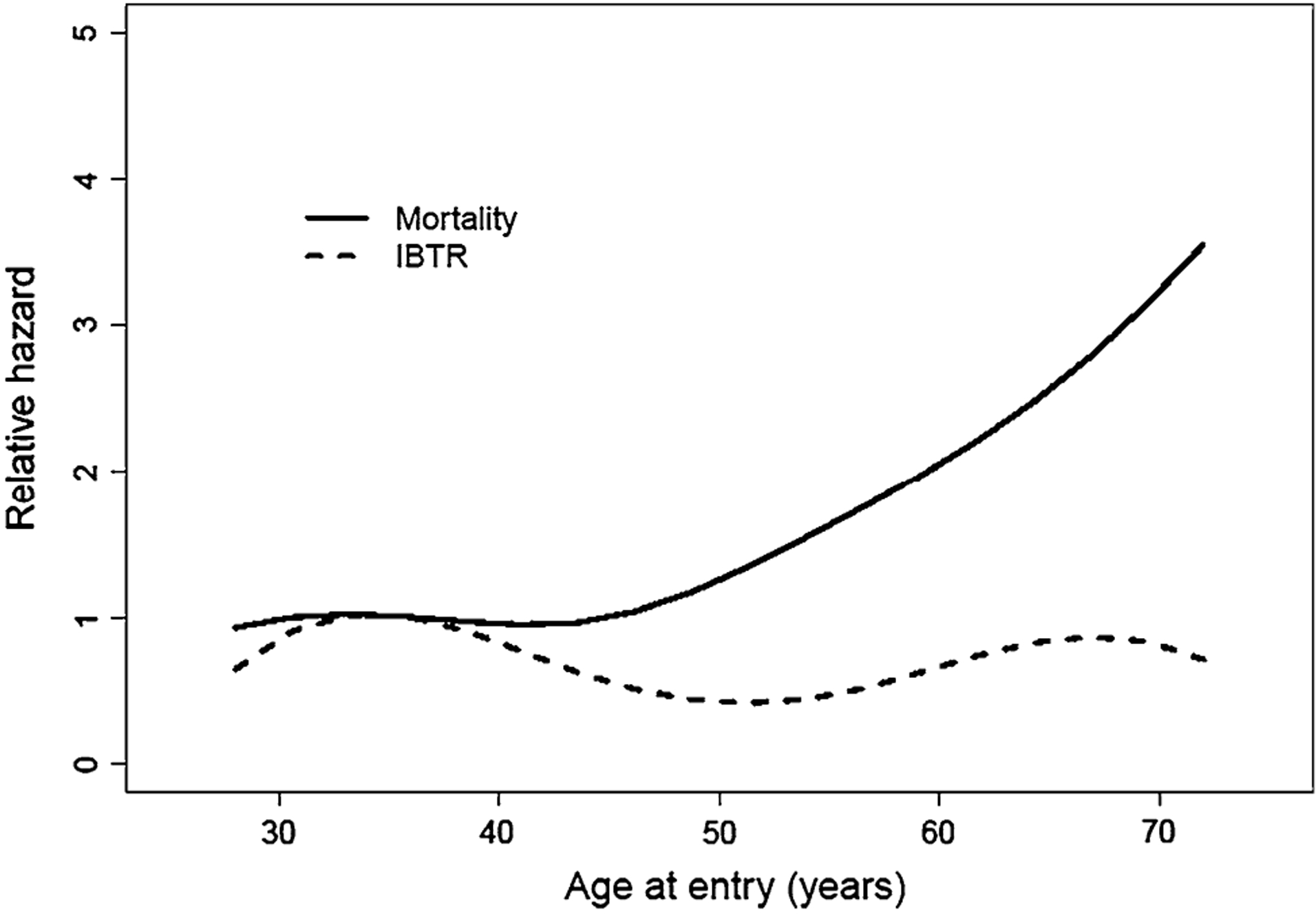
Risk of mortality and ipsilateral breast cancer by age for the BCT treatment group. Age was grouped into 10 years intervals, and Cox proportional hazards model was used to estimate the relative risk of death and ipsilateral breast tumor recurrence (IBTR) in each age group for patients in the BCT treatment group. The reference age group was (30, 40). The smoothed version of these relative hazards was performed by cubine spline interpolation
Distant metastases and other cancers
Distant metastases occurring alone or simultaneously with a local or regional event occurred in 30 patients in the MRM arm and 35 patients in the BCT arm. There was no significant difference in risk of distant metastases (HR = 1.1 with a 95% CI of 0.67–1.79, P = 0.71). Median time to distant metastasis was 3.1 years after MRM and 4.0 years after BCT. The risk of contralateral breast cancer was not increased with the use of radiotherapy, with 11 events in the BCT arm and 15 events in the MRM arm (HR = 0.92 with a 95% CI of 0.62–1.36, P = 0.66). Median time to the development of contralateral cancers was 6.1 years in the MRM arm and 9.4 years in the BCT arm. The incidence of non-breast cancers was similar, with 13 events in the MRM arm and 12 events in the BCT arm (Table 2).
Overall survival and disease-free survival
At a median follow-up of 25.7 years, survival was 43.8% in the MRM group and 37.9% in the BCT group P = 0.38 (Fig. 2). Although the survival curves are superimposed up to 25 years, the MRM arm seems to have a slight advantage there after. There appeared to be a direct correlation between age at enrollment and hazard for death at 25 years (Fig. 3, Supplemental Table 1). Age greater than 50 years old, left sided tumors, tumors >2 cm, and >4 lymph nodes positive were significant predictors of decreased survival on multivariate analysis (Supplemental Table 2). Survival in patients with 1–3 lymph nodes positive was not found to be an independent predictor of survival. The risk of developing a DFS event was significantly different between the MRM and BCT arm (P = 0.0017). The cumulative incidence was 29.0% in the MRM group and 56.4% in the BCT group. Only the treatment arm of BCT and tumors >2 cm were significant on multivariate analysis as independent predictors of disease-free survival (Supplemental Table 3).
Fig. 2.
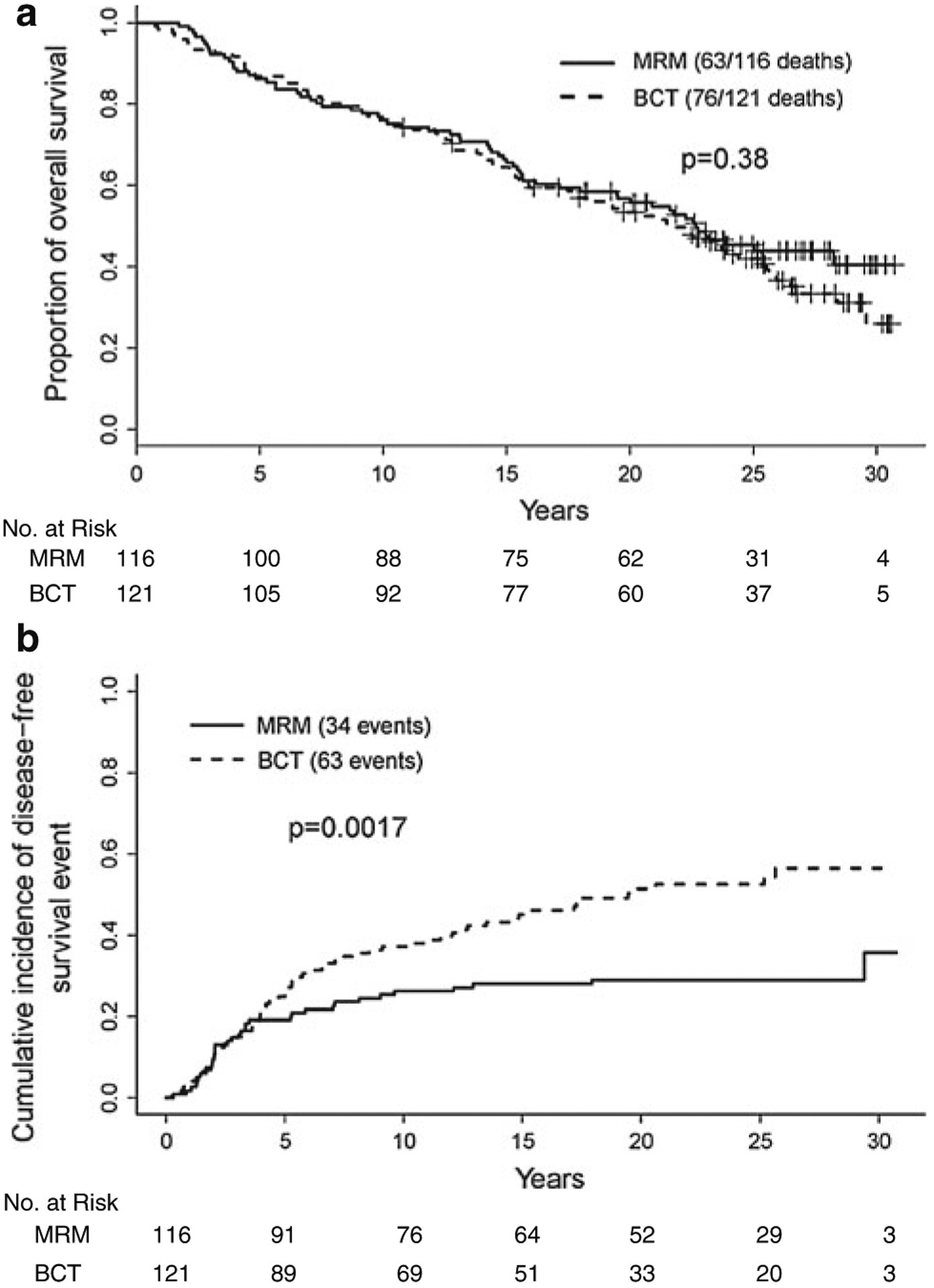
Kaplan–Meier estimates of overall survival and cumulative incidence of disease-free survival between the MRM group compared with the BCT group. (MRM indicates modified radical mastectomy and BCT indicates breast conservation therapy)
Discussion
After over 25 years of follow up, data from our trial continues to demonstrate no survival differences when comparing lumpectomy followed by whole breast radiation versus mastectomy. These results have been recapitulated by the 5 other major trials of this time with extended follow up [3, 4, 8, 17, 18]. Despite differences in eligibility criteria and treatment technique between trials, three iterations of meta-analyses of these trials from the Early Breast Cancer Trialist Group Collaboration (EBCTG) have confirmed noninferiority of breast conservation therapy compared with mastectomy [5, 19].
This single-institution trial differs in methodology from its contemporaries in a number of ways. Although smaller in number than others, it reports the longest follow up and has as theme of the most contemporary treatment characteristics. This was one of the first trials to use CT-based radiotherapy planning to reduce normal tissue toxicity and one of the earliest uses of adjuvant anthracycline chemotherapy in setting of radiotherapy for early breast cancer [14]. However, certain limitations should be noted. Pathological margins were not assessed after tumor excisions in the breast conservation group, allowing for the possibility of microscopic disease. This is especially noteworthy given the less restrictive size criteria of 5 cm or less, which was only shared by the Danish and EORTC trials [4, 17]. In addition, this trial included a particularly extensive level I-III axillary dissection (median 23.0 nodes dissected).
Despite these differences in study design, overall survival and distant metastases in this trial appear to be consistent with other studies. In contrast, however, is the high rate of ipsilateral breast tumor recurrences (IBTR) experienced by patients in the BCT group. The NCI trial has reported the highest cumulative incidence of IBTRs with 23.2% at 25 years, as compared with 14.3% for NASBP B-06 at 20 years, 8.8% for the Milan trial at 20 years, and 9% for the IGR trial at 15 years [3, 8, 18]. Although 23.2% is a high rate of IBTRs, in this study, 11% of patients had contralateral cancers which suggest that if the ipsilateral breast develops new cancers at the same rate, this would account for half of the IBTRs. However, contributing factors to this high rate include the previously described inclusion of larger tumors and inadequate evaluation of margins and residual disease. Trials reporting lower incidence included smaller tumor sizes and more stringent margin evaluation. Furthermore, the high number of these in breast events may also be related to length of follow-up, as 3 of the IBTR events noted in this trial occurred in patients who were free of disease for over 20 years.
In recent years, the significance of IBTRs have come into question as larger pooled analyses have suggested IBTRs may be a significant risk factor for mortality [2, 20]. Related to, and confounding this issue is the difficulty in distinguishing these tumors as true recurrences of initial disease or de novo development of second cancers in patients already at high risk for disease. Molecular analysis regarding these subtypes has indicated they have distinct natural histories, suggesting the heterogeneous nature of these recurrences [16]. At the time of our trial, the distinction of type of recurrence could only be made by pure anatomic localization, making the classification of these tumors unreliable. A central question regarding these in-breast recurrences is their significance as modifiable risk factors, or simply prognostic markers reflecting a proclivity for the development of additional cancer. If they are indeed biologically significant risk factors exclusive to those undergoing BCT, our trial was underpowered to detect them as such. Despite 27 patients in the BCT group experiencing these events, we were unable to detect any survival differences between the two treatments. This is an expected finding, as the most recent EBCTG meta-analysis has estimated that it would take the prevention of four such recurrences to avoid one mortality, indicating the need for larger sample sizes to detect a survival difference due to these events [5].
With extended periods of follow up, it was observed that the risk of competing causes of death becomes a significant factor. At 25 years, approximately one quarter of patients expired without clinical evidence of disease. In these patients, treatment morbidity may have indirectly influenced survival outcomes. The most recent EBCTG meta-analysis has suggested a 1.3% increase in non-breast cancer death in patients receiving radiotherapy after surgery [5]. Although there was an additional 5 non-breast cancer deaths in the BCT arm, this trial was not adequately powered to detect late differences in non-breast mortality. However, multivariate analysis of our cohort did find left-sided disease to be a significant predictor for mortality. Previous reports have suggested left-sided cancers may be a risk factor for cardiac morbidity [6]. To investigate the role of potential cardiotoxicity from treatment, extensive cardiac studies of 60 of the surviving patients in this trial are currently being conducted at the NCI (unpublished data).
As data continues to mature on the long term survival of patients with early stage breast cancer, it is apparent that this issue of treatment morbidity comes into focus. Survival outcomes have improved to the extent that minimizing toxicity of locoregional treatment in selected patients has become a priority. Trends in surgical de-escalation have continued, with a recent report by Giuliano and colleagues demonstrating non-inferiority of sentinel lymph node biopsy when compared to full axillary dissections [10]. Data from this trial seems to support these findings, as no significant survival benefit was noticed when compared to other trials, despite differences in the extent of axillary dissection (Danish trial: median 8.2 nodes dissected, NCI trial: median 23.0 nodes) [4].
This 25 year follow-up of the NCI trial confirms similar long-term survival outcomes between breast conservation therapy and mastectomy in women with early stage breast cancer. Despite an increased number of in-breast recurrences, these events did not seem to translate to an increased risk of distant failure or mortality. However, it should be noted that patients receiving breast conservation may be at risk for additional treatment-related morbidity, of which may occur as a late event. Further studies are required to delineate patients at higher risk for these events, and prolonged follow up should be encouraged in women choosing this therapy.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health. Tu Dan was supported by the Clinical Research Training Program, a public-private partnership supported jointly by NIH and Pfizer, Inc.
Abbreviations
- BCT
Breast conserving therapy
- MRM
Modified radical mastectomy
- IBTR
Ipsilateral breast tumor recurrence
- NIH
National Institutes of Health
- NCI
National Cancer Institute
- EBCTG
Early Breast Cancer Trialists Group
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-011-1867-6) contains supplementary material, which is available to authorized users.
Trial registration: clinicaltrials.gov:NCT00026845.
Contributor Information
Marc E. Lippman, Department of Medicine, Miller School of Medicine, University of Miami, Miami, FL, USA
Eli Glatstein, Radiation Oncology, University of Pennsylvania, 3400 Spruce Street, 2 Donner Building, Philadelphia, PA 19104, USA.
Sandra M. Swain, Washington Hospital Center, Washington Cancer Institute, 110 Irving St. NW, Washington, DC, DC 20010, USA
David N. Danforth, Surgical Oncology Branch, National Cancer Institute, National Institutes of Health, 10 Center, Dr, Bethesda, MD 20892, USA
References
- 1.Anderson JR, Cain KC, Gelber RD (2008) Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol 26:3913–3915. doi: 10.1200/JCO.2008.16.1000 [DOI] [PubMed] [Google Scholar]
- 2.Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, Geyer CE Jr, Wickerham DL, Costantino JP, Wolmark N (2009) Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol 27:2466–2473. doi: 10.1200/JCO.2008.19.8424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arriagada R, Le MG, Rochard F, Contesso G (1996) Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol 14:1558–1564 [DOI] [PubMed] [Google Scholar]
- 4.Blichert-Toft M, Nielsen M, During M, Moller S, Rank F, Overgaard M, Mouridsen HT (2008) Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 47:672–681. doi: 10.1080/02841860801971439 [DOI] [PubMed] [Google Scholar]
- 5.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 6.Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE (2007) Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol 25:3031–3037. doi: 10.1200/JCO.2006.08.6595 [DOI] [PubMed] [Google Scholar]
- 7.Danforth DN Jr, Findlay PA, McDonald HD, Lippman ME, Reichert CM, d’Angelo T, Gorrell CR, Gerber NL, Lichter AS, Rosenberg SA et al. (1986) Complete axillary lymph node dissection for stage I–II carcinoma of the breast. J Clin Oncol 4:655–662 [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241. doi: 10.1056/NEJMoa022152347/16/1233 [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Redmond C, Fisher ER, Bauer M, Wolmark N, Wickerham DL, Deutsch M, Montague E, Margolese R, Foster R (1985) Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med 312:674–681. doi: 10.1056/NEJM198503143121102 [DOI] [PubMed] [Google Scholar]
- 10.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569–575. doi: 10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halsted WS (1894) The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg 20:497–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson JA, Danforth DN, Cowan KH, d’Angelo T, Steinberg SM, Pierce L, Lippman ME, Lichter AS, Glatstein E, Okunieff P (1995) Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 332:907–911. doi: 10.1056/NEJM199504063321402 [DOI] [PubMed] [Google Scholar]
- 13.Lichter AS, Lippman ME, Danforth DN Jr, d’Angelo T, Steinberg SM, de Moss E, MacDonald HD, Reichert CM, Merino M, Swain SM et al. (1992) Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the National Cancer Institute. J Clin Oncol 10:976–983 [DOI] [PubMed] [Google Scholar]
- 14.Lippman ME, Lichter AS, Edwards BK, Gorrell CR, d’Angelo T, DeMoss EV (1984) The impact of primary irradiation treatment of localized breast cancer on the ability to administer systemic adjuvant chemotherapy. J Clin Oncol 2:21–27 [DOI] [PubMed] [Google Scholar]
- 15.Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ, Menard C, Lippman ME, Lichter AS, Altemus RM (2003) Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute randomized trial. Cancer 98:697–702. doi: 10.1002/cncr.11580 [DOI] [PubMed] [Google Scholar]
- 16.Smith TE, Lee D, Turner BC, Carter D, Haffty BG (2000) True recurrence vs new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys 48:1281–1289 [DOI] [PubMed] [Google Scholar]
- 17.van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, van der Schueren E, Helle PA, van Zijl K, Bartelink H (2000) Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 92:1143–1150 [DOI] [PubMed] [Google Scholar]
- 18.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232. doi: 10.1056/NEJMoa020989347/16/1227 [DOI] [PubMed] [Google Scholar]
- 19.Vinh-Hung V, Verschraegen C (2004) Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst 96:115–121 [DOI] [PubMed] [Google Scholar]
- 20.Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE Jr, Jeong JH, Tan-Chiu E, Fisher B, Wolmark N (2006) Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol 24:2028–2037. doi: 10.1200/JCO.2005.04.3273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


