Abstract
Currently, prognostic and therapeutic determinations for canine cutaneous mast cell tumors (MCTs) are primarily based on histologic grade. However, the use of different grading systems by veterinary pathologists and institutional modifications make the prognostic value of histologic grading highly questionable. To evaluate the consistency of microscopic grading among veterinary pathologists and the prognostic significance of the Patnaik grading system, 95 cutaneous MCTs from 95 dogs were graded in a blinded study by 28 veterinary pathologists from 16 institutions. Concordance among veterinary pathologists was 75% for the diagnosis of grade 3 MCTs and less than 64% for the diagnosis of grade 1 and 2 MCTs. To improve concordance among pathologists and to provide better prognostic significance, a 2-tier histologic grading system was devised. The diagnosis of high-grade MCTs is based on the presence of any one of the following criteria: at least 7 mitotic figures in 10 high-power fields (hpf); at least 3 multinucleated (3 or more nuclei) cells in 10 hpf; at least 3 bizarre nuclei in 10 hpf; karyomegaly (ie, nuclear diameters of at least 10% of neoplastic cells vary by at least two-fold). Fields with the highest mitotic activity or with the highest degree of anisokaryosis were selected to assess the different parameters. According to the novel grading system, high-grade MCTs were significantly associated with shorter time to metastasis or new tumor development, and with shorter survival time. The median survival time was less than 4 months for high-grade MCTs but more than 2 years for low-grade MCTs.
Keywords: canine, histologic grading, mast cell tumor
Cutaneous mast cell tumors (MCTs) commonly occur in dogs and account for 7 to 21% of all canine cutaneous neoplasms.2,4,6,12,14 The biological behavior of MCTs varies from benign solitary tumors that can be cured by complete surgical excision to potentially fatal metastatic malignancies.6,9,12 Additionally, MCT degranulation can result in paraneoplastic syndromes varying from local hemorrhage and wound dehiscence to systemic hypotension, hemorrhage, and death.6,9,12 Because of the high incidence of MCTs, their variable biological behavior, the potential fatal outcome attributed to aggressive disease or paraneoplastic syndromes, and the costs and risks associated with treatment, accurate prognostication of MCTs is critical.1,10,11,17
Histologic grading has been the primary tool for veterinary pathologists to assess the potential biological behavior of canine MCTs, and it is commonly used for prognostication and therapeutic determination.1,9–11 The grading systems developed by Bostock in 1973 and Patnaik et al in 1984 are the two most commonly used histologic grading systems for canine cutaneous MCTs.1,10 Both systems are 3-tier grading systems; however, they grade MCTs in reverse order to one another, with well-differentiated MCTs being Patnaik grade 1 and Bostock grade 3, intermediately differentiated tumors being Patnaik and Bostock grade 2, and poorly differentiated tumors being Patnaik grade 3 and Bostock grade 1.1,10 In both systems, poorly differentiated tumors have been associated with decreased survival compared with well-differentiated and intermediately differentiated tumors; however, they vary in histologic grading criteria.1,10 Histologic grading criteria used by Bostock included nuclear:cytoplasmic ratio, mitotic figures, cellular pleomorphism, discreteness of tumor cells, tumor cellularity, and metachromatic granules.1 In contrast, Patnaik et al employed cellular morphology, mitotic index, cellularity, extent of tissue involvement, and the stromal reaction for histologic grading.10
Today, the Patnaik grading system is most commonly used by diagnostic pathologists in the United States to grade canine cutaneous MCTs.6,9 According to this system, grade 1 MCTs are composed of rows or clusters of monomorphic well-differentiated mast cells with round nuclei and medium-sized cytoplasmic granules and are by definition confined to the dermis.10 Grade 1 MCTs have no mitotic figures and minimal stromal reaction or necrosis. Grade 2 MCTs are moderately to highly cellular and composed of moderately pleomorphic mast cells with round to indented nuclei and mostly finely granular cytoplasm that extend to the lower dermis, subcutis, and occasionally into deeper tissues. Grade 2 MCTs have 0 to 2 mitotic figures per high-power field (hpf), and some contain areas of edema, necrosis, and hyalinized collagen. Grade 3 MCTs are highly cellular and composed of pleomorphic mast cells, with indented to round vesicular nuclei and 1 to multiple prominent nucleoli, arranged in sheets that replace the subcutis and underlying tissues. Grade 3 MCTs have 3 to 6 mitotic figures per hpf and contain areas of hemorrhage, edema, necrosis, and hyalinized collagen. According to Patnaik and colleagues’ original study, 93% of dogs with grade 1 MCTs survived longer than 1,500 days, compared with 47% of grade 2 and only 6% of grade 3.10
Although histologic grade is considered the gold standard for MCT prognostication, the predominance of grade 2 MCTs and interobserver variation are repeatedly cited weaknesses of the current histologic grading systems.8,9,11,15,16 An additional criticism of the Patnaik grading schema is that criteria for grade 3 MCT—specifically, the high mitotic rate (3 to 6 mitotic figures per hpf)—exclude some tumors that are likely to be biologically aggressive. We conducted a multi-institutional study in which 95 canine MCTs were histologically graded by 28 experienced surgical veterinary pathologists from institutions in North America and Europe to address these issues.
Material and Methods
This study used 95 cutaneous MCTs from 95 dogs that had been submitted to the Diagnostic Center for Population and Animal Health at Michigan State University between 1998 and 2001. Surgical excision was the only treatment modality for all MCTs. Clinical history and follow-up data were available for all dogs. All cases had sufficient tissue for histologic examination and additional prognostic studies.
History and follow-up information that were obtained for each case included the following: age at diagnosis, sex, breed, weight, diagnostics performed, adjunct medications given at the time of surgery, tumor duration before removal, tumor location, number of tumor masses at the time of diagnosis, additional tumor development, survival time (time from diagnosis of MCT to time of death), and cause of death, if applicable. For surviving dogs, follow-up data were obtained for up to 4 years after surgery. Subsequent tumor development was divided into 2 groups: MCTs recurring at the original surgical site and MCTs occurring elsewhere (outside the original surgical margins). All MCTs were fixed in 10% neutral buffered formalin and paraffin embedded at the time of submission. Five-micron sections were cut and routinely stained with hematoxylin and eosin for histologic evaluation and tumor grading.
Slides were graded by 28 pathologists at American and European surgical pathology services. Pathologists were instructed to grade all MCTs according to the Patnaik grading system. In addition, 6 institutions provided additional data based on internally developed grading systems that were based on cell morphology only or included a subdivision of grade 2 MCTs into grade 2a (more differentiated) and 2b (less differentiated). All pathologists graded blindly without knowledge of other pathologists’ grades or the biological behavior.
For statistical analysis, median grades were computed for each tumor from observations by 28 pathologists using the Patnaik grading system.10 Consistency among pathologists was evaluated with the Cronbach alpha test. To determine to capture interobserver differences, the proportion of pathologists grading each tumor with a specific grade was calculated. A series of yes/no grade index variables was created to describe these proportions. The indices for MCT grades of 3 were as follows: 3–90, at least 90% of pathologists graded the tumor as a 3; 3–80, at least 80% graded as a 3; 3–70, at least 70% graded as 3; and 3–50, at least 50% graded as 3. Comparable indices were generated for grades of 1 and 2. A similar approach was used for the 6 institutional grading systems.
The significance of association between mortality (MCT related or any cause, including euthanasia) and grade indices was assessed with the Fisher exact test, and the direction and strength of association were reported as the odds ratio with 95% confidence intervals (CIs). CIs whose lower limit was greater than 1.0 indicated significant positive association between grade index and mortality; CIs with an upper limit less than 1.0 indicated significant negative association; and CIs that included 1.0 indicated no significant association.
Analysis of variance (ANOVA) was used to assess the association between grade indices and days to additional tumor development/metastasis (MCTs occurring at distant sites, outside the original surgical margins) and the association between grade indices and time to mortality (MCT related or any cause, including euthanasia) or the end of the study. Survival curves were plotted for mortality outcomes by the MCT median grade and by each grade index.
A novel grading system splitting canine cutaneous MCTs into 2 grades—high grade and low grade—was applied to the MCTs of this study in another trial. Based on this grading scheme, a high-grade MCT is characterized by any one of the following criteria: at least 7 mitotic figures in 10 hpf; at least 3 multinucleated (3 or more nuclei) cells in 10 hpf; at least 3 bizarre nuclei (highly atypical with marked indentations, segmentation, and irregular shape) in 10 hpf; karyomegaly (ie, nuclear diameters of at least 10% of neoplastic mast cells vary by at least two-fold). To assess the parameters, fields were selected that were the most mitotically active or had the highest degree of anisokaryosis. MCTs were originally graded by 1 pathologist (M.K.) according to the proposed 2-tier grading system. The assigned grades were used as the gold standard for this grading system. All tumors were then graded in a blind trial by 5 American College of Veterinary Pathologists board certified veterinary pathologists who had not previously graded or examined these MCTs. All 5 pathologists had been provided with the criteria of the new 2-tier grading system but with no microscopic training. The consistency between the grading of the 6 pathologists was assessed as previously described. The association between the novel 2-tier grading system and mortality was assessed with multivariable survival analysis. Initial univariable analyses were undertaken for the following explanatory variables: age, sex, presence of multiple masses, development of additional MCTs or metastasis of tumor, and novel grading system. All variables significant at P < .05 were subsequently included in a multivariable Cox proportional hazards model, with death or euthanasia due to MCT or MCT complication identified as the outcome event. The multivariable model was fitted using backward elimination.
A final analysis was undertaken to compare the use of the Patnaik grading system and the novel 2-tier grading system for prognostication of mortality. Two Cox proportional hazard models were performed with Patnaik grading system and novel grading system included as explanatory variables in both models but fitted in different orders and the 2 ANOVA tables were compared.
Results
Ninety-five dogs in this study ranged from 1 to 14 years of age (mean, 7.3 years; median, 7 years). Fifty dogs were spayed females; 33 dogs were castrated males; 7 were intact females; and 5 were intact males. The dogs represented 22 breeds, including mixed-breed dogs (n = 21), Labrador Retrievers (n = 21), Boxers (n = 11), Golden Retrievers (n = 10), Pugs (n = 4), Boston Terriers (n = 3), Cocker Spaniels (n = 3), Bassett Hounds (n = 3), and 14 other breeds represented by 1 to 2 animals each.
In the 4 years following the first surgical excision of the MCTs, 10 dogs developed a subsequent MCT at the original tumor location and 22 developed a MCT at a distant site. Thirteen dogs died or were euthanized within the follow-up period because of MCT-associated disease. Eleven dogs died within the follow-up period because of non-MCT-associated diseases. Seventy-one dogs were alive at the end of the study.
Based on the Patnaik grading system, there was 74.6% concordance among pathologists for the diagnosis of grade 3 MCTs, 63.0% concordance for the diagnosis of grade 2 MCTs, and 63.1% concordance for the diagnosis of grade 1 MCTs (Table 1). Greater than 50% of pathologists agreed on the diagnosis of grade 3 MCT for 9 tumors. These MCTs were significantly associated with an increased incidence of MCT-related mortality (Fig. 1) and increased development of additional MCTs at different sites, as well as shorter survival times (Tables 2–4). Eighteen MCTs diagnosed as grade 2 MCT by at least 70% of pathologists were also significantly associated with an increased MCT-associated mortality and shorter survival time (Tables 2 and 4). However, when tumors for which more than 50% of pathologists agreed on the diagnosis were included (75 MCTs), grade 2 MCTs were not significantly associated with MCT-associated mortality or shorter survival time (Tables 2 and 4). Grade 2 MCTs were not significantly associated with increased development of additional MCTs at different sites (Table 3). Grade 1 MCTs were not associated with increased incidence of MCT-associated mortality, regardless of the agreement among pathologists (Table 2). When MCTs were categorized on the basis of their median histologic grade, grade 3 MCTs (but not grade 2 or grade 1 MCTs) were significantly associated with decreased overall survival times.
Table 1.
Assignment of Grades for 95 Canine Cutaneous Mast Cell Tumors by 28 Pathologists: n (%)
| Grade | Agree | Disagree | Total |
|---|---|---|---|
| 1 | 389 (63.15) | 227 (36.85) | 616 |
| 2 | 1,129 (63.00) | 663 (37.00) | 1,792 |
| 3 | 188 (74.60) | 64 (25.40) | 252 |
| Total | 1,706 (64.14) | 954 (35.86) | 2,660 |
Figure 1.
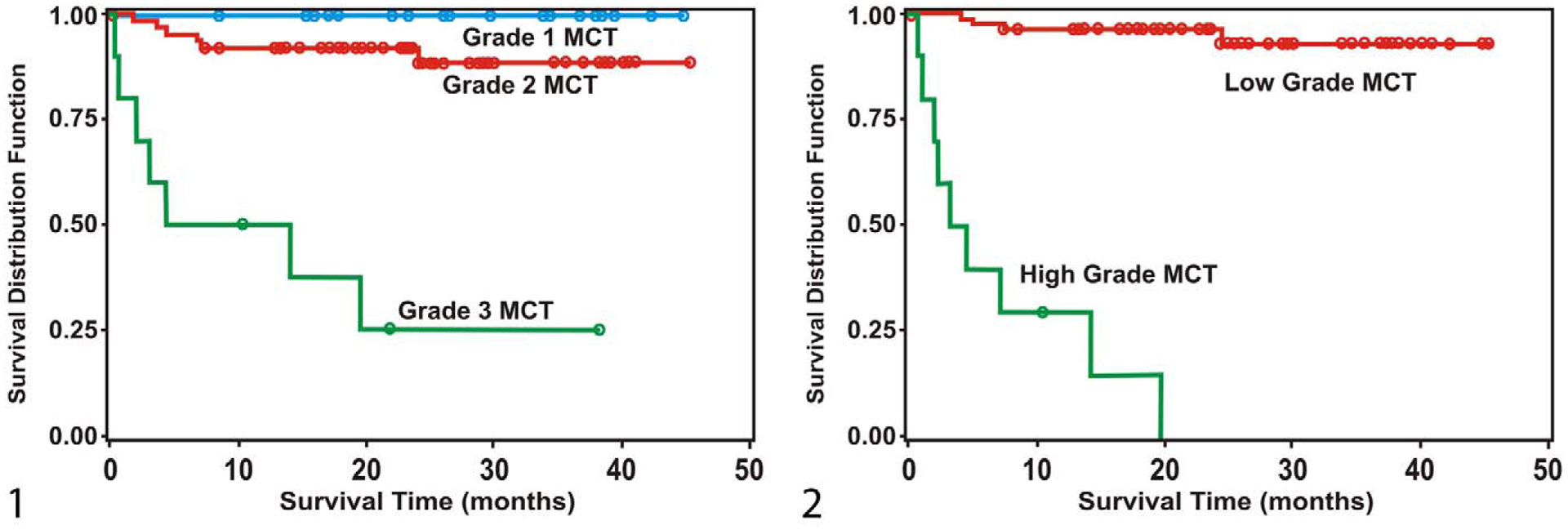
Survival curves for mortality due to mast cell tumors (MCTs), stratified by median histologic grades according to Patnaik et al10 from 95 cases. Figure 2 Survival curves for mortality due to mast cell tumors (MCTs), stratified by histologic grades of proposed 2-tier grading system from 95 cases.
Table 2.
Mast Cell Tumor–Related Mortality by Percentage Class for Histologic Grades According to Patnaik From 95 Cases
| Class | n | Mortality, % | χ2 | P | Fisher P | Odds Ratio | ||
|---|---|---|---|---|---|---|---|---|
| In Class | In Other Classes | Point Estimate | CI | |||||
| Grade 3 | ||||||||
| > 90% | 3 | 100.00 | 10.87 | 19.33 | < .0001 | .0021 | 55.00 | - |
| > 80% | 3 | 100.00 | 10.87 | 19.33 | < .0001 | .0021 | 55.00 | - |
| > 70% | 5 | 100.00 | 8.89 | 32.94 | < .0001 | < .0001 | 106.70 | - |
| > 50% | 9 | 77.78 | 6.98 | 34.21 | < .0001 | < .0001 | 46.67 | 7.9–275.9 |
| Grade 2 | ||||||||
| > 80% | 5 | 60.00 | 11.11 | 9.48 | .0021 | .0174 | 12.00 | 1.8–80.7 |
| > 70% | 18 | 38.89 | 7.79 | 11.81 | .0006 | .0024 | 7.53 | 2.1–26.6 |
| > 50% | 75 | 17.33 | 0.00 | 3.97 | .0462 | .0635 | 8.86 | - |
| Grade 1 | ||||||||
| > 80% | 4 | 0.00 | 14.29 | 0.66 | .4183 | 1.0000 | 0.65 | - |
| > 70% | 5 | 0.00 | 14.44 | 0.83 | .3629 | 1.0000 | 0.52 | - |
| > 50% | 23 | 0.00 | 18.06 | 4.76 | .0291 | .0335 | 0.09 | - |
Confidence interval—reported only where odds ratio is estimable.
Table 4.
Survival Time in Months by Percentage Class for Histologic Grades According to Patnaik From 95 Cases
| Class | n | In Class, Mean | Other Classes, Mean | Analysis of Variance | Wilcoxon Rank-Sum | ||
|---|---|---|---|---|---|---|---|
| F | P | χ2 | P | ||||
| Grade 3 | |||||||
| > 90% | 3 | 1.87 | 23.04 | 10.0 | .0021 | 7.42 | .0064 |
| > 80% | 3 | 1.87 | 23.04 | 10.0 | .0021 | 7.42 | .0064 |
| > 70% | 5 | 5.12 | 23.32 | 12.33 | .0007 | 9.20 | .0024 |
| > 50% | 9 | 10.27 | 23.63 | 11.30 | .0011 | 9.42 | .0021 |
| Grade 2 | |||||||
| > 80% | 5 | 12.14 | 22.93 | 3.99 | .0486 | 2.30 | .1293 |
| > 70% | 18 | 15.57 | 23.95 | 7.70 | .0067 | 5.62 | .0178 |
| > 50% | 75 | 21.41 | 25.96 | 2.33 | .1307 | 1.83 | .1766 |
| Grade 1 | |||||||
| > 80% | 4 | 30.05 | 22.02 | 1.74 | .1902 | 1.86 | .1731 |
| > 70% | 5 | 24.24 | 22.26 | 0.13 | .7207 | 0.24 | .6229 |
| > 50% | 23 | 26.68 | 21.06 | 3.92 | .0506 | 3.39 | .0655 |
Table 3.
Days to Development of Additional Mast Cell Tumors or Metastasis by Percentage Class for Histologic Grades According to Patnaik From 95 cases
| Class | n | In Class, Mean | Other Classes, Mean | Analysis of Variance | Wilcoxon Rank-Sum | ||
|---|---|---|---|---|---|---|---|
| F | P | χ2 | P | ||||
| Grade 3 | |||||||
| > 90% | 3 | 0.87 | 2.42 | 0.22 | .6391 | 1.72 | .1902 |
| > 80% | 3 | 0.87 | 2.42 | 0.22 | .6391 | 1.72 | .1902 |
| > 70% | 5 | 4.42 | 2.25 | 0.71 | .4023 | 6.90 | .0086 |
| > 50% | 9 | 4.12 | 2.18 | 0.98 | .3254 | 7.90 | .0049 |
| Grade 2 | |||||||
| > 80% | 5 | 5.12 | 2.22 | 1.28 | .2606 | 3.30 | .0695 |
| > 70% | 18 | 2.51 | 2.34 | 0.01 | .9085 | 0.15 | .6951 |
| > 50% | 75 | 2.42 | 2.18 | 0.03 | .8631 | 0.11 | .7434 |
| Grade 1 | |||||||
| > 80% | 4 | 4.50 | 2.27 | 0.60 | .4390 | 0.08 | .7827 |
| > 70% | 5 | 3.60 | 2.30 | 0.25 | .6155 | 0.00 | 1.0000 |
| > 50% | 23 | 2.61 | 2.29 | 0.06 | .8109 | 0.01 | .9391 |
None of the individual institutional grading systems provided prognostically useful information in regard to survival times, MCT-associated mortality, or development of additional MCTs at different sites. A consensual subgrade of 2a, representing microscopically well-differentiated grade 2 MCTs, was significantly associated with decreased MCT-associated mortality (Table 5). However, neither grade 2b, representing microscopically less differentiated grade 2 MCTs, or grade 1 MCTs were significantly associated with MCT-associated mortality. The majority of tumors were classified as grade 2a or 3, providing little prognostic differentiation of the studied population (Table 5).
Table 5.
Mast Cell Tumors–Related Mortality by Percentage Class for Histologic Grades According to Institutional Grading System That Uses a Subclassification for Grade 2 Mast Cell Tumors From 95 Cases
| Gradea | n | Mortality, % | χ2 | P | Fisher P | Odds Ratio | ||
|---|---|---|---|---|---|---|---|---|
| In This Class | In Other Classes | Point Estimate | CI | |||||
| 3 | 6 | 66.67 | 10.11 | 15.06 | .0001 | .0029 | 17.78 | 2.85–111.04 |
| 2b | 4 | 25.00 | 13.19 | 0.45 | .5033 | .4506 | 2.19 | 0.21–22.85 |
| 2a | 50 | 2.00 | 26.67 | 12.08 | .0005 | .0005 | 0.06 | 0.01–0.45 |
| 1 | 3 | 0.00 | 14.13 | 0.49 | .4857 | 1.0000 | 0.84 | 0.04–17.22 |
More than 50% of 6 observers reported this grade. CI, confidence interval.
The lack of consistency in grading MCTs, combined with the lack of prognostic significance in differentiating grade 1 from grade 2 MCTs, prompted a review of the current grading criteria and a revised grading system. In review of the morphologic features of those MCTs that had been assigned a median grade 3, alterations in nuclear morphology or a large number of mitotic figures were identified as the primary indicators of a higher grade. Based on the review of the nuclear morphology and the number of mitotic figures per 10 hpf of all cases, criteria were defined to differentiate canine cutaneous MCTs into 1 of 2 grades: high-grade or low-grade MCTs. The choice of these criteria was informed by the results of previous studies that established the importance of the number of mitotic figures per 10 hpf and nuclear morphology for MCT prognosis but did not find an association between depths of infiltration of neoplastic mast cells and prognosis.3,5,13,15,16 High-grade MCTs are characterized by one or more of the following criteria: at least 7 mitotic figures in 10 hpf, at least 3 multinucleated (3 or more nuclei) cells in 10 hpf, at least 3 bizarre nuclei (highly atypical with marked indentations, segmentation, and irregular shape) in 10 hpf; karyomegaly (ie, nuclear diameters of at least 10% of neoplastic mast cells vary by at least 2-fold). The selected fields were those that were most highly mitotically active or had the highest degree of anisokaryosis. All MCTs were graded in another blind trial according to the proposed 2-tier grading system by 6 pathologists (5 that had not previously graded these MCTs). Statistics were applied as previously described.
Using the proposed 2-tier grading system, all 6 pathologists identified the same 10 MCTs as high-grade tumors. Nine of these MCTs had been identified as grade 3 MCTs by 50% of the 28 pathologists who had originally graded them according to Patnaik et al.10 Only 7 of these tumors would have been identified on the basis of the number of mitotic figures alone. Two pathologists each identified 2 additional MCTs (3 cases) as high-grade tumors. The survival times for these 3 cases were between 17 and 19 weeks, and none of these tumors metastasized. The proposed grading system had a 96.8% consistency, and the weighted average of agreements was 99.3%. Based on the consensus grade (10 high-grade MCTs), the proposed 2-tier grading system had a significant association between high-grade MCTs and increased mortality (Table 6, Fig. 2), as well as increased risk of developing additional tumors/metastasis (Table 6). There was no significant association of either grade with the simultaneous occurrence of multiple tumors (Table 6). High-grade MCTs were significantly associated with shorter times to additional tumor development/metastasis (Table 7) and shorter survival times (Table 8). The median survival time was less than 4 months for high-grade MCTs; it was more than 2 years for low-grade MCTs.
Table 6.
Mortality, Mast Cell Tumor–Related Mortality, Occurrence of Multiple Tumors, and New Mast Cell Tumor Development/Metastasis by Percentage Class for Histologic Grades According to the Proposed 2-Tier Grading System From 95 Cases
| Grade | n | Outcome, % | χ2 | P | Fisher P | Odds Ratio | ||
|---|---|---|---|---|---|---|---|---|
| With | With Other | Point Estimate | CI | |||||
| All mortality | ||||||||
| High | 10 | 90.00 | 10.00 | 24.55 | < .0001 | < .0001 | 1.58 | 1.16—2.15 |
| Low | 85 | 17.65 | 82.35 | |||||
| MCT mortality | ||||||||
| High | 10 | 90.00 | 10.00 | 54.53 | < .0001 | < .0001 | 3.21 | 1.42–7.26 |
| Low | 85 | 4.71 | 95.29 | |||||
| Multiple tumors | ||||||||
| High | 10 | 10.00 | 90.00 | 0.00 | .9545 | 1.0000 | 0.94 | 0.11–8.29 |
| Low | 85 | 10.59 | 89.41 | |||||
| New MCT development/metastasis | ||||||||
| High | 10 | 70.00 | 30.00 | 13.64 | .0002 | .0011 | 1.41 | 1.05–1.85 |
| Low | 85 | 17.65 | 82.35 | |||||
Table 7.
Weeks to New Tumor Development or Metastasis by Percentage Class for Histologic Grades According to the Proposed 2-Tier Grading System From 95 Cases
| Grade | n | Weeks | Analysis of Variance | Wilcoxon Rank-Sum | |||
|---|---|---|---|---|---|---|---|
| Mean | Median | F | P | χ2 | P | ||
| High | 8 | 6.30 | 3.00 | 1.50 | .2242 | 10.45 | .0012 |
| Low | 14 | 12.41 | 13.65 | ||||
Table 8.
Survival Time in Months by Percentage Class for Histologic Grades According to Proposed 2-Tier Grading System From 95 Cases
| Score | n | Months | Analysis of Variance | Wilcoxon Rank-Sum | |||
|---|---|---|---|---|---|---|---|
| Mean | Median | F | P | χ2 | P | ||
| High | 10 | 6.29 | 3.65 | 25.53 | < .0001 | 18.59 | < .0001 |
| Low | 85 | 24.26 | 23.00 | ||||
The multivariable model included the novel grading system as the only explanatory variable. The hazard of death for high-grade tumors was 53.8 times (95% CI, 49.83–57.77) that of low-grade tumors. In addition, the ANOVA table (Table 9) from the model fitting the proposed 2-tier grading system first showed that, after fitting this variable, the Patnaik grading system is not statistically significant (ie, does not contribute any more to explaining survival). However, when the Patnaik grading system is fitted first, the proposed 2-tier grading system is still significant in the model, showing that the system contains additional statistically significant information about survival. It is thus a better predictor of survival than the traditional Patnaik grading system.
Table 9.
Analysis of Variance for Cox Proportional Hazard Model Assessing the Association of Proposed 2-Tier Grading System and Patnaik Grading System on Survivala
| Variable | Log Likelihood | χ2 | P | |
|---|---|---|---|---|
| Model 1 | Null | −56.83 | ||
| Proposed grading systema | −37.71 | 38.24 | < .001 | |
| Patnaik grading systemb | −37.61 | 0.20 | .66 | |
| Model 2 | Null | −56.83 | ||
| Patnaik grading systemb | −44.88 | 23.90 | < .001 | |
| Proposed grading systema | −37.61 | 14.54 | < .001 |
df = 1,
df = 2.
Discussion
The results of this study demonstrated significant interobserver variation in MCT grading among pathologists at various institutions. Although 74% of pathologists agreed on the diagnosis of grade 3 MCTs, only 63% agreed on the diagnoses of grade 1 and grade 2 MCTs. This degree of interobserver variation suggests that the current histologic grading system is unreliable, as previously reported.7,8
A second concern highlighted by the results of this study is that grade 2 MCTs accounted for 53% of the grades, which provides little prognostic information. According to the original work by Patnaik et al, 44% of patients with grade 2 MCTs were alive at 1,500 days.10 Therefore, based on these percentages, an animal with a histologic grade 2 MCT has a 56% chance of dying within 5 years of diagnosis and a 44% chance of surviving past 5 years. The diagnosis of grade 2 MCT adds little prognostic value to clinicians, because there is nearly a 50/50 chance of survival by 5 years in these patients. Moreover, there is a tendency for pathologists to opt for a grade 2 when confronted with a tumor that is borderline between grade 1 and 2. Based on these results, in nearly 50% of MCTs diagnosed, histologic grading offers little prognostic value owing to the ambiguity of its histologic grade. Therefore, it was not just a lack of consistency but also a predominance of the ambiguous grade 2 MCT categorization that needed to be addressed in redefining the histologic classification of canine MCTs.
In our opinion, a solution to interobserver variation in histologic classification and the predominance of grade 2 MCTs is a 2-grade histologic classification system based on simple microscopic criteria. According to the data presented here and in previous studies,7,8 the most significant variation in grading was between grade 1 and grade 2 MCTs when based on the Patnaik system. When these tumors were categorized on the basis of median histologic grades of the Patnaik system, neither grade 1 nor grade 2 MCTs were significantly associated with increased incidence of MCT-associated mortality or decreased overall survival. The lack of distinction between grade 1 and grade 2 MCTs (as indicated by the interobserver variation) and the lack of association between grade 1 and grade 2 MCTs and survival suggest that these might not be dichotomous groups but instead represent a spectrum of low-grade MCTs. A 2-grade system of low-grade and high-grade MCTs may be more appropriate and of greater clinical utility. A 2-grade system with clear histologic grading criteria eliminates the ambiguity of intermediate grade MCTs.
A key distinguishing feature between grade 1 and grade 2 MCTs in the Patnaik grading system is the extension of neoplastic mast cells below adnexa into the subcutis.10 Some pathologists use this criterion as the principal differentiating feature between grade 1 and grade 2 MCTs. Tumor invasiveness has been evaluated as an independent prognostic indicator in 2 studies. In one, tumors that had marked invasion into the subcutis and deeper tissues were associated with decreased cancer-free intervals and survival times, compared with tumors that were moderately invasive, as defined by extension into the lower dermis and minimal extension into the subcutis.11 Tumors confined to the superficial dermis, a defining feature of Patnaik grade 1 MCTs, were not included in that study. A second study characterized tumors as being confined to the superficial dermis, extending into the subcutis, isolated in the subcutis, or extending into the underlying musculature.5 That study found no association between tumor depth and patient survival, suggesting that MCTs in the subcutis are not necessarily associated with a worse prognosis.5 Therefore, it may be more appropriate to use morphologic features of neoplastic cells, such as nuclear size, chromatin pattern, nucleoli, the presence of multinucleated (3 or more nuclei) cells, and the number of mitotic figures in histologic grading, while avoiding the use of tumor depth.
The proposed 2-tier grading system is based on the number of mitotic figures in combination with karyomegaly, multinucleated (3 or more nuclei) cells, or bizarre nuclei (highly atypical nuclei with marked indentations, segmentation, and irregular shape). Even though the number of mitotic figures has been documented as a single prognostic parameter,3,14 the novel grading system identified 3 MCTs as high grade that would not have been diagnosed as such based on number of mitotic figures alone. Reported cutoff points for mitotic figures per 10 hpf vary from 5 to 10; unfortunately, those cutoff points were selected rather than statistically determined.1,13 In addition, a validation study identified up to 0.6 mitotic figures per 10 hpf in grade 1 tumors and 7 mitotic figures in grade 3 tumors.3 A conservative cutoff point of 7 mitotic figures per 10 hpf was selected for the new grading system given that light microscopes vary in their field of view, with recent models having wider fields, resulting in a higher mitotic count per hpf. Until prospective studies can refine the cutoff points, the data presented here support the selected value.
Using the proposed 2-tier system, pathologists were able to identify biologically aggressive MCTs with higher consistency and less ambiguity than when applying a 3-tier system. However, the consistency of data are based on a smaller study group, and the newly proposed grading system should be further validated in a prospective trial. Furthermore, as has been previously shown, the morphologic assessment of canine cutaneous MCTs has limitations.11,13,17,18 Especially with the development of novel therapies targeting specific receptors, such as tyrosine kinase inhibitors, other parameters have become more important for determining the most appropriate therapy for canine cutaneous MCTs.17 However, a recent study documented that high-grade MCTs lacking mutations or aberrant KIT expression responded to a chemotherapy protocol composed of vinblastine and prednisone.19
The proposed 2-tier grading system should be applied as an initial screening tool at the time of routine histologic examination and diagnosis of a cutaneous MCT. With this grading system, those tumors that pose a high risk of aggressive behavior can be immediately identified, which would allow the owner and veterinary clinician to request supplemental testing (eg, expression of KIT or screening for c-KIT mutations) to select the most appropriate therapy. Low-grade tumors should be clinically monitored and further prognosticated with proliferation markers, KIT expression, analysis of c-KIT mutations, and so on, to determine the risk of metastases and to select treatment.
Acknowledgements
We thank the clinical faculty who provided follow-up data on dogs. This study represents an initiative of the American College of Veterinary Pathologists’ Oncology Committee; the manuscript was also reviewed and endorsed by the World Small Animal Veterinary Association. We thank both organizations for their support and guidance.
Financial Disclosure/Funding
Funding for this study was provided in part by the Companion Animal Fund of the College of Veterinary Medicine, Michigan State University.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- 1.Bostock DE: The prognosis following surgical removal of mastocytomas in dogs. J Small Anim Pract 14:27–41, 1973. [DOI] [PubMed] [Google Scholar]
- 2.Brodey RS: Canine and feline neoplasia. Adv Vet Sci Comp Med 14:309–354, 1970. [PubMed] [Google Scholar]
- 3.Elston LB, Suerio FAR, Cavalcanti JM, Meze K: The importance of the mitotic index as a prognostic factor for survival of canine cutaneous mast cell tumors: a validation study. Vet Pathol 46:362–365, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Finnie JW, Bostock DE: Skin neoplasia in dogs. Aust Vet J 55:602–604, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Kiupel M, Webster JD, Miller RA, Kaneene JB: Impact of tumour depth, tumour location and multiple synchronous masses on the prognosis of canine cutaneous mast cell tumours. J Vet Med A Physiol Pathol Clin Med 52:280–286, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Misdorp W: Mast cells and canine mast cell tumours: a review. Vet Q 26:156–169, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Northrup NC, Harmon BG, Gieger TL, Brown CA, Carmichael KP, Garcia A, Latimer KS, Munday JS, Rakich PM, Richey LJ, Stedman NL, Cheng AL, Howerth EW: Variation among pathologists in histologic grading of canine cutaneous mast cell tumors. J Vet Diagn Invest 17:245–248, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Northrup NC, Howerth EW, Harmon BG, Brown CA, Carmicheal KP, Garcia AP, Latimer KS, Munday JS, Rakich PM, Richey LJ, Stedman NL, Gieger TL: Variation among pathologists in the histologic grading of canine cutaneous mast cell tumors with uniform use of a single grading reference. J Vet Diagn Invest 17:561–564, 2005. [DOI] [PubMed] [Google Scholar]
- 9.O’Keefe DA: Canine mast cell tumors. Vet Clin N Am Small Anim Pract 20:1105–1115, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Patnaik AK, Ehler WJ, MacEwen EG: Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol 21:469–474, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Preziosi R, Sarli G, Paltrinieri M: Multivariate survival analysis of histologic parameters and clinical presentation in canine cutaneous mast cell tumours. Vet Res Commun 31:287–296, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Priester WA: Skin tumors in domestic animals: data from 12 United States and Canadian colleges of veterinary medicine. J Natl Cancer Inst 50:457–466, 1973. [DOI] [PubMed] [Google Scholar]
- 13.Romansik EM, Reilly CM, Kass PH, Moore PF, London CA: Mitotic index is predictive for survival for canine cutaneous mast cell tumors. Vet Pathol 44:335–341, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell TL, Howlett CR, Middleton DJ, Griffiths DA, Duff BC: Skin neoplasms of dogs in Sydney. Aust Vet J 64:161–164, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Strefezzi RF, Xavier JG, Catão-Dias JL: Morphometry of canine cutaneous mast cell tumors. Vet Pathol 40:268–275, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Strefezzi RF, Xavier JG, Kleeb SR, Catao-Dias JL: Nuclear morphometry in cytopathology: a prognostic indicator for canine cutaneous mast cell tumors. J Vet Diagn Invest 21:821–825, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Webster JD, Yuzbasiyan-Gurkan V, Kaneene JB, Miller R, Resau JH, Kiupel M: The role of c-KIT in tumorigenesis: evaluation in canine cutaneous mast cell tumors. Neoplasia 8:104–111, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster JD, Yuzbasiyan-Gurkan V, Miller RA, Kaneene JB, Kiupel M: Cellular proliferation in canine cutaneous mast cell tumors: associations with c-KIT and its role in prognostication. Vet Pathol 44:298–308, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Webster J, Yuzbasiyan-Gurkan V, Thamm DH, Hamilton E, Kiupel M: Evaluation of prognostic markers for canine mast cell tumors treated with vinblastine and prednisone. BMC Vet Res 4:32, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


