Abstract
The involvement of bile salt–fibroblast growth factor 19 (FGF19) signaling in human liver regeneration (LR) is not well studied. Therefore, we studied aspects of bile salt–FGF19 signaling shortly after liver resection in patients. We compared plasma bile salt and FGF19 levels in arterial, portal and hepatic venous blood, calculated venous‐arterial differences (ΔVA), and determined hepatic transcript levels on two intra‐operative time points: before (< 1 hour) and immediately after (> 2‐3 hours) liver resection (i.e., following surgery). Postoperative bile salt and FGF19 levels were assessed on days 1, 2, and 3. LR was studied by computed tomography (CT)–liver volumetry. Following surgery, the liver, arterial, and portal bile salt levels were elevated (P < 0.05). Furthermore, an increased amount of bile salts was released in portal blood and extracted by the remnant liver (P < 0.05). Postoperatively, bile salt levels were elevated from day 1 onward (P < 0.001). For FGF19, intra‐operative or postoperative changes of ΔVA or plasma levels were not observed. The bile salt–homeostatic regulator farnesoid X receptor (FXR) was markedly up‐regulated following surgery (P < 0.001). Cell‐cycle re‐entry priming factors (interleukin 6 [IL‐6], signal transducer and activator of transcription 3 [STAT3], and cJUN) were up‐regulated following surgery and were positively correlated with FXR expression (P < 0.05). Postoperative hyperbilirubinemia was preceded by postsurgery low FXR and high Na+/Taurocholate cotransporting polypeptide (NTCP) expression in the remnant liver coupled with higher liver bile salt content (P < 0.05). Finally, bile salt levels on postoperative day 1 were an independent predictor of LR (P < 0.05). Conclusion: Systemic, portal, and liver bile salt levels are rapidly elevated after liver resection. Postoperative bile salts were positively associated with liver volume gain. In the studied time frame, FGF19 levels remained unaltered, suggesting that FGF19 plays a minor role in human LR. These findings indicate a more relevant role of bile salts in human LR.
Abbreviations
- A
arterial
- BSEP
bile salt export pump
- CRLM
colorectal liver metastases
- CT
computed tomography
- CYP7A1
cytochrome P450 7A1
- FGF19
fibroblast growth factor 19
- FXR
farnesoid X receptor
- HV
hepatic vein
- IL‐6
interleukin‐6
- IQR
interquartile range
- LR
liver regeneration
- mRNA
messenger RNA
- NTCP
Na+/Taurocholate cotransporting polypeptide
- PDV
portal drained viscera
- PHx
partial hepatectomy
- POD
postoperative day
- PV
portal vein
- SOCS3
suppressor of cytokine signaling 3
- SHP
short heterodimer partner
- STAT3
signal transducer and activator of transcription 3
- TV
tumor volume
- ULN
upper limit of normal
- ΔVA
venous‐arterial difference
Liver regeneration (LR) after partial liver resection is a highly complex process involving a myriad of molecular and metabolic pathways orchestrating the process.( 1 ) Bile salts and the bile salt–regulated enterokine fibroblast growth factor 19 (FGF19, termed Fgf15 in rodents) are critical regulators of LR in rodents.( 2, 3 ) Thus far, limited data are available about the involvement of bile salts or FGF19 in human LR.( 4, 5, 6, 7, 8 )
Bile salts are signaling molecules and ligands of bile salt–sensing receptors, such as the farnesoid X receptor (FXR), which are engaged in regulation of many metabolic and other processes (e.g., energy, bile salt and carbohydrate metabolism, and inflammation).( 9, 10, 11, 12 ) Studies in rodents undergoing partial hepatectomy (PHx) demonstrated that bile salt activation of hepatic Fxr promoted LR by stimulation of hepatocyte proliferation and protection against toxic effects of bile salt overload.( 13, 14 ) In addition, activation of intestinal FXR stimulates production of FGF19/Fgf15, which targets receptors on hepatocytes to repress bile salt synthesis by reducing gene expression of the bile salt synthetic enzyme cytochrome P450 7A1 (CYP7A1).( 15 ) Lowering of the bile salt pool by Fgf15 limits bile salt toxicity and therefore contributes to progression of LR under normal and steatotic conditions in mice.( 3, 16 ) Moreover, Fgf15 directly stimulates hepatocyte proliferation in a bile salt–independent manner through by activation of signaling pathways that promote cell cycle progression (e.g., by activating the signal transducer and activator of transcription 3 [Stat3], a major regulator of LR).( 17 )
Information on the distinctive phases in human LR is not available. Phases of LR are based on animal studies (see Rmilah et al. for a detailed overview of the LR phases in rodents( 18 )). Moreover, the translation of findings from animal studies, in particular data derived from studies on signaling molecules involved, is uncertain. Bearing in mind that FXR/FGF19 pathway–based pharmacotherapy has been evaluated in several clinical trials involving patients with liver disease,( 19, 20, 21, 22 ) and impaired LR contributes to mortality in hepatectomized patients,( 23 ) we studied involvement of the gut–liver axis of bile salt–FGF19 signaling in the initial phase in human LR. To this end, we studied bile salt–FGF19 levels in the vessels supplying and draining the liver, as well as hepatic gene expression, before and shortly after liver resection. Findings were correlated with liver volumetry.
Patients and Methods
Patients and Materials
We studied archived plasma samples and liver tissue of patients who underwent liver surgery for suspected colorectal liver metastases (n = 31) at Maastricht University Medical Center between November 2008 and May 2009. The original study and description of the blood and tissue‐sampling procedure were published previously.( 24, 25 ) Briefly, overnight‐fasted patients were prepared for surgery according to institutional procedures. Intraoperatively, blood was drawn near‐simultaneously from an arterial line (A), the portal (PV), and middle hepatic vein (HV) on two occasions: (1) within 1 hour after abdominal incision and liver mobilization (= baseline) and (2) shortly after liver transection (= following surgery). The average time between the two time points was 2 hours. For analysis of postoperative plasma courses, blood was drawn from an arterial line at postoperative day (POD) 1 and from a peripheral venous catheter at PODs 2 and 3. For 21 patients, postoperative blood samples were available for time‐course analysis. For subgroup analyses, liver resections were classified as minor (< 3 segments, n = 15) or major PHx (≥ 3 segments, n = 16).( 26 ) Concurrent with blood samplings, baseline and postsurgery wedge liver biopsies were taken from normal liver parenchyma of the (future) remnant liver (n = 21 paired specimens). Blood was collected in ethylene diamine tetraacetic acid tubes, and plasma samples and liver specimens were stored at −80°C. To study the postoperative plasma course of bile salts and FGF19 in an independent cohort, age‐matched and sex‐matched patients with colorectal liver metastases (CRLM) (n = 21) were selected from a prospectively collected database of patients who underwent a right hemihepatectomy between 2013 and 2016 at the RWTH University Hospital Aachen (Aachen, Germany).
Analytical Procedures
FGF19 was assayed by sandwich enzyme‐linked immunosorbent assay as described previously (n = 22).( 27 ) Bile salts were extracted from liver homogenates in 75% ethanol as described previously.( 28 ) Total bile salts in plasma (n = 22) and hepatic extracts (n = 18) were determined using an enzymatic cycling method according to the manufacturer’s protocol (Diazyme Laboratories, Poway, CA).
Quantification of Hepatic Gene‐Expression Levels
Total RNA was extracted from liver tissue using Qiazol reagent and RNA‐spin columns (Qiagen, Hilden, Germany). Contaminating genomic DNA was eliminated using gDNA Eliminator Columns (Qiagen). A total of 750 ng RNA was converted to complementary DNA (cDNA) (iScript cDNA synthesis kit; Bio‐Rad, Hercules, CA). Quantitative polymerase chain reactions (PCRs) were conducted in a volume of 10 µL containing cDNA equivalent to 3.75 ng total RNA as template. SYBR Green chemistry (qPCR SYBR Hi‐Rox Green Fluorescein Mix; Bioline, London, United Kingdom) and a LightCycler 384 system (Roche, Basel, Switzerland) were used for real‐time PCR analysis. Data were analyzed with LinReg software and expression was normalized to 36B4.( 29 ) Values are presented relative to the mean expression of the examined transcript in baseline liver specimens. Mean Cq values of the studied genes are reported in Supporting Table S1.
Classification of Patients With Postoperative Hyperbilirubinemia
Postoperative hyperbilirubinemia (POD 1, n = 24) was defined here as total bilirubin levels ≥ 1.5 times upper limit of normal (ULN). Based on this criterion, patients were grouped as normobilirubinemic (n = 10) or hyperbilirubinemic (n = 14). Reduced liver mass following PHx was considered when deviating from the commonly used ≥ 2 times ULN cutoff.
Liver Volumetry
The computed tomography (CT) analysis tool in Siemens Syngo.via version 4.1 (Munich, Germany) was used to perform liver volumetry (n = 28). See a detailed description of the procedure in the Supporting Methods. Briefly, a 3D reconstruction of the liver was acquired (Supporting Fig. S1). Tumor volume (TV, mL), total liver volume (TLV, mL), functional total liver volume (= TLV − TV, mL), and the anticipated future remnant liver volume (FRLV, mL) were assessed. Liver regeneration was estimated from postoperative CT scans (median follow‐up time = 10 [6‐110] days) and assessment of the remnant liver volume (RLV). To determine liver regeneration, the following formula was used: RG (%) = ([RLV at follow‐up − FRLV]/FRLV) × 100.( 30 ) See Supporting Table S2 for liver volumetry parameters.
Statistics and Calculations
Venous‐arterial differences (ΔVA) were calculated across the portal drained viscera (PDV) (= PV − A) and the liver (= HV – [0.7 * PV + 0.3 * A]).( 31 ) Because the actual portal and arterial blood flow were not measured intraoperatively, and it is plausible that flow redistribution occurs after resection, we also calculated ΔVA differences across the liver assuming blood flow to the liver, accounting 90% for portal blood and 10% for arterial flow (liver = HV – [0.9 * PV + 0.1 * A]). A positive value for ΔVA indicates net release or production by the organ/area, and a negative value for ΔVA indicates net uptake. The median ΔVA values were tested against a theoretical value of zero using the Wilcoxon signed‐rank test. Differences between paired samples (bile salt/FGF19 levels, ΔVA, liver bile salts, and hepatic transcripts) were evaluated with the Wilcoxon matched‐pairs signed‐rank test. Significant differences between groups (minor vs. major PHx and normobilirubinemia vs. hyperbilirubinemia) were evaluated with the Mann‐Whitney U test. Correlations were evaluated by the Spearman’s (ρ) correlation coefficient. Data are expressed as median (interquartile range [IQR]) or mean ± SD when appropriate. For graphical purposes, data are displayed as mean ± SEM. To test whether bile salt and FGF19 levels changed postoperatively and to avoid subject deletion because of postoperative missing values, linear mixed models were constructed to study the time course. Akaike’s information criterion was used to fit the best model. The time courses are graphically presented as estimated means ± standard error. Univariable and multivariable regression models were used to assess predictors for LR. Covariates used for multivariable linear regression were follow‐up time (days), weight of resected liver specimen (grams), and bile salt levels on POD1, based on their potential confounding and their significance in univariable analysis (P < 0.05). P values below 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA) and SPSS 22.0 (IBM SPSS Inc., Chicago, IL).
Results
Patients
Thirty‐one patients (mean age 62 years, 58% males) who underwent partial liver resection were studied. Postoperative examination by the pathologist confirmed the diagnosis of CRLM in 28 patients. The clinical and surgical characteristics of the studied patients are listed in Table 1.
TABLE 1.
Patient Characteristics
| Characteristic | Total Cohort (n = 31) |
|---|---|
| Age (years), mean ± SD | 62 ± 11 |
| Male sex (%) | 58 |
| BMI (kg/m2), mean (n = 30) | 26.3 |
| Preoperative liver tests, median [IQR] | |
| Bilirubin (µmol/L) (n = 29) | 13 [11‐15] |
| AST (IU/L) (n = 29) | 23 [16‐31] |
| ALT (IU/L) (n = 29) | 26 [19‐35] |
| GGT (IU/L) (n = 29) | 44 [36‐76] |
| ALP (IU/L) (n = 28) | 100 [76‐127] |
| Liver resection type* | |
| Minor hepatectomy (n = 16) | |
| Posterior sectionectomy (n = 3) | |
| segment resection (n = 7) | |
| Multiple segments resection (<3) (n = 3) | |
| Metastasectomy (n = 3) | |
| Major hepatectomy (n = 15) | |
| Right hemihepatectomy (n = 11) | |
| Left hemihepatectomy (n = 1) | |
| Multiple segments (≥3) (n = 3) | |
| Liver pathology | |
| Metastases (adenocarcinoma), n | 28 |
| Nonmalignant liver parenchyma or no sign of metastases, n | 3 |
Minor hepatectomy: fewer than three segments; major hepatectomy: three or more segments.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; and GGT, gamma glutamyl transferase.
Elevated Arterial and Portal Bile Salt Levels Shortly After Liver Resection
Following resection, bile salt levels were elevated in arterial (P < 0.001) and portal venous blood (P < 0.001), with a tendency toward higher levels in hepatic venous blood (P = 0.085) (Table 2). In contrast to elevated bile salts, FGF19 levels were lower in arterial (P = 0.030), portal (P = 0.026), and hepatic venous blood (P = 0.038) after resection (Table 2). Baseline systemic bile salt levels correlated positively with postsurgery FGF19 levels (ρ = +0.50, P < 0.001), supporting the temporal relationship between bile salts and FGF19 (Supporting Fig. S2).
TABLE 2.
Plasma Bile Salt and FGF19 Level Changes Shortly After Liver Resection
| Item | Before Surgery (n = 22) | After Surgery (n = 22) | P Value |
|---|---|---|---|
| Bile salts (µmol/L) | |||
| A | 2.7 [1.7‐ 6.3] | 5.9 [3.6‐8.1] | <0.001 |
| PV | 7.3 [4.9‐12.4] | 12.3 [9.3‐20.5] | <0.001 |
| Middle HV | 3.7 [2.4‐6.4] | 5.6 [4.6‐8.1] | 0.085 |
| FGF19 (ng/mL) | |||
| A | 0.13 [0.06‐0.18] | 0.08 [0.04‐0.11] | 0.030 |
| PV | 0.16 [0.08‐0.22] | 0.09 [0.05‐0.15] | 0.026 |
| Middle HV | 0.14 [0.08‐0.19] | 0.08 [0.05‐0.14] | 0.038 |
Data are expressed as median [IQR]. P values in bold are less than 0.05 and represent significant statistical differences.
VA Differences of Bile Salts Across the PDV and the Liver Are Altered Shortly After Liver Resection
To assess whether bile salts/FGF19 signaling could be involved in the early events of human LR, we studied the interorgan exchange of bile salts and FGF19 during surgery. For bile salts, the ΔVAPDV at baseline was significantly positive, indicating net release of bile salts. Shortly after liver resection the ΔVAPDV was increased (P = 0.029) (Table 3). There was net hepatic extraction (ΔVALiver negative) of bile salts, which was highest shortly after liver resection (P < 0.001) (Table 3). Intra‐operative changes of ΔVA of FGF19 were not observed. The ΔVAPDV and ΔVALiver remained unaltered (P = 0.633 and P = 0.824, respectively), indicating no net release of FGF19 into the portal circulation and no net hepatic extraction.
TABLE 3.
VA Differences Across the PDV and Liver
| Item | Baseline (n = 22) | Following Surgery (n = 22) | P Value |
|---|---|---|---|
| Bile salts (µmol/L) | |||
| ΔPDV | 4.3 [+2.2 to +8.4]† | +6.7 [+4.2 to +11.8]† | 0.029 |
| ΔLiver * | |||
| 0.7 vs. 0.3 | −2.5 [−5.5 to −1.2]† | −3.4 [−8.2 to −2.7]† | <0.001 |
| 0.9 vs. 0.1 | −3.5 [−7.3 to −1.9]† | −4.7 [−10.5 to −3.6]† | <0.001 |
| FGF19 (ng/mL) | |||
| ΔPDV | +0.02 [+0.01 to +0.03]† | +0.02 [0.00 to +0.03]‡ | 0.633 |
| ΔLiver * | |||
| 0.7 vs. 0.3 | 0.00 [−0.01 to +0.03] | +0.01 [−0.01 to +0.01] | 0.824 |
| 0.9 vs. 0.1 | −0.00 [−0.01 to +0.02] | +0.01 [−0.02 to +0.01] | 0.799 |
Data are expressed as median [IQR]. Positive ΔVA indicates release by the organ/area, and a negative ΔVA indicates uptake. P values in bold are less than 0.05 and represent significant statistical differences.
ΔVA across the liver is calculated assuming different ratios (portal vs. arterial) of blood supply to the liver. Symbols indicate significance against theoretical zero.
P < 0.001 in comparison of VA differences before and after liver resection.
P < 0.01 in comparison of VA differences before and after liver resection.
Postoperative Elevation of Circulating Bile Salts, but Not FGF19
Serum total bilirubin, aspartate transaminase and alanine transaminase were elevated postoperatively, with levels peaking at POD1 and declining thereafter (Supporting Fig. S3). This could indicate moderate impairment of secretory function and (surgically induced) liver damage. Next, we evaluated the postoperative plasma excursion of bile salts and FGF19. We observed a rise in circulating bile salts from POD1 onward (P < 0.001) (Fig. 1A). Plasma bile salt levels were higher on POD1 (6.6 [4.7‐8.4] μmol/L, P = 0.139) and POD2 (10.4 [7.4‐26.4] μmol/L, P < 0.001) compared with baseline values (2.6 [1.6‐2.9] μmol/L) (Fig. 1A). Plasma or systemic bile salt elevation was most pronounced in patients undergoing major liver resection, reaching significance at POD1 (5.2 [3.7‐6.6] μmol/L vs. 8.4 [6.6‐28.4] μmol/L, P = 0.017) and POD2 (8.0 [4.4‐11.8] vs. 22.2 [9.6‐44.8] μmol/L, P = 0.048) (Fig. 1B). Plasma FGF19 levels were not altered in the studied postoperative course (P = 0.058) (Fig. 1C). The extent of liver resection did not affect FGF19 levels (Fig. 1D).
FIG. 1.
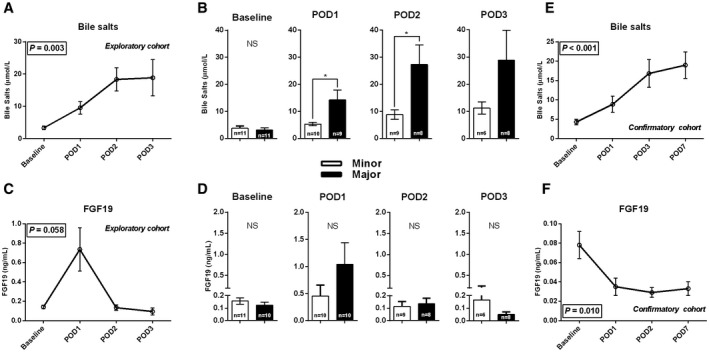
Postoperative time course of bile salts and FGF19. Postoperative time course (n = 21) of systemic bile salts and FGF19 on baseline and PODs 1, 2, and 3. (A) The postoperative time course of bile salts. (B) The differences in systemic bile salts between the minor and major hepatectomy group. (C,D) Findings of FGF19. (E,F) The postoperative bile salt and FGF19 course in an independent cohort of patients with CRLM who underwent a right hemihepatectomy. Asterisks indicates significant differences compared with baseline: ***P < 0.001. Abbreviation: NS, not significant.
We confirmed postoperative courses of bile salts and FGF19 in an independent cohort of 21 patients (mean age = 62 years, 47% males, undergoing right hemihepatectomy for treatment of CRLM; see Supporting Table S3 for additional patient characteristics). Replicating the initial observations, bile salts levels were significantly elevated from postoperative day 1 onward (P < 0.001) (Fig. 1E). In contrast, postoperative FGF19 levels declined in the validation cohort (Fig. 1F).
Transcriptional Alterations Shortly After Liver Resection
Paired specimens of the future remnant (baseline) and remnant liver (following surgery) were available for most of the patients (= 21) and used to study changes in hepatic bile salt content and gene expression. Liver tissue bile salt content was elevated shortly after liver resection (0.15 [0.11‐0.24] µmol/mg vs. 0.21 [0.15‐0.40] µmol/mg liver weight, P = 0.03) (Fig. 2A). To find an explanation for this, we studied gene transcripts related to (regulation of) bile salt synthesis and transport. FXR expression was markedly higher shortly after resection (+5.0 fold, P < 0.001) (Fig. 2B). Note that the intraoperative time correlated significantly with post‐resectional FXR expression (ρ = +0.57, P = 0.008) (Supporting Fig. S4A). Moreover, postsurgery portal bile salt levels correlated with postsurgery hepatic FXR expression (ρ = +0.58, P = 0.002) (Supporting Fig. S4B). The expression of short heterodimer partner (SHP), an FXR target gene, was reduced after resection (−1.5 fold, P = 0.029). Messenger RNA (mRNA) levels of CYP7A1 were also decreased after resection (−1.7 fold, P < 0.01) (Fig. 2B). Expression of oxysterol 7α‐hydroxylase (CYP7B1), participating in the acidic pathway of bile salt synthesis, and sterol 12α‐hydroxylase (CYP8B1) were unaltered shortly after liver surgery (data not shown). Gene expression of BACS and BAAT, coding for enzymes engaged in (re)conjugation of bile acids, did not change after resection (data not shown).
FIG. 2.
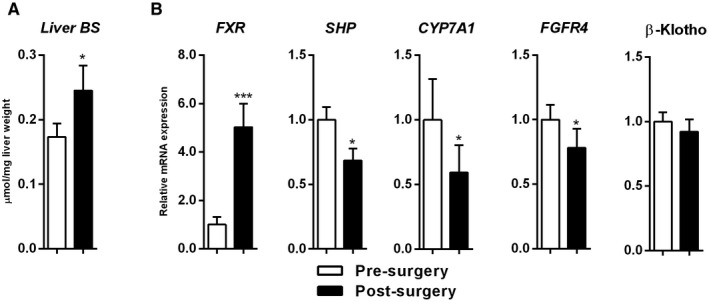
Elevated liver bile salts shortly after liver surgery and up‐regulation of FXR. (A) Paired liver bile salts (n = 18). (B) Paired hepatic expression of genes related to bile salt synthesis (n = 21; bars represent means of fold changes relative to baseline). White bars indicate baseline and black bars indicate after surgery. *P < 0.05 and ***P < 0.001. Abbreviation: BS, bile salt.
Interaction of FGF19 with its hepatic coreceptor complex, FGFR4/β‐Klotho, results in transcriptional repression of CYP7A1.( 32 ) Expression of FGFR4 was decreased following surgery (−1.3 fold, P < 0.05), whereas β‐Klotho expression was not different after resection (Fig. 2B). Hepatic transcript levels of transporters engaged in bile salt uptake or secretion (i.e., the bile salt export pump [BSEP], multidrug resistant‐associated protein‐3 or 4 [MRP3 or 4], and Na+‐taurocholate cotransporting polypeptide [NTCP] and organic anion‐transporting polypeptide 1B3 [OATP1B3]) were all unaltered after liver resection (data not shown). Collectively, higher bile salt content following surgery could be explained by higher portal delivery of bile salts, but unaltered expression of NTCP/BSEP, resulting in bile salt accumulation in hepatocytes (Fig. 2).
Rapid Alterations of Liver, Portal, and Systemic Circulating Bile Salts and Up‐regulated FXR Occurred During the “Priming Phase” of LR
These findings demonstrate that hepatic as well as portal and systemic bile salts, and hepatic FXR expression, were elevated shortly after liver resection. This suggests that bile salt signaling may be involved in the early events (analogous to rodent liver regeneration in the “priming phase”), resulting in regrowth of the human liver. To investigate this notion, we studied hepatic expression of priming factors/immediate‐early genes and their association with bile salt signaling.
Interleukin‐6 (IL‐6) is a pleiotropic cytokine engaged in the priming phase of rodent LR by protecting the liver from fulminant surgical injury.( 33 ) IL‐6 expression was induced after resection (+4.0 fold, P < 0.001) (Fig. 3A). Expression of STAT3, an indirect downstream target gene of IL‐6 signaling and involved in hepatocyte proliferation,( 34 ) was also induced (+1.8 fold, P < 0.001) (Fig. 3A). In addition, c‐JUN, a direct target gene of STAT3 and key regulator of early hepatocyte proliferation,( 35 ) was increased after surgery (+2.0 fold, P = 0.02) (Fig. 3A). Expression of suppressor of cytokine signaling 3 (SOCS3), a negative regulator of IL‐6 signaling and subsequently an indirect repressor of LR, was not altered after resection (P = 0.95) (Fig. 3A). Portal and liver bile salts were significantly correlated with IL‐6 expression (ρ = +0.63, P < 0.001; and ρ = +0.35, P = 0.042; respectively) (Fig. 3B). Furthermore, FXR expression correlated with IL‐6, STAT3, and c‐JUN expression (ρ = +0.70, P < 0.001; ρ = +0.67, P < 0.001; and ρ = +0.44, P = 0.004; respectively), but not with SOCS3 (ρ = +0.13, P = 0.42) (Fig. 3C).
FIG. 3.
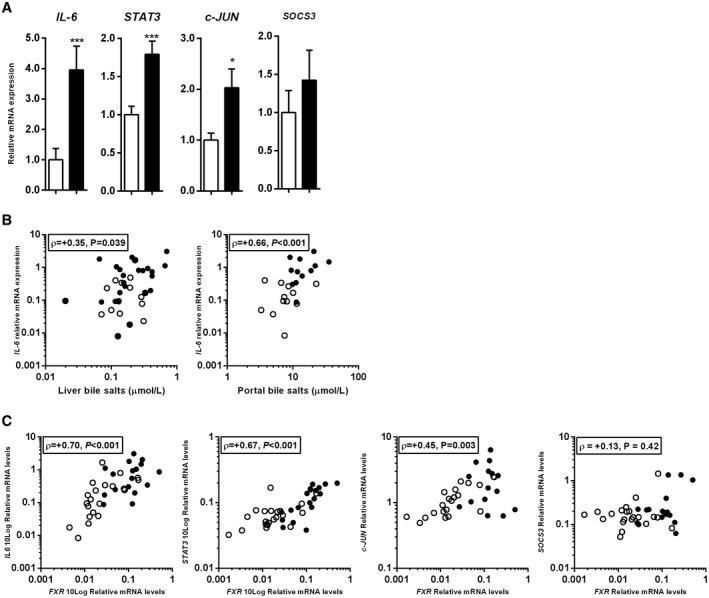
Up‐regulation of early‐phase genes shortly after liver resection. (A) Paired hepatic expression of genes related to IL‐6 signaling pathway. (B) Relation between IL‐6 expression and portal and liver bile salts. (C) Correlation between mRNA expression levels of FXR and IL‐6, STAT3, CJUN, and SOCS3. Bars represent the means of fold changes relative to baseline. White bars indicate before surgery and black bars indicate after surgery. Open circles indicate baseline correlations and filled circles indicate after surgery. *P < 0.05 and ***P < 0.001.
Altered Bile Salt Homeostasis in the Remnant Liver Shortly After Liver Resection Precedes Postoperative Cholestatic Liver Dysfunction
Findings highlight involvement of bile salt signaling early in human liver regeneration. Because some of the patients developed postoperative hyperbilirubinemia, we studied whether these patients had different adaptive responses in the remnant liver compared to patients with normal bilirubin levels. Bilirubin levels on day 1 were substantially higher in the cholestatic group (n = 14) compared with controls (n = 10) (35 [29‐50] vs. 17 [16‐20] µmol/L; P < 0.001) (Fig. 4A). Note, the amount of major hepatectomies in the cholestatic group was not significantly different compared with the noncholestatic group (P = 0.095) (data not shown). FXR gene expression was down‐regulated in the cholestatic group compared with controls (P = 0.029) (Fig. 4B). On the other hand, NTCP was up‐regulated in the cholestatic group (P = 0.010), while BSEP and MRP2 expression were similar between the two groups (P = 0.127 and P = 0.203, respectively) (Fig. 4B). In line with increased NTCP expression, hepatic bile salt content was higher in the cholestatic group (P = 0.047) (Fig. 4C). IL‐6 transcripts were similar between the groups (P = 0.601).
FIG. 4.
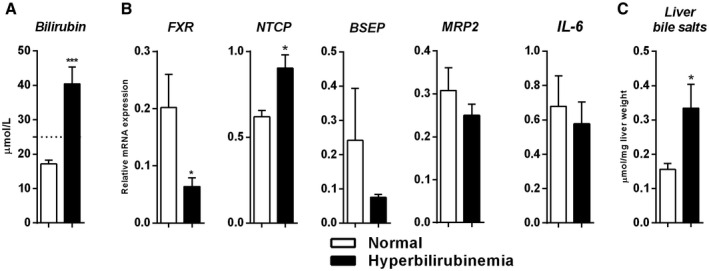
Alteration of bile salt homeostasis shortly after liver resection precedes postoperative hyperbilirubinemia. (A) Bilirubin levels (n = 10 vs. n = 14) on POD1. (B) Gene expression immediately after resection (n = 7 vs. n = 8) of FXR, FXR‐regulated genes engaged in bile salt transport, and IL‐6 between controls and hyperbilirubinemic patients. (C) Hepatic bile salt content (n = 6 vs. n = 7) immediately after resection. Bars represent means and SEMs. White bars indicate healthy patients and black bars indicate hyperbilirubinemic patients. The interrupted line in (A) reflects bilirubin levels ≥ 1.5 times ULN. The significance level is depicted by asterisks and denotes significant differences between the normal and cholestasis groups: *P < 0.05.
Postoperative Bile Salt Levels Are an Independent Predictor of LR
Finally, because our findings indicate that bile salt signaling may occur in the early events of human LR, we investigated whether bile salts and other variables were associated with LR based on pre‐operative and postoperative liver volumetry. The variables studied in univariable and multivariable regression analyses are depicted in Table 4. Predictors of LR were follow‐up time (P = 0.010), weight of resected liver specimen (P = 0.039), and systemic bile salt level on POD1 (P = 0.034) and POD2 (P = 0.010) (Table 4). In a step‐wise multivariable analysis, bile salts at POD1 (P = 0.013) were an independent predictor of LR, when adjusted for follow‐up time and weight of resected liver specimen (Table 4).
TABLE 4.
Univariable and Multivariable Regression Analysis of Association With LR (%) (n = 28)
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| B | SE | P Value | B | SE | P Value | |
| Age at surgery (years) | −530.0 | 0.8 | 0.510 | — | — | — |
| Sex (female) | 30.1 | 17.0 | 0.088 | — | — | — |
| BMI (kg/m2) (n = 27) | −1.2 | 1.9 | 0.549 | — | — | — |
| Follow‐up time (days)* | −0.2 | 0.1 | 0.010 | −0.3 | 0.02 | 0.019 |
| Blood loss during surgery (mL) (n = 10) | 0.0 | 0.0 | 0.896 | — | — | — |
| Weight of resected liver specimen (g) | 0.07 | 0.02 | 0.005 | 0.1 | 0.11 | 0.039 |
| ΔPDV after surgery | ||||||
| Bile salts | −3.7 | 1.5 | 0.201 | — | — | — |
| FGF19 | 103.2 | 282.8 | 0.720 | — | — | — |
| ΔLiver after surgery | ||||||
| Bile salts | 2.7 | 2.2 | 0.239 | — | — | — |
| FGF19 | −25.1 | 292.3 | 0.933 | — | — | — |
| Liver bile salts (n = 16) (µmol/L) | ||||||
| Presurgery | −81.3 | 110.1 | 0.472 | — | — | — |
| Postsurgery | 3.4 | 50.9 | 0.948 | — | — | — |
| Δ surgery† | 82.6 | 72.1 | 0.271 | — | — | — |
| Postoperative bile salt levels (µmol/L) | ||||||
| Day 1 (n = 16) | 2.8 | 1.2 | 0.034 | 2.5 | 0.77 | 0.013 |
| Day 2 (n = 14) | 1.7 | 0.6 | 0.010 | — | — | — |
| Day 3 (n = 12) | 0.56 | 0.59 | 0.365 | — | — | — |
| Postoperative FGF19 levels (ng/mL) | ||||||
| Day 1 (n = 17) | 5.9 | 11.4 | 0.610 | — | — | ‐ |
| Day 2 (n = 14) | 112.6 | 118.9 | 0.362 | — | — | — |
| Day 3 (n = 12) | −50.2 | 128.0 | 0.703 | — | — | — |
P values in bold are less than 0.05 and represent significant statistical differences.
Time between day after surgery and postoperative day of CT scan.
Difference between presurgery and postsurgery levels.
Abbreviations: B, beta; BMI, body mass index; SE, standard error for the beta.
Discussion
The present study in patients demonstrates increased portal delivery of bile salts, enhanced hepatic extraction, and a larger hepatic content of bile salts, shortly after liver resection. These events were associated with up‐regulation of FXR expression in the remnant liver and occurred congruently with up‐regulated expression of cell‐cycle re‐entry priming genes (IL‐6, STAT3, and cJUN). We further provide evidence that postoperative hyperbilirubinemia is preceded by low FXR and high NTCP mRNA expression, and higher hepatic bile salt content in the remnant liver. Finally, circulating bile salts—but not FGF19—rise postoperatively, and independently predict LR in patients after partial liver resection. These findings support a more relevant role of bile salt signaling early in human LR.
In line with earlier studies in animals and humans, the observed rise in systemic bile salts in the postoperative phase was the likely consequence of limited spare capacity of the remnant liver to maintain serum bile salt homeostasis.( 2, 4, 5 ) In addition, our data demonstrate increased portal bile salts and enhanced hepatic extraction shortly after liver resection. Altered enterohepatic dynamics could underlie these observations. In animal experiments, bile flow and biliary bile salt secretion per minute per gram liver weight were increased in partially hepatectomized rats.( 36 ) Together with higher portal delivery, hepatic bile salt content was also increased in the early phase (observations not reported in humans thus far). Because the mRNA expression of major bile salt transporters in the hepatocyte was unaltered, the increased hepatic bile salt content may be explained by higher portal delivery to the periportal zone. It is conceivable that the mechanism for higher portal delivery of bile salts immediately after resection could arise from elevated luminal bile salt input. Intestinal biliary inflow may increase in response to surgical manipulation of the liver. A plausible explanation for the absent changes in expression of the main hepatic bile salt uptake/extraction system could be (1) the short intraoperative timeframe to detect significant changes or (2) alterations in transporter activity through posttranslational modification or membrane insertion. We could not assess these aspects in this study. Intriguingly, hepatic bile salt content and NTCP mRNA expression were both higher in patients who developed postoperative hyperbilirubinemia.
Remarkably, FXR transcripts were 5‐fold higher in the remnant liver shortly after liver resection. Nonetheless, up‐regulated FXR and elevated hepatic bile salt content was not accompanied by induction of FXR‐target genes such as SHP, BSEP, or organic solute transporter‐beta (OSTβ) (data not shown). In rodents, diet‐delivered bile salts up‐regulated Fxr after PHx, whereas treatment with cholestyramine lowered hepatic Fxr transcripts immediately after PHx.( 37 ) These findings suggest that FXR gene transcription is affected by portal influx of bile salts, and such a feed‐forward loop would be in line with the major role of FXR in bile salt homeostasis. Note that portal bile salts were positively correlated with FXR gene expression (Supporting Fig. S4). The actual mechanism for up‐regulation of FXR after resection remains to be elucidated. As FGF19‐induced phosphorylation of hepatic FXR modulates its transcriptional activity, reduced portal FGF19 may underlie the apparent disconnect between higher FXR mRNA expression and lack of FXR target gene induction.( 38 ) FXR has (at least) two major roles in rodent liver regeneration, including (1) maintaining bile salt homeostasis (and thus limiting bile salt toxicity) and (2) promoting hepatocyte proliferation by up‐regulation of hepatic FOXM1b, a key regulator of cell‐cycle progression and induction of mitogenic FGF19/15.( 14 ) However, these events might not be occurring in the studied timeframe. Interestingly, patients who developed hyperbilirubinemia had reduced FXR mRNA expression in the remnant liver. Therefore, the regulation of FXR shortly after liver resection could be important for the functional recovery of hepatocytes in the postoperative phase.
In line with previously reported high intraoperative levels of serum IL‐6, hepatic transcripts of IL‐6 were increased in the remnant liver after liver resection.( 39 ) IL‐6 and other genes related to cell‐cycle re‐entry (STAT3 and cJUN) were positively correlated with FXR expression and portal bile salts. The biological pathways underlying the positive relation between portal bile salts and hepatic mRNA levels of IL‐6 and STAT3 are not understood. A possible explanation may include direct activation of STAT3 signaling by bile salts( 40 ) or the relation may not be causative, but both factors act in parallel in promoting LR. Collectively, this indicates that changes in hepatic bile salt–FXR axis are integrated within the early phase of human LR.
We observed no intraoperative and postoperative changes of FGF19 levels, and levels on the examined time points were not associated with LR. Several key studies demonstrated the pro‐regenerative effects of Fgf15/FGF19 in animal models of LR, including humanized mice.( 3, 41, 42 ) Furthermore, FGF19 promotes the development of HCC.( 43 ) However, we observed maintained (exploratory cohort) or reduced (confirmatory cohort) FGF19 levels during the first postoperative week. Considering that postoperative bile salt levels—but not FGF19—were associated with liver volume gain, we suggest that FGF19 plays a minor role in human LR. Therefore, rising postoperative bile salt levels (reflecting systemic spillover) might be a more relevant physiological biomarker for LR.( 2 ) The study design does not allow us to dismiss a role of FGF19 in human LR. Translation of findings from animal studies may not be straightforward in this aspect. First, rodent liver anatomy allows clean excision of liver lobes with no to minimal inflammation.( 44 ) Human liver resection typically involves dissection along segmental boundaries, causing injury and inflammation in the remnant liver. Roles of FGF19 in these distinct contexts may be different. Second, loss and gain of FGF19/Fgf15‐function approaches in animals result in deteriorated and improved bile salt homeostasis before PHx, respectively, with pronounced consequences for the subsequent course of LR.( 17, 41, 42 ) In addition, the starting point for FGF19 action appears to be different in animals and humans. Further human studies are therefore required to address the role of FGF19 in LR. Ongoing clinical studies with the FGF19 mimetic NGM282/Aldafermin may shed light on the role of FGF19 in recovery of the liver from tissue injury in the context of metabolic liver disease (ClinicalTrials.gov: NCT04210245 and NCT03912532).( 20, 45, 46 )
A couple limitations need to be addressed for this study. First, a total of 31 patients were studied, but blood and tissue samples were not available for all patients. Therefore, subsets of patients were studied. Second, actual portal and hepatic arterial flow were not measured during resection; instead, we used previous reported flow distributions.( 31 ) Importantly, the observed VA differences remain when performing calculations with a different flow ratio.
In summary, this study clearly demonstrates elevation of liver, portal, and systemic circulating bile salts—but not FGF19—shortly after liver resection. Interestingly, changes in FXR and NTCP gene expression, combined with higher bile salt content shortly after liver resection, preceded development of postoperative hyperbilirubinemia. Findings from our work further support a more relevant role of bile salt signaling in the early events in human LR, rather than involvement of FGF19 in this process. Pharmacological activation of FXR could be a potential therapeutic option to enhance regenerative capacity and protect the liver from dysfunctional hepatocytes and postoperative liver failure.
Supporting information
Fig S1‐4
Table S1‐3
Supplementary Material
Acknowledgment
The authors thank Maartje A. van den Broek and Simon A.W.G. Dello, who were engaged in original patient inclusion and sample collection. They also thank Ilke Sauer, who provided assistance in collecting samples and clinical data of patients from the RWTH University Hospital Aachen.
Supported by the Netherlands Organization for Scientific Research (NWO 022.003.011) and the German Research Foundation (Project‐ID 403224013 ‐ SFB 1382).
Potential conflict of interest: Prof. Dr. P.L.M. Jansen advises Falk and Enyo.
References
Author names in bold designate shared co‐first authorship.
- 1.Forbes SJ, Newsome PN. Liver regeneration—mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol 2016;13:473‐485. [DOI] [PubMed] [Google Scholar]
- 2.Naugler WE. Bile acid flux is necessary for normal liver regeneration. PLoS One 2014;9:e97426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uriarte I, Fernandez‐Barrena MG, Monte MJ, Latasa MU, Chang HCY, Carotti S, et al. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post‐resection liver failure in mice. Gut 2013;62:899‐910. [DOI] [PubMed] [Google Scholar]
- 4.Hoekstra LT, van Lienden KP, Schaap FG, Chamuleau RAFM, Bennink RJ, van Gulik TM, et al. Can plasma bile salt, triglycerides, and apoA‐V levels predict liver regeneration? World J Surg 2012;36:2901‐2908. [DOI] [PubMed] [Google Scholar]
- 5.Otao R, Beppu T, Isiko T, Mima K, Okabe H, Hayashi H, et al. External biliary drainage and liver regeneration after major hepatectomy. Br J Surg 2012;99:1569‐1574. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi H, Beppu T, Sugita H, Horino K, Komori H, Masuda T, et al. Increase in the serum bile acid level predicts the effective hypertrophy of the nonembolized hepatic lobe after right portal vein embolization. World J Surg 2009;33:1933‐1940. [DOI] [PubMed] [Google Scholar]
- 7.Stärkel P, Shindano T, Horsmans Y, Gigot JF, Fernandez‐Tagarro M, Marin JJG, et al. Foetal ‘flat’ bile acids reappear during human liver regeneration after surgery. Eur J Clin Invest 2009;39:58‐64. [DOI] [PubMed] [Google Scholar]
- 8.Kurumiya Y, Nagino M, Nozawa K, Kamiya J, Uesaka K, Sano T, et al. Biliary bile acid concentration is a simple and reliable indicator for liver function after hepatobiliary resection for biliary cancer. Surgery 2003;133:512‐520. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki T, Choi M, Moschetta A, Peng LI, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2005;2:217‐225. [DOI] [PubMed] [Google Scholar]
- 10.Kong BO, Wang LI, Chiang JYL, Zhang Y, Klaassen CD, Guo GL, et al. Mechanism of tissue‐specific farnesoid X receptor in suppressing the expression of genes in bile‐acid synthesis in mice. Hepatology 2012;56:1034‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 2015;21:159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5‐mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009;10:167‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, et al. Nuclear receptor‐dependent bile acid signaling is required for normal liver regeneration. Science 2006;312:233‐236. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Wang Y‐D, Chen W‐D, Wang X, Lou G, Liu N, et al. Promotion of liver regeneration/repair by farnesoid X receptor in both liver and intestine in mice. Hepatology 2012;56:2336‐2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JH, Nolan JD, Kennie SL, Johnston IM, Dew T, Dixon PH, et al. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Physiol Gastrointest Liver Physiol 2013;304:G940‐G948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez‐Sola G, Uriarte I, Latasa MU, Fernandez‐Barrena MG, Urtasun R, Elizalde M, et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet‐induced hepatic steatosis: development of an FGF19‐based chimeric molecule to promote fatty liver regeneration. Gut 2017;66:1818‐1828. [DOI] [PubMed] [Google Scholar]
- 17.Kong BO, Sun R, Huang M, Chow MD, Zhong X‐B, Xie W, et al. Fibroblast growth factor 15‐dependent and bile acid‐independent promotion of liver regeneration in mice. Hepatology 2018;68:1961‐1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu Rmilah A, Zhou W, Nelson E, Lin LI, Amiot B, Nyberg SL, et al. Understanding the marvels behind liver regeneration. Wiley Interdiscip Rev Dev Biol 2019;8:e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirschfield GM, Chazouilleres O, Drenth JP, Thorburn D, Harrison SA, Landis CS, et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double‐blind, placebo‐controlled phase II trial. J Hepatol 2018;70:483‐493. [DOI] [PubMed] [Google Scholar]
- 20.Harrison SA, Rinella ME, Abdelmalek MF, Trotter JF, Paredes AH, Arnold HL, et al. NGM282 for treatment of non‐alcoholic steatohepatitis: a multicentre, randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet 2018;391:1174‐1185. [DOI] [PubMed] [Google Scholar]
- 21.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. A placebo‐controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016;375:631‐643. [DOI] [PubMed] [Google Scholar]
- 22.Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco D, Capussotti L, Smadja C, Bouzari H, Meakins J, Kemeny F, et al. Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology 1990;98:733‐738. [PubMed] [Google Scholar]
- 24.van den Broek MAJ, Shiri‐Sverdlov R, Schreurs JJW, Bloemen JG, Bieghs V, Rensen SS, et al. Liver manipulation during liver surgery in humans is associated with hepatocellular damage and hepatic inflammation. Liver Int 2013;33:633‐641. [DOI] [PubMed] [Google Scholar]
- 25.van den Broek MAJ, Bloemen JG, Dello SAWG, van de Poll MCG, Olde Damink SWM, Dejong CHC, et al. Randomized controlled trial analyzing the effect of 15 or 30 min intermittent Pringle maneuver on hepatocellular damage during liver surgery. J Hepatol 2011;55:337‐345. [DOI] [PubMed] [Google Scholar]
- 26.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351‐355. [DOI] [PubMed] [Google Scholar]
- 27.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PLM, et al. High expression of the bile salt‐homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 2009;49:1228‐1235. [DOI] [PubMed] [Google Scholar]
- 28.Modica S, Murzilli S, Moschetta A. Characterizing bile acid and lipid metabolism in the liver and gastrointestinal tract of mice. Curr Protoc Mouse Biol 2011;1:289‐321. [DOI] [PubMed] [Google Scholar]
- 29.Alnouti Y, Csanaky IL, Klaassen CD. Quantitative‐profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC‐MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2008;873:209‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruttadauria S, Parikh V, Pagano D, Tuzzolino F, Cintorino D, Miraglia R, et al. Early regeneration of the remnant liver volume after right hepatectomy for living donation: a multiple regression analysis. Liver Transpl 2012;18:907‐913. [DOI] [PubMed] [Google Scholar]
- 31.van de Poll MCG, Siroen MPC, van Leeuwen PAM, Soeters PB, Melis GC, Boelens PG, et al. Interorgan amino acid exchange in humans: consequences for arginine and citrulline metabolism. Am J Clin Nutr 2007;85:167‐172. [DOI] [PubMed] [Google Scholar]
- 32.Song K‐H, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha‐hydroxylase gene expression. Hepatology 2009;49:297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin‐6‐deficient mice. Science 1996;274:1379‐1383. [DOI] [PubMed] [Google Scholar]
- 34.Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology 1995;21:1443‐1449. [PubMed] [Google Scholar]
- 35.Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, et al. c‐Jun‐N‐terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology 2003;37:824‐832. [DOI] [PubMed] [Google Scholar]
- 36.Hoshino M, Hirano A, Hayakawa T, Kamiya Y, Ohiwa T, Tanaka A, et al. Comparative studies on bile flow and biliary lipid excretion after bile‐acid loading in normal and partially hepatectomized rats. Biochem J 1995;305(Pt 2):367‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong X, Zhao H, Ma X, Wang S. Reduction in bile acid pool causes delayed liver regeneration accompanied by down‐regulated expression of FXR and c‐Jun mRNA in rats. J Huazhong Univ Sci Technolog Med Sci 2010;30:55‐60. [DOI] [PubMed] [Google Scholar]
- 38.Byun S, Kim D‐H, Ryerson D, Kim Y‐C, Sun H, Kong BO, et al. Postprandial FGF19‐induced phosphorylation by Src is critical for FXR function in bile acid homeostasis. Nat Commun 2018;9:2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dello SAWG, Bloemen JG, van de Poll MCG, van Dam RM, Stoot JHMB, van den Broek MAJ, et al. Gut and liver handling of interleukin‐6 during liver resection in man. HPB 2011;13:324‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhat AA, Lu H, Soutto M, Capobianco A, Rai P, Zaika A, et al. Exposure of Barrett's and esophageal adenocarcinoma cells to bile acids activates EGFR‐STAT3 signaling axis via induction of APE1. Oncogene 2018;37:6011‐6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong BO, Huang J, Zhu Y, Li G, Williams J, Shen S, et al. Fibroblast growth factor 15 deficiency impairs liver regeneration in mice. Am J Physiol Gastrointest Liver Physiol 2014;306:G893‐G902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naugler WE, Tarlow BD, Fedorov LM, Taylor M, Pelz C, Li B, et al. Fibroblast growth factor signaling controls liver size in mice with humanized livers. Gastroenterology 2015;149:728‐740.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim RD, Sarker D, Meyer T, Yau T, Macarulla T, Park J‐W, et al. First‐in‐human phase I study of fisogatinib (BLU‐554) validates aberrant FGF19 signaling as a driver event in hepatocellular carcinoma. Cancer Discov 2019;9:1696‐1707. [DOI] [PubMed] [Google Scholar]
- 44.van de Laarschot LFM, Jansen PLM, Schaap FG, Olde Damink SWM. The role of bile salts in liver regeneration. Hepatol Int 2016;10:733‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison SA, Rossi SJ, Paredes AH, Trotter JF, Bashir MR, Guy CD, et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology 2020;71:1198‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayo MJ, Wigg AJ, Leggett BA, Arnold H, Thompson AJ, Weltman M, et al. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double‐blind, placebo‐controlled trial. Hepatol Commun 2018;2:1037‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐4
Table S1‐3
Supplementary Material


