Abstract
Obeticholic acid (OCA) is approved for the treatment of patients with primary biliary cholangitis (PBC) who are partial responders or intolerant to ursodeoxycholic acid. Reports of serious liver injury have raised concerns about its safety in cirrhosis. We investigated the effects of treatment with OCA on hepatic decompensation and liver‐related mortality or transplantation in a cohort with compensated PBC cirrhosis. This was a retrospective cohort study using national data of US veterans with PBC and cirrhosis. We performed a propensity score model using variables associated with OCA prescription to control for baseline risk of decompensation. New OCA users were matched to nonusers. We identified 509 subjects with compensated PBC cirrhosis. We developed a propensity score model using variables associated with OCA prescription; 21 OCA users were matched with 84 nonusers. Over 569 and 3,847 person‐months, respectively, of follow‐up, 5 (23.8%) OCA users and 22 (26.2%) OCA nonusers decompensated. The C‐statistic of the propensity score model was 0.87. On multivariable analysis, after adjusting for potential confounders, OCA use was associated with an increased risk of hepatic decompensation (adjusted hazard ratio, 3.9; 95% confidence interval, 1.33‐11.57; P = 0.01). There was no association between OCA use and liver‐related mortality or transplantation (adjusted hazard ratio, 1.35; 95% confidence interval, 0.35‐5.21; P = 0.66). Conclusion: OCA use was associated with an increase in hepatic decompensation but not liver‐related mortality or transplantation in patients with compensated PBC cirrhosis. Additional studies are recommended to prospectively investigate these findings.
Our data suggest that exposure to OCA in patients with PBC cirrhosis is associated with a 3.9‐fold higher risk of hepatic decompensation compared with a propensity‐matched cohort of PBC cirrhosis with similar baseline risk. Large, prospective studies are needed to confirm our findings. Our findings raise concerns about OCA, and we recommend that clinicians be cautious about using this drug, even in patients with well‐compensated cirrhosis, until we establish safety from ongoing trials.

Abbreviations
- aHR
adjusted hazard ratio
- ALP
alkaline phosphatase
- AMA
antimitochondrial antibody
- AUDIT‐C
Alcohol Use Disorder Identification Test
- BMI
body mass index
- CDW
Corporate Data Warehouse
- CI
confidence interval
- CTP
Child‐Turcotte‐Pugh
- FDA
Food and Drug Administration
- FXR
farnesoid X‐activated receptors
- HCC
hepatocellular carcinoma
- ICD
International Classification of Diseases
- MELD
Model for End‐Stage Liver Disease
- PBC
primary biliary cholangitis
- POISE
PeriOperative ISchemic Evaluation
- PS
propensity score
- UDCA
ursodeoxycholic acid
- VA
Veterans Affairs
Primary biliary cholangitis (PBC) is a chronic autoimmune liver disease that is characterized by the destruction of small intrahepatic bile ducts.( 1 ) Ursodeoxycholic acid (UDCA) has been shown to improve the overall transplant‐free survival in PBC, but approximately 40% of subjects are partial responders to UDCA and have a less favorable outcome.( 1, 2 ) Obeticholic acid (OCA) was approved in the United States in 2016 for the treatment of PBC partial responders or those intolerant to UDCA. The approval was based on demonstration of improvement in alkaline phosphatase (ALP); however, potential benefits on liver‐related events have not been demonstrated. Postmarketing reports of serious liver injury have raised concerns about its safety and risk‐benefit assessment in patients with cirrhosis, leading to a boxed warning by the Food and Drug Administration (FDA).( 3, 4 ) The published experience of OCA in patients with cirrhosis is limited. The initial studies on OCA were powered to demonstrate improvement in ALP rather than clinical events. The phase 3 POISE (PeriOperative ISchemic Evaluation) trial that led to the approval of OCA in the United States included only 20 subjects with cirrhosis, of whom 13 were randomized to OCA and 7 to placebo, with an initially reported follow‐up of 12 months.( 5 )
Bile acids have both pro‐inflammatory and anti‐inflammatory actions through farnesoid X‐activated receptors (FXRs). Upon activation, FXRs regulate both bile acid synthesis and conjugation. In addition, FXRs regulate numerous genes that affect glucose, protein, and lipid metabolism such as peroxisome proliferator‐activated receptor gamma, sterol regulatory binding protein, and cAMP regulatory element‐binding promoter.( 6 ) Furthermore, the presence of both cholestasis and cirrhosis may alter the bioavailability of the drug.
Therefore, there is concern that OCA may be associated with an increased risk of hepatic decompensation. Although recent reports have highlighted individual cases with severe drug‐induced liver toxicity, quantification of increased risk of decompensation related to OCA requires a comparator group of OCA unexposed patients with PBC at similar baseline risk. To address these limitations, we aimed to investigate the effect of OCA use on hepatic decompensation and liver‐related death or transplantation, in a cohort of patients with PBC and well‐compensated cirrhosis and compared outcomes with a propensity‐matched cohort of patients without OCA exposure.
Patients and Methods
Study Design and Subject Identification
This was a retrospective cohort study that first identified subjects using data from the Veterans Affairs (VA) Corporate Data Warehouse (CDW), a clinical data repository that contains patient demographics, International Classification of Diseases (ICD), Clinical Modification diagnosis codes, current procedural terminology codes, laboratory results, imaging, pathology, and prescription records on all patients receiving care within the VA health care system.( 7 ) After charts were identified using ICD codes, manual chart review of each eligible subject was performed centrally at the Bruce W. Carter Miami Veterans Affairs Medical Center to confirm eligibility. Biopsy reports, imaging results, endoscopy reports, clinical details of decompensation, as well as mortality data (including the dates and cause of death) were obtained from direct review of the charts. Institutional review boards at all of the participating VA Medical centers approved the study.
Variables
The data from the manual chart review were combined with laboratory values obtained from the CDW. The following laboratory values were obtained at the time of PBC diagnosis and 2 years after initiation of treatment to evaluate for UDCA response: serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), ALP, and total bilirubin. UDCA response was defined based on Toronto criteria, as ALP less than 1.67 times the upper limit of normal 24 months after initiation of UDCA.
In addition, the following laboratory values were obtained at the time of cirrhosis diagnosis and throughout follow‐up, including the time closest to initiation of OCA: ALT, AST, ALP, albumin, total bilirubin, platelet count, hemoglobin, international normalized ratio (INR), and serum sodium.
Laboratory tests used for baseline values in the unmatched cohort were those obtained closest to the time of diagnosis of cirrhosis from 180 days before to 30 days after. In the matched cohort, laboratory tests used were those obtained closest to the time of the first OCA fill or “matched OCA date” among controls (controls were matched with cases for time from cirrhosis diagnosis). Child Turcotte Pugh (CTP) scores and Model for End‐Stage Liver Disease–Sodium (MELD‐Na) scores were appropriately calculated for patients at baseline and during follow‐up.
All liver biopsy results were obtained by chart review. Tobacco use was characterized as current use, former use, or lifetime nonuse. Alcohol use was defined using ICD codes and Alcohol Use Disorders Identification Test (AUDIT‐C) scores; AUDIT‐C scores ≥ 4 for males and ≥ 3 for females were considered alcohol misuse.( 8 ) We identified comorbidities using the cirrhosis comorbidity (CirCom) score that was first described by Jepsen et al., and defined seven groups based on the presence of one or more of the following comorbidities: acute myocardial infarction, chronic obstructive lung disease, peripheral arterial disease, epilepsy, substance abuse other than alcoholism, congestive heart failure, nonmetastatic or hematologic cancer, metastatic cancer, and chronic kidney disease.( 9 )
Inclusion Criteria
Unique patients with cirrhosis with PBC were initially identified by querying the CDW for one inpatient or two outpatient ICD‐CM Ninth Revision (ICD‐9‐CM) or 10th Revision (ICD‐10‐CM) primary or secondary diagnosis codes for PBC (ICD‐9‐CM, 571.6; ICD‐10‐CM, K74.3) and cirrhosis (ICD‐9‐CM, 571.5; ICD‐10‐CM, K70.3x) from January 2008 to December 2016, with follow‐up to March 31, 2020. Once these subjects were identified, a manual chart review was performed to confirm the diagnosis of PBC and cirrhosis. Patients were included if they met the diagnostic criteria for both PBC and cirrhosis as described as follows: A: Diagnosis of PBC, which was established by confirming two of the three clinical features (ALP greater than 1.5 times the upper limit of normal, a positive antimitochondrial antibody [AMA] or PBC‐specific antinuclear antibody, and a liver biopsy consistent with the diagnosis). Patients were included regardless of whether the diagnosis of PBC was antecedent or concomitant to cirrhosis. B: Diagnosis of cirrhosis, which was confirmed by one of the following: liver biopsy, vibration controlled transient elastography with liver stiffness >16.9 KPa,( 5, 10 ) nodular‐appearing liver, or the presence of portal hypertension. C: Portal hypertension was defined as the presence of thrombocytopenia (<150 × 109/mL) in the absence of alternative explanations, esophageal or gastric varices on upper endoscopy, or collaterals on abdominal imaging.
Exclusion Criteria
Patients were excluded if they did not meet the criteria for PBC based on testing available in the VA, did not meet the criteria for diagnosis of cirrhosis, developed posttransplant PBC cirrhosis, had insufficient data, or had decompensation at the time of initial diagnosis of cirrhosis (documented by the presence of variceal bleeding, ascites, hepatic hydrothorax, spontaneous bacterial peritonitis, or hepatic encephalopathy on chart review). Patients with CTP B or C and patients who were not treated with UDCA or OCA were also excluded.
Propensity Score Matching
Propensity score (PS) matching was performed using the SAS PSMATCH procedure. Patients who received OCA were matched with controls on a 1:4 ratio, matching for gender, race, body mass index (BMI), tobacco use, AUDIT‐C score, CirCom, AST, ALT, ALP, albumin, creatinine, serum sodium, total bilirubin, INR, platelet count, CTP score, MELD‐Na, UDCA response, and untreated time (time from cirrhosis diagnosis to initiation of OCA as described previously). The index date was defined as the date of the first OCA fill for OCA users; untreated time was calculated from the date of cirrhosis diagnosis to the date of OCA initiation. For OCA nonusers, follow‐up time was started from a matched period of untreated time after the diagnosis of cirrhosis, to avoid immortal time biases.
Outcomes
The primary outcome was the risk of hepatic decompensation. This was defined as the development of variceal bleeding, ascites, hepatic hydrothorax, spontaneous bacterial peritonitis, or hepatic encephalopathy on chart review. The secondary outcome was a composite of liver‐related mortality or liver transplantation. Chart review was used to identify the cause of death, which was considered liver‐related if it was attributable to hepatic decompensation or progression of hepatocellular carcinoma (HCC).
Statistical Analysis
Time‐to‐event data were compared using the log‐rank test and Kaplan‐Meier methodology.
The associations of OCA use and risk of decompensation (with death or transplantation as competing risk) and liver‐related mortality or liver transplantation (with non‐liver‐related death as competing risk) were estimated using competing‐risk Cox proportional hazards models (Fine and Gray method), adjusted for the following covariates that were defined a priori, based on previously published data: age,( 11 ) gender,( 12 ) diabetes,( 13 ) UDCA response,( 14 ) and CTP score.( 15 )
We performed a sensitivity analysis including patients with more liberal inclusion criteria who met criteria for PBC and had an ICD code for cirrhosis but did not meet the strict definition for cirrhosis. These patients were often diagnosed to have cirrhosis outside the VA health care system and had ICD code(s) for cirrhosis, and they were excluded from the primary analysis because the diagnosis could not be verified based on testing available within the VA.
Results
Sample Selection
Out of a total of 1,499 subjects identified from the VA CDW, we excluded the following: those who had cirrhosis due to other causes or did not meet the criteria for PBC based on testing available in the VA (n = 317); those who did not meet the criteria for cirrhosis (n = 324); those who developed posttransplant PBC cirrhosis (n = 15); those who did not meet the criteria for either PBC or cirrhosis (n = 30); those with insufficient data (n = 70); those who had decompensation at baseline (n = 203); or those who were not treated with UDCA or OCA (n = 31) (Fig. 1). Therefore, 509 patients with PBC and compensated cirrhosis were included in the primary analysis, of whom 21 received OCA with or without UDCA and 488 received UDCA alone (Fig. 1). The initial and maintenance dose of OCA was 5 mg daily in 95%, and 10 mg daily in 5% of subjects.
Fig. 1.
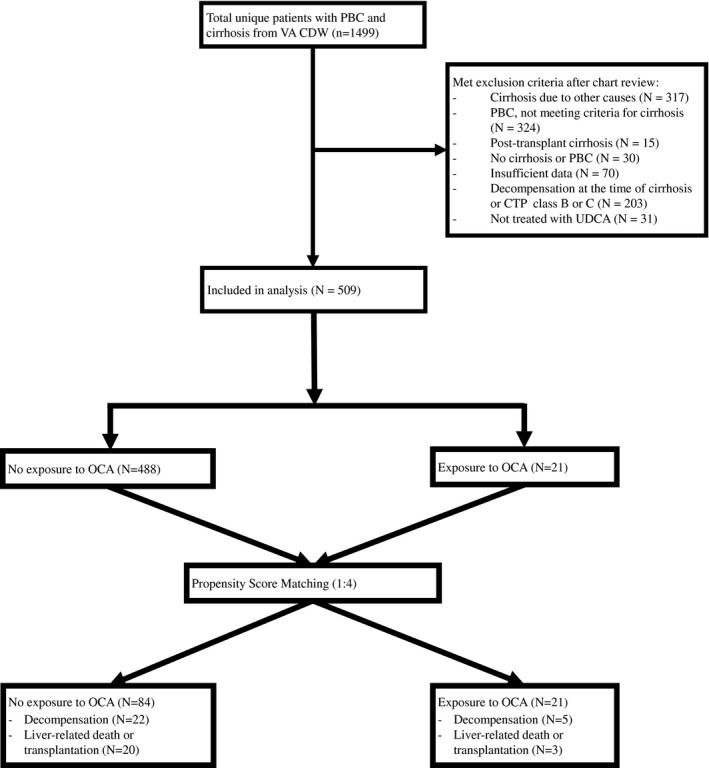
Study flow diagram.
Propensity Matching
We matched 21 OCA users to 84 non‐OCA users. The baseline characteristics of the cohort before and after matching are given in Table 1. As expected in a veteran population, the cohort was predominantly White and male. The PSs of the two groups are shown in Fig. 2. After PS matching, the two groups were well balanced in terms of baseline characteristics (Table 1 and Fig. 2). Although only 70% of OCA exposed patients and 88.0% of OCA unexposed patients who had an available AMA test available for review were AMA‐positive; all subjects with negative or unknown AMA status had a liver biopsy confirming the diagnosis of PBC. The C‐statistic of the propensity model was 0.87.
Table 1.
Baseline Characteristics of Unmatched and Matched Cohort
| Variables | PS‐Matched Sample | ||
|---|---|---|---|
| Nonuser | OCA User | P Value | |
| (n = 84) | (n = 21) | ||
| Age at cirrhosis, median (IQR) | 59.0 (13.5) | 60.0 (9.0) | 0.3196 |
| Gender, n (%) | 0.7689 | ||
| Male | 45 (53.6%) | 12 (57.1%) | |
| Female | 39 (46.3%) | 9 (42.9%) | |
| Race/ethnicity, n (%) | 0.6299 | ||
| Black | 4 (4.8%) | 2 (9.5%) | |
| Other | 7 (8.3%) | 1 (4.8%) | |
| White | 73 (86.9%) | 18 (85.7%) | |
| BMI, median (IQR) | 27.5 (7.3) | 26.5 (7.9) | 0.8460 |
| BMI, n (%) | 0.7407 | ||
| Underweight (less than 18.5) | 1 (1.2%) | 1 (4.8%) | |
| Normal weight (18.5 to 25) | 18 (21.4%) | 5 (23.8%) | |
| Overweight (25 to 30) | 34 (40.5%) | 8 (38.1%) | |
| Obese (more than 30) | 31 (36.9%) | 7 (33.3%) | |
| Tobacco use, n (%) | 0.1918 | ||
| Current smoker | 26 (30.9%) | 3 (14.3%) | |
| Former smoker | 25 (29.8%) | 10 (47.6%) | |
| Never smoker | 33 (39.3%) | 8 (38.1%) | |
| AUDIT score 1 year after cirrhosis, n (%) | 0.9999 | ||
| Low | 76 (90.5%) | 19 (90.5%) | |
| High | 8 (9.5%) | 2 (9.5%) | |
| Cirrhosis comorbidity index, n (%) | 0.6511 | ||
| 0 | 20 (23.8%) | 6 (28.6%) | |
| 1 + 0/3 + 1 | 64 (76.2%) | 15 (71.4%) | |
| UDCA responder, n (%) | 0.3480 | ||
| No | 55 (65.5%) | 16 (76.2%) | |
| Yes | 29 (34.5%) | 5 (23.8%) | |
| Portal hypertension at baseline, n (%) | 0.2666 | ||
| Yes | 55 (65.5%) | 12 (57.1%) | |
| No | 29 (34.5%) | 9 (42.9%) | |
| AMA, n (%) | 0.0642 | ||
| Positive | 71 (84.5%) | 14 (66.7%) | |
| Negative | 9 (10.7%) | 6 (28.6%) | |
| Unknown | 4 (4.8%) | 1 (4.8%) | |
| Varices, n (%) | 0.3312 | ||
| Yes | 22 (26.2%) | 9 (42.9%) | |
| No | 58 (69.1%) | 11 (52.4%) | |
| Unknown | 4 (4.8%) | 1 (4.8%) | |
| Cirrhosis diagnosis, n (%) | 0.6798 | ||
| Biopsy proven | 27 (32.1%) | 7 (33.3%) | |
| FibroScan | 3 (3.6%) | ‐ | |
| Abnormal liver imaging | 54 (64.3%) | 14 (66.7%) | |
| AST (IU/mL), median (IQR) | 38.0 (22.5) | 48.0 (17.0) | 0.1255 |
| ALT (IU/mL), median (IQR) | 37.5 (32.5) | 49.0 (27.0) | 0.1266 |
| ALP (IU/mL), median (IQR) | 226.5 (184.0) | 281.0 (187.0) | 0.2067 |
| Albumin (g/dL), median (IQR) | 3.8 (0.5) | 3.8 (0.7) | 0.8353 |
| Platelet count (x10E9/L), median (IQR) | 196.0 (104.5) | 166.0 (97.0) | 0.0987 |
| Serum sodium (mEq/L), median (IQR) | 138.0 (4.0) | 138.0 (3.0) | 0.4766 |
| Creatinine (mg/dL), median (IQR) | 0.9 (0.3) | 0.9 (0.4) | 0.7708 |
| Total bilirubin (mg/dL), median (IQR) | 0.7 (0.4) | 0.9 (0.3) | 0.0505 |
| International normalized ratio, median (IQR) | 1.0 (0.2) | 1.0 (0.1) | 0.3492 |
| CTP score, median (IQR) | 5.0 (0.0) | 5.0 (0.0) | 0.6583 |
| MELD score, median (IQR) | 6.0 (0.0) | 6.0 (0.0) | 0.8925 |
Laboratory tests in the unmatched cohort were those obtained closest to the time of diagnosis of cirrhosis. In the matched cohort, laboratory tests used were those obtained at the time of OCA initiation or “matched OCA date” (matched for time from cirrhosis diagnosis).
Abbreviation: IQR, interquartile range.
Fig. 2.
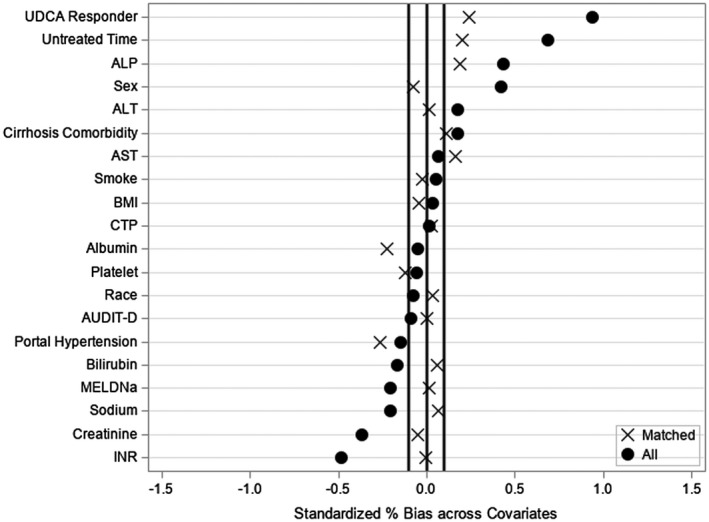
Standardized variable differences plot between subjects exposed to OCA and controls with PBC cirrhosis, before (o) and after (x) PS matching.
Association of OCA Use and Hepatic Decompensation
Over 569 and 3,847 person‐months, respectively, of follow‐up, 5 (23.8%) OCA users and 22 (26.2%) OCA nonusers decompensated. The time to decompensation was not statistically different between the two groups (median 20.0 vs. 46.0 months; P = 0.31). On multivariable analysis, after adjusting for potential confounders, OCA use was associated with an increased risk of hepatic decompensation (adjusted hazard ratio [aHR], 3.9; 95% confidence interval [CI], 1.33‐11.57; P = 0.01) (Table 2 and Fig. 3A).
Table 2.
Univariable and Multivariable aHRs for the Risk of Decompensation and Liver‐Related Death or Transplantation
| Variable | Decompensation | Liver‐Related Death or Transplantation | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| OCA exposure | ||||||||
| No | REF | REF | REF | REF | ||||
| Yes | 2.52 (0.85, 7.44) | 0.0946 | 3.92 (1.33, 11.57) | 0.0135 | 1.29 (0.39,4.34) | 0.6752 | 1.35 (0.35, 5.21) | 0.6599 |
| Age | 1.07 (1.03, 1.11) | 0.0004 | 1.11 (1.05, 1.18) | 0.0002 | 1.04 (1.01,1.08) | 0.0188 | 1.08 (1.04, 1.13) | 0.0004 |
| Gender | ||||||||
| Male | REF | REF | REF | REF | ||||
| Female | 0.16 (0.05, 0.57) | 0.0044 | 0.77 (0.18, 3.24) | 0.7237 | 0.63 (0.33,1.17) | 0.1439 | 0.39 (0.16, 0.95) | 0.0391 |
| Diabetes | ||||||||
| No | REF | REF | REF | REF | ||||
| Yes | 1.72 (0.70, 4.21) | 0.2379 | 4.66 (1.19, 8.25) | 0.0273 | 1.27 (0.67,2.43) | 0.4657 | 2.46 (1.04, 5.82) | 0.0404 |
| UDCA response | ||||||||
| Yes | REF | REF | REF | REF | ||||
| No | 1.38 (0.52, 3.69) | 0.5158 | 1.27 (0.55, 2.96) | 0.5803 | 2.53 (1.20,5.36) | 0.0152 | 4.11 (1.57, 10.77) | 0.0040 |
| CTP score | ||||||||
| CTP A6 | REF | REF | REF | REF | ||||
| CTP A5 | 0.10 (0.03, 0.34) | 0.0002 | 0.03 (0.01, 0.14) | <.0001 | 0.41 (0.15,1.10) | 0.0759 | 0.30 (0.10, 0.89) | 0.0302 |
Bold‐face values indicates P values <0.05.
Fig. 3.
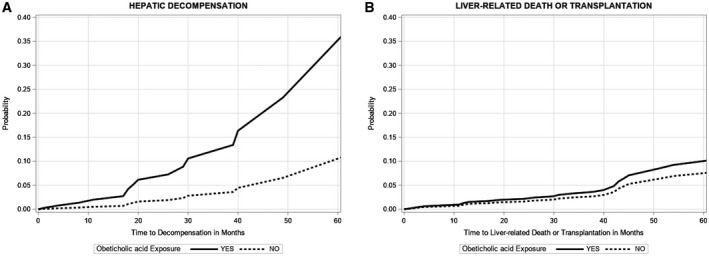
(A) Cumulative incidence of decompensation by OCA exposure in subjects with PBC cirrhosis. (B) Cumulative incidence of liver related death or transplantation by OCA exposure in subjects with PBC cirrhosis.
Hepatic decompensation was associated with CTP (A5 vs. A6; aHR, 0.03; 95% CI, 0.01‐0.14; P < 0.001), higher age (aHR, 1.11; 95% CI, 1.05‐1.18; P = 0.0002), and diabetes mellitus (aHR, 4.66; 95% CI, 1.19‐8.25; P = 0.03), but not UDCA response (vs. partial responders: aHR, 1.27; 95% CI, 0.55‐2.96; P = 0.58). The decompensating event in the OCA group was medically controlled ascites in 2 patients, refractory ascites in 1 patient, gastric variceal bleed in 1 patient, and hepatic encephalopathy in 1 patient. No other precipitating factor such as an infection or an alternative drug was identified in patients who decompensated. Two other patients in the OCA group developed HCC. None of the patients developed bilirubin >3 mg/dL or coagulopathy, and none received a liver transplant during the study period. Seven patients discontinued OCA during the study period, 3 for severe pruritus, 1 due to nonresponse to treatment, 1 because of gastrointestinal side effects and increased sweating, and 2 for unknown reasons.
Of the 21 exposed subjects with OCA, 20 received 5 mg daily and 1 received 10 mg daily. There was no relationship between the dose of OCA and decompensation. The median duration of OCA exposure was 17 months (interquartile range: 19 months). On logistic regression analysis, duration of OCA exposure was not associated with decompensation (adjusted odds ratio, 0.92; 95% CI, 0.83‐1.03; P = 0.13).
Association of OCA Use and Liver‐Related Death or Transplantation
We also explored the association between OCA use and liver‐related death or transplantation (Table 2 and Fig. 3B). Liver‐related causes of death were documented in 3 (14.3%) of OCA users (during 607 person‐months of follow‐up) and 20 (23.8%) of OCA nonusers (during 4,504 person‐months of follow‐up). The median time to liver‐related death was not different between the two groups (18.0 vs. 47.0 months; P = 0.19). Of the 3 patients who died of liver‐related causes, 1 was related to HCC, 1 had decompensated cirrhosis, and the third patient had HCC and hepatic decompensation. All 3 patients were ineligible for liver transplantation due to psychosocial reasons. Among the controls, 15 subjects developed liver‐related death (including 2 from HCC), and 5 underwent liver transplantation. On multivariable analysis, OCA exposure was not associated with liver‐related death or transplantation (aHR, 1.35; 95% CI, 0.35‐5.21; P = 0.66).
Sensitivity Analysis
We did a sensitivity analysis by including 833 subjects (additional 324 above the main analysis) by including those who met the criteria for PBC and had an ICD diagnosis of cirrhosis but did not meet the criteria for cirrhosis based on tests available within the VA system. Of these, 29 received OCA with or without UDCA, and 804 received UDCA alone (Supporting Fig. S1).
We matched 29 OCA users to 116 non‐OCA users using the same method as the primary analysis. The two groups were well‐balanced in terms of all baseline characteristics. Over 845 and 5,225 person‐months, respectively, of follow‐up, 5 (17.2%) OCA users and 27 (23.3%) OCA nonusers decompensated (Supporting Fig. S1). After adjusting for potential confounders, OCA exposure was associated with an increase in the hazard of decompensation (aHR, 4.65; 95% CI, 1.30‐16.57; P = 0.02) (Supporting Table S2).
Three subjects in the OCA group (10.3%) and 25 (21.2%) controls had a liver‐related death or transplantation. After adjusting for potential confounders, OCA exposure was not associated with liver‐related death or transplantation (aHR, 4.26; 95% CI, 0.75‐24.36; P = 0.10) (Supporting Table S2). On post hoc analysis, the power to detect differences in death or transplantation was only 14.6%. We calculated the sample size needed to detect statistical significances in death or transplantation with 80% power, on 424 subjects, including 85 with OCA exposure.
Discussion
We found that among patients with compensated PBC cirrhosis, OCA use was associated with an increased risk of hepatic decompensation compared with a propensity‐matched group of subjects without OCA exposure.
Although Eaton et al. first reported OCA‐associated hepatic decompensation, the data were descriptive, with a series of patients from multiple institutions, some of whom had PBC, while others had PSC.( 3 ) Our data build on their hypothesis‐generating work by demonstrating that OCA is associated with increased decompensation when compared to a cohort with similar baseline risk. Because not all subjects underwent a liver biopsy, it is possible that some subjects with PBC may have portal hypertension secondary to nodular regenerative hyperplasia (likely to be equally distributed in the OCA exposed and unexposed groups). Decompensation that occurred in this subgroup of subjects is more likely to be medication‐related rather than worsening portal hypertension due to PBC progression.
We also note that only 70% (14 of 20 with AMA results available) in the OCA group were AMA‐positive. This is not completely surprising, because AMA‐negative PBC has been described to have a worse outcome compared with AMA‐positive disease.( 16 ) This study only included subjects who progressed to cirrhosis, and the OCA‐exposed group is further enriched with patients who are UDCA partial responders. Therefore, the prevalence of AMA‐negative disease is high in the overall study population and even higher in the OCA‐exposed subjects with PBC cirrhosis, compared to a cohort of patients with PBC without cirrhosis. We adjusted for this variable in the PS matching.
We included consecutive patients with a diagnosis of PBC cirrhosis, a propensity‐matched control group, and excluded patients with PSC and those with elevated bilirubin or decompensation at baseline. Based on multiple reported cases of severe hepatic decompensation, the FDA issued a warning in September 2017 that patients with CTP B or C cirrhosis should be started on lower doses of OCA. This warning did not recommend dose adjustment in patients with well‐compensated cirrhosis.
It is likely that our data demonstrated an association when two prospective clinical trials did not, because of the sample size and duration of follow‐up. The phase 3 POISE trial included only 20 subjects with cirrhosis, of whom 13 were randomized to OCA and 7 to placebo, and were followed for 12 months for their initial publication. A subset of the initial population continued into an open‐label extension study, and the 36‐month interim results show that 3 patients developed variceal bleed and 1 developed ascites.( 17 ) Although these studies did not demonstrate an increased risk of decompensation with OCA compared with placebo, the total follow‐up of patients with cirrhosis of 240 and 720 person‐months, respectively, was likely underpowered to detect significant differences.
Potential Mechanisms
The findings are not completely unexpected, considering the complex and pleiotropic role that FXR plays in the regulation of numerous hepatic pathways.( 6 ) In a recent review, Hoofnagle discussed the role played by FXR on glucose, protein, and lipid metabolism that can have unpredictable effects on the liver.( 6 ) Semmler et al. described the effects of single nucleotide polymorphisms in the FXR gene that are associated with severe portal hypertension in a cohort of patients with cirrhosis and clinically significant portal hypertension.( 18 ) We also found that 2 of 21 OCA users developed HCC. This is interesting, because OCA is an inducer of fibroblast growth factor (FGF) 19, a growth factor that is associated with hepatocellular carcinogenesis.( 19 ) Although the FGF expression of HCC in these 2 patients was unavailable, this association would be worth exploring in future studies.
Limitations
We acknowledge several limitations. First, the requirement that every patient included meet strict documentation of criteria for both PBC and cirrhosis with tests done within the VA system eliminated many potentially eligible subjects. This reduced our sample size and may have decreased the power to detect differences in liver‐related mortality or transplantation. However, the benefit of the strict inclusion criteria is the greater reliability of our data and the enrichment of the cohort for the development of clinical events. Despite strict inclusion criteria, the total number of subjects with cirrhosis in our cohort was similar to the multicenter Global PBC study group. Second, consistent with a veteran population, our population had more males compared to traditional cohorts of patients with PBC. Although the propensity matching helped to balance the gender distribution, our findings are generalizable only to a population that is male and White. Third, we acknowledge the limitations of a retrospective study, and the potential for residual confounding despite adjustment for known confounders. We did not adjust for baseline pruritus, as this was not well‐documented in charts. Fourth, we acknowledge that despite including over 500 subjects with PBC cirrhosis, the numbers of OCA‐exposed patients and events in the OCA‐exposed cohort are low (21 and 5, respectively).
It is possible that the FDA warnings of hepatotoxicity may have tempered the use of OCA in patients with cirrhosis who are partial or nonresponders to UDCA. We believe that these limitations are outweighed by strengths, including a well‐characterized cohort of patients with both PBC and compensated cirrhosis assembled in a real‐world setting without a referral center bias. The data were obtained from patients receiving care from a national health care system with uniform practices across its facilities. We recognized and adjusted for bias by matching for UDCA partial response as well as for untreated time (to avoid immortal time biases).
In summary, our data suggest that exposure to OCA in patients with PBC cirrhosis is associated with a 3.9‐fold higher risk of hepatic decompensation. Large, prospective studies are needed to confirm our findings, and these are currently underway (NCT02308111 and NCT04076527). As OCA is being evaluated for the treatment of nonalcoholic steatohepatitis, our findings may have an impact beyond patients with PBC. Our findings raise concerns about OCA, and we recommend that clinicians be cautious about using this drug, even in patients with well‐compensated cirrhosis, until we establish safety from ongoing trials.
Supporting information
Fig S1
Fig S2
Table S1
Supported by the VCU Massey Cancer Center Biostatistics Shared Resource, which is supported, in part, with funding from National Institutes of Health–National Cancer Institute Cancer Center Support (Grant No. P30 CA016059).
Potential conflict of interest: Dr. Levy consults for and advises and received grants from CymaBay, Genfit, GSK, Pliant, Cara Therapeutics, and Mirum. She consults for and advises Escient. She received grant from Gilead, Intercept, Novartis, High Tide, Zydus, and Target Pharmasolutions. She received royalties from Up‐To‐Date. Dr. John advises Dova Therapeutics. He receives institutional grant funding from Bristol Myers Squibb, Exelixis, Glaxo SmithKline, Glycotest, Inc, H3B biosciences, and Viking therapeutics. Dr. Martin consults and received grants from AbbVie. He consults for Mallinckrodt and CLDF. He received grants from GenFit, Durect, Grifols, Sera Trials, TARGET, Viking, and Enanta.
Disclaimer: The authors prepared this work in their personal capacity. The opinions expressed in this article are the authors’ own and do not reflect the view of Department of Veterans Affairs, or the United States government.
REFERENCES
- 1.Goel A, Kim WR. Natural history of primary biliary cholangitis in the ursodeoxycholic acid era: role of scoring systems. Clin Liver Dis 2018;22:563‐578. [DOI] [PubMed] [Google Scholar]
- 2.Harms MH, van Buuren HR, Corpechot C, Thorburn D, Janssen HLA, Lindor KD, et al. Ursodeoxycholic acid therapy and liver transplant‐free survival in patients with primary biliary cholangitis. J Hepatol 2019;71:357‐365. [DOI] [PubMed] [Google Scholar]
- 3.Eaton JE, Vuppalanchi R, Reddy R, Satapathy SK, Ali B, Kamath PS, et al. Liver injury in patients with cholestatic liver disease treated with obeticholic acid. Hepatology 2020;71:1511‐1514. [DOI] [PubMed] [Google Scholar]
- 4.Center for Drug Evaluation and Research . FDA Drug Safety Communication: FDA warns about serious liver injury with Ocaliva (obeticholic acid) for rare chronic liver disease. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐drug‐safety‐communication‐fda‐warns‐about‐serious‐liver‐injury‐ocaliva‐obeticholic‐acid‐rare. Accessed June 24, 2020. [Google Scholar]
- 5.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. A placebo‐controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016;375:631‐643. [DOI] [PubMed] [Google Scholar]
- 6.Hoofnagle JH. FXR agonists as therapy for liver disease. Hepatology 2020;72:1‐3. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan DE, Serper MA, Mehta R, Fox R, John B, Aytaman A, et al. Effects of hypercholesterolemia and statin exposure on survival in a large national cohort of patients with cirrhosis. Gastroenterology 2019;156:1693‐1706. [DOI] [PubMed] [Google Scholar]
- 8.Williams EC, Rubinsky AD, Lapham GT, Chavez LJ, Rittmueller SE, Hawkins EJ, et al. Prevalence of clinically recognized alcohol and other substance use disorders among VA outpatients with unhealthy alcohol use identified by routine alcohol screening. Drug Alcohol Depend 2014;135:95‐103. [DOI] [PubMed] [Google Scholar]
- 9.Jepsen P, Vilstrup H, Lash TL. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology 2014;146:147‐156. [DOI] [PubMed] [Google Scholar]
- 10.Corpechot C, Carrat F, Poujol‐Robert A, Gaouar F, Wendum D, Chazouillères O, et al. Noninvasive elastography‐based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012;56:198‐208. [DOI] [PubMed] [Google Scholar]
- 11.Marschall H‐U, Henriksson I, Lindberg S, Söderdahl F, Thuresson M, Wahlin S, et al. Incidence, prevalence, and outcome of primary biliary cholangitis in a nationwide Swedish population‐based cohort. Sci Rep 2019;9:11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John B, Khakoo NS, Aitcheson G, Levy C, Goldberg D, Bhamidimarri KR, et al. Male gender is associated with a high rate of decompensation and mortality in primary biliary cholangitis with well compensated cirrhosis. J Hepatol 2021;73:S463. [Google Scholar]
- 13.Híndi M, Levy C, Couto CA, Bejarano P, Mendes F. Primary biliary cirrhosis is more severe in overweight patients. J Clin Gastroenterol. 2013;47:e28‐e32. [DOI] [PubMed] [Google Scholar]
- 14.Reisman Y, van Dam GM, Gips CH, Lavelle SM, Euricterus PM. Survival probabilities of Pugh‐Child‐PBC classified patients in the euricterus primary biliary cirrhosis population, based on the Mayo clinic prognostic model. Hepatogastroenterology 1997;44:982‐989. [PubMed] [Google Scholar]
- 15.Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HLA, Invernizzi P, Mason AL, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow‐up study. Gastroenterology 2014;147:1338‐1349. [DOI] [PubMed] [Google Scholar]
- 16.Juliusson G, Imam M, Björnsson ES, Talwalkar JA, Lindor KD. Long‐term outcomes in antimitochondrial antibody negative primary biliary cirrhosis. Scand J Gastroenterol. 2016;51:745‐752. [DOI] [PubMed] [Google Scholar]
- 17.Trauner M, Nevens F, Shiffman ML, Drenth JPH, Bowlus CL, Vargas V, et al. Long‐term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3‐year results of an international open‐label extension study. Lancet Gastroenterol Hepatol 2019;4:445‐453. [DOI] [PubMed] [Google Scholar]
- 18.Semmler G, Simbrunner B, Scheiner B, Schwabl P, Paternostro R, Bucsics T, et al. Impact of farnesoid X receptor single nucleotide polymorphisms on hepatic decompensation and mortality in cirrhotic patients with portal hypertension. J Gastroenterol Hepatol 2019;34:2164‐2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng N, Wei W, Wang Z. Emerging roles of FGF signaling in hepatocellular carcinoma. Transl Cancer Res 2016;5:1‐6. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1


