Abstract
Background
Myocardial injury and inflammation at cardiac MRI in patients with COVID-19 have been described in recent publications. Concurrently, a chronic COVID-19 syndrome (CCS) after SARS-CoV-2 infection has been observed and manifests with symptoms such as fatigue and exertional dyspnea.
Purpose
To explore the relationship between CCS and myocardial injury and inflammation as an underlying cause of the persistent complaints in previously healthy individuals.
Materials and Methods
In this prospective study from January 2021 to April 2021, study participants without known cardiac or pulmonary diseases prior to SARS-CoV-2 infection who had persistent CCS symptoms such as fatigue or exertional dyspnea after convalescence and healthy control participants underwent cardiac MRI. The cardiac MRI protocol included evaluating the T1 and T2 relaxation times, extracellular volume, T2 signal intensity ratio, and late gadolinium enhancement (LGE). Student t tests, Mann-Whitney U tests, and χ2 tests were used for statistical analysis.
Results
Forty-one participants with CCS (mean age, 39 years ± 13 [standard deviation]; 18 men) and 42 control participants (mean age, 39 years ± 16; 26 men) were evaluated. The median time between the initial incidence of mild to moderate COVID-19 not requiring hospitalization and undergoing cardiac MRI was 103 days (interquartile range, 88–158 days). Troponin T levels were normal. Parameters indicating myocardial inflammation and edema were comparable between participants with CCS and control participants (T1 relaxation times: 978 msec ± 23 vs 971 msec ± 25 [P = .17]; T2 relaxation times: 53 msec ± 2 vs 52 msec ± 2 [P = .47]; T2 signal intensity ratios: 1.6 ± 0.2 vs 1.6 ± 0.3 [P = .10]). Visible myocardial edema was present in none of the participants. Three of 41 (7%) participants with CCS demonstrated nonischemic LGE, whereas no participants in the control group demonstrated nonischemic LGE (0 of 42 [0%]; P = .07). None of the participants fulfilled the 2018 Lake Louise criteria for the diagnosis of myocarditis.
Conclusion
Individuals with chronic COVID-19 syndrome who did not undergo hospitalization for COVID-19 did not demonstrate signs of active myocardial injury or inflammation at cardiac MRI.
© RSNA, 2021
Online supplemental material is available for this article.
See also the editorial by Lima and Bluemke in this issue.
Summary
Previously healthy individuals with prolonged cardiorespiratory symptoms after SARS-CoV-2 infection who were not hospitalized at any disease stage had no signs of active cardiac inflammation at cardiac MRI.
Key Results
■ In this prospective study of 41 participants with cardiorespiratory chronic COVID-19 syndrome (CCS) and 42 control participants, cardiac MRI mapping parameters indicating myocardial inflammation were comparable (T1 relaxation times: 978 msec ± 23 vs 971 msec ± 25 [P = .17]; T2 relaxation times: 53 msec ± 2 vs 52 msec ± 2 [P = .47]).
■ In three of 41 (7%) participants with CCS, nonischemic myocardial late gadolinium enhancement lesions were present, whereas none were observed in the control group.
Introduction
With the ongoing COVID-19 pandemic, the number of scientific studies as well as our knowledge regarding novel SARS-CoV-2 has steadily increased. It is now known that the virus not only affects the lower respiratory airways but can also affect other systems, such as the central nervous or cardiovascular systems (1–3). Since the beginning of the COVID-19 pandemic at the end of 2019, reports have been emerging of patients experiencing persistent COVID-19 symptoms such as fatigue or exertional dyspnea after having tested negative for SARS-CoV-2 through polymerase chain reaction testing. This has led to a new pathologic condition termed chronic COVID-19 syndrome (CCS) or long COVID (4,5). Unfortunately, the exact etiology of CCS remains unknown.
Recent studies have reported on structural myocardial damage in patients who experienced acute myocarditis or myocardial scar formation as a result of SARS-CoV-2 infection (2,6–11). Puntmann et al (8) demonstrated that about 78% of patients who had recently recovered from COVID-19 had abnormal cardiac MRI findings, including increased native T1 and T2 myocardial relaxation times, decreased ejection fractions, and increased left ventricular volumes. A study from China (12) showed that 7%–15% of hospitalized patients with COVID-19 had elevated troponin T levels as an indirect sign of myocardial infarction. On the other hand, a more recent study of 149 health care workers (13) demonstrated no detectable difference at MRI regarding cardiovascular abnormalities between a seropositive cohort with mild COVID-19 symptoms 6 months after the initial diagnosis and a healthy seronegative control group. Although cardiac injury at cardiac MRI was also found to be a component of the systemic immune response to SARS-CoV-2 or the direct myocardial damage from SARS-CoV-2 usually observed in severely ill patients (14), the possible long-term effects of SARS-CoV-2 on recovered but still symptomatic patients with CCS has not been sufficiently determined.
In this prospective study, previously healthy patients who had recovered from mild to moderate COVID-19 but continued to experience CCS symptoms such as chest pain, exertional dyspnea, or fatigue underwent multiparametric cardiac MRI. The purpose of our explorative study was to evaluate to what extent inflammatory or structural changes in the myocardium are present in patients with convalescent SARS-CoV-2 infection who continue to experience cardiorespiratory symptoms.
Materials and Methods
This prospective study was performed in concordance with the Declaration of Helsinki and International Conference on Harmonization of Good Clinical Practice. The study design, information processing, and study implementation were approved by the institutional review board (application no. 039/21). Written informed consent was obtained from each patient after patients were provided with information regarding the study and the potential risks of participation.
Study Participants
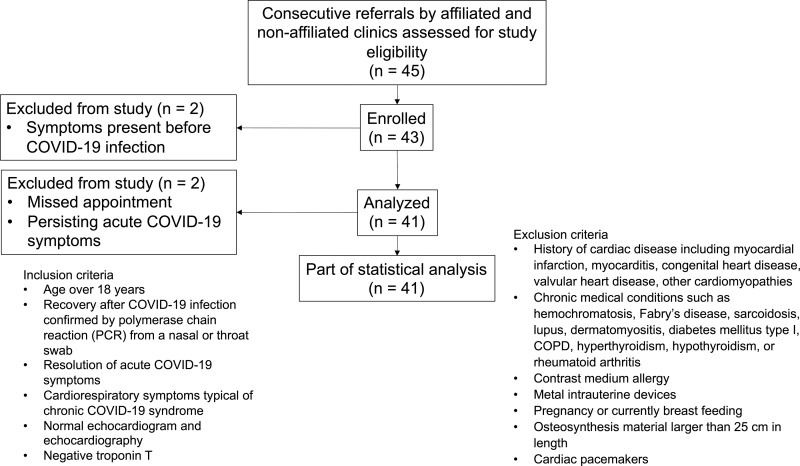
Recruitment occurred consecutively between January 2021 and April 2021. Participants over the age of 18 without known cardiac or pulmonary diseases, as outlined in Figure 1, who also reported persistent fatigue, exertional dyspnea, or cardiac symptoms after COVID-19 convalescence were included in this single-center case–control study. As viral load clearance in mild to moderate infections typically requires 10–13 days, leading to a negative polymerase chain reaction test result, we defined CCS as failure of symptom resolution within 30 days of the initial diagnosis (15,16). Cardiac symptoms were defined as being persistent, as being occasional, or as provoking chest pain, tachycardia, or shortness of breath. Participants with CCS must have had a negative polymerase chain reaction test result and must have experienced resolution of acute COVID-19 symptoms for at least 2 weeks before cardiac MRI was performed. Acute COVID-19 symptoms were defined as fever, dry or wet cough, changes in taste and smell with or without dyspnea, and a positive polymerase chain reaction test result. Participants were referred by local medical offices and university centers. Clinical manifestations of initial COVID-19 were classified as previously described (17). All participants with CCS had unremarkable results from previous examinations (including normal echocardiographic results, normal electrocardiographic results, and normal troponin T levels), which ruled out other causes for their complaints after SARS-CoV-2 infection. The stringent recruitment process and the inclusion and exclusion criteria are summarized in Figure 1. The control group consisted of age-matched healthy participants without prior SARS-CoV-2 infection. All control participants had an unremarkable past medical history regarding cardiovascular disease. Electrocardiographic results were unremarkable, and no cardiac risk factors were present. The initial date of COVID-19 diagnosis was defined as the day of the first positive polymerase chain reaction test result.
Figure 1:
Flowchart depicts the recruitment process, inclusion and exclusion criteria, and included participants with chronic COVID-19 syndrome. COPD = chronic obstructive pulmonary disease, PCR = polymerase chain reaction.
Cardiac MRI Protocol
Each participant underwent multiparametric cardiac MRI, and each examination was performed by using the same clinical whole-body MRI system (Ingenia 1.5-T imager; Philips Healthcare). A 32-channel torso coil with a digital interface was used for signal reception. A signal intensity correction algorithm (constant level appearance, Philips Medical Systems) was used to correct for torso coil–related signal inhomogeneities. Electrocardiogram-gated steady-state free-precession cine images were obtained in short-axis, two-chamber, and four-chamber views for functional analysis. T2-weighted short-tau inversion-recovery sequences in short-axis and transversal views were performed for visualization of myocardial edema and calculation of the T2 signal intensity ratio. Segmented inversion-recovery gradient-echo sequences were performed to acquire late gadolinium enhancement (LGE) images, which were obtained in short-axis, two-chamber, four-chamber, and transversal views. The Look-Locker method was used to determine the optimal inversion time for LGE image acquisition (18). Myocardial T1 and T2 mapping was performed in end-diastolic short-axis views, and apical, midventricular, and basal sections were acquired. For myocardial T1 mapping, a standard 3(3)3(3)5 modified Look-Locker inversion-recovery acquisition scheme was applied (19). Postcontrast T1 maps were obtained by using the same acquisition scheme 10 minutes after contrast material administration. For myocardial T2 mapping, a six-echo gradient spin-echo sequence was applied (20). For contrast enhancement, a bolus of 0.2 mmol of gadoterate meglumine (Clariscan, GE Healthcare) per kilogram of body weight was administered. Directly prior to every cardiac MRI examination, blood samples were drawn for blood count and hematocrit assessment. Additionally, a transversal respiratory-gated T2-weighted fast spin-echo sequence (Philips MultiVane XD, Philips Healthcare) for the assessment of lung pathologic findings was performed. A detailed description of the sequence parameters is provided in the supplemental material (Table E1 [online]).
Image Analysis
Image analysis was performed by a board-certified radiologist (J.A.L., with 8 years of experience in cardiac MRI) and a radiologic resident (D. Kravchenko, with 2 years of experience in cardiac MRI) by using dedicated software (IntelliSpace Portal, version 10.6.32.82.; Philips Medical Systems). Readers were blinded to the clinical information. Papillary muscles were included in the volumetric quantification of the left ventricle. The presence of focal areas of regional high signal intensities in a nonischemic distribution pattern on T2 short-tau inversion-recovery images and on LGE images was visually assessed through the consensus of the two readers. Semiquantitative markers of myocardial edema (the T2 signal intensity ratio) and myocardial injury and fibrosis (an enhanced volume percentage on short-axis LGE images acquired by using a full-width half-maximum technique) were calculated as previously reported (21). Myocardial T1 and T2 relaxation maps were motion corrected by using a software-implemented algorithm (fast elastic image registration, IntelliSpace Portal, version 10.1), and global T1 and T2 relaxation times as well as hematocrit-corrected global extracellular volume values were calculated as previously described (22,23). For the assessment of the 2018 Lake Louise criteria, institution-specific cutoffs were used as previously described (23). The cutoffs for the diagnosis of myocardial inflammation were greater than or equal to 1000 msec for myocardial T1 relaxation times and greater than or equal to 55.9 msec for myocardial T2 relaxation times. The imaging protocol in this study was the same as that used in our previous studies of patients with suspected acute myocarditis (22,23).
Symptom Questionnaire
Participants completed a questionnaire pertaining to novel symptoms they experienced during and after acute COVID-19 immediately before undergoing the cardiac MRI examination. Each symptom was then rated on a 0–10 numeric rating scale for the subjective symptom burden during acute infection and the current symptom status. No symptoms were prescribed, such that each participant was free to fill out their own symptom constellation. A copy of the questionnaire is provided in the supplemental material (Fig E1 [online]).
Statistical Analysis
Prism (version 8.4.1; GraphPad Software) was used for statistical analysis. Participant characteristics are given as means ± standard deviations or as absolute frequencies with percentages. Data were checked for normal distribution by using the Shapiro-Wilk test. Dichotomous variables were compared by using the χ2 test. For the comparison of continuous interindividual variables, the Student t test was used. The Mann-Whitney U test was used for non–normally distributed data. For intraindividual comparisons, the Wilcoxon rank test was used. The threshold for statistical significance was set to a P value of less than .05.
Results
Participant Characteristics
A total of 83 participants were included in this prospective study: 41 participants with CCS (mean age, 39 years ± 13; 18 men) and 42 control participants (mean age, 39 years ± 16; 26 men). There were no significant differences between the groups regarding age (P = .88), sex (P = .10), weight (P = .78), height (P = .28), or body mass index (P = .20). The initial severity level of COVID-19 in participants with CCS was mild (37 of 41, 90%) or moderate (four of 41, 10%). Participant characteristics are summarized in Table 1. Excluded patients are summarized in Figure 1; two referred participants had to be excluded because of having a date of symptom onset before SARS-CoV-2 infection, one participant did not show up to their appointment, and one participant had to be excluded because of demonstrating a persistent pulmonary infection that was most likely due to COVID-19. No participant with CCS required hospitalization during acute COVID-19. Two participants with CCS received dexamethasone and fenoterol for treatment of symptoms.
Table 1:
Clinical and Cardiac MRI Characteristics of Participants with CCS and Control Participants
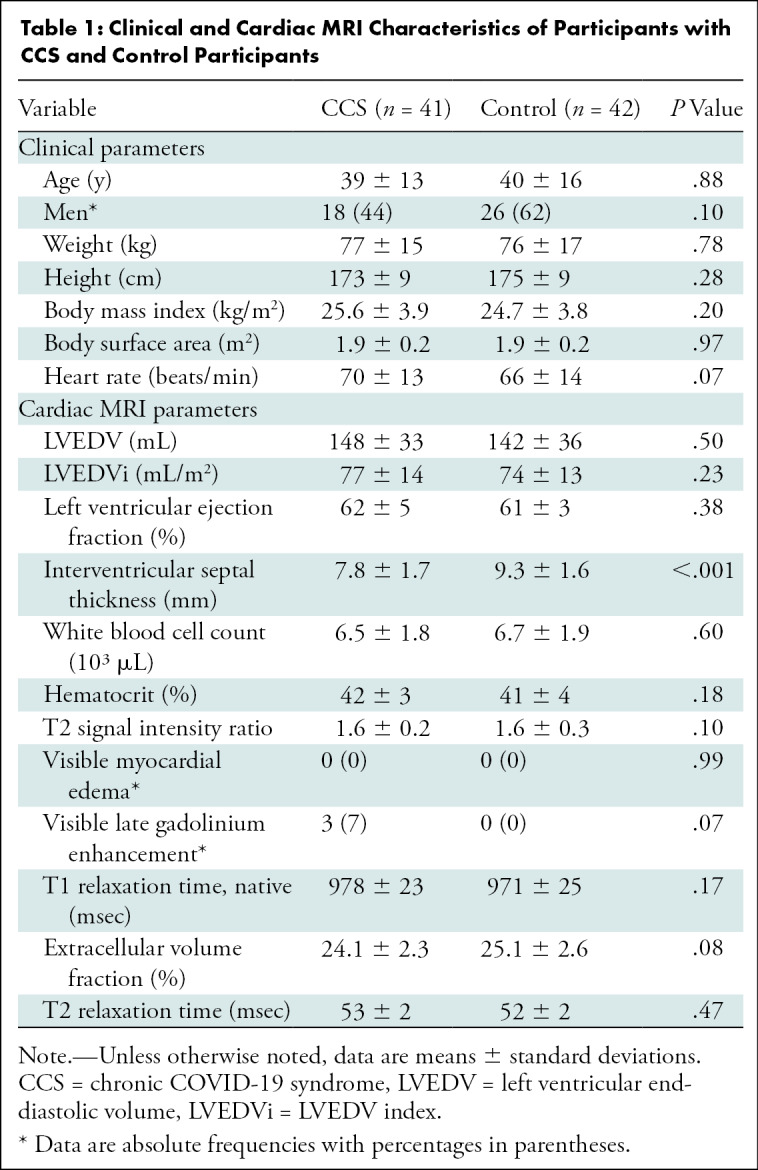
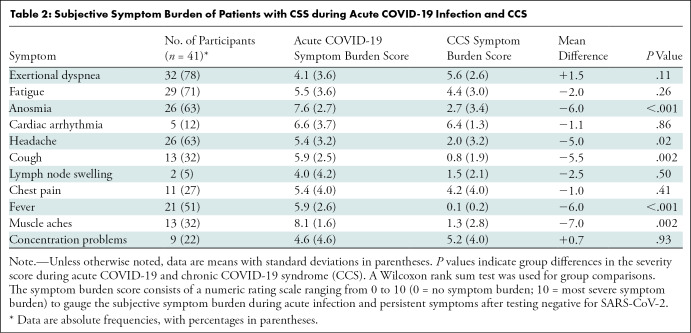
The median time between the initial COVID-19 diagnosis and cardiac MRI was 103 days (interquartile range, 88–158 days). Participants with CCS reported dyspnea (32 of 41, 78%) and fatigue (29 of 41, 71%) as well as improving anosmia (26 of 41, 63%), headaches (26 of 41, 63%), cough (13 of 41, 32%), and fever (21 of 41, 51%). The only preexisting medical conditions in participants with CCS were allergic asthma (one of 41, 2%), a past medical history of pituitary adenoma (one of 41, 2%), and arterial hypertension (two of 41, 5%). The results of the symptom burden questionnaire are summarized in Table 2.
Table 2:
Subjective Symptom Burden of Patients with CSS during Acute COVID-19 Infection and CCS
Cardiac MRI Results
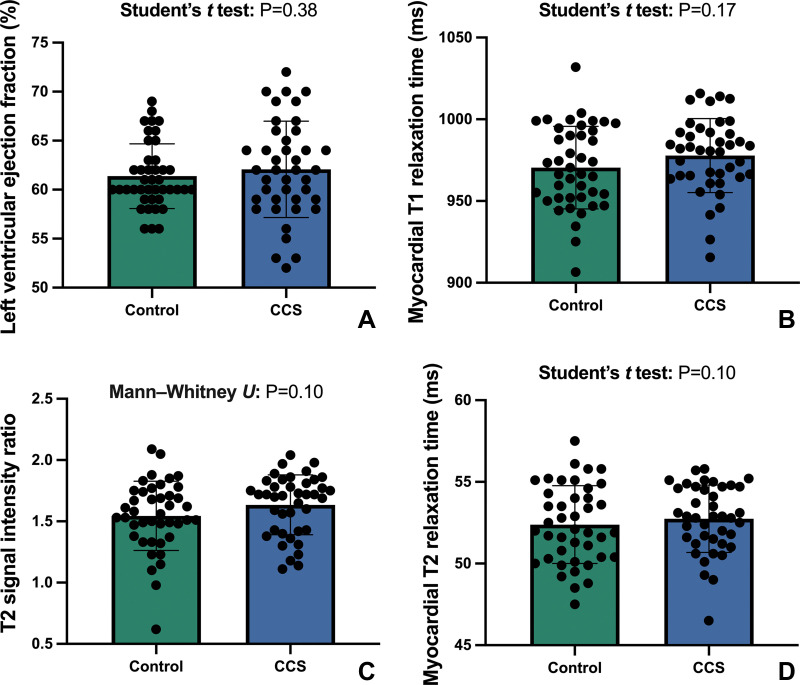
All control participants had normal cardiac MRI results without structural abnormalities or signs of previous myocarditis. No differences in the left ventricular ejection fraction (62% ± 5 vs 61% ± 3; P = .38) or the left ventricular end-diastolic volume index (77 mL/m2 ± 14 vs 74 mL/m2 ± 13; P = .23) were observed between the groups. No regional wall motion abnormalities were observed in any group. The T2 signal intensity ratio was within the normal range for both groups (see Table 1). No focal myocardial edema was visually observed.
No differences were found in the native T1 relaxation times (978 msec ± 23 vs 971 msec ± 25 ; P = .17) or the T2 relaxation times (53 msec ± 2 vs 52 msec ± 2; P = .47) between participants with CCS and control participants (see Fig 2).
Figure 2:
Column graphs with individual plotted values show the distribution of MRI parameters in the control group and in the group with chronic COVID-19 syndrome (CCS). The means of the data are represented by bars. Whiskers represent standard deviations. The distribution is shown for the (A) left ventricular ejection fraction, (B) myocardial T1 relaxation time, (C) T2 signal intensity ratio, and (D) myocardial T2 relaxation time.
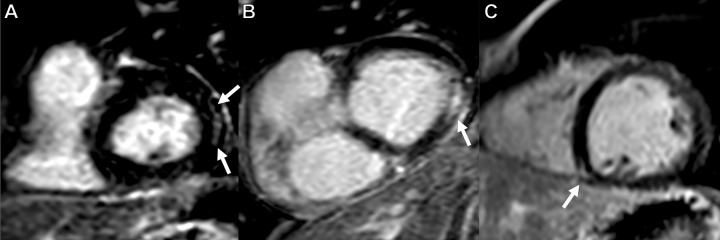
Focal nonischemic LGE lesions were present in three of 41 (7%) participants with CCS. Two of three (67%) LGE lesions were found in the subepicardium of the basal inferolateral wall, and one of three (33%) LGE lesions was midventricular at the right ventricular attachment (see Fig 3). Extracellular volume values did not differ between participants with CCS and control participants (24.1% ± 2.3 vs 25.1% ± 2.6; P = .15). None of the participants fulfilled the 2018 Lake Louise criteria for the diagnosis of inflammatory cardiomyopathies.
Figure 3:
Late gadolinium enhancement (LGE) short-axis views demonstrate LGE in three of 41 patients (7%). Subepicardial LGE along the basal inferolateral wall (arrows) in (A) a 63-year-old man and (B) a 54-year-old man. (C) LGE at the right ventricular attachment (arrow) in a 19-year-old man.
Six of 41 (15%) participants with CCS had incidental findings at cardiac MRI that could potentially have had an impact on the prolonged cardiorespiratory symptoms, whereas the control group did not demonstrate any incidental findings. These findings were as follows: a small aberrant accessory lung (one of 41, 2%), pericardial effusion without signs of pericarditis (one of 41, 2%), visual signs of right ventricular high-pressure overload (one of 41, 2%), persistent slight pulmonary opacities in the lower left lobe (one of 41, 2%), and discrete pleural effusions (two of 41, 5%) with maximum anterior–posterior distances of 5 and 7 mm, respectively.
Discussion
COVID-19 has now been established to be a multisystem disease, affecting many parts of the human body. Fatigue and dyspnea have been described to be some of the most common COVID-19 symptoms (4,24,25). Unfortunately, some of these symptoms persist in patients who test negative for SARS-CoV-2, leading to chronic COVID-19 syndrome (CCS). The long-term risks and costs of CCS remain unknown, and the exact etiology of CCS is poorly understood. We hypothesized that CCS may be caused by ongoing myocardial injury and inflammation. However, none of our patients fulfilled the diagnostic criteria of ongoing myocardial inflammation, and markers of myocardial edema were not elevated. Myocardial T1 and T2 relaxation times did not differ between participants with CCS and control participants (myocardial T1 relaxation times: 978 msec ± 23 vs 971 msec ± 25 [P = .17]; myocardial T2 relaxation times: 53 msec ± 2 vs 52 msec ± 2 [P = .47]). Three of 41 (7%) analyzed participants with CCS showed myocardial changes on images with late gadolinium enhancement (LGE) (eg, changes consistent with myocardial scarring), although none of these participants fulfilled the 2018 Lake Louise criteria for active inflammatory cardiomyopathy. Furthermore, one lesion was an unspecific lesion at the right ventricular insertion point, which is an uncommon finding in myocarditis. As the burden of LGE lesions was very low and the participants showed no signs of ongoing myocardial inflammation (ie, signs of myocardial edema at cardiac MRI), these findings are likely not the cause of the described CCS symptoms. Our percentage of images positive for cardiac findings is considerably lower than those previously reported by Puntmann et al (8) (78%), Wang et al (10) (30%), and Huang et al (26) (58%).
We did not find any evidence to support the hypothesis that CCS in young, previously healthy patients who have had COVID-19 is caused by structural myocardial damage. Puntmann et al (8) analyzed 100 patients who had recovered from COVID-19, irrespective of preexisting conditions, and showed that up to 78% of patients had myocardial involvement (8). This is a difference in incidence of more than tenfold compared with our observed 7% of positive LGE findings. The patient population from Puntmann et al (8) differed from ours regarding selection in two major ways. First, they did not screen for CSS but recruited participants by using broad selection criteria. Second, 33 (33%) of their patients had a severe course of disease requiring hospitalization. Only one of our participants required hospitalization, but this participant was excluded from the final analysis because of continuous signs of active pneumonia that were most likely due to COVID-19. Additionally, we excluded patients with preexisting cardiac or respiratory conditions, whereas other research groups (eg, Wang et al [10] and Huang et al and [26]) recruited recovered previously hospitalized patients and included these patients for analysis. This naturally begs the question of whether or not these reported cardiac findings were present before SARS-CoV-2 infection. The discrepancy in findings between our study and the above-mentioned studies is most likely due to patient selection, more specifically disease severity as reflected by the hospitalization rate. Most participants in the CCS group had a mild initial course of COVID-19. In another cardiac MRI study by Joy et al (13) of health care workers who had recovered from asymptomatic or mild COVID-19, cardiovascular abnormalities were also no more common than in the control group, supporting our findings. Interestingly, the only significant difference we found between the two groups was the septal thickness at end-diastole (7.8 mm ± 1.7 among participants with CSS vs 9.3 ± 1.6 mm among control participants; P = <.001), but both groups were within normal limits (27). Six participants demonstrated incidental findings at MRI that theoretically could have also impacted cardiopulmonary symptoms. However, no distinct pattern among the incidental findings that might be specific to the CCS symptoms described in our study was present.
There was no significant difference between those with an active infection and those who were only symptomatic at the time of the questionnaire in terms of the subjective symptom burdens of dyspnea (4.1 ± 3.6 vs 5.6 ± 2.6; P = .11) and fatigue (5.5 ± 3.6 vs 4.4 ± 3.0; P = .26), even with a worsening of the symptom burden of dyspnea (mean difference, 1.5 points) and an improvement in fatigue-like symptoms (mean difference, 2.0 points) being shown over the time course. This suggests that overall, most participants did not perceive a significant difference between their symptom burden during acute infection and their symptom burden during CCS in terms of fatigue and exertional dyspnea. However, other symptoms such as anosmia, headache, cough, myalgia, and fever improved markedly.
Our study had a few limitations. For one, because a single cardiac MRI examination was performed after symptom onset for each patient, we cannot be sure that the reported cardiac MRI findings were not also present before SARS-CoV-2 infection. In this regard, it is important to mention that the nonischemic LGE lesions found could have been nonspecific to CCS and may have been due to previously unperceived myocarditis that was present before SARS-CoV-2 infection (21). In addition, no histopathologic analysis was performed regarding the presence of active myocarditis. However, the quantitative MRI techniques that were applied have been reported to enable sensitive detection of even subclinical myocardial edema and inflammation in various medical conditions (28). The questionnaire was administered only once at the time of the cardiac MRI examination, thus possibly leading to a recall bias for acute symptoms. Finally, our findings are not applicable to patients currently experiencing acute COVID-19.
In conclusion, we found no evidence to support that chronic COVID-19 syndrome (CSS) in participants who were not hospitalized for COVID-19 is caused by active myocardial inflammation. Our observed incidence of cardiac MRI studies positive for cardiac-specific findings was lower than that previously reported. CCS symptoms might not be caused by myocardial inflammation induced by COVID-19.
Acknowledgments
Acknowledgments
We offer a special thank you to all staff, including Christina Ritt, Tino Winter, Ulrike Schröder, Anna Otto, Olga Ramig, Antje Stascheit, Linda Crone, Carla Lambertz, and Martina Ferro. Without you, this study would not have been possible.
Supported in part by the German Heart Foundation and German Foundation of Heart Research (F/28/20). A.F. supported by a research grant from the BONFOR research program of the University of Bonn (2020-2A-04).
Disclosures of Conflicts of Interest: D. Kravchenko Stocks in Pfizer, Johnson & Johnson, and Abvvie. A.I. No relevant relationships. S.Z. No relevant relationships. N.M. No relevant relationships. M.R. No relevant relationships. A.F. No relevant relationships. C.C.P. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Guerbet, Bayer, Vital, Philips Healthcare, and Julius Zorn. A.H. No relevant relationships. M.V. No relevant relationships. J.N. No relevant relationships. D. Kuetting No relevant relationships. G.D.D. No relevant relationships. U.I.A. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and/or travel from Siemens Healthineers. J.A.L. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Philips Healthcare.
Abbreviations:
- CCS
- chronic COVID-19 syndrome
- LGE
- late gadolinium enhancement
References
- 1. Gavriatopoulou M , Korompoki E , Fotiou D , et al . Organ-specific manifestations of COVID-19 infection . Clin Exp Med 2020. ; 20 ( 4 ): 493 – 506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang WT , Toh HS , Liao CT , Yu WL . Cardiac involvement of COVID-19: a comprehensive review . Am J Med Sci 2021. ; 361 ( 1 ): 14 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta A , Madhavan MV , Sehgal K , et al . Extrapulmonary manifestations of COVID-19 . Nat Med 2020. ; 26 ( 7 ): 1017 – 1032 . [DOI] [PubMed] [Google Scholar]
- 4. Townsend L , Dyer AH , Jones K , et al . Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection . PLoS One 2020. ; 15 ( 11 ): e0240784 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wostyn P . COVID-19 and chronic fatigue syndrome: Is the worst yet to come? Med Hypotheses 2021. ; 146 110469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wenzel P , Kopp S , Göbel S , et al . Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab . Cardiovasc Res 2020. ; 116 ( 10 ): 1661 – 1663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pirzada A , Mokhtar AT , Moeller AD . COVID-19 and myocarditis: what do we know so far? CJC Open 2020. ; 2 ( 4 ): 278 – 285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puntmann VO , Carerj ML , Wieters I , et al . Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) . JAMA Cardiol 2020. ; 5 ( 11 ): 1265 – 1273 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanghvi SK , Schwarzman LS , Nazir NT . Cardiac MRI and myocardial injury in COVID-19: diagnosis, risk stratification and prognosis . Diagnostics (Basel) 2021. ; 11 ( 1 ): 130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang H , Li R , Zhou Z , et al . Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance . J Cardiovasc Magn Reson 2021. ; 23 ( 1 ): 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen BH , Shi NN , Wu CW , et al . Early cardiac involvement in patients with acute COVID-19 infection identified by multiparametric cardiovascular magnetic resonance imaging . Eur Heart J Cardiovasc Imaging 2021. ; 22 ( 8 ): 844 – 851 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X , Wang H , Zhao R , et al . Elevated extracellular volume fraction and reduced global longitudinal strains in participants recovered from COVID-19 without clinical cardiac findings . Radiology 2021. ; 299 ( 2 ): E230 – E240 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joy G , Artico J , Kurdi H , et al . Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers . JACC Cardiovasc Imaging doi: 10.1016/j.jcmg.2021.04.011. Published online May 5, 2021. Accessed August 26, 2021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luetkens JA , Isaak A , Öztürk C , et al . Cardiac MRI in Suspected Acute COVID-19 Myocarditis . Radiology: Cardiothoracic Imaging 2021. ; 3 ( 2 ): e200628 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang D , Mo G , Yuan X , et al . Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection . Am J Respir Crit Care Med 2020. ; 201 ( 9 ): 1150 – 1152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samrah SM , Al-Mistarehi AH , Kewan T , et al . Viral clearance course of COVID-19 outbreaks . J Multidiscip Healthc 2021. ; 14 ( 555 ): 565 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gandhi RT , Lynch JB , Del Rio C . Mild or moderate COVID-19 . N Engl J Med 2020. ; 383 ( 18 ): 1757 – 1766 . [DOI] [PubMed] [Google Scholar]
- 18. Look DC , Locker DR . Time saving in measurement of NMR and EPR relaxation times . Rev Sci Instrum 1970. ; 41 ( 2 ): 250 – 251 . [Google Scholar]
- 19. Messroghli DR , Radjenovic A , Kozerke S , Higgins DM , Sivananthan MU , Ridgway JP . Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart . Magn Reson Med 2004. ; 52 ( 1 ): 141 – 146 . [DOI] [PubMed] [Google Scholar]
- 20. Sprinkart AM , Luetkens JA , Träber F , et al . Gradient spin echo (GraSE) imaging for fast myocardial T2 mapping . J Cardiovasc Magn Reson 2015. ; 17 ( 1 ): 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luetkens JA , Doerner J , Thomas DK , et al . Acute myocarditis: multiparametric cardiac MR imaging . Radiology 2014. ; 273 ( 2 ): 383 – 392 . [DOI] [PubMed] [Google Scholar]
- 22. Luetkens JA , Faron A , Isaak A , et al . Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: results of a validation cohort . Radiol Cardiothorac Imaging 2019. ; 1 ( 3 ): e190010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luetkens JA , Homsi R , Sprinkart AM , et al . Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis . Eur Heart J Cardiovasc Imaging 2016. ; 17 ( 2 ): 154 – 161 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang C , Wang Y , Li X , et al . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China . Lancet 2020. ; 395 ( 10223 ): 497 – 506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D , Hu B , Hu C , et al . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China . JAMA 2020. ; 323 ( 11 ): 1061 – 1069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang L , Zhao P , Tang D , et al . Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging . JACC Cardiovasc Imaging 2020. ; 13 ( 11 ): 2330 – 2339 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Missiri AM , El Meniawy KAL , Sakr SAS , Mohamed ASE . Normal reference values of echocardiographic measurements in young Egyptian adults . Egypt Heart J 2016. ; 68 ( 4 ): 209 – 215 . [Google Scholar]
- 28. Luetkens JA , Doerner J , Schwarze-Zander C , et al . Cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV-infected patients . Circ Cardiovasc Imaging 2016. ; 9 ( 3 ): e004091 . [DOI] [PubMed] [Google Scholar]