Abstract
Background
Guillain-Barré syndrome (GBS) is the main cause of acute and subacute flaccid paralysis in western nations since the eradication of poliomyelitis.
Objective
The aim of this study is to investigate epidemiology and mortality characteristics of GBS in the north of Iran.
Material and methods
In this study, the hospital information system (HIS) was used to access each patient’s information. The final 174 cases were examined in terms of age, sex, place of residence, the year of referral, the month of referral, the season of referral, client city, accompanying background disease, and the type of GBS.
Results
The mean incidence rate in Guilan province was about 0.69 in 100,000 persons, and the case fatality rate was 10.34%. The most reported type of GBS was AIDP (33.90%), and the most common symptom was upper and lower limbs paresis in 65 cases (37%). Respiratory distress (P = < 0.001), complications during hospitalization (P = 0.0001), and ICU requirement (P = 0.001) were significantly higher in dead patients.
Conclusion
In this study, the incidence of GBS was higher in men than women and the highest number of cases was in the age group of 60 to 75 years. The significant point was the high-case fatality rate in Guilan province compared to the previous studies. The complications during hospitalization such as respiratory distress, ICU requirement, and underlying disease had a significant relation with the fatality of GBS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10072-021-05562-y.
Keywords: Guillain-Barré syndrome, Epidemiology, Mortality, Guilan, Iran, Incidence rate
Introduction
One of the most acute inflammatory neuropathy is GBS that can occur at any age. The pathogenesis of GBS is still unknown, but evidence suggests that it is an autoimmune disease, most commonly caused by infection, with upper respiratory tract infections and diarrhea being the most common presentation [1]. Since GBS is an acute inflammatory polyneuropathy, it leads to limb weakness and progresses over a short time ranging from a few days to a maximum of 4 weeks [2, 3]. According to some laboratory data and a combination of clinical manifestations including bilateral flaccid weakness of extremities, absent or decreased deep tendon reflexes (DTRs), monophasic course of the disease, and usual findings on cerebrospinal fluid (CSF) and nerve conduction studies, GBS can be diagnosed [4].
Acute motor and sensory axonal neuropathy (AMSAN), inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy (AMAN), and the Miller Fisher syndrome (MFS) are the most common types of GBS [5].
GBS is a global disease, but its epidemiological characteristics can vary from region to region. Therefore, to better understand GBS, it is necessary to identify the epidemiological characteristics, including age, sex, geographical location, and other environmental risk factors [6]. The general population’s overall GBS incidence is estimated at 0.81 to 1.89 cases, and children under 15 years of age 1–2 cases per 100,000 [4, 6]. Although conflicting views exist in the identification of risk factors, infectious agents such as H1N1 influenza vaccination [7], Campylobacter jejuni [8, 9], cytomegalovirus [10], and Zika virus [11] have been identified in recent studies. However, some studies have rejected the risk of infection with the influenza virus [12].
Neuromuscular respiratory failure is one the most severe complication of GBS that results in shallow breathing, low tidal volume, and poor gas exchange, so it would cause tachypnea and delayed hypercapnia [13]. As another critical manifestation of GBS, dysautonomia may lead to extreme blood pressure fluctuations, cardiac arrhythmia, hypersecretions, gastrointestinal tract dysfunction, and bladder dysfunction. Both conditions can cause severe symptoms and death in patients [14]. Two proven treatments are intravenous immunoglobulin (IVIG) and plasma exchange (PE). However, the IVIG is the first treatment choice because of the fewer side effect and easy prescription and access [15, 16].
Despite the effective treatments such as plasma exchange (PE), IV immunoglobulins (IVIG), and current understanding of the pathophysiology, the case fatality rate is high in acute cases [17], so in some studies, the case fatality rate fluctuates from 2.8 to 12.1% (14,15). The case fatality rate of patients admitted to the intensive care unit (ICU) is above 50% [17]. This study aims to evaluate the epidemiology and mortality characteristics of GBS in Guilan from 2009 to 2018 based on the local database and to contribute to a better estimation of the exact incidence of GBS in Iran.
Methods
Sample
This retrospective study was conducted in the north of Iran (Guilan province) based on a comprehensive review of medical records at the children (17-Sharvar) and adult (Poursina) referral public hospitals. Patients of all age groups diagnosed with the onset of GBS were enrolled in this study from 1 January 2009 to 31 December 2018.
Retrieving hospitals’ information database analysis provided a list of patients with a primary diagnosis of GBS (code 357.0 in the International Classification of Diseases, ninth revision (ICD-9)).
Death certification review
Inclusion criteria include (a) being as a citizen, (b) GBS diagnosis had been made by a neurologist, (c) the case fulfilled the criteria of Asbury and Cornblath or with a GBS diagnosis is indicated by the code G61.1 ICD-10, and (d) GBS must be the significant and first diagnosis, in case of physical comorbidity.
Exclusion criteria include (a) diagnosis was not definite, even after the initial diagnosis, (b) registered patients outside the province.
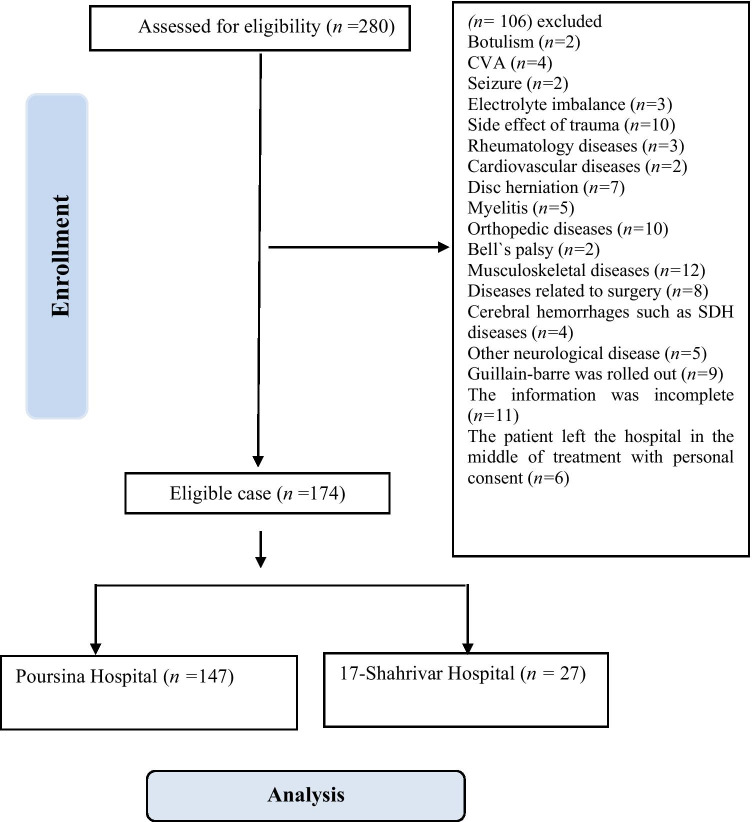
The consort diagram is illustrated in Fig. 1. Primarily, 280 potential patients were identified. Then, potential participants were subjected to a comprehensive assessment. At this step, 106 patients who had been diagnosed with the other medical condition like Botulism, electrolyte imbalance, CVA, seizure, traumatic complications, rheumatology disease, cardiovascular disease, disc herniation, myelitis, orthopedic disorders, Bell’s palsy, musculoskeletal disease, surgical-related diseases, cerebral hemorrhage such as SDH, other neurological condition, GBS was ruled out, incomplete medical data, incomplete treatment due to discharged by personal consent were excluded.
Fig. 1.
Flowchart related to the patient selection of GBS from HIS of two referral hospitals in Guilan province from 2009 to 2019
Altogether, 40 cases of the 17-Shahrivar Hospital and 240 cases of the Poursina Hospital with GBS diagnosis from 2009 to 2019 with G61.1 code extracted from the HIS system.
The medical files of all patients with GBS diagnosis who have been admitted to the 17 Shahrivar and Poursina hospitals’ neurological departments were reviewed to fulfill the inclusion criteria separately. Patients’ age, sex, place of residence, the year of referral, the month of referral, the season of referral, the client city, the accompanying background disease, and the type of GBS were extracted using their HIS records. In the end, 147 cases in adults and 27 cases in children were obtained (Fig. 1).
Data analysis
A total sample of 174 patients who met the study criteria was assessed in terms of age, sex, place of residence, the year of referral, the month of referral, the season of referral, client city, accompanying background disease, and the type of GBS. Then, they were statistically analyzed using IBM SPSS statistics version 24. Mean and the standard deviation was used to examine quantitative variables (such as age), and frequency and percentage were used to examine qualitative variables (such as gender and place of residence). Finally, the incidence rate and the case fatality rate were assessed. We used new cases of each year divided by the year’s populations (per 100,000 person-years) based on the Statistical Center of Iran report to access the incidence rate. For the case fatality rate, all expired cases were divided by the number of individuals diagnosed with GBS for 10 years; the resulting ratio was then multiplied by 100 to yield a percentage. The binary logistic regression test was used to determine the relation of the discharge status with other variables.
Ethical aspect
This study was approved in the Neuroscience Research Center of the Poursina Hospital of the Guilan University of Medical Sciences. Also, the study was approved by the Research Ethics Committee of the Guilan University of Medical Sciences (Code: IR.GUMS.REC.1398.340).
Result
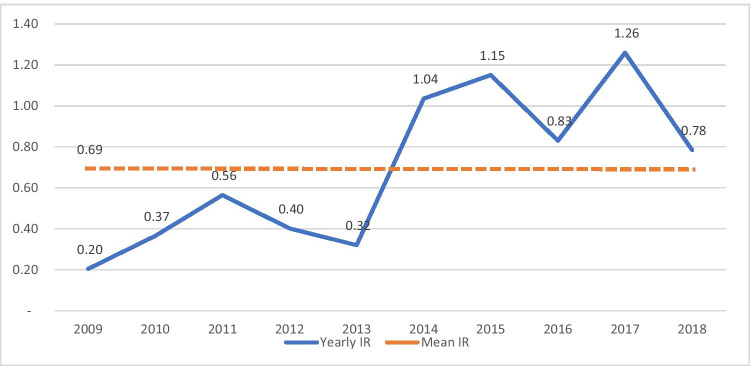
Among 174 patients diagnosed with GBS between 2009 and 2019, 105 (60.34%) were men, and 69 (39.65%) were women. As shown in Fig. 2, the lowest number of patients was recorded in 2009 (5 (2.87%)) with the incidence rate (IR) = 0.20 per 100,000 person-years, and the highest number of patients was recorded in 2017 (32 (18.42%)) with IR = 1.26. Also, the seasonal incidence in order belongs to spring with 53 cases (30.45%) followed by summer with 50 cases (28.77%), winter with 37 cases (21.36%), and autumn 34 cases (19.54%).
Fig. 2.
The linear chart of the yearly incidence rate of GBS compared to the mean incidence rate in Guilan province from 2009 to 2018
The sodium serum level was normal in 137 cases (78.73%), 3 cases (1.72%) had hypernatremia, and 34 cases (19.55%) had hyponatremia. The potassium serum level was normal in 139 cases (79.88%), 27 cases (15.51%) had hyperkalemia, and 8 cases (4.61%) had hypokalemia. Cerebrospinal fluid (CSF) was analyzed in 73 patients, among which 24 patients had normal CSF analysis, 42 cases had increased CSF protein alone, and seven patients had increased CSF protein and increased white blood cell (WBC) count. The most common symptom was upper and lower limb paresis in 65 cases (37.35%), followed by 52 cases (29.88%) of lower limb paresis alone. According to Table 1, patients’ other common symptoms are upper and lower limb paresthesia, lower limb paresthesia, respiratory distress, headache, dysphagia, vertigo, nausea and vomiting, facial paresis, and upper limb paresthesia.
Table 1.
Frequency of GBS symptoms at the onset of diagnosis
| Symptom | Frequency | Percentage |
|---|---|---|
| Upper and lower limb paresis | 65 | 37.35% |
| Lower limb paresis | 52 | 29.88% |
| Upper and lower limb paresthesia | 37 | 21.26% |
| Respiratory distress | 16 | 9.19% |
| Headache | 13 | 7.47% |
| Vertigo | 13 | 7.47% |
| Lower limb paresthesia | 11 | 6.32% |
| Dysphagia | 11 | 6.32% |
| Nausea and vomiting | 11 | 6.32% |
| Facial paresis | 11 | 6.32% |
| Fever | 8 | 4.59% |
| Urinary incontinence | 8 | 4.59% |
| Dysarthria | 7 | 4.02% |
| Diplopia | 6 | 3.44% |
| Backache | 5 | 2.87% |
| Blurred vision | 5 | 2.87% |
| Urinary retention | 4 | 2.29% |
| Fatigue and weakness | 4 | 2.29% |
| Dysphonia | 3 | 1.72% |
| Neck paresis | 2 | 1.14% |
| Tachycardia and diaphoresis | 2 | 1.14% |
| Chest pain | 2 | 1.14% |
| Xerostomia | 2 | 1.14% |
| Ophthalmoplegia | 2 | 1.14% |
| Facial paresthesia | 2 | 1.14% |
| Quadriplegia | 1 | 0.57% |
| Diarrhea | 1 | 0.57% |
| Limb edema | 1 | 0.57% |
| Fecal incontinence | 1 | 0.57% |
Based on the examination, 141 cases (81.03%) had decreased distal lower extremity strength, and the distal lower extremity strength was not detected in 5 cases. One hundred twenty-six cases (72.41%) had lower limb areflexia, 28 cases (16.09%) had decreased lower extremity deep tendon reflex (DTR), and 20 cases (11.49%) had normal lower limb DTR. The plantar reflex was also normal in 121 cases (69.54%). Among the 174 studied patients, gait was impaired in 141 cases (81.03%). Also, pain sensation was impaired in 3 cases (1.72%), heat sensation in 1 case (0.57%), and position sensation in 6 cases (2.29%). Thus, a well-demarcated sensory level was not found in any of the patients. During the hospitalization of patients in the study, 27 cases of respiratory complications, 5 cases of dysphagia, 3 cases of renal and urinary tract complications (hematuria, urinary retention, and acute tubular necrosis), and one case of other complications such as abdominal distension, rectorrhagia, orbital cellulitis, liver enzymes rise, seizures, and lower extremities edema were reported. Also, 132 cases did not have any complications during hospitalization.
Among the included patients, 120 cases (68.96%) were treated with intravenous immunoglobulin (IVIG), 29 cases (16.67%) with plasma exchange, 18 cases (10.35%) with supportive treatment, and 7 cases (4.02%) with plasma exchange and IVIG. The average hospital length of stay was 15.88 days, with most patients (112 cases (64.36%)) between 0 and 15 days, followed by 28 cases between 15 and 30 days. Among the patients, 78 cases (44.82%) required consulting for intensive care unit (ICU) admission. Among 174 patients, 88 cases had increased erythrocyte sedimentation rate (ESR) serum level (50.58%), 10 cases had unreported ESR (5.75%), and 76 cases (43.67%) had normal ESR.
Out of 174 patients, electromyography and nerve conduction velocity (EMG-NCV) findings were available in 113 patients, which revealed that the most common type of GBS was (AIDP) with 63 cases (36.20%) followed by AMSAN with 28 cases (16.09%), AMAN with 20 cases (11.49%), and Miller Fisher with 2 cases (1.14%).
Thirty-one percent of patients had recent URI, 20.11% had hypertension, 10.34% had hyperlipemia, 7.47% had a history of GBS, 6.32% had thyroid disease, 6.32% had a neurologic disease, 4.59% had a psychological disorder, 4.59% had anemia, 4.59% had a gastrointestinal disease, 4.02% had a musculoskeletal disease, 2.29% had previous GI surgery, 2.29% had cancer, 1.14% had a rheumatologic disease, and 1.14% had Down syndrome.
Among 29 pediatric patients (age < 12 years old), 13 (44.83%) were boys, and 16 (55.17%) were girls. The mean age was 6.62 years old, and the highest number of patients was recorded in spring. Twenty-eight cases (96.55%) were recovered successfully, and 1 case (3.45%) died. The most common type of GBS was AIDP in 13 cases (44.83%). The most common complication during hospitalization was respiratory distress in 2 cases (6.89%), and 11 cases (37.93%) required consulting for ICU admission, but only five cases were transferred. Sixteen patients (55.17%) had a history of prodromal gastroenteritis and upper respiratory tract infection, 2–4 weeks before developing GBS. Thus, one case (3.45%) had a history of infection by varicella-zoster. The most common initial symptom was lower limb weakness, followed by gait impairment and upper limb weakness. Based on the examination, 24 patients (82.75%) had decreased distal and proximal lower extremity strength, nine patients (31.03%) had developed lower limb areflexia, and 15 ones (51.72%) had decreased lower extremity deep tendon reflex. Among the patients, 28 cases (96.55%) were treated with intravenous immunoglobulin (IVIG) and one patient (3.45%) with supportive treatment.
Findings of the current study demonstrated that underlying disease ([OR = 0.33 (95% CI 0.12–0.89), P = 0.03]) was more common in the cured people (Table 2).
Table 2.
Comparison of the demographic data with discharge status
| Variable | Dimensions | Discharge status n (%) | Univariate | ||
|---|---|---|---|---|---|
| Cured | Death | OR (95% CI) | P | ||
| Age | Children (0–12yrs) | 28 (96.56) | 1 (3.44) | 0.28 (0.01–5.09) | 0.39 |
| Younger adults (13–30yrs) | 26 (89.65) | 3 (10.35) | 0.92 (0.08–10.15) | 0.94 | |
| Middle-aged (31–50yrs) | 43 (95.56) | 2 (4.44) | 0.37 (0.03–4.60) | 0.44 | |
| Senior adults (51–75yrs) | 51 (82.26) | 11 (17.74) | 1.72 (0.19–15.24) | 0.62 | |
| Old (76yrs and older) | 8 (88.89) | 1 (11.11) | Ref | ||
| Gender | Male | 94 (89.52) | 11 (10.48) | 1.03 (0.38–2.81) | 0.94 |
| Female | 62 (89.86) | 7 (10.14) | Ref | ||
| Underlying disease | Yes | 117 (92.86) | 9 (7.14) | 0.33 (0.12–0.89) | 0.03 |
| No | 39 (81.25) | 9 (18.75) | Ref | ||
| Seasons | Spring | 47 (88.68) | 6 (11.32) | 1.44 (0.33–6.19) | 0.61 |
| Summer | 45 (90) | 5 (10) | 1.25 (0.28–5.63) | 0.76 | |
| Autumn | 30 (88.24) | 4 (11.76) | 1.51 (0.31–7.30) | 0.60 | |
| Winter | 34 (91.89) | 3 (8.11) | Ref | ||
Findings demonstrated that respiratory distress ([OR = 34.40 (95% CI 8.71–135. 82), P = < 0.001]), and other complications during hospitalization ([OR = 21.50 (95% CI 3.56–129.73), P = 0.0001]) were higher in the deceased cases compared with uncomplicated patients. Also ICU care requirement ([OR = 12.12 (95% CI 2.69–54.60), P = 0.001]) were higher in the deceased cases (Table 3).
Table 3.
Comparison of the clinical dominoes with discharge status
| Variable | Dimensions | Discharge status n (%) | Univariate | ||
|---|---|---|---|---|---|
| Cured | Death | OR (95% CI) | P | ||
| Type of GBS* | AIDP | 59 (93.65) | 4 (6.35) | 0.29 (0.07–1.22) | 0.09 |
| AMSAN | 26 (92.86) | 2 (7.14) | 0.84 (0.27–2.62) | 0.77 | |
| AMAN | 19 (95) | 1 (5) | Ref | ||
| Type of treatment | IVIG | 110 (91.67) | 10 (8.33) | 0.72 (0.14–3.62) | 0.69 |
| Plasmapheresis | 24 (82.76) | 5 (17.24) | 1.66 (0.28–9.66) | 0.56 | |
| Plasmapheresis + IVIG | 6 (85.71) | 1 (14.29) | 1.33 (0.10–17.54) | 0.82 | |
| Conservative | 16 (88.89) | 2 (11.11) | Ref | ||
| Duration of hospitalization | 1 day to a week | 47 (95.92) | 2 (4.08) | 0.34 (0.06–1.89) | 0.22 |
| Two weeks | 68 (86.08) | 11 (13.92) | 1.32 (0.43–4.09) | 0.62 | |
| > Two weeks | 41 (89.13) | 5 (10.87) | Ref | ||
| Cerebrospinal fluid (CSF) | Normal | 22 (91.67) | 2 (8.33) | 0.92 (0.18–4.60) | 0.92 |
| Increased WBC | 6 (85.71) | 1 (14.29) | 1.70 (0.18–15.76) | 0.63 | |
| Increased Protein | 36 (85.71) | 6 (14.29) | 1.70 (0.56–5.13) | 0.34 | |
| Not reported | 92 (91.09) | 9 (8.91) | Ref | ||
| ESR | Increased | 80 (90.90) | 8 (9.10) | 0.85 (0.30–2.38) | 0.75 |
| Not reported | 8 (80) | 2 (20) | 2.12 (0.38–11.79) | 0.38 | |
| Normal | 68 (89.47) | 8 (10.53) | Ref | ||
| Sodium level | Hyper | 3 (100) | 0 (0) | – | - |
| Hypo | 31 (91.18) | 3 (8.82) | 0.78 (0.21–2.89) | 0.71 | |
| Normal | 122 (89.05) | 15 (10.95) | Ref | ||
| Potassium level | Normal | 124 (89.20) | 15 (10.80) | 1.29 (0.35–4.73) | 0.70 |
| Hyperkalemia | 32 (91.43) | 3 (8.57) | Ref | ||
| Gait | Normal | 32 (96.97) | 1 (3.03) | 0.22 (0.02–1.77) | 0.15 |
| Abnormal | 124 (87.94) | 17 (12.06) | Ref | ||
| Proximal lower extremity force | No force | 5 (83.33) | 1 (16.67) | 1.61 (0.17–14.68) | 0.67 |
| Normal | 22 (95.65) | 1 (4.35) | 0.36 (0.04–2.90) | 0.34 | |
| Decreased | 129 (88.97) | 16 (11.03) | Ref | ||
| Distal lower extremity force | No force | 4 (80) | 1 (20) | 1.95 (0.20–18.57) | 0.56 |
| Normal | 27 (96.43) | 1 (3.57) | 0.28 (0.03–2.27) | 0.23 | |
| Decreased | 125 (88.65) | 16 (11.35) | Ref | ||
| Lower extremity deep tendon reflex | No force | 18 (90) | 2 (10) | 1.44 (0.18–11.22) | 0.72 |
| Normal | 112 (88.89) | 14 (11.11) | 1.62 (0.34–7.59) | 0.53 | |
| Decreased | 26 (92.86) | 2 (7.14) | Ref | ||
| Plantar reflex | Mute | 39 (82.98) | 8 (17.02) | 2.27 (0.83–6.18) | 0.10 |
| Upward | 6 (100) | 0 (0) | - | - | |
| Downward | 111 (91.74) | 10 (8.26) | Ref | ||
| Complications during hospitalization | Respiratory distress + other complications | 6 (100) | 0 (0) | - | - |
| Respiratory distress | 15 (55.56) | 12 (44.44) | 34.40 (8.71–135.80) | < 0.001 | |
| Other | 6 (66.67) | 3 (33.33) | 21.50 (3.56–129.70) | 0.001 | |
| No complication | 129 (97.73) | 3 (2.27) | Ref | ||
| ICU care requirement | Yes | 62 (79.49) | 16 (20.51) | 12.12 (2.69–54.60) | 0.001 |
| No | 94 (97.92) | 2 (2.08) | Ref | ||
Note: *n = 111
Discussion
This study is the first survey on GBS in Guilan province and a few studies on GBS disease epidemiology in Iran. As previously mentioned, in this retrospective study, 174 patients were observed for 10 years, from 2009 to 2018. Therefore, this study’s primary aim was to determine the GBS incidence rate, case fatality rate, and epidemiology.
This study showed that the mean incidence rate of GBS was 0.69 cases per 100,000 person-years in Guilan province, which was lower compared with the global incidence rate (less than 1.89) based on the previous studies. The highest mean incidence rate belonged to Rasht, and the lowest was rate belonged to Shaft in Guilan province. Two main factors seem to be responsible for the lower incidence rate in our study than the global incidence rate; first, the target hospitals were the only referral hospitals in Guilan province, and some patients in other regions of this province may not be diagnosed and died or cured in regional hospitals without being diagnosed with GBS. According to Fig. 2, the GBS incidence rate became more after 2013 comparing to the previous years. Before 2014, due to the lack of an organized documentary system, some data were missed. Also, the patient’s recording system has been updated since 2014, that provides better patient’s record for us. In addition, during recent years, because of the increasing number of neurologists in Guilan province, and access to more diagnostic facilities, GBS cases can be diagnosed easily.
The results of the present study showed that the case fatality rate was 10.34%. The previous studies indicated the case fatality rates of 2.58%, 8%, 2.8%, 2.4%, 8%, and 8%, in the USA, the UK, Greece, Pakistan, and Jordan, respectively [18–22]. The total evidence indicated that the case fatality rate was higher in Guilan compared with other cities in Iran and other countries, which might be due to the duration of this study which included 10 years at both adult and pediatric referral hospitals since we were not able to assess patient’s information and the underlying factors due to the lack of some medical records. Another reason was that the number of patients with GBS was not very high compared to the death cases due to some missed cases, especially in 2013. Thus, further studies are required to investigate this issue.
This study showed that men were more vulnerable to developing the disease than women (approximately 1.5 to 1), consistent with other past studies. For example, in Blockbashi et al.’s study (2019), the GBS development ratio was reported to be 1.5 to 1 in men to women [15, 23, 24].
This study showed that the highest number of patients aged 45–60 years followed by 60–75 years in age groups. Previous studies have also shown that the incidence increases with age [25]. In Al-Hakim et al.’s study (2018), the highest incidence rate of GBS was observed in the population aged 60–69 years (2.69 per 100,000 person-years). Also, in Lilo et al.’s study (2015), the highest incidence rate was reported in patients aged 70–79. On the other hand, in the patients aged 80 and above, the GBS incidence rate decreased, which is similar to the present study in that the lowest incidence rate was observed in patients aged 75–90 (5.74%) [26]. Various reasons may explain the reducing incidence rate of GBS in patients aged over 80, including reduced survival rate and the decreased diagnosis ability in this age group due to GBS’s unusual and mild manifestations [26].
In the present study, the most reported cases were in spring and winter, though there was no significant difference between seasons. The most reported type of GBS was AIDP in this study, and previous researches have shown that this GBS type is more common in the spring, especially in April and March; hence, it can justify the higher number of reported GBS cases in spring in Guilan province. Also, previous studies have shown the incidence rise of AIDP type in the European countries, North America, and the Middle East [17]. Each type of GBS frequency followed a similar pattern in different seasons, and after AIDP, the most common type of GBS was ASMAN and AMAN in each season, respectively. However, one case of MFS was reported in summer, and the other one was found in winter. According to Table 3, the relation between the type of GBS and discharge status was not significant. In this statistical analysis, the Miller fisher type and unavailable cases were not analyzed, so only 111 cases were considered in the analysis. Further investigation is needed in this field because the information about GBS type was only available in 113 patients.
According to Table 3, there was no significant relationship between the type of treatment and discharge status. Thirteen cases did not meet the criteria to receive IVIG and plasmapheresis treatment, such as inability to walk unassisted, dysphagia, and respiratory distress [27]. Five patients had severe symptoms, but they did not have immunotherapy treatment due to the drug side effects, obstacles of accessing drugs, diagnostic delay, and rapid progression of the disease.
Based on the result of this study, seven cases had an increased number of WBC (more than 10) in the CSF. Previous studies showed that the number of WBC could be up to 50 (or infrequently more) in some cases, but another differential diagnosis should be rolled out during hospitalization [28]. Five of them received IVIG, and only two cases were treated with supportive care. Among these seven cases, only one of them died but was treated with IVIG. According to the documents of these patients, to find the source of increase in the level of WBC in CSF, such as aseptic meningitis, all necessary tests such as HIV and the other test were done. However, due to limitations in technologies and diagnostic tools, GBS was considered the best diagnosis for these patients based on the clinical symptoms, examination, and EMG-NCV. Also, two cases received antibiotics during their hospitalization because of pneumonia, and one patient had diabetes and its association with increase infection rate.
We found most pediatric patients had a history of prodromal infection 2–4 weeks before the onset of GBS, gastroenteritis, and varicella-zoster, respectively. Faruk Incecik et al. [1] reported 60.9% prodromal infections: upper respiratory tract infection 82.1%, gastroenteritis 10.7%, and varicella-zoster 7.2% [29]. In our study, the most common initial presentation was lower limb weakness. Also, in the previous studies, all their patients reported limb weakness as their initial presentation [30]. We found no cranial nerve involvement, but the other studies reported 50.8% [31] and 39.5% [30] of cases that developed cranial nerve involvement. In this study, one pediatric patient died. He was 11 years old with a history of mental retardation and upper respiratory tract infection 2 weeks before his first presentation. He had developed lower limb paralysis and then upper limb paralysis for 1 week before hospitalization. On the admission day, he had a speech problem and dysphagia, his level of consciousness was decreased, and he was intubated. He was initially admitted to the pediatric intensive care unit. The hospitalization duration was 20 days, and he was treated with IVIg. The CSF cell count, serum sodium level, serum potassium level, and ESR were normal. The case fatality rate in our study was 3% compared to the study of Faruk Incecik et al. that was 2.1% [29].
The second aim of this study was to evaluate the leading mortality causes, comorbidities, complications during the hospitalization, necessity of ICU care, and the underlying factor that could affect the course of the disease and its mortality.
Our study had fewer underlying diseases, such as recent respiratory infection, high blood pressure, hyperlipidemia, diabetes, a history of Guillain-Barré syndrome, thyroid disorders, neurological disease, psychiatric disease, heart disease, and anemia in the deceased cases compared to the cured cases. This finding could be due to the lack of data in our retrospective study, and some underlying diseases may have been missed in the patient information recording process. Also, these underlying diseases were hypertension and hyperlipidemia, which seem to be just a common coexistence and have not been effective in the course of the disease. Additionally, the number of deceased cases was markedly lower than the cured cases; thus, further investigations are required to find the relationship between each underlying disease and GBS mortality. It was not possible in our study because some of the patients had different cases underlying diseases at the same time. For example, there was a patient with cardiovascular disorder, hypertension, previous history of urinary bladder carcinoma, and GI surgery in the cured group. Also, in the deceased group, psychological disorders, gastrointestinal disorders, and hypertension were found in one patient. Therefore, it is recommended that the effect of underlying diseases on GBS mortality be surveyed separately in future studies.
In the present study, a significant relation was observed between respiratory complications during hospitalization and the case fatality rate (P = 0.001), and respiratory failure was reported in 15 cases of 18 cases of mortality. In line with the present study’s findings, Alshekhlee et al.’s study showed that respiratory failure was the most common GBS complication (11.3%) [19]. In addition, a significant relationship was observed between ICU requirement and the case fatality rate (P = 0.001). In this study, 78 patients were consulted for ICU admission, 26 of whom were transferred to ICU, 16 of whom (61.58%) died. In the study of Wang et al., nearly 25% of patients had dyspnea and needed mechanical ventilation. However, the case fatality rate in the mechanical ventilator-dependent patients was 4–15%, significantly lower than the present study. Therefore, it seems that respiratory failure management was not as effective in our hospitals compared to the previous study, and further investigation is required to find the factors associated with this high-case fatality rate [32].
In this study, most patients had a recent history of respiratory tract infection, and past studies have also shown a significant association between recent respiratory infection and gastroenteritis with GBS [8]. Ogawara et al. mentioned that 45% of patients had prior infections, most of which showed upper respiratory tract symptoms. In addition, they found there is a substantial connection between AMAN, IgG antibodies against anti-ganglioside antibodies, and C. jejuni infection [9]. One case with a history of influenzas vaccine injection 3 weeks before the onset of his symptoms, one case with a history of influenzas infection, and two cases with chickenpox infection were reported in our study. As we know, coronaviruses are neurotrophic, and they tend neuroinvasion [33]. Neurological complications of SARS-COV2 could be expected in the pandemic [34]. Some case reports from different countries worldwide reported GBS as a complication of SARS-COV2 infection [35–39], though case–control studies are required to prove this relationship. Also, GBS is one of the reported complications of influenza vaccination [40]. Thus, due to COVID-19 extended vaccination worldwide, the GBS incidence rate could increase, though no GBS has been reported following COVID-19 vaccination yet [41, 42].
Limitation
This study was a retrospective review. Thus, some medical records might be incomplete or incorrect. This study was focused on two referral hospitals in Rasht. As a routine because of the developed technologies and more efficient treatment modalities such as plasma exchange and IVIG, most GBS patients in Guilan province are referred to these hospitals. On the other hand, some private hospitals in Rasht may also diagnose and treat some patients, so they have not been included in the present study. Since the current study was a retrospective study, there was some lack of data about new cases each year, especially in 2013. In this case, it is better to consider the mean incidence rate in the present study as the least mean incidence rate. It is recommended that the epidemiology of GBS be surveyed every year since the new GBS cases occur every year, and accurate analysis of GBS may be achieved by studying the annual incidence rate of this syndrome.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate the support of the Neuroscience Research Center of Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
Author contribution
Mozaffar Hosseininezhad and Seyed Sepehr Khatami developed the original idea and drafted the manuscript. Sajjad Saadat did critical revision of the manuscript for important intellectual content and study supervision. Mona Asghari, Hoora Ghovvati Choshal, and Zahra Gholipour Soleimani collected the data. Alireza Hooshmand Marvasti analyzed the data.
Declarations
Ethical approval
We received ethical exemption status from the Research Ethics Committee of Guilan University of Medical Sciences.
Conflict of interest
The authors declare no competing interests.
Informed consent
In this study, we collected data of the patient with Guilain barre disease from their profile stored in the two referral hospitals’ data bank archives. To achieve this data, we got authorization access to this data directly from the hospital’s Chief executive officer(CEO). In this case, we got permission to use patients’ medical history and demographic information without revealing their names and personal information. Besides that, we surveyed the prevalence of GBS for the past ten years, so our research has not had any effect on patients’ diagnosis and treatment. Also, our study was accepted in the Neuroscience Research Center of the Poursina Hospital of the Guilan University of Medical Sciences and approved by the Research Ethics Committee of the Guilan University of Medical Sciences (Code: IR.GUMS.REC.1398.340).
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mozaffar Hosseininezhad, Email: hosseininezhadm@gmail.com.
Seyed Sepehr Khatami, Email: seyedsepehrkhatami@yahoo.com.
Sajjad Saadat, Email: sajjadsaadat69@gmail.com.
Mona Asghari, Email: monaasghari.1996@gmail.com.
Hoora Ghovvati Choshal, Email: hooraghovvatichoshal@gmail.com.
Alireza Hooshmand Marvasti, Email: halireza5654@gmail.com.
Zahra Gholipour Soleimani, Email: elahe.gholipour20@hotmail.com.
References
- 1.Pithadia AB, Kakadia N. Guillain-Barré syndrome (GBS) Pharmacol Rep. 2010;62(2):220–232. doi: 10.1016/S1734-1140(10)70261-9. [DOI] [PubMed] [Google Scholar]
- 2.Sipilä JO, et al. Epidemiology of Guillain-Barré syndrome in Finland 2004–2014. J Peripher Nerv Syst. 2017;22(4):440–445. doi: 10.1111/jns.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Beleidy AS, et al. Antiganglioside antibodies determine the clinical severity and predict response to therapy in Egyptian children with Guillain-Barré Syndrome. Pediatr Pol. 2013;88(3):224–229. doi: 10.1016/j.pepo.2013.02.006. [DOI] [Google Scholar]
- 4.Jacobs BC, et al. International Guillain-Barré Syndrome Outcome Study: protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain-Barré syndrome. J Peripher Nerv Syst. 2017;22(2):68–76. doi: 10.1111/jns.12209. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti MD, et al. A multicentric prospective incidence study of Guillain-Barré syndrome in Italy. the ITANG study. Neuroepidemiology. 2015;45(2):90–99. doi: 10.1159/000438752. [DOI] [PubMed] [Google Scholar]
- 6.Momen AA, Shakurnia A. The epidemiology of guillain-barré syndrome in children under 15 years old in Southwest Iran. Biomed Hub. 2017;2(3):1–8. doi: 10.1159/000480693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwong JC, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis. 2013;13(9):769–776. doi: 10.1016/S1473-3099(13)70104-X. [DOI] [PubMed] [Google Scholar]
- 8.Wachira VK, Peixoto HM, de Oliveira MRF. Systematic review of factors associated with the development of Guillain-Barré syndrome 2007–2017: what has changed? Tropical Med Int Health. 2019;24(2):132–142. doi: 10.1111/tmi.13181. [DOI] [PubMed] [Google Scholar]
- 9.Ogawara K, et al. Axonal Guillain-Barré syndrome: relation to anti-ganglioside antibodies and Campylobacter jejuni infection in Japan. Ann Neurol. 2000;48(4):624–631. doi: 10.1002/1531-8249(200010)48:4<624::AID-ANA9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Kobori S, et al. Coexisting infectious diseases on admission as a risk factor for mechanical ventilation in patients with Guillain-Barré syndrome. J Epidemiol. 2017;27(7):311–316. doi: 10.1016/j.je.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parra B, et al. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375(16):1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 12.Grave C, et al. Seasonal influenza vaccine and Guillain-Barré syndrome: a self-controlled case series study. Neurology. 2020;94(20):e2168–e2179. doi: 10.1212/WNL.0000000000009180. [DOI] [PubMed] [Google Scholar]
- 13.Racca F, et al. Practical approach to respiratory emergencies in neurological diseases. Neurol Sci. 2020;41(3):497–508. doi: 10.1007/s10072-019-04163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijdicks EF, Klein CJ (2017) Guillain-barré syndrome. In Mayo clinic proceedings, vol 92, No. 3. Elsevier, pp 467–479 [DOI] [PubMed]
- 15.Hughes RA, Swan AV, van Doorn PA (2014) Intravenous immunoglobulin for Guillain-Barré syndrome. Cochr Database Syst Rev 2014(9):CD002063. 10.1002/14651858.CD002063.pub6 [DOI] [PMC free article] [PubMed]
- 16.Li C, et al. The effects of IVIg therapy on serum levels of neuropeptide Y and cytokines in Guillain-Barré syndrome. Neurol Sci. 2020;41(2):295–303. doi: 10.1007/s10072-019-04063-3. [DOI] [PubMed] [Google Scholar]
- 17.Arami MA, Yazdchi M, Khandaghi R. Epidemiology and characteristics of Guillain-Barre syndrome in the northwest of Iran. Ann Saudi Med. 2006;26(1):22–27. doi: 10.5144/0256-4947.2006.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadegari S, Kazemi N, Nafissi S. Clinical and electrophysiological features of Guillain-Barré syndrome in Iran. J Clin Neurosci. 2014;21(9):1554–1557. doi: 10.1016/j.jocn.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Alshekhlee A, et al. Guillain-Barré syndrome: incidence and mortality rates in US hospitals. Neurology. 2008;70(18):1608–1613. doi: 10.1212/01.wnl.0000310983.38724.d4. [DOI] [PubMed] [Google Scholar]
- 20.Yakoob MY, et al. Characteristics of patients with Guillain Barre Syndrome at a tertiary care centre in Pakistan, 1995–2003. J Pak Med Assoc. 2005;55(11):493. [PubMed] [Google Scholar]
- 21.Said SD. Clinical experience with Gullain Barre syndrome over a 6-year period in one hospital in the Middle East. Jordan Med J. 2009;43(4):280–285. [Google Scholar]
- 22.Radhakrishnan K, El-Mangoush M, Gerryo S. Descriptive epidemiology of selected neuromuscular disorders in Benghazi, Libya. Acta Neurol Scand. 1987;75(2):95–100. doi: 10.1111/j.1600-0404.1987.tb07901.x. [DOI] [PubMed] [Google Scholar]
- 23.Bölükbaşi F, et al. Guillain-barré syndrome and its variants: Clinical course and prognostic factors. Arch Neuropsychiatry. 2019;56(1):71. doi: 10.5152/npa.2017.18091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benamer HT, Bredan A. Guillain-Barré syndrome in Arab countries: a systematic review. J Neurol Sci. 2014;343(1–2):221–223. doi: 10.1016/j.jns.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 25.Granieri E, et al. Incidence study of Guillain-Barré syndrome in the province of Ferrara, Northern Italy, between 2003 and 2017. A 40-year follow-up. Neurol Sci. 2019;40(3):603–609. doi: 10.1007/s10072-018-3688-4. [DOI] [PubMed] [Google Scholar]
- 26.Al-Hakem H, et al. Guillain-Barré syndrome in Denmark: a population-based study on epidemiology, diagnosis and clinical severity. J Neurol. 2019;266(2):440–449. doi: 10.1007/s00415-018-9151-x. [DOI] [PubMed] [Google Scholar]
- 27.van Doorn PA. Diagnosis, treatment and prognosis of Guillain-Barré syndrome (GBS) La Presse Médicale. 2013;42(6 Part 2):e193–e201. doi: 10.1016/j.lpm.2013.02.328. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard J. Guillain-Barré syndrome. Clin Med (Lond) 2010;10(4):399–401. doi: 10.7861/clinmedicine.10-4-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Incecik F, Hergüner MO, Altunbasak S. Guillain-Barré syndrome in children. Neurol Sci. 2011;32(3):381–385. doi: 10.1007/s10072-010-0434-y. [DOI] [PubMed] [Google Scholar]
- 30.Lin J-J, et al. Clinical variants of Guillain-Barré syndrome in children. Pediatr Neurol. 2012;47(2):91–96. doi: 10.1016/j.pediatrneurol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Koul R, et al. Clinical characteristics of childhood guillain-barré syndrome. Oman Med J. 2008;23(3):158. [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Complications of Guillain-Barré syndrome. Expert Rev Clin Immunol. 2016;12(4):439–448. doi: 10.1586/1744666X.2016.1128829. [DOI] [PubMed] [Google Scholar]
- 33.Kim J-E, et al. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol (Seoul, Korea) 2017;13(3):227. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahimi K. Guillain-Barre syndrome during COVID-19 pandemic: an overview of the reports. Neurol Sci. 2020;41(11):3149–3156. doi: 10.1007/s10072-020-04693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M (2021) Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol 268(4):1133–1170 [DOI] [PMC free article] [PubMed]
- 36.Alberti P, Beretta S, Piatti M, Karantzoulis A, Piatti ML, Santoro P, Ferrarese C (2020) Guillain-Barré syndrome related to COVID-19 infection. Neurol-Neuroimmunol Neuroinflam 7(4) [DOI] [PMC free article] [PubMed]
- 37.Caress JB, et al. COVID-19–associated Guillain-Barré syndrome: the early pandemic experience. Muscle Nerve. 2020;62(4):485–491. doi: 10.1002/mus.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uncini A, Vallat J-M, Jacobs BC. Guillain-Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry. 2020;91(10):1105–1110. doi: 10.1136/jnnp-2020-324491. [DOI] [PubMed] [Google Scholar]
- 40.Haber P, et al. Guillain-Barré syndrome following influenza vaccination. JAMA. 2004;292(20):2478–2481. doi: 10.1001/jama.292.20.2478. [DOI] [PubMed] [Google Scholar]
- 41.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliver SE (2020) The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine—United States, December 2020. MMWR. Morbid Mortal Week Rep 69 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.