Abstract
In patients with cirrhosis, sarcopenia is a critical reduction in skeletal muscle mass and frailty represents a status of global physical dysfunction caused by under nutrition, muscle wasting, and functional impairment. Both are prevalent conditions in liver transplant candidates and have shown to be independent predictors of adverse outcome. Evidence supports their incorporation into clinical practice both as a prognostic factor guiding clinical decision making and as a tool to identify candidates for physical and nutritional interventions. The wide heterogeneity of instruments used for sarcopenia and frailty measurement, the absence of a single suitable instrument for sarcopenia and frailty assessment in the outpatient versus inpatient acute‐on‐chronic clinical scenario, and the lack of strong evidence showing a beneficial effect of sarcopenia and frailty improvement on outcomes before and after transplantation are some of the questions that remain unanswered.
Frailty and sarcopenia are prevalent conditions in liver transplant candidates and have shown to be independent predictor of adverse outcome.Evidence supports their incorporation into clinical practice both as a prognostic factor guiding clinical decision‐making, and as a tool to identify candidates for physical and nutritional interventions.The wide heterogeneity of instruments used for sarcopenia and frailty measurement, the absence of a single suitable instrument for sarcopenia and frailty assessment in the outpatient vs inpatient acute‐on‐chronic clinical scenario and the lack of strong evidence showing a beneficial effect of sarcopenia and frailty improvement on outcomes before and after transplantation are some of the questions that remain unanswered.
Abbreviations
- 6MWD
6‐minute walk distance
- ACLF
acute‐on‐chronic liver failure
- ADL
activities of daily living
- CLIF‐C
Chronic Liver Failure Consortium
- CT
computed tomography
- CTP
Child‐Turcotte‐Pugh
- FrAILT
Functional Assessment in Liver Transplantation
- HFRS
hospital frailty risk score
- ICU
intensive care unit
- KPS
Karnofsky scale
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- NASH
nonalcoholic steatohepatitis
- SMI
skeletal muscle index
- TAM
transplantation for acute‐on‐chronic liver failure‐3 model
Most liver transplant (LT) centers worldwide currently prioritize organ allocation based on the Model for End‐Stage Liver Disease (MELD) score with or without the addition of serum sodium concentration (MELD‐Na). This system has been widely recognized for its objectivity, fairness, and success in reducing mortality on LT waiting lists.( 1, 2 ) Spain has the highest donation rate in the world, with waiting times, mortality, and drop‐offs from the lists that are better than in many other countries. Yet, despite these impressive achievements, out of 1,686 adults in 2019 included in the Spanish LT wait list, 45 (3%) individuals died and 85 (5%) were delisted due to worsening of their clinical condition (n = 28) and/or development of medical contraindications (n = 57).( 3 ) These data suggest that factors other than MELD should be considered in the decision‐making process regarding transplantation. Indeed, in recent years we have witnessed a change in the profile of LT candidates that is characterized by an increase in both the proportion of elderly patients (between 60 and 69 and over 70 years old) and proportion of those in whom cirrhosis is secondary to nonalcoholic steatohepatitis (NASH), which is frequently associated with present or past histories of cardiovascular risk factors.( 3 ) Altogether, there is an emerging phenotype of the typical LT candidate, increasingly described as sicker, medically more complex, and frail.( 4 )
The aim of this manuscript is to present a comprehensive (not systematic) review of currently available evidence of the implications of sarcopenia and frailty in patients with cirrhosis evaluated for LT. We conducted an exhaustive search of the PubMed database from early 2010 to September 2020. Keywords used were sarcopenia, frailty, skeletal muscle mass, acute‐on‐chronic liver failure (ACLF), cirrhosis, LT, and prehabilitation. Language was restricted to English. We included prospective and retrospective observational studies, clinical trials (randomized controlled trials and open noncontrolled trials), and expert opinion statements from recognized hepatology/LT international organizations. A total of 57 studies were included.
Definition of Sarcopenia and Frailty in Patients With Cirrhosis
Sarcopenia is a critical reduction in skeletal muscle mass. Initially considered aging related, it was later described in a wide variety of pathological states outside of the elderly population and in association with clinical negative effects.( 5 )
Frailty was first defined in the field of geriatrics as “a state of increased vulnerability to physical stress (i.e., surgery) and decreased physiologic reserve”.( 6 ) The frailty phenotype originally described by Fried et al.( 7 ) included sarcopenia, progressive immobility, decreased energy expenditure, and malnutrition and was considered to be a multidimensional concept that results from the compromise of multiple systems, including cardiovascular, neurologic, endocrine, and musculoskeletal as well as psychosocial.( 4 )
In hepatology, both frailty and sarcopenia are attracting growing interest due to their high prevalence in LT candidates and their robust association with adverse outcomes in cirrhosis and LT.( 4, 5 ) The need to find an objective measurement of frailty in patients with cirrhosis has led researchers to focus on physical frailty related to functional impairment, that is, loss of the ability to perform everyday activities and maintain health/wellness, excluding cognitive, social, or emotional factors.( 4 ) Therefore, while sarcopenia and physical limitation might be components of frailty in LT candidates, they are different conditions. The frailty syndrome encompasses more than loss of muscle mass as it integrates the concepts of functional performance, functional capacity, and disability and represents a situation of global physical dysfunction.( 5 )
In LT candidates, physical frailty and sarcopenia are partly influenced by liver disease severity because liver failure itself inevitably leads to this process. Other factors, such as age, nutritional status, and non‐liver related comorbidities (e.g., diabetes, heart disease, kidney failure) also contribute to the development of sarcopenia and the frailty phenotype in patients with cirrhosis( 6 ) (Fig. 1). In some patients, comorbidities, malnutrition, and sarcopenia have better prognostic value than the severity of liver disease as measured by the MELD score.( 5 ) Professionals who take care of those with liver failure have largely noticed that the same MELD score encompasses a group of patients with highly heterogeneous clinical manifestations and outcomes. In fact, the concept of frailty has always been taken into subjective consideration in the decision‐making process related to LT; yet, to date its integration into clinical practice has been anecdotal, hampered by the lack of consensus on its definition, assessment tools, and implications for decision making on LT.( 8 )
FIG. 1.
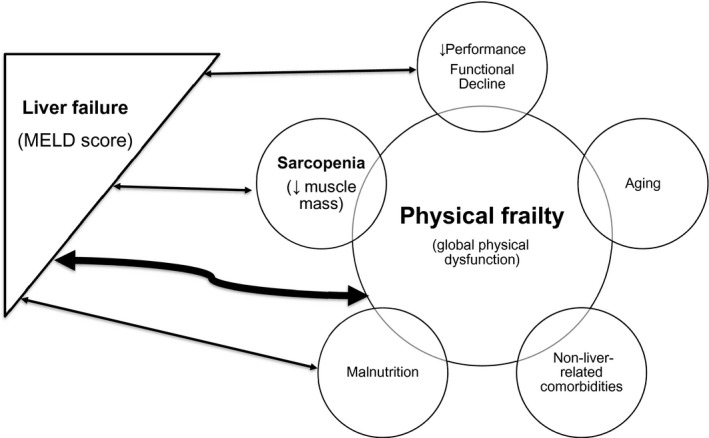
Contributors to sarcopenia and physical frailty in patients with end‐stage liver disease.
Clinical Vignette
To better illustrate the issues above mentioned, the present review will address the case of a 67 years old Caucasian male with cirrhosis of mixed aetiology (alcoholic and non‐alcoholic steatohepatitis ‐ASH/NASH‐) referred for LT evaluation.
Outpatient clinical scenario
His past medical history revealed moderate alcohol intake (80g weekly, abstinent for 6 months), obesity (body mass index 30.2), and long‐standing arterial hypertension and type 2 diabetes without micro or macrovascular complications.
Regarding the liver disease, the patient had well‐controlled ascites, had never been admitted for any hepatic decompensation event, had a MELD score of 15 and a B8 Child‐Turcotte‐Pugh (CTP) classification. He lived with his wife, was retired for 2 years, and was currently taking care of his grandchild on weekdays. The hepatologist’s general impression about the patient overall health was good but given his age and extra hepatic comorbidities he/she wondered whether to perform a frailty assessment as part of the pre‐transplant protocol.
Tests of physical frailty recommended in the literature were ordered and the following scores were obtained: Liver Frailty Index 3.1 (mild frailty); Six‐minute walk test 352 meters (absence of frailty, low limit of normality); KPS 80 (absence of frailty, low limit of normality) and no difficulties with ADLs. Once the remaining pre‐transplant tests were completed, the patient was discussed by the LT Selection Committee and after ruling out formal contra‐indications, he was listed with a MELD score of 15 and a CTP‐B8.
Acute on Chronic inpatient clinical scenario
Unfortunately, one month later the patient was admitted in the Intensive Care Unit (ICU) with septic shock (mean arterial pressure of 78 mmHg despite adequate resuscitation and cardiac output) probably from urinary origin (urine white blood cell >15/high power field and isolation of extended spectrum beta‐lactamase Escherichia coli in urine culture). 24h‐48h after admission the patient developed ACLF with jaundice (total bilirubin, 12 mg/dl), INR of 2.5, MELD score of 36, CTP‐C13, development of West‐Haven grade II‐III hepatic encephalopathy and acute kidney injury (creatinine, 1.8 mg/dl) without renal replacement therapy requirement. The patient had 3 organ failures with an ACLF grade 3 and a CLIF‐C ACLF Score of 65 at diagnosis. He was temporally inactive on the transplant list due to infection.
One week after admission, the urinary tract infection was controlled with antibiotics but the final ACLF grade and CLIF‐C ACLF Score at 7 days remained steady (grade 3 and 64 points, respectively). A TAM score and a frailty assessment were done to help determining possible LT futility. The TAM score of 2 points was compatible with low risk. However, as the patient was still in the ICU, with renal and neurological dysfunction, the sole feasible instruments to assess his frailty status were the KPS (10 to 20 points) and the SMI measurement on an CT scan (40 cm2/ m2), both compatible with severe frailty and sarcopenia.
In brief, a 67‐years old LT candidate, with ASH/NASH cirrhosis, extra‐hepatic comorbidities and mild/absent frailty at baseline is admitted in the ICU with an infection‐related ACLF grade 3 at diagnosis. One week after the onset of this acute decompensation, the patient’s clinical course tended to stabilization with a persistent ACLF grade 3 with 3 organ failures, MELD score 36, CTP C13 and sarcopenia. Ten days after admission, the infectious process was under control and a decision of whether to proceed with LT was needed.
Our patient was carefully discussed at the LT Selection Committee who recommended intensive nutritional therapy given that the clinical situation did not allow physical intervention. The patient was maintained temporally inactive on the waitlist with close clinical monitoring and frailty severity reassessment every 2‐4 weeks. Two weeks after admission clinical improvement was confirmed without the need for hemodynamic support and the patient was discharged from the ICU to the hepatology ward with a MELD score of 35, without signs of infection and undergoing intensive rehabilitation and nutritional therapies (tailored to the patient’s clinical situation). Due to persistent signs of hepatic encephalopathy, frailty was reassessed using the KPS (20‐30 points) and the SMI measurement on a new CT scan with a result of 40 cm2/ m2 (as prior), compatible with severe frailty and sarcopenia.
Post‐Tranplant clinical scenario
One month after patient’s admission, the LT Committee decided to proceed with LT due to clinical improvement and no further deterioration of frailty status. The postoperative course following LT from a brain death young donor was uneventful, with excellent graft function and total recovery of renal function.
Twelve months post‐transplantation the patient was in good clinical condition, with normal liver chemistry tests yet maintaining impaired frailty status, which assessed on the last clinic visit, had only improved modestly with a SMI on a CT abdominal scan of 45 cm2/ m2 (still compatible with sarcopenia) and a Liver Frailty Index of 3,5 (moderate frailty). Of note, post‐transplant rehabilitation was not carried out.
Sarcopenia and Physical Frailty as Predictors of Outcome in Outpatient Versus Inpatient
In the last 5‐10 years, multiple publications have demonstrated an association between sarcopenia/frailty and adverse outcomes both before and after LT (Table 1).
Table 1.
Association between sarcopenia and frailty and adverse outcomes before and after LT
| Time | Outcome | Authors | Study Design | Sample Size (n) | Performance Tool | Hazard Ratio (95% CI)* |
|---|---|---|---|---|---|---|
| Before transplant | Mortality/wait‐list mortality | Carey et al.( 13 ) | Prospective, single center (USA) | n = 121 | 6MWD | 0.58 (0.37‐0.93); P = 0.02 |
| Lai et al.( 6 ) | Prospective, single center (USA) | n = 294 | ADL | 1.18 (0.87‐1.58); P = 0.28 | ||
| per point decrease | ||||||
| Montano‐Loza et al.( 22 ) | Prospective, single center (Canada) | n = 669 | L3‐SMI | 0.97 (0.96‐0.99); P < 0.001 | ||
| Tapper et al.( 31 ) | Retrospective, single center (USA) | n = 734 | ADL | 1.83 (1.05‐3.20) | ||
| Orman et al.( 32 ) | Retrospective, multicenter (USA) | n = 7,9092 | KPS | Category B (KPS 50%‐70%) 1.14 (1.11‐1.18) | ||
| Category C (KPS 10%‐40%) 1.63 (1.55‐1.72) | ||||||
| Tandon et al.( 33 ) | Prospective, multicenter (USA + Canada) | n = 954 | KPS | 0.97 (0.96‐0.98); P = 0.03 | ||
| Kardashian et al.( 15 ) | Prospective, single center (USA) | n = 1,405 | Liver frailty index | 0.046 (0.030‐0.068) | ||
| wait‐list mortality at 3 months | ||||||
| Hospitalization for ACLF | Praktiknjo et al.( 20 ) | Prospective, single center (Germany) | n = 186 | Psoas muscle thickness | 0.808 (0.736‐0.886); P < 0.001 | |
| Shah et al.( 36 ) | Retrospective, multicenter (USA) | n = 16,561 | HFRS | 1.03 (1.02‐1.03); P < 0.001 | ||
| per 1‐unit increase | ||||||
| After transplant | Overall complications | Lee et al.( 25 ) | Retrospective, single center (USA) | n = 325 | Dorsal muscle group area | 0.48 (0.32‐0,72); P = 0.001 |
| per 1‐unit increase | ||||||
| Valero et al.( 26 ) | Retrospective, single center (USA) | n = 96 | Psoas area | 3.01 (1.19–7.63); P = 0.02 | ||
| Posttransplant hospital stay | Di Martini et al.( 24 ) | Retrospective, single center (USA) | n = 338 | L3‐SMI | Men, –0.02; (P < 0.001) | |
| Women, –0.01; (P < 0.05) | ||||||
| Montano‐Loza et al.( 21 ) | Retrospective, single center (Canada) | n = 248 | L3‐SMI | 40 ± 4 days with sarcopeniavs. 25 ± 3 days without sarcopenia; (P = 0.005) | ||
| Frailty phenotype | Lai et al.( 16 ) | Prospective, single center (USA) | n = 214 | Liver frailty index | Worsened 3 months post‐LT, 3.9 (3.5‐4.4); P = 0.02 | |
| Similar 6 months post‐LT, 3.7 (3.2‐4.1); P = 0.07) | ||||||
| Improved 12 months post‐LT, 3.4 (3.0‐3.9); P < 0.001) |
For each frailty measure P value; results adjusted for covariates.
Abbreviation: CI, confidence interval.
Currently, a large portion of the decision to list a patient for transplant is based largely on subjective judgement.( 9 ) The clinician assessment (i.e., the “eye‐ball test,” the clinical frailty scale, or the Braden scale) has been shown to predict wait‐list mortality in patients with cirrhosis.( 10 ) Despite its subjectivity and interobserver variability, this approach is part of life and death decisions. Therefore, the need to find more objective measures of frailty and sarcopenia in this patient population cannot be overemphasized. Indeed, the North American expert opinion statements on sarcopenia and frailty in LT recommends that every patient with cirrhosis waiting for LT should undergo performance assessments at baseline and longitudinally to guide clinical decision making and to identify patients for intervention.( 4, 5 ) However, there is significant heterogeneity in the metrics used to quantify sarcopenia and frailty in the published literature (Table 2). The great challenges in identifying a single standard test are many different modalities available for muscle mass and frailty measurement and the lack of evidence supporting a single tool as suitable for frailty and sarcopenia assessment in different clinical settings related to LT, mainly inpatient versus outpatient. Considering the need for objectivity, speed, and low cost, the experts selected a battery of tools of choice depending on the patient's clinical scenario, available resources, and the clinical decision to be made based on the test results( 4, 5 ) (Table 3).
Table 2.
Instruments used to measure sarcopenia and physical frailty
| Measure | Type of Measurement | Frailty Tools | Characteristics | |
|---|---|---|---|---|
| Anatomical measures | Muscle mass (sarcopenia) | Anthropometry( 5 ) | Size, weight, BMI, tricipital fold, mid‐arm and calf circumference | |
| Bioimpedance( 5 ) | Lean mass + fat mass + fat BMI + phase angle | |||
| Densitometry( 5 ) | Lean and fat mass indices | |||
| Cross‐sectional imaging tests (CT/MRI) | Psoas muscle area( 29 ) | Sum of the areas of the two psoas at the level of the third or fourth lumbar vertebra | ||
| Psoas thickness( 29 ) | Transversal diameter measured at the level of the umbilicus | |||
| SMI( 27, 28 ) | Sum of the area of all skeletal muscles at the level of the third or fourth vertebra | |||
| Functional measures | Capacity for physical performance | Self‐reported tests | ADL( 11 ) | Daily self‐care activities (feeding, showering, personal and toilet hygiene, dressing, grooming, transferring) |
| Instrumental ADL( 11, 15 ) | Activities that let an individual live independently (e.g., homemaking, cooking, shopping, taking medications, using telephone) | |||
| KPS( 11 ) | Functional scale running from 100 to 0 in 10 by 10 intervals. 100, perfect health; 90, minor signs and symptoms; 80, some signs or symptoms; 70, unable to work; 60, occasional assistance; 50, considerable assistance; 40, disabled; 30, hospitalization indicated; 20, hospitalization necessary; 10, moribund | |||
| Performance‐based tests | Fried frailty instrument( 7, 11 ) | Physical inactivity + weakness + slowness + exhaustion + weight loss | ||
| Short physical performance battery( 11 ) | Balance + chair stand + gait speed | |||
| 6MWD( 11 ) | Distance covered by walk in 6 minutes | |||
| Liver frailty index( 12, 15 ) | Grip strength + chair stands + balance. Specific for cirrhosis. | |||
| Clinical measures | Hospital admissions | Administrative data | HFRS( 35 ) | From the International Classification of Diseases coding system; not specifically validated in patients with cirrhosis. Research studies. |
Abbreviations: BMI, body mass index; MRI, magnetic resonance imaging.
Table 3.
Selection of sarcopenia/frailty instruments and sarcopenia/frailty staging criteria depending on clinical setting in patients with cirrhosis*
| Patient Type | Sarcopenia/Frailty Tools | Stages of Sarcopenia/Frailty | |||
|---|---|---|---|---|---|
| Severe | Moderate | Mild/Absent | |||
| Outpatient | Performance‐based tests (frailty tools) | Liver frailty index | ≥4.2 | 3.2‐4.1 | <3.2 |
| 6MWD | <250 m | 250‐350 m | >350 m | ||
| Inpatient | Self‐reported tests (frailty tools) | ADL | Difficulty with ≥2 ADL | Difficulty with 1 ABVD | No difficulty with ABVD |
| KPS | 0‐40 | 50‐60 | ≥80 | ||
| Abdominal CT scan (sarcopenia tool) | SMI | Men <50 cm2/m2 | Men >50 cm2/m2 | ||
| Women <39 cm2/m2 | Women >39 cm2/m2 | ||||
Adapted from Lai et al.( 4 )
Outpatient prognostic tools
From a functional point of view, frailty can be measured as the impaired capacity for physical performance by performance‐based tests (Fried frailty instrument, short physical performance battery, 6‐minute walk distance [6MWD], and liver frailty index), which have mostly been originally used in the field of geriatrics; they have been investigated in the outpatient liver disease setting where patients are in a relatively stable clinical situation and have been useful in predicting mortality in LT candidates.( 11 )
To date, the liver frailty index and the 6MWD are the frailty instruments with the wider applicability in the outpatient clinical scenario given their objectivity and ease of use at baseline and longitudinally.( 4 )The 6MWD is the maximum distance (in meters) covered by walking on a flat and straight surface in 6 minutes,( 11 ) and the recently published liver frailty index is a continuous index that is specific for patients with cirrhosis. The liver frailty index consists of three simple performance‐based tests: (i) dominant grip strength, measured by a dynamometer (in kilograms); (ii) chair stands (time in seconds) that it takes for a patient to stand up and sit down in a chair 5 times without using the arms; (iii) balance, which is the patient’s ability of balancing while holding three positions (side, semitandem, and tandem) for 10 seconds each.( 12 ) An online calculator is available (https://liverfrailtyindex.ucsf.edu/) to facilitate its determination.
Results from two large prospective cohort studies have contributed to the emergence of these constructs of frailty as important drivers of mortality in patients with end‐stage liver disease. In an American single‐center study that included 121 patients waiting for LT, a 6MWD <250 m was identified as a risk factor for mortality on the wait list independent of liver disease severity.( 13 ) The ongoing Functional Assessment in Liver Transplantation (FrAILT) Study, from which the liver frailty index was derived, includes outpatients with cirrhosis listed for LT at nine LT centers in the United States. Subjects enrolled undergo frailty assessments using performance‐based and self‐reported tests previously employed in the field of geriatrics.( 12 ) Results from a subset of patients in the FrAILT cohort robustly showed that the liver frailty index predicts wait‐list mortality independently of liver disease severity, ascites, and hepatic encephalopathy.( 5 , 14 ) Optimal liver frailty index cutoffs have been established to identify “frail” LT candidates with increased risk for LT wait‐list mortality (4.4 for 3‐month mortality prediction and 4.2 for prognostication at 6 and 12 months), and the addition of the liver frailty index to the MELD‐Na score has been found to more accurately predict wait‐list mortality over MELD‐Na alone.( 15 ) However, studies are scarce in the posttransplant setting. Unlike the liver frailty index, there is no evidence proving the 6MWD predictive validity for posttransplant outcomes.( 4 ) Data from 214 LT recipients included in the FrAILT Study who had serial liver frailty index testing (pretransplant and at 3, 6, or 12 months posttransplant) and were stratified at each time point as being robust, prefrail, and frail showed that LT candidates who were frail had longer lengths of hospitalizations after LT. Unexpectedly, frailty scores in all groups worsened at 3 months posttransplant, and less than 40% of patients (even those deemed robust pretransplant) had achieved robustness by 1 year.( 16 ) The results of this well‐designed prospective study underscore the need to incorporate measures to prevent frailty before LT to achieve full recovery potential posttransplant.
A limitation of these physical tests is the fact that they may be affected by specific situations that may not reflect the functional reality of the patient. For example, a patient with a large volume of ascites may have greater difficulty in performing the tests and may obtain different measurements of frailty before and after paracentesis. However, published studies that include patients with refractory ascites suggest that the lower scores obtained in the frailty tests actually reflect their scarce muscle mass and deficient nutritional status rather than an inability to follow test instructions.( 11, 12 )
Inpatient prognostic tools
During hospital admission, patients usually present transient alterations in their physical and cognitive function, limiting the ability of the previously mentioned performance‐based frailty assessments to estimate their physiological reserve. Sarcopenia metrics and several scales of physical frailty have been evaluated in the inpatient context (Table 3).
In the last decade, strong evidence has demonstrated that the measurement of sarcopenia is associated with poor outcomes in hepatology/LT and is useful in the hospital setting given the low likelihood of being affected by acute changes in functional performance.( 5 ) In patients with cirrhosis waiting for LT, sarcopenia is associated with decreased quality of life,( 17 ) increased rate of liver‐related complications,( 18 ) and length of hospital stay.( 18 ) Indeed, skeletal muscle plays an integral role in ammonium detoxification, and sarcopenia has been identified as an independent risk factor for hepatic encephalopathy in patients with cirrhosis.( 19 ) Other complications, such as ascites, infections, and ACLF, are also more frequent in patients with cirrhosis with sarcopenia than in patients with cirrhosis without sarcopenia.( 20, 21 ) A Canadian single‐center study that included more than 600 patients waiting for LT showed that the inclusion of sarcopenia (defined from specific cutoffs of the skeletal muscle index [SMI] at the third lumbar vertebra in a computed tomography [CT] scan) within MELD (MELD‐sarcopenia score) enhanced the prediction of mortality in patients with cirrhosis, especially in LT candidates with low MELD scores.( 22 ) After LT, sarcopenia is associated with a higher probability of death,( 23 ) a longer duration of hospital stay,( 24 ) and overall increased rate of complications.( 25, 26 ) Another study from Canada that enrolled 248 patients with cirrhosis who had a CT scan before LT and defined sarcopenia from specific cutoffs of the SMI at the third lumbar vertebra confirmed that sarcopenia increases the risk of perioperative bacterial infections.( 21 )
The North American group of experts in sarcopenia advocates for the use of CT‐based skeletal muscle area measured at the third lumbar vertebra on an abdominal CT scan using body segmentation software (e.g., ImageJ, sliceOmatic)( 5, 27 ) and sex‐specific cut‐off values (SMI <50 cm2/m2 in men and <39 cm2/m2 in women) to standardize sarcopenia measurement in LT candidates.( 5, 28 ) Reasons presented by this consortium are (i) SMI appears to be a better measurement than the individual measure of the psoas muscle or the psoas muscle index, especially in men with cirrhosis( 29 ); (ii) cross‐sectional imaging tests are routinely used in most LT centers as part of the pretransplant protocol (to assess both biliary and vascular anatomy) and to monitor LT candidates with hepatocellular carcinoma; (iii) preliminary studies with magnetic resonance imaging‐based images have found similar results to those based on a CT scan,( 30 ) but the latter is cheaper, more widely available, and faster to perform in clinical practice. Notwithstanding, measurement of SMI on an abdominal CT scan has some limitations, including that the need to add specific software to the CT imaging in updated equipment may result in centers doing an SMI assessment with standard CT scanners and that simply determining the SMI does not equate to a full evaluation of frailty. In addition, some important aspects remain unclear regarding the use of a CT‐based estimation of skeletal muscle mass, such as (i) the validity of the measure of only the psoas muscle versus the total muscle area; (ii) the utility of values below some specific percentiles (i.e., fifth percentile); and (iii) the sensitivity of change in values over time.( 5 )
Self‐reported tests (Karnofsky performance status [KPS], activities of daily living [ADL]) are provider‐ and patient‐assessed frailty instruments that were initially used in the geriatric population but have also been evaluated in the context of liver disease. They are functional scales that are simple and quick to perform systematically in the inpatient setting. For frailty classifications, established cutoffs are used to define robust (KPS ≥80; no difficulty with ADL), prefrail (KPS between 50 and 60; difficulty with one ADL), and frail (KPS ≤40; difficulty with two or more ADL)( 4 ) (Table 3). KPS and ADL have been demonstrated to predict nontransplant‐related mortality,( 31, 32, 33 ) re‐admissions,( 31, 34 ) and mortality after LT( 32 ) in both large prospective and retrospective studies; yet, these self‐reported tests include subjective aspects and thus may differ between operators and be insensitive to subtle but prognostic increments of the frailty spectrum.( 4 )
Finally, in the research domain, the hospital frailty risk score (HFRS) has been developed using the International Classification of Diseases coding, a system largely implemented in administrative hospital databases. This score was recently used to evaluate frailty in patients with ACLF but remains to be validated specifically for patients with cirrhosis.( 35, 36 )
Prognostic tools have been developed to improve outcome prediction in patients with ACLF.( 37, 38, 39, 40, 41 ) According to the large observational study carried out by the European Association for the Study of the Liver–Chronic Liver Failure (EASL‐CLIF) Consortium and called the EASL‐CLIF Acute‐on Chronic Liver Failure in Cirrhosis (CANONIC) study, the initial grade of ACLF coupled with disease severity and a specifically developed score (CLIF‐Consortium [CLIF‐C] ACLF score [with web calculator at http://www.efclif.com]) seem to better predict short (28‐day) and medium‐term (90‐day) mortality when compared to the classical scores (MELD, MELD‐Na, and CTP) in this patient population.( 38 ) Of note, the grade of ACLF is defined by the number of organ failures (liver, kidney, brain, coagulation, circulatory, respiratory), while the CLIF‐C ACLF score and probability of dying is calculated by adding age and white cell count.( 37 )
In the absence of multiple organ failures (four or more organ failures), posttransplant survival in patients with ACLF is good.( 40 ) A recent multicenter study validated a prognostic model as a predictor of posttransplant survival in patients with ACLF grade 3, the transplantation for ACLF‐3 model (TAM) score. It is based on four independent pre‐LT risk factors (age ≥53 years, arterial lactate level ≥4 mml/L, mechanical ventilation with the ratio of arterial oxygen partial pressure to fractional inspired oxygen ≤200 mm Hg, and white cell count ≤10 g/L) and distinguishes a high‐risk from a low‐risk group of individuals, identifying an optimal window for transplantation.( 41 ) Of note, sarcopenia and functional status likely play an additional role in determining outcome in ACLF. To date, only two retrospective cohort studies have evaluated the importance of frailty/sarcopenia in the context of ACLF.( 20, 36 ) Recent research that included 16,561 hospitalized patients with cirrhosis found that frailty (ascertained using the HFRS, based on population‐level data) was significantly associated with a higher risk of ACLF‐related hospitalizations and that this risk increased with frailty severity. Surprisingly, frailty did not impact short‐term ACLF mortality despite predicting poorer survival among all cirrhosis hospitalizations.( 36 ) In a recent multicenter study that enrolled 186 patients with decompensated cirrhosis during the first year following transjugular intrahepatic portosystemic shunt, sarcopenia (defined using sex‐specific cutoffs of the psoas muscle thickness) was associated with an increased risk of developing ACLF, especially fatal ACLF ending with death (50% in patients with sarcopenia vs. 11% in patients without sarcopenia).( 20 ) Given that disbalanced inflammation has been identified as central to the pathogenesis and outcome of ACLF, the authors suggested that sarcopenia could be considered a clinical manifestation of an underlying chronic systemic inflammation.( 42 )
To sum up, the overarching conclusion emerging from the literature is that sarcopenia and physical frailty are key determinants of adverse outcomes in the LT setting and strongly predict pretransplant mortality independent of liver disease severity (Table 1). The results were mostly derived from large prospective studies. Conversely, data in the posttransplant population are less robust and mostly based on retrospective investigations (Table 1). The main limitation of published studies is the vast heterogeneity of instruments used to assess sarcopenia and physical frailty. Moreover, most of the studies are North American single‐center studies, which jeopardize the generalization of results. There is lack of consensus on a single index to unify decision making and to allow an equitable evaluation of outcomes for all patients with cirrhosis across all transplant centers.( 8 ) The liver frailty index and the 6MWD seem to be the more suitable instruments to use in the outpatient context; yet, there are no convincing data on their performance for inpatients.( 4 ) Measurement of SMI on an abdominal CT scan and self‐reported tests (KPS, ADL) have a key utility in the inpatient ACLF clinical scenario where performance‐based frailty assessments may be compromised due to acute changes in functional performance and therefore may not appropriately reflect the patient’s underlying physiological reserve in a clinical stable situation (Table 3).
Utility of Sarcopenia and Physical Frailty in Clinical Practice
Prognostic factors guiding clinical decision making
The MELD score can accurately predict 90‐day wait‐list mortality for most patients with cirrhosis, and because it has widespread implementation, reductions in the number of patients listed for LT, wait‐list time, and deaths on the wait list have been widely proven.( 43, 44 ) Nevertheless, one of the major limitations of the MELD score is that it does not include an assessment of the nutritional and functional status of LT candidates.( 22 ) Serum creatinine values (included into the MELD score calculation formula) depend on both renal function and muscle mass; thus, frailty/sarcopenia might potentially penalize patients while hindering their access to LT with the current prioritization system.
Data on the ability of the MELD score to predict survival following LT are also conflicting.( 45 ) The advantages of using a frailty‐based score are that it incorporates the extrahepatic manifestations of cirrhosis (malnutrition and functional decline) as well as comorbidities not related to liver disease (e.g., age, diabetes, heart disease), thus becoming a useful predictor of outcome both before and after LT. Of note, the experts on sarcopenia and frailty emphasize that a single assessment should never be the only reason for not including or removing a patient from the LT wait list because there is no evidence to support a specific sarcopenia or frailty cutoff beyond which a person should not undergo LT.( 4, 5 ) The patient’s frailty/sarcopenia status should be handled as one of many objective criteria (medical, functional, or psychosocial) routinely considered when determining transplant candidacy. Given that patients with sarcopenia and frailty waiting for LT have a higher risk of death than predicted by their MELD score and an increased risk of ACLF requiring hospitalization, sarcopenia and physical frailty could be incorporated into clinical practice as a predictor of outcome complementary to the MELD score. In fact, it has been proven that adding frailty/sarcopenia to the MELD score improves the prediction of mortality in patients with cirrhosis, mainly in patients with low MELD scores who are the most disadvantaged by the current prioritization system.( 15, 22 ) The recent expert frailty consensus statement recommends serial frailty assessments in all LT candidates every 2 to 12 weeks while on the wait list, and the implications regarding LT will depend on the severity of frailty (Table 4).( 4 ) Early identification of frail patients might prompt shorter follow‐up periods to better monitor health status, possibly expedite transplant evaluation, and start strategies to mitigate the risk, if needed.( 20, 36 ) Studies investigating modifications of the MELD score where serum creatinine is replaced by a parameter not influenced by the patient’s muscle mass should also be promoted.
Table 4.
Recommendations for frailty assessment and prehabilitation based on frailty status in LT candidates*
| Type | Frailty Assessment | ||
|---|---|---|---|
| Severe | Moderate | Mild/Absent | |
| Type of prehabilitation | Intensive inpatient prehabilitation (2‐4 weeks) | Supervised home‐based exercise programs (4‐12 weeks) | Standard physical exercise recommendations |
| Wait list follow‐up | Consider temporal wait‐list inactivation | Close monitoring | Regular follow‐up |
| Frailty reassessment (every 2‐4 weeks) | Frailty reassessment (every 4‐12 weeks) | Frailty reassessment (every 12 weeks) | |
| LT | LT if frailty reverses | LT if frailty stabilizes | LT as usual |
| Posttransplant rehabilitation | Yes | Yes | No |
Adapted from Lai et al.( 4 )
Intervention
Sarcopenia and frailty are risk factors for patients with cirrhosis that differ from other more traditional risk factors, such as age, sex, or the MELD score, in that they are potentially modifiable with nutritional interventions and exercise.( 46, 47 )
Objective tools to measure physical frailty can identify the best timing to refer a patient before frailty to a specific physical and nutritional intervention as well as to access the benefits of a specific program.( 11, 48 )
In recent years, there has been a growing interest in the concept of “prehabilitation” in the field of surgery.( 49 ) This term refers to multidisciplinary training focused on physical and nutritional status aimed at enhancing a patient’s physiological reserve before surgery.( 4 ) The literature regarding major abdominal surgeries has shown that prehabilitation programs (such as comprehensive physical activity programs, home‐based supervised exercises, educational and lifestyle interventions, and/or nutritional advice) improve results and decrease the cost of the procedure.( 4 ) In the LT setting, published data on the impact of prehabilitation are limited to small single‐center cohorts, but results point to a slowdown in progression and even a potential reversibility of sarcopenia and physical frailty( 50, 51, 52, 53, 54, 55, 56, 57 ) (Table 5). A Spanish study investigated changes in maximal strength, aerobic capacity, and health‐related quality of life in LT recipients after a combination of supervised resistance and aerobic training and observed a significant improvement in physical condition.( 56 ) More recently, in a British pilot study, patients with cirrhosis waiting for LT were involved in an intensive aerobic exercise program highlighting that these types of programs are also feasible and possibly beneficial at improving fitness in patients with advanced liver disease.( 57 ) Likewise, there are several ongoing prospective studies aimed at assessing the effects of different prehabilitation programs on outcomes in LT candidates and recipients (i.e., trial code NCT02367092). Expert groups currently recommend including sarcopenia and frailty measures in clinical practice to identify patients who are candidates for prehabilitation programs focused on nutritional status and physical activity optimization.( 4, 5, 58 ) Expert consensus documents emphasize that all LT candidates should be provided with recommendations on exercise and nutrition. Overall, therapeutic interventions to target sarcopenia and frailty include (i) nutritional counseling with a caloric intake based on an ideal body weight that favors protein containing key amino acids and avoids starvation; (ii) combination in a 3:2 ratio of aerobic and resistance training; and (iii) pharmacologic treatment with vitamin D3 as vitamin D deficiency is a well‐known sarcopenia driver; L‐carnitine to suppress muscle loss; and/or testosterone replacement for male patients with hypogonadal cirrhosis to preserve muscle mass and function.( 4, 5 ) The effects of L‐carnitine and testosterone supplementation on muscle mass have not been widely assessed in patients with cirrhosis, but recent evidence has shown their potential utility to treat and/or prevent sarcopenia in this population. A retrospective case‐control study from Japan enrolled 35 patients with cirrhosis who received L‐carnitine supplementation during at least 6 months and 35 propensity score‐matched patients who did not receive the study drug. Authors demonstrated L‐carnitine had a preventive effect on skeletal muscle depletion (assessed by the psoas muscle mass index on CT images) without significant adverse events reported.( 59 ) Similarly, one randomized, double‐blinded, placebo‐controlled clinical trial of intramuscular testosterone undecanoate in 101 men with cirrhosis and low baseline testosterone showed testosterone safely increased muscle mass, as evaluated by measurement of both appendicular and total lean body mass using dual energy X‐ray absorptiometry.( 60 ) However, given the scarcity of health resources, an algorithm to tailor prehabilitation intensity to the sarcopenia and frailty status of the LT candidate has been developed to benefit the most vulnerable patients (Table 4). Of note, there is a need to further assess the impact of frailty improvement following prehabilitation programs on outcomes before and after LT.
Table 5.
Studies investigating exercise intervention in patients with cirrhosis and LT recipients*
| Type | Author | Study Design, Duration | Population | Study Groups | Intervention | Outcome† | ||
|---|---|---|---|---|---|---|---|---|
| Pretransplant | Román et al.( 50 ) | RCT, 12 weeks | n = 17 | Exercise (n = 8) | Supervised moderate exercise (treadmill or cycle ergometry 1 hour, 3 days/week) | Exercise capacity | Within exercise group comparison relative to baseline measures | Increase in 6MWD, 365 vs. 445 m (P = 0.01) |
| MELD 7‐13, | Control | All received leucine (10 g/day) | Increase in number of steps climbed during 2‐minute step tests (P = 0.02) | |||||
| CTP A 82% | (n = 9) | Muscle thigh circumference | Increase in lower thigh circumference, 41 vs. 46 cm (P = 0.02) | |||||
| Health‐related quality of life (SF‐36) | Improvement in three domains of SF‐36: | |||||||
| general health (P = 0.03); | ||||||||
| vitality (P = 0.01); | ||||||||
| social function (P = 0.04) | ||||||||
| Zenith et al.( 51 ) | RCT, 8 weeks | n = 20 | Exercise (n = 9) | Supervised moderate exercise (cycle ergometry 40 minutes, 3 days/week) | Exercise capacity | Between‐group comparison | VO2 peak, 5.3 mL/kg/minute higher in exercise group (P = 0.001). | |
| MELD 10 | Control (n = 10) | Muscle thigh circumference | (Exercise better than control) | Increase in quadriceps muscle thickness (P = 0.01) | ||||
| CTP A 84% | Health‐related quality of life (EQ‐VAS) | EQ‐VAS increase by a mean of 20.4 points on a 0‐100 scale (P = 0.01) | ||||||
| Debette‐Gratien et al.( 52 ) | Open noncontrolled clinical trial, 12 weeks | n = 13 | Exercise (n = 8) | Supervised moderate exercise (cycle ergometry ≥20 minutes + resistance training 20 minutes, 2 days/week) | Exercise capacity | Comparison relative to baseline measures | VO2 peak increase in 1.7 mL/kg/minute | |
| Dropouts n = 6 | No control | (P < 0.008) | ||||||
| MELD 7‐21 | 6MWD increase in 40 m (P < 0.02) | |||||||
| CTP A 63% | Muscular strength | Mean quadriceps strength improved 7 kg force (P < 0.008) | ||||||
| Macías‐Rodríguez et al.( 53 ) | RCT, 14 weeks | n = 22 | Exercise (n = 14) | Supervised moderate exercise (cycle ergometry 40 minutes, 3 days/week) | Portal hypertension | Between‐groups comparison. | HVPG, 6.5 mm Hg lower than controls in exercise group (P = 0.009) | |
| Control (n = 15) | Functional capacity (CPET) | Within‐exercise group comparison relative to baseline | Improvement in ventilatory efficiency (P = 0.033) | |||||
| All nutritional therapy | ||||||||
| Román et al.( 54 ) | RCT, 12 weeks | n = 25 | Exercise (n = 15) | Supervised exercise (1 hour, 3 days/week) | Functional capacity (CPET) | Within exercise group comparison relative to baseline | Higher total effort time (P < 0.001) | |
| Control, relaxation program (n = 10) | Body composition (DXA) | Fat body mass decrease (P = 0.003) and lean body mass increase (P = 0.01) | ||||||
| Risk of falls | Risk of fall decrease (P = 0.02) | |||||||
| Kruger et al.( 55 ) | RCT, 8 weeks | n = 40 | Exercise (n = 20) | Home‐based exercise (moderate to high intensity cycling exercise, 3 days/week) | Exercise capacity | Between‐groups comparison (exercise better than control) | In the exercise‐adherent group (≥80% training sessions): | |
| Control, usual care (n = 20) | 6MWD increase by 46.4 m (P = 0.009) | |||||||
| VO2 peak increase by 2.8 mL/kg/minute (P = 0.02) | ||||||||
| Morkane et al.( 57 ) | Prospective cohort study, 6 weeks | n = 16 | Exercise (n = 16) | Supervised exercise on a static bike 3 days/week | Exercise capacity (CPET) | Comparison relative to baseline measures | VO2 peak increase by 2.3 mL/kg/minute (P = 0.02) | |
| Dropouts (n = 7) | No control | |||||||
| LT candidates | ||||||||
| Posttransplant | Moya‐Nájera et al.( 56 ) | RCT, 24 weeks | n = 54 | Exercise (n = 22) | Supervised exercise (cycloergometer incremental ramp protocol, 6 minutes, 2 days/week) | Exercise capacity | Between‐groups comparison (exercise better than control) | VO2 peak increase 15% vs. 7% (P = 0.04) |
| Dropouts (n = 4) | Control (n = 28) | Muscle strength | Maximal global strength increase 31% vs. 9% (P = 0.001) | |||||
| Health‐related quality of life | Improvement in physical functioning and vitality (P = 0.03) | |||||||
Adapted from Tandon et al.( 47 )
None of the studies showed that exercise intervention improved transplant outcomes.
Abbreviations: CPET, cardiopulmonary exercise test; DXA, dual energy X‐ray absorptiometry; EQ‐VAS, EuroQol‐visual analogue scale; HVPG, hepatic venous pressure gradient; RCT, randomized controlled trial; SF‐36, Short Form 36; VO2, peak exercise oxygen uptake.
Research Needed
Important research questions regarding sarcopenia and frailty assessment in patients with cirrhosis evaluated for LT remain unsolved. These include (i) the impact of sarcopenia and frailty status on mortality following LT, particularly in the inpatient ACLF setting; (ii) the impact of longitudinal changes in sarcopenia and frailty status in LT outcomes; and (iii) the relationship between sarcopenia/frailty and liver disease progression. Further research in this field is greatly needed, particularly large multicenter studies.
Conclusion
While sarcopenia is an excessive loss of muscle mass, frailty in patients with cirrhosis results from malnutrition, muscle wasting, and functional decline.
Sarcopenia and frailty are predictors of adverse outcome in the LT setting, and there is robust evidence they both predict the risk of pretransplant mortality independent of the severity of liver disease. Data are less robust in the posttransplant setting where there is a need for well‐designed, prospective, multicenter studies.
One of the challenges in incorporating sarcopenia and frailty in clinical practice is the wide heterogeneity of instruments used for their measurement. Considering different patient clinical settings and the need for high‐speed, low‐cost, and objectivity in the LT field, the liver frailty index and the 6MWD in outpatients and self‐reported tests (KPS, ADL) and the SMI measurement on an abdominal CT scan in hospitalized patients (particularly those with ACLF) have the broadest applicability among the performance tools.
In summary, sarcopenia and frailty metrics should be incorporated into clinical practice as prognostic factors guiding clinical decision making and intervention tools to identify candidates for prehabilitation programs and to establish nutrition and physical status optimization before LT. A single sarcopenia and/or frailty assessment should never be the only criteria for not including or removing a patient from the LT wait list but should be handled as one of many objective criteria routinely considered when determining transplant candidacy. Standardized sarcopenia and frailty measures could be used to guide the type and intensity of physical and nutritional interventions in LT candidates and recipients; they should be contemplated as a vital sign and measured systematically and routinely during clinic visits.
The understanding of the relevance of sarcopenia and frailty in the LT setting is growing rapidly; however, some important questions require further investigation. The potential to modify and even to reverse some of the components of the frailty status could result in improved transplant outcomes and better patient care.
Acknowledgment
We would like to thank hepatologist Ester Coelho Little (MD) from the University of Arizona, College of Medicine, USA for her help in English proofreading.
Supported by the Carlos III Institute of Health (grants CM17/00006 to L.P.R., PI19/01360 to M.B., and PI20/00731 to M.A.C.I.).
Potential conflict of interest: Nothing to report.
References
- 1.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33:464‐470. [DOI] [PubMed] [Google Scholar]
- 2.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R; Network for Organ Sharing Organ Procurement and Transplantation Network Liver and Transplantation Committee . Results of the first year of the new liver allocation plan. Liver Transpl 2004;10:7‐15. [DOI] [PubMed] [Google Scholar]
- 3.Organización Nacional de Trasplantes . Actividad de donación Y trasplante hepático e intestinal España 2019. [in Spanish]. http://www.ont.es/infesp/Memorias/Actividad%20de%20Donaci%C3%B3n%20y%20Trasplante%20Hep%C3%A1tico%20e%20Intestinal%202019.pdf.
- 4.Lai JC, Sonnenday CJ, Tapper EB, Duarte‐Rojo A, Dunn MA, Bernal W, et al. Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant 2019;19:1896‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte‐Rojo A, et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology 2019;70:1816‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870‐1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146‐M156. [DOI] [PubMed] [Google Scholar]
- 8.Lai JC. Editorial: advancing adoption of frailty to improve the care of patients with cirrhosis: time for a consensus on a frailty index. Am J Gastroenterol 2016;111:1776‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai JC, Covinsky KE, McCulloch CE, Feng S. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol 2018;113:235‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai JC, Covinsky KE, Hayssen H, Lizaola B, Dodge JL, Roberts JP, et al. Clinician assessments of health status predict mortality in patients with end‐stage liver disease awaiting liver transplantation. Liver Int 2015;35:2167‐2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai JC, Volk ML, Strasburg D, Alexander N. Performance‐based measures associate with frailty in patients with end‐stage liver disease. Transplantation 2016;100:2656‐2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, et al. Development of a novel frailty index to predict mortality in patients with end‐stage liver disease. Hepatology 2017;66:564‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, et al. Six‐minute walk distance predicts mortality in liver transplant candidates. Liver Transpl 2010;16:1373‐1378. [DOI] [PubMed] [Google Scholar]
- 14.Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams‐DeMarco M, et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology 2019;156:1675‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kardashian A, Ge J, McCulloch CE, Kappus MR, Dunn MA, Duarte‐Rojo A, et al. Identifying an optimal liver frailty index cutoff to predict waitlist mortality in liver transplant candidates. Hepatology 2021;73:1132‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai JC, Segev DL, McCulloch CE, Covinsky KE, Dodge JL, Feng S. Physical frailty after liver transplantation. Am J Transplant 2018;18:1986‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norman K, Kirchner H, Lochs H, Pirlich M. Malnutrition affects quality of life in gastroenterology patients. World J Gastroenterol 2006;12:3380‐3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvares‐da‐Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005;21:113‐117. [DOI] [PubMed] [Google Scholar]
- 19.Bhanji RA, Moctezuma‐Velazquez C, Duarte‐Rojo A, Ebadi M, Ghosh S, Rose C, et al. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int 2018;12:377‐386. [DOI] [PubMed] [Google Scholar]
- 20.Praktiknjo M, Clees C, Pigliacelli A, Fischer S, Jansen C, Lehmann J, et al. Sarcopenia is associated with development of acute‐on‐chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol 2019;10:e00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montano‐Loza AJ, Meza‐Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 2014;20:640‐648. [DOI] [PubMed] [Google Scholar]
- 22.Montano‐Loza AJ, Duarte‐Rojo A, Meza‐Junco J, Baracos VE, Sawyer MB, Pang JX, et al. Inclusion of sarcopenia within MELD (MELD‐Sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol 2015;6:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. Systematic review and meta‐analysis of the impact of computed tomography‐assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant 2016;16:2277‐2292. [DOI] [PubMed] [Google Scholar]
- 24.DiMartini A, Cruz RJ Jr, Dew MA, Myaskovsky L, Goodpaster B, Fox K, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl 2013;19:1172‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CS, Cron DC, Terjimanian MN, Canvasser LD, Mazurek AA, Vonfoerster E, et al. Dorsal muscle group area and surgical outcomes in liver transplantation. Clin Transplant 2014;28:1092‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valero V 3rd, Amini N, Spolverato G, Weiss MJ, Hirose K, Dagher NN, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg 2015;19:272‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano‐Loza AJ, et al; Life Enhancement, and Exercise in Liver Transplantation Consortium . A multicenter study to define sarcopenia in patients with end‐stage liver disease. Liver Transpl 2017;23:625‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golse N, Bucur PO, Ciacio O, Pittau G, Sa Cunha A, Adam R, et al. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl 2017;23:143‐154. [DOI] [PubMed] [Google Scholar]
- 29.Ebadi M, Wang CW, Lai JC, Dasarathy S, Kappus MR, Dunn MA, et al.; Fitness, Life Enhancement, and Exercise in Liver Transplantation (FLEXIT) Consortium . Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle 2018;9:1053‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tandon P, Mourtzakis M, Low G, Zenith L, Ney M, Carbonneau M, et al. Comparing the variability between measurements for sarcopenia using magnetic resonance imaging and computed tomography imaging. Am J Transplant 2016;16:2766‐2767. [DOI] [PubMed] [Google Scholar]
- 31.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology 2015;62:584‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orman ES, Ghabril M, Chalasani N. Poor performance status is associated with increased mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14:1189‐1195.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tandon P, Reddy KR, O'Leary JG, Garcia‐Tsao G, Abraldes JG, Wong F, et al.; North American Consortium for the Study of End‐Stage Liver Disease . A Karnofsky performance status‐based score predicts death after hospital discharge in patients with cirrhosis. Hepatology 2017;65:217‐224. [DOI] [PubMed] [Google Scholar]
- 34.Tandon P, Tangri N, Thomas L, Zenith L, Shaikh T, Carbonneau M, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol 2016;111:1759‐1767. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775‐1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah S, Goldberg DS, Kaplan DE, Sundaram V, Taddei TH, Mahmud N. Patient frailty is independently associated with the risk of hospitalization for acute‐on‐chronic liver failure. Liver Transpl 2021;27:16‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al.; CANONIC Study Investigators of the EASL–CLIF Consortium . Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426‐1437, 1437.e1‐e9. [DOI] [PubMed] [Google Scholar]
- 38.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, et al.; CANONIC study investigators of the EASL‐CLIF Consortium . Development and validation of a prognostic score to predict mortality in patients with acute‐on‐chronic liver failure. J Hepatol 2014;61:1038‐1047. [DOI] [PubMed] [Google Scholar]
- 39.Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria A, et al.; CANONIC Study Investigators of the EASL‐CLIF Consortium . Clinical course of acute‐on‐chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243‐252. [DOI] [PubMed] [Google Scholar]
- 40.Bahirwani R, Shaked O, Bewtra M, Forde K, Reddy KR. Acute‐on‐chronic liver failure before liver transplantation: impact on posttransplant outcomes. Transplantation 2011;92:952‐957. [DOI] [PubMed] [Google Scholar]
- 41.Artzner T, Michard B, Weiss E, Barbier L, Noorah Z, Merle JC, et al. Liver transplantation for critically ill cirrhotic patients: stratifying utility based on pretransplant factors. Am J Transplant 2020;20:2437‐2448. [DOI] [PubMed] [Google Scholar]
- 42.Laleman W, Claria J, Van der Merwe S, Moreau R, Trebicka J. Systemic inflammation and acute‐on‐chronic liver failure: too much, not enough. Can J Gastroenterol Hepatol 2018;2018:1027152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al.; United Network for Organ Sharing Liver Disease Severity Score Committee . Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91‐96. [DOI] [PubMed] [Google Scholar]
- 44.Brown RS Jr, Lake JR. The survival impact of liver transplantation in the MELD era, and the future for organ allocation and distribution. Am J Transplant 2005;5:203‐204. [DOI] [PubMed] [Google Scholar]
- 45.Habib S, Berk B, Chang CC, Demetris AJ, Fontes P, Dvorchik I, et al. MELD and prediction of post‐liver transplantation survival. Liver Transpl 2006;12:440‐447. [DOI] [PubMed] [Google Scholar]
- 46.Duarte‐Rojo A, Ruiz‐Margáin A, Montano‐Loza AJ, Macías‐Rodríguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end‐stage liver disease: improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24:122‐139. [DOI] [PubMed] [Google Scholar]
- 47.Tandon P, Ismond KP, Riess K, Duarte‐Rojo A, Al‐Judaibi B, Dunn MA, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol 2018;69:1164‐1177. [DOI] [PubMed] [Google Scholar]
- 48.Williams AM, Waits S, Englesbe MJ. The importance of prehabilitation in liver transplantation. Curr Transplant Rep 2015;2:312‐315. [Google Scholar]
- 49.Volk ML, Sonnenday C. Patient‐centered liver transplantation. Clin Liver Dis (Hoboken) 2016;8:24‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Román E, Torrades MT, Nadal MJ, Cárdenas G, Nieto JC, Vidal S, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci 2014;59:1966‐1975. [DOI] [PubMed] [Google Scholar]
- 51.Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1920‐1926.e2. [DOI] [PubMed] [Google Scholar]
- 52.Debette‐Gratien M, Tabouret T, Antonini M‐T, Dalmay F, Carrier P, Legros R, et al. Personalized adapted physical activity before liver transplantation: acceptability and results. Transplantation 2015;99:145‐150. [DOI] [PubMed] [Google Scholar]
- 53.Macías‐Rodríguez RU, Ilarraza‐Lomelí H, Ruiz‐Margáin A, Ponce‐de‐León‐Rosales S, Vargas‐Vorácková F, García‐Flores O, et al. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol 2016;7:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Román E, García‐Galcerán C, Torrades T, Herrera S, Marín A, Doñate M, et al. Effects of an exercise programme on functional capacity, body composition and risk of falls in patients with cirrhosis: a randomized clinical trial. PLoS One 2016;11:e0151652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kruger C, McNeely ML, Bailey RJ, Yavari M, Abraldes JG, Carbonneau M, et al. Home exercise training improves exercise capacity in cirrhosis patients: role of exercise adherence. Sci Rep 2018;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moya‐Nájera D, Moya‐Herraiz Á, Compte‐Torrero L, Hervás D, Borreani S, Calatayud J, et al. Combined resistance and endurance training at a moderate‐to‐high intensity improves physical condition and quality of life in liver transplant patients. Liver Transpl 2017;23:1273‐1281. [DOI] [PubMed] [Google Scholar]
- 57.Morkane CM, Kearney O, Bruce D, Melikian C, Martin DS. An outpatient hospital‐based exercise training programme for patients with cirrhotic liver disease awaiting transplantation: a feasibility trial. Transplantation 2020;104:97‐103. [DOI] [PubMed] [Google Scholar]
- 58.Waits SA, Englesbe MJ. Making progress toward frailty remediation in end‐stage liver disease. Transplantation 2016;100:2526. [DOI] [PubMed] [Google Scholar]
- 59.Ohara M, Ogawa K, Suda G, Kimura M, Maehara O, Shimazaki T, et al. L‐Carnitine suppresses loss of skeletal muscle mass in patients with liver cirrhosis. Hepatol Commun 2018;2:906‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol 2016;65:906‐913. [DOI] [PubMed] [Google Scholar]


