Abstract
The prevalence of myopia is increasing extensively worldwide. The number of people with myopia in 2020 is predicted to be 2.6 billion globally, which is expected to rise up to 4.9 billion by 2050, unless preventive actions and interventions are taken. The number of individuals with high myopia is also increasing substantially and pathological myopia is predicted to become the most common cause of irreversible vision impairment and blindness worldwide and also in Europe. These prevalence estimates indicate the importance of reducing the burden of myopia by means of myopia control interventions to prevent myopia onset and to slow down myopia progression. Due to the urgency of the situation, the European Society of Ophthalmology decided to publish this update of the current information and guidance on management of myopia. The pathogenesis and genetics of myopia are also summarized and epidemiology, risk factors, preventive and treatment options are discussed in details.
Keywords: Myopia, pathologic myopia, preventive medicine, blindness, time spent outdoors, myopia reduction interventions, atropine, orthokeratology
Introduction
Myopia is recognized as a significant global public health issue, expected to affect an increasing number of people in the next decades.1 Pathology such as myopic maculopathy and optic neuropathy in highly myopic eyes can cause significant irreversible visual impairment and blindness.1–3 Besides, myopia increases the risk of other pathological ocular changes such as cataract, glaucoma, and retinal detachment, all of which can cause irreversible vision loss.4,5 Significant disease associations exist even at low and moderate levels of myopia. Current evidence suggests that there is no safe threshold level of myopia for any of the known ocular diseases linked to myopia.6,7
The importance of the increase in the prevalence of myopia and its sequelae has been well documented particularly in East Asian countries that have experienced the most pronounced increase in myopia prevalence. Consequently, the experience in preventing the development and progression of myopia in children and adolescents is more advanced in East Asia. The prevalence of myopia also increased in Europe in recent decades and reached the level of 45–50% in the 25–29 years old age group.8 Thus, Europe is becoming aware of the importance of myopia both as a public health issue and as a significant socioeconomic burden.
The International Myopia Institute (IMI) with its group of 85 multidisciplinary experts in the field has recently published a series of white papers on the pathogenesis of myopia including the results of experimental studies,9 genetics,10 and the results of clinical studies including the outcome of randomized controlled trials.11 Based on the IMI White Papers,11 the main aim of this article is to increase awareness and to provide recommendations for European ophthalmologists to prevent the development and progression of myopia in children and adolescents.
Definition and classification
To be consistent with international standards, the definitions and classification of myopia as used in this article follow those described by the International Myopia Institute (IMI) in the IMI White Papers (Tables 1 and 2).12
Table 1.
Summary of proposed general and quantitative thresholds for myopia12 (with permission from IMI).
| Term | Definition |
|---|---|
| Qualitative definitions | |
| Myopia | A refractive error in which rays of light entering the eye parallel to the optic axis are brought to a focus in front of the retina when ocular accommodation is relaxed. This usually results from the eyeball being too long from front to back, but can be caused by an overly curved cornea and/or a lens with increased optical power. It also is called nearsightedness. |
| Axial myopia | A myopic refractive state primarily resulting from a greater than normal axial length. |
| Refractive myopia | A myopic refractive state that can be attributed to changes in the structure or location of the image forming structures of the eye, that is, the cornea and lens. |
| Secondary myopia | A myopic refractive state for which a single, specific cause (e.g. drug, corneal disease, or systemic clinical syndrome) can be identified that is not a recognized population risk factor for myopia development. |
| Quantitative definitions | |
| Myopia | A condition in which the spherical equivalent refractive error of an eye is ⩽−0.50 D when ocular accommodation is relaxed. |
| Low myopia | A condition in which the spherical equivalent refractive error of an eye is ⩽−0.50 and >−6.00 D when ocular accommodation is relaxed. |
| High myopia | A condition in which the spherical equivalent refractive error of an eye is ⩽−6.00 D when ocular accommodation is relaxed. |
| Pre-myopia | A refractive state of an eye of ⩽+0.75 D and >−0.50 D in children where a combination of baseline refraction, age, and other quantifiable risk factors provide a sufficient likelihood of the future development of myopia to merit preventative interventions. |
Table 2.
Definitions for the structural complications of myopia12 (with permission from IMI).
| Term | Definition |
|---|---|
| Descriptive definitions | |
| Pathologic myopia | Excessive axial elongation associated with myopia that leads to structural changes in the posterior segment of the eye (including posterior staphyloma, myopic maculopathy, and high myopia-associated optic neuropathy) and that can lead to loss of best-corrected visual acuity. |
| Myopic macular degeneration (MMD) | A vision-threatening condition occurring in people with myopia, usually high myopia that comprises diffuse or patchy macular atrophy with or without lacquer cracks, macular Bruch’s membrane defects, CNV, and Fuchs spot. |
| Diagnostic subdivisions of MMD | |
| Myopic maculopathy | Category 0: no myopic retinal degenerative lesion. Category 1: tessellated fundus Category 2: diffuse chorioretinal atrophy. Category 3: patchy chorioretinal atrophy. Category 4: macular atrophy. ‘‘Plus’’ features (can be applied to any category): lacquer cracks, myopic choroidal neovascularization, and Fuchs spot. |
| Presumed myopic macular degeneration | A person who has vision impairment and vision acuity that is not improved by pinhole, which cannot be attributed to other causes, and: • The direct ophthalmoscopy records a supplementary lens <−5.00 D and shows changes such as “patchy atrophy” in the retina or, • The direct ophthalmoscopy records a supplementary lens <−10.00 D. |
| Specific clinical conditions characteristic of pathologic myopia | |
| Myopic traction maculopathy (MTM) | A combination of macular retinoschisis, lamellar macula hole and/or foveal retinal detachment (FRD) in eyes with high myopic attributable to traction forces arising from adherent vitreous cortex, epiretinal membrane, internal limiting membrane, retinal vessels, and posterior staphyloma. |
| Myopia-associated glaucoma-like optic neuropathy | Optic neuropathy characterized by a loss of neuroretinal rim and enlargement of the optic cup, occurring in eyes with high myopia eyes with a secondary macrodisc or peripapillary delta zone at a normal IOP. |
Pathogenesis
More than 50 years ago, myopia was believed to be mostly genetic in origin,13 although epidemiological studies have long ago shown the connection with education, near-work and higher occupational status.14–16 Subsequently, experimental models have provided evidence that myopia may develop as an adaptation to environmental visual conditions through the same mechanisms used in emmetropization. Thus, myopia onset and progression is now understood to result from a complex interplay of visual/environmental conditions and genetic factors that modulate the visually guided eye growth so that the control mechanisms are no longer able to coordinate growth with the development of the optical components of the eye.9,17
Most recent articles on the pathomechanism of accelerated eye growth refer to the influence of peripheral retinal defocus.18–24 Animal and human studies have also examined additional ocular and environmental factors that may affect retinal image quality and influence eye growth. These factors include accommodation,22,25–28 higher-order aberrations (HOA),29–31 circadian rhythms,32–34 light intensity and spectral composition,35–37 and overstimulation of retinal OFF pathways38 – for an overview see Figure 1.
Figure 1.
Model of the visually regulated control of eye growth and refractive state9 (with permission from IMI).
Work from animal models suggests that form deprivation and retinal defocus initiate a signaling cascade that leads to a number of cellular and biochemical changes in the retina and the retinal pigment epithelium (RPE). These chemical signals are transmitted through the choroid, causing changes in scleral extracellular matrix (ECM) synthesis which alters the biomechanical properties of the sclera, leading to increases in ocular growth and a more myopic refractive state.39–41 The animal studies/models have show that the choroid plays an active role in emmetropization, both by modulation of its thickness to adjust the retina to the focal plane of the eye (choroidal accommodation), and well as through the release of growth factors that have the potential to regulate scleral extracellular matrix remodeling.42 Experimental studies have identified several biochemical compounds, such as retinal dopamine,43 retinoic acid44, and nitric oxide45 that are involved in the modulation of axial length (AL) changes.
Epidemiology
According to Holden et al.1 quoted in the World Report on Vision published by the World Health Organization (WHO) in October 2019, the estimated number of people globally with myopia in 2020 was predicted to be 2620 million, with a further expected increase to 3361 million by 2030. The number of individuals with high myopia was also expected to increase substantially from 399 million in 2020 to 516 million by 2030.1,46 Both these estimates assume no impact of interventions intended to slow down myopia progression.
This means that pathological myopia is predicted to become the most common cause of irreversible vision impairment and blindness worldwide, and the prevalence estimates indicate the importance of reducing the global burden of myopia by means of myopia reduction interventions.
The predicted prevalence of myopia by 2050 is 65% of the population in Asia, 56% in Western Europe, 54% in Central Europe, and 50% in Eastern Europe1 (Figure 2).
Figure 2.

Estimated increase in the prevalence (%) of myopia in three European regions. Adapted from article of Holden et al.1
Many studies have reported substantial variations in the prevalence of myopia between different ethnic groups and different age groups.47
Prevalence of myopia in different ethnic groups
The myopia burden is highest in East Asia and the high-income countries of the Asia-Pacific region (51.6% and 53.4% prevalence in 2020, respectively) but the prevalence is also high in Europe (Western Europe: 36.7%, Central Europe: 34.6%, and Eastern Europe: 32.2%).1,48 An earlier meta-analysis from fifteen population-based adult cohorts and cross-sectional studies across Europe determined an age-standardised prevalence of 30.6% for myopia.49 The peak prevalence of myopia was identified in the 25–29 years age group (47.2%) although the prevalence of high myopia was relatively low in Europe, with an age-standardised estimate of 2.7%.49
A recent review by Grzybowski et al. showed that the prevalence of myopia in school-aged children was 73% in East Asia and 42% in North America. A low prevalence (under 10%) was described in African and South American children.8 In groups of White ethnicity there was no clear evidence of differences in myopia prevalence between studies of recent decades from Europe, the USA and Oceania.50 (Tables 3 and 4).
Table 3.
Prevalence of myopia in children in Europe.
| Authors | Publication time | Age (years) | Location | Number of participants | Definition (diopter) (D) | Myopia prevalence (%) |
|---|---|---|---|---|---|---|
| Matamoros51 | 2015 | 0–9 | France | 1781 | ⩽−0.5 | 19.6 |
| Tideman52 | 2017 | 6 | Netherlands | 5711 | ⩽−0.5 | 2.4 |
| Enthoven et al.53 | 2020 | 9 | Netherlands | 5074 | ⩽−0.5 | 11.5 |
| Rudnicka et al.54 | 2010 | 10–11 | United Kingdom | 233 | ⩽−0.5 | 3.4 |
| O’Donoghue et al.55 | 2015 | 12–13 | Northern Ireland | 661 | ⩽−0.5 | 17.7 |
| Tideman et al.56 | 2020 | 13 | Netherlands | 3600 | ⩽−0.5 | 22.2 |
| Matamoros51 | 2015 | 10–19 | France | 8289 | ⩽−0.5 | 42.7 |
| Lundberg57 | 2017 | Mean: 15.4 | Denmark | 307 | ⩽−0.5 | 33.6 cycloplegia: 17.9 |
| Hagen et al.58 | 2018 | 16–19 | Norway | 393 | ⩽−0.5 | 13 |
Table 4.
Prevalence of myopia in teenage Asian children.
| Authors | Publication time | Age (years) | Location | Number of participants | Definition (diopter) (D) | Myopia prevalence (%) |
|---|---|---|---|---|---|---|
| Lam et al.59 | 2004 | 13–15 | Hong Kong | 289 | ⩽−0.5 | 87.2 |
| Matsumara et al.60 | 1999 | 17 | Japan | 346 | ⩽−0.5 | 66.0 |
| Wu et al.61 | 2013 | 17 | China | 6026 | ⩽−0.5 | 84.6 |
| Lin et al.62 | 2004 | 16–18 | Taiwan | 2474 | <−0.25 | 84.0 |
| Jung63 | 2012 | 19 | South Korea | 23,616 | <−0.5 | 96.5 |
| Lee et al.64 | 2013 | 19 | South Korea | 2805 | ⩽−0.5 | 83.3 |
| Koh et al.65 | 2014 | Mean: 19.8 | Singapore | 28,908 | <−0.5 | 81.6 |
Progression of myopia in different ethnic groups
Studies on the pattern of myopia progression in Asian and European children give contradicting results.66–70
A meta-analysis determined 0.27 D/year faster progression in 1-year follow-up among 9-year-old Asian children than in age-matched European children.69 However, an Australian study examining children of European White and East Asian ethnicity living in the same geographic location, found the progression of myopia to be similar between the two groups, primarily because the rate of progression was lower in the group of East Asian children living in Australia compared with children living in East Asia, suggesting that environmental differences can influence myopia progression.70 Interestingly, in a recent study comparing Finnish and Singaporean children of the same age group, the 3-year myopia progression was faster among Finnish children.71
Prevalence of myopia across age groups
An early age of onset of myopia appears to be the strongest predictor of high myopia in both Asians and White children.71–74
It was recently shown that parental myopia was associated with a greater risk of early-onset myopia in Asian, Hispanic, non-Hispanic white, and African American children.75
In children younger than 6 years of age the prevalence of myopia is low. This is the case even in Asia where the prevalence of myopia is considered to be critically high in young adults (Table 5).
Table 5.
Prevalence of myopia in younger ages (<9 years).
The prevalence of myopia increases markedly from approximately 6 years of age (Table 6, Figure 3). When comparing the indicators for the last 45 years (from 1971 to 2016), the frequency of myopia in Novosibirsk schoolchildren aged 7–10 years increased 5.1 times (from 4.5% to 23.0%), 11–14 years increased 3.8 times (from 10.5% to 40.0%), and 15–18 years increased 2.1 times (from 21.5% to 45.0%). It follows that the highest rate of onset of myopia is currently observed in children aged 7 to 10 years.79,80
Table 6.
Increased prevalence of myopia with age.
| Authors | Publication time | Location | Number of participants | Definition (diopter) (D) | Myopia prevalence changes (years of age: prevalence) |
|---|---|---|---|---|---|
| Ma et al.76 | 2016 | Shanghai, China | 8267 | ⩽−0.5 | 3 years: 1.8% 10 years: 52.2% |
| Guo et al.77 | 2017 | China | 1127 | ⩽−0.5 | 3 years: 0.0% 6 years: 3.7% |
| Wu et al.61 | 2013 | China | 6026 | ⩽−0.5 | 4 years: 1.2% 17 years: 84.6% |
| Giordano et al.81 | 2004 | Hong Kong | 2546 | ⩽−1.0 | 5 years: 4.6% 10 years: 43.5% |
| Matsumara et al.60 | 1999 | Japan | 346 | ⩽−0.5 | 12 years: 43.5% 17 years: 66.0% |
| He et al.82 | 2007 | Southern China | 2400 | ⩽−0.5 | 13 years: 36.8% 17 years: 53.9% |
Figure 3.
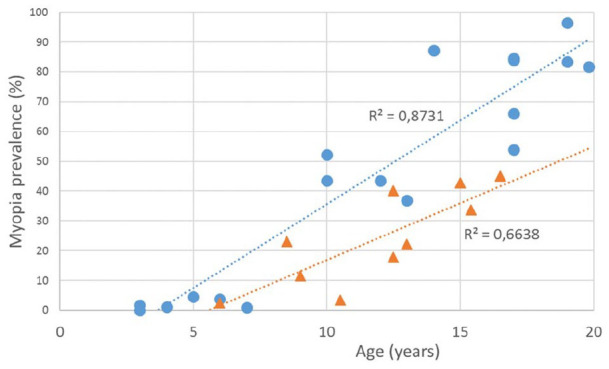
Myopia prevalence in East Asia (dot) and in Europe (triangle) in different age groups of children (linear trend lines) (data published 1999–2020).51–65,76–78,81,82
In the Correction of Myopia Evaluation Trial (COMET) study 426 ethnically diverse (African, Asian, Hispanic, Mixed, and White) myopic children were followed-up annually for at least 6 and up to 11 years to assess, amongst other things, when myopia seemed to stabilize. Nearly half (48%) of the children had stable myopia by age 15 years, 77% by 18 years and 90% by the age 21 years. There is evidence for myopia to have stabilized in most myopic individuals by the age 24 years, except in high myopes.83–85 In a 23-year follow-up study of Finnish myopic children from mean age of 11 years at baseline, the adulthood myopic progression from the mean age of 24 years 8 years onwards was ⩾1.00 D in 17.9% of cases and mean annual change was -0.05 ± 0.09 D.86 Other studies show a decline in progression rate with increasing age in young myopes of both European and Asian ethnicity.69,87,88 In White European children, average yearly progression rates for myopia and axial length were −0.41 D and −0.30 mm respectively between 6 and 16 years of age and −0.16 D and 0.15 mm respectively between 12 and 22 years.88
In 2000, Holden et al. showed that the greatest proportion of myopic people was between 10 and 39 years of age.1 A European study also reported that myopia was most common in younger participants (47.2%), with those aged 25–29 years having a prevalence almost double (27.5%) that of those of middle and older age (55–59 years).89
However, given the predicted increases in prevalence discussed above, the distribution of myopia in the population is expected to widen by 2050, with a significant proportion of the population exhibiting myopia from 10 years of age all the way through to 79 years of age; with the bulk of late onset (16 years or older) myopia, reflecting the significant lifestyle changes, mostly intensive near work over the past 10 to 25 years.1,73,90 This may well be exacerbated by changes in working patterns following the Covid-19 pandemic (increased time indoors, increased time on electronic devices, etc.).
Based on a meta-analysis from 2015, there is a clear trend of higher myopia prevalence in the last 20 to 30 years across Western and Northern Europe.89,91 In contrast, while the prevalence of myopia is reported to have been rising around the world, a similar trend in Southeast Norway appears to be absent.58 Neither in Denmark, where nearly 140 years of myopia research did not find a convincing change in prevalence of myopia.92 Asian ancestry does not inevitably lead to myopia, since the prevalence of myopia in these areas was much lower two or three generations ago.88,93 This suggests that environmental and social factors must be involved in the promotion of myopia in modern populations.
At the current time, the incidence of myopia is increasing in younger age groups, which means that prevalence rates in older adults are generally lower.47,51,91,94 However, a bimodal pattern was observed in the prevalence of myopia among subjects of African-American ethnicity and in the population of Singapore. In both groups aged 40+ years, the prevalence was also highest among individuals in their forties and seventies.95,96 A similar bimodal pattern was found in France, but the peak of myopia prevalence was in people in their twenties and above eighties (Figure 4).51 The bimodal distribution is probably due to the increase of the axial myopia among younger people, and secondary refractive myopization due to nuclear cataract in elderly people.47
Figure 4.
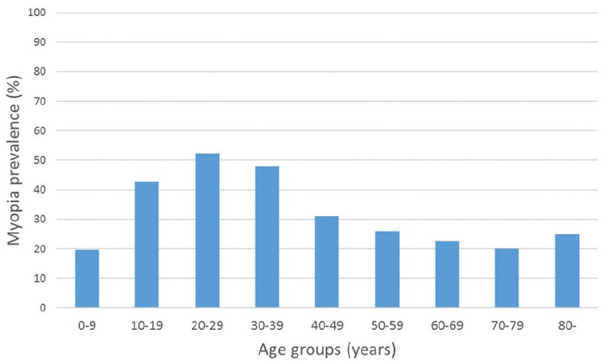
Myopia prevalence in France 2012–2013 across the different age groups. Adapted from article of Matamaros et al.51
Genetics
Myopia is caused by a complex interplay between genetic and environmental factors associated with exposure to the life of a school-child which may limit outdoor exposure. Myopia heritability estimates vary, but are mostly high. Oligogenic and polygenic risk scores indicate that persons at high genetic risk have an up to 40 times increased risk of myopia compared with persons at low genetic risk.10
To date, more than 600 genetic loci have been identified for refraction and myopia.97 Risk variants mostly confer low risk but are highly prevalent in the general population. Several genes for secondary syndromic myopia overlap with those for common myopia. Annotated genes have a wide variety of functions, and all retinal layers appear to be sites of expression.10,97 Pathway analyses indicated a light-induced retina-to-sclera signaling pathway for myopia development. The involved genes appeared to play a role in synaptic transmission, cell-cell adhesions, calcium ion binding, cation channel activity, and plasma membrane function. Many are light dependent and related to the cell cycle and growth pathways.10
Secondary syndromic forms of high myopia, such as Marfan’s syndrome, congenital stationary night blindness, Stickler’s syndrome, and the Donnai-Barrow syndrome, are inherited predominantly in a Mendelian fashion.98 These forms of myopia are rare. Furthermore, some retinal dystrophies have a myopia phenotype. Therefore, a clinician should be aware of their syndromic features and extensive ocular examinations should be performed in case of a young onset of myopia or clinical suspicion.
To date, there is no robust evidence to suggest that there are fundamental differences in the genetic background of myopia risk between European and Asian individuals.10
The recent global rise in myopia prevalence is unlikely to be due to genetic factors alone. Multiple searches of the 1966–2001 PubMed database clearly point to a strong environmental impact on the development and progression of myopia. Changes in environmental factors in Singapore have been so great that large numbers of children with non-myopic parents are now in the high myopia category, and hence at greater risk of developing pathological myopia. Environmental pressures increase the risk of myopia across the population to a similar extent, irrespective of parental refractive error,99–101 although parental myopia is associated with a greater risk of early-onset of myopia.71,75
Risk factors
Studies have identified a number of non-genetic risk factors which affect the prevalence, progression or prevention of myopia. Environmental factors (time spent outdoors), education, personal characteristics, and binocularity play important roles in the onset and progression of myopia.
Personal characteristics
Ethnicity
Epidemiological evidence regarding the prevalence of myopia shows major differences between ethnic groups, although the burden of available evidence for this may be explained primarily by environmental influences.52,88,102
Gender
Females show faster progression than males,69,103–107 however, this difference has not been observed in all studies.108–110 In the ethnic groups studied (Whites and Asians), sex differences emerge in the myopia prevalence at approximately 9 years of age. In one study, by late adolescence, white females as compared to white males were twice as likely to be myopic.50
Parental myopia
Parental history of myopia correlates with the rate of axial elongation and increase in myopic refractive error (myopia progression).52,111–113 Studies from different ethnic groups have shown that having one or two myopic parents increased the risk of myopia114–116 and with a significant association between a strong family history and the incidence of myopia.117 However, the number of myopic parents appears to have a lower predictive value for the development and progression of childhood myopia in some studies118,119 with the amount of myopia in the family having stronger predictive value.10,120 The effect of parental myopia on myopia in their offsprings may not be taken as proof for a genetic contribution to myopia, since the correlation might also be the result of a shared lifestyle121 and their higher education.71 However, parental myopia was associated with a greater risk of early-onset myopia in a recent study.75
Cognitive functions and education
Education seems to be important in triggering the onset of myopia, but less important in determining the degree to which myopia progresses.122,123 Study among 31–35 year-old Finnish men showed that myopic men scored better in all four cognitive tests done and their reaction and movement times were faster than non-myopic men.124 It was recently shown that refractive error genetic risk was significantly correlated with intelligence, both in childhood and adulthood, and educational attainment (defined as the number of years spent in formal education).97 In the Singapore Cohort Study of the Risk Factors for Myopia (SCORM) both academic grades and intelligence quotient (IQ) scores appear to be independently associated with myopia in Singaporean children. Interestingly nonverbal IQ could be a stronger risk factor for myopia than books read per week.125 Both verbal and non-verbal components of the cognitive function were strongly and consistently associated with myopia among more than one million Israeli adolescents.101 Recent studies have gone beyond simply observing an association of myopia and education to providing evidence for a causal role.126–128 However, it is challenging to disentangle the risk of myopia due to education and less time outdoors.
Physical attributes
The connection between physical attributes and myopia is not definite. Jung et al. found that body stature (height, weight) of 19 years old males from Seoul was not significantly associated with myopia.63
In contrast, a recent study reported that in Caucasian children increase in body height and axial elongation were correlated in emmetropia. AL increased at a greater rate than body height in myopia. This indicates that at a time when body growth is stabilising, axial elongation is unregulated.113 In Japanese elementary school children aged 8 to 9 years, body weight and body mass index (BMI) were significantly and positively associated with myopia.129 Another study from Europe determined that in Finnish men BMI was about 5% smaller, and fat content was lower among the myopic than non-myopic men.124
Birth circumstances
Very low birth weight significantly impacts on the refractive state in the long term. By age 10–12 years, individuals with very low birthweight have an increased prevalence of all refractive errors with a shift toward myopia of 1 diopter.130 Significant prematurity that is associated the development of retinopathy of prematurity is also a well recognised cause of myopia.
Studies assessing the association between myopia and birth month indicated that there was a higher prevalence of myopia in subjects born during summer or autumn months compared to the winter.131,132 The exact mechanism is unclear but may be related to the level of exposure to natural light during the early perinatal period.131 The prevalence of myopia is higher in first-born versus non-first-born individuals.133,134
Binocular vision/accommodation
Myopia onset and progression were found to be related to an elevated response accommodation-convergence/accommodation (AC/A) ratio which could be observed before the onset of myopia. The theory was proposed that poor or inaccurate accommodative response with increased (accommodative lag) and consequential hyperopic retinal blur during near viewing activities may be a stimulant to axial growth.107,135–137
Myopia onset
Mutti and colleagues found that an increased AC/A ratio was a predictor of myopia onset and was associated with a greater accommodative lag.136 In a 3-year follow-up study among myopic children, mean accommodation stimulus was significantly lower among the faster progressing myopes (0.3 D) than among the slower progressing myopes (1.5 D).138 AC/A ratios of those individuals who became myopic began to increase approximately 4 years before the diagnosis of myopia was made, continued to increase until the diagnosis was made, but did not affect the rate of eventual myopia progression.136
Myopia progression
Children and young adults with myopia also show reduced accommodative facility and greater accommodative convergence compared with age-matched emmetropic individuals. Accommodative deficits in myopia may be the functional consequences of the anatomy of any equatorial enlargement in the eye.135,139,140 Still, some studies indicate that higher accommodative lag may be predictive of myopia progression in children and adults141,66 whereas others do not.142–144
Although abnormal binocularity might be a risk factor for myopia progression,66,145,146 none of the studies has shown an additional effect on risk assessment compared to refractive error and axial length, genetics, or environmental effects.11
Environment
The weight of scientific research over the last 5 to 6 decades suggests that environmental factors are driving the observed rise in the prevalence of myopia.147,148
Time spent outdoors
To date, the most influential and consistent environmental factor associated to the onset of myopia is more time spent indoors versus outdoors. There are different theories about whether the beneficial effect of time spent outdoors is due to the brightness of light exposure,149,150 to increased short-wavelength exposure (360–400 nm) and/or ultraviolet light exposure,151,152 or to other mechanisms.
Increasing time outdoors is effective in preventing the onset of myopia as well as in slowing the myopic shift in refractive error in non-myopic eyes. But amount of time spent outdoors was not associated with a slowing of the myopic progression in eyes that were already myopic.153 However, the latest review in this topic concluded that outdoor time helps not only to reduce the risk of development of myopia in non-myopic children, but also to slow down the speed of change in refractive error and axial length in myopic children.154 A more recent prospective study suggested that a lower amount of time spent outdoors among Taiwan schoolchildren might be compensated by a higher bright light intensity (10,000 lux) indoors to achieve the same protective effects against development and progression of myopia.155
Near work
Spending more time at school or other near work activities is associated with a higher amount of indoors time.11,156 Several further studies have confirmed these connections. In a 3-year follow-up study more time spent reading and performing close work and less time spent outdoors were both connected with faster myopic progression.138 There is strong evidence of rapid, environmentally induced change in the prevalence of myopia, associated with increased education and urbanisation.102 Based on the landmark studies by Mutti et al.115 and Rose et al.,157 Huang and colleagues found more time spent on near-work activities was associated with a higher odds of becoming myopic, increasing by 2% for every additional 1 diopter-hour more of near work per week.25 In a recent Chinese multivariate logistic analysis the time spent within a working distance of <20 cm was a risk factor for myopia.158
In Europe as compared to East Asia, the prevalence of myopia has remained markedly lower possibly because of differences in the intensity of education from an early age.148,159 Increasing educational achievement associated with a higher prevalence of myopia can be observed not only in Asia, but also in Europe.148 A recent study from Israel showed an increase in the prevalence of myopia which could be associated with urbanization- and higher education-related factors among several subpopulations.160
In a German study, higher levels of school and post-school professional education were associated with a more myopic refraction,161 and a study on discordant monozygotic twins from the United Kingdom (UK) has confirmed known environmental risk factors for myopia, namely higher occupational status, being resident in an urban area, and undertaking more close work.162,104 Previous studies have linked the increase in myopia prevalence with an increasing intensity of the education system, without strong evidence for that it is near work that is the culprit, rather than the fact that an indoor environment lacks visual information necessary for healthy development.163
The Consortium for Refractive Error and Myopia (CREAM) studies, using data from European and Asian participants from different age strata, observed that the overall risk of myopia was significantly affected by the educational level. Time spent performing near work and years of education carried a far greater risk for myopia than genetic factors alone.127,164,165 Overall, it would seem clear that environmental and genetic factors interact which each other.
The mechanism linking education to myopia may be defocus signals in the central and peripheral retina6,18,39–41 and persistent lags of accommodation,22,25–28 which may stimulate axial elongation. A recent alternative hypothesis suggests that the problem may be associated with the use of black text on a white background, which heavily overstimulates retinal OFF pathways.38 White text on black paper leads to an opposite situation, with an overstimulation of ON pathways in the retina. In young humans, the choroid became thinner in only 1 h when subjects read black text on white background but became thicker when they read white text on a black background.38 Previous studies have shown in experimental condition that thinner choroids are associated with myopia development and thicker choroids with myopia inhibition.39,40,42 Therefore, reading white text from a black screen or tablet may inhibit myopia, while conventional black text on white background may stimulate myopia.38
Use of computers and smart phones
Digital devices nowadays constitute a significant form of near work, and correlate with myopia. Some recent studies have documented significant associations between myopia and digital screen time.49,53,121,166,167 However, a recent systematic review found mixed results.168 It has to be taken into account that digital devices may favour indoor lifestyles, and it has remained elusive whether it was a primary or secondary effect. It is also clear that the sharp rise in myopia prevalence was reported before such devices became ubiquitous in childhood. Nonetheless, the increased availability and use of digital screens for both leisure and recreation by very young children may be further promoting myopia onset and progression. Quantitative data relating to screen use and other environmental factors in prospective studies of childhood eye growth and refractive error are needed to fully understand the influence of these ‘essentials’ of modern life on our children’s refractive outcomes.
Location of residency
Both country and location of residency (urban vs rural) of an individual are associated with the likelihood of myopia.
Children from urban environments have higher odds of developing myopia than those from rural environments.50,163 In a Hong Kong study, ocular axial length was found to be significantly longer among those living in areas with a higher population density and in a smaller home as compared to those who were living in a low-population density and larger-size home.169 Living in a flat or room on a lower floor was associated with a lower prevalence of myopia compared to living on a higher floor among school-aged children in China.170
Socioeconomic status
The socioeconomic status (monthly house income, parental education) has been linked to the likelihood of myopia, with varying strengths of association.
A study examining Korean children demonstrated that being in the highest tertial of household monthly income, living in a home owned by parents, living in an urban area, and having a disability were significantly associated with myopia.171 Myopic children were also found to have a stronger parental history of myopia in families with higher parental level of education,47 although parental income and occupation had weaker associations with childhood myopia in a study conducted by Xiang et al.116
Interestingly, in a sample from the Netherlands 6 year old children with myopia were more likely to live with unmarried parents and in a rental home. Families with low income and a low maternal education level showed an increased risk for myopia.52
Interventions for controlling myopia
The main measures that can be taken for the prevention of the development of myopia and for the reduction of the progression of myopia include: (1) Public health (lifestyle) interventions – optimization of environmental influences, (2) Pharmacological approach with the topical application of atropine eye drops, (3) Optical devices including multifocal spectacles and multifocal contact lenses that can have aspheric or discrete dual-focus designs, and orthokeratology.
There is high quality evidence that all methods slow the development or progression of myopia although the efficacy is different for the various interventions.
Optimization of environmental influences
Outdoor activities
Many studies (including randomized clinical trials) highlight the protective role of increased outdoor/sport time on myopia prevention.115,155,157,172–177 In a meta-analysis, every additional hour of outdoor time per week lead to a reduction in the risk of myopia by 2%.178 The chance of becoming myopic is reduced by around one third if time spent outdoors is increased from 0 to 5 h per week to 14 or more hours per week.172,179
The mechanism of increased outdoor time as an intervention for myopia control is not completely clear. Spending time outdoors itself, instead of physical activities outdoors, has been suggested to be the protective factor.150,180 Patterns of defocus on the retina by three-dimensional structures of the environment have also been proposed as a possible mechanism of protection during outdoor activities.6
The protective effect of outdoor activity on myopia development in children seems to be partly mediated by the light-stimulated release of dopamine from the retina, since increased dopamine release appears to inhibit increased axial elongation.179,181 The absence of ultraviolet (UV) light may provoke axial myopia.182 According to Flitcoft et al., compared to the spatial properties of the natural world, man-made (urban) environments and indoor environments have spatial features similar to those than created by diffusing filters that induce form deprivation myopia in animal models.163 The spatial frequency composition of the constructed environment, both indoors and outdoors, is therefore different from the natural world. Enhancing spatial frequency content of the visual scene may help to limit myopia.
Evidence linking time outdoors to the prevention of myopia is stronger than that linking it to slowing the progression of existing myopia.179
Wu et al. have shown that participation in outdoor activities during school recess (10–20 min in both the morning and afternoon) has a significant effect on myopic shift in non-myopic children but not on the myopic progression of children with myopia.175 Confirming the above relationship, another study did not detect an effect of near work or time outdoors on the progression of myopia in those with established myopia.174 However, other studies have shown faster myopia progression during the darker winter than the brighter summer months.183,184
Vitamin D
A number of studies have reported lower levels of serum vitamin D in myopes compared with non-myopes.185–189
Lower 25-hydroxyvitamin D concentration in serum was associated with longer AL and a higher risk of myopia in young children, and the effect was independent of outdoor exposure time. Associations were not different between European and non-European children.185 In another study, total vitamin D and D3 were biomarkers for time spent outdoors, however there was no evidence they were independently associated with future myopia.190
In a study by the CREAM consortium, a Mendelian randomization analysis did not support a direct involvement of vitamin D with myopic refractive error, as individuals genetically predisposed to lower 25(OH)D levels were not more myopic.152
Indoor lighting
In a Chinese study, increasing the light levels from approximately 100 to 500 lux in school classrooms had a significant effect on myopia onset, refraction and axial elongation.149 Another more recent multivariate logistic analysis reported that time spent with a light intensity of >3000 lux was a protective factor for myopia in China.158
Studies are investigating if achieving light levels indoors similar to the outdoor environment can reduce the incidence and progression of myopia.163,191 Torii et al. examined short wavelength violet (360–400 nm wavelength) light which is absent in indoor environments and may play a role in the inhibition of myopia progression.151 They showed that over a 1-year period, children who wore violet light transmitting contact lenses had significantly less axial length elongation compared to those wearing violet light blocking eyeglasses.151
During the last few years, light-emitting diode (LED) lights have been designed as a new generation of task lights instead of traditional light sources. A cross-sectional-study, based in China, determined the association of the types of lamp for homework (including incandescent lamp, fluorescent lamp, and LED lamp) with the prevalence of myopia in young teenagers. Using LED lamps was associated with more myopic refractive error and longer axial length.192
Moreover, the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) recommended avoiding the use of LED light sources emitting cold-white light with a strong blue component in places frequently used by children, to prevent possible photochemical damages and photoreceptors loss. ANSES recommends limitation of the sale of LEDs for domestic use.193
Conclusion environmental influences: Near work indoor and outdoor activity play important roles in the development of myopia and in the prevention of myopia, respectively. There is strong evidence that less near work and more outdoor activity provide protection against myopia development in the human eye.
Time outdoors itself, rather than physical activity outdoors, has been suggested to be the protective factor.150,180 The link between time outdoors in the prevention of myopia is stronger than the link between time outdoors and slowing of the progression of existing myopia.179
Optical
Spectacles
Wearing spectacles is non-invasive and generally well-tolerated.
Undercorrection
Undercorrection of myopia with spectacles has been common practice for many years. The theory is to reduce myopia progression by reducing the accommodative demand during near work. Current evidence suggests this is not beneficial and can be harmful.
An early non-randomized trial from 1965 found that undercorrection slowed the progression of myopia.194 In another study from 2017, over a period of 2 years, 12-year-old Chinese children with no correction had slower myopia progression (diff: 0.29 D) and less axial elongation (0.08 mm) than children with full correction suggesting myopic defocus might act as an inhibitor of eye growth in humans.195
However, other studies examining undercorrection found just the opposite, namely either an increase in myopia progression or significantly more baseline myopia and longer axial length in children with undercorrection than in children with full correction.196–198
A 1-year study of myopic Chinese children, wearing spectacles which either under- or fully corrected their myopia did not show any differences in myopia progression or axial elongation.198
Undercorrection strategies do not provide optimal distance visual acuity and may also lead to behavioural changes, such as a reduction in outdoor activities in some children which, as noted above, may promote myopia progression.199
As also summarized in the recent Cochrane and other systematic reviews, an over-correction or under-correction of the myopic refractive error had no strong evidence of benefits and instead possible risks for myopia progression200–202 and should be avoided.
Peripheral defocus-correcting spectacle lenses
Studies have assessed different types of novel spectacle lens designs aimed at modulating the relative peripheral defocus in Asian children, with no differences in the rate of progression of myopia or axial elongation when compared with single vision (SV) control groups.203 Aspherization of the distance zone added to progressive additional lenses (PALs) did not enhance their therapeutic efficacy in slowing myopia progression.204
Moreover, novel spectacle lens design to reduce peripheral hyperopic defocus was reported to demonstrate a reduction in myopia progression in the younger subgroup of children aged 6 to 12 years with a parental history of myopia, in a 1-year trial.205 However, this beneficial effect was only observed in an exploratory subgroup analysis that had insufficient statistical power to produce definitive results.
More recently, a specially designed ‘competing defocus’ spectacle lens, called Defocus Incorporated Multiple Segments (DIMS) spectacle lens has been used for myopia control in a 2-year randomized trial by Lam et al.206 This lens design has a central optical zone for correcting refractive error and multiple segments of constant myopic defocus (+3.50 D) surrounding the central zone. This enables the lens to provide clear vision and myopic defocus simultaneously for distance, intermediate or near. The results from the clinical trial showed that children of East Asian ethnicity wearing DIMS lenses had 52% less myopia progression (average −0.41 ± 0.06 D in the DIMS group vs. average −0.85 ± 0.08 D in the single vision group) and 62% less axial elongation (mean difference 0.34 ± 0.04 mm) compared with single vision spectacle lenses and about 21.5% of the DIMS lens wearers had no myopia progression during the 2-year long study period while among the controls this was the case in only 6%.206
Bifocal spectacles and progressive additional lenses (PALs)
Bifocals and progressive addition lenses, which allow the wearer to see objects clearly in the distance and at near, have been used in an attempt to retard myopia progression by reducing accommodative effort and lag during extended near work.207 Studies with progressive addition lenses have typically shown a small but clinically insignificant effect on slowing myopia progression200,204,208,209 and two different European clinical treatment trials did not find bifocals to prevent myopia progression.210,211 A meta-analysis noted small reductions in myopia progression (0.25 D) and axial elongation (−0.12 mm).204 This effect was greater for children with a higher level of myopia (<−3.0 D), accommodative lag, or near esophoria.144,207,212–215
Cheng et al. found that, over 3 years, executive bifocal lenses slowed myopia progression by 39% and up to 51% with base-in prisms incorporated in a selected group of fast progressing myopic children when compared with single vision spectacles. For children with low lags of accommodation the prismatic bifocal lenses had a greater benefit.145
Conclusion spectacle lenses: Undercorrection of myopia is not recommended as it increased myopia progression slightly (low-certainty evidence, Cochrane-2020)201 and did not slow myopia progression as previously thought. Bifocal spectacles or progressive addition lenses versus single vision lenses (SVLs) yielded a small effect in slowing myopia progression (moderate-certainty evidence).201 Studies evaluating different peripheral defocus-correcting lenses versus SVLs reported inconsistent results for refractive error and axial length outcomes (low-certainty evidence)201 although results for DIMS spectacles are promising.201
Contact lenses
Soft contact lens (SCL)
There is no substantial evidence in the literature that conventional soft contact lens wear leads to either slower or faster myopia progression than spectacle wear.199,216–218
Rigid gas permeable (RGP)
In some studies, rigid gas permeable lenses were reported to slow myopia progression in children,219–221 but more recent, well-designed studies showed that the use of these lenses did not impact axial elongation and that the apparent control of myopia progression observed with RGPs was most likely induced by temporary corneal flattening.222,223
Bifocal/multifocal soft contact lens (BFSCL/MFSCL)
Bifocal contact lens designs often include a central distance focus, and peripheral rings with near add, creating a peripheral myopic defocus. In these designs, the peripheral region of the lens has relatively more positive power, incorporated a gradual increase toward the periphery (progressive design) or presented in distinct zones (concentric ring design). Concentric ring designs show better control over axial elongation than progressive designs (44.4% vs 31.6%), whereas their effects on refraction changes were similar (36.3% vs 36.4%).199 Studies exploring the effect of these bifocal soft contact lenses224–226 indicate slowing of myopia progression (refraction) by 30–38% and axial length by 31–51% over a period of 24 months.227
In a recent randomized controlled trial, MiSight, a multizone design contact lens produced lower myopia progression (59%) and lower axial growth of the eye (52%) at 3 years compared to spectacle use.228,229
Different studies suggest that bifocal contact lens efficacy may improve with increase in wear time, in children with faster rates of progression,230 near esophoria,146 and with designs possessing a higher hyperopic power in the mid-periphery.207
Soft radial refractive gradient (SRRG) experimental contact lenses increase the higher-order aberrations and relatively decrease the peripheral hyperopia to produce a peripheral myopic defocus. A myopia control study by Paune et al. showed a potential to decrease the accommodation lag, which is a factor in regulation of axial elongation.231
The Cambridge Anti-Myopia Study (CAMS) randomised clinical trial (14–22 years of age participants) used aberration-controlled contact lenses to reduce the lag of accommodation and vision training to improve accommodative facility. The study was unable to demonstrate that the progression of myopia could be reduced over a 2-year period by improving accommodative function.232
The recent 3-year multicenter, randomized clinical BLINK (Bifocal Lenses in Nearsighted Kids) study use contact lenses with a central correction for myopia plus a high add (+2.50 diopters) or medium add (+1.50 diopters) power in peripheral concentric zones. These lenses were compared to conventional single-vision contact lenses. Contact lenses with a high add power slowed myopia progression by 0.45 D and eye growth by 0.23 mm compared with single-vision contact lenses, and slowed myopia progression by 0.29 D and eye growth by 0.16 mm compared with medium add power multifocal contact lenses.233
Orthokeratology (ortho-K)
Orthokeratology lenses are specially designed RGP contact lenses that are worn overnight. The redistribution of corneal epithelial cells temporarily corrects myopia the next day after the removal of the lens.234
Various clinical studies have demonstrated the effectiveness of inhibiting myopic progression with ortho-K (Table 7). The effect of slowing axial length elongation ranges from 30% to 63%. The overall treatment effect is around 50%. Ortho-K also has been shown to induce relative myopic shifts in peripheral refractive errors in all meridians,235 consistent with the most popular hypothesis for this myopia control effect236 although a role for altered higher-order aberrations cannot be excluded.237,238 Another hypothesis of the mechanism behind the myopia control effect of ortho-K is that the changes in lag of accommodation may be due to increasing positive spherical aberration and changes in choroidal thickness.239,240
Table 7.
Myopia control studies using ortho-K lenses.
| Author (year) | Location | Number of participants (OK/control) | Study design | Study duration (years) | Control group | Reduction effect (%) |
|---|---|---|---|---|---|---|
| Cho et al.244 | Hong Kong | 35/35 | Self-selected prospective, early study control | 2 | SV | 46 |
| Walline et al. (2009)245 | USA | 28/28 | Prospective and historical control | 2 | SVCL | 56 |
| Kakita et al.246 | Japan | 42/50 | Self-selected retrospective | 2 | SV | 36 |
| Cho and Cheung247 | Hong Kong | 37/41 | Randomized single-masked | 2 | SV | 43 |
| Hiraoka et al.248 | Japan | 22/21 | Self-selected retrospective | 5 | SV | 30 |
| Santodomingo- Rubido et al.249 | Spain | 31/30 | Self-selected prospective | 2 | SV | 32 |
| Charm and Cho250 | Hong Kong | 20/16 | Randomized single-masked | 2 | SV | 63 |
| Chen et al.251 | Hong Kong | 35/23 | Self-selected prospective toric ortho-K | 2 | SV | 52 |
| Zhu et al.252 | China | 65/63 | Self-selected retrospective | 2 | SV | 51 |
| Na and Yoo253 | Korea | 9/9 | Retrospective, monocular myopia | 2 | CLE | 58 |
SV: single vision spectacle lens; SVCL: single vision contact lens; CLE: contralateral eye.
Several meta-analyses241–243 have confirmed the effectiveness of ortho-K for myopia control, although Si et al.241,244 recommended further research, given that five of the seven studies included in their meta-analysis were from Asia.
In orthokeratology studies, the parameters of older age, earlier onset of myopia, female sex, lower myopia at baseline, longer anterior chamber depth, greater corneal power, more prolate corneal shape, larger iris, and pupil diameters, and lower levels of parental myopia have been linked to slower axial elongation in children.200,247,254–260
Myopia progression in orthokeratology was significantly associated with the peripheral myopization and asymmetric optical changes mostly induced by third-order aberrations.261
In a few studies, early termination of ortho-K treatment has been suggested to lead to an increased rate of axial elongation in children (a rebound effect).262,263 Some studies also suggest that relative treatment efficacy may decrease over time.248,264,265
Overnight use of any contact lens is associated with a higher risk of microbial keratitis (MK) than daily use.266 Practitioners should be aware of this infectious risk because it is an important part of the risk-benefit ratio.267
A 12-month, population-based study estimated the risk of contact lens-related MK.266 The authors identified 285 eligible cases of contact lens-related MK and 1798 controls. For daily wear of rigid gas-permeable contact lenses, the annualized incidence was 1.2 per 10,000 wearers (95% CI = 1.1 to 1.5) and the incidence for overnight wear of soft contact lenses was higher: 19.5 per 10,000 wearers (95% CI = 14.6 to 29.5) for conventional hydrogels and 25.4 per 10,000 wearers (95% CI = 21.2 to 31.5) for silicone hydrogels.266
In comparison, the most in-depth attempts to quantify the risk of MK associated with overnight corneal reshaping (ortho-K) lenses with 2599 patient-years of wear reported the overall estimated incidence of MK, which was 7.7 per 10,000 years of wear. For children, the estimated incidence of MK was 13.9 per 10,000 patient-years and for adults the estimated incidence of MK is 0 per 10,000 patient-years.267
A systematic review, which analysed clinical studies from 1980 to 2015, incorporated a total of 170 publications, summarized the most common complication of ortho-K treatment, which was corneal staining. Other clinically significant side effects included epithelial iron deposit, prominent fibrillary lines and transient changes of corneal biomechanical properties, but no long-term effect on corneal endothelium. Evidences suggest that ortho-K is a safe option for myopia retardation and the risk of microbial keratitis was similar to other overnight modalities (194,183,308).255,265,267 In another meta-analysis, the dropout rate in ortho-K studies was found to be between 6.7 and 30.0%, similar as in the controls at 2-year follow-up.268
Future research for contact lens design
A recent randomized clinical trial has reported 2-year results of novel contact lenses that either imposed myopic defocus at the retina or modulated retinal image quality.269 The first design principle aimed to reduce hyperopic defocus and induce myopic defocus across a large portion of the retina. The second design principle used extended depth of focus contact lenses that were designed to result in a global retinal image quality, which was improved for points on and anterior, and degraded for points posterior to the retina to prevent axial elongation.
At 2 years, the new lenses slowed myopia progression by 32% and 26% and reduced axial length elongation by 25% and 27%, respectively. Thus, these lens types resulted in slower eye growth compared to use of conventional, single vision contact lenses.269
Conclusion contact lenses
Ineffective: Rigid gas permeable contact lenses showed inconsistent results in myopia progression (very low-certainty evidence).201 Comparing spherical aberration SCLs with single vision SCLs reported no difference in myopia (refractive) progression nor in axial length elongation (low-certainty evidence).201
Effective: Axial elongation was slightly less for bifocal SCL wearers than for single vision SCL wearers (low-certainty evidence).201 Orthokeratology contact lenses were more effective than SVLs in slowing axial elongation (moderate-certainty evidence).201 There is evidence of myopia control with soft multifocal contact lenses (low-certainty evidence),201 specific myopia control soft lens designs (moderate-certainty evidence)201 and orthokeratology (moderate-certainty evidence).201
Auditory biofeedback training
Current investigations demonstrated the efficacy of auditory biofeedback training to improve the accommodation response in myopic young adults. The training may cause a reduction of the accommodative lag, which can lead to a slowdown of myopia progression,270 and may enhance the therapeutic effect of multifocal contact lenses in myopia control.271
Pharmacological
Atropine
Atropine is a nonselective muscarinic receptor antagonist. Atropine is reported to stimulate extracellular matrix (ECM) biosynthesis in scleral fibroblast cells, thus thickening the scleral tissue and reducing its elasticity and tendency to elongation. In addition, atropine may decrease ECM biosynthesis in other tissues such as choroidal fibroblasts thus improving scleral blood perfusion through the choroid, due to a higher permeability of its ECM and slowing down myopia progression.272
There is also evidence from studies on chickens for atropine to increase the release of the neurotransmitter dopamine into the extracellular space and the vitreous, which may cancel out a presumed retinal signal that controls eye growth and through this, myopia.273 Furthermore, it has been shown that dopamine could act directly on the cornea, as some dopaminergic receptor activity is located in rabbit and bovine corneas.274,275 Thus, the primary site of action of atropine is controversial; some authors have even hypothesized that 0.01% atropine may primarily act on the cornea.276
Atropine has been reported to have a dose dependent inhibitory effect on myopia progression. The initial use of high doses of atropine (0.5%, 1.0%) slowed myopia progression by more than 75% over 2 years with essentially no change in mean axial length in the atropine-treated eyes compared to the placebo-treated eyes and the untreated fellow eyes in both atropine and placebo groups.277 Lower doses (0.1%, and 0.01%) can also slow myopia by up to 67% and have fewer side effects.243,277–279
Data from the Atropine in the Treatment of Myopia (ATOM) two study showed that after a 1-year washout, there was a myopic rebound when atropine was stopped, especially for higher doses and in younger children.280,281 After 36 months, treatment with 0.01% atropine showed the slowest progression of myopia,278 and over 5 years, 0.01% atropine eyedrops were more effective in slowing myopia progression with less visual side effects compared with higher doses of atropine.282
Nevertheless, in recent studies examining the rate of axial elongation, 0.01% atropine had minimal benefit.283,284
These conflicting study results above are examples of conflicting evidence which seems to depend upon whether axial length or refractive change are used as outcome measures.
Brennan et al, examined the apparent discrepancy in refractive error change and axial elongation in studies and concluded that the relation between the two is confounded by use of atropine.285 To compare subjects from studies wearing spectacles alone and studies where atropine was used, utilizing best-fit slopes the two lines differ substantially with the slope for untreated spectacle wearers being −2.05 D/mm and that for studies using atropine being −0.83 D/mm. They felt their observation could result from the fact that atropine produces changes in the anterior optical structures of the eye or leads to an extreme cycloplegia in treated eyes thereby producing apparent reductions in refractive progression in the absence of corresponding reduction in axial elongation.285
In the Low-Concentration Atropine for Myopia Progression (LAMP) study involving children treated with concentrations of 0.01%, 0.025%, and 0.05% atropine for a 1 year, there was a reduction of spherical equivalent (SE) progression of 27%, 43%, and 67%, and a slowing of axial length growth of 12%, 29%, and 51%, respectively. Overall, the effect on spherical equivalent refraction was larger than that on axial length.279
In the LAMP study, compared with the first year of follow-up, the second-year efficacy of 0.05% atropine eye drops and 0.025% atropine eye drops remained similar (p > 0.1) and improved slightly in the 0.01% atropine group (p = 0.04). In the LAMP-II Study, the efficacy of 0.05% atropine eye drops was double that of the 0.01% eye drops with respect to the reduction of myopic progression, and therefore the 0.05% atropine concentration was considered by the authors to be the optimal concentration among the studied atropine concentrations for slowing the progression of myopia.286
Around 10% of children show a fast rate of myopia progression even on high-dose atropine. The studies performed to date cannot distinguish if this indicates that certain children respond less well to atropine than others, or if there is a limit to how much of a reduction in progression can be achieved. A poorer response was associated with younger age, a higher degree of myopia at baseline, starting spectacle wear at a younger age and a history of parental myopia.282,287,288
A recent study in school children tested a novel 1% atropine treatment regimen in which one eye was treated at one time point and the other eye at another time point (one eye received treatment at day 1, the other eye received treatment at day 16) achieving a frequency of once a month in the first 2 years. Gradual withdrawal of the atropine to once every 2 months for 12 months, followed by no drops for 12 months, could effectively retard the progression of moderate myopia with a significant reduction in myopic rebound, while minimizing the side effects.289
Atropine has been shown to be effective in treating myopia in Europe suggesting that intervention with atropine could work irrespective of ethnicity.290–295
Primary ocular side effects of topical atropine are due to the inhibitory actions of atropine on the iris sphincter and ciliary muscles, resulting in mydriasis, photophobia and reduced accommodation, with symptoms of glare and blur at near. Prescription of photochromatic and progressive spectacles may help. A report from the Erasmus Medical Center in Rotterdam, the Netherlands, has shown that in a real world setting, 72% of children stayed on 0.5% atropine therapy for 3 years, despite the side effects.295 More severe topical reactions such as allergic keratoconjunctivitis and lid erythema and rashes may occur277,278,296,297 and could lead to discontinuation of the eye drops. Other possible side effects include dry skin, mouth, and throat, drowsiness, restlessness, irritability, delirium, tachycardia, and flushing of the face or neck.199,298 Nonetheless, in two of the largest clinical trials of topical atropine, the ATOM1 and ATOM2 studies, none of the reported serious adverse events were thought to be associated with atropine and there have been no significant adverse systemic side effects.277,278 No differences in the incidence of adverse effects between Asian and White patients were identified.297
Pirenzepine
Pirenzepine is an M1 muscarinic receptor antagonist. In a 12-month study in an Asian population, 2% pirenzepine gel applied locally to the eye twice daily reduced myopia progression by 44% and axial elongation by 39% compared with the control group; adverse events were observed in 11%.299
Another 2-year, double masked, placebo controlled parallel trial with 2% pirenzepine from the USA yielded a 41% reduction in myopia progression with 2% pirenzepine compared to the placebo treatment, however, the difference in axial elongation between the groups did not reach statistical significance.300 As with atropine, the antimuscarinic properties of pirenzepine may lead to blurred near vision, sensitivity to light, some discomfort and itching, and medication residue on the eyelids or eyelashes. Some children may develop small nodules or bumps under the eyelid.201,224,300
At this point in time, pirenzepine is not available as a treatment option for myopia control.199
Seven-methylxantine (7-MX)
Oral 7-MX is an adenosine antagonist and a metabolite of caffeine and theobromine.
Recently, 7-MX has been shown to reduce the axial myopia produced by the hyperopic defocus in rhesus monkey and augmented hyperopic shifts in response to myopic defocus.301
In a pilot study from Denmark, systemic treatment with 7-MX appeared to be efficient in retarding axial elongation and myopia progression among myopic children with relatively few adverse effects. At 24 months, axial elongation was reduced by 0.1 mm and refractive error by 0.22 D in the 7-MX group compared to the placebo group. The drug appears to be safe and without side effects.302 Thus, it provides consolidated basis for further investigation to develop it into a drug for clinical use.303
Intraocular pressure (IOP) lowering eyedrops
Timolol
Timolol is a relatively nonselective beta-adrenergic antagonist. Jensen looked at the effect of 0.25% timolol maleate eyedrops used twice a day in a 2-year study.210 This was compared with bifocal spectacles and SV spectacles. There was no evidence to suggest that timolol reduced the rate of myopia progression.210
Latanoprost
In an analysis by El-Nimri et al., the efficacy of topical latanoprost was examined as a representative prostaglandin analog for controlling myopia progression in a form-deprived guinea pig model of myopia.304 The results showed that topically applied latanoprost was effective in both lowering IOP and slowing myopia progression in that model.304
Alpha 2-adrenergic agonists
A recent study reported evidence that form-deprivation myopia could be inhibited by high concentrations of brimonidine, clonidine, and guanfacine in the chick. The data suggested that α-adrenoceptors are valid target receptors for anti-myopia therapies.305
Future research for antimyopia drug development
The latest research focuses on the recent advances in genome-wide studies of the signaling pathways underlying myopia development and discusses the potential of systems genetics and pharmacogenomic approaches for the development of antimyopia drugs.306
Conclusion pharmaceutical agents: Antimuscarinic eye drugs such as atropine eye drop or pirenzepine eye gel may slow the progression of myopia (moderate-certainty evidence).201 Axial elongation was lower for children treated with atropine than for those treated with placebo (moderate-certainty evidence)201 in studies using higher doses. However, there is a weaker association between refractive error and axial length changes than optical studies. According to Cochrane summary, systematic seven-methylxanthine had a small effect on myopic progression and axial elongation compared with placebo in one study (moderate-certainty evidence).201 One study did not find slowed myopia progression when comparing timolol eye drops with no drops (low-certainty evidence).201
Surgical interventions
Posterior scleral reinforcement (PSR)/contraction (PSC)
PSR is a surgical approach modifying the sclera remodeling causing direct mechanical reinforcement of the eyeball wall, to slow down myopia progression and prevent the formation of a staphyloma.307 PSR involves surgical implantation under general anaesthesia. A variety of materials having been used, ranging from fascia lata, as well as lyophilized dura, strips of tendon, aorta, and donor sclera.199
Several studies have shown that PSR can effectively limit the progression of axial elongation in highly myopic children with varying efficiency.308–312
The non-crosslinked material has limited efficacy in preventing sclera from expanding into high myopia. A new surgical technique uses sclera treated by genipin (a natural crosslinker) to increase its strength in order to enhance AL shortening; this technique is referred to as posterior scleral contraction. Genipin has emerged as a safer choice as a crosslinking agent due to its stability, biocompatibility, and general safety.313,314
Based on a recent study examining 26 clinical trials, postoperative complications of PSR are mainly ocular hypertension, conjunctival tissue oedema, vitreous haemorrhage, retinal, or choroidal haemorrhage, diplopia or eye movement disorder, retinal detachment, and optic atrophy. Reinforcement material expulsion, symblepharon, and choroidal effusions may also occur. Intraoperative complications may include injury of vortex vein and penetration of sclera. However, the common complications were transient.307
Currently, PSR for high myopia is mainly performed in Russia, Eastern Europe, and China, although there are also publications from the United States310 and a case report on complications from Australia.315
The use and safety of PSR is controversial, and more studies are needed to confirm its therapeutic benefits.307
Injection-based scleral strengthening (SSI)
SSI involves the injection under Tenon’s capsule of chemical reagents intended to biomechanically stabilize the extracellular (collagen) matrix of the sclera.
According to Golychev et al.316 myopia was reported to stabilize with this method in 61% of cases after a follow up period of approximately 2 years.
In a study from Russia, a polymer gel containing a mixture of polyvinylpyrrolidone, acrilamidehydrazide, and ethylacrylate was delivered monocularly by a sub-Tenon’s capsule injection. Refractions are reported to have remained stable in 79.6% of eyes 1 year after the SSI intervention, and in 52.9% cases, after 4 to 9 years.317
Another approach is the intravitreal injection of Aquaporin-1 (AQP-1), which is a membrane-locating protein that contributes to the water transmembrane transportation leading to a thicker choroid. A thicker choroid will impede the progression of axial length through modulating the expression of sclera-related growth factor and scleral fibril synthesis. On this topic there are only animal experiments.318
In recent years, scientists have also proposed the concept of subscleral injection of mesenchymal stem cells and dopamine injection representing a promising new strategy against the progression of myopia.319
Collagen cross-linking (CCL)
CCL is used worldwide for corneal tissue strengthening by using riboflavin as a photosensitizer and ultraviolet A (UVA) to increase the formation of intra- and interfibrillar covalent bonds by photosensitized oxidation, mainly in keratoconus patients. The use of this approach for stabilizing the sclera in pathological myopia has to-date been limited to experimental animals (rabbit models).320,321
When using CCL treatment for myopia control in animal models, histologically serious side effects were found in the entire posterior globe with almost complete loss of the photoreceptors, the outer nuclear layer and the retinal pigment epithelium.199,321,322
An alternative approach is non-enzymatic glycation using a sugar molecule, such as ribose or glucose, without the controlling action of an enzyme. Carbohydrate-based collagen crosslinking is advantageous because it requires a less invasive application procedure, does not use UVA, and reduces scleral toxicity since it does not require UV exposure.323
Conclusion surgical interventions: Because of the invasive nature and lack of large randomized trials, surgical interventions should not be recommended as first line treatment modalities for the prevention of the progression of myopia, neither for moderate myopia nor for high myopia.
Combination of interventions
To improve the efficacy of therapies against myopia progression, the combined effects of two or more interventions have been evaluated. Leshno et al. collected data from paediatric ophthalmologists related to the choice of treatment modalities (pharmacological, optical and behavioural) to slow down the progression of myopia according to geographical regions. Most respondents used a combination of either two (38%) or three modalities of treatment (56%); behavioural treatment was used by the highest number of respondents (92%). A combination of all three modalities was the most popular in most regions, apart from Central-Asia where the prevalence of optical and behavioural combinations was higher.324
Currently, numerous publications provide the evidence that combined treatment with atropine and ortho-K lenses provides an additive benefit in myopia control.325–328 In a preliminary study, during a 1-year follow-up, the combination of ortho-K and atropine 0.01% ophthalmic solution was more effective in slowing axial elongation than ortho-K monotherapy in 8–12 years old children. The increase in axial length over 1 year was 0.09 ± 0.12 mm in the combination group and 0.19 ± 0.15 mm in the ortho-K monotherapy group.325
Atropine and ortho-K seem to slow the progression of myopia through different mechanisms.325 Atropine-induced pupil dilation increase retinal illumination and may expose more of the retinal periphery to relative myopic defocus, potentially enhancing the effect of the ortho-K lens to slow axial growth.328 A recent study showed an additive effect between 0.01% atropine and ortho-K over 1 year, with mean axial elongation in the atropin with orthokeratology group 0.09 mm/year slower than that in the ortho-K group.329 A review reported that all included studies improved myopia control by the synergistic effect of ortho-K with low-dose atropine, compared with orthokeratology treatment alone.330
Another pharmacological and optical combination therapy is the use of multifocal spectacles with 0.5% atropine. The combination treatment was found to slow the progression of myopia significantly more than each treatment alone.331
The Bifocal & Atropine in Myopia (BAM) study, which started in 2017, is designed to investigate whether 0.01% atropine and +2.50-diopter add canter-distance soft bifocal contact lenses (SBCL) slows myopia progression more than SBCL alone. The study completion date was June 2020 and therefore the findings should be available soon.332
In a recent study, several daily disposable and multifocal contact lenses were investigated for their potential to release two anti-myopia drugs. All lenses showed some degree of drug release in an uncontrolled manner. A contact lens-based drug delivery system is an option worthy of further evaluation.333
Conclusion combination therapies: Myopia progression was slower in children treated with combinations of atropine eye drops and multifocal spectacles than in children treated with placebo eye drops and single vision lenses (moderate-certainty evidence).201 Orthokeratology with low-dose atropine improved myopia control by the synergistic effect compared with orthokeratology treatment alone. Further studies are needed to fully assess the efficacy and safety of atropine and orthokeratology or bi- or multifocal soft contact lens combination therapy.
Guidelines for clinical management and control of myopia in children
Management of premyopes
Having one or two myopic parents or family members increases the risk and progression rates of myopia.10,115,116,118–120 There is a positive correlation between the number of myopic parents and the risk of developing myopia.117 Environmental factors such as excessive near work/indoor time and insufficient outdoor exposure are factors driving the recent epidemic rise in the prevalence of myopia.147,148 It draws attention to the fact that these children need to be observed more closely.
To determine the first refraction in a child, cycloplegia must be used.334 Lack of cycloplegia in refractive error measurement increases the risk of misclassification for both myopia and hyperopia335 and makes application of an evidence-based approach to myopia management challenging; research studies from which evidence-based practices are derived have primarily used cycloplegic methods to define refractive error.
The presence of +0.75 D or less of hyperopia at the age of 6 years indicates that myopia is likely to develop in the near future.334,336,338 In prospective data from White European children, McCullough et al. demonstrated that children presenting with a refractive error of <+0.63 D at 6–7 years and with at least one myopic parent were likely to develop myopia by age 13 years and those with no myopic parents were likely to develop myopia by 16 years.338
Other work suggests that premyopes may also show specific binocular vision disorders.136 Since the visual profile of the myopic child is characterized by higher accommodative lag,26,27 high AC/A ratio (esophoria at near)135,139 and reduced accommodative flexibility,26,27 it would be important to include tests that evaluate the binocular vision and not only refraction. More attention needs to be paid to children who have a strong family history of myopia and the management of binocular vision disorders is recommended.
The visual complications of myopia are strongly related to axial length growth, thereby monitoring the axial length changes should be primary target for myopia management. Where axial biometry measures are available, these can also be informative in identifying children at risk for myopia who should be provided behavioural advice and monitored closely for the onset of myopia in order that anti-myopia therapies can be applied.93,337,338
Percentile growth curves for White European children are available for axial length in childhood and can be used to identify patients whose eye size puts them at increased risk for future myopia.93,337,338 In addition to identifying risk for future myopia, these centiles can be used after the onset of myopia to monitor growth trajectories with/without anti-myopia intervention.
According to Tideman and Klaver, axial length does not have a stable growth rate with age, nor is it similar among the sexes and ethnicities. Their studies generated axial length growth curves as a function of age based on data from children with European ethnicity. These curves (boys/girls) give information to estimate the risk of developing high myopia in adulthood.93,337
Data from McCullough et al. demonstrate that axial lengths greater than 23.07 mm at 6–7 years are associated with a strong risk of future myopia.338
Based on these findings, it is suggested to screen children before the age of 6 years or in the first school year for family history of myopia, time spent outdoors, time performing close activities (like, cell phone or tablet use, playing with toys, handwork, reading, drawing, etc.), and binocular vision.
Children with higher risk should be encouraged to spend more time outdoors as the key evidence-based strategy that appears effective in reducing the incidence of myopia.339
Selection of myopia control methods
Based on the child’s individual and parental factors, it should be possible to offer a strategy against myopia progression. Decision to treat should be based on age of onset and axial length or refraction at a given age.285 If there is a suspicion of any underlying ocular disease, additional examinations are recommended (corneal topography, electrophysiology, retinal imaging, or genetic testing).337
Lifestyle advices
Indoor and near work activity
Excessive near work may influence the development and progression of myopia.11,100,340 Close reading distance (20–25 cm) and continuous reading (>45 min), head tilt, closer nib-to-fingertip distance (which means greater head tilt) have been associated with greater odds of myopia progression.158,340–342
In a 23-year follow-up study of Pärssinen, myopic progression was highest among those whose reading posture in childhood was sitting and lowest among those who reported reading in suppine position.343 Short reading distance in childhood predicted higher adulthood myopia among females. Time spent on reading and close work in childhood was related to myopic progression during the first 3 years but did not predict adulthood myopia.86
Children should not be prevented from participating in near work activity, but attention should be given to the following measures: regular breaks, appropriate reading distances without head tilt, and near-to-distance fixation changes while reading with sufficient outdoor activities.339 The Erasmus Myopia Research Group in the Netherlands recommends complete absence of close-up screen use for children up to 2 years old; maximum 1 h day, for children up to 5 years, and a maximum of 2 h day for children aged 5–12 years.179,337,344
Time spent outdoors and lighting
Spending time outdoors without requiring physical activity or direct sunlight exposure appears to have a protective effect against myopia onset but not for myopic progression.86,153,339
Every additional 1-h of outdoor time per week is associated with a reduction in the risk of myopia by 2%.178 The chance of becoming myopic is reduced by around one third if time spent outdoors is increased from 0 to 5 h per week to 14 or more per week.172,179
A minimum of 8 to 15 h of outdoor activity per week is recommended for school aged children to achieve clinically meaningful protection from myopiagenic stimuli.157,172,174,176,178,179,345 Individuals who are at risk of developing myopia should try to maximise natural lighting and to increase time spent outdoors.149,150,158,339 To maximise indoor lighting149,150,339 use incandescent light bulbs rather than fluorescent or LED lighting.192,334
Nutritional advice
Nowadays a lot of health issues may be connected to nutritional habits. Therefore, parents may enquire whether a change in dietary habits could decrease the probability of eye diseases.
In Chinese schoolchildren higher saturated fat and cholesterol intake were associated with longer axial length.346 As noted above, treatment with caffeine metabolite seven-methylxanthine has small effect on eye growth in children.302
Although caffeine-like stimulants may be part of nutritional advice for myopes in the future, there is no current high-level evidence to support nutritional treatments for myopia control.339
Refractive corrections
Spectacle lenses are non-invasive, simple, and affordable technique for optical correction of refractive errors, such as myopia.
Children should be encouraged to wear their myopic correction full time, as undercorrection of myopia has been shown in some studies to increase myopia progression.196,197,198
Decreasing full distance myopic refractive error correction during near work will reduce accommodative demand and accommodative lag.339 However, in a 3-year randomized controlled clinical trial mildly myopic school children aged 9–11 years showed significantly less myopia progression when they wore full correction continuously than wearing spectacles only for distant vision. Neither the use of bifocals nor avoiding the use of spectacles in reading slows myopia progression.211 Spectacle with peripheral defocus designs such as the DIMS lenses206 should be considered over SV lenses in progressing myopes.
Contact lenses play an important role in myopia control. This includes ortho-K and regulatory approved soft contact lenses for myopia control, and studies are ongoing comparing the effect on myopia control of various recently developed contact lens types. Based on a meta-analysis, ortho-k and soft lenses for myopia control offer similar levels of axial length control.243
A recent report of American Academy of Ophthalmology concluded that ortho-K may be effective in slowing myopic progression for children and adolescents, however, safety remains a concern because of the risk of potentially blinding microbial keratitis from contact lens wear.347
Customizing ortho-K lens designs to limit the central treatment zone may help to bring more plus power inside the pupil and achieve a greater shift in relative peripheral myopia.348–350 However, such approaches need to be evaluating in randomized controlled trials.
If customization of the ortho-K lenses is not possible, soft multifocal lenses are preferable for any patient who has less than 2.00 D of refractive error. Also, patients who have a photopic pupil size smaller than 4.5 mm will be better served by a soft multifocal lens with a design that is independent of pupil size. Using soft multifocal contact lenses, the highest plus power that does not generate blur at distance without over-minusing the original cycloplegic refractive power, was recommended by a recent myopia control summary.334
For orthokeratology lens wear should be encouraged every night for a minimum of 8 h per night to maximize correction for best-unaided vision during waking hours.339
The treatment effect of multifocal soft contact lens (MFSCL) is likely to be positively correlated with wearing time.230 Full time use of MFSCLs is recommended during school hours and for schoolwork at home, providing greater myopia control efficacy.339 Preferably, regulatory approved myopia controlling new designs, bifocal, progressive additional lenses (PAL), or single-vision spectacles may be prescribed for when children are not wearing their contact lenses.339
Another possibility is to add spectacles to supplement contact lens wear when accommodation is deficient.334
Children, who are intolerant of contact lenses or showing high exophoria at near, could be prescribed (prismatic) bifocal or anti-myopia spectacles. Fast progressors may not be treated sufficiently with low-add-power lenses, particularly in case of accommodative dysfunction. Spectacle lenses are also the first option of care in very young children (who are unable to wear contact lenses due to access or cost), any situations that associated with poor hygiene conditions, or if children grew up in locations with no or only limited access specialized eye care.351
Atropine therapy
A report from American Academy of Ophthalmology concluded that the use of atropine to prevent myopic progression is supported by Level I evidence.352 The World Society of Pediatric Ophthalmology and Strabismus in its Myopia Consesnus Statement argued that atropine 0.01% appears to offer an appropriate risk-benefit ratio, with no clinically significant visual side effects balanced against a reasonable and clinically significant 50% reduction in myopia progression (https://www.wspos.org/wspos-myopia-consensus-statement/ accessed 24 November 2020).
In a recent protocol developed by Chia and Tay,353 children are first started on a lower dose of atropine with a plan to increase the dose as necessary. Once medication is started, progression (in terms of refraction and axial length) should be monitored every 6 months, for at least 2 years. Based on the protocol from the Netherland,93,337 axial length and gender-specific growth curve charts are used to evaluate the risk of myopipa/high myopia. Children with risk of myopia at the 75th percentile or above are then started on atropine 0.5% eye drops.337
The ATOM 2 study showed that 0.01% atropine resulted in a 60% risk of a refractive error rebound effect in children aged 8–10 years, compared to 30% at age 10–12 years and 8% after the age of 12 years.281 The change in spherical equivalent was greater than the change in axial length and not directly associated with the change in axial length alone.281 This suggests that in children younger than 12 years who showed no progression in the past year, atropine 0.01% may be slowly tapered by reducing drop frequency (by 1–2 days/week each year). However, if children are older than 12 years, then the frequency of eye drops could be tapered more quickly (by 1–2 days/week every 6 months). Using this regime, most children will be off medication by about 14–15 years of age.353
The Low-Concentration Atropine for Myopia Progression (LAMP) study recommends the use of 0.05% atropine rather than 0.01%, as the lower concentration allowed unacceptable levels of axial length progression.279
In children who progress on low-dose atropine, the frequency of application, or dose could be increased (using atropine 0.01% twice a day; or using a higher concentration, 0.05%, 0.1%, 0.5%, or 1%). Increasing the dose of atropine needs to be balanced against side effects of loss of accommodation and glare/aberrations from large pupil. Once an adequate control of myopia is achieved, medication can be continued till the child reaches teenage years and then tapered as required. There are some children (11%), however, who may progress rapidly even on 0.5% atropine.331 If this occurs, then the possibility of stopping treatment or trying other treatment modalities should be discussed. Even after stopping treatment, it may be necessary to monitor children for a further 6–12 months to ensure that there is no further rebound.353
Patients undergoing atropine therapy will require distance refractive error correction. It is recommended that patients be prescribed their full distance refractive correction; however, patients may require near addition correction to alleviate near visual symptoms and photochromic lenses or additional sunglasses to relieve glare issues if necessary.339
Combination therapy in practice
For patients using mono-therapy in the form of atropine or ortho-K and who still experience progression of myopia and axial elongation at a faster rate than expected, combination therapy should be considered. Ortho-K with low-dose atropine improved myopia control by the synergistic effect compared with orthokeratology treatment alone, presumably because it increases the pupil area and, therefore, allows more plus power to reach the peripheral retina.328,330,334 The effectiveness and side effects of combination therapy with atropine and soft myopia control contact lens is unknown, and should be first evaluated in prospective clinical trials before being used in clinical practice.
Treatment duration
Axial length is the most important metric to monitor in pre-myopic and myopic children.93,285,334,337,338
Myopia generally progresses most rapidly during preteenager years (7–12 years), subsequently slowing through adolescence and adulthood.67,91,354 The mean age of myopia stabilization is around 15.6 years of age, and 95% of myopes stabilize by age of 24 years.83
There are some publications of myopia onset and progression at a later age among specific occupational groups, during university education courses such as medicine, law or engineering.355–357
The efficacy of some treatments may wane after the first 6 months to 2 years of treatment.248,285,358–360 There is insufficient evidence that faster progressors, or younger myopes, derive greater benefit from treatment.285 The same treatments and protocols as applied in childhood may be applicable in later-onset myopia, although the available evidence is limited.339
In case of atropine treatment parents and patients should be made aware that myopia progression may accelerate after stopping higher-dose atropine usage, but despite this rebound effect, the level of myopia post-treatment will be less than it would have been without treatment.280,282 The long-term use of atropine should only be undertaken with caution as long-term side effects have not been evaluated.339 It may be beneficial to tail off dosage or dose frequency at the end of treatment to minimize rebound effects.
Although the results of the ATOM studies point to some loss of treatment efficacy with time, at least with the higher concentrations of atropine, a study by Wu and colleagues which involved concentrations between 0.05% and 0.1%, suggested that treatment effects with low-dose atropine can be maintained for up to 4.5 years.287
Discontinuation of ortho-K lens wear before age 14 has been shown to lead to a more rapid increase in axial length over a 7-month period, faster than concurrent single vision spectacle wearing controls; however, this slows again with resumed lens wear after another 6 months. This suggests that ortho-K wear should not be discontinued before age 14.262
Long-term use of soft myopia control contact lens and ortho-K is not contraindicated if ocular health is maintained through regular aftercares and strong compliance.264,339,361 Progressive additional lenses can also be used for vision correction, but the long-term, clinically meaningful myopia control effect of such lenses is small in comparison with contact lens corrections, except in specific populations.145,214 Bifocal spectacle lenses might be a good solution for longevity treatment.362,363 A study of children wearing progressive addition lenses for 1 year, then switched to single vision glasses for 1 year showed no rebound.143 No rebound effect was reported with soft contact lens for myopia control.364
Compliance and safety issues may require a change in treatment modality or a halting of treatment. Poor tolerance of visual side effects may also prompt cessation or change of myopia control therapy.339
In conclusion, outdoor time is the most promising intervention method. There is consistent evidence of a benefit of slowing myopia development by the use of atropine eye drops, while the optimum concentration of atropine and the value of a combined use of atropine eye drops with optical devices are yet to be fully explored. There is also evidence of myopia control with soft multifocal contact lenses, orthokeratology, and new types of multifocal spectacle lenses. Information is constantly evolving, so it is important to stay abreast of studies published in the peer-reviewed literature.
Acknowledgments
The authors would like to thank Monica Jong, Executive Manager of the International Myopia Institute for the arrangement of the cooperation and the financial support for open access and Jaakko Kaprio for reviewing the genetic aspects of the manuscript.
Footnotes
Abbreviations: 7-MX: Seven-methylxantine
AC/A: accommodation-convergence/accommodation ratio
ANSES: Agency for Food, Environmental and Occupational Health and Safety
AL: axial length
AQP-1: Aquaporin-1
ATOM: Atropine in the Treatment of Myopia
BAM: Bifocal & Atropine in Myopia
BFSCL: bifocal soft contact lens
BMI: Body Mass Index
BLINK: Bifocal Lenses In Nearsighted Kids
CAMS: Cambridge Anti-Myopia Study
CCL: collagen cross-linking
CI: confidence interval
CLE: contralateral eye
COMET: Correction of Myopia Evaluation Trial
CREAM: Consortium for Refractive Error and Myopia
D: diopter
DIMS: Defocus Incorporated Multiple Segments
ECM: extracellular matrix
HOA: higher-order aberration
IMI: International Myopia Institute
IOP: intraocular pressure
IQ: intelligence quotient
LAMP: Low-Concentration Atropine for Myopia Progression
LED: light-emitting diode
MFSCL: multifocal soft contact lens
MK: microbial keratitis
OH: hydroxy
OK: orthokeratology
Ortho-K: orthokeratology
PAL: progressive additional lens
PSC: posterior scleral contraction
PSR: posterior scleral reinforcement
RGP: rigid gas permeable lens
RPE: retinal pigment epithelium
SBCL: soft bifocal contact lenses
SCL: soft contact lens
SCORM: Cohort Study of the Risk Factors for Myopia
SE: spherical equivalent
SRRG: soft radial refractive gradient
SSI: Injection-based scleral strengthening
SV: single vision
SVCL: single vision contact lens
SVL: single vision lens
UK: United Kingdom
USA: United States of America
UV: ultraviolet
UVA: ultraviolet A
WHO: World Health Organization
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JBJ discloses patent application: “Agents for use in the therapeutic or prophylactic treatment of myopia or hyperopia” (European patent application 16 720 043.5 and US patent application US 2019 0085065 A1). AG reports grants from Alcon, Zeiss, Topcon, Bausch, personal fees, and non-financial support from Santen, Thea, Polpharma, and Pfizer, outside the submitted work. JAG is unpaid Consultant for CooperVision Inc. KJS is funded by Nevakar Inc as part of their EU/US low dose atropine trial. JRP is consultant for Nevakar. JSW is on the advisory board of Alcon, Nevakar, and Novars. SW is employee of Carl Zeiss Vision International GmbH. The other authors declared no potential conflicts of interest with respect to the authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AG is funded by Institute for Research in Ophthalmology (Number: Unrestricted Grant 1/2020). JAG is funded by Welsh Government and Fight for Sight (Number: 24WG201). The author(s) disclosed receipt of the following financial support for the online publication of this article: The open access fee for this publication is paid by the International Myopia Institute (IMI).
ORCID iDs: János Németh  https://orcid.org/0000-0001-8575-4888
https://orcid.org/0000-0001-8575-4888
Ingrida Januleviciene  https://orcid.org/0000-0002-2660-7922
https://orcid.org/0000-0002-2660-7922
Kathryn J Saunders  https://orcid.org/0000-0002-9289-5731
https://orcid.org/0000-0002-9289-5731
Annechien EG Haarman  https://orcid.org/0000-0002-1452-5700
https://orcid.org/0000-0002-1452-5700
Siegfried Wahl  https://orcid.org/0000-0003-3437-6711
https://orcid.org/0000-0003-3437-6711
Serge Resnikoff  https://orcid.org/0000-0002-5866-4446
https://orcid.org/0000-0002-5866-4446
References
- 1.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 2.Morgan IG, Ohno-Matsui K, Saw S-M.Myopia. Lancet 2012; 379: 1739–1748. [DOI] [PubMed] [Google Scholar]
- 3.Resnikoff S, Jonas JB, Friedman D, et al. Myopia- a 21st century public health issue. Invest Ophthalmol Vis Sci 2019; 60: Mi–Mii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fricke T, Holden B, Wilson D, et al. Global cost of correcting vision impairment from uncorrected refractive error. Bull World Health Org 2012; 90: 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu L, Sun X, Zhou X, et al. Causes and 3-year-incidence of blindness in Jing-An District, Shanghai, China 2001–2009. BMC Ophthalmol 2011; 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flitcroft DI.The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res 2012; 31: 622–660. [DOI] [PubMed] [Google Scholar]
- 7.Haarman AEG, Enthoven CA, Tideman JWL, et al. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci 2020; 61: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grzybowski A, Kanclerz P, Tsubota K, et al. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol 2020; 20: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troilo D, Smith EL, III, Nickla DL, et al. IMI-experimental models of emmetropization and myopia. Invest Ophthalmol Vis Sci 2019; 60: M31–M88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tedja MS, Haarman AEG, Meester-Smoor MA, et al. IMI-myopia genetics report. Invest Ophthalmol Vis Sci 2019; 60: 89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolffsohn JS, Flitcroft DI, Gifford KL, et al. IMI-myopia control reports overview and introduction. Invest Ophthalmol Vis Sci 2019; 60: M1–M17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flitcroft DI, He M, Jonas JB, et al. IMI-defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci 2019; 60: M20–M30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorsby A.Refraction and its components in twins. Privy council, Medical research council, Special report series, no. 303. London: HM Stationery Office, 1962. pp. 136–137. [Google Scholar]
- 14.Kepler J.Dioptrice, Agsburg (1611): Cited by Duke-Elder S, Abrams D. Ophthalmic optics and refraction. In: Duke-Elder S. (ed.) System of ophthalmology. Vol V. London: Henry Kimpton, 1970, p.341. [Google Scholar]
- 15.Cohn H. (1867): Untersuchungen der Augen von 10060 Schoolkindern nebst Vorschlägen 3 fur Verbesserung der Augen nachtheiligen Schul-Einrichtungen, Leipzig 1867. Cited by Rehm DS. The myopia myth. Ligonier, IN: IMPA, 1981, p.57. [Google Scholar]
- 16.Tscherning M.Studien uber die Aetiologie der Myopie. Albrcht. Von Graefe’s Arvch Ophthalmol 1883; 29: 201–202. [Google Scholar]
- 17.Chakraborty R, Read SA, Vincent SJ.Understanding myopia: pathogenesis and mechanisms. In: Ang M, Wong TY. (eds.) Updates on myopia. A clinical perspective. 1st ed. Singapore: Springer, 2020, pp.65–95. [Google Scholar]
- 18.Smith EL, III, Hung LF.Form-deprivation myopia in monkeys is a graded phenomenon. Vis Res 2000; 40: 371–381. [DOI] [PubMed] [Google Scholar]
- 19.Smith EL, III, Hung LF, Huang J, et al. Effect of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthtlmol Vis Sci 2010; 51: 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wildsoet C, Pettigrew JD.Experimental myopia and anomalous eye growth patterns unaffected by optic nerve section in chickens: evidence for local control of eye growth. Clin Vis Sci 1988; 3: 99–107. [Google Scholar]
- 21.Smith EL, III, Ramamirtham R, Qiao-Grider Y, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci 2007; 48: 3914–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaeffel F, Glasser A, Howland HC.Accommodation, refractive error and eye growth in chickens. Vision Res 1988; 28: 639–657. [DOI] [PubMed] [Google Scholar]
- 23.Smith EL, III, Kee C, Ramamirtam R, et al. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthtlmol Vis Sci 2005; 46: 3965–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charman WN, Radhakrishnan H.Peripheral refraction and the development of refractive error: a review. Ophthalmic Physiol Opt 2010; 30: 321–338. [DOI] [PubMed] [Google Scholar]
- 25.Huang HM, Chang DS, Wu PC.The association between near work activities and myopia in children-a systematic review and meta-analysis. PLoS One 2015; 10: e0140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gwiazda J, Thorn F, Bauer J, et al. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci 1993; 34: 690–694. [PubMed] [Google Scholar]
- 27.Gwiazda J, Bauer J, Thorn F, et al. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vis Res 1995; 35: 1299–1304. [DOI] [PubMed] [Google Scholar]
- 28.Abbott ML, Schmid KL, Strang NC.Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic Physiol Opt 1998; 18: 13–20. [PubMed] [Google Scholar]
- 29.Charman WN.Aberrations and myopia. Ophthalmic Physiol Opt 2005; 25: 285–301. [DOI] [PubMed] [Google Scholar]
- 30.Lau JK, Vincent SJ, Collins MJ, et al. Ocular higher-order aberrations and axial eye growth in young Hong Kong children. Sci Rep 2018; 8: 6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiraoka T, Kotsuka J, Kakita T, et al. Relationship between higher-order wavefront aberrations and natural progression of myopia in schoolchildren. Sci Rep 2017; 7: 7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nickla DL.The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A 2006; 192: 399–407. [DOI] [PubMed] [Google Scholar]
- 33.Kearney S, O’Donoghue L, Pourshahidi LK, et al. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthalmic Physiol Opt 2017; 37: 557–567. [DOI] [PubMed] [Google Scholar]
- 34.Jee D, Morgan IG, Kim EC.Inverse relationship between sleep duration and myopia. Acta Ophthalmol 2016; 94: 204–210. [DOI] [PubMed] [Google Scholar]
- 35.Cohen Y, Belkin M, Yehezkel O, et al. Dependency between light intensity and refractive development under lightdark cycles. Exp Eye Res 2011; 92: 40–46. [DOI] [PubMed] [Google Scholar]
- 36.Norton TT.What do animal studies tell us about the mechanism of myopia-protection by light? Optom Vis Sci 2016; 93: 1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M, Schaeffel F, Jiang B, et al. Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Invest Ophthalmol Vis Sci 2018; 59: 4413–4424. [DOI] [PubMed] [Google Scholar]
- 38.Aleman AC, Wang M, Schaeffel F.Reading and myopia: contrast polarity matters. Sci Rep 2018; 8: 10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wildsoet C, Wallman J.Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vis Res 1995; 35: 1175–1194. [DOI] [PubMed] [Google Scholar]
- 40.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vis Res 1995; 35: 37–50. [DOI] [PubMed] [Google Scholar]
- 41.Harper AR, Summers JA.The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp Eye Res 2015; 133: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Summers JA.The choroid as a sclera growth regulator. Exp Eye Res 2013; 114: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone RA, Lin T, Laties AM, et al. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA 1989; 86: 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seko Y, Shimokawa H, Tokoro T.In vivo and in vitro association of retinoic acid with form-deprivation myopia in the chick. Exp Eye Res 1996; 63: 443–452. [DOI] [PubMed] [Google Scholar]
- 45.Fujikado T, Kawasaki Y, Fujii J, et al. The effect of nitric oxide synthase inhibitor on form-deprivation myopia. Curr Eye Res 1997; 16: 992–996. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organisation. World report of vision. Geneva, Switzerland: WHO, 2019, p. 42. [Google Scholar]
- 47.Foster PJ, Jiang Y.Epidemiology of myopia. Eye (Lond) 2014; 28: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organisation. World report of vision. Geneva, Switzerland: WHO, 2019, p. 154. [Google Scholar]
- 49.McCrann S, Loughman J, Butler JS, et al. Smartphone use as a possible risk factor for myopia. Clin Exp Optom 2020; 104(1): 13092. [DOI] [PubMed] [Google Scholar]
- 50.Rudnicka AR, Kapetanakis VV, Wathern AK, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol 2016; 100: 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matamoros E, Ingrand P, Pelen F, et al. Prevalence of myopia in France: a cross-sectional analysis. Medicine 2015; 94: e1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tideman JWL, Polling JR, Hofman A, et al. Environmental factors explain socioeconomic prevalence differences in myopia in 6-year-old children. Br J Ophthalmol 2017; 102: 243–247. [DOI] [PubMed] [Google Scholar]
- 53.Enthoven CA, Tideman JWL, Polling JR, et al. The impact of computer use on myopia development in childhood: the Generation R study. Prev Med 2020; 132: 105988. [DOI] [PubMed] [Google Scholar]
- 54.Rudnicka AR, Owen CG, Nightingale CM, et al. Ethnic differences in the prevalence of myopia and ocular biometry in 10-and 11-year-old children: The Child Heart and Health Study in England (CHASE). Invest Ophthalmol Vis Sci 2010; 51: 6270–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Donoghue L, Kapetanankis VV, McClelland JF, et al. Risk factors for childhood myopia: findings from the NICER Study. Invest Ophthalmol Vis Sci 2015; 56: 1524–1530. [DOI] [PubMed] [Google Scholar]
- 56.Tideman JWL, Enthoven C, Jaddoe V, et al. Axial length growth from 6 to 13 years of age and risk of myopia at age 13: the Generation R study. Invest Ophthalmol Vis Sci 2020; 61: 852. [Google Scholar]
- 57.Lundberg K, Suhr Thykjaer A, Søgaard Hansen R, et al. Physical activity and myopia in Danish children-The CHAMPS Eye Study. Acta Ophthalmol 2017; 96: 134–141. [DOI] [PubMed] [Google Scholar]
- 58.Hagen LA, Gjelle JVB, Arnegard S, et al. Prevalence and possible factors of myopia in Norwegian adolescents. Sci Rep 2018; 8: 13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lam CS, Goldschmidt E, Edwards MH.Prevalence of myopia in local and international schools in Hong Kong. Optom Vis Sci 2004; 81: 317–322. [DOI] [PubMed] [Google Scholar]
- 60.Matsumura H, Hirai H.Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv Ophthalmol 1999; 44(suppl 1): S109–S115. [DOI] [PubMed] [Google Scholar]
- 61.Wu JF, Bi HS, Wang SM, et al. Refractive error, visual acuity and causes of vision loss in children in Shandong, China: The Shandong Children Eye Study. PLoS One 2013; 8: e82763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin LL, Shih YF, Hsiao CK, et al. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singapore 2004; 33: 27–33. [PubMed] [Google Scholar]
- 63.Jung SK, Lee JH, Kakizaki H, et al. Prevalence of myopia and its association with body stature and educational level in 19- year-old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci 2012; 53: 5579–5583. [DOI] [PubMed] [Google Scholar]
- 64.Lee JH, Jee D, Kwon JW, et al. Prevalence and risk factors for myopia in a rural Korean population. Invest Ophthalmol Vis Sci 2013; 54: 5466–5471. [DOI] [PubMed] [Google Scholar]
- 65.Koh V, Yang A, Saw SM, et al. Differences in prevalence of refractive errors in young Asian males in Singapore between 1996-1997 and 2009-2010. Ophthalmic Epidemiol 2014; 21: 247–255. [DOI] [PubMed] [Google Scholar]
- 66.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci 2003; 44: 1492–1500. [DOI] [PubMed] [Google Scholar]
- 67.Saw SM, Tong L, Chua WH, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci 2005; 46: 51–57. [DOI] [PubMed] [Google Scholar]
- 68.Lam CS, Edwards M, Millodot M, et al. A 2-year longitudinal study of myopia progression and optical component changes among Hong Kong schoolchildren. Optom Vis Sci 1999; 76:370–380. [DOI] [PubMed] [Google Scholar]
- 69.Donovan L, Sankaridurg P, Ho A, et al. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci 2012; 89: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.French AN, Morgan IG, Burlutsky G, et al. Prevalence and 5- to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology 2013; 120: 1482–1491. [DOI] [PubMed] [Google Scholar]
- 71.Pärssinen O, Soh ZD, Tan CS, et al. Comparison of myopic progression in Finnish and Singaporean children. Acta Ophthalmol 2021; 99: 171–180. [DOI] [PubMed] [Google Scholar]
- 72.Chua SY, Sabanayagam C, Cheung YB, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt 2016; 36: 388–394. [DOI] [PubMed] [Google Scholar]
- 73.Bullimore MA, Reuter KS, Jones LA, et al. The study of progression of adult nearsightedness (SPAN): design and baseline characteristics. Optom Vis Sci 2006; 83: 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorn F, Gwiazda J, Held R.Myopia progression is specified by a double exponential growth function. Optom Vis Sci 2005; 82: 286–297. [DOI] [PubMed] [Google Scholar]
- 75.Jiang X, Tarczy-Hornoch K, Cotter SA, et al. Association of parental myopia with higher risk of myopia among multiethnic children before school age. JAMA Ophthalmol 2020; 138: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma Y, Qu X, Zhu X, et al. Age-specific prevalence of visual impairment and refractive error in children aged 3-10 years in Shanghai, China. Invest Ophthalmol Vis Sci 2016; 57: 6188–6196. [DOI] [PubMed] [Google Scholar]
- 77.Guo X, Fu M, Ding X, et al. Significant axial elongation with minimal change in refraction in 3- to 6- year-old Chinese preschoolers: the Shenzhen Kindergarten Eye Study. Ophthalmology 2017; 124: 1826–1838. [DOI] [PubMed] [Google Scholar]
- 78.Li Z, Xu K, Wu S, et al. Population-based survey of refractive error among school-aged children in rural northern China: the Heilongjiang Eye Study. Clin Exp Ophthalmol 2014; 42: 379–384. [DOI] [PubMed] [Google Scholar]
- 79.Smirnova I, Prediger V, Potykova JU.Epidemiology of disorders of refraction, accommodation and convergence, at schoolchildren of Siberia. Mod Optom 2017; 102: 19–28. [Google Scholar]
- 80.Logan N.Modern trends in refractogenesis in Siberian schoolchildren. Poster abstract P 003. Ophthalmic Physiol Opt 2018; 38(3): 215–216. [DOI] [PubMed] [Google Scholar]
- 81.Giordano L, Friedman DS, Repka MX, et al. Prevalence of refractive error among preschool children in an urban population: the Baltimore Pediatric Eye Disease Study. Ophthalmology 2009; 116: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He M, Zeng J, Liu Y, et al. Refractive error and visual impairment in urban children in Southern China. Invest Ophthalmol Vis Sci 2004; 45: 793–799. [DOI] [PubMed] [Google Scholar]
- 83.COMET Group. Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET). Invest Ophthalmol Vis Sci 2013; 54: 7871–7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee MW, Lee SE, Lim HB, et al. Longitudinal changes in axial length in high myopia: a 4-year prospective study. Br J Ophthalmol 2019; 104: 600–603. [DOI] [PubMed] [Google Scholar]
- 85.Lee JTL, Guo X, Li Z, et al. Progression and longitudinal biometric changes in highly myopic eyes. Invest Ophthalmol Vis Sci 2020; 61: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pärssinen O, Kauppinen M, Viljanen A.The progression of myopia from its onset at age 8-12 to adulthood and the influence of heredity and external factors on myopic progression. A 23-year follow-up study. Acta Ophthalmol 2014; 92: 730–739. [DOI] [PubMed] [Google Scholar]
- 87.Bullimore MA, Jones LA, Moeschberger ML, et al. A retrospective study of myopia progression in adult contact lens wearers. Invest Ophthalmol Vis Sci 2002; 43: 2110–2113. [PubMed] [Google Scholar]
- 88.Morgan IG, Rose KA.Is the nature-nurture debate finally over? Clin Exp Optom 2019; 102: 3–17. [DOI] [PubMed] [Google Scholar]
- 89.Williams KM, Verhoeven VJM, Cumberland P, et al. Prevalence of refractive error in Europe: the European Eye Epidemiology (E3) Consortium. Eur J Epidemiol 2015; 30: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinge B, Midelfart A, Jacobsen G, et al. The influence of near-work on development of myopia among university students: a three-year longitudinal study among engineering students in Norway. Acta Ophthalmol Scand 2000; 78: 26–29. [DOI] [PubMed] [Google Scholar]
- 91.McCullough SJ, O’Donoghue L, Saunders KJ.Six year refractive change among white children and young adults: evidence for significant increase in myopia among white UK children. PLoS One 2016; 11: e0146332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansen MH, Hvid-Hansen A, Jacobsen N, et al. Myopia prevalence in Denmark - a review of 140 years of myopia research. Acta Ophthalmol 2021; 99: 118–127. [DOI] [PubMed] [Google Scholar]
- 93.Tideman JWL, Polling JR, Vingerling JR, et al. Axial in length growth and the risk of developing myopia European children. Acta Ophthalmol 2018; 96: 301–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Q, Klein BE, Klein R, et al. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci 1994; 35: 4344–4347. [PubMed] [Google Scholar]
- 95.Katz J, Tielsch JM, Sommer A.Prevalence and risk factors for refractive errors in an adult inner city population. Invest Ophthalmol Vis Sci 1997; 38: 334–340. [PubMed] [Google Scholar]
- 96.Wong TY, Foster PJ, Hee J, et al. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci 2000; 41: 2486–2494. [PubMed] [Google Scholar]
- 97.Hysi PG, Choquet H, Khawaja AP, et al. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat Genet 2020; 52: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J, Zhang Q.Insight into the molecular genetics of myopia. Mol Vis 2017; 23: 1048–1080. [PMC free article] [PubMed] [Google Scholar]
- 99.Rose KA, Morgan IG, Smith W, et al. High heritability of myopia does not preclude rapid changes in prevalence. Clin Exp Ophthalmol 2002; 30: 168–172. [DOI] [PubMed] [Google Scholar]
- 100.Zylbermann R, Landau D, Berson D.The influence of study habits on myopia in Jewish children. J Pediatr Ophthalmol Strabismus 1993; 30: 319–322. [DOI] [PubMed] [Google Scholar]
- 101.Megreli J, Barak A, Bez M, et al. Association of myopia with cognitive function among one million adolescents. BMC Public Health 2020; 20: 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morgan I, Rose K.How genetic is ‘school myopia’? Prog Retin Eye Res 2005; 24: 1–38. [DOI] [PubMed] [Google Scholar]
- 103.Zhou WJ, Zhang YY, Li H, et al. Five-year progression of refractive errors and incidence of myopia in school-aged children in Western China. J Epidemiol 2016; 26: 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pärssinen O.Relation between refraction, education, occupation, and age among 26- and 46-year-old Finns. Am J Optom Physiol Opt 1987; 64: 136–143. [DOI] [PubMed] [Google Scholar]
- 105.Midelfart A, Kinge B, Midelfart S, et al. Prevalence of refractive errors in young and middle-aged adults in Norway. Acta Ophthalmol Scand 2002; 80: 501–505. [DOI] [PubMed] [Google Scholar]
- 106.Sun J, Zhou J, Zhao P, et al. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Invest Ophthalmol Vis Sci 2012; 53: 7504–7509. [DOI] [PubMed] [Google Scholar]
- 107.Mutti DO, Mitchell L, Hayes JR, et al. Accommodation lag before and after the onset of myopia. Invest Ophthtlmol Vis Sci 2006; 47: 837–846. [DOI] [PubMed] [Google Scholar]
- 108.Gwiazda J, Hyman L, Dong LM, et al. Factors associated with high myopia after 7 years of follow-up in the Correction of Myopia Evaluation Trial (COMET) cohort. Ophthalmic Epidemiol 2007; 14: 230–237. [DOI] [PubMed] [Google Scholar]
- 109.Shrestha SP, Bhat KS, Binu VS, et al. Pattern of refractive errors among the Nepalese population: a retrospective study. Nepal J Ophthalmol 2010; 2: 87–96. [DOI] [PubMed] [Google Scholar]
- 110.Rezvan F, Khabazkhoob M, Fotouhi A, et al. Prevalence of refractive errors among school children in Northeastern Iran. Ophthalmic Physiol Opt 2012; 32: 25–30. [DOI] [PubMed] [Google Scholar]
- 111.Zadnik K, Satariano WA, Mutti DO, et al. The effect of parental history of myopia on children’s eye size. JAMA 1994; 271: 1323–1327. [PubMed] [Google Scholar]
- 112.Lee YY, Lo CT, Sheu SJ, et al. Risk factors for and progression of myopia in young Taiwanese men. Ophthalmic Epidemiol 2015; 22: 66–73. [DOI] [PubMed] [Google Scholar]
- 113.Kearney S, Strang NC, Cagnolati B, et al. Change in body height, axial length and refractive status over a four-year period in Caucasian children and young adults. J Optom 2020; 13: 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ip JM, Huynh SC, Robaei D, et al. Ethnic differences in the impact of parental myopia: findings from a population-based study of 12-year-old Australian children. Invest Ophthalmol Vis Sci 2007; 48: 2520–2528. [DOI] [PubMed] [Google Scholar]
- 115.Mutti DO, Mitchell GL, Moeschberger ML, et al. Parental myopia, near work, school achievement, and children’s refractive error. Invest Ophthalmol Vis Sci 2002; 43: 3633–3640. [PubMed] [Google Scholar]
- 116.Xiang F, He M, Morgan IG.The impact of parental myopia on myopia in Chinese children: population-based evidence. Optom Vis Sci. 2012; 89: 1487–1496. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X, Qu X, Zhou X.Association between parental myopia and the risk of myopia in a child. Exp Ther Med 2015; 9: 2420–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wenbo L, Congxia B, Hui L.Genetic and environmental-genetic interaction rules for the myopia based on a family exposed to risk from a myopic environment. Gene 2017; 626: 305–308. [DOI] [PubMed] [Google Scholar]
- 119.Jones-Jordan LA, Sinnott LT, Manny RE, et al. Early childhood refractive error and parental history of myopia as predictors of myopia. Invest Ophthalmol Vis Sci 2010; 51: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McMonnies CW.Clinical prediction of the need for interventions for the control of myopia. Clin Exp Optom 2015; 98: 518–526. [DOI] [PubMed] [Google Scholar]
- 121.Morgan IG, French AN, Rose KA. Risk factors for myopia: putting causal pathways into a social context. In: Ang M, Wong TY. (eds.) Updates on myopia. A clinical perspective. 1st ed. Singapore: Springer, 2020, pp.133–170. [Google Scholar]
- 122.Teasdale TW, Fuchs J, Goldschmidt E.Degree of myopia in relation to intelligence and educational level. Lancet 1988; 2: 1351–1354. [DOI] [PubMed] [Google Scholar]
- 123.Rosner M, Belkin M.Intelligence, education, and myopia in males. Arch Ophthalmol 1987; 105: 1508–1511. [DOI] [PubMed] [Google Scholar]
- 124.Pärssinen O, Era P, Leskinen AL.Some physiological and psychological characteristics of myopic and non-myopic men. Acta Ophthalmol 1985; 173: 85–87. [DOI] [PubMed] [Google Scholar]
- 125.Saw SM, Tan SB, Fung D, et al. IQ and the association with myopia in children. Invest Ophthalmol Vis Sci. 2004; 45: 2943–2948. [DOI] [PubMed] [Google Scholar]
- 126.Cuellar-Partida G, Lu Y, Kho PF, et al. Assessing the genetic predisposition of education on myopia: a mendelian randomization study. Genet Epidemiol 2016; 40: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mountjoy E, Davies NM, Plotnikov D, et al. Education and myopia: assessing the direction of causality by mendelian randomisation. BMJ 2018; 361: k2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Plotnikov D, Williams C, Atan D, et al. Effect of education on myopia: evidence from the United Kingdom ROSLA 1972 reform. Invest Ophthalmol Vis Sci 2020; 61: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Terasaki H, Yamashita T, Yoshihara N, et al. Association of lifestyle and body structure to ocular axial length in Japanese elementary school children. BMC Ophthalmol 2017; 17: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.O’Connor AR, Stephenson TJ, Johnson A, et al. Change of refractive state and eye size in children of birth weight less than 1701 g. Br J Ophthalmol 2006; 90: 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mandel Y, Grotto I, El-Yaniv R, et al. Season of birth, natural light, and myopia. Ophthalmology 2008; 115: 686–692. [DOI] [PubMed] [Google Scholar]
- 132.McMahon G, Zayats T, Chen YP, et al. Season of birth, daylight hours at birth, and high myopia. Ophthalmology 2009; 116: 468–473. [DOI] [PubMed] [Google Scholar]
- 133.Guggenheim JA, McMahon G, Northstone K, et al. Birth order and myopia. Ophthalmic Epidemiol 2013; 20: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guggenheim JA, Williams C. and UK Biobank Eye and Vision Consortium. Role of educational exposure in the association between myopia and birth order. JAMA Ophthalmol 2015; 133: 1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gwiazda J, Thorn F, Held R.Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci 2005; 82: 273–278. [DOI] [PubMed] [Google Scholar]
- 136.Mutti DO, Mitchell GL, Jones-Jordan LA, et al. The response AC/A ratio before and after the onset of myopia. Invest Ophthalmol Vis Sci 2017; 58: 1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nakatsuka S, Hasebe F, Nonak F, et al. Accommodative lag under habitual seeing conditions: comparison between myopic and emmetropic children. Jpn J Ophthalmol 2005; 49: 189–194. [DOI] [PubMed] [Google Scholar]
- 138.Pärssinen O, Lyyra AL.Myopia and myopic progression among schoolchildren: a three-year follow-up study. Invest Ophthalmol Vis Sci 1993; 34: 2794–2802. [PubMed] [Google Scholar]
- 139.Gwiazda J, Grice K, Thorn F.Response AC/A ratios are elevated in myopic children. Ophthalmic Physiol Opt 1999; 19: 173–179. [DOI] [PubMed] [Google Scholar]
- 140.Mutti DO, Jones LA, Moeschberger ML, et al. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci 2000; 41: 2469–2478. [PubMed] [Google Scholar]
- 141.Allen PM, O’Leary DJ.Accommodation functions: co-dependency and relationship to refractive error. Vision Res 2006; 46: 491–505. [DOI] [PubMed] [Google Scholar]
- 142.Rosenfield M, Desai R, Portello JK.Do progressing myopes show reduced accommodative responses? Optom Vis Sci 2002; 79: 268–273. [DOI] [PubMed] [Google Scholar]
- 143.Berntsen DA, Sinnott LT, Mutti DO, et al. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci 2012; 53: 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Berntsen DA, Sinnott LT, Mutti DO, et al. Accommodative lag and juvenile-onset myopia progression in children wearing refractive correction. Vision Res 2011; 51: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cheng D, Woo GC, Drobe B, et al. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three-year results of a randomized clinical trial. JAMA Ophthalmol 2014; 132: 258–264. [DOI] [PubMed] [Google Scholar]
- 146.Aller TA, Liu M, Wildsoet CF.Myopia control with bifocal contact lenses: a randomized clinical trial. Optom Vis Sci 2016; 93: 344–352. [DOI] [PubMed] [Google Scholar]
- 147.Dolgin E.The myopia boom. Nature 2015; 519: 276–278. [DOI] [PubMed] [Google Scholar]
- 148.Williams KM, Bertelsen G, Cumberland P, et al. Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology 2015; 122: 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hua WJ, Jin JX, Wu XY, et al. Elevated light levels in schools have a protective effect on myopia. Ophthalmic Physiol Opt 2015; 35: 252–262. [DOI] [PubMed] [Google Scholar]
- 150.Read SA, Collins MJ, Vincent SJ.Light exposure and physical activity in myopic and emmetropic children. Optom Vis Sci 2014; 91: 330–341. [DOI] [PubMed] [Google Scholar]
- 151.Torii H, Ohnuma K, Kurihara T, et al. Violet light transmission is related to myopia progression in adult high myopia. Sci Rep 2017; 7: 14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Williams KM, Bentham GC, Young IS, et al. Association between myopia, ultraviolet B radiation exposure, serum vita min D concentrations, and genetic polymorphisms in vitamin D metabolic pathways in a multicountry European study. JAMA Ophthalmol 2017; 135: 47–53. [DOI] [PubMed] [Google Scholar]
- 153.Xiong S, Sankaridurg P, Naduvilath T, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol 2017; 95: 551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cao K, Wan Y, Yusufu M, et al. Significance of outdoor time for myopia prevention: a systematic review and meta-analysis based on randomized controlled trials. Ophthalmic Res 2020; 63: 97–105. [DOI] [PubMed] [Google Scholar]
- 155.Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125:1239–1250. [DOI] [PubMed] [Google Scholar]
- 156.Pärssinen O.The wearing of spectacles and occurrence of myopia. In: Acta Universitatis Tamperensis ser A. Vol 207. Tampere, Finland: University of Tampere, 1986. [Google Scholar]
- 157.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008; 115: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 158.Wen L, Cao Y, Cheng Q, et al. Objectively measured near work, outdoor exposure and myopia in children. Br J Ophthalmol 2020; 104: 1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morgan IG, Rose KA.Myopia and international educational performance. Ophthalmic Physiol Opt 2013; 33: 329–338. [DOI] [PubMed] [Google Scholar]
- 160.Shapira Y, Mimouni M, Machluf Y, et al. The increasing burden of myopia in Israel among young adults over a generation: analysis of predisposing factors. Ophthalmology 2019; 126: 1617–1626. [DOI] [PubMed] [Google Scholar]
- 161.Mirshahi A, Ponto KA, Hoehn R, et al. Myopia and level of education: results from the Gutenberg Health Study. Ophthalmology 2014; 121: 2047–2052. [DOI] [PubMed] [Google Scholar]
- 162.Ramessur R, Williams KM, Hammond CJ.Risk factors for myopia in a discordant monozygotic twin study. Ophthalmic Physiol Opt 2015; 35: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Flitcroft DI, Harb EN, Wildsoet CF. The spatial frequency content of urban and indoor environments as a potential risk factor for myopia development. Invest Ophthalmol Vis Sci 2020; 61: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Verhoeven VJM, Buitendijk GHS, Rivadeneira F, et al. Consortium for refractive error and myopia (CREAM). Education influences the role of genetics in myopia. Eur J Epidemiol 2013; 28: 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fan Q, Guo X, Tideman JWL, et al. Childhood gene-environment interactions and age-dependent effects of genetic variants associated with refractive error and myopia: the CREAM Consortium. Sci Rep 2016; 6: 25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Williams KM, Kraphol E, Yonova-Doing E, et al. Early life factors for myopia in the British twins early development study. Br J Ophthalmol 2019; 103: 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saxena R, Vashist P, Tandon R, et al. Incidence and progression of myopia and associated factors in urban school children in Delhi: The North India Myopia Study (NIM Study). PLoS One 2017; 12: e0189774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lanca C, Saw SM.The association between digital screen time and myopia: a systematic review. Ophthalmic Physiol Opt 2020; 40: 216–229. [DOI] [PubMed] [Google Scholar]
- 169.Choi KY, Yu WY, Lam CHI, et al. Childhood exposure to constricted living space: a possible environmental threat for myopia development. Ophthalmic Physiol Opt 2017;37: 568–575. [DOI] [PubMed] [Google Scholar]
- 170.Wu X, Gao G, Jin J, et al. Housing type and myopia: the mediating role of parental myopia. BMC Ophthalmol 2016; 16: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lim HT, Yoon JS, Hwang SS, et al. Prevalence and associated sociodemographic factors of myopia in Korean children: the 2005 Third Korea National Health and Nutrition Examination Survey (KNHANES III). Jpn J Ophthalmol 2012; 56: 76–81. [DOI] [PubMed] [Google Scholar]
- 172.Jones LA, Sinnott LT, Mutti DO, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci 2007; 48: 3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jones-Jordan LA, Mitchell GL, Cotter SA, et al. Visual activity prior to and following the onset of juvenile myopia. Invest Ophthalmol Vis Sci 2011; 52: 1841–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jones-Jordan LA, Sinnott LT, Cotter SA, et al. Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci 2012; 53: 7169–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu PC, Tsai CL, Wu HL, et al. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology 2013; 120: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 176.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA Ophthalmol 2015; 314: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 177.Jin JX, Hua WJ, Jiang X, et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast china: The Sujiatun Eye Care Study. BMC Ophthalmol 2015; 15: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sherwin JC, Reacher MH, Keogh RH, et al. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology 2012; 119: 2141–2151. [DOI] [PubMed] [Google Scholar]
- 179.French AN, Ashby RS, Morgan IG, et al. Time outdoors and the prevention of myopia. Exp Eye Res 2013; 114: 58–68. [DOI] [PubMed] [Google Scholar]
- 180.Jacobsen N, Jensen H, Goldschmidt E.Does the level of physical activity in university students influence development and progression of myopia? A 2-year prospective cohort study. Invest Ophthalmol Vis Sci 2008; 49: 1322–1327. [DOI] [PubMed] [Google Scholar]
- 181.Feldkaemper M, Schaeffel F.An updated view on the role of dopamine in myopia. Exp Eye Res 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 182.Prepas SB.Light, literacy and the absence of ultraviolet radiation in the development of myopia. Med Hypotheses 2008; 70: 635–637. [DOI] [PubMed] [Google Scholar]
- 183.Donovan L, Sankaridurg P, Ho A, et al. Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci 2012; 89: 1196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gwiazda JE, Deng L, Manny RE, et al. Seasonal variations in the progression of myopia in children enrolled in the correction of myopia evaluation trial. Invest Ophthalmol Vis Sci 2014; 55: 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tideman JWL, Polling JR, Voortman T, et al. Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur J Epidemiol 2016; 31: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mutti DO, Marks AR.Blood levels of vitamin D in teens and young adults with myopia. Optom Vis Sci 2011; 88: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Choi JA, Han K, Park YM, et al. Low serum 25-hydroxyvitamin D is associated with myopia in Korean adolescents. Invest Ophthalmol Vis Sci 2014; 55: 2041–2047. [DOI] [PubMed] [Google Scholar]
- 188.Yazar S, Hewitt AW, Black LJ, et al. Myopia is associated with lower vitamin D status in young adults. Invest Ophthalmol Vis Sci 2014; 55: 4552–4559. [DOI] [PubMed] [Google Scholar]
- 189.Kwon JW, Choi JA, La TY.Serum 25-hydroxyvitamin D level is associated with myopia in the Korea national health and nutrition examination survey. Medicine 2016; 95: e5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Guggenheim JA, Williams C, Northstone K, et al. Does vitamin D mediate the protective effects of time outdoors on myopia? Findings from a prospective birth cohort. Invest Ophthalmol Vis Sci 2015; 55: 8550–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou Z, Chen T, Wang M, et al. Pilot study of a novel classroom designed to prevent myopia by increasing children’s exposure to outdoor light. PLoS One 2017; 12: e0181772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pan CW, Wu RK, Liu H, et al. Types of lamp for homework and myopia among Chinese school-aged children. Ophthal Epidemiol 2018; 25: 250–256. [DOI] [PubMed] [Google Scholar]
- 193.Behar-Cohen F, Martinsons C, Viénot F, et al. Light-emitting diodes (LED) for domestic lighting: any risks for the eye? Prog Retin Eye Res 2011; 30: 239–257. [DOI] [PubMed] [Google Scholar]
- 194.Tokoro T, Kabe S. Treatment of the myopia and the changes in optical components. Report II. Full- or under-correction of myopia by glasses [in Japanese]. Nippon Ganka Gakkai Zasshi 1965; 69: 140–144. [PubMed] [Google Scholar]
- 195.Sun YY, Li SM, Li SY, et al. Effect of uncorrection versus full correction on myopia progression in 12-year-old children. Graefes Arch Clin Exp Ophthalmol 2017; 255: 189–195. [DOI] [PubMed] [Google Scholar]
- 196.Chung K, Mohidin N, O’Leary DJ.Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res 2002; 42: 2555–2559. [DOI] [PubMed] [Google Scholar]
- 197.Adler D, Millodot M.The possible effect of undercorrection on myopic progression in children. Clin Exp Optom 2006; 89: 315–321. [DOI] [PubMed] [Google Scholar]
- 198.Li SY, Li SM, Zhou YH, et al. Effect of undercorrection on myopia progression in 12-year-old children. Graefes Arch Clin Exp Ophthalmol 2015; 253: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 199.Wildsoet CF, Chia A, Cho P, et al. IMI-interventions for controlling myopia onset and progression report. Invest Ophthtlmol Vis Sci 2019; 60: M106–M131. [DOI] [PubMed] [Google Scholar]
- 200.Walline JJ, Lindsley K, Vedula SS, et al. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev 2011; 12: CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Walline JJ, Lindsley KB, Vedula SS, et al. Interventions to slow progression of myopia in children. Cochrane Database of Syst Rev 2020; 1: CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Logan NS, Wolffsohn JS.Role of un-correction, under-correction and over-correction of myopia as a strategy for slowing myopic progression. Clin Exp Optom 2020; 103: 133–137. [DOI] [PubMed] [Google Scholar]
- 203.Kanda H, Oshika T, Hiraoka T, et al. Effect of spectacle lenses designed to reduce relative peripheral hyperopia on myopia progression in Japanese children: a 2-year multicenter randomized controlled trial. Jpn J Ophthalmol 2018; 62: 537–543. [DOI] [PubMed] [Google Scholar]
- 204.Hasebe S, Jun J, Varnas SR.Myopia control with positively aspherized progressive addition lenses: a 2-year, multicenter, randomized, controlled trial. Invest Ophthalmol Vis Sci 2014; 55: 7177–7188. [DOI] [PubMed] [Google Scholar]
- 205.Sankaridurg P, Donovan L, Varnas S, et al. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optom Vis Sci 2010; 87: 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lam CSY, Tang WC, Tse DY, et al. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol 2020; 104: 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tang WC, Leung M, Wong ACK, et al. Optical interventions for myopia control. In: Ang M, Wong TY. (eds.) Updates on myopia. A clinical perspective. 1st ed. Singapore: Springer, 2020, pp.289–305. [Google Scholar]
- 208.Gwiazda JE, Hyman L, Everett D, et al. Five–year results from the correction of myopia evaluation trial (COMET). Invest Ophthalmol Vis Sci 2006; 47: 1166. [Google Scholar]
- 209.Hasebe S, Ohtsuki H, Nonaka T, et al. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci 2008; 49: 2781–2789. [DOI] [PubMed] [Google Scholar]
- 210.Jensen H.Myopia progression in young school children. A prospective study of myopia progression and the effect of a trial with bifocal lenses and beta blocker eye drops. Acta Ophthalmol Suppl 1991; 200: 1–79. [PubMed] [Google Scholar]
- 211.Pärssinen O, Hemminki E, Klemetti A.Effect of spectacle use and accommodation on myopic progression: final results of three-year randomised clinical trial among schoolchildren. Br Med J 1989; 73: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li SM, Ji YZ, Wu SS, et al. Multifocal versus single vision lenses intervention to slow progression of myopia in school-age children: a meta-analysis. Surv Ophthalmol 2011; 56: 451–460. [DOI] [PubMed] [Google Scholar]
- 213.Gwiazda JE, Hyman L, Norton TT, et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci 2004; 45: 2143–2151. [DOI] [PubMed] [Google Scholar]
- 214.Correction of Myopia Evaluation Trial 2 Study Group for the Pediatric Eye Disease Investigator Group. Progressive-addition lenses versus single-vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria. Invest Ophthalmol Vis Sci 2011; 52: 2749–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yang Z, Lan W, Ge J, et al. The effectiveness of progressive addition lenses on the progression of myopia in Chinese children. Ophthalmic Physiol Opt 2009; 29: 41–48. [DOI] [PubMed] [Google Scholar]
- 216.Horner DG, Soni PS, Salmon TO, et al. Myopia progression in adolescent wearers of soft contact lenses and spectacles. Optom Vis Sci 1999; 76: 474–479. [DOI] [PubMed] [Google Scholar]
- 217.Walline JJ, Jones LA, Sinnott L, et al. A randomized trial of the effect of soft contact lenses on myopia progression in children. Invest Ophthalmol Vis Sci 2008; 49: 4702–4706. [DOI] [PubMed] [Google Scholar]
- 218.Marsh-Tootle WL, Dong LM, Hyman L, et al. Myopia progression in children wearing spectacles vs. switching to contact lenses. Optom Vis Sci 2009; 86: 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kelly TS, Chatfield C, Tustin G.Clinical assessment of the arrest of myopia. Br J Ophthalmol 1975; 59: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Perrigin J, Perrigin D, Quintero S, et al. Silicone-acrylate contact lenses for myopia control: 3-year results. Optom Vis Sci 1990; 67: 764–769. [DOI] [PubMed] [Google Scholar]
- 221.Stone J.The possible influence of contact lenses on myopia. Br J Physiol Opt 1976; 31: 89–114. [PubMed] [Google Scholar]
- 222.Katz J, Schein OD, Levy B, et al. A randomized trial of rigid gas permeable contact lenses to reduce progression of children’s myopia. Am J Ophthalmol 2003; 136: 82–90. [DOI] [PubMed] [Google Scholar]
- 223.Walline JJ, Jones LA, Mutti DO, et al. A randomized trial of the effects of rigid contact lenses on myopia progression. Arch Ophthalmol 2004; 122: 1760–1766. [DOI] [PubMed] [Google Scholar]
- 224.Walline JJ.Myopia control: a review. Eye Contact Lens 2016; 42: 3–8. [DOI] [PubMed] [Google Scholar]
- 225.Anstice NS, Phillips JR.Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology 2011; 118: 1152–1161. [DOI] [PubMed] [Google Scholar]
- 226.Paune J, Morales H, Armengol J, et al. Myopia control with a novel peripheral gradient soft lens and orthokeratology: a 2-year clinical trial. Biomed Res Int 2015; 2015: 507572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li SM, Kang MT, Wu SS, et al. Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: a meta-analysis. Ophthalmic Physiol Opt 2017; 37: 51–59. [DOI] [PubMed] [Google Scholar]
- 228.Ruiz-Pomeda A, Perez-Sanchez B, Valls I, et al. MiSight Assessment Study Spain (MASS). A 2-year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol 2018; 256: 1011–1021. [DOI] [PubMed] [Google Scholar]
- 229.Chamberlain P, Peixoto-de-Matos SC, Logan NS, et al. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci 2019; 96: 556–567. [DOI] [PubMed] [Google Scholar]
- 230.Lam CSY, Tang WC, Tse DY-Y, et al. Defocus incorporated soft contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2-year randomised clinical trial. Br J Ophthalmol 2014; 98: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Paune J, Thivent S, Armengol J, et al. Changes in peripheral refraction, higher-order aberrations, and accommodative lag with a radial refractive gradient contact lens in young myopes. Eye Contact Lens 2016; 42: 380–387. [DOI] [PubMed] [Google Scholar]
- 232.Allen PM, Radhakrishnan H, Price H, et al. A randomised clinical trial to assess the effect of a dual treatment on myopia progression: The Cambridge Anti-Myopia Study. Ophthalmic Physiol Opt 2013; 33: 267–276. [DOI] [PubMed] [Google Scholar]
- 233.Walline JJ, Walker MK, Mutti DO, et al. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: The BLINK Randomized Clinical Trial. JAMA 2020; 324: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Choo JD, Caroline PJ, Harlin DD, et al. Morphologic changes in cat epithelium following continuous wear of orthokeratology lenses: a pilot study. Cont Lens Anterior Eye 2008; 31: 29–37. [DOI] [PubMed] [Google Scholar]
- 235.Queiros A, Amorim-de-Sousa A, Lope-Ferreira D, et al. Relative peripheral refraction across 4 meridians after orthokeratology and LASIK surgery. Eye Vis (Lond) 2018; 5: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Smith EL, III. Prentice award lecture 2010: a case for peripheral optical treatment strategies for myopia. Optom Vis Sci 2011; 88: 1029–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hiraoka T, Kakita T, Okamoto F, et al. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology 2015; 122: 93–100. [DOI] [PubMed] [Google Scholar]
- 238.Tarrant J.Spherical aberration, accommodation and myopia. PhD Thesis, University of California, Berkeley, CA, 2010. [Google Scholar]
- 239.Han X, Xu D, Ge W, et al. A comparison of the effects of orthokeratology lens, medcall lens, and ordinary frame glasses on the accommodative response in myopic children. Eye Contact Lens 2018; 44: 268–271. [DOI] [PubMed] [Google Scholar]
- 240.Chen Z, Xue F, Zhou J, et al. Effects of orthokeratology on choroidal thickness and axial length. Optom Vis Sci 2016; 93: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 241.Si JK, Tang K, Bi HS, et al. Orthokeratology for myopia control: a meta-analysis. Optom Vis Sci 2015; 92: 252–257. [DOI] [PubMed] [Google Scholar]
- 242.Sun Y, Xu F, Zhang T, et al. Orthokeratology to control myopia progression: a meta-analysis. PLoS One 2015; 10: e0124535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology 2016; 123: 697–708. [DOI] [PubMed] [Google Scholar]
- 244.Cho P, Cheung SW, Edwards M.The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res 2005; 30: 71–80. [DOI] [PubMed] [Google Scholar]
- 245.Walline JJ, Jones LA, Sinnott LT.Corneal reshaping and myopia progression. Br J Ophthalmol 2009; 93: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 246.Kakita T, Hiraoka T, Oshika T.Influence of overnight orthokeratology on axial elongation in childhood myopia. Invest Ophthalmol Vis Sci 2011; 52: 2170–2174. [DOI] [PubMed] [Google Scholar]
- 247.Cho P, Cheung S-W.Retardation of myopia in orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci 2012; 53: 7077–7085. [DOI] [PubMed] [Google Scholar]
- 248.Hiraoka T, Kakita T, Okamoto F, et al. Long- term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci 2012; 53: 3913–3919. [DOI] [PubMed] [Google Scholar]
- 249.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, et al. Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Invest Ophthalmol Vis Sci 2012; 53: 5060–5065. [DOI] [PubMed] [Google Scholar]
- 250.Charm J, Cho P.High myopia-partial reduction ortho-k: a 2-year randomized study. Optom Vis Sci 2013; 90: 530–539. [DOI] [PubMed] [Google Scholar]
- 251.Chen C, Cheung SW, Cho P.Myopia control using toric orthokeratology (TO-SEE study). Invest Ophthalmol Vis Sci 2013; 54: 6510–6517. [DOI] [PubMed] [Google Scholar]
- 252.Zhu MJ, Feng HY, He XG, et al. The control effect of orthokeratology on axial length elongation in Chinese children with myopia. BMC Ophthalmol 2014; 14: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Na M, Yoo A.The effect of orthokeratology on axial length elongation in children with myopia: contralateral comparison study. Jpn J Ophthalmol 2018; 62: 327–334. [DOI] [PubMed] [Google Scholar]
- 254.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, et al. Factors preventing myopia progression with orthokeratology correction. Optom Vis Sci 2013; 90: 1225–1236. [DOI] [PubMed] [Google Scholar]
- 255.Lipson MJ, Brooks MM, Koffler BH.The role of orthokeratology in myopia control: a review. Eye Contact Lens 2018; 44: 224–230. [DOI] [PubMed] [Google Scholar]
- 256.Wang B, Naidu RK, Qu X.Factors related to axial length elongation and myopia progression in orthokeratology practice. PLoS One 2017; 12: e0175913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhong Y, Chen Z, Xue F, et al. Corneal power change is predictive of myopia progression in orthokeratology. Optom Vis Sci 2014; 91: 404–411. [DOI] [PubMed] [Google Scholar]
- 258.Chen Z, Niu L, Xue F, et al. Impact of pupil diameter on axial growth in orthokeratology. Optom Vis Sci 2012; 89: 1636–1640. [DOI] [PubMed] [Google Scholar]
- 259.Lee YC, Wang JH, Chiu CJ.Effect of orthokeratology on myopia progression: twelve-year results of a retrospective cohort study. BMC Ophthalmol 2017; 17: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fu AC, Chen XL, Lv Y, et al. Higher spherical equivalent refractive errors is associated with slower axial elongation wearing orthokeratology. Cont Lens Anterior Eye 2016; 39: 62–66. [DOI] [PubMed] [Google Scholar]
- 261.Kim J, Lim DH, Han SH, et al. Predictive factors associated with axial length growth and myopia progression in orthokeratology. PLoS One 2019; 14: e0218140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cho P, Cheung SW.Discontinuation of orthokeratology on eyeball elongation (DOEE). Cont Lens Anterior Eye 2017; 40: 82–87. [DOI] [PubMed] [Google Scholar]
- 263.Lee TT, Cho P.Discontinuation of orthokeratology and myopic progression. Optom Vis Sci 2010; 87: 1053–1056. [DOI] [PubMed] [Google Scholar]
- 264.Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, et al. Long-term efficacy of orthokeratology contact lens wear in controlling the progression of childhood myopia. Curr Eye Res 2017; 42: 713–720. [DOI] [PubMed] [Google Scholar]
- 265.Liu YM, Xie P.The safety of orthokeratology-a systematic review. Eye Contact Lens 2016; 42: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 2008; 115: 1655–1662. [DOI] [PubMed] [Google Scholar]
- 267.Bullimore MA, Sinnott LT, Lones-Jordan LA.The risk of microbial keratitis with overnight corneal reshaping lenses. Optom Vis Sci 2013; 90: 937–944. [DOI] [PubMed] [Google Scholar]
- 268.Wen D, Huang J, Chen H, et al. Efficacy and acceptability of orthokeratology for slowing myopic progression in children: a systematic review and meta-analysis. J Ophthalmol 2015; 2015: 360806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sankaridurg P, Bakaraju RC, Naduvilath T, et al. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic Physiol Opt 2019; 39: 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wagner S, Ohlendorf A, Schaeffel F, et al. Reducing the lag of accommodation by auditory biofeedback: a pilot study. Vision Res 2016; 129: 50–60. [DOI] [PubMed] [Google Scholar]
- 271.Wagner S, Schaeffel F, Troilo D.Changing accommodation behaviour during multifocal soft contact lens wear using auditory biofeedback training. Sci Rep 2020; 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cristaldi M, Olivieri M, Pezzino S, et al. Atropine differentially modulates ECM production by ocular fibroblasts, and its ocular surface toxicity is blunted by colostrum. Biomedicines 2020; 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schwahn HN, Kaymak H, Schaeffel F.Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis Neurosci 2000; 17: 165–176. [DOI] [PubMed] [Google Scholar]
- 274.Cavallotti C, Pescosolido N, Artico M, et al. Localization of dopamine receptors in the rabbit cornea. Cornea 1999; 18: 721–728. [DOI] [PubMed] [Google Scholar]
- 275.Grüb M, Mielke J, Rohrbach M, et al. Dopamine receptors of the corneal epithelium and endothelium in German. Klin Monbl Augenheilkd 2012; 229: 822–825. [DOI] [PubMed] [Google Scholar]
- 276.Gong Q, Janowski M, Liu L. Low-dose atropine for myopia control: considering all the data. comment and response. In reply. JAMA Ophthalmol 2018; 136: 303–304. [DOI] [PubMed] [Google Scholar]
- 277.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology 2006; 113: 2285–2291. [DOI] [PubMed] [Google Scholar]
- 278.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology 2012; 119: 347–354. [DOI] [PubMed] [Google Scholar]
- 279.Yam JC, Jiang Y, Tang SM, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 2019; 126: 113–124. [DOI] [PubMed] [Google Scholar]
- 280.Tong L, Huang XL, Koh AL, et al. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology 2009; 116: 572–579. [DOI] [PubMed] [Google Scholar]
- 281.Chia A, Chua WH, Wen L, et al. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol 2014; 157: 451–457. [DOI] [PubMed] [Google Scholar]
- 282.Chia A, Lu QS, Tan D.Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology 2016; 123: 391–399. [DOI] [PubMed] [Google Scholar]
- 283.Bullimore MA, Berntsen DA.Low-dose atropine for myopia control: considering all the data. JAMA Ophthalmol 2018; 136: 303. [DOI] [PubMed] [Google Scholar]
- 284.Bullimore MA, Richdale K.Myopia control 2020: where are we and where are we heading? Ophthalmic Physiop Opt 2020; 40: 254–270. [DOI] [PubMed] [Google Scholar]
- 285.Brennan N, Toubouti Y, Cheng X, et al. Efficacy in myopia control. Prog Retin Eye Res. Epub ahead of print 27 November 2020. DOI: 10.1016/j.preteyeres.2020.100923. [DOI] [PubMed] [Google Scholar]
- 286.Yam JC, Li FF, Zhang X, et al. Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study: phase 2 report. Ophthalmology 2020; 127: 910–919. [DOI] [PubMed] [Google Scholar]
- 287.Wu PC, Yang YH, Fang PC.The long-term results of using low-concentration atropine eye drops for controlling myopia progression in schoolchildren. J Ocul Pharmacol Ther 2011; 27: 461–466. [DOI] [PubMed] [Google Scholar]
- 288.Loh KL, Lu Q, Tan D, et al. Risk factors for progressive myopia in the atropine therapy for myopia study. Am J Ophthalmol 2015; 159: 945–949. [DOI] [PubMed] [Google Scholar]
- 289.Zhu Q, et al. Efficacy and safety of 1% atropine on retardation of moderate myopia progression in Chinese school children. Int J Med Sci 2020; 17: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Brodstein RS, Brodstein DE, Olson RJ, et al. The treatment of myopia with atropine and bifocals. A long-term prospective study. Ophthalmology 1984; 91: 1373–1379. [DOI] [PubMed] [Google Scholar]
- 291.Kennedy RH, Dyer JA, Kennedy MA, et al. Reducing the progression of myopia with atropine: a long term cohort study of Olmsted County students. Binocul Vis Strabismus Q 2000; 15(3 Suppl): 281–304. [PubMed] [Google Scholar]
- 292.Syniuta LA, Isenberg SJ.Atropine and bifocals can slow the progression of myopia in children. Binocul Vis Strabismus Q 2001; 16: 203–208. [PubMed] [Google Scholar]
- 293.Polling JR, Kok R, Tideman JWL, et al. Effectiveness study of atropine for progressive myopia in Europeans. Eye 2016; 30: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Diaz-Llopis M, Pinazo-Durán MD.Superdiluted atropine at 0.01% reduces progression in children and adolescents. A 5 year study of safety and effectiveness. Arch De la Soc Esp De Oftalmol (Engl Ed) 2018; 93: 182–185. [DOI] [PubMed] [Google Scholar]
- 295.Polling JR, Tan E, Driessen S, et al. A 3-year follow-up study of atropine treatment for progressive myopia in Europeans. Eye (Lond) 2020; 34: 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Shih YF, Chen CH, Chou AC, et al. Effects of different concentrations of atropine on controlling myopia in myopic children. J Ocul Pharmacol Ther 1999; 15: 85–90. [DOI] [PubMed] [Google Scholar]
- 297.Gong Q, Janowski M, Luo M, et al. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol 2017; 135: 624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.North R, Kelly ME.A review of the uses and adverse effects of topical administration of atropine. Ophthalmic Physiol Opt 1987; 7: 109–114. [DOI] [PubMed] [Google Scholar]
- 299.Tan DT, Lam DS, Chua WH, et al. One-year multicenter, double- masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology 2005; 112: 84–91. [DOI] [PubMed] [Google Scholar]
- 300.Siatkowski RM, Cotter SA, Crockett RS, et al. Two-year multicenter, randomized, double-masked, placebo-con- trolled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS 2008; 12: 332–339. [DOI] [PubMed] [Google Scholar]
- 301.Hung LF, Arumugab B, Ostrin L, et al. The adenosine receptor antagonist, 7-methylxanthine, alters emmetropizing responses in infant macaques. Invest Opthalmol Vis Sci 2018; 59: 472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Trier K, Munk Ribel-Madsen S, Cui D, et al. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. J Ocul Biol Dis Infor 2008; 1: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Singh H, Sahajpal NS, Singh H, et al. Pre-clinical and cellular toxicity evaluation of 7-methylxanthine: an investigational drug for the treatment of myopia. 2019; 12: 1–10. [DOI] [PubMed] [Google Scholar]
- 304.El-Nimri NW, Wildsoet CF.Effects of topical latanoprost on intraocular pressure and myopia progression in young guinea pigs. Invest Ophthalmol Vis Sci 2018; 59: 2644–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Carr BJ, Nguyen CT, Stell WK.Alpha 2-adrenoceptor agonists inhibit form-deprivation myopia in the chick. Clin Exp Optom 2019; 102: 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tkatchenko TV, Tkatchenko AV.Pharmacogenomic approach to antimyopia drug development: pathways lead the way. Trends Pharmacol Sci 2019; 40: 833–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Huang V, Duan A, Qi Y.Posterior scleral reinforcement to prevent progression of high myopia. Asia Pac J Ophthalmol 2019; 8: 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Xue A, Bao F, Zheng L, et al. Posterior scleral reinforcement on progressive high myopic young patients. Optom Vis Sci 2014; 91: 412–418. [DOI] [PubMed] [Google Scholar]
- 309.Li XJ, Yang XP, Li QM, et al. Posterior scleral reinforcement for the treatment of pathological myopia. Int J Ophthalmol 2016; 9: 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ward B, Tarutta EP, Mayer MJ.The efficacy and safety of posterior pole buckles in the control of progressive high myopia. Eye 2009; 23: 2169–2174. [DOI] [PubMed] [Google Scholar]
- 311.Coroneo MT, Beaumont JT, Hollows FC.Scleral reinforcement in the treatment of pathologic myopia. Aust N Z J Ophthalmol 1988; 16: 317–320. [DOI] [PubMed] [Google Scholar]
- 312.Dong X, Liu J, Bu J.The efficacy of modified posterior scleral reinforcement with round scleral patches in Chinese children with high myopia. Graefes Arch Clin Exp Ophthalmol 2020; 258: 1543–1547. [DOI] [PubMed] [Google Scholar]
- 313.Su Y, Pan A, Wu Y, et al. The efficacy of posterior scleral contraction in controlling high myopia in young people. Am J Trans Les 2018; 10: 3628–3634. [PMC free article] [PubMed] [Google Scholar]
- 314.Xue A, Zheng L, Tan G, et al. Genipin-crosslinked donor sclera for posterior scleral contraction/reinforcement to fight progressive myopia. Invest Ophthalmol Vis Sci 2018; 59: 3564–3573. [DOI] [PubMed] [Google Scholar]
- 315.Park JJ, Gole GA.Corticosteroid-induced glaucoma in a child after a scleral reinforcement procedure. Clin Exp Ophthalmol 2002; 30: 372–374. [DOI] [PubMed] [Google Scholar]
- 316.Golychev VN, Medvetskaia GA, Golubeva LA, et al. Our experience with the use of sclera-strengthening injections in the prevention of progressive myopia [in German]. Vestnik Oftalmologii 1989; 105: 26–27. [PubMed] [Google Scholar]
- 317.Avetisov ES, Tarutta EP, Iomdina EN, et al. Nonsurgical and surgical methods of sclera reinforcement in progressive myopia. Acta Ophthalmol Scand 1997; 75: 618–623. [DOI] [PubMed] [Google Scholar]
- 318.Li Z, Chen W, Zhang H, et al. The aquaporin-1 depletion downregulates the sclera biomechanical strength. Curr Eye Res 2020; 20: 1–5. [DOI] [PubMed] [Google Scholar]
- 319.Janowski M, Bulte JW, Handa JT, et al. Concise review: using stem cells to prevent the progression of myopia-a concept. Stem Cells 2015; 33: 2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Dotan A, Kremer I, Livnat T, et al. Scleral cross-linking using riboflavin and ultraviolet-a radiation for prevention of progressive myopia in a rabbit model. Exp Eye Res 2014; 127: 190–195. [DOI] [PubMed] [Google Scholar]
- 321.Wollensak G, Iomdina E, Dittert DD, et al. Cross-linking of scleral collagen in the rabbit using riboflavin and UVA. Acta Ophthalmol Scand 2005; 83: 477–482. [DOI] [PubMed] [Google Scholar]
- 322.Saw SM, Matsumura S, Hoang QV.Prevention and Management of Myopia and Myopic Pathology. Invest Ophthalmol Vis Sci 2019; 60: 488–499. [DOI] [PubMed] [Google Scholar]
- 323.Kim TG, Kim W, Choi S, et al. Effects of scleral collagen crosslinking with different carbohydrate on chemical bond and ultrastructure of rabbit sclera: future treatment for myopia progression. PLoS One 2019; 14: e0216425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Leshno A, Farzavandi SK, Gomez-de-Liano R, et al. Practice patterns to decrease myopia progression differ among paediatric ophthalmologists around the word. Br J Ophthalmol 2020; 104: 535–540. [DOI] [PubMed] [Google Scholar]
- 325.Kinoshita N, Konno Y, Hamada N, et al. Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: first year results. Jpn J Ophthalmol 2018; 62: 544–553. [DOI] [PubMed] [Google Scholar]
- 326.Tan Q, Ng A LK, Cheng GPM. Combined atropin with orthokeratology for myopia control: study design and preliminary results. Curr Eye Res 2019; 44: 671–678. [DOI] [PubMed] [Google Scholar]
- 327.Chen Z, Huang S, Zhou J, et al. Adjunctive effect of orthokeratology and low dose atropine on axial elongation in fast-progressing myopic children-a preliminary retrospective study. Cont Lens Anterior Eye 2019; 42: 439–442. [DOI] [PubMed] [Google Scholar]
- 328.Wan L, Wei C-C, Chen CS, et al. The synergistic effects of orthokeratology and atropine in slowing the progression of myopia. J Clin Med 2018; 7: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tan Q, Ng AL, Choy BN, et al. One-year results of 0.01% atropine with orthokeratology (AOK) study: a randomised clinical trial. Ophthalmic Physiol Opt 2020; 40: 557–566. [DOI] [PubMed] [Google Scholar]
- 330.Sánchez-González JM, De-Hita-Cantalejo C, Baustita-Llamas MJ, et al. The combined effect of low-dose atropine with orthokeratology in pediatric myopia control: review of the current treatment status for myopia. J Clin Med 2020; 9: 2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Shih YF, Hsiao CK, Chen CJ, et al. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand 2001; 79: 233–236. [DOI] [PubMed] [Google Scholar]
- 332.Huang J, Mutti DO, Jones-Jordan LA, et al. Bifocal & atropine in myopia study: baseline data and methods. Optom Vis Sci 2019; 96: 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hui A, Bajgrowitz-Cieslak M, Phan CM, et al. In vitro release of two anti-muscarinic drugs from soft contact lenses. Clin Ophthalmol 2017; 11: 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Michaud L.Myopia management: how to get started. Contact Lens Spectrum 2020; 1: 25–30. [Google Scholar]
- 335.Zhu D, Wang Y, Yang X, et al. Pre- and postcycloplegic refractions in children and adolescents. PLoS One 2016; 11: e0167628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zadnik K, Sinnott LT, Cotter SA, et al. Prediction of juvenile-onset myopia. JAMA Ophthalmol 2015; 133: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Klaver C, Polling JR. and Erasmus Myopia Research Group. Myopia management in the Netherlands. Ophthalmic Physiol Opt 2020; 40: 230–240. [DOI] [PubMed] [Google Scholar]
- 338.McCullough S, Adamson G, Breslin KMM, et al. Axial growth and refractive change in white European children and young adults: predictive factors for myopia. Sci Rep 2020; 10: 15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Gifford KL, Richdale K, Kang P, et al. IMI-clinical management guidelines report. Invest Ophthtlmol Vis Sci 2019; 60: M184–M203. [DOI] [PubMed] [Google Scholar]
- 340.Li SM, Li SY, Kang MT, et al. Near work related parameters and myopia in Chinese children: the Anyang Childhood Eye Study. PLoS One 2015; 10: e0134514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Charman WN.Myopia, posture and the visual environment. Ophthalmic Physiol Opt 2011; 31: 494–501. [DOI] [PubMed] [Google Scholar]
- 342.Guo L, Yang J, Mai J, et al. Prevalence and associated factors of myopia among primary and middle school-aged students: a school-based study in Guangzhou. Eye 2016; 30: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Pärssinen O, Kauppinen M.Associations of reading posture, gaze angle and reading distance with myopia and myopic progression. Acta Ophthalmol 2016; 94: 775–779. [DOI] [PubMed] [Google Scholar]
- 344.French AN, Morgan IG, Mitchell P, et al. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology 2013; 120: 2100–2108. [DOI] [PubMed] [Google Scholar]
- 345.Guggenheim JA, Northstone K, McMahon G, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci 2012; 53: 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lim LS, Gazzard G, Low YL, et al. Dietary factors, myopia, and axial dimensions in children. Ophthalmology 2010; 117: 993–997. [DOI] [PubMed] [Google Scholar]
- 347.VanderVeen DK, Kraker RT, Pineles SL, et al. Use of orthokeratology for the prevention of myopic progression in children: a report by the American Academy of Ophthalmology. Ophthalmology 2019; 126: 623–636. [DOI] [PubMed] [Google Scholar]
- 348.Marcotte-Collard R, Simard P, Michaud L.Analysis of two orthokeratology lens designs and comparison of their optical effects on the cornea. Eye Contact Lens 2018; 44: 322–329. [DOI] [PubMed] [Google Scholar]
- 349.Gifford P, Tran M, Priestley C, et al. Reducing treatment zone diameter in orthokeratology and its effect on peripheral ocular refraction. Cont Lens Anterior Eye 2020; 43: 54–59. [DOI] [PubMed] [Google Scholar]
- 350.Smith EL, III. Optical treatment strategies to slow myopia progression: effects of the visual extent of the optical treatment zone. Exp Eye Res 2013; 114: 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Jones L, Drobe B, González-Méijome JM, et al. IMI-industry guidelines and ethical considerations for myopia control report. Invest Ophthtlmol Vis Sci 2019; 28; 60: 161–183. [DOI] [PubMed] [Google Scholar]
- 352.Pineles SL, Kraker RT, VanderVeen DK, et al. Atropine for the prevention of myopia progression in children: a report by the American Academy of Ophthalmology. Ophthalmology 2017; 124: 1857–1866. [DOI] [PubMed] [Google Scholar]
- 353.Chia A, Tay SA.Clinical management and control of myopia in children. In: Ang M, Wong TY. (eds) Updates on myopia. A clinical perspective. 1st ed. Singapore: Springer, 2020, pp.187–200. [Google Scholar]
- 354.Wong HB, Machin D, Tan SB, et al. Ocular component growth curves among Singaporean children with different refractive error status. Invest Ophthalmol Vis Sci 2010; 51: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 355.Zadnik K, Mutti DO.Refractive error changes in law students. Am J Optom Physiol Opt 1987; 64: 558–561. [DOI] [PubMed] [Google Scholar]
- 356.Kinge B, Midelfart A.Refractive errors among engineering students in Norway. Ophthalmic Epidemiol 1994; 1: 5–13. [DOI] [PubMed] [Google Scholar]
- 357.Lv L, Zhang Z.Pattern of myopia progression in Chinese medical students: a two-year follow-up study. Graefes Arch Clin Exp Ophthalmol 2013; 251: 163–168. [DOI] [PubMed] [Google Scholar]
- 358.Ganesan P, Wildsoet CF.Pharmaceutical intervention for myopia control. Expert Rev Ophthalmol 2010; 5: 759–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Cheng X, Xu J, Chehab K, et al. Soft contact lenses with positive spherical aberration for myopia control. Optom Vis Sci 2016; 93: 353–366. [DOI] [PubMed] [Google Scholar]
- 360.Aller TA.Clinical management of progressive myopia. Eye 2014; 28: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Walline JJ, Greiner KL, McVey ME, et al. Multifocal contact lens myopia control. Optom Vis Sci 2013; 90: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 362.Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci 2000; 77: 395–401. [DOI] [PubMed] [Google Scholar]
- 363.Cheng D, Schmid KL, Woo GC, et al. Randomized trial of effect of bifocal and prismatic bifocal spectacles on myopic progression: two-year results. Arch Ophthalmol 2010; 128: 12–19. [DOI] [PubMed] [Google Scholar]
- 364.Zhu Q, Liu Y, Tighe S, et al. Retardation of myopia progression by multifocal soft contact lenses. Int J Med Sci 2019; 16: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]



