Abstract
BACKGROUND:
Thioredoxin-1 (TRX1), a ubiquitous 12 kDa protein, exerts anti-oxidant and anti-inflammatory effects. In contrast, the truncated form, called TRX80, produced by macrophages induces up-regulation of pro-inflammatory cytokines. TRX80 also promotes the differentiation of mouse peritoneal and human macrophages toward a pro-inflammatory M1 phenotype.
METHODS:
TRX1 and TRX80 plasma levels were determined using specific ELISA. ADAM-17 and ADAM-10 activities were measured using respectively SensoLyte® 520 ADAM10 Activity Assay Kit *Fluorimetric* and InnoZyme™ TACE Activity Kit. Western immunoblots were performed using specific antibodies to ADAM-10 or ADAM-17. Angiogenesis study was evaluated in vitro using Human Microvascular Endothelial Cells-1 and in vivo using the matrigel plug angiogenesis assay in mice. The expression of macrophage phenotype markers were investigated using real-time polymerase chain reaction. Phosphorylation of Akt, mTOR and 70S6K was determined using specific antibodies. The effect of TRX80 on NLRP3 inflammasome activity was evaluated by measuring the level of IL-1β and IL-18 in the supernatants of activated macrophages using ELISA. Hearts were used for lesion surface evaluation and immunohistochemical studies and whole descending aorta were stained with oil-red-O. For transgenic mice generation, the human scavenger receptor (SR-A) promoter/enhancer was used to drive macrophage-specific expression of human TRX80 in mice.
RESULTS:
In this study, we observed a significant increase of plasma levels of TRX80 in aged subjects compared to healthy young subjects. In parallel, an increase in expression and activity of ADAM-10 and ADAM-17 in aged peripheral blood mononuclear cells compared to young subjects were observed. Furthermore, TRX80 was found to colocalize with TNF−α, a macrophage M1 marker, in human atherosclerotic plaque. In addition, TRX80 induced the expression of murine M1 macrophages markers through Akt-2/mTOR-C1/70S6K pathway and activated the inflammasome NLRP3, leading to IL-1β and IL-18 release, potent atherogenic cytokines. Moreover, TRX80 exerts a powerful angiogenic effect both in vitro and inmouse. Finally, transgenic mice that overexpress human TRX80 specifically in macrophages of apoE−/− mice have a significant increase of aortic atherosclerotic lesions.
CONCLUSION:
TRX80 showed an age-dependent increase in human plasma. In mouse models, TRX80 was associated with a pro-inflammatory status and increased atherosclerosis.
Keywords: Macrophage, truncated thioredoxin, atherosclerosis, inflammation, inflammasome, IL-1β, angiogenesis
Introduction
The development of atherosclerosis is dependent upon both innate and adaptive arms of the immune response, which have been shown to modulate lesion initiation, progression, and potentially devastating thrombotic complications 1, 2. In this process, macrophages play a central role in accumulation of lipids in atherosclerotic plaques 3, immune responses 4 and the maintenance of inflammation 5. Therefore, macrophage recruitment and plasticity are key components of atherosclerosis.
Interestingly, macrophages residing within atherosclerotic lesions represent a heterogeneous cell population, whose activation and function are influenced by various cytokines and microbial products. For example, interleukin-1β (IL-1β), interferon-γ, and endotoxin lipopolysaccharide (LPS) increase the inflammatory activation profile, yielding the so-called M1 macrophages 6 that produce proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-6, and IL-12, as well as reactive oxygen species (ROS) and nitrogen intermediates 7. Consistently, M1 macrophages are associated with increased inflammation and tissue damage. In contrast, IL-4, IL-13 8 peroxisome proliferator–activated receptor-γ (PPAR-γ) activators 8 and adiponectin 9, 10 promote polarization of macrophages into the anti-inflammatory, alternative M2 type. M2 macrophages secrete the anti- inflammatory cytokine IL-10, transforming growth factor-β, and IL-1 receptor antagonist, and upregulate scavenger receptors, the mannose receptor CD206, and arginase-1. Overall, the accumulation of M2 macrophages leads to reduction of inflammation.
Growing evidence indicates that overproduction of ROS under pathophysiological conditions is an integral component in the development of cardiovascular diseases (CVDs), such as atherosclerosis, ischemic heart disease, hypertension, cardiomyopathies, cardiac hypertrophy and congestive heart failure 11, 12. An imbalance favoring the cellular production of free radicals in amounts exceeding the cellular defense capacities is referred to as oxidative stress 11, 13, which markedly contributes to arterial inflammation 14. Therefore, cells have evolved several strategies, both enzymatic and non-enzymatic, to overcome free radical-induced oxidative stress including preventive and repairing mechanisms, physical barriers, and antioxidant defenses. Non-enzymatic antioxidants are ascorbic acid (vitamin C), α-tocopherol (vitamin E), glutathione, carotenoids, flavonoids, and other antioxidants. Enzymatic antioxidant defenses include superoxide dismutase, glutathione peroxidase, catalase, glutaredoxin, and thioredoxin system 12.
The thioredoxin system comprises thioredoxin (TRX), truncated TRX (TRX80), thioredoxin reductase (TRXR), and NADPH. In addition a natural TRX inhibitor, the thioredoxin-interacting protein (TXNIP), is also an important part of this system. The thioredoxin system is essential for maintaining the balance of the cellular redox status and is involved in the regulation of redox signaling. It is also pivotal for growth promotion, neuroprotection, inflammatory modulation, anti-apoptosis, immune function, and atherosclerosis. As an ubiquitous and multifunctional protein, TRX1, a 12-kDa highly conserved protein, is expressed in all forms of life and executes its function through its anti-oxidative, protein reducing, signal transducing activities 15. Several studies reported the beneficial role of TRX1 in CVDs 16–19. In contrast, the C-terminal truncated form, composed of 1–80 or 1–84 N-terminal amino acids (TRX80), which is either secreted from cells already truncated or cleaved by 2α−secretases (ADAM-10 and ADAM-17) at the cell surface 20, possesses a pro-inflammatory cytokine-like activity 21. Macrophages have been reported to cleave full-length TRX1 to yield TRX80 22.
The level of TRX80 in plasma has been reported to vary from 2 to 175 ng/ml and is markedly increased under inflammatory conditions 23, 24. TRX80 activates monocytes and induces up-regulation of cell surface pathogen recognition receptors, molecules essential for T-cell activation and function 25, as well as for the release of the pro-inflammatory cytokines 26. In contrast to the full length TRX1, which down-regulates the expression of a number of inflammatory genes 27, TRX80 promotes mouse peritoneal and human macrophages toward a pro-inflammatory M1 phenotype and significantly increases aortic lesion surface area in mice 28. Therefore, it is thought that these two different forms of TRX may use different signaling pathway or regulate the same signalling pathway but in different ways. Among the candidate signalling pathways, Akt pathway is most likely to be used by TRX1 and/or TRX80.
Akt, also known as PKB, is a family of three serine/threonine protein kinases (Akt1, Akt2, and Akt3) that regulate a host of cellular functions, including cell survival, proliferation, differentiation, and intermediary metabolism 29. In the vascular wall, Akt plays an important role in the proliferation and migration of endothelial cells, regulation of vascular permeability, and angiogenesis 30–32. Recent studies of Akt knockout mice have shown that despite significant sequence homology, the three Akt isoforms have some non-redundant functions. Although Akt1-deficient mice exhibit overall growth impairment 33, Akt2 knockout mice have impaired glucose tolerance and insulin resistance 34, and Akt3 nulls display a selective reduction in brain size 35.
Additionally, it has been reported that a global absence of Akt1 in vivo enhances atherosclerotic lesion burden and promotes coronary atherosclerosis in a mouse model of atherosclerosis, indicating that Akt1 exerts vascular protection against atherogenesis 29. Interestingly, it has been demonstrated that Akt2 ablation results in the M2 polarization of macrophages, whereas Akt1 ablation promotes their M1 polarization 36. Therefore, the implication of Akt pathway has been studied to explore the signalling pathway used by TRX1 and/or TRX80 to induce the macrophages polarization. Because we have observed a significant increase of the plasma level of TRX80 in aged subjects in comparison to young people, we conducted a series of in vitro and in vivo experiments to determine the mechanism underlying the cleavage of the TRX1 and the generation of the TRX80. We also investigated the in vivo role of TRX80 on angiogenesis, inflammation, oxidative process and arterial lesion development in mice.
Methods
Cohort constitution
A cohort of young and aged male and female was collected in collaboration with the Health Professional Department in Ksar Hellal Hospital (Monastir, Tunisia). A total of 283 subjects (male and female) divided into two groups, young (n=144 from 20 to 40 years old; (male n= 98; female n=46)) and aged (n=139, ≥ 65 years old (male n=94; female n=45)) were recruited after a routine health checkup and were included in the study if they were free of any history of obesity, hypertension, dyslipidemia, diabetes mellitus, smoking or coronary artery diseases. In addition, subjects who had inflammatory diseases, valvular heart disease, cancers or rheumatoid arthritis were also excluded. Heparinized plasma was collected after overnight fasting, centrifuged and stored at −80°C. TRX1 levels were determined using ELISA kit from International office IBL, Hamburg, Germany. TRX80 levels were determined using an ELISA kit developed in our laboratory (Supplemental Methods). For α-secretases activity, peripheral blood mononuclear cells (PBMC) were purified from 5 young and 5 aged subjects and ADAM-17 and ADAM-10 activities were measured using respectively SensoLyte® 520 ADAM10 Activity Assay Kit *Fluorimetric* (Anaspec, France) and InnoZyme™ TACE Activity Kit (Merck, France). Aliquots of cell lysates were also used to perform Western immunoblots, using specific antibodies to ADAM-10 or ADAM-17 (NeoBiotech, France) (Supplemental methods). All subjects provided written informed consent for the collection and storage of plasma aliquots and peripheral blood mononuclear cells consistent with the current study. Colocalization of TRX80 and TNF-α on M1 macrophages in human atherosclerotic lesions was conducted on 5 μm sections of vessel specimens obtained from patients undergoing vascular surgery for atherosclerotic complications (Online Supplemental Methods). For the angiogenesis study, the effect of TRX80 was evaluated in vitro using Human Microvascular Endothelial Cells-1 (HMEC-1) and in vivo using the matrigel plug angiogenesis assay in mice (Supplemental Methods). The data are presented as mean ± SD.
In vitro study on mouse peritoneal macrophages
In this study, murine peritoneal macrophages were left untreated or treated with LPS, IL-4, TRX80, or a combination thereof. The expression of macrophage phenotype markers, CD206, MCP-1, IL-10, and TNF-α, were investigated at the transcription level, using real-time polymerase chain reaction (Supplemental Table 1). For TRX80 signal pathway determination, murine peritoneal macrophages were untreated or treated with TRX80, PI3K inhibitor, Akt inhibitor, mTOR inhibitor, or a combination thereof. Phosphorylation of Akt, mTOR and 70S6K was determined using specific antibodies (Supplemental Methods). The effect of TRX80 on NLRP3 inflammasome activity was evaluated by measuring the level of IL-1β and IL-18 in the supernatants of activated macrophages (see figure legends) isolated from C57Bl/6.Nlrp3+/+ or C57Bl/6.Nlrp3−/− using ELISA. The data are presented as mean ± SD.
In vivo study on C57Bl/6.apoE−/− mice.
In vivo study was performed on C57Bl/ 6.apoE−/− mice. Heart and descending aortas were excised and fixed. Hearts were used for lesion surface evaluation and immunohistochemical studies and whole descending aorta were stained (en face) with oil-red-O and the lesion area per animal was quantified (Supplemental Methods) and the data are presented as mean ± SD. All procedures involving animal handling and their care were in accordance with the University of Pierre and Marie Curie Guidelines for Husbandry of Laboratory Mice.
Transgenic mice generation
The human scavenger receptor (SR-A) promoter/enhancer was used to drive macrophage-specific expression of human TRX80 in mice (Supplemental Figure 1A). In the created transgenic line the heterozygous mice had 50 copies of the TRX80 transgene per genome. Expression of human TRX80 was measured in peritoneal macrophages (Supplemental Figure 1B). For the study on the effect of TRX80 transgene on development of atherosclerosis, the transgene was transferred onto an apoE-KO background by crossing the transgenic mice with apoE-KO mice, which were also on a C57Bl/6N genetic background (Jackson Lab, stock 2052).
Statistical analyses
Statistical significance was calculated with Mann-Whitney test, unpaired t-test or ANOVA and reported in the legend of each figure and table. P<0.05 was considered significant.
Results
Baseline clinical characteristics
Different parameters of subjects included in this study such as age, body mass index (BMI), glycemia, Blood Pressure, LDL-Cholesterol (LDL-c), HDL-Cholesterol (HDL-c), Total Cholesterol (Total-c) and Triglycerides (TG) were reported in Table 1.
Table 1.
Baseline clinical characteristics and TRX1 and TRX80 levels in the plasma of young and aged subjects.
| Male | Female | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Young (n=98) | Old (n=94) | Fold changes | Young (n=46) | Old (n=45) | Fold changes | |
| Age (year) | 32.20 ± 6.51 | 75.10 ± 5.04 | 2.33 | 30.72 ± 4.71 | 71.09 ± 10.93 | 2.31 |
| BMI (kg/m2) | 23.45 ± 1.91 | 24.69 ± 1.42 | 1.05 | 22.94 ± 3.95 | 25.49 ± 2.22 | 1.11 |
| Blood Pressure (mmHg) | 12.74 ± 0.75 | 13.29 ± 0.76 | 1.04 | 11.41 ± 0.97 | 13.56 ± 1.12 | 1.19 |
| Glycemia (mmol/l) | 5.15 ± 0.37 | 5.17 ± 0.44 | 1.00 | 4.25 ± 0.71 | 5.04 ± 0.50 | 1.27 |
| LDL-c (mmol/l) | 2.11 ± 0.38 | 2.74 ± 0.48 | 1.30 | 2.27 ± 0.22 | 2.51 ± 0.28 | 1.11 |
| HDL-c (mmol/l) | 1.29 ± 0.18 | 1.16 ± 0.19 | 0.90 | 1.61 ± 0.25 | 1.49 ± 0.10 | 0.92 |
| Total Cholesterol (mmol/l) | 3.75 ± 0.43 | 4.35 ± 0.54 | 1.16 | 4.15 ± 0.57 | 4.96 ± 0.36 | 1.19 |
| Triglycerides (mmol/l) | 0.97 ± 0.27 | 1.17 ± 0.28 | 1.21 | 0.96 ± 0.34 | 1.18 ± 0.17 | 1.23 |
| Thioredoxin-1 (ng/ml) | 71.96 ± 13.36 | 24.84 ± 9.26 | 0.35 | 45.42 ± 14.49 | 25.90 ± 10.05 | 0.57 |
| Thioredoxin-80 (ng/ml) | 9.00 ± 9.01 | 60.25 ± 18.50 | 6.69 | 11.22 ± 15.30 | 40.41 ± 24.41 | 3.60 |
Statistical tests using Generalized Linear Model (GLM – R Software – ‘car’ library with Gaussian family) using the following model: TRX1Age + BMI + Blood Pressure + Glycemia + LDL-c + HDL-c + Total Cholesterol + TG and TRX80 Age + BMI + Blood Pressure + Glycemia + LDL-c + HDL-c + Total Cholesterol + TG. Then ANOVA was performed on the results of the GLM. For males, the results stand for both molecules TRX1/TRX80 with p-values < 0.001 in both cases. Similarly, for females, the results stand for TRX1/TRX80 with p-value < 0.05
Plasma levels of TRX1 and TRX80 in young and aged subjects
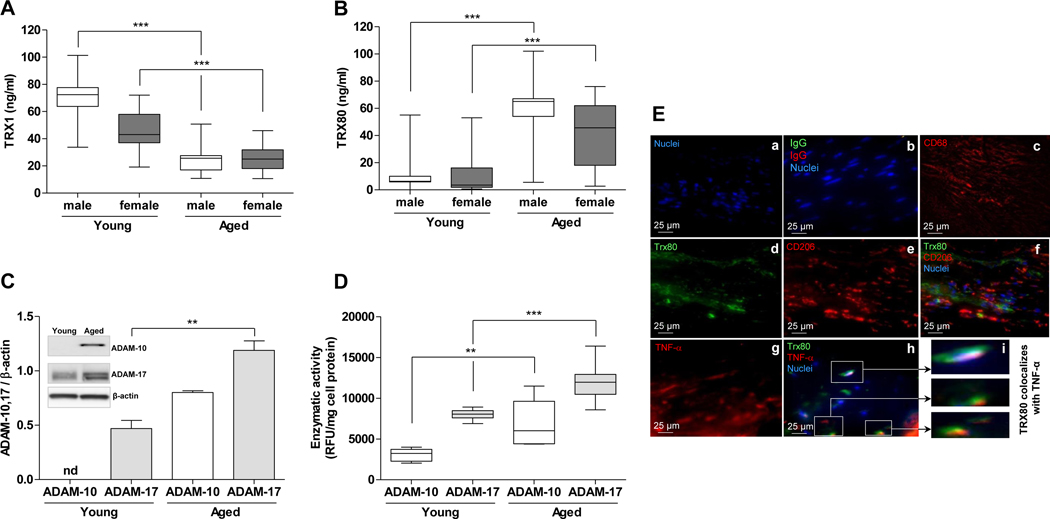
TRX1 is known to be cleaved at its C-terminal 21, producing a truncated protein called TRX80, which accumulates in plasma 24. The conversion of TRX1 to TRX80 appears to be a molecular switch from an anti- to pro-inflammatory molecule. Two α-secretases (ADAM-10 and ADAM-17) were recently reported to be responsible for its cleavage 20. Nevertheless, the physiological or pathological condition in which the cleavage process occurs is not known. Because inflammation often increases with age and age is an independent risk factor for cardiovascular diseases, we evaluated the level of both TRX1 and TRX80 in the plasma of young and aged male and female subjects. Higher levels of full length TRX1 were found in young male versus old male subjects (71.96 ± 13.36 ng/ml vs. 24.84 ± 9.26 ng/ml respectively, P< 0.001), as well as in young female versus old female subjects (45.42 ± 14.49 vs 25.90 ± 10.05 ng/ml respectively, P< 0.001) (Fig. 1A). In contrast, TRX80 was lower in young males vs. aged subjects (9.00 ± 9.01 vs. 60.21 ± 18.50 ng/ml, P<0.001) and in young females than in aged respective subjects (11.22 ± 15.13 vs 40.41 ± 24.41 ng/ml, P< 0.001) (Fig. 1B). Mean ± SD, Mann-Whitney test was used. In order to assess cofounding effects, we performed an additional statistical tests using Generalized Linear Model (GLM - R software - ‘car’ library with Gaussian family) using the following model: TRX1 ~ Age + BMI + Blood Pressure + glycemia + LDLc + HDLc + Totalc + TG and TRX80 ~ Age + BMI + Blood Pressure + glycemia + LDLc + HDLc + Totalc + TG. Then ANOVA was performed on the results of the GLM. For males, the results stand for both molecules TRX1/TRX80 with p-values <0.001 in both cases. For females, the results stand for TRX1/TRX80 with P-value <0.05.
Figure 1. Plasma TRX1 and TRX80, ADAM-10 and ADAM-17 expression and activity and immunohistochemical studies.
Plasma levels of TRX1 and TRX80 in young and aged subjects. Plasma levels of TRX1 (A) and TRX80 (B) in young (male (n=98) and female (n=46)) and aged (male (n=94) and female (n=45)) subjects were determined by ELISA and are represented in box plots indicating the median and the lower and upper quartiles. Statistically significant differences are indicated (male (young vs aged; ***P<0.001; Mann-Whitney); C- ADAM-10 and ADAM-17 expression in PBMC from young and aged subjects. Peripheral blood mononuclear cells from young (n=5) and aged (n=5) subjects were cultured, lysed, centrifuged and 20 μg protein/lane were separated on 4–15% gels and electroblotting onto PVDF membrane as described in the methods section. Specific antibodies against either ADAM-10 (1:500), ADAM-17 (1:500), or β-actin for normalization were added, Membranes were washed, incubated with HRP conjugated secondary antibody (1:5000) and specific bands were visualized and scanned. Data are presented as mean ± SD of five young and five aged subjects. Statistically significant differences are indicated (**P<0.01; Mann-Whitney). One representative Western immunoblot was inserted. D- Measurement of ADAM-10 and ADAM-17 activities. Peripheral Blood Mononuclear Cells were isolated from healthy young and aged donors, washed, lysed, centrifuged and the supernatant was collected and used to assay ADAM-10 and ADAM-17 activities using respectively SensoLyte® 520 ADAM10 Activity Assay Kit *Fluorimetric* and InnoZyme™ TACE Activity Kit. The results are represented in box plots indicating the median, the lower and upper quartiles. Statistically significant differences are indicated (**P<0.01; ***P<0.001; Mann-Whitney). E- TRX80 colocalizes with M1 macrophages in human atherosclerotic lesions. Serial sections of paraffin-embedded human atherosclerotic vessel specimens from three patients undergoing vascular surgery for atherosclerotic complications were stained with DAPI for nuclei visualization (a), the primary antibodies were substituted with control IgG (b), anti-CD68 (a specific marker for macrophages) (c), TRX80 monoclonal mouse antibody, that reacts exclusively with truncated protein, was stained with Cy-5-conjugated secondary antibody (d), anti-human CD206 (e), double stained withanti-TRX80 and anti-CD206 (f), anti-TNFα (g) and double stained with anti-TRX80 and anti-TNF-α primary antibodies (h). Magnification of selected area in panel h (i). Representative images are shown. Original magnifications 400X.
ADAM-10 and ADAM-17 expression and activity increased in PBMC in aged subjects
The cleavage process of TRX1 into TRX80 could possibly be attributed to the increase of the two α-secretases expression and/or activities. Indeed, we showed that ADAM-10 and ADAM-17 expression (Fig. 1C) and activities (Fig. 1D) increased in peripheral blood mononuclear cells (PBMC) isolated from aged subjects in comparison to PBMC isolated from young people. Because oxidative stress, inflammatory cytokines and oxidized LDL could increase with age, we examined whether H2O2, IL-6, IL-1β, TNF-α or oxLDL were responsible for the induction of the expression of the two α-secretases in cultured murine peritoneal macrophages. None of these treatments, however, had any appreciable effect on the level of either α-secretase (Supplemental Figure 2).
TRX80 colocalizes with M1 macrophages in human atherosclerotic lesions
The staining of serial sections of human atherosclerotic plaques confirmed that TRX80 expressed by macrophages colocalized with the M1 macrophage marker TNF-α (Fig. 1E (h, i). In contrast, TRX80 does not colocalize with CD206, a marker of M2 macrophages (Fig. 1E, f), indicating that TRX80 could promote the M1 polarization in human atherosclerotic vessels.
TRX80 and NLRP3 inflammasome activation
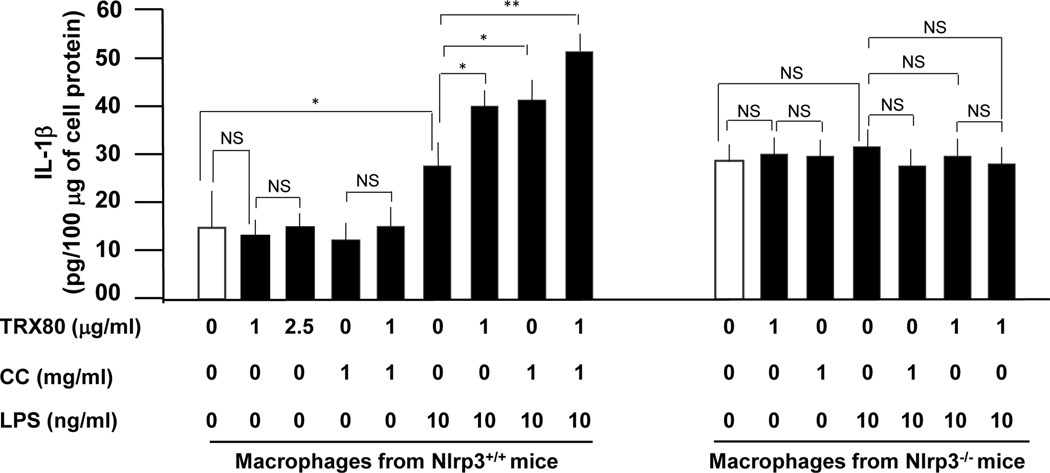
Because the NLRP3 inflammasome is involved in the pro-IL-1β and pro-IL-18 maturation into biological active IL-1β and IL-18, we determined whether TRX80 is able to activate the NLRP3 inflammasome. TRX80, however, did not stimulate the generation of mature IL-1β and IL-18 in the absence of LPS (Fig. 2 and Supplemental Figure 3 respectively). Of note, LPS is known to activate NF-κB, leading to proIL-1β and proIL-18 generation. Therefore, cholesterol crystals (CC), which are specific activator of NLRP3 inflammasome 37, induced the maturation of proIL-1β and proIL-18 into IL-1β and IL-18. In the presence of TRX80 (1 or 2.5 μg/ml), CC (1 mg/ml) or a combination of TRX80 and CC, the generation of mature IL-1β and IL-18 was comparable to control cells indicating that neither TRX80 nor CC affect NF-κB. (Fig. 2 and Supplemental Figure 3). Treatment of macrophages with LPS (10 ng/ml) enhanced the expression of IL-1β (24.4 ± 5.2 vs 15.2 ± 4.4 pg/100 μg cell protein for the control; P<0.05) (Fig. 2) and IL-18 (32.3 ± 6.1 vs; 18.6 ± 0.8 pg/100 μg cell protein for the control; P<0.05) (Supplemental Figure 3). Treatment of macrophages with LPS (10 ng/ml) for 4 h and then with TRX80 (1 μg/ml) for additional 24 h, potentiated the level of IL-1β (41 ± 4.0 vs 24.4 ± 5.2 pg/100 μg cell protein for LPS-treated cells; P<0.05) (Fig. 2) and IL-18 (49.0 ± 6.0 vs 32.3 ± 6.1 pg/100 μg cell protein for LPS-treated cells; P<0.05) (Supplemental Figure 3). Likewise, a marked induction of IL-1β (44.0 ± 4.0 pg/100 μg cell protein vs LPS-stimulated cells; P<0.05) (Fig. 2) and IL-18 (58.2 ± 4.0 pg/100 μg cell protein vs LPS-stimulated cells; P<0.05) (Supplemental Figure 3) was observed when cells were first activated with LPS (10 ng/ml) for 4 h and then with CC (1 mg/mL) for additional 24 h. Finally, treatment of macrophages with LPS (10 ng/ml) for 4 h followed by treatment with CC (1 mg/ml) and TRX80 (1 μg/ml) for additional 24 h, further potentiated the secretion of IL-1β (52.1 ± 6 pg/100 μg cell protein vs LPS-stimulated cells; P<0.01) (Fig. 2) and IL-18 (75.9 ± 3.0 pg/100 μg cell protein vs LPS-stimulated cells; P<0.01) (Supplemental Figure 3). In contrast, treatment of macrophages isolated from Nlrp3−/− with either, TRX80, LPS, CC or a combination thereof did not induce generation of mature IL-1β (Fig. 2) or IL-18 (Supplemental Figure 3).
Figure 2. Effect of TRX80 on inflammasome NLRP3 activation.
Peritoneal macrophages were purified and cultured at 0.5×106 cells/ml. The cells were incubated with hrTRX80 in the absence or presence of either CC (1 mg/ml) or LPS (10 ng/ml) or both. Data are presented as mean ± SD of IL-1β (pg/100 μg cell protein) of three independent experiments performed in duplicate. Statistically significant differences, using Mann-Whitney test, are indicated (*P<0.05; **P<0.01). We have also used ANOVA for multiple comparisons to analyse the data obtained on macrophages isolated from Nlrp3−/− mice. In all cases; there is no significance between samples.
Correlation between the plasma levels of TRX80 and IL-1β
Since the plasma levels of TRX80 vary with age and because TRX80 contributes to the generation of IL-1β, we examined whether a correlation exists between the plasma levels of IL-1β observed in young and aged subjects and the plasma levels of TRX80 in the same subjects. We did not observe, however, any significant correlation between TRX80 and IL-1β (Supplemental Figure 4).
Both TRX1 and TRX80 activate Akt
TRX1 and TRX80 up-regulated Akt activation in non-treated macrophages, as demonstrated by an in vitro kinase assay performed on total cellular extracts after exposure to TRX1 (1 μg/ml) or TRX80 (1 μg/ml) for 15 min. These data were confirmed by Western blots, which showed increased levels of the activated form of pAkt (Supplemental Figure 5). The TRX1 did not dampen the down-regulation of Akt activity by LY294002 unlike TRX80. When cells were primed with either IL-4 (15 ng/ml) or LPS (100 ng/ml), treatment with TRX1 or TRX80 did not show any further activation of Akt (Supplemental Figure 5). It is possible to suggest that they could activate different isoforms of Akt.
TRX80, but not TRX1, activates mTOR
To determine which isoform has been activated by TRX1 and TRX80, a specific inhibitor of Akt1/2 kinase (A6730, Sigma), was used. With this inhibitor, we observed an IC50 = 58 nM, 210 nM, and 2.12 mM for Akt1, Akt2, and Akt3, respectively. Accordingly, either Akt1 or Akt2 has been inhibited then the cells were treated with different concentrations (0, 1, 2, and 5 μg/ml) of TRX1 or TRX80 for 15 min; TRX80 significantly activated mTOR in a dose-dependent manner only when Akt1 is inhibited whereas TRX1 did not activate it (Supplemental Figure 6).
TRX80 activates mTOR in dose-dependent manner
The p70S6K (Thr389) is known to be a direct substrate of mTORC1. It is often used as functional readout of mTORC1 activity, as phosphorylation of this site by mTORC1 has been confirmed both in vitro and in vivo and is inhibited by rapamycin treatment. Therefore, to confirm our previous results, murine peritoneal macrophages were pretreated with rapamycin (20 nM) for 1 h followed by exposure to different concentrations of TRX80 for 30 min. Results of Western blot analysis, using phospho-p70S6K and p70S6K specific antibodies, showed that TRX80 activates mTOR in a dose-dependent manner (Supplemental Figure 7).
Inhibition of mTOR downregulated the expression of inflammatory cytokines
To further explore the role of mTOR in macrophage polarization, isolated murine peritoneal macrophages were pretreated with or without rapamycin (20 nM) for 6 h and/or exposed to LPS (100 ng/ml) and/or TRX80 (1 μg/ml) stimulation. Rapamycin attenuated the expression of inflammatory cytokines, IL-6, IL-1β, and TNF-α, revealing the role of mTOR signaling in macrophage phenotype orientation. Interestingly, TRX80 positively regulates the expression of these cytokines most probably by activation of the mTOR pathway (Supplemental Figure 8).
mTOR inhibition orients resting macrophages toward M2 phenotype
Freshly isolated murine peritoneal macrophages were pretreated with or without rapamycin (20 nM) for 6 h and/or exposed to IL-4 (15 ng/ml) and/or TRX80 (1 μg/ml) stimulation. Rapamycin stimulated the expression of anti-inflammatory cytokines, IL-10 and CD206 indicating the implication of mTOR signaling in macrophage polarization. Of note, TRX80 treatment attenuated the expression of these cytokines maybe through reactivating mTOR (Supplemental Figure 9).
TRX80 induced angiogenesis
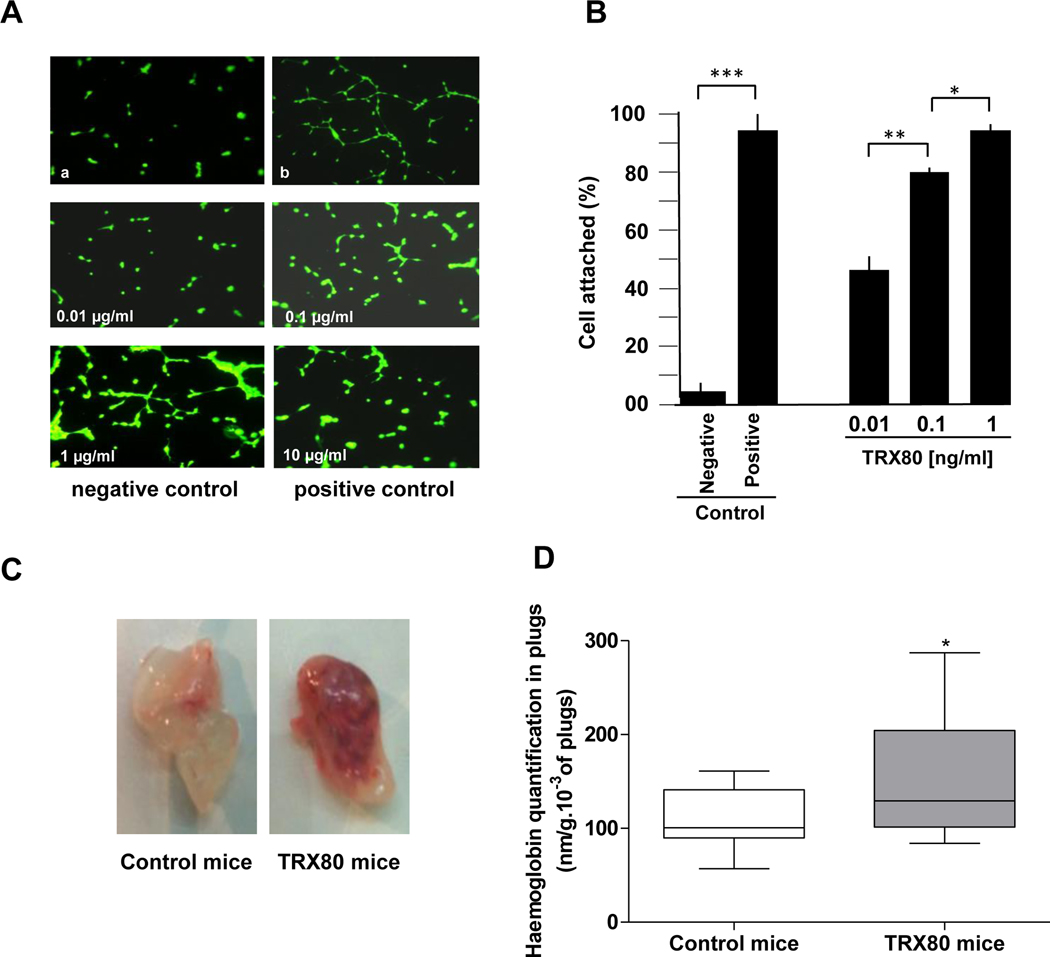
TRX80 was also found to be angiogenic in vitro and in vivo. Indeed, cultured HMEC-1 cells treated or not with TRX80 showed a significant increase, in a dose-dependent manner, of angiogenesis, as measured by capillary tube formation (40 ± 5%; 73 ± 10% and 87 ± 5% respectively for TRX80 at 0.01, 0.1 and 1 μg/ml; P<0.001 vs. negative control) (Fig. 3A and 3B). Of note, the percentage of tube formation obtained with the positive control was 90 ± 3% and the negative control was 4 ± 1% (Fig. 3B). Similarly, the Matrigel plug angiogenesis assay in mice was used to investigate the pro-angiogenic effect of TRX80 in vivo. The result in Figure 3C and 3D indicated a significant increase of angiogenesis (157.10 ± 66.92 for TRX80 (n=17) vs. 109.30 ± 30.08 for the control (n=17), P=0.02.
Figure 3. Effect of TRX80 on angiogenesis.
A,B- Treatment of cultured HMEC-1 cells with TRX80 showed a significant increase, in a dose-dependent manner, of angiogenesis as measured by capillary tube formation. Similarly, the Matrigel plug angiogenesis assay in mice (C,D) was used to investigate in vivo the pro-angiogenic effect of TRX80. The data are presented as mean ± SD. Statistically significant differences, calculated with the unpaired t-test, are indicated (*P<0.05; **P<0.01; ***P<0.001).
M1 macrophages number increased in arterial lesion of TRX80 transgenic mice
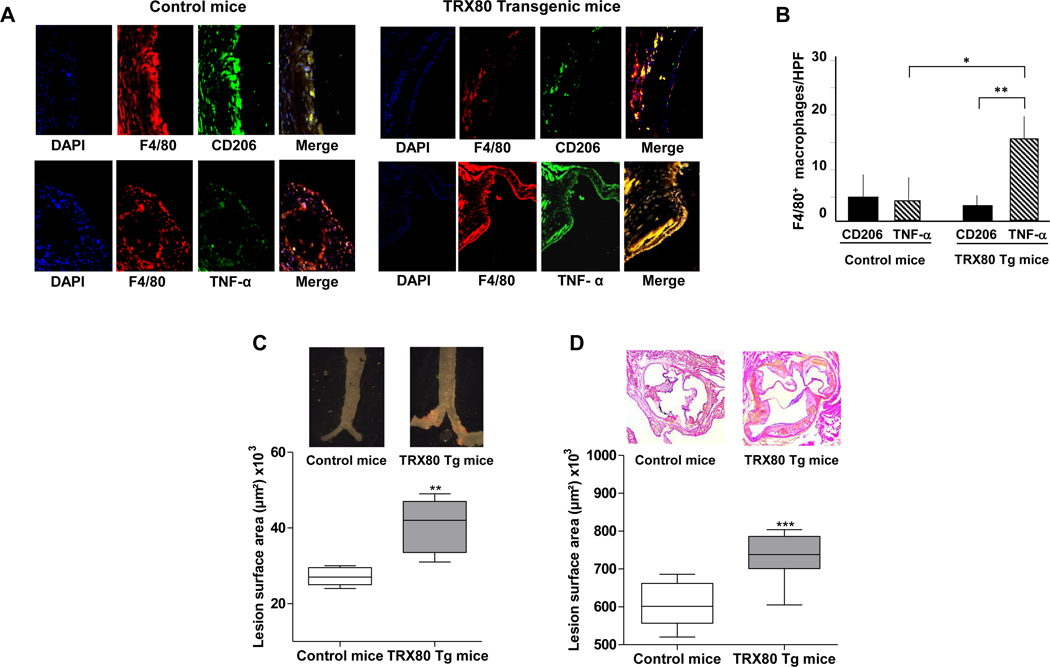
Immunohistochemical analysis showed that the number of macrophages expressing TNF-α is greater than the number of macrophages expressing CD206 in transgenic mice compared to control mice (Fig.4A and 4B).
Figure 4. Macrophages phenotype in atherosclerotic lesions of TRX80 transgenic mice and the lesions surface in the aortic sinus and in the whole aortas (‘en face’).
C57Bl/6.ApoE−/− mice (control) and TRX80 transgenic mice in C57Bl/6.ApoE−/− background were sacrificed. Paraffin serial sections (7 μm) of proximal aortas were analyzed by immunohistochemistry using double immunostaining with antibodies either against F4/80 and TNF-α (M1 marker) or CD206 (M2 marker). A- Representative images. B- Quantification of the number of macrophages (F4/80) that express TNF-α or CD206. Data are mean ± SD of 8 mice. Statistically significant differences, are indicated (*P<0.05; **P<0.01; unpaired t-test).
At 8 months of age, female ApoE−/− and female TRX80 Tg mice on ApoE−/− background were sacrificed, hearts and proximal aortas were removed and fixed. Hearts were cut directly under and parallel to the leaflet, and the upper portions were embedded in OCT medium. One hundred sections of 7-μm thickness were prepared from the top of the left ventricle. The sections were stained for lipids with Oil-Red O and counterstained with Hematoxylin Harris. Sections were used for specific morphometric evaluation of intimal lesions. C-Ascendant aortas were stained with Sudan IV and total surface lesion area was evaluated (n=5; **P<0.01). D- The mean lesion size ± SD in these sections was determined (n=5; ***P<0.001; Mann-Whitney test).
Human TRX80, specifically expressed in murine macrophages, increased atherosclerotic lesions in apoE.KO mice.
The overexpression of human TRX80, specifically in macrophages of apoE.KO mice, does not change significantly change the phenotype of mice. Indeed, we did not observe any obvious differences between control and transgenic mice (e.g; body weight, longevity, apparent neurological disorder, hair color or any visible anatomic differences). It does not increase either the level of TRX80 in the plasma (undetectable human TRX80). Of note, the endogenous levels of murine TRX80 cannot be determined because of the lack of specific antibodies. Overexpression of human TRX80 also did not affect the plasma levels of pro-inflammatory cytokines, such as IL-33, IL-6, MCP-1 nor the plasma levels of autoantibodies against oxLDL (Supplemental Figure 10). However, we observed a difference in aortic atherosclerotic lesions in the transgenic mice in comparison to the control (Fig. 4C and 4D). Indeed, the mean total surface lesion area (aortic root, thoracic and bifurcation) increased significantly only in homozygous for TRX80 transgene females, which had 100 copies of the transgene per genome (n=5) vs control mice (n=5) (40866 μm2±7077 vs. 27511±2285 μm2; P=0.0079) (Fig. 4C). In addition, in sinus aorta the mean lesion area in transgenic mice (female 100 copies) was significantly higher than in control mice (735072±61899; n=8 vs. 606894±58098; n=9; P=0.0016) (Fig. 4D).
Discussion
In this study, we have shown that the level of the TRX80 increased in human plasma with age, whereas the level of full length TRX1 decreased in the same plasma (Fig. 1A and 1B). Such an increase in the level of TRX80 can be attributed to the increase of the expression and activity of ADAM-10 and ADAM-17 on peripheral blood mononuclear cells (PBMC) (Fig. 1C and 1D). We have also shown that TRX80 can up-regulate the expression of several pro-inflammatory genes, including IL-1β in human macrophages 28. This could, at least in part, explain the “inflam’ageing” phenomenon observed in aged subjects. Therefore, we have identified a TRX80 signal pathway involving macrophage M1 phenotype polarization and found that TRX80 induces the expression of murine M1 macrophages markers through Akt-2/mTOR-C1/70S6K pathway (Supplemental Figure 5 to Supplemental Figure 9). In addition, we observed a colocalization of TRX80 with TNF-α, a marker of M1 macrophages, in human atherosclerotic plaque (Fig. 1E (h,i)). This may be due to the TRX80-induced activation of pro-inflammatory Akt-2/mTOR-C1/70S6K signaling pathways.
Both TRX1 and TRX80 activate Akt in resting macrophages, whereas in already polarized macrophages toward either M1 or M2, they could not further activate Akt. Previously, it was documented that TRX1 can activate Akt 38, 39, but in this manuscript, for the first time, we show the effect of TRX80 on Akt/mTOR pathway. As mentioned earlier, we hypothesized that each molecule may activate a different isoform of Akt. Therefore, we have inhibited Akt isoforms separately. It has been found that only TRX80 specifically activates mTOR only when Akt1 is inhibited. Thus, in this study, we established that TRX80 induced macrophage M1 phenotype through the phosphorylation of Akt-2, mTOR-C1 and 70S6K. In contrast, TRX1 induced macrophage M2 phenotype through Akt-1, mTOR-C2 and 4E-BP1. Taken together, these studies indicate that TRX80 functions as regulator of macrophage phenotype, tipping the balance towards the pro-inflammatory M1 state. As a consequence, atherosclerotic plaques become larger and probably more unstable.
Based on these findings, inhibition of mTOR activity appears to orient resting macrophages toward M2 phenotype and impairs the polarizing effect of LPS. These results are consistent with recent works by Byles V. et al 40 and Pan H. et al 41. In these studies, it was shown that LPS-induced expression of IL-1β and IL-6 was markedly suppressed by INK128, an mTOR inhibitor, at both mRNA and protein levels 41. Another recent study showed that rapamycin treatment restores Akt activation simultaneously with rescue of M2 gene expression and arginase activity. Moreover, rapamycin treatment of control bone marrow derived macrophages modestly increased Akt signaling and M2 response 40.
Furthermore, our results show that TRX80 uses mTOR signaling pathway to exert its effect in polarizing macrophages toward M1 phenotype, since it induced mTOR activity in a dose-dependent manner, as demonstrated by the increased phosphorylation of P70S6K. The molecular mechanism by which TRX80 could rescue mTOR from the inhibitory action of rapamycin is still unclear. It may prevent the formation of rapamycin- FK506 binding protein (FKBP12) complex since rapamycin first binds to its cellular receptor, FKBP12, and it is the rapamycin-FKBP12 complex that inhibits TOR function. This mechanism of action is conserved in eukaryotes 42. Further downstream proteins need to be carefully investigated in order to find out the exact signaling pathway employed by either TRX1 or TRX80 to stimulate macrophage polarization, since specific macrophage-targeted therapies are now taking the first steps into the clinical arena.
Angiogenesis is a physiological process required for embryonic vascular development, which is also involved in wound healing and in pathophysiological progress of some diseases, such as diabetic retinopathy, cancer and atherosclerosis 43. In the normal arterial wall, the vasa vasorum constitute a microvascular network in the adventitia. While no capillaries are found in the intima and the media of normal arteries, neovascularization is seen in the intima of human atherosclerotic lesions 44, 45. These neocapillaries are thought to favor the progression of the plaque, promote plaque instability and intraplaque haemorrhage and finally increase the risk of athero-thrombotic events 45–47. Thus, the finding from this study that TRX80-induced angiogenesis both in vitro in a HMEC-1 tube formation model and in vivo in the murine Matrigel plug model (Fig. 3A and 3B) may be critical in understanding the link between ageing and CVD. Indeed, our results on TRX80 transgenic mice indicated an increase in lesion surface area even in the absence of high fat diet. This pathological effect can possibly be attributed to the increase number of the macrophages with the M1 phenotype, as well as the neovascularization in atherosclerotic lesions in the transgenic mice.
To date, the biological roles of TRX80 are still largely unknown. Previously, TRX80 was termed eosinophil cytotoxicity-enhancing factor due to its eosinophil cytotoxicity and was first detected in the plasma of patients suffering from severe schistosomiasis 48–50. Of note, these patients also developed atherosclerosis. It may be valuable to identify other pathologies associated with TRX80 generation and to study in greater details the molecular mechanisms leading to its generation.
Finally, because IL-1β and IL-18 maturation are under the control of inflammasome NLRP3 and the plasma level of these cytokines increased in elderly subjects, we investigated the effect of TRX80 on inflammasome NLRP3 activation and IL-1β and IL-18 generation. Our data showed that TRX80 exerts its effect, at least in part, through the activation of inflammasome NLRP3 (Fig. 2 and Supplemental Figure 3). However, we did not observe any correlation between the plasma levels of TRX80 and IL-1β. Furthermore, we have generated transgenic mice that overexpress human TRX80 specifically in macrophages of apoE.KO mice. These mice had a significant increase of aortic surface lesion (Fig. 4C and 4D). Taken together, we have identified, TRX80, as a new pro-inflammatory and pro-angiogenic actor, which, in contrast to TRX1, increases its plasma concentration with age and thus could explain increased associated with ageing.
Supplementary Material
Clinical Perspective.
What is new?
A significant increase of plasma levels of TRX80 in healthy aged subjects.
A significant increase of ADAM-10 and ADAM-17 activities, two α-secretases responsible for the TRX1 cleavage, in aged peripheral blood mononuclear cells.
A significant activation of the inflammasome NLRP3 by TRX80 leading to IL-1β and IL-18 release.
Transgenic mice that overexpress TRX80, specifically in macrophages, have a significant increase of aortic atherosclerotic lesions.
What are the clinical implications?
The loss of TRX1 and the increase of TRX80 with age explain, at least in part, the occurrence of oxidative stress and inflammation in aged subjects.
The plasma level of TRX80 could be considered as a new parameter for the evaluation of inflammation, angiogenesis and atherosclerotic lesions development.
Acknowledgment:
We thank Prof. Christopher Glass for providing the pAL1 vector, Prof. Onnik Agbulut for performing the mice heart section, Prof. Sonia Karabina for providing oxLDL and Prof. Hédi Soula for performing statistical tests using Generalized Linear Model.
Sources of Funding: Work by AR and BV for this study was supported by intramural National Health Lung and Blood Institute research funds.
Work by DC, DFDM, MMH, FC, VD, KEH and MR was supported by Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique and Université Pierre et Marie Curie.
Footnotes
Disclosures: None
References
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. [DOI] [PubMed] [Google Scholar]
- 2.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. [DOI] [PubMed] [Google Scholar]
- 3.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibata N, Glass CK. Regulation of macrophage function in inflammation and atherosclerosis. J Lipid Res. 2009;50 Suppl:S277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. [DOI] [PubMed] [Google Scholar]
- 6.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. [DOI] [PubMed] [Google Scholar]
- 8.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. [DOI] [PubMed] [Google Scholar]
- 9.Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299:H656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. [DOI] [PubMed] [Google Scholar]
- 13.Avery SV. Molecular targets of oxidative stress. Biochem J. 2011;434:201–210. [DOI] [PubMed] [Google Scholar]
- 14.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–939. [DOI] [PubMed] [Google Scholar]
- 15.El Hadri K, Mahmood DF, Couchie D, Jguirim-Souissi I, Genze F, Diderot V, Syrovets T, Lunov O, Simmet T, Rouis M. Thioredoxin-1 promotes anti-inflammatory macrophages of the M2 phenotype and antagonizes atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1445–1452. [DOI] [PubMed] [Google Scholar]
- 16.Yamawaki H, Haendeler J, Berk BC: Thioredoxin. a key regulator of cardiovascular homeostasis. Circ Res. 2003;93:1029–1033. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Holmgren A. Thioredoxin system in cell death progression. Antioxid Redox Signal. 2012;17:1738–1747. [DOI] [PubMed] [Google Scholar]
- 18.Ebrahimian T, Touyz RM. Thioredoxin in vascular biology: role in hypertension. Antioxid Redox Signal. 2008;10:1127–1136. [DOI] [PubMed] [Google Scholar]
- 19.World CJ, Yamawaki H, Berk BC. Thioredoxin in the cardiovascular system. J Mol Med (Berl). 2006;84:997–1003. [DOI] [PubMed] [Google Scholar]
- 20.Gil-Bea F, Akterin S, Persson T, Mateos L, Sandebring A, Avila-Carino J, Gutierrez-Rodriguez A, Sundstrom E, Holmgren A, Winblad B, Cedazo-Minguez A. Thioredoxin-80 is a product of alpha-secretase cleavage that inhibits amyloid-beta aggregation and is decreased in Alzheimer’s disease brain. EMBO Mol Med. 2012;4:1097–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pekkari K, Holmgren A. Truncated thioredoxin: physiological functions and mechanism. Antioxid Redox Signal. 2004;6:53–61. [DOI] [PubMed] [Google Scholar]
- 22.Bizzarri C, Holmgren A, Pekkari K, Chang G, Colotta F, Ghezzi P, Bertini R. Requirements for the different cysteines in the chemotactic and desensitizing activity of human thioredoxin. Antioxid Redox Signal. 2005;7:1189–1194. [DOI] [PubMed] [Google Scholar]
- 23.Cortes-Bratti X, Basseres E, Herrera-Rodriguez F, Botero-Kleiven S, Coppotelli G, Andersen JB, Masucci MG, Holmgren A, Chaves-Olarte E, Frisan T, Avila-Carino J. Thioredoxin 80-activated-monocytes (TAMs) inhibit the replication of intracellular pathogens. PLoS One. 2011;6:e16960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pekkari K, Gurunath R, Arner ES, Holmgren A. Truncated thioredoxin is a mitogenic cytokine for resting human peripheral blood mononuclear cells and is present in human plasma. J Biol Chem. 2000;275:37474–37480. [DOI] [PubMed] [Google Scholar]
- 25.Lemarechal H, Anract P, Beaudeux JL, Bonnefont-Rousselot D, Ekindjian OG, Borderie D. Impairment of thioredoxin reductase activity by oxidative stress in human rheumatoid synoviocytes. Free Radic Res. 2007;41:688–698. [DOI] [PubMed] [Google Scholar]
- 26.Bertini R, Howard OM, Dong HF, Oppenheim JJ, Bizzarri C, Sergi R, Caselli G, Pagliei S, Romines B, Wilshire JA, Mengozzi M, Nakamura H, Yodoi J, Pekkari K, Gurunath R, Holmgren A, Herzenberg LA, Ghezzi P. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med. 1999;189:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billiet L, Furman C, Larigauderie G, Copin C, Brand K, Fruchart JC, Rouis M. Extracellular human thioredoxin-1 inhibits lipopolysaccharide-induced interleukin-1beta expression in human monocyte-derived macrophages. J Biol Chem. 2005;280:40310–40318. [DOI] [PubMed] [Google Scholar]
- 28.Mahmood DF, Abderrazak A, Couchie D, Lunov O, Diderot V, Syrovets T, Slimane MN, Gosselet F, Simmet T, Rouis M, El Hadri K. Truncated thioredoxin (Trx-80) promotes pro-inflammatory macrophages of the M1 phenotype and enhances atherosclerosis. J Cell Physiol. 2013;228:1577–1583. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science. 2001;292:1728–1731. [DOI] [PubMed] [Google Scholar]
- 35.Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. [DOI] [PubMed] [Google Scholar]
- 36.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, Stathopoulos EN, Tsichlis PN, Tsatsanis C. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109:9517–9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaimul Ahsan M, Nakamura H, Tanito M, Yamada K, Utsumi H, Yodoi J. Thioredoxin-1 suppresses lung injury and apoptosis induced by diesel exhaust particles (DEP) by scavenging reactive oxygen species and by inhibiting DEP-induced downregulation of Akt. Free Radic Biol Med. 2005;39:1549–1559. [DOI] [PubMed] [Google Scholar]
- 39.Sartelet H, Rougemont AL, Fabre M, Castaing M, Duval M, Fetni R, Michiels S, Beaunoyer M, Vassal G. Activation of the phosphatidylinositol 3’-kinase/AKT pathway in neuroblastoma and its regulation by thioredoxin 1: Hum Pathol. 2011;42:1727–1739. [DOI] [PubMed] [Google Scholar]
- 40.Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, Horng T. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan H, Xu LH, Ouyang DY, Wang Y, Zha QB, Hou XF, He XH. The Second-Generation mTOR Kinase Inhibitor INK128 Exhibits Anti-inflammatory Activity in Lipopolysaccharide-Activated RAW 264.7 Cells. Inflammation 2014;37:756–765. [DOI] [PubMed] [Google Scholar]
- 42.Sandsmark DK, Pelletier C, Weber JD, Gutmann DH. Mammalian target of rapamycin: master regulator of cell growth in the nervous system. Histol Histopathol. 2007;22:895–903. [DOI] [PubMed] [Google Scholar]
- 43.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. [DOI] [PubMed] [Google Scholar]
- 45.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. [DOI] [PubMed] [Google Scholar]
- 46.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. [DOI] [PubMed] [Google Scholar]
- 47.Michel JB, Virmani R, Arbustini E, Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J. 2011;32:1977–1985, 1985a, 1985b, 1985c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dessein AJ, Lenzi HL, Bina JC, Carvalho EM, Weiser WY, Andrade ZA, David JR. Modulation of eosinophil cytotoxicity by blood mononuclear cells from healthy subjects and patients with chronic schistosomiasis mansoni. Cell Immunol. 1984;85:100–113. [DOI] [PubMed] [Google Scholar]
- 49.Lenzi HL, Mednis AD, Dessein AJ. Activation of human eosinophils by monokines and lymphokines: source and biochemical characteristics of the eosinophil cytotoxicity-enhancing activity produced by blood mononuclear cells. Cell Immunol. 1985;94:333–346. [DOI] [PubMed] [Google Scholar]
- 50.Silberstein DS, Ali MH, Baker SL, David JR. Human eosinophil cytotoxicity-enhancing factor. Purification, physical characteristics, and partial amino acid sequence of an active polypeptide. J Immunol. 1989;143:979–983. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.