Abstract
Summary
This study was performed to evaluate whether the use of drugs in the treatment of osteoporosis in women is associated with COVID-19 outcomes. The results showed that the risk of hospitalization, intensive care unit admission, and mortality was not altered in individuals taking anti-osteoporosis drugs, suggesting no safety issues during a COVID-19 infection.
Introduction
Whether patients with COVID-19 receiving anti-osteoporosis drugs have lower risk of worse outcomes has not been reported yet. The aim of this study was to evaluate the association of anti-osteoporosis drug use with COVID-19 outcomes in women.
Methods
Data obtained from a nationwide, multicenter, retrospective cohort of patients diagnosed with COVID-19 from March 11th to May 30th, 2020 was retrieved from the Turkish Ministry of Health Database. Women 50 years or older with confirmed COVID-19 who were receiving anti-osteoporosis drugs were compared with a 1:1 propensity score-matched COVID-19 positive women who were not receiving these drugs. The primary outcomes were hospitalization, ICU (intensive care unit) admission, and mortality.
Results
A total of 1997 women on anti-osteoporosis drugs and 1997 control patients were analyzed. In the treatment group, 1787 (89.5%) women were receiving bisphosphonates, 197 (9.9%) denosumab, and 17 (0.9%) teriparatide for the last 12 months. Hospitalization and mortality rates were similar between the treatment and control groups. ICU admission rate was lower in the treatment group (23.0% vs 27.0%, p = 0.013). However, multivariate analysis showed that anti-osteoporosis drug use was not an independent associate of any outcome. Hospitalization, ICU admission, and mortality rates were similar among bisphosphonate, denosumab, or teriparatide users.
Conclusion
Results of this nationwide study showed that preexisting use of anti-osteoporosis drugs in women did not alter the COVID-19-related risk of hospitalization, ICU admission, and mortality. These results do not suggest discontinuation of these drugs during a COVID-19 infection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-021-06067-2.
Keywords: Bisphosphonates, COVID-19, Denosumab, Mortality, Osteoporosis, Teriparatide
Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) is a novel coronavirus that emerged in China in late 2019 [1, 2]. The disease that it causes, COVID-19, was announced as a global pandemic on March 11th, 2020 by World Health Organization (WHO) [3]. By June 13th, 2021, COVID-19 had infected 175,306,598 people and caused 3,792,777 deaths worldwide [4]. COVID-19 represents a clinical spectrum ranging from asymptomatic or mild/moderate illness which resembles upper respiratory tract infection to severe disease evolving to pneumonia, acute respiratory distress syndrome, and death [1, 5, 6].
Both inborn and acquired immunological responses play important roles in the severity and progression of COVID-19. Replication of SARS-CoV-2 followed by destruction of cells triggers recruitment of monocytes and macrophages and release of cytokines [7, 8]. The immune system is dysregulated in severe forms of COVID-19 with exaggerated release of cytokines and inflammatory markers making them appropriate biomarkers for monitoring disease progression [8, 9]. In this context, some immune-modulating drugs used in chronic inflammatory diseases which reduce immune response may provide protection against COVID-19 by decreasing its incidence, severity, and mortality [10, 11].
Osteoporosis is a systemic skeletal disease characterized by low bone mass and deterioration of bone microarchitecture with a consequent increase in bone fragility and susceptibility to fractures [12]. It is a chronic disease resulting in increased morbidity, mortality, and treatment costs [13]. Currently, most organizations do not recommend discontinuation of drugs used in the treatment of osteoporosis during the management of COVID-19, assuming that these drugs do not have an impact on prognosis [14–17]. However, the effects of osteoporosis and drugs used in its treatment on the incidence or prognosis of COVID-19 are largely unknown. In one study, osteoporosis was among the factors that increased the risk of COVID-19 [18]. In another study, zoledronate, denosumab, and supplemental calcium users had a tendency for a lower incidence of COVID-19 [19]. Denosumab and zoledronate may exert their protective effects by immune-modulating actions [10, 11, 19]. The aim of the present study was to evaluate whether the use of anti-osteoporosis drugs was associated with the risk of worse COVID-19 outcomes in women.
Materials and methods
Study design and participants
In this nationwide, multicenter, retrospective cohort study, we used the data retrieved from the Turkish Ministry of Health National Electronic Database. This database includes information of all COVID-19 patients across the country who were confirmed with a positive polymerase chain reaction (PCR) test between March 11th, 2020 (diagnosis of the first COVID-19 patient in Turkey) and May 30th, 2020. This was the period in which nearly total lockdown was enforced in Turkey. The study was conducted in accordance with 1964 Helsinki Declaration, as revised in 2013 and approved by the COVID-19 Investigation Review Board (IRB) designated by the Ministry of Health, which waived the requirement of informed consent due to the retrospective study design and anonymity of the database (IRB number: 95741342–020:186,404/28.10.2020).
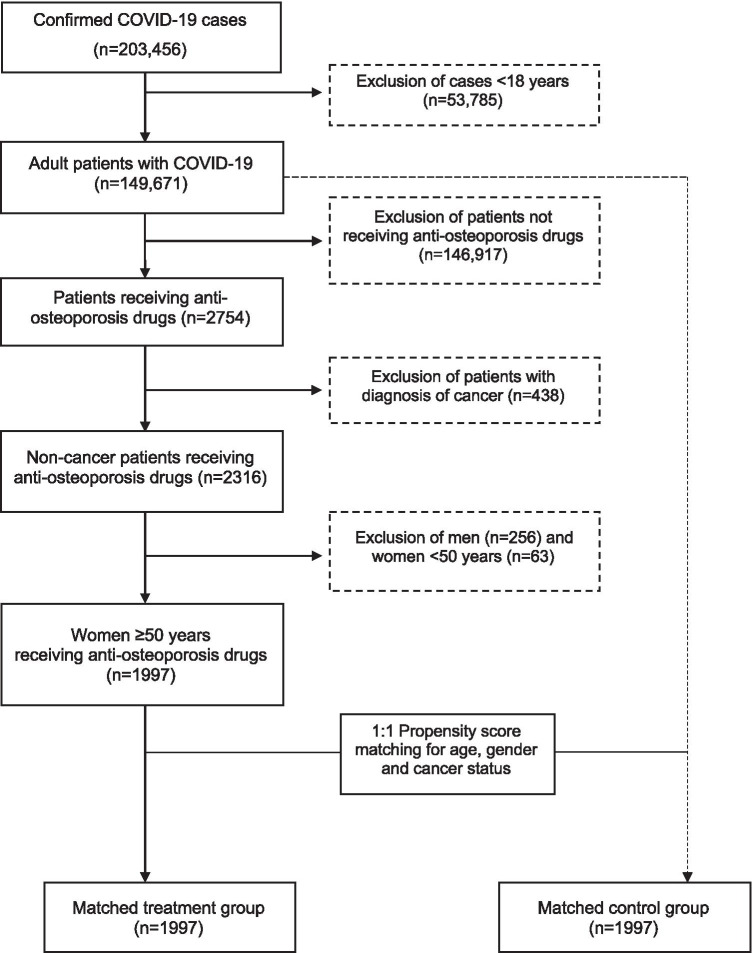
Of the 203,456 COVID-19 cases 53,785 (26.4%) were excluded since they were younger than 18 years. The remaining 149,671 adult cases who were followed ambulatory or in an inpatient setting were eligible for inclusion. Among this population, 2754 patients were identified with anti-osteoporosis drug (bisphosphonates, denosumab, and teriparatide) prescription in the last 12 months. Patients receiving these drugs for cancer treatment (n = 438) were excluded. Men (n = 256) and women younger than 50 years of age (n = 63) were also excluded. Women 50 years or older who were receiving anti-osteoporosis drugs (treatment group, n = 1997) were matched for age by 1:1 propensity score matching (PSM) with women who were not receiving these drugs (control group, n = 1997) (Fig. 1).
Fig. 1.
Flowchart of the study population selection
Data collection
Gender, age, attained level of education, smoking, comorbidities, body mass index (BMI), chest computerized tomography (CT) on admission, laboratory tests, and medications were obtained from the dataset.
Type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, obesity, asthma, chronic obstructive pulmonary disease (COPD), cardiovascular disease, rheumatic diseases, and chronic kidney disease (CKD) were noted as comorbidities.
Serum calcium, albumin, phosphorus, alkaline phosphatase (ALP), magnesium, 25-hydroxyvitamin D (25(OH)D), estimated glomerular filtration rate (eGFR), liver function tests (AST and ALT), D-dimer, C-reactive protein (CRP), procalcitonin, lactate dehydrogenase (LDH), ferritin, fibrinogen, and lymphocyte count were recorded. All blood tests were performed in hospital laboratories certificated by the Turkish Ministry of Health. Test results that were available within the last 10 days before the diagnosis of COVID-19 and 25(OH)D results available in the last 3 months were included.
Information about the use of anti-osteoporosis drugs (bisphosphonates, denosumab, and teriparatide), drugs for neuropathic pain, antidepressants, nonsteroidal anti-inflammatory drugs (NSAIDs), antidiabetic and antihypertensive medications, statins and acetylsalicylic acid was obtained from the Ministry of Health database. Anatomical Therapeutic Chemical (ATC) classification system was used for listing the drugs [20] (Supplementary Table 1).
Definitions
The term “treatment group” refers to women 50 years or older, receiving anti-osteoporosis drugs without diagnosis of cancer. Since menopausal status was not available in the database, the selection of this age as cutoff was based on the average menopause age of Turkish women in different cohorts [21, 22]. The term “control group” refers to age-matched women not receiving any osteoporosis treatment and not registered with diagnosis of osteoporosis and cancer. Higher education was defined as receiving formal education for 9 or more years. Smoking was defined as currently smoking at the time of diagnosis. BMI was calculated as the ratio of weight to the square of height (kg/m2). Calcium, albumin, phosphorus, ALP, magnesium, and 25(OH)D were expressed in units. eGFR was calculated based on the CKD-EPI equation [23]. Lymphopenia was defined as lymphocyte count less than 1000/μL, and percentage of patients with lymphopenia was used in the analyses. Other laboratory results were analyzed using the percentage of patients who exceed the upper limit of normal (ULN). T2DM, hypertension, dyslipidemia, obesity, asthma, COPD, cardiovascular disease, rheumatic diseases, and CKD were defined based on the International Classification of Diseases System-10 (ICD-10) codes.
Study outcomes
The study outcomes were COVID-19-related hospitalization, ICU admission, and mortality. Hospitalization and ICU admission were defined according to COVID-19 Guidelines prepared by the Turkish Ministry of Health Scientific Board [23]. Patients with moderate to severe Covid-19 and those who had underlying diseases such as cardiovascular disease, diabetes mellitus, hypertension, cancer, chronic lung, and other immunosuppressive diseases were hospitalized in hospitals reserved for the pandemic (Ministry of Health hospitals, State and Foundation University hospitals, and private hospitals). Patients with severe disease like pneumonia with respiratory failure, acute respiratory distress syndrome, sepsis, septic shock, myocarditis, arrhythmia, cardiogenic shock, metabolic acidosis, coagulation dysfunction, and multiple organ failure were followed in ICU [23].
Statistical analyses
All data were analyzed using Statistical Package for the Social Sciences (SPSS) for Windows 25.0 (SPSS Inc. 111 Chicago, IL). Numerical variables were expressed as mean ± standard deviation (SD) or medians and interquartile range (IQR) and categorical variables as counts (n) and percentages (%). Normality of distribution was assessed using Kolmogorov–Smirnov test. Differences between the groups were assessed using Chi-square test for categorical variables and Student’s t test or Mann–Whitney U test for numerical variables. Multivariate logistic regression analysis was performed to study the independent predictors of hospitalization, ICU admission, and mortality. Statistical significance was defined as a two-sided p value of ≤ 0.05.
Results
Basic and demographic characteristics of the treatment and control groups are shown in Table 1. Both groups were 1:1 matched for age (median 59 years, IQR 13). BMI (26.6 vs 31.2 kg/m2, p < 0.001) was lower and 25(OH)D level (22.64 vs 19.65 ng/mL, p = 0.043) was higher in the treatment group. Education, smoking, chest CT findings on admission, and other laboratory findings including markers of COVID-19 severity were similar in the two groups.
Table. 1.
Clinical characteristics and outcomes of women 50 years or older diagnosed with COVID-19 who were receiving and not receiving anti-osteoporosis drugs
| Treatment groupa (n = 1997) |
Control groupb (n = 1997) |
Available data (n/n, treatment group/control group) | pd | |
|---|---|---|---|---|
| Age, years, median (IQR) | 59 (13) | 59 (13) | 1997/1997 | 1.000 |
| Follow-up center, n (%) | ||||
| Public hospitals | 1575 (78.9) | 1512 (78.9) | 1997/1997 | 0.055 |
| University hospitals | 138 (6.9) | 153 (7.7) | ||
| Private centers | 284 (14.2) | 332 (16.6) | ||
| Education, 9 years and over, n (%) | 12 (4.5) | 12 (4.2) | 265/284 | 0.862 |
| Smoking, current smoker, n (%) | 91 (7.4) | 95 (7.5) | 1237/1260 | 0.862 |
| BMI, kg/m2, median (IQR) | 26.6 (6.8) | 31.2 (6.9) | 224/235 | < 0.001 |
| Chest CT on admission consistent with COVID-19, n (%) | 805 (41.7) | 849 (43.7) | 1929/1945 | 0.227 |
| Comorbid conditions | ||||
| T2DM, n (%) | 949 (48.2) | 1038 (52.8) | 1969/1966 | 0.004 |
| Hypertension, n (%) | 1747 (87.5) | 1752 (87.2) | 1997/1997 | 0.810 |
| Dyslipidemia, n (%) | 859 (43.0) | 835 (41.8) | 1997/1997 | 0.442 |
| Obesity, n (%) | 95 (42.4) | 142 (60.4) | 224/235 | < 0.001 |
| Asthma/COPD, n (%) | 955 (47.8) | 829 (41.5) | 1997/1997 | < 0.001 |
| Cardiovascular disease, n (%) | 1047 (52.4) | 990 (49.6) | 1997/1997 | 0.071 |
| Rheumatic diseases, n (%) | 46 (2.3) | 13 (0.7) | 1997/1997 | < 0.001 |
| CKD, n (%) | 236 (28.7) | 272 (35.4) | 823/768 | 0.012 |
| Laboratory values | ||||
| Calcium, mg/dL, median (IQR) | 8.90 (1.05) | 8.80 (1.02) | 253/258 | 0.202 |
| Albumin, g/dL, median (IQR) | 3.63 (0.78) | 3.70 (1.00) | 154/159 | 0.687 |
| Phophorus, mg/dL, median (IQR) | 3.20 (0.92) | 3.33 (1.44) | 65/80 | 0.358 |
| ALP, IU/L, median (IQR) | 72.50 (43.75) | 72.50 (32.25) | 118/138 | 0.398 |
| Magnesium, mg/dL, median (IQR) | 1.97 (0.45) | 1.92 (0.38) | 76/99 | 0.556 |
| 25(OH)D, ng/mL, median (IQR) | 22.64 (21.96) | 19.65 (16.27) | 15/116 | 0.043 |
| eGFR (mL/kg/min/1.73 m2), median (IQR) | 88.95 (54.20) | 85.47 (54.77) | 568/631 | 0.085 |
| AST, > ULN, n (%) | 61 (17.9) | 58 (16.6) | 340/350 | 0.634 |
| ALT, > ULN, n (%) | 31 (9.0) | 35 (10.1) | 343/348 | 0.648 |
| D-dimer > ULN, n (%) | 128 (69.9) | 139 (69.8) | 183/199 | 0.984 |
| CRP, > ULN, n (%) | 492 (74.0) | 462 (74.4) | 665/621 | 0.866 |
| Procalcitonin, > ULN, n (%) | 5 (4.9) | 14 (11.1) | 102/126 | 0.147 |
| LDH, > ULN, n (%) | 222 (56.3) | 197 (50.9) | 394/387 | 0.127 |
| Ferritin, > 100 ng/mL, n (%) | 208 (62.3) | 256 (67.2) | 334/381 | 0.169 |
| Fibrinogen, > ULN, n (%) | 44 (75.9) | 38 (67.9) | 58/56 | 0.342 |
| Lymphopenia, Lym# < 1000, n (%) | 337 (26.8) | 316 (25.1) | 1258/1260 | 0.328 |
| Treatments | ||||
| Bisphophonates, n (%)c | 1787 (89.5) | NA | 1997/–– | ––- |
| Denosumab, n (%)c | 197 (9.9) | NA | 1997/–– | ––- |
| Teriparatide, n (%)c | 17 (0.9) | NA | 1997/–– | ––- |
| Neuropathic pain medications, n (%) | 1058 (53.0) | 693 (34.7) | 1997/1997 | < 0.001 |
| Antidepressant drugs, n (%) | 1037 (51.9) | 850 (42.6) | 1997/1997 | < 0.001 |
| NSAIDs, n (%) | 1943 (97.3) | 1784 (89.3) | 1997/1997 | < 0.001 |
| Antidiabetic drugs, n (%) | 640 (32.0) | 800 (40.1) | 1997/1997 | < 0.001 |
| Antihypertensive drugs, n (%) | 1561 (78.2) | 1551 (77.7) | 1997/1997 | 0.703 |
| Statins, n (%) | 525 (26.3) | 554 (27.7) | 1997/1997 | 0.301 |
| Acetylsalicylic acid, n (%) | 887 (44.4) | 792 (39.7) | 1997/1997 | 0.002 |
| Hospitalization, n (%) | 1411 (70.7) | 1451 (72.7) | 1997/1997 | 0.160 |
| ICU admission, n (%) | 324 (23.0) | 391 (27.0) | 1407/1446 | 0.013 |
| Mortality, n (%) | 237 (11.9) | 255 (12.8) | 1997/1997 | 0.386 |
aWomen 50 years or older who were receiving anti-osteoporosis drugs, bAge-matched women who were not receiving anti-osteoporosis drugs, cSome patients received two anti-osteoporosis drugs at different times, dp values in bold show statistically significant results
25(OH)D, 25-hydroxy vitamin D; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CT, computerized tomography; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; IQR, interquartile range; LDH, lactate dehydrogenase; Lym#, Lymphocyte count per mililiter; NA, non available; NSAIDs, nonsteroid anti-inflammatory drugs; T2DM, type 2 diabetes mellitus; ULN, upper limit of normal
Asthma/COPD (47.8% vs 41.5%, p < 0.001) and rheumatic diseases (2.3% vs 0.7%, p < 0.001) were more common, and T2DM (48.2% vs 52.8%, p = 0.004), obesity (42.4% vs 60.4%, p < 0.001), and CKD (28.7% vs 35.4%, p = 0.012) were less common in the treatment group. The prevalence of hypertension, dyslipidemia, and cardiovascular diseases were not different between the groups (Table 1).
Among the treatment groups, 1787 (89.5%) women received bisphosphonates, 197 (9.9%) received denosumab, and 17 (0.9%) received teriparatide. A few patients were identified to receive both drugs at different times. Neuropathic pain medications (53.0% vs 34.7%, p < 0.001), antidepressants (51.9% vs 42.6%, p < 0.001), NSAIDs (97.3% vs 89.3%, p < 0.001), and acetylsalicylic acid (44.4% vs 39.7%, p = 0.002) were more commonly; and antidiabetic drugs (32.0% vs 40.1%, p < 0.001) were less commonly used in the treatment group. No significant difference was found regarding the use of antihypertensive drugs and statins between the groups (Table 1).
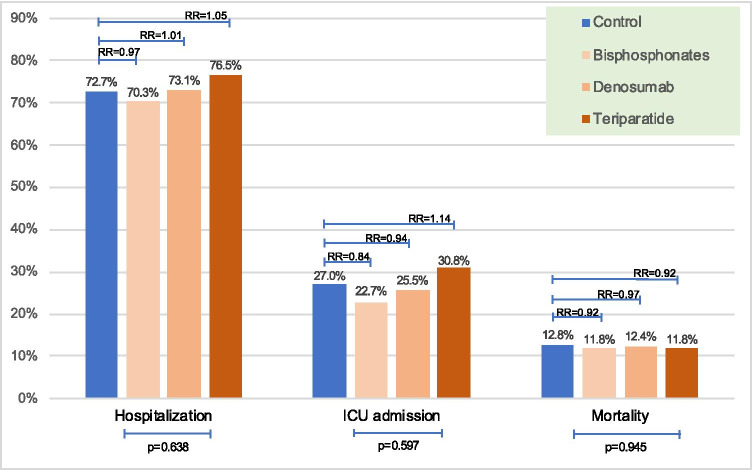
As shown in Table 1, hospitalization and mortality rates were similar between the treatment and control groups (70.7% vs 72.7%, p = 0.16 for hospitalization and 11.9% vs 12.8%, p = 0.386 for mortality). However, ICU admission rate was lower in the treatment group (23.0% vs 27.0%, p = 0.013). There were no significant differences in hospitalization, ICU admission, and mortality rates of women according to the type of anti-osteoporosis drug (Fig. 2).
Fig. 2.
Comparison of COVID-19 outcomes among the three anti-osteoporosis drug users and relative risks of outcomes compared to control group (ICU, intensive care unit)
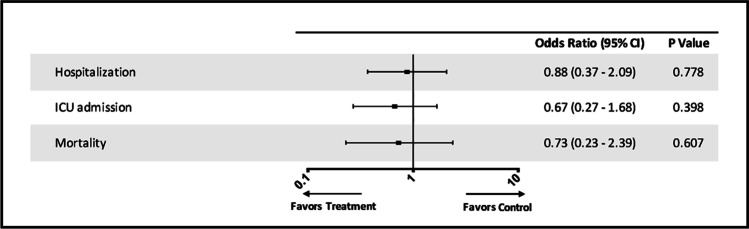
The results of the multivariate logistic regression analysis are shown in Table 2. When age, T2DM, hypertension, dyslipidemia, obesity, asthma/COPD, cardiovascular disease, CKD, and anti-osteoporosis drug use were entered as independent variables into the regression model, hypertension (OR: 3.92, 95% CI: 1.05–14.56, p = 0.042) was significantly associated with hospitalization, and age (OR: 1.07, 95% CI: 1.02–1.13, p = 0.01) was significantly associated with ICU admission. None of the variables was associated with mortality. Multivariate analysis showed that anti-osteoporosis drug use was not significantly associated with hospitalization, ICU admission, or mortality in adjusted analyses (Table 2 and Fig. 3).
Table. 2.
Multivariate logistic regression analysis of treatment (women 50 years or older who were receiving anti-osteoporosis drugs) and control (age-matched women who were not receiving anti-osteoporosis drugs) groups (dependent variable: hospitalization, ICU admission, mortality)
| Hospitalization | ICU admission | Mortality | ||||
|---|---|---|---|---|---|---|
| OR, CI (95%) | a | OR, CI (95%) | a | OR, CI (95%) | p | |
| Age | 0.98 (0.94–1.04) | 0.633 | 1.07 (1.02–1.13) | 0.010 | 1.06 (0.99–1.14) | 0.104 |
| T2DM | 1.06 (0.43–2.67) | 0.894 | 2.62 (0.95–7.19) | 0.062 | 2.82 (0.65–12.23) | 0.167 |
| Hypertension | 3.92 (1.05–14.56) | 0.042 | 0.28 (0.04–1.95) | 0.200 | 0.47 (0.04–5.46) | 0.545 |
| Dyslipidemia | 1.42 (0.59–3.42) | 0.428 | 1.84 (0.72–4.70) | 0.202 | 1.23 (0.37–4.05) | 0.736 |
| Obesity | 0.55 (0.23–1.31) | 0.177 | 0.85 (0.35–2.04) | 0.709 | 1.36 (0.43–4.31) | 0.603 |
| Asthma/COPD | 0.94 (0.41–2.20) | 0.894 | 0.83 (0.35–1.93) | 0.658 | 0.46 (0.15–1.47) | 0.192 |
| Cardiovascular disease | 1.42 (0.58–3.48) | 0.432 | 1.34 (0.52–3.46) | 0.548 | 2.22 (0.60–8.17) | 0.232 |
| CKD | 0.73 (0.30–1.77) | 0.484 | 1.35 (0.56–3.26) | 0.509 | 2.84 (0.92–8.81) | 0.071 |
| Anti-osteoporosis drug use | 0.88 (0.37–2.08) | 0.778 | 0.67 (0.27–1.68) | 0.398 | 0.73 (0.23–2.39) | 0.607 |
ap values in bold show stattistically significant result
CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; OR, odds ratio; T2DM, type 2 diabetes mellitus
Fig. 3.
Multivariate adjusted odds ratio of outcomes. The term “Treatment” refers to women 50 years or older who were receiving anti-osteoporosis drugs; “Control” refers to age-matched women who were not receiving anti-osteoporosis drugs (ICU, intensive care unit)
Discussion
This is one of the few studies focusing on COVID-19 outcomes in women who were receiving anti-osteoporosis drugs. The results revealed that hospitalization and mortality rates were similar between the treatment and control groups except for the ICU admission rate that was lower in the treatment group. In multivariate analysis, however, anti-osteoporosis drug use was not significantly associated with any of the three study outcomes. Also, women receiving bisphosphonates, denosumab, and teriparatide had similar rates for COVID-19 outcomes.
Most organizations do not recommend discontinuing anti-osteoporosis drugs during the COVID-19 era [14–17]. However, disruption of osteoporosis treatment may occur during the adoption of strict measures such as social distancing and lockdowns during any pandemic. In such a case, delay of intravenous bisphosphonates even for several months may not be harmful [15]. On the other hand, when denosumab treatment is not ensured within 7 months of the last injection, and the delay in treatment with teriparatide exceeds 2 to 3 months, temporarily transitioning to an oral bisphosphonate is suggested [15].
To date, there are no published studies on the potential effects of drugs used in the treatment of osteoporosis on significant outcomes such as hospitalization, ICU admission, and mortality in patients with COVID-19. In a study that evaluated the comorbidity burden among COVID-19 patients, osteoporosis was associated with an increased incidence of COVID-19, and it was identified as an independent risk factor in adjusted analysis [18]. On the other hand, a previous diagnosis of osteoporosis was not associated with disease severity in COVID-19 patients. The relation of anti-osteoporosis drugs with outcomes of COVID-19 was not evaluated in that study. Another study showed that preexisting use of denosumab and zoledronate was associated with a trend towards reduced incidence of COVID-19, although not statistically significant [19]. The authors proposed several mechanisms of action for zoledronate and denosumab, including their favorable role in the regulation of immune responses. The results of these two studies suggest that osteoporosis as a comorbidity may increase the incidence but anti-osteoporosis drugs may decrease the risk of COVID-19.
The studies mentioned above included both women and men, and the only outcome of interest was the incidence of COVID-19 [18, 19]. In the current study, only women 50 years or older who were receiving anti-osteoporosis drugs were included as a more homogenous group, and they were compared with age-matched women with COVID-19 who were not on anti-osteoporosis drugs. Therefore, the confounding effect of gender on study outcomes was eliminated. Women receiving anti-osteoporosis drugs had similar hospitalization and mortality but lower ICU admission rate than those who were not on an anti-osteoporosis drug. Thus, taken together with the results of the previous study [19], it is likely that the preexisting use of anti-osteoporosis drugs did not worsen the clinical course of COVID-19.
Obesity, T2DM, and CKD were less common whereas rheumatic diseases and asthma/COPD were more common in the treatment group in the sample of the current study. When these comorbidities were analyzed in multivariate analysis, none of them was found to be independent associates of the outcomes. Hypertension and age were significantly associated with hospitalization and ICU admission, respectively. However, mean age and frequency of hypertension did not differ between the groups. Therefore, we assume that our findings were not confounded by the chronic comorbidities. In accordance with disease states, more women in the treatment group received neuropathic pain medications, antidepressants, and NSAIDs, and fewer women received antidiabetic drugs. Although vitamin D status was available only in 3.3% of the total study population, women in the treatment group had higher 25(OH)D levels. They might be receiving vitamin D supplements as part of their osteoporosis management. We were unable to analyze if the differences in 25(OH)D levels and vitamin D replacement status had a confounding effect on our study outcomes. It is also likely that the preexisting use of several drugs such as analgesics and antidepressants may have some effects opposite to those of anti-osteoporosis drugs on the immune system [19]. However, the design of this study did not allow us to further investigate all medications of the patients.
Bisphosphonates are antiresorptive agents which bind to hydroxyapatite and inhibit farnesyl-pyrophosphate synthase, an enzyme in the mevalonate pathway [19, 24, 25]. They inhibit prenylation of intracellular signaling proteins which are essential for osteoclast function and survival and thereby they induce apoptosis of osteoclasts [24, 25]. Intravenous zoledronate was shown to decrease mortality by 28% after hip fracture but only 8% of this reduction was due to reductions in recurrent fractures [26, 27]. Zoledronate-treated patients were less likely to die from pneumonia and cardiac arrhythmias [27]. In SARS-CoV-2 infection, T cells are depleted, and the dendritic cells are abundant in the lungs [28]. The dendritic cells internalize inhaled SARS-CoV-2 particles. Virion release from the dendritic cells depends on the prenylation signal from the mevalonate pathway. Amino-bisphosphonates were shown to increase T cells and disrupt dendritic cells by inhibiting the mevalonate pathway [28].
Denosumab, another antiresorptive agent, has a different mechanism of action [24]. It prevents RANKL from binding its receptor RANK on osteoclasts and eventually inhibits osteoclastogenesis. RANK/RANKL system is also involved in immune responses. Apart from its effects on bone, denosumab acts as an immune-modulating agent and decreases inflammatory cytokines [19, 24, 29]. There are many studies evaluating the protective and therapeutic effects of denosumab on microbial diseases, especially viral infections [30, 31]. Recently, the role of innate immune system and T cell activation in host responses against SARS-CoV-2 was demonstrated in rhesus macaques [32]. RANKL is expressed in the immune cells of humans and plays an important role in T cell activation [33]. These findings imply that denosumab may have an effect on the course of COVID-19. A recent meta-analysis showed an increased risk of the ear, nose, and throat, and gastrointestinal infections with denosumab [34]. However, the overall risks of any infections and mortality were not increased [34]. In a survey study, women older than 50 years receiving treatment for postmenopausal osteoporosis were not at high risk for severe COVID-19, and denosumab was not a risk factor for COVID-19 [35].
Teriparatide is a parathormone analogue and an anabolic agent which increases bone formation. In vitro and in vivo studies evaluating the effects of parathormone on the immune system have shown inconsistent results mostly due to differences in the methodology of the studies [36, 37]. Most studies were performed in patients with CKD who had secondary hyperparathyroidism. In vitro and in vivo studies on the effects of high parathormone levels on T and B lymphocytes were summarized in a review article [36]. It appears that there are controversial reports about the effects of parathormone on the proliferation of T lymphocytes and the immunoglobulin production of the B lymphocytes [36]. Another study reported that parathormone had no effect on interleukin-6 and interleukin-8 production in activated leukocytes in healthy donors [37]. The clinical relevance of these findings is not clear, and laboratory studies are not perfect models to show the effects of chronic exposure to parathormone on the immune system. Besides, we cannot extrapolate these findings to patients receiving parathormone analogs for osteoporosis.
In this study, ICU admission rate was lower in the treatment group. This result may imply a less severe clinical course of COVID-19 infection in patients receiving anti-osteoporosis medications. The immune-modulating effects of anti-osteoporosis drugs, mainly bisphosphonates, may explain the lower rate. Since most of the patients in the treatment group received bisphosphonates (89.5%) and few of them received denosumab (9.9%) and teriparatide (0.9%), the effects of bisphosphonates on the immune system and hence COVID-19 course reflected by the ICU admission rate may be more prominent than the other drugs. However, hospitalization, ICU admission, and mortality rates were similar among women on three different drugs, and multivariate analysis showed that anti-osteoporosis drug use was not independently associated with any outcome.
This study has several limitations. Due to its retrospective design, the effects of missing data and confounding factors such as the use of drugs like steroids, calcium, and vitamin D supplements cannot be interpreted. Some patients in the control group may have osteoporosis although they were not receiving anti-osteoporosis drugs. In this regard, we relied on ICD-10 codes and included women without registered diagnosis of osteoporosis. Also, we were not able to separately analyze oral and intravenous bisphosphonate users, although some distinct immunologic properties of bisphosphonate class of drugs may cause differences in COVID-19 outcomes. Strengths of the study include a nationwide representation covering all seven geographical regions, involvement of all types of care hospitals in charge of government or private bodies, and the large number of patients. Besides, data of patients diagnosed in the first 3 months of the COVID-19 pandemic were collected. This is the period in which nearly total lockdown was enforced and the same treatment guideline prepared by the Ministry of Health was implemented for all patients diagnosed with COVID-19. Finally, the control group was selected by using 1:1 PSM approach to exclude the possible confounding effects of age, gender, and cancer status.
In conclusion, this is the first study evaluating the association of anti-osteoporosis drugs and COVID-19 outcomes in women. The results showed that the preexisting use of anti-osteoporosis drugs was not associated with a worse clinical course of COVID-19. Therefore, it appears that the use of anti-osteoporosis drugs during the COVID-19 era is safe, and we do not recommend discontinuation of osteoporosis treatment in this period.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge all the healthcare professionals who devoted their lives to the management of patients with COVID-19 disease and produced this database.
Data availability
The Turkish Ministry of Health Database covers the data for management of COVID-19 patients. The data is available to researchers who are permitted by the General Directorate of Health Information Systems. The authors are not allowed to share these data.
Code availability
No custom code or mathematical algorithm was used during the preparation of the manuscript.
Declarations
Ethics approval and consent to participate
The study was approved by the COVID-19 Investigation Review Board (IRB) designated by the Ministry of Health, which waived the requirement of informed consent due to the retrospective study design and anonymity of the database (IRB number: 95741342–020:186404/28.10.2020).
Informed consent was not required due to the retrospective design of the study.
Consent for publication
All authors approved the final version and publication of the manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng SQ, Peng HJ. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9:575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease 2019 (COVID-19) [Internet]. https://www.who.int/ Accessed 13 June 2021
- 4.WHO Coronavirus Disease (COVID-19) Dashboard. Available at ‘https://covid19.who.int/’. Accessed 13 June 2021
- 5.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z, Li T, Liang L, Wang H, Wei F, Meng S, Cai M, Zhang Y, Xu H, Zhang J, Jin R (2020) Clinical characteristics of coronavirus disease 2019 patients in Beijing. China PLoS One 15:e0234764. 10.1371/journal.pone.0234764 [DOI] [PMC free article] [PubMed]
- 7.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, Deng G. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michelena X, Borrell H, López-Corbeto M, López- Lasanta M, Moreno E, Pascual-Pastor M, Erra A, Serrat M, Espartal E, Antón S, Añez GA, Caparrós-Ruiz R, Pluma A, Trallero-Araguaz E, Barcelo-Bru M, Almirall M, De Agustin JJ, Llados J, Julia A, Marsal S. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. 2020;50:564–570. doi: 10.1016/j.semarthrit.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, Izadi Z, Jacobsohn L, Katz P, Lawson-Tovey S, Mateus EF, Rush S, Schmajuk G, Simard J, Strangfeld A, Trupin L, Wysham KD, Bhana S, Costello W, Grainger R, Hausmann JS, Liew JW, Sirotich E, Sufka P, Wallace ZS, Yazdany J, Machado PM, Robinson PC, COVID-19 Global Rheumatology Alliance (2020) Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 79:859–866. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed]
- 12.Consensus development conference diagnosis, prophylaxis, and treatment of osteoporosis (1993) Am J Med 94:646–650. 10.1016/0002-9343(93)90218-e [DOI] [PubMed]
- 13.Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF) Executive summary of the European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Calcif Tissue Int. 2019;104:235–238. doi: 10.1007/s00223-018-00512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gittoes NJ, Criseno S, Appelman-Dijkstra NM, Bollerslev J, Canalis E, Rejnmark L, Hassan-Smith Z. Endocrinology in the time of COVID-19: management of calcium metabolic disorders and osteoporosis. Eur J Endocrinol. 2020;183:G57–G65. doi: 10.1530/EJE-20-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu EW, Tsourdi E, Clarke BL, Bauer DC, Drake MT. Osteoporosis management in the era of COVID-19. J Bone Miner Res. 2020;35:1009–1013. doi: 10.1002/jbmr.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landewé RB, Machado PM, Kroon F, Bijlsma HW, Burmester GR, Carmona L, Combe B, Galli M, Gossec L, Iagnocco A, Isaacs JD, Mariette X, McInnes I, Mueller-Ladner U, Openshaw P, Smolen JS, Stamm TA, Wiek D, Schulze-Koops H. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79:851–858. doi: 10.1136/annrheumdis-2020-217877. [DOI] [PubMed] [Google Scholar]
- 17.Girgis CM, Clifton-Brigh RJ. Osteoporosis in the age of COVID-19. Osteoporos Int. 2020;31:1189–1191. doi: 10.1007/s00198-020-05413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji W, Huh K, Kang M, Hong J, Bae GH, Lee R, Na Y, Choi H, Gong SY, Choi YH, Ko KP, Im JS, Jung J. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35:e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanch-Rubió J, Soldevila-Domenech N, Tío L, Llorente-Onaindia J, Ciria-Recasens M, Polino L, Gurt A, de la Torre R, Maldonado R, Monfort J, Group TCS Influence of anti-osteoporosis treatments on the incidence of COVID-19 in patients with non-inflammatory rheumatic conditions. Aging (Albany NY) 2020;12:19923–19937. doi: 10.18632/aging.104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.https://www.who.int/tools/atc-ddd-toolkit/methodology. Accessed 13 June 2021
- 21.Ayranci U, Orsal O, Orsal O, Arslan G, Emeksiz DF. Menopause status and attitudes in Turkish midlife female population: an epidemiological study. BMC Womens Health. 2010;10:1. doi: 10.1186/1472-6874-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuzun S, Eskiyurt N, Akarirmak U, Saridogan M, Senocak M, Johansson H, Kanis JA, Turkish Osteoporosis Society Incidence of hip fracture and prevalence of osteoporosis in Turkey: the FRACTURK study. Osteoporos Int. 2012;23:949–955. doi: 10.1007/s00198-011-1655-5. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed]
- 24.Baron R, Ferrari S, Russell GG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 26.Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S, Recurrent Fracture Trial HORIZON. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colón-Emeric CS, Mesenbrink P, Lyles KW, Pieper CF, Boonen S, Delmas P, Eriksen EF, Magaziner J. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res. 2010;25:91–97. doi: 10.1359/jbmr.090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brufsky A, Marti JLG, Nasrazadani A, Lotze MT. Boning up: amino-bisphophonates as immunostimulants and endosomal disruptors of dendritic cell in SARS-CoV-2 infection. J Transl Med. 2020;18:261. doi: 10.1186/s12967-020-02433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu YG, Ritchlin CT. Denosumab: targeting the RANKL pathway to treat rheumatoid arthritis. Expert Opin Biol Ther. 2017;17:119–128. doi: 10.1080/14712598.2017.1263614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng ML, Fong L. Effects of RANKL-targeted therapy in immunity and cancer. Front Oncol. 2014;3:329. doi: 10.3389/fonc.2013.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi-Sakamoto M, Tamai R, Kiyoura Y. Beyond bone remodeling–emerging functions of osteoprotegerin in host defense and microbial infection. Integr Mol Med. 2015;2:384–390. doi: 10.15761/IMM.1000173. [DOI] [Google Scholar]
- 32.McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, Liu J, Peter L, Atyeo C, Zhu A, Bondzie EA, Dagotto G, Gebre MS, Jacob-Dolan C, Li Z, Nampanya F, Patel S, Pessaint L, Van Ry A, Blade K, Yalley-Ogunro J, Cabus M, Brown R, Cook A, Teow E, Andersen H, Lewis MG, Lauffenburger DA, Alter G, Barouch DH. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobacchi C, Menale C, Villa A (2019) The RANKL-RANK axis: a bone to thymus round trip. Front Immunol 10:629. 10.3389/fimmu.2019.00629 [DOI] [PMC free article] [PubMed]
- 34.Diker-Cohen T, Rosenberg D, Avni T, Shepshelovich D, Tsvetov G, Gafter-Gvili A. Risk for infections during treatment with denosumab for osteoporosis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105:dgz322. doi: 10.1210/clinem/dgz322. [DOI] [PubMed] [Google Scholar]
- 35.Formenti AM, Pedone E, di Filippo L, Ulivieri FM, Giustina A. Are women with osteoporosis treated with denosumab at risk of severe COVID-19? Endocrine. 2020;70:203–205. doi: 10.1007/s12020-020-02500-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geara AS, Castellanos MR, Bassil C, Schuller-Levis G, Park E, Smith M, Goldman M, Elsayegh S. Effects of parathyroid hormone on immune function. Clin Dev Immunol. 2010;2010:418695. doi: 10.1155/2010/418695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castellanos M, Jung E, Park SY, Schuller-Levis G, Odaimi M, Elsayegh S, Kleiner M, Elsoueidi R, Shtaynberg N, Park E. Effect of parathyroid hormone and teriparatide on immune function of human adherent and non-adherent leukocytes. Clin Nephrol. 2010;74:83–90. doi: 10.5414/cnp74083. [DOI] [PubMed] [Google Scholar]
- 38.https://hsgm.saglik.gov.tr/depo/birimler/goc_sagligi/covid19/rehber/COVID-19_Rehberi20200414_eng_v4_002_14.05.2020.pdf. Accessed 13 June 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Turkish Ministry of Health Database covers the data for management of COVID-19 patients. The data is available to researchers who are permitted by the General Directorate of Health Information Systems. The authors are not allowed to share these data.
No custom code or mathematical algorithm was used during the preparation of the manuscript.