Abstract
Background
Inflammation is pathognomonic of all stages of tumor formation, and therefore, there is renewed interest in systemic inflammatory response (SIR) markers including haematological inflammatory markers such as neutrophil-to-lymphocyte ratio (NLR) as prognostic predictors in several cancers.
Aim
This study was aimed to investigate the effect of pretreatment peripheral blood NLR on the survival prognosis of patients with epithelial ovarian cancer (EOC).
Methods
We identified 93 patients with a complete clinical record from a cohort of 155 patients who received treatment for EOC between 2009 and 2018. Patients’ sociodemographic and clinicopathologic characteristics, and updated three-year follow-up status were extracted from medical records. Pretreatment peripheral blood NLR was calculated by dividing the neutrophil count by the lymphocyte count. We employed the receiver operating characteristic (ROC) curve to identify the optimal cut-off value of the NLR in estimating progression-free survival (PFS) and overall survival (OS). The PFS and OS were assessed using the Kaplan-Meier method, and survival differences were compared using the Log Rank (Mantel-Cox) test. Independent prognostic predictors were determined using Cox regression analysis.
Results
According to the ROC curves, the optimal cut-off values for the NLR were 2.23 and 1.93 for PFS and OS, respectively. A high NLR was associated with poor PFS (P = 0.033) and OS (P = 0.013) in the univariate analyses. In the multivariate analyses, a high NLR was still an independent predictor of OS (hazard ratio [HR] = 2.23; 95% CI, 1.08 to 4.61) but not PFS (hazard ratio [HR] = 2.43; 95% CI, 0.95 to 6.27).
Conclusion
The NLR at an optimum cut-off value of 1.93 is an independent prognostic predictor of OS in patients with EOC.
Keywords: neutrophil-to-lymphocyte ratio, epithelial ovarian cancer, overall survival, progression - free survival, systemic inflammatory response syndrome
Introduction
About 90% of all histological types of ovarian cancer are of epithelial origin [1,2] with over 70% of epithelial ovarian cancer (EOC) being diagnosed at the advanced stage of the disease [3]. Despite the great progress in surgical intervention and chemotherapy, the prognosis of EOC remains poor due to the absence of specific symptoms in the early stage and the tendency of the disease to metastasize over a short period.
The interaction between inflammation and cancer development has gained significant research interest in the recent past [4]. Various hematological markers of inflammation could be useful in the prediction of survival in patients with different types of cancer [5-8]. Inflammation is seen in all stages of tumor formation including the initiation, promotion, progression, invasion, and metastasis of a tumor [9,10], and there is currently a renewed interest in systemic inflammatory response (SIR) markers including hematological inflammatory markers. One of such hematological inflammatory markers that have shown the potential to be a credible prognostic marker in various cancers including EOC is the neutrophil-to-lymphocyte ratio (NLR) [11-14].
NLR reflects the balance of the inflammatory and immune systems, and also indicates the balance between pro-tumor and anti-tumor status, making it a useful predictor of cancer prognosis [15]. NLR is a useful marker for evaluating the systemic balance between the pro-tumor inflammatory effect of neutrophil and the anti-tumor immune response of lymphocytes [16,17]. The use of biomarkers like NLR, which are inexpensive and readily available, as reliable prognostic markers are necessary for the identification of high-risk patients who may benefit from maintenance therapy following their upfront primary treatment with primary debulking surgery and adjuvant chemotherapy or neoadjuvant chemotherapy and interval debulking surgery. To our knowledge, there have been no studies to date among black African women on the prognostic significance of NLR in EOC. Hence, our study was aimed to evaluate the role of pretreatment peripheral blood NLR on the three-year prognostic outcomes of EOC patients managed in a public tertiary center in Lagos, Nigeria over a 10-year review period.
Materials and methods
Study design and clinical setting
We designed a retrospective observational study involving a review of the ward register and medical records of women diagnosed with epithelial ovarian cancer (EOC) and who had their complete primary treatment at the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria between March 2009, and February 2018. LUTH is the teaching hospital of the College of Medicine, University of Lagos. It acts as a tertiary referral center for other hospitals in Lagos and its surrounding states.
Eligibility criteria
Ninety-three patients with complete clinical records were included in the analysis (Fig 1) Exclusion criteria were: (1) non-EOC; (2) Eastern Cooperative Oncology Group (ECOG) performance status of 2-4 [18]; (3) failure of completion of primary treatment or loss to follow-up; (4) unavailable pretreatment hematologic parameters; (5) record of disease complications such as an active infection; (6) any hematologic disease; or (7) medication with any immunosuppressive agent. Relevant data extracted from the ward register and patients’ clinical records were age, parity, body mass index (BMI), menopausal status, serum cancer antigen-125 (CA-125) concentration, comorbidities such as diabetes mellitus, hypertension, cardiac, liver, and kidney disease), complete blood count, presence of ascites, International Federation of Gynecology and Obstetrics (FIGO) stage, type of upfront treatment, surgical debulking status [19], histological subtypes [20], progression-free survival, and overall survival. We selected the pretreatment peripheral neutrophil and lymphocyte counts from the complete blood counts. The NLR was then estimated by dividing the absolute neutrophil count by the lymphocyte count [21].
Study outcomes
Two outcomes were assessed: (1) progression-free survival (PFS), determined by estimating the time interval from the completion of primary treatment to the first relapse as evidenced by clinical examination, elevated tumor marker (serum cancer antigen-25 levels) and/or radiological studies; and (2) overall survival (OS), defined by determining the time interval from the completion of primary treatment until the death of the patients from all causes or last follow-up for patients who were still alive [22]. We censored the survival data after three-year follow-up.
Sample size determination
We used G*Power for Windows version 3.1.9.2 (Kiel University, Germany) to calculate the sample size. The study was powered for a two-sided test with a Type I error rate of 5% (Zα=1.96) and power of 80% (Zβ=0.84), adjusted for a 10% attrition rate. Given a variability of 0.5 in the hazard ratio (HR) of death and progression of the disease from a high NLR of 1.91 and 1.82, respectively [23], the number of deaths was 75 and while that of disease relapse was 88. However, being a retrospective study, records of all eligible EOC patients during the study period were reviewed and included for data analyses.
Statistical analysis
Data were analyzed using SPSS version 27.0 statistical package for Windows (manufactured by IBM Corp., Armonk, NY, United States) and descriptive statistics were computed for all patients’ baseline characteristics. Patients’ sociodemographic and clinicopathologic characteristics were described if normally distributed using mean and standard deviation (SD) and if skewed using median and interquartile range (IQR), for continuous variables. Categorical variables were presented using frequencies and percentages. The optimum cut-off values for the NLR were estimated using the Receiver operating characteristic (ROC) curve analysis with Youden's index. Estimates of PFS and OS time stratified by NLR ratios were determined using the Kaplan-Meier (KP) curve analysis and then compared by the use of Log Rank (Mantel-Cox) test statistic [24]. We censored patients without tumor recurrence or those who were alive at the last follow-up. Hazard ratios (HR) were determined using the multivariate Cox regression models while adjusting for other potential covariates. We built the final multivariate models to include the patients’ age and other variables using a P-value < 0.5 to remain in the model. Statistical significance was reported at P-value < 0.05.
Ethical considerations
This study was conducted with consideration for the provisions of the Declaration of Helsinki. The Health Research Ethics committee of the Lagos University Teaching Hospital approved the protocol (ADM/DCST/HREC/APP/3699) before access to the patients’ case records for data collection and analysis.
Results
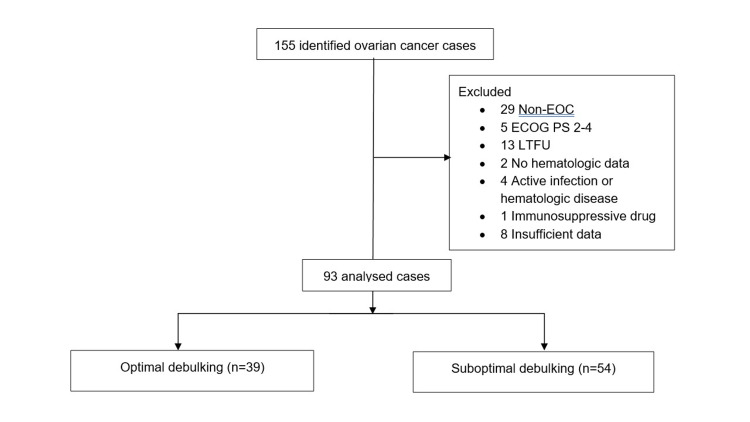
Out of the 155 patients with ovarian cancer who were managed in the hospital during the period under review, we included 93 patients’ data in the final analyses. We excluded 29 women with non-EOC, 5 with an ECOG performance status of between 2 and 4, 13 who were lost to follow-up or failed to complete their primary treatment, 2 with unavailable pretreatment hematologic parameters, 4 with an active infection or hematologic disease, 1 on medication with an immunosuppressive agent and 8 with insufficient clinical data (Figure 1).
Figure 1. Flowchart of patient selection process.
EOC: epithelial ovarian cancer; ECOG: Eastern Cooperative Oncology Group; PS: performance status; LTFU: lost to follow-up.
The mean age of the patients at presentation was 47.1 years (SD: 13.9 years). A total of 57 (63.1%) women had primary surgical debulking as their upfront primary treatment with the majority diagnosed with advanced FIGO stages of the disease (n = 65, 69.8%), and high-grade serous carcinomas (n = 54, 58.1%). Twenty-nine (31.2%) of the patients had documented tumor relapse while 36 (38.7%) died during the follow-up period (Table 1).
Table 1. Characteristics of patients with epithelial ovarian cancer (n = 93).
HGSC: high-grade serous carcinomas; IQR: interquartile range; NLR: neutrophil-to-lymphocyte ratio; LGSC: low-grade serous carcinomas; NACT: neoadjuvant chemotherapy; PDS: primary debulking surgery; SD: standard deviation.
| Characteristics | Number (%) |
| Mean age (SD) in years | 47.1 (13.9) |
| Mean BMI (SD) in kg/m2 | 23.6 (5.2) |
| Median serum CA-125 levels (IQR) in U/mL | 112.4 (44.2, 582.1) |
| Median NLR (IQR) | 1.89 (1.21, 3.12) |
| Parity | |
| Nulliparous | 43 (46.2) |
| Multiparous | 50 (53.8) |
| Menopausal status | |
| Premenopause | 52 (55.9) |
| Postmenopause | 41 (44.1) |
| Comorbidity | |
| Yes | 16 (17.2) |
| No | 77 (82.8) |
| Upfront primary treatment | |
| PDS | 57 (61.3) |
| NACT | 36 (38.7) |
| Ascites | |
| Yes | 37 (39.8) |
| No | 56 (60.2) |
| FIGO stage | |
| Early (I & II) | 28 (30.2) |
| Advanced (III & IV) | 65 (69.8) |
| Surgical debulking status | |
| Optimal | 39 (41.9) |
| Suboptimal | 54 (58.1) |
| Histological subtype | |
| Type I (LGSC and others) | 33 (35.5) |
| Type II (HGSC) | 60 (64.5) |
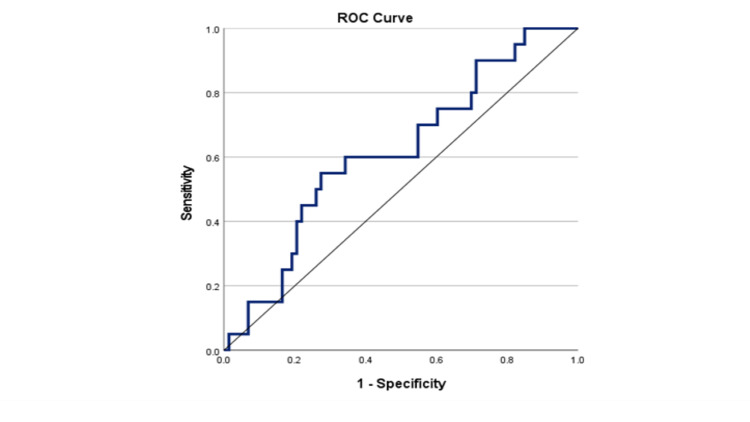
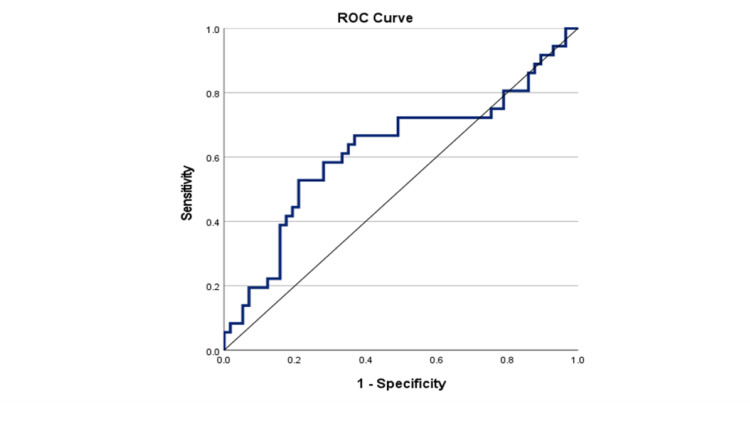
As shown in Figure 2, the area under the curve (AUC) was 0.68 (95% CI: 0.57-0.80; P = 0.005), and the optimal NLR cut-off value for PFS was 2.23, with the sensitivity and specificity being 0.66 and 0.72, respectively. There were 55 (59.1%) patients with an NLR < 2.23 and 68 (44.2%) patients with an NLR ≥ 2.23. In Figure 3, we recorded the AUC as 0.62 (95% CI: 0.50-0.75; P = 0.049), and the optimal NLR cut-off value for OS was 1.93, with the sensitivity and specificity being 0.67 and 0.63, respectively. Using this cut-off value, there were 48 (51.6%) patients with an NLR<1.93 and 45 patients (48.4%) with an NLR≥1.93.
Figure 2. Receiver operating curve of pre-operative NLR for predicting PFS in patients with EOC.
ROC: receiver operating curve; NLR: neutrophil-to-lymphocyte ratio; PFS: progression-free survival; EOC: epithelial ovarian cancer.
Figure 3. Receiver operating curve of pre-operative NLR for predicting OS in patients with EOC.
ROC: receiver operating curve; NLR: neutrophil-to-lymphocyte ratio; OS: overall survival; EOC: epithelial ovarian cancer.
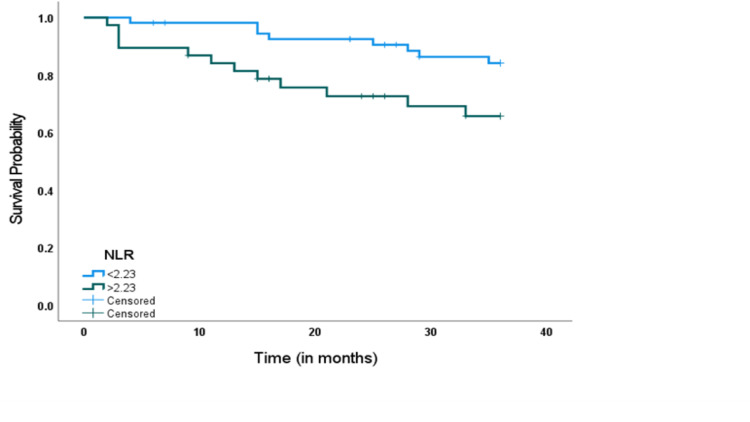
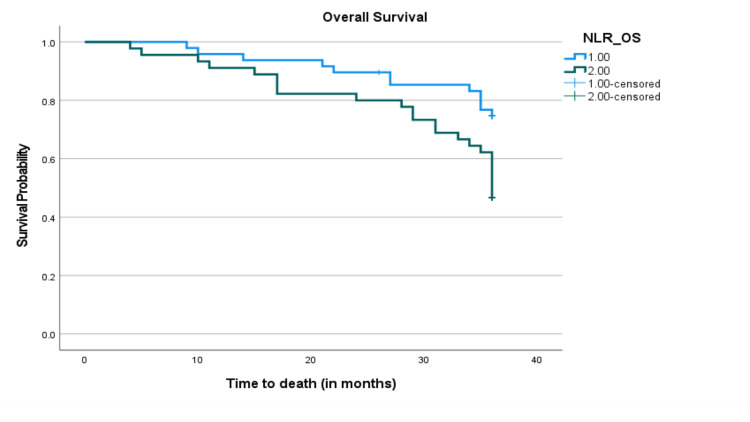
In the Kaplan-Meier (KP) survival curve stratified by the NLR, we recorded statistically shorter PFS and OS in EOC patients with NLR values above the optimal cut-offs than in those with an NLR below the cut-offs (Figures 4, 5).
Figure 4. Kaplan-Meier survival curves for PFS in patients with EOC after surgical resection. Progression-free survival of patients with NLR > 2.23 was shorter than those with NLR ≤ 2.23 (P = 0.033).
NLR: neutrophil-to-lymphocyte ratio; PFS: progression-free survival; EOC: epithelial ovarian cancer.
Figure 5. Kaplan-Meier survival curves for OS in patients with EOC after surgical resection. Progression-free survival of patients with NLR > 1.93 was shorter than those with NLR ≤ 1.93 (P = 0.013).
NLR: neutrophil-to-lymphocyte ratio; OS: overall survival; EOC: epithelial ovarian cancer.
In the univariate Cox regression analysis, parity (P = 0.013) and pretreatment NLR (P = 0.033) were significant prognostic factors for PFS, whereas, in the multivariate analysis, only parity (HR: 4.63, 95% CI: 1.39-15.39, P = 0.012) was an independent predictor of reduced PFS (Table 2). In Table 3, parity (P = 0.031) and pretreatment NLR (P = 0.013) were recorded to be significant prognostic predictors of OS in the univariate analyses, and following adjustment in the final multivariate model both parity (HR: 2.32, 95% CI: 1.11-4.84, P = 0.025) and NLR (HR: 2.23, 95% CI: 1.08-4.61, P = 0.031) were also recorded to be independent risk predictors of reduced OS.
Table 2. Univariate and multivariate analyses for progression-free survival.
BMI, body mass index; HR, hazard ratio; NACT; neoadjuvant chemotherapy; NLR, neutrophil-to-lymphocyte ratio; PDS; primary debulking surgery; FIGO: International Federation of Gynecology and Obstetrics; CA-125: cancer antigen-125; LG includes endometrioid carcinoma, clear cell carcinoma, mucinous carcinoma, and low-grade serous carcinomas; HG includes high-grade serous carcinomas.
| Characteristics | Category | Univariate | Multivariate | |
| P-value | HR (95% CI) | P-value | ||
| Age | ≥47 vs. <47 years | 0.894 | 0.60 (0.22-1.63) | 0.316 |
| Parity | Multiparous vs. nulliparous | 0.013 | 4.63 (1.39-15.39) | 0.012 |
| Menopausal status | Postmenopause vs. premenopause | 0.632 | - | - |
| BMI | ≥24.0 vs. <24.0 kg/m2 | 0.990 | - | - |
| Serum CA-125 levels | ≥112.0 vs. <112.0 U/mL | 0.401 | 1.30 (0.36-4.68) | 0.687 |
| Comorbidity | Yes vs. No | 0.069 | 2.77 (0.91-8.40) | 0.072 |
| Upfront primary treatment | NACT vs. PDS | 0.366 | 0.71 (0.23-2.15) | 0.539 |
| Ascites | Yes vs. No | 0.294 | 0.91 (0.34-2.40) | 0.848 |
| FIGO stage | Advanced vs. early | 0.296 | 1.19 (0.25-5.74) | 0.824 |
| Surgical debulking status | Optimal vs. suboptimal | 0.952 | - | - |
| Histological subtype | HG vs. LG | 0.958 | - | - |
| Pretreatment NLR | ≥2.23 vs. <2.23 years | 0.033 | 2.43 (0.95-6.27) | 0.065 |
Table 3. Univariate and multivariate analyses for overall survival.
BMI, body mass index; HR, hazard ratio; NACT; neoadjuvant chemotherapy; NLR, neutrophil-to-lymphocyte ratio; PDS; primary debulking surgery; FIGO: International Federation of Gynecology and Obstetrics; CA-125: cancer antigen-125; LG includes endometrioid carcinoma, clear cell carcinoma, mucinous carcinoma, and low-grade serous carcinomas; HG includes high-grade serous carcinomas.
| Characteristics | Category | Univariate | Multivariate | |
| P-value | HR (95% CI) | P-value | ||
| Age | ≥47 vs. <47 years | 0.712 | 0.65 (0.32-1.32) | 0.230 |
| Parity | Multiparous vs. nulliparous | 0.031 | 2.32 (1.11-4.84) | 0.025 |
| Menopausal status | Postmenopause vs. premenopause | 0.927 | - | - |
| BMI | ≥24.0 vs. <24.0 kg/m2 | 0.795 | - | - |
| Serum CA-125 levels | ≥112.0 vs. <112.0 U/mL | 0.662 | - | - |
| Comorbidity | Yes vs. no | 0.749 | - | - |
| Upfront primary treatment | NACT vs. PDS | 0.657 | - | - |
| Ascites | Yes vs. No | 0.284 | 1.05 (0.53-2.07) | 0.900 |
| FIGO stage | Advanced vs. early | 0.507 | - | - |
| Surgical debulking status | Optimal vs. suboptimal | 0.944 | - | - |
| Histological subtype | HG vs. LG | 0.470 | 1.22 (0.57-2.60) | 0.615 |
| Pretreatment NLR | ≥1.93 vs. <1.93 years | 0.013 | 2.23 (1.08-4.61) | 0.031 |
Discussion
Currently, the estimate of the survival outcomes of patients with EOC relies mainly on various clinicopathological variables, such as the extent of tumor resectability during debulking surgery, FIGO stage, presence of ascites and serum cancer antigen 125 levels [22]. While reflecting the behavior and presentation of cancer in biology, these parameters may not necessarily represent the actual burden of the disease in patients with EOC. Recently, there is increased attention on NLR, which is easily obtained from the routine pretreatment peripheral blood tests, as an important prognostic marker in multiple cancers Including EOC [11-14]. Therefore, in the present study, we evaluated the impact of pretreatment peripheral blood NLR on the short-term survival outcomes of EOC patients managed over a 10-year review period at LUTH, and we found that a high pretreatment NLR was an independent predictor of reduced OS, whereas its prognostic effect on PFS was only dependent on the patient’s parity.
The NLR is an inflammation- and immunity-related marker calculated using the ratio of peripheral absolute neutrophil count to lymphocyte count. This reflects both the neutrophilia and lymphopenia within the tumor microenvironment. The increase in neutrophils supports cancer cell invasion, migration, and angiogenesis which lead to cancer progression [25] while the reduction in lymphocytes results from a poor and inadequate immunologic response to the tumor [26]. In corroboration with the findings of other published studies [27,28] suggesting that the NLR is an independent prognostic predictor of survival, our study found that the different survival endpoints have different cut-off values and that the NLR has a significant prognostic value in EOC patients. The lack of significant independent prognostic effect of NLR on the OS may likely due to the relatively short follow-up period of three years used in our current study compared to the average five-year follow-up used in other similar studies [27-29]. This may also be due to the optimum cutoff value selected in the current study to stratify NLR into the high-NLR and low-NLR groups. In previous studies, the cutoff values of NLR were estimated using methods such as the median levels or ROC analysis for PFS and OS [27,28]. Thus, it is still difficult to determine which of these is the best method. The NLR cutoff value in our study was selected from the ROC analysis, and this was used to stratify the EOC patients into low- and high-NLR groups. However, our finding was also in line with that of Raungkaewmanee et al. [29] who reported no significant association between NLR and PFS or OS in ovarian cancer patients. Williams et al. [30] reported in their study conducted that higher NLR levels were associated with various clinicopathological characteristics including the presence of ascites, tumor stage and grade; however, similar associations were not seen in our study.
The major limitations of this study were the poor clinical data management system in our center resulting in the high number of ovarian cancer cases with incomplete or missing data. Furthermore, as this is an institutional-based study, we may not be able to generalize the findings to other centers or geopolitical zones in the country. Therefore, there is a need to further evaluate the clinical applicability and identify gaps in this study through a long-term prospective, multicenter study with a larger sample size.
Conclusions
Our study reported that pretreatment peripheral blood neutrophil-lymphocyte ratio at optimum cut-off is an important prognostic predictor of PFS in epithelial ovarian cancer. However, a prevalence study among healthy cancer-free women should be conducted to determine the normal range of NLR, as this would help to define the optimum cut-off for NLR levels in subsequent studies. In addition, there is a need to provide additional evidence to corroborate the findings of this study through a prospective multicenter study with a larger sample size among black women in sub-Saharan Africa.
Acknowledgments
The authors thank the staff of the medical records department of Lagos University Teaching Hospital who participated in this study.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Funding Statement
The research reported in this article was supported by the Fogarty International Center of the National Institutes of Health under (Award Numbers D43TW010934 and D43TW010543); and the Conquer Cancer International Innovation Grant under (Project ID 16576)
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Lagos University Teaching Hospital Health Research Ethics Committee issued approval ADM/DCST/HREC/APP/3699. Notice of expedited review and approval.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Cancer statistics, 2020. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.A five-year review of ovarian cancer at a tertiary institution in Lagos, South-West, Nigeria. Okunade KS, Okunola H, Okunowo AA, Anorlu RI. Niger J Gen Pract. 2016;14:23–27. [Google Scholar]
- 4.Hallmarks of cancer: the next generation. Hanahan D, Weinberg RA. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Ann Surg Oncol. 2016;23:900–907. doi: 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- 6.Prognostic role of Glasgow prognostic score in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Li MX, Bi XY, Li ZY, et al. Medicine. 2015;94:0. doi: 10.1097/MD.0000000000002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pre-treatment white blood cell subtypes as prognostic indicators in ovarian cancer. Bishara S, Griffin M, Cargill A, et al. Eur J Obstet Gynecol Reprod Biol. 2008;138:71–75. doi: 10.1016/j.ejogrb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 8.The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Cancer-related inflammation. Mantovani A, Allavena P, Sica A, Balkwill F. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 10.Inflammation and cancer. Coussens LM, Werb Z. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elevated C-reactive protein and hypoalbuminemia measured before resection of colorectal liver metastases predict postoperative survival. Kobayashi T, Teruya M, Kishiki T, et al. Dig Surg. 2010;27:285–290. doi: 10.1159/000280021. [DOI] [PubMed] [Google Scholar]
- 12.The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Wu M, Guo J, Guo L, Zuo Q. Tumour Biol. 2016;37:12525–12533. doi: 10.1007/s13277-016-5122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pretreatment neutrophil-to-lymphocyte ratio: a predictor of advanced prostate cancer and biochemical recurrence in patients receiving radical prostatectomy. Zhang GM, Zhu Y, Ma XC, et al. Medicine. 2015;94:0. doi: 10.1097/MD.0000000000001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Templeton AJ, Ace O, McNamara MG, et al. Cancer Epidemiol Biomarkers Prev. 2014;23:1204–1212. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- 15.Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: a meta-analysis. Xue TC, Zhang L, Xie XY, et al. PLoS One. 2014;9:0. doi: 10.1371/journal.pone.0096072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Moses K, Brandau S. Semin Immunol. 2016;28:187–196. doi: 10.1016/j.smim.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Donskov F. Semin Cancer Biol. 2013;23:200–207. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Azam F, Latif MF, Farooq A, Tirmazy SH, AlShahrani S, Bashir S, Bukhari N. Case Rep Oncol. 2019;12:728–736. doi: 10.1159/000503095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Chi DS, Eisenhauer EL, Zivanovic O, et al. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Subtypes of ovarian cancer and ovarian cancer screening. Koshiyama M, Matsumura N, Konishi I. Diagnostics. 2017;7:12. doi: 10.3390/diagnostics7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neutrophil/lymphocyte ratio and lymphocyte/monocyte ratio in ulcerative colitis as non-invasive biomarkers of disease activity and severity. Okba AM, Amin MM, Abdelmoaty AS, Ebada HE, Kamel AH, Allam AS, Sobhy OM. Auto Immun Highlights. 2019;10:4. doi: 10.1186/s13317-019-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Predictors of survival outcomes after primary treatment of epithelial ovarian cancer in Lagos, Nigeria. Okunade KS, Adejimi AA, Ohazurike EO, et al. JCO Glob Oncol. 2021;7:89–98. doi: 10.1200/GO.20.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prognostic significance of neutrophil-lymphocyte ratio (NLR) in patients with ovarian cancer: a systematic review and meta-analysis. Yin X, Wu L, Yang H, Yang H. Medicine. 2019;98:0. doi: 10.1097/MD.0000000000017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nonparametric estimation from incomplete observations. Kaplan E, Meier P. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Neutrophils in the tumor microenvironment. Powell DR, Huttenlocher A. Trends Immunol. 2016;37:41–52. doi: 10.1016/j.it.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elevated Fas expression is related to increased apoptosis of circulating CD8+ T cell in patients with gastric cancer. Yoshikawa T, Saito H, Osaki T, Matsumoto S, Tsujitani S, Ikeguchi M. J Surg Res. 2008;148:143–151. doi: 10.1016/j.jss.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Influence of CA125, platelet count and neutrophil to lymphocyte ratio on the immune system of ovarian cancer patients. Baert T, Van Camp J, Vanbrabant L, et al. Gynecol Oncol. 2018;150:31–37. doi: 10.1016/j.ygyno.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. J Gynecol Oncol. 2012;23:265–273. doi: 10.3802/jgo.2012.23.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, Cramer DW. Gynecol Oncol. 2014;132:542–550. doi: 10.1016/j.ygyno.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]