Abstract
Atherosclerotic stenosis of the internal carotid artery is an important cause of stroke. The aim of this guideline is to analyse the evidence pertaining to medical, surgical and endovascular treatment of patients with carotid stenosis. These guidelines were developed based on the ESO standard operating procedure and followed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach. The working group identified relevant questions, performed systematic reviews and meta-analyses of the literature, assessed the quality of the available evidence, and wrote recommendations. Based on moderate quality evidence, we recommend carotid endarterectomy (CEA) in patients with ≥60–99% asymptomatic carotid stenosis considered to be at increased risk of stroke on best medical treatment (BMT) alone. We also recommend CEA for patients with ≥70–99% symptomatic stenosis, and we suggest CEA for patients with 50–69% symptomatic stenosis. Based on high quality evidence, we recommend CEA should be performed early, ideally within two weeks of the last retinal or cerebral ischaemic event in patients with ≥50–99% symptomatic stenosis. Based on low quality evidence, carotid artery stenting (CAS) may be considered in patients < 70 years old with symptomatic ≥50–99% carotid stenosis. Several randomised trials supporting these recommendations were started decades ago, and BMT, CEA and CAS have evolved since. The results of another large trial comparing outcomes after CAS versus CEA in patients with asymptomatic stenosis are anticipated in the near future. Further trials are needed to reassess the benefits of carotid revascularisation in combination with modern BMT in subgroups of patients with carotid stenosis.
Keywords: Carotid stenosis, endarterectomy, stenting, medical therapy, stroke, transient ischaemic attack
Introduction
Atherosclerotic carotid artery disease is one of the major causes of ischaemic stroke and transient ischaemic attack (TIA), accounting for about 10–15% of cases, depending on the method of aetiological classification and the patient population studied.1 Atherosclerotic carotid stenosis mostly occurs at the carotid bifurcation, involving the distal common and the proximal internal carotid artery.2 Other sites which are predisposed to develop atherosclerotic stenosis are the origin of the common carotid artery and the cavernous segment of the intracranial carotid artery. The prevalence of atherosclerotic carotid disease increases with age and is higher in men than in women. In Caucasian populations, ≥50% stenosis of the carotid artery was identified in 2.3% of men in the sixth decade, in 6.0% in the seventh decade and in 7.5% of men aged 80 years; in women, the corresponding pre-valence figures were 2.0%, 3.6% and 5.0% in these age groups, respectively.3
This guideline provides recommendations on the use of carotid endarterectomy (CEA) and carotid artery stenting (CAS) in patients with symptomatic or asymptomatic stenosis of the extracranial carotid bifurcation caused by atherosclerosis. We did not review the available evidence regarding management of proximal common carotid artery or intracranial internal carotid artery stenosis, or non-atherosclerotic causes of stenosis, such as secondary to dissection, fibromuscular dysplasia, arteritis etc. Furthermore, we did not include aspects of diagnostic imaging, peri-procedural management, technical aspects of CEA and CAS, or medical therapy. Guidance on these topics can be found in other guidelines.4–6
Methods
This guideline document was commissioned by the European Stroke Organisation (ESO). A multi-disciplinary Module Working Group (MWG) was established, consisting of experts in the field from vascular neurology, vascular surgery and neuroradiology, who are represented as authors of this guideline document. The composition of this group was approved by the ESO Guidelines Board and the ESO Executive Committee, based on a review of the intellectual and financial disclosures of the proposed members.
The guidelines were developed using GRADE methodology7 and the ESO Standard Operating Procedure.8 In brief, we defined the patient population, the interventions and comparators, the outcomes of clinical interest (PICOs), and the design of studies to be included. The outcomes were rated as critical, important or of limited importance according to the GRADE criteria.7,8
Population
This guideline makes recommendations on treatment of patients with symptomatic or asymptomatic atherosclerotic carotid stenosis. Carotid stenosis was defined as symptomatic if it had caused ischaemic cerebrovascular events in the ipsilateral eye (transient monocular blindness or retinal infarction) or cerebral hemisphere (transient ischaemic attack (TIA) or stroke) in the preceding six months. Asymptomatic carotid stenosis was defined as a stenosis which was not associated with any ocular or cerebral ischaemic events in the ipsilateral carotid territory within the preceding six months.
Patient subgroups
PICO questions were additionally analysed for the following pre-specified patient subgroups when data were available:
Age (</≥70 years)
Sex
Degree of stenosis, according to the method used in the NASCET study9 or its non-invasive equivalent (mild: <50%, moderate: 50–69%, severe: 70–99%, near occlusion (defined as collapse of the distal lumen))
Time since most recent ischaemic event (for symptomatic carotid stenosis)
Type of most recent ischaemic event (for symptoma-tic carotid stenosis): stroke, transient ischaemic attack, ocular ischaemia (including transient monocular blindness or amaurosis fugax and retinal infarction).
Interventions and comparators
Interventions and comparators are CAS, CEA, and contemporary best medical therapy (as defined by the study authors at the time of the study). The guideline does not address carotid revascularisation done as part of acute stroke therapy, or carotid angioplasty without insertion of a stent.
Outcomes
We graded outcomes occurring in the peri-procedural period of carotid artery revascularisation, as well as outcomes occurring in the post-procedural period on a scale of 0–9 to classify them as either critical for decision making (grade 7–9; Table 1); important, but not critical for making a decision (grade 4–6; Table 1); or of limited importance for making a decision (grade 0–3). Critical and important outcomes were included in the evidence profile.
Table 1.
Outcomes.
| Peri-procedural outcomes graded as critical for decision making | -Death -Any stroke (ischaemic or haemorrhagic), defined as an acute onset of focal neurological dysfunction, with symptoms lasting for longer than 24 h or leading to death within 24 h, of non-traumatic vascular aetiology. Retinal infarction with visual loss lasting for longer than 24 h, was included within the definition of stroke. -Major stroke, defined as resulting in substantial impairment or disability (measured by a modified Rankin scale10 score of >2, typically 30 days or more after the event, if available), or death |
| Peri-procedural outcomes graded as important for decision making | -Myocardial infarction, according to the definitions used in the individual trials -Cranial nerve injury |
| Post-procedural outcomes graded as critical for decision making | -Ipsilateral stroke, occurring in the territory of the anterior or middle cerebral artery on the side of the randomised artery. -Any stroke -Major stroke, defined as resulting in substantial impairment or disability (measured by a modified Rankin Scale score10 (mRS) of >2, if available), or death |
| Post-procedural outcomes graded as important for decision making | -Death -Severe residual or recurrent stenosis (≥70% according to the NASCET method of grading stenosis9 or its non-invasive equivalent) or occlusion of the treated artery. |
The peri-procedural period was defined as the period between randomisation in the trial and 30 days after treatment, or as the first 30 days after randomisation in patients who did not undergo revascularisation (unless different definitions were used in individual trials in question). Peri-procedural outcomes were included as a measure of treatment safety. Post-procedural outcomes (i.e. outcomes occurring beyond the peri-procedural period) were included as a measure of treatment efficacy.
Formation of PICO questions
A series of PICO (Population, Intervention, Comparator, Outcome) questions were developed and subsequently approved by the ESO Guidelines board and the ESO Executive Committee. The PICO questions were based on the peri-procedural and post-procedural outcomes, graded as critical or important for decision making, as well as combinations of these outcomes. We only compared peri-procedural outcomes on their own in trials of CAS versus CEA. This resulted in 4 PICO questions for the comparison of CEA versus medical therapy alone, 4 PICO questions for the comparison of CAS versus medical therapy alone and 11 PICO questions for the comparison of CAS versus CEA in separate trials in patients with asymptomatic carotid stenosis and in patients with symptomatic carotid stenosis. We also formulated one PICO question concerning the risk of restenosis after CAS or CEA which was addressed using combined data from patients with symptomatic and asymptomatic carotid stenosis; these data are reported in the section on symptomatic carotid stenosis. Subgroup analyses for these PICO questions were also performed in the aforementioned pre-specified patient subgroups, where data were available.
Literature search, data extraction and synthesis
Literature searches were restricted to reports of randomised controlled trials (RCTs). We identified three systematic reviews of RCTs in the Cochrane Database of Systematic Reviews, which were of relevance to this guideline, one comparing CEA with medical therapy alone for asymptomatic carotid stenosis,11 one comparing CEA with medical therapy alone for symptomatic carotid stenosis12 and one comparing CAS with CEA for asymptomatic or symptomatic carotid stenosis.13 For the comparisons of CEA versus medical therapy, and CAS versus CEA, systematic searches of the MEDLINE, EMBASE, and Cochrane databases (from the date of the last search in the Cochrane reviews to 10 August 2020) were conducted by two ESO Guidelines methodologists (AL and MTR) using the same search terms which were defined in the Cochrane reviews. For the comparison of CAS versus best medical therapy, a de novo search of the literature was performed using the MEDLINE, EMBASE and Cochrane databases from their inception until 10 August 2020, using the search terms provided in the Online Appendix. To reduce the number of duplicate references identified, we simultaneously searched for relevant data in patients with asymptomatic and symptomatic carotid stenosis.
For each of the three main comparisons, a group of MWG members (a ‘PICO group’) was formed to select the studies for inclusion and to evaluate the available evidence. Within each PICO group, two MWG members independently screened the titles and abstracts of publications identified from the searches (first level selection), and subsequently assessed the full text of potentially relevant studies (second level selection). Data were extracted independently by AL and MTR from studies which met criteria for second level selection, separately for patients with asymptomatic and those with symptomatic carotid stenosis. At least one additional MWG member checked the extracted data results for accuracy.
For some PICO questions (PICO 6.1 and 6.9), we included outcomes in pre-defined patient subgroups derived from pooled analyses of individual patient data (IPD) from the EVA-3S, SPACE, ICSS and CREST trials which were performed by the Carotid Stenosis Trialists’ Collaboration (CSTC).
The risks of selection, performance, detection, attrition and reporting bias in each randomised trial were assessed using the Cochrane Collaboration’s tool.14 Heterogeneity across studies was assessed using Cochran’s Q (reported as a p value) and I2 statistics.15 For each PICO question and each outcome, the quality of evidence was rated using the GRADEpro Guideline Development Tool (McMaster University, 2015; developed by Evidence Prime, Inc.) as high, moderate, low or very low.8
The relevant PICO group was responsible for analysing the available data and formulating an evidence-based recommendation according to the GRADE evidence profiles and the ESO standard operating procedure. Random-effect metanalyses were conducted and relative intervention effects were summarised as risk ratios (RR) and their 95% confidence interval. The absolute measure of intervention effects was calculated as the difference between the baseline risk of an outcome (patients receiving control intervention) and the risk of outcome after the intervention was applied (risk of an outcome in patients who received an intervention). Absolute effects are based on the relative magnitude of an effect with respect to the baseline risk, which is similar to risk differences. The fewer value represents any value below 1 per 1000 and the more value represents any value more than 1 per 1000.
The wording and the rating of the strength of each recommendation was passed by majority voting by all MWG members. An Expert Consensus Statement, based on voting by all MWG members, was presented where the PICO group considered that there was insufficient evidence available to provide clear evidence-based recommendations for situations in which practical guidance was needed for everyday clinical practice. Importantly, these Expert Consensus Statements should not be regarded as evidence-based recommendations since they only reflect the opinion of the majority of the members of the MWG.
The Guideline document was subsequently reviewed by all MWG members and modified until a consensus was reached. Finally, the guideline document was peer-reviewed and approved by external reviewers and members of the ESO Guidelines Board and ESO Executive Committee.
Results
Endarterectomy or medical therapy for asymptomatic carotid stenosis
Description of studies
The Veterans Administration (VA) asymptomatic carotid stenosis cooperative study randomised 444 men with ≥50% asymptomatic carotid stenosis on angiography to CEA (n = 211) or medical therapy alone (n = 233) between 1983 and 1987.16 Five percent of patients turned out to have <50% stenosis after centralised analysis of the angiograms. Patients had never experienced any prior ipsilateral cerebrovascular events and were followed up for a mean of 47.9 months. The results were reported in 1993.
The Asymptomatic Carotid Atherosclerosis Study (ACAS) randomly allocated 1662 patients with ≥60% asymptomatic carotid artery stenosis to CEA (n = 825) or medical therapy alone (n = 834) between 1987 and 1993. Patients were defined as being ‘asymptomatic’ if they never had cerebrovascular symptoms in the distribution of the ‘study’ carotid artery or vertebrobasilar territory. Patients with contralateral cerebral hemispheric symptoms within the previous 45 days were excluded. Outcomes after a median follow-up period of 2.7 years were reported in 1995.17 The definition of haemodynamically-significant carotid stenosis was based on meeting at least one of three pre-specified criteria from an ocular pneumoplethysmographic (OPG-Gee) examination, an ultrasound of carotid arteries and/or catheter angiography indicating a diameter stenosis of ≥60% (NASCET methodology). Patients randomised to surgery on the basis of ultrasound findings, or ultrasound combined with OPG-Gee were also required to have a catheter angiogram prior to CEA. If a post-randomisation angiogram revealed that the contralateral carotid artery was more severely stenosed, that artery then became the allocated ‘study artery’.
The Asymptomatic Carotid Surgery Trial (ACST-1) randomised 3120 patients with ≥60% asymptomatic carotid stenosis on ultrasound to immediate CEA (n = 1560, median delay one month (IQR: 0.3–2.5)) or initial medical therapy with the option of deferred CEA (n = 1560) between 1993 and 2003.18,19 The first ACST-1 report in 2004 provided data on outcomes during follow-up for up to five years (mean 3.4 years) after randomisation.18 A subsequent report in 2010 included outcomes over a median follow-up period of nine years (IQR 6–11 years) after randomisation.19
The Aggressive Medical Treatment Evaluation for Asymptomatic Carotid Artery Stenosis (AMTEC) study randomised 55 patients with 70–79% carotid stenosis to receive CEA (n = 31) or medical therapy alone (n = 24) between 2009 and 2013.20 Stenosis was graded by ultrasound examinations, but had to be confirmed by computed tomographic or magnetic resonance angiography (CTA/MRA) or catheter angiography. The trial was stopped prematurely by the independent data and safety monitoring board because of a high rate of the primary endpoint in the medical arm after a median follow-up period of 3.3 years (maximum, 5.0 years); results were reported in 2015.
Data from patients with 50–99% asymptomatic carotid stenosis randomly assigned to CEA (n = 203) or medical therapy alone (n = 113) between 2009 and 2013 in the three-arm Stent-protected Angioplasty in Asymptomatic Carotid Artery Stenosis vs. Endarterectomy (SPACE-2) trial were also included in the present section.21,22 A detailed description of the SPACE-2 trial is provided in section ‘Stenting or medical therapy for asymptomatic carotid stenosis.
The effects of treatment are presented with medical therapy alone as the reference group. A summary of findings is provided in Table 2.
Table 2.
Summary of findings for endarterectomy versus medical therapy for asymptomatic carotid stenosis (PICO 1.1–1.4).
| Certainty assessment |
№ of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Endarterectomy | Medical therapy | Relative(95% CI) | Absolute(95% CI) | ||
| PICO 1.1: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death | ||||||||||||
| 5 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 139/2830(4.9%) | 190/2764(6.9%) | RR 0.73(0.59–0.90) | 19 fewer per 1000(from 28 fewer to 7 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 1.2: Long-term risk of stroke in any territory, including peri-procedural death | ||||||||||||
| 5 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 238/2830(8.4%) | 326/2764(11.8%) | RR 0.74(0.59–0.92) | 31 fewer per 1000(from 48 fewer to 9 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 1.2.1a: Long-term risk of stroke in any territory, including peri-procedural death. Subgroup: Men | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 89/1021(8.7%) | 134/1023(13.1%) | RR 0.67(0.52–0.86) | 43 fewer per 1000(from 63 fewer to 18 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 1.2.1b: Long-term risk of stroke in any territory, including peri-procedural death. Subgroup: Women | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 40/539(7.4%) | 65/537(12.1%) | RR 0.61(0.42–0.89) | 47 fewer per 1000(from 70 fewer to 13 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 1.2.2a: Long-term risk of stroke in any territory, including peri-procedural death. Subgroup: Age <75 years | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 98/1231(8.0%) | 160/1239(12.9%) | RR 0.62(0.49 to 0.78) | 49 fewer per 1000(from 66 fewer to 28 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 1.2.2b: Long-term risk of stroke in any territory, including peri-procedural death. Subgroup: Age <75 years | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | 41/329(12.5%) | 39/321(12.1%) | RR 1.03(0.68–1.55) | 4 more per 1000(from 39 fewer to 67 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 1.2.3a: Long-term risk of stroke in any territory, including peri-procedural death. Subgroup: <80% Stenosis | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 56/641(8.7%) | 86/643(13.4%) | RR 0.65(0.48–0.90) | 47 fewer per 1000(from 70 fewer to 13 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 1.2.3b: Long-term risk of stroke in any territory, including peri-procedural death. Subgroup: ≥80% Stenosis | ||||||||||||
| 1 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 83/919(9.0%) | 113/917(12.3%) | RR 0.73(0.56–0.96) | 33 fewer per 1000(from 54 fewer to 5 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 1.3: Long-term risk of major stroke, including peri-procedural death | ||||||||||||
| 4 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 119/2619(4.5%) | 153/2531(6.0%) | RR 0.77(0.61–0.98) | 14 fewer per 1000(from 24 fewer to 1 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 1.4: Long-term risk of death | ||||||||||||
| 4 | Randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | 699/2619(26.7%) | 667/2531(26.4%) | RR 1.02(0.88–1.20) | 5 more per 1000(from 32 fewer to 53 more) | ⨁⨁◯◯ LOW |
IMPORTANT |
CI: confidence interval; RR: risk ratio.
aEndarterectomy and medical therapy have evolved since the trials contributing the evidence were performed.
bFew events and wide confidence intervals.
PICO 1.1: In patients with asymptomatic carotid stenosis, does endarterectomy compared with medical therapy alone reduce the long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death?
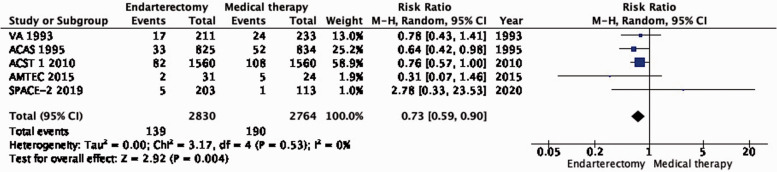
There is moderate quality evidence that endarterectomy reduces the long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death compared with medical therapy alone (RR: 0.73, 95% CI: 0.59–0.90; equivalent to 19 fewer events with CEA per 1000, from 28 fewer to 7 fewer; Figure 1.1).
Figure 1.1.
Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death in endarterectomy versus medical therapy for asymptomatic carotid stenosis.
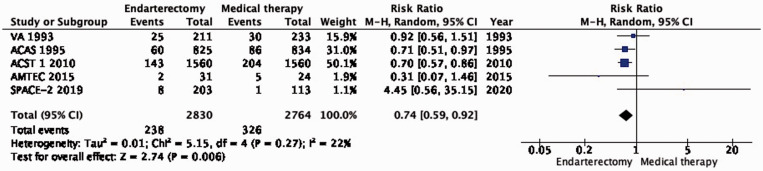
PICO 1.2: In patients with asymptomatic carotid stenosis, does endarterectomy compared with medical therapy alone reduce the long-term risk of stroke in any territory, including peri-procedural death?
There is also moderate quality evidence that endarterectomy reduces the long-term risk of stroke in any territory, including peri-procedural death, compared with medical therapy alone (RR: 0.74, 0.59–0.92; 31 fewer events with CEA per 1000 patients; from 48 fewer to 9 fewer; Figure 1.2). Comparison of the data on the estimated number of ipsilateral strokes (PICO 1.1) and strokes in any territory (PICO 1.2) suggests that CEA might also prevent strokes occurring outside the territory supplied by the operated carotid artery.
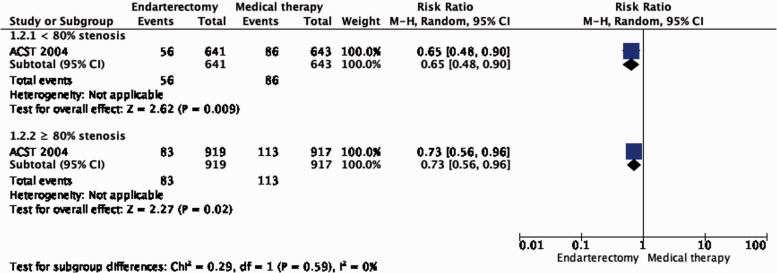
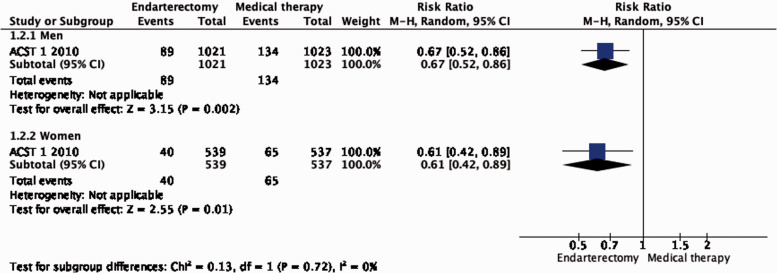
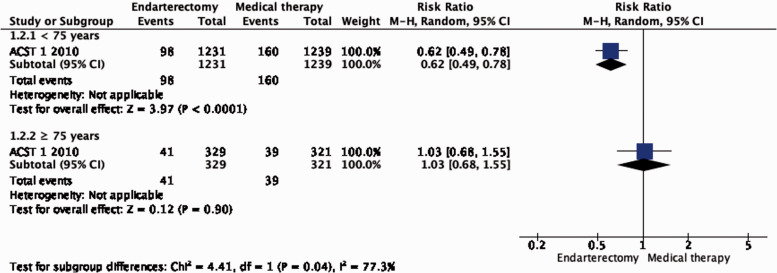
Subgroup data regarding age, sex and severity of stenosis were derived from ACST-1 only. The effect of CEA is significantly modified by age (interaction p = 0.04): there is moderate evidence of a benefit of CEA in patients younger than 75 years (RR: 0.62, 0.49–0.78; Figure 1.2.2), but no evidence of benefit observed in patients ≥75 years old (RR: 1.03, 95% CI: 0.68–1.55, low quality evidence). There is no evidence of a modification of the effect of CEA according to sex (Figure 1.2.1) or severity of stenosis (Figure 1.2.3).
Figure 1.2.3.
Long-term risk of stroke in any territory, including peri-procedural death in endarterectomy versus medical therapy for asymptomatic carotid stenosis. Subgroup: Severity of carotid stenosis.
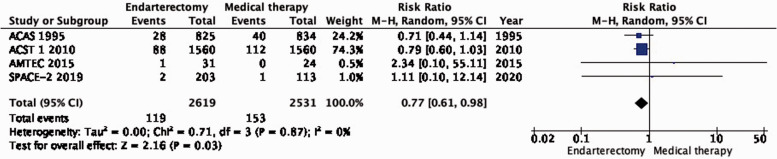
PICO 1.3: In patients with asymptomatic carotid stenosis, does endarterectomy compared with medical therapy alone reduce the long-term risk of major stroke, including peri-procedural death?
There is moderate quality evidence that endarterectomy reduces the long-term risk of major stroke, including peri-procedural death compared with medical therapy alone (RR: 0.77: 0.61–0.98; 14 fewer events with CEA per 1000; from 24 fewer to 1 fewer; Figure 1.3).
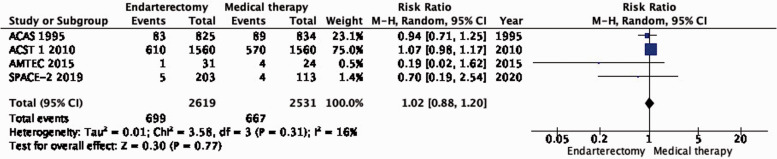
PICO 1.4: In patients with asymptomatic carotid stenosis, does endarterectomy compared with medical therapy alone reduce the long-term risk of death?
There is no difference in long-term risk of death between patients assigned to endarterectomy and those assigned to medical therapy alone (RR: 1.02, 95% CI: 0.88–1.20; 5 more events with CEA per 1000 patients, from 32 fewer to 53 more; low quality evidence; Figure 1.4).
Figure 1.4.
Long-term risk of death in endarterectomy versus medical therapy for asymptomatic carotid stenosis.
Analysis of current evidence and evidence-based recommendation
Data to assess the benefit of endarterectomy compared with medical therapy alone in patients with asymptomatic carotid stenosis were available from five RCTs which included a total of 5791 patients with mainly ≥60% stenosis. We found moderate quality evidence that CEA reduces the risk of ipsilateral stroke and the risk of stroke in any territory in these patients. Based on the results of a single trial, we found no evidence that the benefit of CEA varied significantly between men and women, or according to the severity of the carotid stenosis. We did not find evidence of an increase of the benefit of surgery with increasing degree of asymptomatic carotid stenosis. However, a recent population-based study and systematic review suggested an increase in stroke risk with increasing degrees of asymptomatic carotid stenosis amongst patients receiving contemporary medical therapy.23 Age influenced the effect of surgery in ACST-1, with benefits only observed in patients < 75 years of age. As the effect of age on treatment was only reported in a subgroup analysis of a single trial and taking into account the fact that cardiovascular disease mortality is decreasing and life expectancy is increasing in these patients, we refrained from making recommendations for CEA in patients with asymptomatic carotid stenosis based on fixed age limits.
The two largest trials contributing data were performed two to three decades ago. Best medical management of patients with atherosclerotic disease has evolved since, with more widespread use of statins and other lipid-lowering agents, and stricter control of blood pressure. Annual risks of ipsilateral stroke in more recent observational studies of patients with asymptomatic carotid stenosis range from 0.34 to 1.4%, which is lower than in the medical arms of the RCTs.24–26 However, surgical techniques and peri-operative management have also improved since these landmark trials were completed. For these reasons, we downgraded the overall quality of evidence for indirectness.
Additional information
The question of whether carotid revascularisation confers additional benefits over modern medical therapy is being investigated in ongoing RCTs: the Second European Carotid Surgery Trial (ECST-2) enrolled 429 patients with asymptomatic or low-to-intermediate risk symptomatic carotid stenosis; follow-up is ongoing.27 The Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2) includes two parallel trials of stenting vs. medical therapy and endarterectomy vs. medical therapy in patients with ≥70% asymptomatic carotid stenosis.28
There is debate about whether CEA should only be performed in patients with asymptomatic carotid stenosis who are considered to be at ‘higher risk’ of stroke on best medical treatment (BMT) alone. The guidelines published by the European Society for Vascular Surgery (ESVS) have proposed that surgery should be considered in selected patients with 60–99% asymptomatic carotid stenosis with one or more imaging or clinical characteristics that may be associated with an increased risk of late ipsilateral stroke.4 These characteristics may include, among others, silent infarction on neuroimaging,29 high degree23 and progression of stenosis,30,31 echolucent plaque on ultrasound,32,33 intra-plaque haemorrhage on MRI34,35 and micro-emboli24 or reduced cerebrovascular reserve36 on trans-cranial Doppler. This concept is currently being investigated in the Endarterectomy Combined With Optimal Medical Therapy (OMT) vs OMT Alone in Patients With Asymptomatic Severe Atherosclerotic Carotid Artery Stenosis at Higher-than-average Risk of Ipsilateral Stroke (ACTRIS) trial, which is including patients with asymptomatic carotid stenosis who have imaging features believed to confer an increased risk of stroke.
Expert consensus statement
Expert consensus statement:
12/12 experts concluded that in selected patients 75 years of age or older with ≥60% asymptomatic carotid artery stenosis and an expected survival of at least five years, who are considered to be at an increased risk of stroke on best medical therapy alone, carotid endarterectomy is suggested after careful consideration of the risks and benefits at a multi-disciplinary team meeting.
Stenting or medical therapy for asymptomatic carotid stenosis
Description of studies
The Stent-protected Angioplasty in Asymptomatic Carotid Artery Stenosis vs. Endarterectomy (SPACE-2) trial was a randomised multi-centre study in Germany, Austria and Switzerland which aimed to assess the safety and efficacy of CAS or CEA compared with best medical therapy (BMT) alone in patients with asymptomatic ≥50% common or internal carotid artery stenosis.22 Stenoses were considered asymptomatic if patients had not experienced ipsilateral amaurosis fugax, a TIA or stroke within the preceding 180 days. SPACE-2 started in 2009 as a three-arm trial randomly assigning patients to CEA+BMT, CAS+BMT, or BMT alone in a 3:3:1 ratio, with a target sample size of 3550 patients. For CAS, the use of protection devices was not mandatory. The trial design was changed in 2013 to a two-arm trial of CEA+BMT versus CAS+BMT. Due to slow recruitment, the trial was stopped prematurely in 2014 after 513 patients had been randomised to CEA (n = 203), CAS (n = 197) or BMT (n = 113). This section of the guidelines only includes outcomes of patients in the CAS and BMT groups. Results after one year of follow-up were previously published. The primary efficacy endpoint (the cumulative risk of any stroke or death from any cause within 30 days, plus any ipsilateral ischaemic stroke within five years of follow-up) is yet to be reported.
We excluded two smaller RCTs because these studies did not report outcomes by symptom status,37,38 or patients were treated with primary balloon angioplasty.38 Therefore, the SPACE-2 data were the only data which could be used to address the PICO questions in this section.
The effects of treatment are presented with medical therapy alone as the reference group. A summary of findings is provided in Table 3.
Table 3.
Summary of findings for stenting versus medical therapy for asymptomatic carotid stenosis (PICO 2.1–2.4).
| Certainty assessment |
№ of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Stenting | Medical therapy | Relative(95% CI) | Absolute(95% CI) | ||
| PICO 2.1: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death | ||||||||||||
| 1 | Randomised trials | Seriousa | Not serious | Seriousb | Very seriousc | None | 6/197(3.0%) | 1/113(0.9%) | RR 3.44(0.42–28.23) | 22 more per 1000(from 5 fewer to 241 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 2.2: Long-term risk of stroke in any territory, including peri-procedural death | ||||||||||||
| 1 | Randomised trials | Seriousa | Not serious | Seriousb | Very seriousc | None | 8/197(4.1%) | 1/113(0.9%) | RR 4.59(0.58–36.22) | 32 more per 1000(from 4 fewer to 312 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 2.3: Long-term risk of major stroke, including peri-procedural death | ||||||||||||
| 1 | Randomised trials | Seriousa | Not serious | Seriousb | Not seriousc | None | 1/197(0.5%) | 1/113(0.9%) | RR 0.57(0.04–9.08) | 4 fewer per 1000(from 8 fewer to 72 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 2.4: Long-term risk of death | ||||||||||||
| 1 | Randomised trials | Seriousa | Not serious | Seriousb | Very seriousc | None | 2/197(1.0%) | 4/113(3.5%) | RR 0.29(0.05–1.54) | 25 fewer per 1000(from 34 fewer to 19 more) | ⨁◯◯◯ VERY LOW |
IMPORTANT |
CI: confidence interval; RR: risk ratio.
aTrial was stopped early.
bInsufficient length of follow-up to assess long-term effects.
cVery wide confidence intervals.
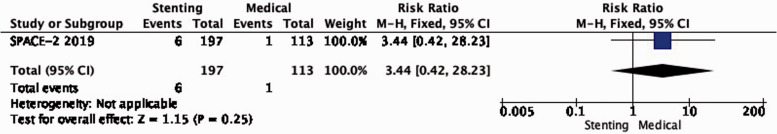
PICO 2.1: In patients with asymptomatic carotid stenosis, does stenting compared with medical therapy alone reduce the long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death?
There is very low quality of evidence from SPACE-2 of a non-significant increase in the risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death with stenting compared with medical therapy alone (RR: 3.44, 95% CI: 0.42–28.23; equivalent to 22 more events with CAS per 1000 patients, from 5 fewer to 241 more; Figure 2.1).
Figure 1.2.
Long-term risk of stroke in any territory, including peri-procedural death in endarterectomy versus medical therapy for asymptomatic carotid stenosis.
Figure 2.1.
Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death in stenting versus medical therapy for asymptomatic carotid stenosis.
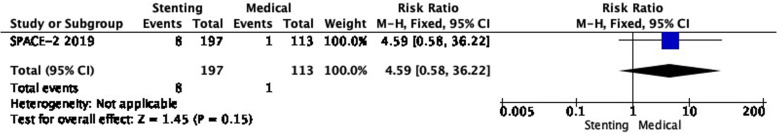
PICO 2.2: In patients with asymptomatic carotid stenosis, does stenting compared with medical therapy alone reduce the long-term risk of stroke in any territory, including peri-procedural death?
There is also very low quality evidence from SPACE-2 of a non-significantly higher risk of stroke in any territory, including peri-procedural death with stenting compared with medical therapy (RR: 4.59, 0.58–36.22; 32 more events with CAS per 1000 patients, from 4 fewer to 312 more; Figure 2.2).
Figure 2.2.
Long-term risk of stroke in any territory, including peri-procedural death in stenting versus medical therapy for asymptomatic carotid stenosis.
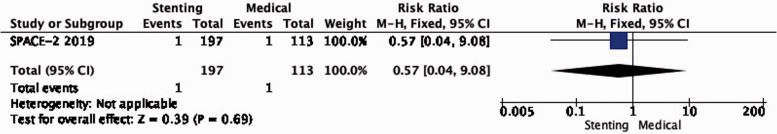
PICO 2.3: In patients with asymptomatic carotid stenosis, does stenting compared with medical therapy alone reduce the long-term risk of major stroke, including peri-procedural death?
Only one such composite event occurred in each of the stenting and medical therapy groups in SPACE-2 (RR: 0.57, 0.04–9.08; low quality evidence; Figure 2.3).
Figure 2.3.
Long-term risk of major stroke, including peri-procedural death in stenting versus medical therapy for asymptomatic carotid stenosis.
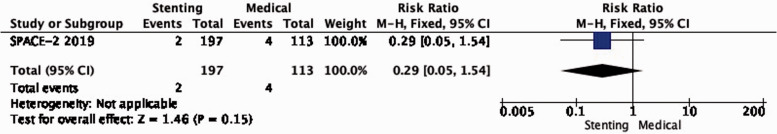
PICO 2.4: In patients with asymptomatic carotid stenosis, does stenting compared with medical therapy alone reduce the long-term risk of death?
There is very low quality of evidence that the long-term risk of death did not differ between patients treated with stenting and medical therapy in SPACE-2 (RR: 0.29, 0.05–1.54; Figure 2.4).
Figure 2.4.
Long-term risk of death in stenting versus medical therapy for asymptomatic carotid stenosis.
Analysis of current evidence and evidence-based recommendations
The evidence from this single, prematurely terminated RCT is very limited. The recruited study population is too small, and the available follow-up period is too short to reliably compare data between treatment groups. We downgraded the evidence for the risk of bias (due to the early termination), imprecision, and indirectness (insufficient length of follow-up), resulting in a very low quality of evidence.
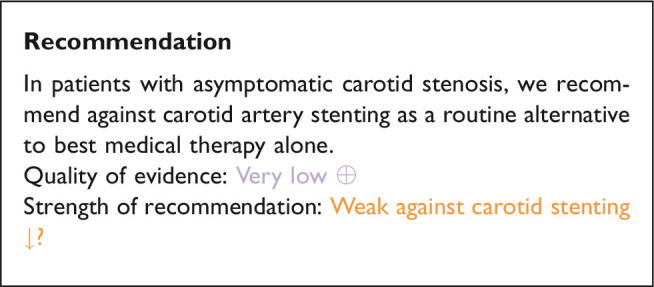
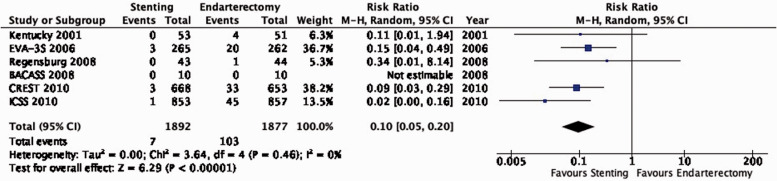
Recommendations regarding the choice between stenting and endarterectomy in patients with asymptomatic carotid stenosis, in whom revascularisation is considered to be appropriate are provided in section “Stenting or endarterectomy for asymptomatic carotid stenosis”.
Additional information
Carotid artery stenting versus best medical therapy alone are being compared in one of the two parallel study arms in the ongoing Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2).28
Stenting or endarterectomy for asymptomatic carotid stenosis
Description of studies
A single-centre trial in Lexington, Kentucky, USA randomised 85 participants with ≥80% asymptomatic carotid stenosis to receive either CAS without a cerebral protection device (CPD) or CEA and reported results up to four years after randomisation in 2004.39 A further report in 2014 combined long-term outcomes for up to 10 years in both asymptomatic and symptomatic patients who were enrolled in another trial at the same institution, but the authors did not present separate data according to symptom status.40 Therefore, we chose the 2004 report to extract outcome data from patients with asymptomatic stenosis to address our PICO questions.
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), a multicentre trial in the USA and Canada, randomised 1321 patients with ≥50% symptomatic carotid stenosis and 1181 patients with ≥60% asymptomatic carotid stenosis to CAS or CEA between 2000 and 2008.41–48 Interventionists with an experience of < 30 CAS procedures were required to complete a training programme. The use of a CPD was mandatory during stenting. Initial results were published in 2010; the final trial results were published in 2016 with follow-up data for up to 10 years after randomisation (median of 7.4 years). Only data from asymptomatic patients were extracted for our analyses to address these PICO questions.
A single-centre trial in Houston, Texas, USA randomised 60 patients with ≥80% asymptomatic carotid stenosis to receive CAS (with mandatory use of a CPD) or CEA. The primary outcome was ‘cognitive performance’ after treatment; this and other clinical outcome data for up to 6 months after randomisation were reported in 2014.49 No data were available for five patients who withdrew consent or were lost to follow-up.
A single-centre trial conducted in Ostrava, Czech Republic, randomised 63 patients with asymptomatic and 87 patients with symptomatic ≥70% carotid stenosis to undergo CAS (with the use of a CPD, where possible) or CEA and reported results in 2014.50 The primary outcome was the occurrence of new ischaemic brain lesions on magnetic resonance imaging after treatment. Clinical outcome events up to 30 days after treatment were also reported, and these were made available and categorised according to symptom status following correspondence with the investigators.
The Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis (ACT-1) allocated 1453 participants <80 years of age with ≥70% asymptomatic carotid stenosis in a 3:1 ratio to undergo CAS (with mandatory use of a CPD) or CEA between 2005 and 2013.51 A prior experience of ≥25 procedures was required from surgeons and interventionists. The initially planned sample size was 1658 participants, but the study was stopped prematurely due to slow enrolment. Results up to five years after randomisation were previously published.
A single-centre trial at Carmel Medical Center in Israel randomised 136 participants with ≥70% asymptomatic carotid stenosis to receive CAS (with mandatory use of a CPD) or CEA. Results up to five years after randomisation were reported in 2017.52 Three patients were lost to follow-up.
Events occurring up to one year after treatment were also extracted from the CAS and CEA groups of the 3-arm SPACE-2 trial (described in section ‘Stenting or medical therapy for asymptomatic carotid stenosis’).22
We did not include data from the multi-centre Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial conducted in the USA,53–55 from one Chinese multi-centre trial,56 and two single-centre studies conducted in Beijing, China.57,58 Reasons for exclusion of these randomised studies were the inclusion of patients with both asymptomatic and symptomatic carotid stenosis without reporting of separate outcome data according to symptomatic status, inclusion of ‘high surgical risk’ patients only, or results in the English language only being available as a conference abstract.
The effects of treatment are presented with endarterectomy as the reference group. A summary of findings is provided in Table 4.
Table 4.
Summary of findings for stenting versus endarterectomy for asymptomatic carotid stenosis (PICO 3.1–3.11).
| Certainty assessment |
№ of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Stenting | Endarterectomy | Relative(95% CI) | Absolute(95% CI) | ||
| PICO 3.1: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Not serious | Seriousa | None | 86/2018(4.3%) | 46/1292(3.6%) | RR 1.25(0.88–1.79) | 9 more per 1000(from 4 fewer to 28 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 3.2. Long-term risk of post-procedural ipsilateral stroke | ||||||||||||
| 5 | Randomised trials | Not serious | Not serious | Not serious | Very seriousb | None | 23/926(2.5%) | 20/927(2.2%) | RR 1.12(0.62–2.00) | 3 more per 1000(from 8 fewer to 22 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 3.3. Long-term risk of stroke in any territory, including peri-procedural death | ||||||||||||
| 5 | Randomised trials | Not serious | Not serious | Not serious | Seriousa | None | 68/929(7.3%) | 55/928(5.9%) | RR 1.22(0.87–1.71) | 13 more per 1000(from 8 fewer to 42 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 3.4. Long-term risk of major stroke, including peri-procedural death | ||||||||||||
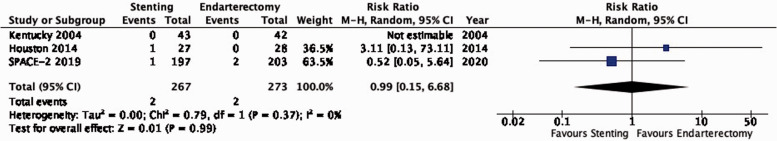
| 3 | Randomised trials | Not serious | Not serious | Not serious | Very seriousb | None | 2/267(0.7%) | 2/273(0.7%) | RR 0.99(0.15–6.68) | 0 fewer per 1000(from 20 fewer to 20 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 3.5: Long-term risk of death | ||||||||||||
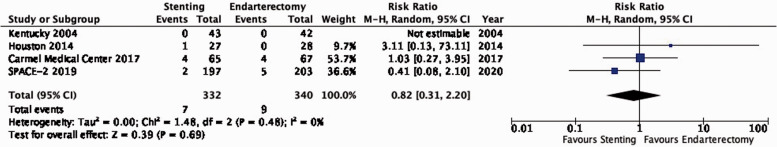
| 4 | Randomised trials | Not serious | Not serious | Not serious | Very seriousb | None | 7/332(2.1%) | 9/340(2.6%) | RR 0.82(0.31–2.20) | 5 fewer per 1000(from 18 fewer to 32 more) | ⨁⨁◯◯ LOW |
IMPORTANT |
| PICO 3.6: Risk of peri-procedural stroke | ||||||||||||
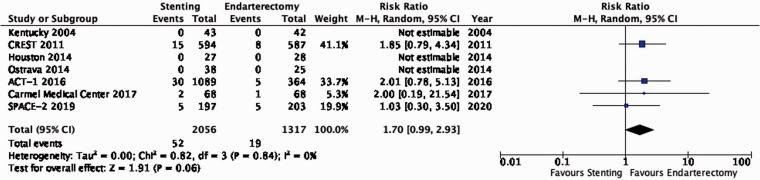
| 7 | Randomised trials | Not serious | Not serious | Not serious | Seriousa | None | 52/2056(2.5%) | 19/1317(1.4%) | RR 1.70(0.99–2.93) | 10 more per 1000(from 0 fewer to 28 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 3.7: Risk of peri-procedural death | ||||||||||||
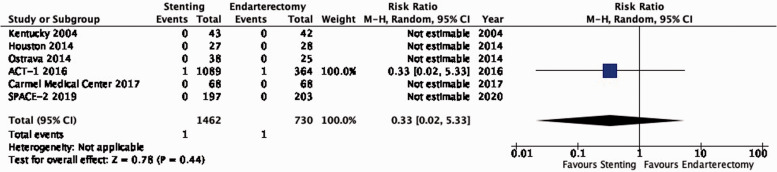
| 6 | Randomised trials | Not serious | Not serious | Not serious | Not serious | None | 1/1462(0.1%) | 1/730(0.1%) | RR 0.33(0.02–5.33) | 1 fewer per 1000(from 1 fewer to 6 more) | ⨁⨁⨁⨁ HIGH |
CRITICAL |
| PICO 3.8: Risk of peri-procedural stroke or death | ||||||||||||
| 7 | Randomised trials | Not serious | Not serious | Not serious | Seriousa | None | 53/2058(2.6%) | 20/1320(1.5%) | RR 1.62(0.96–2.76) | 9 more per 1000(from 1 fewer to 27 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 3.9: Risk of peri-procedural major stroke or death | ||||||||||||
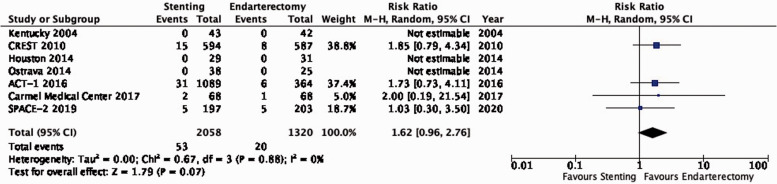
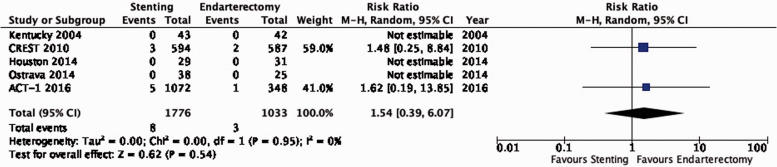
| 5 | Randomised trials | Not serious | Not serious | Not serious | Seriousa | None | 8/1776(0.5%) | 3/1033(0.3%) | RR 1.54(0.39–6.07) | 2 more per 1000(from 2 fewer to 15 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 3.10: Risk of peri-procedural myocardial infarction | ||||||||||||
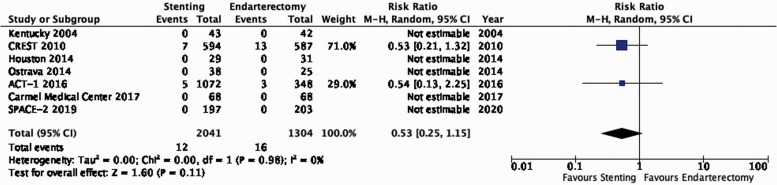
| 7 | Randomised trials | Not serious | Not serious | Seriousc | Seriousa | None | 12/2041(0.6%) | 16/1304(1.2%) | RR 0.53(0.25–1.15) | 6 fewer per 1000(from 9 fewer to 2 more) | ⨁⨁◯◯ LOW |
IMPORTANT |
| PICO 3.11: Risk of peri-procedural cranial nerve injury | ||||||||||||
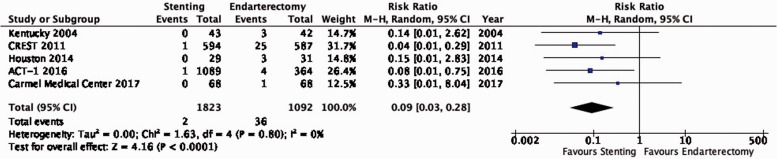
| 5 | Randomised trials | Not serious | Not serious | Not serious | Not serious | Very strong associationd | 2/1823(0.1%) | 36/1092(3.3%) | RR 0.09(0.03–0.28) | 30 fewer per 1000(from 32 fewer to 24 fewer) | ⨁⨁⨁⨁ HIGH |
IMPORTANT |
CI: confidence interval; RR: risk ratio.
aFew events, wide confidence intervals.
bVery wide confidence intervals.
cDefinition of myocardial infarction differed across trials.
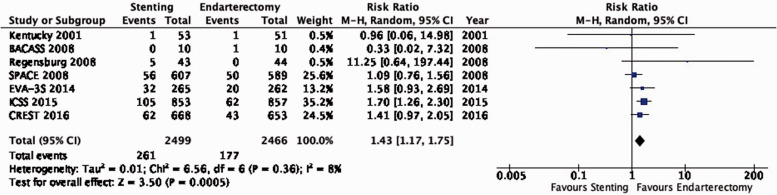
dVery large effect.
PICO 3.1: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death?
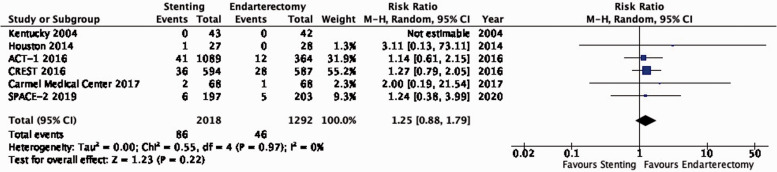
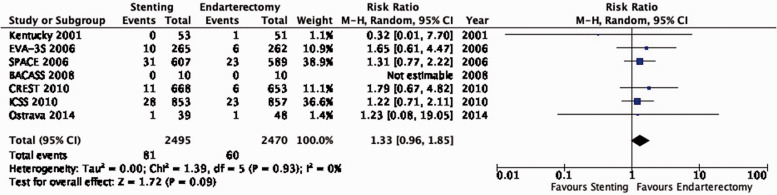
There is moderate quality evidence that stenting is likely associated with an increased long-term risk of post-procedural ipsilateral stroke, peri-procedural stroke in any territory, or peri-procedural death (RR: 1.25, 95% CI: 0.88–1.79; equivalent to 9 more events with CAS per 1000 patients, from 4 fewer to 28 more; Figure 3.1).
Figure 1.2.1.
Long-term risk of stroke in any territory, including peri-procedural death in endarterectomy versus medical therapy for asymptomatic carotid stenosis. Subgroup: Sex.
Figure 3.1.
Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death in stenting versus endarterectomy for asymptomatic carotid stenosis.
PICO 3.2: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of post-procedural ipsilateral stroke?
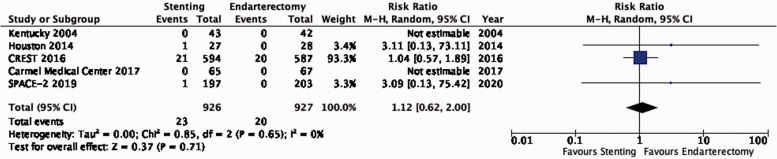
There is low quality evidence that endarterectomy and stenting do not differ in preventing post-procedural ipsilateral stroke, excluding peri-operative events (RR: 1.12, 0.62–2.00; 3 more events with stenting per 1000 patients, from 8 fewer to 22 more; Figure 3.2).
Figure 3.2.
Long-term risk of post-procedural ipsilateral stroke in stenting versus endarterectomy for asymptomatic carotid stenosis.
PICO 3.3: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of stroke in any territory, including peri-procedural death?
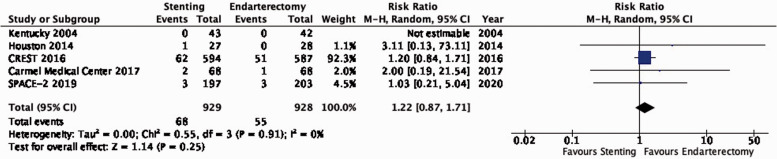
There is moderate quality evidence that stenting is likely associated with an increased long-term risk of stroke in any territory or peri-procedural death (RR: 1.22, 0.87–1.71; 13 more events with stenting per 1000 patients, from 8 fewer to 42 more; Figure 3.3).
Figure 3.3.
Long-term risk of stroke in any territory, including peri-procedural death in stenting versus endarterectomy for asymptomatic carotid stenosis.
PICO 3.4: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of major stroke, including peri-procedural death?
There is low quality evidence that endarterectomy and stenting do not differ in the long-term risk of major stroke or peri-procedural death (RR: 0.99, 0.15–6.68; 0 fewer events with stenting per 1000 patients, from 20 fewer to 20 more; Figure 3.4).
Figure 3.4.
Long-term risk of major stroke, including peri-procedural death in stenting versus endarterectomy for asymptomatic carotid stenosis.
PICO 3.5: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of death?
There is low quality evidence that endarterectomy and stenting do not differ in the long-term risk of death (RR: 0.82, 0.31–2.20; 5 fewer events with stenting per 1000 patients, from 18 fewer to 32 more; Figure 3.5).
Figure 3.5.
Long-term risk of death in stenting versus endarterectomy for asymptomatic carotid stenosis.
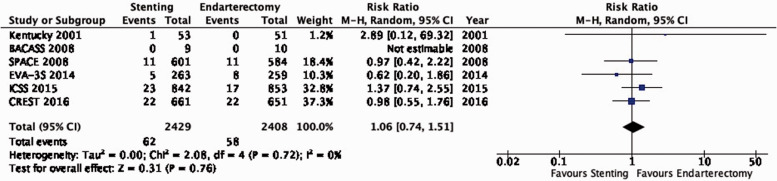
PICO 3.6: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural stroke?
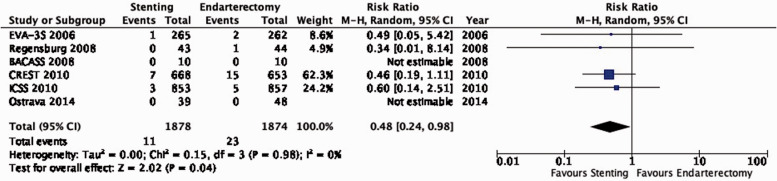
There is moderate quality evidence that stenting is likely associated with an increased risk of peri-procedural stroke (RR: 1.70, 0.99–2.93; 10 more events with stenting per 1000 patients, from 0 fewer to 28 more; Figure 3.6).
Figure 3.6.
Risk of peri-procedural stroke in stenting versus endarterectomy for asymptomatic carotid stenosis.
PICO 3.7: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural death?
There is high quality evidence that endarterectomy and stenting do not differ in the risk of peri-procedural death (RR: 0.33, 0.02–5.33; 1 less event with stenting per 1000 patients, from 1 less to 6 more; Figure 3.7). We did not downgrade the quality of evidence for imprecision because only a single event occurred in each treatment group.
Figure 3.7.
Risk of peri-procedural death in stenting versus endarterectomy for asymptomatic carotid stenosis.
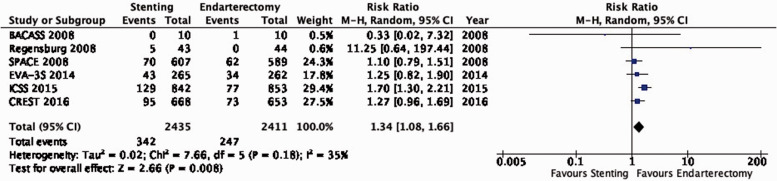
PICO 3.8: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural stroke or death?
There is moderate quality evidence that stenting is likely associated with an increased risk of peri-procedural stroke or death as compared to endarterectomy (RR: 1.62, 0.96–2.76; 9 more events per 1000 patients, from 1 less to 27 more; Figure 3.8).
Figure 3.8.
Risk of peri-procedural stroke or death in stenting versus endarterectomy for asymptomatic carotid stenosis.
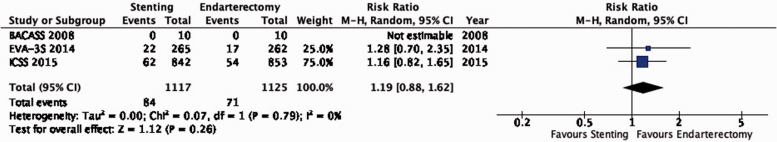
PICO 3.9: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural major stroke or death?
There is moderate quality evidence that stenting is likely associated with a slight increase of the risk of major peri-procedural stroke or death (RR: 1.54, 0.39–6.07; 2 more events with stenting per 1000 patients, from 2 fewer to 15 more; Figure 3.9).
Figure 3.9.
Risk of peri-procedural major stroke or death in stenting versus endarterectomy for asymptomatic carotid stenosis.
PICO 3.10: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural myocardial infarction?
There is low quality evidence that stenting is likely associated with a lower risk of peri-procedural myocardial infarction as compared to endarterectomy (RR: 0.53, 0.25–1.15; 6 fewer events with stenting per 1000 patients, from 9 fewer to 2 more; Figure 3.10). We additionally downgraded the quality of evidence for indirectness because all extracted events originated from the CREST and ACT-1 trials, where screening with ECG and cardiac enzymes of all patients was performed before and after treatment; the definition of myocardial infarction included elevation of cardiac enzymes alone, or in combination with ECG changes only (without clinical symptoms).
Figure 3.10.
Risk of peri-procedural myocardial infarction in stenting versus endarterectomy for asymptomatic carotid stenosis.
PICO 3.11: In patients with asymptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural cranial nerve injury?
There is high quality evidence that stenting is associated with a lower risk of peri-procedural cranial nerve injury than endarterectomy (RR: 0.09, 95% CI: 0.03–0.28; 30 fewer events per 1000 patients with stenting, from 32 fewer to 24 fewer; Figure 3.11). We upgraded the quality of evidence by two levels for strength of effect.
Figure 3.11.
Risk of peri-procedural cranial nerve injury in stenting versus endarterectomy for asymptomatic carotid stenosis.
Analysis of current evidence and evidence-based recommendation
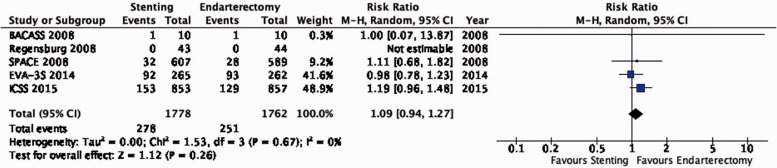
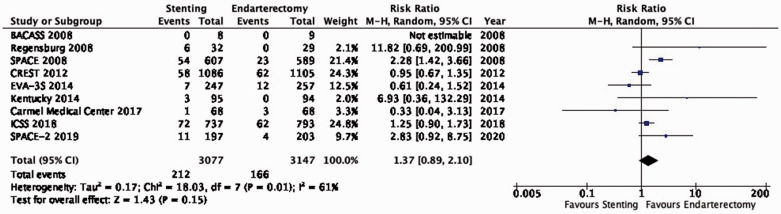
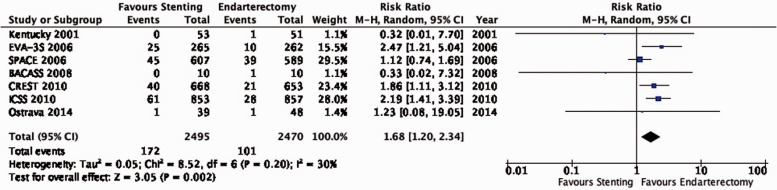
Data comparing the short-term risks and long-term effects between stenting and endarterectomy for asymptomatic carotid stenosis were available from seven trials including a total of 3373 patients. Most studies required patients to have ≥ 60% carotid stenosis for inclusion. Duration of follow-up in the largest trials was for five years or more. The risks of most outcome events were low, which led us to downgrade the level of evidence for imprecision. Low event rates also precluded meaningful subgroup analyses. Overall, we found no clear evidence of statistically significant differences in outcomes between endarterectomy or stenting that were rated as critical for decision making when treating patients with asymptomatic carotid stenosis (low to moderate quality evidence). As the available evidence is not sufficient to recommend stenting as an alternative to endarterectomy, carotid endarterectomy presently remains the treatment of choice for patients with asymptomatic carotid stenosis considered to require revascularisation.

Additional information
The Asymptomatic Carotid Surgery Trial-2 (ACST-2) has recently completed recruitment of 3.638 patients with asymptomatic carotid stenosis who were randomly assigned to CAS or CEA.59 First results are expected in late 2021 and will considerably increase the evidence base, which may lead to updates to the above recommendation.
Expert consensus statements
Expert consensus statement:
12/12 experts concluded that in patients with asymptomatic carotid stenosis in whom revascularisation is considered to be appropriate and who are less suitable for surgery, stenting may be suggested. We recommend careful consideration of the risks and benefits at a multi-disciplinary team meeting.
Expert consensus statement:
12/12 experts concluded that the independently assessed risk of in-hospital stroke or death following endarterectomy or stenting for asymptomatic carotid stenosis should be as low as possible, ideally below 2%.6
Endarterectomy or medical therapy for symptomatic carotid stenosis
Description of studies
There are three RCTs which randomly assigned patients with symptomatic carotid artery stenosis to CEA or medical therapy alone in a 1:1 ratio. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) separately reported results in patients with severe (70–99%), moderate (50–69%) or mild (<50%) symptomatic carotid stenosis.9 The first report in 1991 included outcomes in 659 patients with severe stenosis who had experienced a hemispheric or retinal transient ischaemic attack (TIA) or a non-disabling stroke within the 120 days before enrolment.60 The second report in 1998 included outcomes in 858 patients with moderate stenosis and 1368 patients with mild stenosis with a transient ischaemic attack or non-disabling stroke within 180 days before study entry.61 The 1998 report also provided long-term follow-up data for up to eight years in patients with severe stenosis included in the first report.
The MRC European Carotid Surgery Trial (ECST) reported results in 778 patients with severe (70–99%) and 374 patients with very mild (0–29%) symptomatic carotid stenosis in 1991,62,63 the results in 1599 patients with mild to moderate (30–69%) symptomatic carotid stenosis in 1996, and the final results with follow-up for up to eight years in all 3024 patients with symptomatic carotid stenosis in 1998.64 Eligible patients had a non-disabling ischaemic stroke, TIA or retinal infarction attributable to the carotid stenosis in the preceding six months. In the publication from which data for the current guideline were extracted, degrees of stenosis had been re-measured according to the method used in the NASCET trial.12
The Veterans Affairs Cooperative Studies Program (VACSP) symptomatic carotid stenosis trial included 189 patients with >50% symptomatic carotid stenosis and followed them up for a maximum of 33 months.65 Eligible patients had an ischaemic stroke, TIA or transient monocular blindness in the preceding 120 days. Results were reported in 1991.
The effects of treatment are presented with medical therapy alone as the reference group. A summary of findings is provided in Table 5.
Table 5.
Summary of findings for endarterectomy versus medical therapy for symptomatic carotid stenosis (PICO 4.1–4.4).
| Certainty assessment |
№ of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Endarterectomy | Medical therapy | Relative(95% CI) | Absolute(95% CI) | ||
| PICO 4.1: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death (30-99% stenosis) | ||||||||||||
| 3 | Randomised trials | Not serious | Seriousa | Seriousb | Seriousc | None | 394/3336(11.8%) | 415/2754(15.1%) | RR 0.83(0.61–1.14) | 26 fewer per 1000(from 59 fewer to 21 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 4.1.1a: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death (50-99% stenosis): Age < 65 years | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | None | 86/731(11.8%) | 93/550(16.9%) | RR 0.70(0.53–0.92) | 51 fewer per 1000(from 79 fewer to 14 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 4.1.1b: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death (50-99% stenosis): Age ≥ 65 years | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | None | 89/743(12.0%) | 151/694(21.8%) | RR 0.57(0.44–0.73) | 94 fewer per 1000(from 122 fewer to 59 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 4.1.2a. Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death (50-99% stenosis): Men | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | None | 112/1013(11.1%) | 184/873(21.1%) | RR 0.54(0.44–0.67) | 97 fewer per 1000(from 118 fewer to 70 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 4.1.2.b.: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death (50-99% stenosis): Women | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Seriousc | None | 63/461(13.7%) | 60/371(16.2%) | RR 0.85(0.58–1.23) | 24 fewer per 1000(from 68 fewer to 37 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 4.1.3a: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death (50-99% stenosis): <2 weeks since most recent ischaemic event | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | Strong associationd | 40/325(12.3%) | 88/299(29.4%) | RR 0.41(0.30–0.58) | 174 fewer per 1000(from 206 fewer to 124 fewer) | ⨁⨁⨁⨁ HIGH |
CRITICAL |
| PICO 4.1.3b: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death (50-99% stenosis): 2–4 weeks since most recent ischaemic event | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | None | 31/268(11.6%) | 44/215(20.5%) | RR 0.58(0.35–0.98) | 86 fewer per 1000(from 133 fewer to 4 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 4.1.3c: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death (50-99% stenosis): 4–12 weeks since most recent ischaemic event | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | None | 63/560(11.3%) | 81/498(16.3%) | RR 0.70(0.51–0.95) | 49 fewer per 1000(from 80 fewer to 8 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 4.1.3d: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death (50-99% stenosis): >12 weeks since most recent ischaemic event | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Very seriousd | None | 41/321(12.8%) | 31/232(13.4%) | RR 1.01(0.66–1.57) | 1 more per 1000(from 45 fewer to 76 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 4.1.4a: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Near occlusion | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Very seriousd | None | 24/157(15.3%) | 17/114(14.9%) | RR 1.03(0.57–1.84) | 4 more per 1000(from 64 fewer to 125 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 4.1.4b: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Severe (70–99%) stenosis | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | Strong associationd | 50/518(9.7%) | 117/436(26.8%) | RR 0.37(0.27–0.50) | 169 fewer per 1000(from 196 fewer to 134 fewer) | ⨁⨁⨁⨁ HIGH |
CRITICAL |
| PICO 4.1.4c: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Moderate (50–69%) stenosis | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Seriousc | None | 101/808(12.5%) | 110/694(15.9%) | RR 0.82(0.58–1.15) | 29 fewer per 1000(from 67 fewer to 24 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 4.1.4d: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Mild (<50%) stenosis | ||||||||||||
| 2 | Randomised trials | Not serious | Seriousa | Seriousb | Seriousc | None | 212/1762(12.0%) | 164/1413(11.6%) | RR 1.09(0.64–1.85) | 10 more per 1000(from 42 fewer to 99 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 4.2: Long-term risk of stroke in any territory, including peri-procedural death (30-99% stenosis) | ||||||||||||
| 3 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | None | 586/3336(17.6%) | 584/2754(21.2%) | RR 0.85(0.77–0.94) | 32 fewer per 1000(from 49 fewer to 13 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 4.2.1a: Long-term risk of stroke in any territory, including peri-procedural death: Near-occlusion | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Very seriousd | None | 32/157(20.4%) | 25/114(21.9%) | RR 1.00(0.46–2.21) | 0 fewer per 1000(from 118 fewer to 265 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 4.2.1b: Long-term risk of stroke in any territory, including peri-procedural death: Severe (70–99%) stenosis | ||||||||||||
| 2 | Randomised trials | Not serious | Seriousa | Seriousa | Not serious | Strong associationd | 84/518(16.2%) | 143/436(32.8%) | RR 0.48(0.29–0.81) | 171 fewer per 1000(from 233 fewer to 62 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 4.2.1c: Long-term risk of stroke in any territory, including peri-procedural death: Moderate (50–69%) stenosis | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | None | 144/808(17.8%) | 165/694(23.8%) | RR 0.77(0.63–0.94) | 55 fewer per 1000(from 88 fewer to 14 fewer) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 4.2.1d: Long-term risk of stroke in any territory, including peri-procedural death: Mild (<50%) stenosis | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Seriousc | None | 318/1762(18.0%) | 244/1413(17.3%) | RR 1.09(0.89–1.34) | 16 more per 1000(from 19 fewer to 59 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 4.3: Long-term risk of major stroke, including peri-procedural death (30-99% stenosis) | ||||||||||||
| 3 | Randomised trials | Not serious | Not serious | Seriousb | Seriousc | None | 150/3336(4.5%) | 152/2754(5.5%) | RR 0.79(0.51–1.22) | 12 fewer per 1000(from 27 fewer to 12 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 4.3.1a: Long-term risk of major stroke, including peri-procedural death: Near-occlusion | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Very seriousd | None | 12/157(7.6%) | 7/114(6.1%) | RR 1.33(0.35–5.08) | 20 more per 1000(from 40 fewer to 251 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 4.3.1b: Long-term risk of major stroke, including peri-procedural death: Severe (70–99%) stenosis | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Not serious | Strong associationd | 22/518(4.2%) | 53/436(12.2%) | RR 0.35(0.22–0.57) | 79 fewer per 1000(from 95 fewer to 52 fewer) | ⨁⨁⨁⨁ HIGH |
CRITICAL |
| PICO 4.3.1c: Long-term risk of major stroke, including peri-procedural death: Moderate (50–69%) stenosis | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Seriousc | None | 35/808(4.3%) | 39/694(5.6%) | RR 0.73(0.41–1.27) | 15 fewer per 1000(from 33 fewer to 15 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 4.3.1d: Long-term risk of major stroke, including peri-procedural death: Mild (<50%) stenosis | ||||||||||||
| 2 | Randomised trials | Not serious | Not serious | Seriousb | Seriousc | None | 79/1762(4.5%) | 50/1413(3.5%) | RR 1.24(0.82–1.87) | 8 more per 1000(from 6 fewer to 31 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 4.4: Long-term risk of death (30-99% stenosis) | ||||||||||||
| 3 | Randomised trials | Not serious | Not serious | Seriousb | Seriousc | None | 738/3335(22.1%) | 576/2758(20.9%) | RR 1.00(0.85–1.19) | 0 fewer per 1000(from 31 fewer to 40 more) | ⨁⨁◯◯ LOW |
IMPORTANT |
CI: confidence interval; RR: risk ratio.
aSignificant heterogeneity, I2 > 70%.
bEndarterectomy and medical therapy have evolved since the trials contributing the evidence were performed.
cFew events, wide confidence intervals.
dLarge effect.
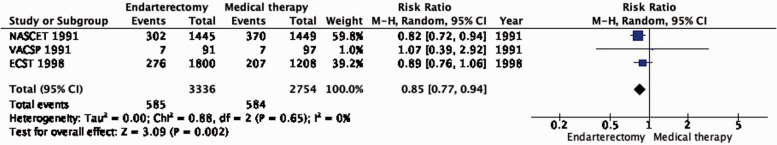
PICO 4.1: In patients with symptomatic carotid stenosis, does endarterectomy compared with medical therapy alone reduce the long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death?
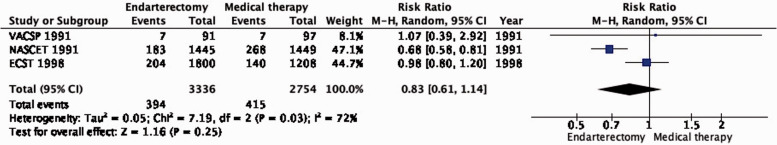
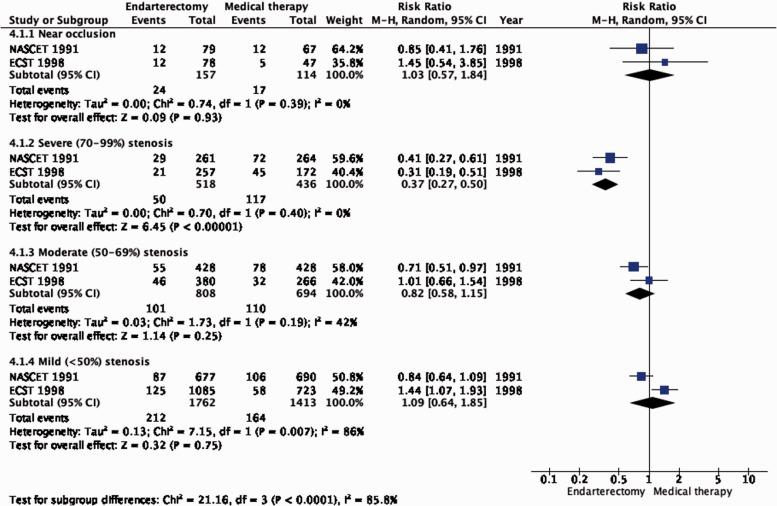
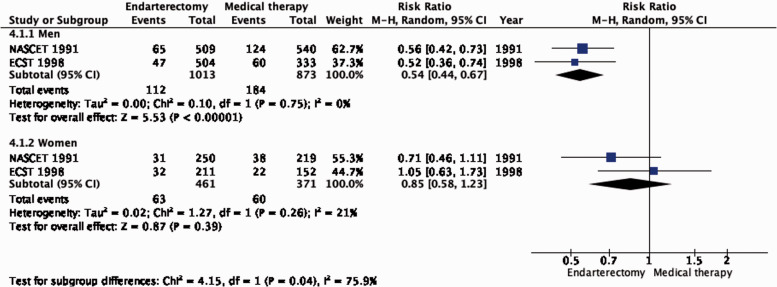
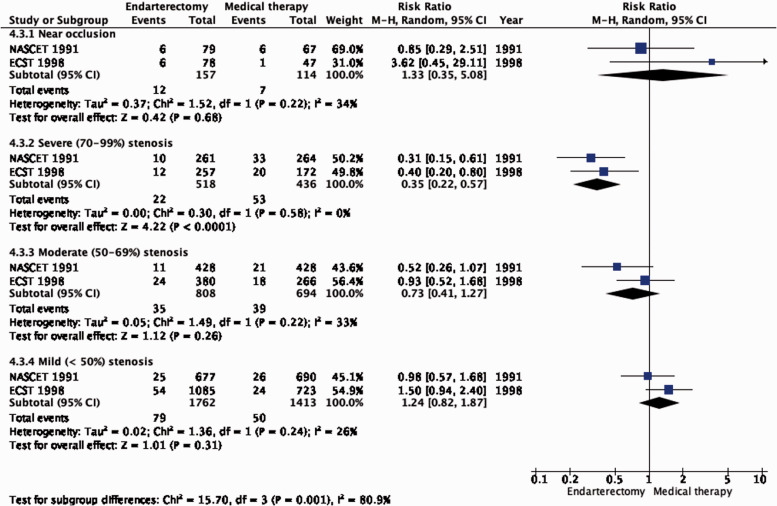
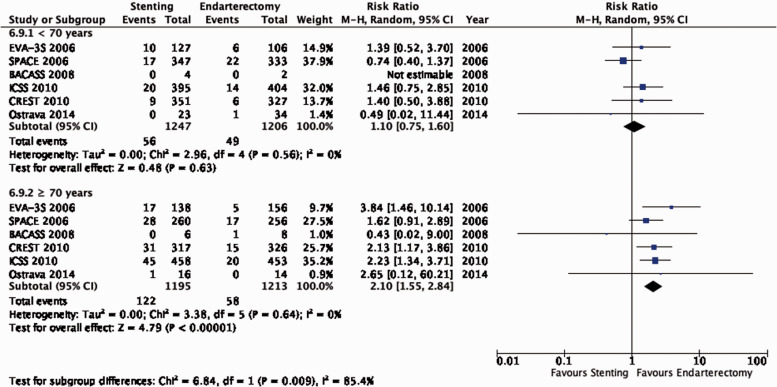
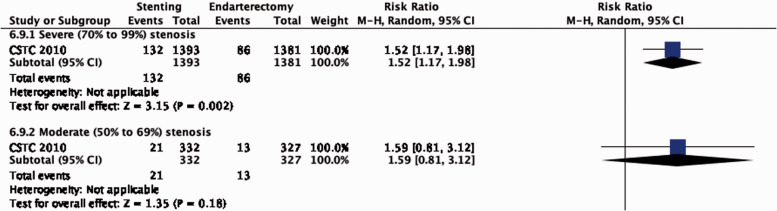
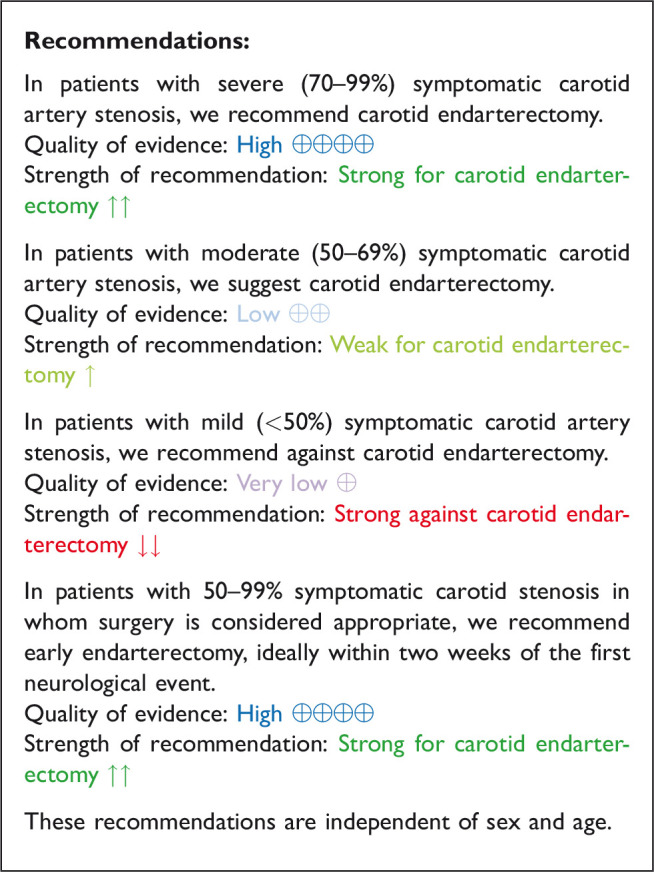
The reduction in the long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death, with endarterectomy is strongly dependent on the degree of the symptomatic stenosis and the time interval between the index neurological event and randomisation. There is very low quality evidence for a benefit of CEA if data from all symptomatic patients, regardless of the severity of their stenosis, are grouped and analysed together (RR: 0.83, 95% CI: 0.61–1.14; equivalent to 26 fewer events with CEA per 1000 patients, from 59 fewer to 21 more; Figure 4.1). The level of evidence was additionally downgraded for inconsistency due to statistical heterogeneity between trials. Stratifying results by degree of stenosis, there is high quality evidence of a meaningful benefit of CEA in patients with 70–99% stenosis (RR: 0.37, 0.27–0.50; 169 fewer events per 1000 patients, from 196 fewer to 134 fewer; Figure 4.1.4); low quality evidence of potential benefit in an overall population of patients with 50–69% stenosis (RR: 0.82, 0.58–1.15; 29 fewer events per 1000 patients, from 67 fewer to 24 more); and no evidence of benefit amongst patients with <50% stenosis (RR: 1.09, 0.64–1.85) or near-occlusion (RR: 1.03, 0.57–1.84; very low grade evidence each). The interaction between degree of stenosis and the effect of CEA was significant (p < 0.0001).
Figure 1.2.2.
Long-term risk of stroke in any territory, including peri-procedural death in endarterectomy versus medical therapy for asymptomatic carotid stenosis. Subgroup: Age.
Figure 4.1.
Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death in endarterectomy versus medical therapy for 30–99% symptomatic carotid stenosis.
Figure 4.1.1.
Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death in endarterectomy versus medical therapy for 50–99% symptomatic carotid stenosis. Subgroup: Age.
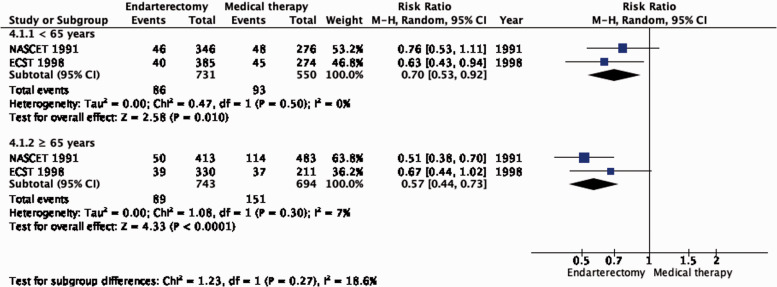
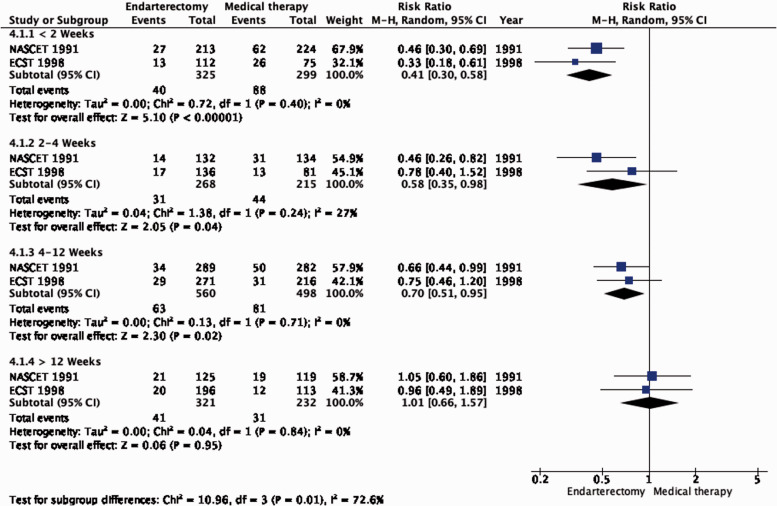
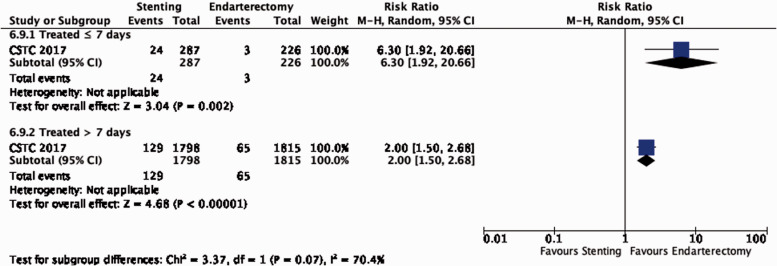
The benefit of CEA in patients with ≥50% stenosis was most pronounced amongst patients randomised within two weeks of the index neurological event (RR: 0.41, 0.30–0.58, 174 fewer events per 1000 patients, from 206 fewer to 124 fewer, high quality evidence; Figure 4.1.3), but benefit was still present up to 12 weeks (p = 0.001 for interaction with time).
Figure 4.1.3.
Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death in endarterectomy versus medical therapy for 50–99% symptomatic carotid stenosis. Subgroup: Time since last ischaemic event.
Figure 4.1.4.
Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death in endarterectomy versus medical therapy for symptomatic carotid stenosis. Subgroup: Severity of stenosis.
An individual patient data meta-analysis of all three trials showed that the degree of stenosis and time since the last event modified the effect of CEA in an additive manner. There was a significant 14.8% (95% CI: 6.2–23.4%) absolute reduction in the five-year risk of ipsilateral carotid territory ischaemic stroke or any stroke or death within 30 days of CEA in patients with moderate (50–69%) stenosis who were randomised within 14 days of their index ischaemic event (data not included in SoF table or figure).66
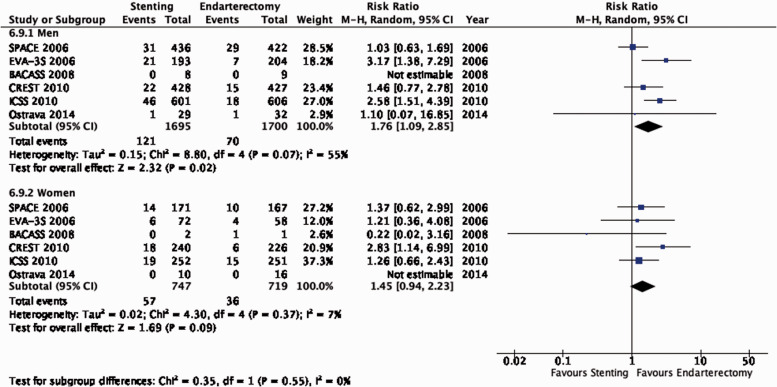
There is no evidence that the benefit of CEA differs with age (Figure 4.1.1). Although the reduction in the combined outcome was not statistically significant in women (Figure 4.1.2), this was likely due to the low number of women included in the trials (n = 832).
Figure 4.1.2.
Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death in endarterectomy versus medical therapy for 50–99% symptomatic carotid stenosis. Subgroup: Sex.
PICO 4.2: In patients with symptomatic carotid stenosis, does endarterectomy compared with medical therapy alone reduce the long-term risk of stroke in any territory, including peri-procedural death?
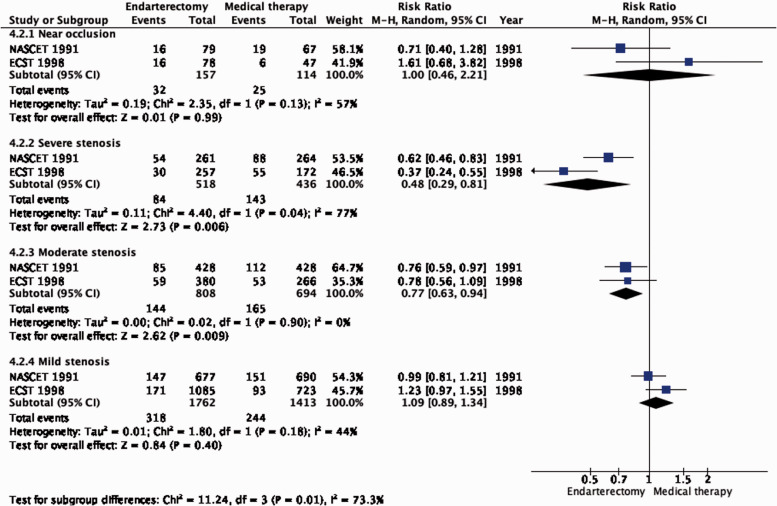
Amongst patients with all degrees of stenosis combined, there is moderate quality of evidence that CEA reduced the long-term risk of stroke in any territory, including peri-procedural death, compared with medical therapy alone (RR: 0.85, 95% CI: 0.77–0.94; 32 fewer events per 1000 patients, from 49 fewer to 13 fewer; Figure 4.2). The evidence for a beneficial effect of CEA was of moderate quality in patients with 70–99% stenosis (RR: 0.48, 95% CI: 0.29–0.81; 171 fewer events per 1000 patients, from 233 fewer to 62 fewer; Figure 4.2.1) and in patients with 50–59% stenosis (RR: 0.77, 95% CI: 0.63–0.94; 55 fewer events per 1000 patients, from 88 fewer to 14 fewer). Comparing the number of events prevented between PICO 4.1 and PICO 4.2 within each stenosis category, it can be inferred that CEA mainly prevents ipsilateral stroke.
Figure 4.2.
Long-term risk of stroke in any territory, including peri-procedural death in endarterectomy versus medical therapy for 30–99% symptomatic carotid stenosis.
Figure 4.2.1.
Long-term risk of stroke in any territory, including peri-procedural death in endarterectomy versus medical therapy for symptomatic carotid stenosis. Subgroup: Severity of stenosis.
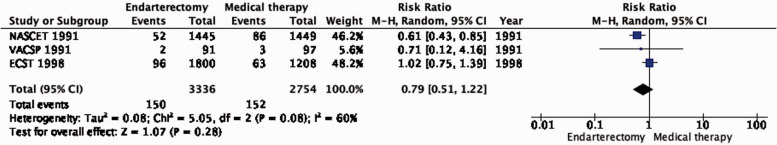
PICO 4.3: In patients with symptomatic carotid stenosis, does endarterectomy compared with medical therapy alone reduce the long-term risk of major stroke, including peri-procedural death?
Amongst patients with all degrees of stenosis combined, endarterectomy did not significantly reduce the long-term risk of major stroke, including peri-procedural death (RR: 0.79, 95% CI: 0.51–1.22; 12 fewer events per 1000 patients, from 27 fewer to 12 more; low quality evidence; Figure 4.3). However, once again, the benefit of CEA varies according to the degree of stenosis. In patients with 70–99% stenosis, there is high quality evidence of benefit (RR: 0.35, 95% CI: 0.22–0.57; 79 fewer events per 1000 patients, from 95 fewer to 52 fewer; Figure 4.3.1). Conversely, there was low quality evidence of potential benefit in patients with 50–69% stenosis (RR: 0.73, 95% CI: 0.41–1.27; 15 fewer events per 1000 patients, from 33 fewer to 15 more), low quality evidence of harm in patients with <50% stenosis (RR: 1.24, 95% CI: 0.82–1.87), and very low quality evidence of harm in patients with near occlusion (RR: 1.33, 95% CI: 0.35–5.08).
Figure 4.3.
Long-term risk of major stroke, including peri-procedural death in endarterectomy versus medical therapy for 30–99% symptomatic carotid stenosis.
Figure 4.3.1.
Long-term risk of major stroke, including peri-procedural death in endarterectomy versus medical therapy for symptomatic carotid stenosis. Subgroup: Severity of Stenosis.
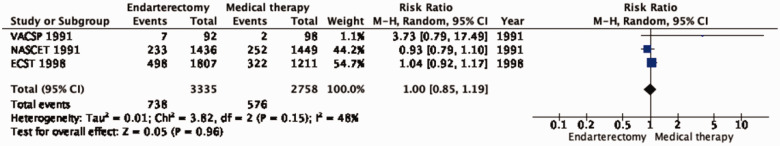
PICO 4.4: In patients with symptomatic carotid stenosis, does endarterectomy compared with medical therapy alone reduce the long-term risk of death?
Endarterectomy does not reduce the long-term risk of death compared with medical therapy alone (RR: 1.00, 95% CI: 0.85–1.19; 0 fewer events per 1000 patients, from 31 fewer to 40 more; low quality of evidence; Figure 4.4).
Figure 4.4.
Long-term risk of death in endarterectomy versus medical therapy for 30–99% symptomatic carotid stenosis.
Analysis of current evidence and evidence-based recommendation
Evidence of the effect of CEA compared with medical therapy alone for symptomatic carotid stenosis was available from three trials, which included 6098 patients. Symptomatic carotid stenosis was defined by the occurrence of ischaemic ocular or cerebral events attributable to the stenosis within four to six months before enrolment, depending on the trial and the severity of stenosis. The evidence provided relates to the time when these trials were performed three decades ago. Medical treatment of patients with atherosclerotic carotid stenosis has improved, with widespread use of statins, the availability of better antiplatelet treatment regimens and stricter control of blood pressure. However, surgical techniques and perioperative management have also improved since these trials were completed. We therefore downgraded the overall quality of evidence for indirectness.
The benefits of CEA in patients with symptomatic carotid stenosis strongly depends on the degree of stenosis. Amongst patients with severe (70–99%) stenosis, there is high quality evidence that CEA prevents ipsilateral stroke, moderate quality evidence that it prevents stroke in any territory, and high quality evidence that it prevents major stroke, taking into account the combined risks of peri-operative stroke or death. In patients with moderate (50–69%) carotid stenosis, there is low quality evidence that CEA prevents ipsilateral stroke and major stroke, and moderate quality evidence for prevention of stroke in any territory, again taking into account the peri-operative stroke or death risk, if patients are operated upon within 14 days of their presenting cerebrovascular event. There is no evidence that CEA prevents stroke in patients with mild (<50%) stenosis or near-occlusion of the carotid artery. However, the definition of near-occlusion in the early endarterectomy trials depended on intra-arterial angiography, and there are no widely-accepted standardised criteria for near-occlusion on ultrasound or non-invasive angiography.67 We therefore could not make any clear recommendations on the treatment of carotid near-occlusion in this guideline. The benefit of CEA also strongly depends on the timing of treatment, with the greatest reduction in stroke risk achieved if surgery is performed <14 days of the index event. We found no evidence that the benefit of CEA varies substantially between men and women or between older and younger patients.
The optimal management of patients with distal tandem stenosis is uncertain. In NASCET, patients who had 85–99% extracranial ICA stenosis and any degree of co-existing, ipsilateral intracranial atherosclerotic disease (IAD) had an increased risk of ipsilateral stroke over three years if they were treated with best medical therapy alone compared with those without IAD (45.7% vs. 25.3%, relative risk 1.8, 95% CI: 1.1–3.2).68 However, the three-year risk of ipsilateral stroke in surgically-treated patients with 85–99% extracranial ICA stenosis was similar in those with and those without IAD (8.6% vs. 10%, relative risk 0.9; 95% CI: 0.2–3.0). Therefore, IAD should not deter one from proceeding to CEA in suitable patients, whilst acknowledging that only a very small number of patients with severe stenosis were included in this subgroup analysis of the NASCET data.
Additional information
The Second European Carotid Surgery Trial (ECST-2) is comparing optimised medical therapy (OMT) alone versus OMT and carotid revascularisation in patients with symptomatic carotid stenosis estimated to be at low or intermediate risk of stroke using ‘clinical risk modelling’, and in patients with asymptomatic carotid stenosis. ECST-2 discontinued recruitment after inclusion of 429 patients in its pilot phase and results are awaited (www.ecst2.com, last accessed 2 February 2021).
Stenting or medical therapy for symptomatic carotid stenosis
Description of studies
We identified no RCTs comparing stenting versus medical therapy alone in patients with symptomatic carotid stenosis that fulfilled our inclusion criteria. We excluded two small RCTs because these studies did not report outcomes according to symptom status,37,38 or patients were treated with primary balloon angioplasty.38
Stenting or endarterectomy for symptomatic carotid stenosis
Description of studies
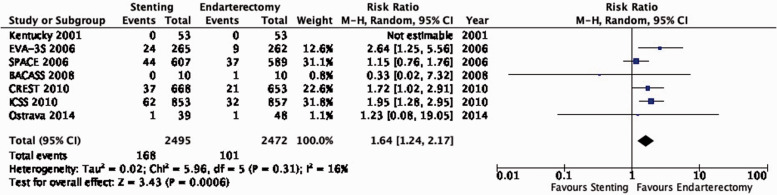
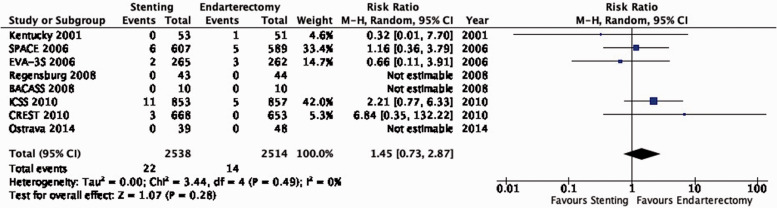
A single-centre trial in Lexington, Kentucky, USA randomised 104 patients with ≥70% symptomatic carotid stenosis to receive either CAS without a cerebral protection device (CPD) or CEA and reported results up to two years after randomisation in 2001.69
The French multi-centre Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial randomised 527 patients with ≥60% symptomatic carotid stenosis to undergo CAS or CEA between 2000 and 2005.70–75 Interventionists were required to have performed at least 12 CAS procedures, or at least 35 stenting procedures in the supra-aortic trunks, of which at least 5 involved the carotid artery. The use of CPDs during stenting was made mandatory after an interim analysis raised safety concerns amongst patients treated without CPDs. The trial was stopped early for safety and futility reasons. Initial results were published in 2006, and final results with available data over a median follow-up period of 7.1 years were reported in 2014.
The multi-centre Stent-supported Percutaneous Angioplasty of the Carotid artery versus Endarterectomy (SPACE) trial randomised 1214 patients with ≥50% symptomatic carotid stenosis between CAS and CEA in Germany, Austria, and Switzerland between 2001 and 2006.76–78 Interventionists had to show proof of at least 25 successful, consecutive percutaneous transluminal angioplasty or stent procedures in the carotid artery. The use of a CPD was not mandatory. The trial was stopped early for reasons of futility and lack of funding. Initial results were published in 2006 and final results up to two years after randomisation were published in 2008.
A single-centre trial in Regensburg, Germany, randomised 87 patients with ≥70% symptomatic carotid stenosis to undergo CAS without a CPD or CEA between 1999 and 2002.79 Recruitment was stopped when the multi-centre SPACE trial, which had a similar study design, was commenced. Results over a median follow-up period of >5 years were published in 2008.
The multi-centre International Carotid Stenting Study (ICSS) randomised 1713 patients with ≥50% symptomatic carotid stenosis to receive either CAS or CEA in Europe, Australia, New Zealand, and Canada between 2001 and 2008.80–83 Eligible patients had symptoms attributable to their carotid stenosis within 12 months before randomisation; however, only 4% had symptoms which occurred more than 6 months before randomisation. Interventionists were required to have carried out at least 50 stenting procedures, at least 10 of which were in the carotid artery. Use of CPDs was recommended but not mandatory. Initial results were published in 2011 and final results with data over a median follow-up period of 4.2 years were reported in 2015.
The single-centre Basel Carotid Artery Stenting Study (BACASS) randomised 20 patients with ≥50% symptomatic carotid stenosis to CAS with routine use of a CPD or CEA between 1998 and 2002.84 Recruitment was stopped when the centre started recruiting patients in ICSS. Results including follow-up data over a median of four years after randomisation were published in 2008.
We also extracted relevant outcomes in symptomatic patients from the Ostrava and CREST trials, which are described in results section ‘Stenting or endarterectomy for asymptomatic carotid stenosis’. Furthermore, we included outcomes in pre-defined patient subgroups derived from pooled analyses of individual patient data (IPD) from the EVA-3S, SPACE, ICSS and CREST trials which were performed by the Carotid Stenosis Trialists´ Collaboration (CSTC).85–87
We excluded one industry-funded multi-centre randomised trial because the results were only reported in a conference abstract,88 and also excluded one single-centre and one multicentre randomised trial in which the majority of patients in the endovascular group were treated with primary balloon angioplasty.89,90
The effects of treatment are presented with endarterectomy as the reference group. A summary of findings is provided in Table 6.
Table 6.
Summary of findings for stenting or endarterectomy for symptomatic carotid stenosis (PICO 6.1–6.12).
| Certainty assessment |
№ of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Stenting | Endarterectomy | Relative(95% CI) | Absolute(95% CI) | ||
| PICO 6.1: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death | ||||||||||||
| 7 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 261/2499(10.4%) | 177/2466(7.2%) | RR 1.43(1.17–1.75) | 31 more per 1000(from 12 more to 54 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.1.1a: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Age < 65 years | ||||||||||||
| 4 | IPD of randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | HR 0.83(0.56–1.21) | ⨁⨁◯◯ LOW |
CRITICAL | |||
| PICO 6.1.1b: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Age 65–74 years | ||||||||||||
| 4 | IPD of randomised trials | Not serious | Not serious | Seriousa | Not serious | None | HR 1.67(1.23–2.27) | ⨁⨁⨁◯ MODERATE |
CRITICAL | |||
| PICO 6.1.1c: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Age ≥ 75 years | ||||||||||||
| 4 | IPD of randomised trials | Not serious | Not serious | Seriousa | Not serious | None | HR 1.85(1.35–2.53) | ⨁⨁⨁◯ MODERATE |
CRITICAL | |||
| PICO 6.1.2a: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Men | ||||||||||||
| 4 | IPD of randomised trials | Not serious | Not serious | Seriousa | Not serious | None | HR 1.54(1.23–1.95) | ⨁⨁⨁◯ MODERATE |
CRITICAL | |||
| PICO 6.1.2b: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Women | ||||||||||||
| 4 | IPD of randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | HR 1.25(0.90–1.74) | ⨁⨁◯◯ LOW |
CRITICAL | |||
| PICO 6.1.3a: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Severe (70-99%) stenosis | ||||||||||||
| 4 | IPD of randomised trials | Not serious | Not serious | Seriousa | Not serious | None | HR 1.48(1.20–1.81) | ⨁⨁⨁◯ MODERATE |
CRITICAL | |||
| PICO 6.1.3b: Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death: Moderate (50-69%) stenosis | ||||||||||||
| 4 | IPD of randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | HR 1.33(0.84–2.10) | ⨁⨁◯◯ LOW |
CRITICAL | |||
| PICO 6.2: Long-term risk of post-procedural ipsilateral stroke | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 62/2429(2.6%) | 58/2408(2.4%) | RR 1.06(0.74–1.51) | 1 more per 1000(from 6 fewer to 12 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.3. Long-term risk of stroke in any territory, including peri-procedural death | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 352/2435(14.5%) | 247/2411(10.2%) | RR 1.34(1.08–1.66) | 35 more per 1000(from 8 more to 68 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.4. Long-term risk of major stroke, including peri-procedural death | ||||||||||||
| 3 | Randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | 84/1117(7.5%) | 71/1125(6.3%) | RR 1.19(0.88–1.62) | 12 more per 1000(from 8 fewer to 39 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 6.5. Long-term risk of death | ||||||||||||
| 5 | Randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | 278/1778(15.6%) | 251/1762(14.2%) | RR 1.09(0.94–1.27) | 13 more per 1000(from 9 fewer to 38 more) | ⨁⨁◯◯ LOW |
IMPORTANT |
| PICO 6.6: Long-term risk of severe restenosis | ||||||||||||
| 9 | Randomised trials | Not serious | Seriousc | Seriousa | Seriousb | None | 212/3077(6.9%) | 166/3147(5.3%) | RR 1.37(0.89–2.10) | 20 more per 1000(from 6 fewer to 58 more) | ⨁◯◯◯ VERY LOW |
IMPORTANT |
| PICO 6.7: Risk of peri-procedural stroke | ||||||||||||
| 7 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 168/2495(6.7%) | 101/2472(4.1%) | RR 1.64(1.24–2.17) | 26 more per 1000(from 10 more to 48 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.8: Risk of peri-procedural death | ||||||||||||
| 8 | Randomised trials | Not serious | Not serious | Seriousa | Very seriousd | None | 22/2538(0.9%) | 14/2514(0.6%) | RR 1.45(0.73–2.87) | 3 more per 1000(from 2 fewer to 10 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 6.9: Risk of peri-procedural stroke or death | ||||||||||||
| 7 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 172/2495(6.9%) | 101/2470(4.1%) | RR 1.68(1.20–2.34) | 28 more per 1000(from 8 more to 55 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.9.1a: Risk of peri-procedural stroke or death: Age < 70 years | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | 56/1247(4.5%) | 49/1206(4.1%) | RR 1.10(0.75–1.60) | 4 more per 1000(from 10 fewer to 24 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 6.9.1b: Risk of peri-procedural stroke or death: Age ≥ 70 years | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | Strong associatione | 122/1195(10.2%) | 58/1213(4.8%) | RR 2.10(1.55–2.84) | 53 more per 1000(from 26 more to 88 more) | ⨁⨁⨁⨁ HIGH |
CRITICAL |
| PICO 6.9.2a: Risk of peri-procedural stroke or death: Men | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 121/1695(7.1%) | 70/1700(4.1%) | RR 1.76(1.09–2.85) | 31 more per 1000(from 4 more to 76 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.9.2b: Risk of peri-procedural stroke or death: Women | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | 57/747(7.6%) | 36/719(5.0%) | RR 1.45(0.94–2.23) | 23 more per 1000(from 3 fewer to 62 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 6.9.3a: Risk of peri-procedural stroke or death: Severe (70–99%) stenosis | ||||||||||||
| 3 | IPD of randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 132/1393(9.5%) | 86/1381(6.2%) | RR 1.52(1.17–1.98) | 32 more per 1000(from 11 more to 61 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.9.3b: Risk of peri-procedural stroke or death: Moderate (50–69%) stenosis | ||||||||||||
| 3 | IPD of randomised trials | Not serious | Not serious | Seriousa | Seriousd | None | 21/332(6.3%) | 13/327(4.0%) | RR 1.59(0.81–3.12) | 23 more per 1000(from 8 fewer to 84 more) | ⨁⨁◯◯ LOW |
CRITICAL |
| PICO 6.9.4a: Risk of peri-procedural stroke or death: ≤ 7 days since most recent ischaemic event | ||||||||||||
| 4 | IPD of randomised trials | Not serious | Not serious | Seriousa | Not serious | Very strong associatione | 24/287(8.4%) | 3/226(1.3%) | RR 6.30(1.92–20.66) | 70 more per 1000(from 12 more to 261 more) | ⨁⨁⨁⨁ HIGH |
CRITICAL |
| PICO 6.9.4b: Risk of peri-procedural stroke or death: > 7 days since most recent ischaemic event | ||||||||||||
| 4 | IPD of randomised trials | Not serious | Not serious | Serious a | Not serious | None | 129/1798(7.2%) | 65/1815(3.6%) | RR 2.00(1.50–2.68) | 36 more per 1000(from 18 more to 60 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.9.5a: Risk of peri-procedural stroke or death: Hemispheric stroke as most recent ischaemic event | ||||||||||||
| 3 | IPD of randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 85/813(10.5%) | 52/797(6.5%) | RR 1.60(1.15–2.23) | 39 more per 1000(from 10 more to 80 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.9.5b: Risk of peri-procedural stroke or death: TIA as most recent ischaemic event | ||||||||||||
| 3 | IPD of randomised trials | Not serious | Not serious | Seriousa | Not serious | None | 53/589(9.0%) | 31/601(5.2%) | RR 1.74(1.14–2.68) | 38 more per 1000(from 7 more to 87 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL |
| PICO 6.9.5c: Risk of peri-procedural stroke or death: Retinal ischaemia as most recent ischaemic event | ||||||||||||
| 3 | IPD of randomised trials | Not serious | Not serious | Seriousa | Very seriousd | None | 15/310(4.8%) | 14/297(4.7%) | RR 1.03(0.50–2.09) | 1 more per 1000(from 24 fewer to 51 more) | ⨁◯◯◯ VERY LOW |
CRITICAL |
| PICO 6.10: Risk of peri-procedural major stroke or death | ||||||||||||
| 7 | Randomised trials | Not serious | Not serious | Seriousa | Seriousb | None | 81/2495(3.2%) | 60/2470(2.4%) | RR 1.33(0.96–1.85) | 8 more per 1000(from 1 fewer to 21 more) | ⨁⨁◯◯ LOW |
|
| PICO 6.11: Risk of peri-procedural myocardial infarction | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Seriousa | Seriousb | Strong associatione | 11/1878(0.6%) | 23/1874(1.2%) | RR 0.48(0.24–0.98) | 6 fewer per 1000(from 9 fewer to 0 fewer) | ⨁⨁⨁◯ MODERATE |
IMPORTANT |
| PICO 6.12: Risk of peri-procedural cranial nerve injury | ||||||||||||
| 6 | Randomised trials | Not serious | Not serious | Seriousa | Not serious | Very strong associatione | 7/1892(0.4%) | 103/1877(5.5%) | RR 0.10(0.05–0.20) | 49 fewer per 1000(from 52 fewer to 44 fewer) | ⨁⨁⨁⨁ HIGH |
IMPORTANT |
CI: confidence interval; RR: risk ratio.
aStenting and endarterectomy have evolved since the time of the contributing trials.
bFew events, wide confidence intervals.
cSignificant heterogeneity, I2 > 60%.
dVery wide confidence intervals.
eLarge effect.
PICO 6.1: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death?
There is moderate quality of evidence that endarterectomy is superior to stenting in preventing the combined outcome of post-procedural ipsilateral stroke, peri-procedural stroke in any territory, or peri-procedural death (RR: 1.43, 95% CI: 1.17–1.75; equivalent to 31 more events with stenting per 1000 patients, from 12 more to 54 more; Figure 6.1). In a pooled IPD analysis from the EVA-3S, SPACE, ICSS and CREST trials, the relative risk of this outcome varied with age87: this analysis provided moderate quality evidence that CEA was superior to CAS in patients aged 65–74 years (hazard ratio (HR): 1.67, 95% CI: 1.23–2.27) and ≥75 years (HR: 1.85, 95% CI: 1.35–2.53), and low quality evidence that there was no difference in outcomes between stenting and endarterectomy amongst patients <65 years old (HR: 0.83, 95% CI: 0.56–1.21), with a significant interaction between age and treatment effect (p = 0.003; data not shown in figure). There was no evidence of an interaction with sex or severity of stenosis.
Figure 1.3.
Long-term risk of major stroke, including peri-procedural death in endarterectomy versus medical therapy for asymptomatic carotid stenosis.
Figure 6.1.
Long-term risk of ipsilateral stroke, including peri-procedural stroke in any territory or peri-procedural death in stenting versus endarterectomy for symptomatic carotid stenosis.
PICO 6.2: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of post-procedural ipsilateral stroke?
There is moderate quality of evidence that stenting and endarterectomy do not differ in their ability to prevent long-term post-procedural ipsilateral stroke (RR: 1.06, 95% CI: 0.74–1.51; equivalent to 1 more event with stenting per 1000 patients, from 6 fewer to 12 more; Figure 6.2).
Figure 6.2.
Long-term risk of post-procedural ipsilateral stroke in stenting versus endarterectomy for symptomatic carotid stenosis.
PICO 6.3: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of stroke in any territory, including peri-procedural death?
There is moderate quality of evidence that endarterectomy is superior to stenting in preventing the combined long-term outcome of stroke in any territory or peri-procedural death (RR: 1.34, 95% CI: 1.08–1.66; 35 more events with stenting per 1000 patients, from 8 more to 68 more; Figure 6.3).
Figure 6.3.
Long-term risk of stroke in any territory, including peri-procedural death in stenting versus endarterectomy for symptomatic carotid stenosis.
PICO 6.4: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of major stroke, including peri-procedural death?
There is low quality of evidence that endarterectomy and stenting do not differ in the long-term risk of major stroke or peri-procedural death (RR: 1.19, 95% CI: 0.88–1.62; 12 more events with stenting per 1000 patients, from 8 fewer to 39 more; Figure 6.4).
Figure 6.4.
Long-term risk of major stroke, including peri-procedural death in stenting versus endarterectomy for symptomatic carotid stenosis.
PICO 6.5: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of death?
There is low quality of evidence that endarterectomy and stenting do not differ in the long-term risk of death (RR: 1.09, 95% CI: 0.94–1.27; 13 more events with stenting per 1000 patients, from 9 fewer to 38 more; Figure 6.5).
Figure 6.5.
Long-term risk of death in stenting versus endarterectomy for symptomatic carotid stenosis.
PICO 6.6: In patients with asymptomatic or symptomatic carotid stenosis, do endarterectomy and stenting differ in the long-term risk of severe restenosis?
For the analysis of restenosis, we combined the data from trials including patients with asymptomatic carotid stenosis, symptomatic stenosis, or both. There is very low quality evidence that endarterectomy and stenting do not differ in the long-term risk of severe restenosis (RR: 1.37, 95% CI: 0.89–2.10; Figure 6.6). We additionally downgraded the evidence for inconsistency, as there was evidence of substantial heterogeneity between trials (I2 = 57%).
Figure 6.6.
Long-term risk of severe restenosis in stenting versus endarterectomy for symptomatic or asymptomatic carotid stenosis.
PICO 6.7: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural stroke?
There is moderate quality of evidence that stenting is associated with a higher risk of peri-procedural stroke than endarterectomy (RR: 1.64, 95% CI: 1.24–2.17; 26 more events with stenting per 1000 patients, from 10 more to 48 more; Figure 6.7).
Figure 6.7.
Risk of peri-procedural stroke in stenting versus endarterectomy for symptomatic carotid stenosis.
PICO 6.8: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural death?
There is very low quality of evidence that stenting and endarterectomy do not differ in the risk of peri-procedural death (RR: 1.45, 95% CI: 0.73–2.87; 3 more events per 1000 patients with stenting, from 2 fewer to 10 more; Figure 6.8).
Figure 6.8.
Risk of peri-procedural death in stenting versus endarterectomy for symptomatic carotid stenosis.
PICO 6.9: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural stroke or death?
There is moderate quality evidence that stenting is associated with a higher risk of peri-procedural stroke or death than endarterectomy overall (RR: 1.68, 95% CI: 1.20–2.34; 28 more events with stenting per 1000 patients, from 8 more to 55 more; Figure 6.9). However, these results vary with age. Amongst patients ≥70 years, there is high quality evidence that CAS is associated with a higher risk of this composite outcome compared with CEA (RR: 2.10, 95% CI: 1.55–2.84; 53 more events with stenting per 1000 patients, from 26 more to 88 more; Figure 6.9.1). Amongst patients <70 years, there is low quality evidence that the risk of this combined outcome does not differ between the two treatment modalities (RR: 1.10, 95% CI: 0.75–1.60; 4 more events with stenting per 1000 patients, from 10 fewer to 24 more). The interaction between age and treatment effect is significant (p = 0.009). There is no evidence of an interaction with sex (Figure 6.9.2).
Figure 6.9.
Risk of peri-procedural stroke or death in stenting versus endarterectomy for symptomatic carotid stenosis.
Figure 6.9.1.
Risk of peri-procedural stroke or death in stenting versus endarterectomy for symptomatic carotid stenosis. Subgroup: Age.
Figure 6.9.2.
Risk of peri-procedural stroke or death in stenting versus endarterectomy for symptomatic carotid stenosis. Subgroup: Sex.
A pooled analysis of IPD from EVA-3S, SPACE and ICSS provides no evidence for a modification of the effect of CAS versus CEA on the risk of peri-procedural stroke or death by the severity of stenosis (Figure 6.9.3) or type of most recent ischaemic event (hemispheric stroke, transient ischaemic attack or ocular ischaemia; Figure 6.9.5).85
Figure 6.9.3.
Risk of peri-procedural stroke or death in stenting versus endarterectomy for symptomatic carotid stenosis. Subgroup: Severity of stenosis.
Another pooled analysis of IPD from EVA-3S, SPACE, ICSS and CREST provides high-quality evidence of an increased risk of peri-procedural stroke or death with CAS compared with CEA amongst patients treated <7 days after their most recent ischaemic event (RR: 6.30, 95% CI: 1.92–20.66; 70 more events with CAS per 1000 patients, from 12 more to 261 more; Figure 6.9.4), and moderate quality evidence for this difference amongst patients treated >7 days after the event (RR: 2.00, 95% CI: 1.50–2.68; 36 more events with stenting per 1000 patients, from 18 more to 60 more).86 The unadjusted p-value for the interaction between timing and treatment effect was 0.07, the adjusted p-value in the original publication was 0.06.
Figure 6.9.4.
Risk of peri-procedural stroke or death in stenting versus endarterectomy for symptomatic carotid stenosis. Subgroup: Time since last ischaemic event.
Figure 6.9.5.
Risk of peri-procedural stroke or death in stenting versus endarterectomy for symptomatic carotid stenosis. Subgroup: Type of last ischaemic event.
PICO 6.10: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural major stroke or death?
There is low quality of evidence that stenting is likely associated with an increased risk of peri-procedural major stroke or death (RR: 1.33, 95% CI: 0.96–1.85; 8 more events with stenting per 1000 patients, from 1 fewer to 21 more; Figure 6.10).
Figure 6.10.
Risk of peri-procedural major stroke or death in stenting versus endarterectomy for symptomatic carotid stenosis.
PICO 6.11: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural myocardial infarction?
There is moderate quality of evidence that stenting is associated with a lower risk of peri-procedural myocardial infarction than endarterectomy (RR: 0.48, 95% CI: 0.24–0.98; 6 fewer events with stenting per 1000 patients, from 9 fewer to 0 fewer; Figure 6.11). Even though the relative effect was large, there were a limited number of clinically relevant cardiac outcome events observed. Furthermore, we had additional concerns about ‘indirectness’ due to the definition of myocardial infarction used in the CREST trial which contributed to two thirds of the cardiac outcome events included in the aggregate analysis (see results section ‘Stenting or endarterectomy for asymptomatic carotid stenosis’).
Figure 6.11.
Risk of peri-procedural myocardial infarction in stenting versus endarterectomy for symptomatic carotid stenosis.
PICO 6.12: In patients with symptomatic carotid stenosis, do endarterectomy and stenting differ in the risk of peri-procedural cranial nerve injury?
There is strong evidence that stenting is associated with a lower risk of peri-procedural cranial nerve injury than endarterectomy (RR: 0.10, 95% CI: 0.05–0.20; 49 fewer events with stenting per 1000 patients, from 52 fewer to 44 fewer; Figure 6.12). We upgraded the quality of the evidence by two levels for strength of effect.
Figure 6.12.
Risk of peri-procedural cranial nerve injury in stenting versus endarterectomy for symptomatic carotid stenosis.
Analysis of current evidence and evidence-based recommendation
Evidence to compare short-term risks and long-term effects of CAS versus CEA for the treatment of symptomatic carotid stenosis was derived from 7 RCTs which included a total of 4893 patients. It is important to note that the available evidence for CAS relates to percutaneous trans-femoral stenting only. There are no available data from RCTs on the safety of trans-carotid stenting. As such, all recommendations included in this guideline refer to trans-femoral CAS. All studies included patients with ≥50% stenosis. Symptomatic carotid stenosis was defined by the occurrence of ischaemic ocular or cerebral events attributable to the stenosis within six months prior to enrolment, except in ICSS where a very small minority of patients were enrolled 6–12 months after symptom onset. Amongst the four largest trials contributing to the evidence, the median duration of follow-up was four to seven years in three studies and two years in one study. When recruitment in these trials started 20 years ago, carotid artery stenting was still at a relatively early stage of technical development, peri-procedural medication regimens were not standardised, and there was limited experience with the procedure. In addition, only a minority of patients included in these trials were treated within the recommended 14 days of their index ischaemic event. We therefore downgraded the quality of evidence for indirectness.
Overall, there is moderate quality evidence that endarterectomy is superior to stenting when one considers peri-procedural and post-procedural outcomes that were rated as ‘critical’ for decision making. The differences between stenting and endarterectomy are mainly apparent in the peri-procedural period: stenting is associated with a higher risk of peri-procedural stroke than endarterectomy (critical for decision making), whereas endarterectomy is associated with higher risks of myocardial infarction and mostly transient cranial nerve palsy (important for decision making).
The risks of peri-procedural stroke or death differ between patient subgroups: there is high quality evidence that stenting is associated with a higher risk of this outcome in patients ≥70 years, and low quality evidence that the risk of this outcome is similar in patients <70 years. The higher risk of peri-procedural stroke or death after carotid artery stenting compared with endarterectomy is also more evident amongst patients treated within seven days of their index cerebrovascular event. After the peri-procedural period, there is moderate grade evidence that stenting and endarterectomy do not differ in their ability to prevent stroke.
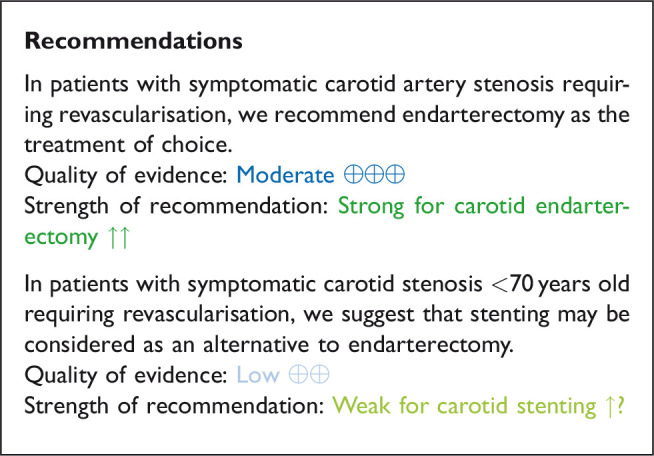
Additional information
In light of technical developments in stent design and cerebral protection devices, and alternative (trans-brachial and trans-carotid) access routes which are now available, new trials of stenting in selected patients with symptomatic carotid stenosis are warranted.
Expert consensus statements
12/12 experts concluded that the suitability of a patient with symptomatic carotid stenosis for carotid endarterectomy versus stenting should also take into account the interval since their last ischaemic cerebrovascular event, as well as anatomical and morphological features, including the atherosclerotic burden of the aortic arch.
11/12 experts concluded that the independently assessed risk of in-hospital stroke or death following endarterectomy or stenting for symptomatic carotid stenosis should not exceed 4%.6
12/12 experts concluded that where possible, the indication for carotid endarterectomy or carotid artery stenting should be discussed at a multi-disciplinary team meeting. Consensus decisions can be made in between meetings, in order not to delay urgent revascularisations.
12/12 experts concluded that the establishment of validated local, regional or national registries, including audit systems for carotid interventions to monitor complication rates in patients with asymptomatic and symptomatic carotid stenosis is recommended.
Discussion
This evidence-based guideline was developed following the GRADE process and provides recommendations for the treatment of symptomatic and asymptomatic carotid stenosis by endarterectomy (CEA) or stenting (CAS) versus best medical therapy alone. All recommendations and expert consensus statements are summarised in Tables 7 and 8.
Table 7.
Synoptic table of all recommendations.
| Recommendations | Quality of evidence | Strength of recommendation |
|---|---|---|
| In patients with ≥60% asymptomatic carotid artery stenosis considered to be at increased risk of stroke on best medical therapy alone, we recommend carotid endarterectomy. | Moderate ⊕⊕⊕ | Strong for carotid endarterectomy ↑↑ |
| In patients with asymptomatic carotid stenosis, recommend against carotid artery stenting as a routine alternative to best medical therapy alone. | Very low ⊕ | Weak against carotid stenting ↓ |
| In patients with asymptomatic carotid stenosis in whom revascularisation is considered to be appropriate, we suggest endarterectomy as the current treatment of choice. | Moderate ⊕⊕⊕ | Weak for carotid endarterectomy ↑ |
| In patients with severe (70–99%) symptomatic carotid artery stenosis, we recommend carotid endarterectomy. | Moderate ⊕⊕⊕ | Strong for carotid endarterectomy ↑↑ |
| In patients with moderate (50–69%) symptomatic carotid artery stenosis, we suggest carotid endarterectomy. | Low ⊕⊕ | Weak for carotid endarterectomy ↑ |
| In patients with mild (<50%) symptomatic carotid artery stenosis, we recommend against carotid endarterectomy. | Very low ⊕ | Strong against carotid endarterectomy ↓↓ |
| In patients with 50–99% symptomatic carotid stenosis in whom surgery is considered appropriate, we recommend early endarterectomy, ideally within two weeks of the last neurological event. | High ⊕⊕⊕⊕ | Strong for carotid endarterectomy ↑↑ |
| In patients with symptomatic carotid artery stenosis requiring revascularisation, we recommend endarterectomy as the treatment of choice. | Moderate ⊕⊕⊕ | Strong for carotid endarterectomy ↑↑ |
| In patients with symptomatic carotid stenosis <70 years old requiring revascularisation, we suggest that stenting may be considered as an alternative to endarterectomy. | Low ⊕⊕ | Weak for carotid stenting ↑ |
Table 8.
Synoptic table of all expert consensus statements.
| Expert consensus statements Based on voting by all MWG members | Voting results |
|---|---|
| In selected patients 75 years of age or older with ≥60% asymptomatic carotid artery stenosis and an expected survival of at least five years, who are considered to be at an increased risk of stroke on best medical therapy alone, carotid endarterectomy is suggested after careful consideration of the risks and benefits at a multi-disciplinary team meeting. | 12/12 |
| In patients with asymptomatic carotid stenosis in whom revascularisation is considered to be appropriate and who are less suitable for surgery, stenting may be suggested. We recommend careful consideration of the risks and benefits at a multi-disciplinary team meeting. | 12/12 |
| The independently assessed risk of in-hospital stroke or death following endarterectomy or stenting for asymptomatic carotid stenosis should be as low as possible, ideally below 2%. | 12/12 |
| The suitability of a patient with symptomatic carotid stenosis for carotid endarterectomy versus stenting should also take into account the interval since their last ischaemic cerebrovascular event, as well as anatomical and morphological features, including the atherosclerotic burden of the aortic arch. | 12/12 |
| The independently assessed risk of in-hospital stroke or death following endarterectomy or stenting for symptomatic carotid stenosis should be as low as possible, ideally below 4%. | 11/12 |
| Where possible, the indication for carotid endarterectomy or carotid artery stenting should be discussed at a multi-disciplinary team meeting. Consensus decisions can be made in between meetings, in order not to delay urgent revascularisations. | 12/12 |
| 12/12 experts concluded that the establishment of validated local, regional or national registries, including audit systems for carotid interventions to monitor complication rates in patients with asymptomatic and symptomatic carotid stenosis is recommended. | 12/12 |
MWG: Module Working Group.
Carotid revascularisation has been studied in randomised clinical trials for more than three decades, providing a wealth of evidence. Observational case series and large-scale registries are important to advance treatments and provide contemporary data on risks in real-world settings, but ultimately, the choice between treatment options should be informed by evidence from high quality RCTs, where such trials are available. We therefore based our recommendations in this guideline document for the choice between medical therapy alone, CEA or CAS on the evidence derived from randomised clinical trials only.
In some areas, particularly for stenting of asymptomatic carotid stenosis, the available evidence from clinical trials is still limited. However, additional data from large trials in asymptomatic carotid stenosis which are currently ongoing are expected in the near future and should provide a stronger evidence base to guide management of these patients.
CAS and CEA differ in treatment-associated risks, such as myocardial infarction and stroke. To fully determine the overall clinical impact of these outcomes in patients, additional measures such as quality of life and level of dependency should be systematically assessed in future trials.
We also acknowledge the fact that many of the trials providing the evidence for these guidelines were performed two to three decades ago. There have been important advances in the medical management of patients with atherosclerosis, and technical developments have also improved the safety of CEA and CAS since then. Because we had some concerns – especially in patients with asymptomatic carotid stenosis – that the applicability of the findings obtained in earlier trials may not apply to current clinical practice with contemporary medical and interventional treatment, we reduced the grade of some of the evidence for ‘indirectness’.
Any benefit of CEA or CAS is closely related to peri-procedural complication rates. Since the in-hospital complication rates of CEA and CAS have improved in recent years, expert consensus statements were prepared which suggested that the independently-assessed peri-operative stroke and death rates after CEA or CAS should ideally be below 2% in patients with asymptomatic carotid stenosis and below 4% in patients symptomatic carotid stenosis. In randomised trials, about two thirds of these events occurred in the first two days after treatment, when patients were typically still in hospital.91 Therefore these proposed acceptable in-hospital thresholds of 2% and 4% correspond with the traditionally-recommended 30-day thresholds of 3% and 6% for patients with asymptomatic and symptomatic stenosis, respectively. In-hospital thresholds may be more easily applicable to routine clinical practice because many patients will not be independently assessed by a neurologist or stroke physician 30 days after intervention. Moreover, outcomes following CEA and CAS should ideally be analysed at a local, regional and national level.
With modern medical management aiming for lower targets for lipid and blood pressure control, and more effective antiplatelet regimens (especially in patients with recent symptoms), the risk of stroke in asymptomatic and symptomatic carotid stenosis is expected to be lower than in the medical arms of some prior published trials. Ongoing trials are investigating whether contemporary medical therapy may obviate the need for invasive revascularisation in selected patient groups.
There have been a number of developments in the field of carotid artery stenting since the first trials which compared stenting with endarterectomy were completed, including the design of closed-cell and mesh-design stents,92,93 newer approaches to cerebral protection (involving reversal or arrest of blood flow),94–105 and alternative access routes which avoid the aortic arch (including trans-brachial and trans-carotid access.106–110 In addition, quality assurance programmes for stenting have been introduced in some countries.111 For patients with symptomatic stenosis, the restriction to the evidence from past randomised trials may underestimate the role of CAS in experienced centres who are able to maintain low peri-procedural complication rates. Although stenting using more modern state-of-the-art techniques might reduce the peri-procedural risk of stroke, this needs to be tested in randomised trials of CAS versus CEA. Until further evidence is available, in patients requiring carotid revascularisation, the current weight of evidence is in favour of recommending CEA over CAS in most patient subgroups.
Plain language summary
Carotid stenosis refers to narrowing of a major blood vessel in the neck (the carotid artery) which carries blood to the eye and brain and is caused by fatty and calcium deposits in the blood vessel wall (atherosclero-tic plaque). Carotid stenosis may cause a transient ischaemic attack (TIA or ‘warning stroke’) or a stroke. The narrowing can be removed by a surgical procedure called ‘carotid endarterectomy’, during which the surgeon opens the artery and removes the carotid plaque. An alternative treatment, called ‘caro-tid artery stenting’, involves passing a fine wire and tube through the skin and into the narrowed artery in the neck. A metal tube (stent) is placed inside the carotid artery to open it up with a view to preventing it from narrowing again. In patients who have not experienced recent symptoms (such as stroke, TIA, or ocular (eye) symptoms) from their carotid stenosis (‘asymptomatic patients’), but who are still considered to be at risk of stroke on medication alone, we recommend carotid endarterectomy. In patients who have recently experienced these symptoms (‘symptomatic patients’), we recommend carotid endarterectomy if the stenosis is severe, and suggest carotid endarterectomy may be considered if the stenosis is moderate. If surgery is recommended, we advise that carotid endarterectomy should be carried out as early as possible after the patient’s initial symptoms, preferably within two weeks. Carotid artery stenting can be considered as an option to carotid endarterectomy in patients with symptomatic carotid stenosis, especially in patients younger than 70 years of age.
| Name | Conflicts of interest |
|---|---|
| Leo H Bonati | Co-Principal Investigator of the ECST-2 and ACST-2 trial. Grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the University of Basel and AstraZeneca for research related to carotid artery disease. Consultancy fees from AstraZeneca. |
| Joachim Berkefeld | Unrelated grants for angiographic imaging research from Siemens. |
| Gert J de Borst | Member of the writing committee of the ESVS guideline on the management of carotid atherosclerotic disease; EU Horizon 2020 grant for stratification of carotid disease; consultation fees from BAYER; Steering Committee Member ECST-2, CSTC. |
| Richard Bulbulia | Co-PI ACST-2, Co-Chair, ESC Position Paper on Carotid Disease |
| Hans-Henning Eckstein | Steering Committee SPACE-1, Co-PI SPACE-2, CSTC, Co-Investigator ROADSTER 1 and ROADSTER 2, Co-Investigator ACST-2, EU Horizon 2020 grant for stratification of carotid disease |
| Alison Halliday | PI for the ACST trials, Steering committee member for ECST-2, past TSC member ICSS. Chair-Elect of ESC Council on Stroke, co-author on the ESVS Carotid Guidelines, Co-author ESC Guidelines on Dyslipidaemias, CSTC |
| Isabelle van Herzeele | Consulting and Research Grants from Silkroad Medical, Medtronic and Impact APV Europe. |
| Stavros Kakkos | Member of the writing committee of the ESVS guideline on the management of carotid atherosclerotic disease. |
| Igor Koncar | EU Horizon 2020 grant for stratification of carotid disease, Member of the writing committee of the ESVS guideline on the management of carotid atherosclerotic disease |
| Dominick JH McCabe | Prior grant funding from: The Meath Foundation; The Irish Institute of Clinical Neuroscience; The Irish Heart Foundation Stroke Prevention Bursary programme; The Trinity College Dublin Innovation Bursary; The Vascular Neurology Research Foundation, Ireland. Unrestricted educational grant funding from: Biogen Idec, Ireland; Verum Diagnostica, GmbH; Bayer HealthCare, Ireland; and SINNOWA Medical Science & Technology Co., China for unrelated translational research studies. Member of the writing committee for the ESVS guidelines for the management of patients with atherosclerotic carotid and vertebral artery stenosis (2017 and 2022). |
| Jean-Baptiste Ricco | Grant and consultation fees from BAYER, Member of the writing committee of the ESVS guideline on the management of carotid atherosclerotic disease |
| Peter Ringleb | Steering Committee Member SPACE-2, CSTC. Unrelated grant from Boehringer Ingelheim (ECASS-4). Unrelated lecture fees from Boehringer Ingelheim, Bayer, Pfizer, Daiichi Sankyo. |
Acknowledgements
We would like to acknowledge Guillaume Turc (chair of the ESO guidelines board), Thorsten Steiner (former chair of the ESO guidelines board), Anne Hege Aamodt and Terry Quinn for reviewing the PICOs; and Bart van der Worp (past president of the ESO), Simona Sacco (the co-chair of the ESO guidelines board), Anne Hege Aamodt, Christian Nolte, Laura Capoccia and George Hamilton for reviewing the final text. We would also like to acknowledge Luzia Balmer for her excellent administrative support.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the development of these guidelines was provided by the European Stroke Organisation, Basel, Switzerland.
Informed consent: Not applicable.
Ethical approval: Ethical approval was not necessary for the work described in this paper.
Guarantor: A specific guarantor does not exist. The Module Working Group has jointly developed the manuscript.
Contributorship: All listed authors have contributed to the preparation and writing of the manuscript, researched literature and conceived the guideline, were involved in protocol development, reviewed and edited the manuscript and approved the final version of the manuscript. LHB, SK and JB wrote the first draft of the manuscript.
ORCID iD: Isabelle van Herzeele https://orcid.org/0000-0002-1754-7390
Supplemental material: Supplemental material for this article is available online.
References
- 1.Petty GW, Brown RD, Jr, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999; 30: 2513–2516. [DOI] [PubMed] [Google Scholar]
- 2.Fisher CM, Gore I, Okabe N, et al. Atherosclerosis of the carotid and vertebral arteries—extracranial and intracranial. J Neuropathol Exp Neurol 1965; 24: 455–476. [Google Scholar]
- 3.de Weerd M, Greving JP, Hedblad B, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke 2010; 41: 1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naylor AR, Ricco JB, de Borst GJ, et al. Editor’s choice – management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55: 3–81. [DOI] [PubMed] [Google Scholar]
- 5.Aboyans V, Ricco JB, Bartelink MEL, et al.; ESC Scientific Document Group. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European stroke organization (ESO) the task force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (ESC) and of the European society for vascular surgery (ESVS). Eur Heart J 2018; 39: 763–816. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein HH, Kühnl A, Berkefeld J, et al. Diagnosis, treatment and follow-up in extracranial carotid stenosis. Dtsch Arztebl Int 2020; 117: 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol 2011; 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 8.Ntaios G, Bornstein NM, Caso V, et al.; European Stroke Organisation. The European stroke organisation guidelines: a standard operating procedure. Int J Stroke 2015; 10Suppl A100: 128–135. [DOI] [PubMed] [Google Scholar]
- 9.North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991; 22: 711–720. [DOI] [PubMed] [Google Scholar]
- 10.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 11.Chambers BR, Donnan GA.Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 2005: CD001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rerkasem A, Orrapin S, Howard DP, et al. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev 2020; 9: CD001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller MD, Lyrer P, Brown MM, et al. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. Cochrane Database Syst Rev 2020; 2: CD000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Altman DG, Gøtzsche PC, et al.; Cochrane Statistical Methods Group. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, www.training.cochrane.org./handbook (2019, accessed 16 April 2021).
- 16.Hobson RWI, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. N Engl J Med 1993; 328: 221–227. [DOI] [PubMed] [Google Scholar]
- 17.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995; 273: 1421–1428. [PubMed] [Google Scholar]
- 18.MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004; 363: 1491–1502. [DOI] [PubMed] [Google Scholar]
- 19.Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376: 1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolos I, Troitskiy A, Balakhonova T, et al.; Aggressive Medical Treatment Evaluation for Asymptomatic Carotid Artery Stenosis (AMTEC) Study Group. Modern medical treatment with or without carotid endarterectomy for severe asymptomatic carotid atherosclerosis. J Vasc Surg 2015; 62: 914–922. [DOI] [PubMed] [Google Scholar]
- 21.Reiff T, Stingele R, Eckstein HH, et al. Stent-protected angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy: SPACE2 – a three-arm randomised-controlled clinical trial. Int J Stroke 2009; 4: 294–299. [DOI] [PubMed] [Google Scholar]
- 22.Reiff T, Eckstein HH, Mansmann U, et al. Angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy compared to best medical treatment: one-year interim results of SPACE-2. Int J Stroke2020; 15: 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard DPJ, Gaziano L, Rothwell PM.Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol 2021; 20: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the asymptomatic carotid emboli study (ACES): a prospective observational study. Lancet Neurol 2010; 9: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquardt L, Geraghty OC, Mehta Z, et al. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41: e11–e17. [DOI] [PubMed] [Google Scholar]
- 26.Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67: 180–186. [DOI] [PubMed] [Google Scholar]
- 27.Cheng SF, Brown MM.Contemporary medical therapies of atherosclerotic carotid artery disease. Semin Vasc Surg 2017; 30: 8–16. [DOI] [PubMed] [Google Scholar]
- 28.Mott M, Koroshetz W, Wright CB.CREST-2: identifying the best method of stroke prevention for carotid artery stenosis. Stroke 2017; 48: e130–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakkos SK, Sabetai M, Tegos T, et al. Silent embolic infarcts on computed tomography brain scans and risk of ipsilateral hemispheric events in patients with asymptomatic internal carotid artery stenosis. J Vasc Surg 2009; 49: 902–909. [DOI] [PubMed] [Google Scholar]
- 30.Kakkos SK, Nicolaides AN, Charalambous I, et al.; Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Predictors and clinical significance of progression or regression of asymptomatic carotid stenosis. J Vasc Surg 2014; 59: 956.e1–967.e1. [DOI] [PubMed] [Google Scholar]
- 31.Hirt LS.Progression rate and ipsilateral neurological events in asymptomatic carotid stenosis. Stroke 2014; 45: 702–706. [DOI] [PubMed] [Google Scholar]
- 32.Kakkos SK, Griffin MB, Nicolaides AN, et al. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg 2013; 57: 609–618. [DOI] [PubMed] [Google Scholar]
- 33.Nicolaides AN, Kakkos SK, Kyriacou E, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010; 52: 1486–1496. [DOI] [PubMed] [Google Scholar]
- 34.Gupta A, Kesavabhotla K, Baradaran H, et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke 2015; 46: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schindler A, Schinner R, Altaf N, et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: meta-analysis of individual patient data. JACC Cardiovascular Imaging 2020; 13: 395–406. [DOI] [PubMed] [Google Scholar]
- 36.King A, Serena J, Bornstein NM, et al.; ACES Investigators. Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis? A prospective substudy of the asymptomatic carotid emboli study. Stroke 2011; 42: 1550–1555. [DOI] [PubMed] [Google Scholar]
- 37.Zhao XL, Jia JP Ji XM, et al. A follow-up: stroke in patients with bilateral severe carotid stenosis after intervention treatment. Chin J Clin Rehabil 2003; 7: 2714–2715. [Google Scholar]
- 38.Ederle J, Featherstone RL, Brown MM; CAVATAS Orators. Long-term outcome of endovascular treatment versus medical care for carotid artery stenosis in patients not suitable for surgery and randomised in the carotid and vertebral artery transluminal angioplasty study (CAVATAS). Cerebrovasc Dis 2009; 28: 1–7. [DOI] [PubMed] [Google Scholar]
- 39.Brooks WH, McClure RR, Jones MR, et al. Carotid angioplasty and stenting versus carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery 2004; 54: 318–324. [DOI] [PubMed] [Google Scholar]
- 40.Brooks WH, Jones MR, Gisler P, et al. Carotid angioplasty with stenting versus endarterectomy: 10-year randomized trial in a community hospital. JACC Cardiovasc Interv 2014; 7: 163–168. [DOI] [PubMed] [Google Scholar]
- 41.Hobson RW.Update on the carotid revascularization endarterectomy versus stent trial (CREST) protocol. J Am Coll Surg 2002; 194: S9–S14. [DOI] [PubMed] [Google Scholar]
- 42.Hobson RW.CREST (carotid revascularization endarterectomy versus stent trial): background, design, and current status. Semin Vasc Surg 2000; 13: 139–143. [PubMed] [Google Scholar]
- 43.Sheffet AJ, Roubin G, Howard G, et al. Design of the carotid revascularization endarterectomy vs. stenting trial (CREST). Int J Stroke 2010; 5: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brott TG, Hobson RW, Howard G, et al.; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silver FL, Mackey A, Clark WM, et al. Safety of stenting and endarterectomy by symptomatic status in the carotid revascularization endarterectomy versus stenting trial (CREST). Stroke 2011; 42: 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard VJ, Lutsep HL, Mackey A, et al.; CREST investigators. Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the carotid revascularization endarterectomy versus stenting trial (CREST). Lancet Neurol 2011; 10: 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lal BK, Beach KW, Roubin GS, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol 2012; 11: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brott TG, Howard G, Roubin GS, et al.; CREST Investigators. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med 2016; 374: 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kougias P, Collins R, Pastorek N, et al. Comparison of domain-specific cognitive function after carotid endarterectomy and stenting. J Vasc Surg 2015; 62: 355–361. [DOI] [PubMed] [Google Scholar]
- 50.Kuliha M, Roubec M, Prochazka V, et al. Randomized clinical trial comparing neurological outcomes after carotid endarterectomy or stenting. Br J Surg 2015; 102: 194–201. [DOI] [PubMed] [Google Scholar]
- 51.Rosenfield K, Matsumura JS, Chaturvedi S, et al.; ACT I Investigators. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med 2016; 374: 1011–1020. [DOI] [PubMed] [Google Scholar]
- 52.Mannheim D, Karmeli R.A prospective randomized trial comparing endarterectomy to stenting in severe asymptomatic carotid stenosis. J Cardiovasc Surg 2017; 58: 814–817. [DOI] [PubMed] [Google Scholar]
- 53.Gurm HS, Yadav JS, Fayad P, et al.; SAPPHIRE Investigators. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med 2008; 358: 1572–1579. [DOI] [PubMed] [Google Scholar]
- 54.Yadav JS, Wholey MH, Kuntz RE, et al.; Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351: 1493–1501. [DOI] [PubMed] [Google Scholar]
- 55.Yadav JS.Carotid stenting in high-risk patients: design and rationale of the SAPPHIRE trial. Cleve Clin J Med 2004; 71Suppl 1: S45–S46. [DOI] [PubMed] [Google Scholar]
- 56.Ling F, Jiao LQ.Preliminary report of trial of endarterectomy versus stenting for the treatment of carotid atherosclerotic stenosis in China (TESCAS-C). Chin J Cerebrovasc Dis 2006; 3: 4–8. [Google Scholar]
- 57.Liu CW, Liu B, Ye W, et al. Carotid endarterectomy versus carotid stenting: a prospective randomized trial. Zhonghua Wai KeZa Zhi 2009; 47: 267–270. [PubMed] [Google Scholar]
- 58.Wang P, Liang C, Du J, et al. Effects of carotid endarterectomy and carotid artery stenting on high-risk carotid stenosis patients. Pak J Med Sci 2013; 29: 1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudarakanchana N, Dialynas M, Halliday A.Asymptomatic carotid surgery trial-2 (ACST-2): rationale for a randomised clinical trial comparing carotid endarterectomy with carotid artery stenting in patients with asymptomatic carotid artery stenosis. Eur J Vasc Endovasc Surg 2009; 38: 239–242. [DOI] [PubMed] [Google Scholar]
- 60.Barnett HJM, Taylor DW, Haynes RB, et al.; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 61.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American symptomatic carotid endarterectomy trial collaborators. N Engl J Med 1998; 339: 1415–1425. [DOI] [PubMed] [Google Scholar]
- 62.European Carotid Surgery Trialists’ Collaborative Group. MRC European carotid surgery trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991; 337: 1235–1243. [PubMed] [Google Scholar]
- 63.European Carotid Surgery Trialists’ Collaborative Group. Endarterectomy for moderate symptomatic carotid stenosis: interim results from the MRC European carotid surgery trial. Lancet 1996; 347: 1591–1593. [PubMed] [Google Scholar]
- 64.European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European carotid surgery trial (ECST). Lancet 1998; 351: 1379–1387. [PubMed] [Google Scholar]
- 65.Mayberg MR, Wilson SE, Yatsu F, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA 1991; 266: 3289–3294. [PubMed] [Google Scholar]
- 66.Rothwell PM, Eliasziw M, Gutnikov SA, et al.; Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004; 363: 915–924. [DOI] [PubMed] [Google Scholar]
- 67.Johansson E, Fox AJ.Carotid near-occlusion: a comprehensive review, part 1–definition, terminology, and diagnosis. AJNR Am J Neuroradiol 2016; 37: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kappelle LJ, Eliasziw M, Fox AJ, et al. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. The North American symptomatic carotid endarterectomy trail. Stroke 1999; 30: 282–286. [DOI] [PubMed] [Google Scholar]
- 69.Brooks WH, McClure RR, Jones MR, et al. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol 2001; 38: 1589–1595. [DOI] [PubMed] [Google Scholar]
- 70.Mas JL, Chatellier G, Beyssen B; EVA-3S Investigators. Carotid angioplasty and stenting with and without cerebral protection: clinical alert from the endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial. Stroke 2004; 35: e18–e20. [DOI] [PubMed] [Google Scholar]
- 71.EVA-3S Investigators. Endarterectomy vs. Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial . Cerebrovasc Dis 2004; 18: 62–65. [DOI] [PubMed] [Google Scholar]
- 72.Mas JL, Chatellier G, Beyssen B, et al.; EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 2006; 355: 1660–1671. [DOI] [PubMed] [Google Scholar]
- 73.Mas JL, Trinquart L, Leys D, et al.; EVA-3S investigators. Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol 2008; 7: 885–892. [DOI] [PubMed] [Google Scholar]
- 74.Arquizan C, Trinquart L, Touboul PJ, et al.; EVA-3S Investigators. Restenosis is more frequent after carotid stenting than after endarterectomy: the EVA-3S study. Stroke 2011; 42: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 75.Mas JL, Arquizan C, Calvet D, et al.; EVA-3S Investigators. Long-term follow-up study of endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis trial. Stroke 2014; 45: 2750–2756. [DOI] [PubMed] [Google Scholar]
- 76.Ringleb PA, Kunze A, Allenberg JR, et al.; Steering Committee of the SPACE Study. The stent-supported percutaneous angioplasty of the carotid artery vs. endarterectomy trial. Cerebrovasc Dis 2004; 18: 66–68. [DOI] [PubMed] [Google Scholar]
- 77.Ringleb PA, Allenberg J, Bruckmann H, et al. 30 Day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 2006; 368: 1239–1247. [DOI] [PubMed] [Google Scholar]
- 78.Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the stent-protected angioplasty versus carotid endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008; 7: 893–902. [DOI] [PubMed] [Google Scholar]
- 79.Steinbauer MG, Pfister K, Greindl M, et al. Alert for increased long-term follow-up after carotid artery stenting: results of a prospective, randomized, single-center trial of carotid artery stenting vs carotidendarterectomy. Journal of Vascular Surgery 2008; 48: 93–8. [DOI] [PubMed] [Google Scholar]
- 80.Featherstone RL, Brown MM, Coward LJ; ICSS Investigators. International carotid stenting study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis 2004; 18: 69–74. [DOI] [PubMed] [Google Scholar]
- 81.Ederle J, Dobson J, Featherstone RL, et al.; International Carotid Stenting Study investigators. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (international carotid stenting study): an interim analysis of a randomised controlled trial. Lancet 2010; 375: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the international carotid stenting study (ICSS) randomised trial. Lancet 2015; 385: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonati LH, Gregson J, Dobson J, et al. Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the international carotid stenting study (ICSS): secondary analysis of a randomised trial. Lancet Neurol 2018; 17: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffmann A, Taschner C, Engelter ST, et al. Carotid artery stenting versus carotid endarterectomy. A prospective, randomised trial with long termfollow up (BACASS). SchweizerArchiv Für Neurologie Und Psychiatrie 2006; 157: 191. [Google Scholar]
- 85.Bonati LH, Dobson J, Algra A, et al.; Carotid Stenting Trialists' Collaboration. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010; 376: 1062–1073. [DOI] [PubMed] [Google Scholar]
- 86.Rantner B, Kollerits B, Roubin GS, et al.; Carotid Stenosis Trialists’ Collaboration. Early endarterectomy carries a lower procedural risk than early stenting in patients with symptomatic stenosis of the internal carotid artery: results from 4 randomized controlled trials. Stroke 2017; 48: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 87.Brott TG, Calvet D, Howard G, et al.; Carotid Stenosis Trialists’ Collaboration. Long-term outcomes of stenting and endarterectomy for symptomatic carotid stenosis: a preplanned pooled analysis of individual patient data. Lancet Neurol 2019; 18: 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alberts MJ.Results of a multicentre prospective randomized trial of carotid artery stenting vs carotid endarterectomy. Stroke 2001; 32: 325. [Google Scholar]
- 89.Investigators C.Endovascular versus surgical treatment in patients with carotid stenosis in the carotid and vertebral artery transluminal angioplasty study (CAVATAS): a randomised trial. Lancet 2001; 357: 1729–1737. [PubMed] [Google Scholar]
- 90.Naylor AR, Bolia A, Abbott RJ, et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg 1998; 28: 326–334. [DOI] [PubMed] [Google Scholar]
- 91.Muller MD, von Felten S, Algra A, et al.; Carotid Stenosis Trialists’ Collaboration. Immediate and delayed procedural stroke or death in stenting versus endarterectomy for symptomatic carotid stenosis. Stroke 2018; 49: 2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Speziale F, Capoccia L, Sirignano P, et al. Thirty-day results from prospective multi-specialty evaluation of carotid artery stenting using the CGuard MicroNet-covered embolic prevention system in real-world multicentre clinical practice: the IRON-Guard study. EuroIntervention 2018; 13: 1714–1720. [DOI] [PubMed] [Google Scholar]
- 93.Broussalis E, Griessenauer C, Mutzenbach S, et al. Reduction of cerebral DWI lesion burden after carotid artery stenting using the CASPER stent system. J Neurointerv Surg 2019; 11: 62–67. [DOI] [PubMed] [Google Scholar]
- 94.Parodi JC, Schonholz C, Parodi FE, et al. Initial 200 cases of carotid artery stenting using a reversal-of-flow cerebral protection device. J Cardiovasc Surg (Torino) 2007; 48: 117–124. [PubMed] [Google Scholar]
- 95.Asakura F, Kawaguchi K, Sakaida H, et al. Diffusion-weighted MR imaging in carotid angioplasty and stenting with protection by the reversed carotid arterial flow. AJNR Am J Neuroradiol 2006; 27: 753–758. [PMC free article] [PubMed] [Google Scholar]
- 96.El-Koussy M, Schroth G, Do DD, et al. Periprocedural embolic events related to carotid artery stenting detected by diffusion-weighted MRI: comparison between proximal and distal embolus protection devices. J Endovasc Ther 2007; 14: 293–303. [DOI] [PubMed] [Google Scholar]
- 97.Faraglia V, Palombo G, Stella N, et al. Cerebral embolization during transcervical carotid stenting with flow reversal: a diffusion-weighted magnetic resonance study. Ann Vasc Surg 2009; 23: 429–435. [DOI] [PubMed] [Google Scholar]
- 98.Leal JI, Orgaz A, Fontcuberta J, et al. A prospective evaluation of cerebral infarction following transcervical carotid stenting with carotid flow reversal. Eur J Vasc Endovasc Surg 2010; 39: 661–666. [DOI] [PubMed] [Google Scholar]
- 99.Pinter L, Ribo M, Loh C, et al. Safety and feasibility of a novel transcervical access neuroprotection system for carotid artery stenting in the PROOF study. J Vasc Surg 2011; 54: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 100.Clair DG, Hopkins LN, Mehta M, et al.; EMPiRE Clinical Study Investigators. Neuroprotection during carotid artery stenting using the GORE flow reversal system: 30-day outcomes in the EMPiRE clinical study. Catheter Cardiovasc Interv 2011; 77: 420–429. [DOI] [PubMed] [Google Scholar]
- 101.Nikas D, Reith W, Schmidt A, et al. Prospective, multicenter european study of the GORE flow reversal system for providing neuroprotection during carotid artery stenting. Catheter Cardiovasc Interv 2012; 80: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 102.Leal I, Orgaz A, Flores A, et al. A diffusion-weighted magnetic resonance imaging-based study of transcervical carotid stenting with flow reversal versus transfemoral filter protection. J Vasc Surg 2012; 56: 1585–1590. [DOI] [PubMed] [Google Scholar]
- 103.Mokin M, Dumont TM, Chi JM, et al. Proximal versus distal protection during carotid artery stenting: analysis of the two treatment approaches and associated clinical outcomes. World Neurosurg 2014; 81: 543–548. [DOI] [PubMed] [Google Scholar]
- 104.Bijuklic K, Wandler A, Hazizi F, et al. The PROFI study (prevention of cerebral embolization by proximal balloon occlusion compared to filter protection during carotid artery stenting): a prospective randomized trial. J Am Coll Cardiol 2012; 59: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 105.Ansel GM, Hopkins LN, Jaff MR, et al.; Investigators for the ARMOUR Pivotal Trial. Safety and effectiveness of the INVATEC MO.MA proximal cerebral protection device during carotid artery stenting: results from the ARMOUR pivotal trial. Catheter Cardiovasc Interv 2010; 76: 1–8. [DOI] [PubMed] [Google Scholar]
- 106.Kühn AL, Singh J, Moholkar VM, et al. Distal radial artery (snuffbox) access for carotid artery stenting – technical pearls and procedural set-up. Interv Neuroradiol 2021; 27: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palombo G, Stella N, Faraglia V, et al. Cervical access for filter-protected carotid artery stenting: a useful tool to reduce cerebral embolisation. Eur J Vasc Endovasc Surg 2010; 39: 252–257. [DOI] [PubMed] [Google Scholar]
- 108.Kwolek CJ, Jaff MR, Leal JI, et al. Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal. J Vasc Surg 2015; 62: 1227–1234. [DOI] [PubMed] [Google Scholar]
- 109.Plessers M, Van Herzeele I, Hemelsoet D, et al. Transcervical carotid stenting with dynamic flow reversal demonstrates embolization rates comparable to carotid endarterectomy. J Endovasc Ther 2016; 23: 249–254. [DOI] [PubMed] [Google Scholar]
- 110.Malas MB, Dakour-Aridi H, Wang GJ, et al. Transcarotid artery revascularization versus transfemoral carotid artery stenting in the society for vascular surgery vascular quality initiative. J Vasc Surg 2019; 69: 92.e2–103.e2. [DOI] [PubMed] [Google Scholar]
- 111.Kallmayer MA, Tsantilas P, Knappich C, et al. Patient characteristics and outcomes of carotid endarterectomy and carotid artery stenting: analysis of the German mandatory national quality assurance registry – 2003 to 2014. J Cardiovasc Surg (Torino) 2015; 56: 827–836. [PubMed] [Google Scholar]