Abstract
Space-occupying brain oedema is a potentially life-threatening complication in the first days after large hemispheric or cerebellar infarction. Several treatment strategies for this complication are available, but the size and quality of the scientific evidence on which these strategies are based vary considerably. The aim of this Guideline document is to assist physicians in their management decisions when treating patients with space-occupying hemispheric or cerebellar infarction. These Guidelines were developed based on the European Stroke Organisation (ESO) standard operating procedure and followed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach. A working group identified 13 relevant questions, performed systematic reviews and meta-analyses of the literature, assessed the quality of the available evidence, and wrote evidence-based recommendations. An expert consensus statement was provided if not enough evidence was available to provide recommendations based on the GRADE approach. We found high-quality evidence to recommend surgical decompression to reduce the risk of death and to increase the chance of a favourable outcome in adult patients aged up to and including 60 years with space-occupying hemispheric infarction who can be treated within 48 hours of stroke onset, and low-quality evidence to support this treatment in older patients. There is continued uncertainty about the benefit and risks of surgical decompression in patients with space-occupying hemispheric infarction if this is done after the first 48 hours. There is also continued uncertainty about the selection of patients with space-occupying cerebellar infarction for surgical decompression or drainage of cerebrospinal fluid. These Guidelines further provide details on the management of specific subgroups of patients with space-occupying hemispheric infarction, on the value of monitoring of intracranial pressure, and on the benefits and risks of medical treatment options. We encourage new high-quality studies assessing the risks and benefits of different treatment strategies for patients with space-occupying brain infarction.
Keywords: Guideline, acute ischaemic stroke, hemicraniectomy, oedema
Introduction
Space-occupying brain oedema is a potentially life-threatening complication in the first few days after large hemispheric or cerebellar infarction.1,2 With conservative treatment alone, death rates of up to 80% have been reported.1–3 Randomised trials have shown that surgical decompression, consisting of a large hemicraniectomy and duraplasty, reduces the risk of death in patients with space-occupying hemispheric infarction.4–12 In meta-analyses of these trials, surgery also increased the chance of a favourable outcome, defined as a score on the modified Rankin Scale (mRS) of 3 or lower.3,13 However, debate on the use of surgical decompression in specific patient subgroups, such as those with aphasia or aged over 60 years, still continues. This also applies to the use of surgical options for space-occupying cerebellar infarction, to monitoring of intracranial pressure (ICP), and to medical treatment options, such as osmotic therapy.
The European Stroke Organisation (ESO) decided to provide guidelines on the management of space-occupying brain infarction based on a systematic literature review and on the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system.14,15 The aim of this Guideline document is to assist physicians in the management of patients with space-occupying brain infarction.
Methods
A module working group (MWG) composed of JH, EJ, PM, PS, SS, TS, GT, and HBvdW (MWG leader) was formed and approved by the ESO Guideline Board and Executive Committee based on the review of the intellectual and financial disclosures of all MWG members (Supplemental Table 1). AL participated as methodologist. These guidelines were prepared based on the GRADE methodology and the ESO standard operating procedure, the last with modifications.14,15
The steps undertaken by the working group are summarised as follows:
A list of topics of clinical interest for Guidelines’ users was produced and agreed by the MWG members.
For each of these topics, a list of relevant outcomes was produced and rated according to GRADE definitions as critical, important or of limited importance.14,15 Functional outcome as assessed with the mRS and survival ultimately were the only outcomes rated as of critical importance. ‘Favourable outcome’ was defined as a score on the mRS ≤3 at one year, or earlier if data at one year were not available, and ‘poor outcome’ as mRS ≥4.
The MWG formulated 13 Population, Intervention, Comparator, Outcome (PICO) questions, which were reviewed and subsequently approved by the ESO Guidelines Board and Executive Committee. Because the first evidence of benefit of surgical decompression for space-occupying hemispheric infarction was limited to adult patients aged 60 years or younger who were treated within 48 hours of stroke onset,4–6,13 and because this had an impact on earlier guidelines, we chose to assess the effects of surgical decompression in three PICOs (1 – 3) addressing these cut-offs.
For each PICO question, a systematic review of the PubMed, EMBASE, CINAHL, and Cochrane Library databases was conducted with the support of the ESO Guidelines methodologist (AL). AL and HBvdW agreed on the search terms for each PICO question. The literature search was conducted from the inception of each database up to and including 30 September 2020 and subsequently updated with information from an individual-patient data meta-analysis published in February 2021.3 Only English-language articles were included. For the development of recommendations, the literature search was limited to randomised clinical trials (RCTs). Expert consensus statements could also be based on information from observational studies.
The articles were imported into the Covidence software (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia) and duplicate articles were removed. For each PICO question, two authors (different for each PICO) independently screened titles and abstracts for eligibility (first level selection). Full texts of all potentially relevant articles were loaded into Covidence software. Second level selection or full text screening of these articles was performed independently by the same authors. Discrepancies or conflicts in selection or rejection of studies were then resolved by consensus. In studies with duplicate data (companion publications), the original study or the study reporting detailed or more recent data (with a greater number of subjects) was included.
AL extracted the data for the selected articles. Data were checked by one of the authors.
The risk of bias of RCTs was assessed using the COCHRANE Collaboration’s tool.16 We assessed randomisation (random sequence generation), allocation concealment, blinding of participants, outcome assessment, attrition bias (incomplete outcome data), reporting bias (selective reporting) and other biases in each study.
Meta-analysis was performed with Review Manager (RevMan) 5.3 COCHRANE Collaboration software when more than one study reported the outcome and the number of subjects was ≥ 6 in each group. Risk ratios (RR) and 95% confidence intervals (CIs) for dichotomous variables were calculated. The I2 statistic, an expression of inconsistency of the studies’ results and describing the percentage of variation across studies due to heterogeneity rather than by chance, was calculated. An I2 >50% and p value <0.05 were considered to indicate statistically significant heterogeneity among the studies for an outcome. The reasons for high heterogeneity were explored. A random effects model was used for all outcomes. Where appropriate, subgroup analyses based on age of the patients, time to intervention were performed.
The results of data analysis were imported into the GRADEpro Guideline Development Tool (McMaster University, 2015; developed by Evidence Prime, Inc.). For each PICO question and each outcome, the quality of evidence (QoE) was rated as high, moderate, low or very low based on the type of available evidence (RCTs or observational studies) and considerations on risk of bias, inconsistency of results, indirectness of evidence, imprecision of results, and publication bias.14,15 GRADE evidence profiles/summary of findings tables were generated using GRADEPro.
For each PICO question, one author addressed the questions by writing up to four distinct paragraphs. In the first paragraph ‘analysis of current evidence’ the relevant RCTs were summarised and briefly discussed. Whenever no RCT was available, this was reported. Secondly, an ‘additional information’ paragraph could be added to provide more details on RCTs mentioned in the first paragraph and to summarise relevant results of observational studies. Thirdly, an evidence-based recommendation was provided, based on the GRADE methodology. The direction, the strength and the formulation of the recommendation were determined according to the GRADE evidence profiles and the ESO standard operating procedure. Fourthly, an ‘expert consensus statement’ was added whenever the authors considered that not enough evidence was available to provide evidence-based recommendations for situations in which practical guidance is needed for the everyday clinical practice. In that particular case, a pragmatic suggestion was provided. Importantly, the ‘expert consensus statements’ should not be mistaken as ‘evidence-based recommendations.’
The guidelines document was subsequently reviewed several times by all MWG members and modified until a consensus was reached. The Delphi method was used for the expert consensus statements.
Finally, the guideline document was reviewed and approved by external reviewers and members of the ESO Guideline Board and ESO Executive Committee.
Results
PICO 1: In patients with space-occupying hemispheric infarction aged 18 up to and including 60 years, does surgical decompression initiated within 48 hours of stroke onset as compared to no surgical decompression reduce the risk of death or poor outcome?
Analysis of current evidence
The literature search identified eight RCTs comparing surgical decompression with no surgical decompression,4–11 one additional RCT published in the supplement of an individual patient data meta-analysis3 but later published separately12 (Supplemental Figure 1), one earlier and smaller individual patient data meta-analysis,13 and 10 systematic reviews and meta-analyses.17–27 One RCT8 could not be included in the quantitative meta-analyses because no information was available on effects in patients in the two different age groups.
Three early RCTs included adult patients ≤ 60 years: DEcompressive Craniectomy In MALignant middle cerebral artery infarct (DECIMAL), DEcompressive Surgery for the Treatment of malignant INfarction of the middle cerebral arterY (DESTINY), and Hemicraniectomy After Middle cerebral artery infarction with Life-threatening Edema Trial (HAMLET).4–6 Five RCTs included adult patients up to and including 80 years: Zhao et al., Slezins et al., Hemicraniectomy And Durotomy upon Deterioration From Infarction Related Swelling (HeADDFIRST), Hemicraniectomy for Malignant Middle cerebral Infarction (HeMMI), and DEcompressive surgery for the treatment of Malignant Infarction of the middle cerebral artery: a randomised, controlled trial in a Turkish population (DEMITUR).7,8,10–12
In DECIMAL, DESTINY, Zhao et al., Slezins et al., and DEMITUR patients had to be treated up to 48 hours after symptom onset.4,5,7,8,12 HAMLET, HeADDFIRST, and HeMMI allowed treatment beyond 48 hours of symptom onset.6,10,11 Of note, the definition of ‘symptom onset’ in patients in whom the exact time of onset was unknown was not clarified in any of the articles reporting the main results. All trials included previously independent patients, i.e. a mRS ≤ 2, patients with very severe strokes, defined as a score on the National Institutes of Health Stroke Scale (NIHSS) of 15 or more (with different thresholds in the individual trials), a reduced consciousness, and infarcts involving either two thirds or more of the territory of the middle cerebral artery (MCA; DESTINY, HAMLET, DEMITUR, Zhao et al.),4,6,7,9,12 more than half of the MCA territory (HeADDFIRST, HeMMI),10,11 or more than half of the MCA territory and larger than 140 mL (DECIMAL, Slezins et al.).5,8
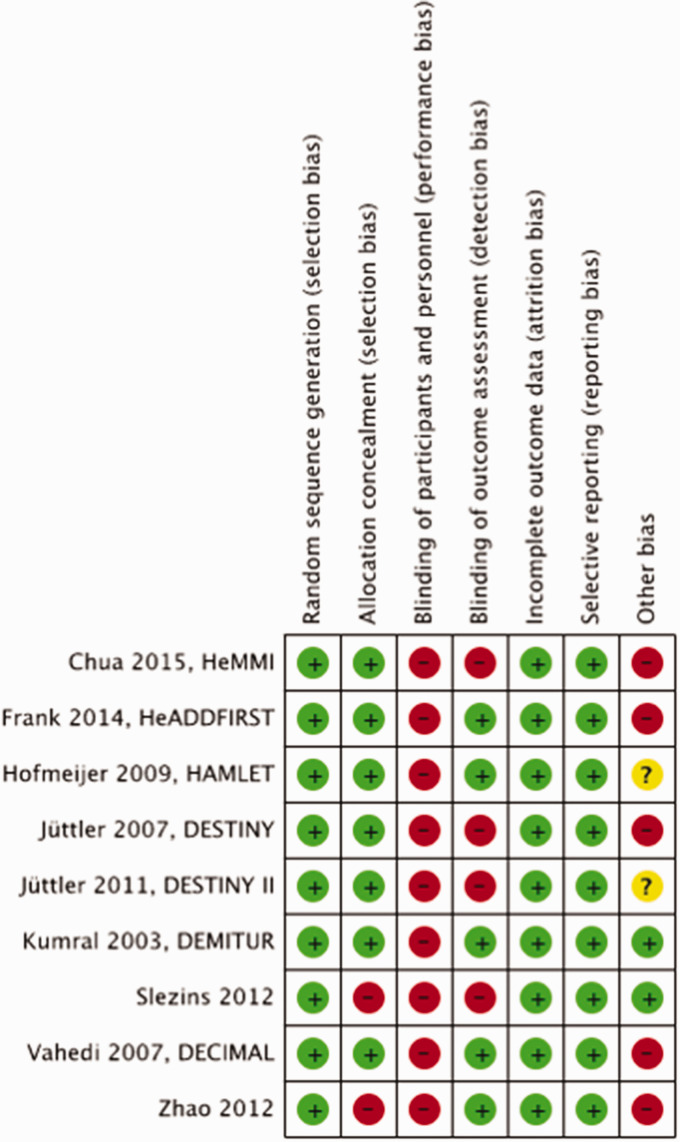
Most RCTs were small and suffered from a certain amount of bias. Blinding of participants and personnel to the procedure could not be performed in any trial, and often blinding of the procedure in outcome assessment was not applied or was unclear in many trials. In addition, allocation concealment was not always clear, and several trials were stopped early (Figure 1).
Figure 1.
Risk of bias in each trial of surgical decompression for space-occupying hemispheric infarction.
Before the first publication of any individual RCT, a pooled analysis of three European trials was published in 2007.13 In this pooled analysis, surgical decompression reduced the risks of death or a poor outcome (defined here as mRS of 5 or death at one year) in adult patients ≤ 60 years of age if treated within 48 hours of stroke onset.13 Another pooled analysis of individual patient data including 488 patients from DECIMAL, DESTINY, DESTINY II, HAMLET, Zhao et al., Slezins et al., and DEMITUR confirmed the reduction in mortality and also demonstrated an increase in the chance of a favourable outcome (defined as mRS ≤3 at one year) with surgical decompression in adult patients ≤ 60 years of age.3
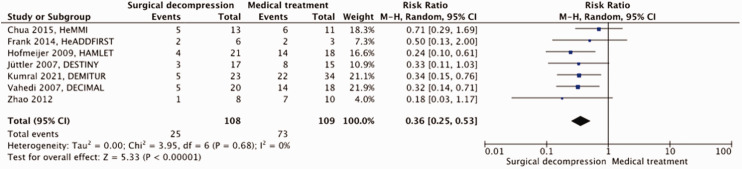
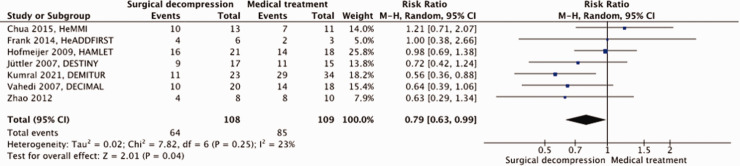
A total of 217 patients were included in the meta-analysis. Surgical decompression reduced the risk of death by an absolute 44% (RR, 0.36; 95% CI, 0.25 – 0.53); p < 0.00001; Figure 2) and that of a poor outcome by an absolute 19% (RR, 0.79; 95% CI, 0.63 – 0.99; p = 0.04; Figure 3). Surgical decompression increased the chance of survival with moderately severe to severe disability (mRS 4 or 5) from 11% to 36% (RR, 2.49; 95% CI, 1.23 – 5.04; Supplemental Figure 2). The quality of the evidence was rated as moderate (Table 1).
Figure 2.
Risk of death with surgical decompression vs. medical treatment in patients aged 18 up to and including 60 years with space-occupying hemispheric infarction who can be treated within 48 hours of stroke onset.
Figure 3.
Risk of a poor outcome (mRS ≥4) with surgical decompression vs. medical treatment in patients aged 18 up to and including 60 years with space-occupying hemispheric infarction who can be treated within 48 hours of stroke onset.
Table 1.
GRADE evidence profile table for PICO 1.
|
Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Surgical decompression | Medical management | Relative (95% CI) | Absolute (95% CI) | ||
| PICO 1: Death, Age 18-60 years, ≤ 48 hr | ||||||||||||
| 7 | randomised trials | seriousa | not serious | not serious | not serious | large effect | 25/108 (23.1%) | 73/109 (67.0%) | RR 0.36(0.25 to 0.53) | 429 fewer per 1,000(from 502 fewer to 315 fewer) | ⨁⨁⨁⨁HIGH | critical |
| PICO 1: Death or moderately severe disability (mRS 4-6), Age 18-60 years, ≤ 48 hr | ||||||||||||
| 7 | randomised trials | seriousa, b | not serious | not serious | not serious | large effect | 64/108 (59.3%) | 85/109 (78.0%) | RR 0.79(0.63 to 0.99) | 164 fewer per 1,000(from 289 fewer to 8 fewer) | ⨁⨁⨁◯MODERATE | critical |
CI: confidence interval; RR, risk ratio.
aFour trials terminated prematurely.
bLack of blinding.
Additional information
In an individual patient data meta-analysis of randomised trials, there was no difference in the benefit of surgical decompression with regard to mortality or functional outcome between patients with and those without aphasia.3 Reported rates of neuropsychological deficits or depression are essentially equal with or without aphasia, and quality of life appears not to depend on the presence or absence of aphasia.27,28 However, reliable data from randomised trials considering these outcomes are lacking.
In an individual patient data meta-analysis of randomised trials, the benefit of surgical decompression with regard to mortality or functional outcome did not depend on the presence of an infarct in the territory of the anterior or posterior cerebral artery in addition to that of the middle cerebral artery.3
HeADDFIRST excluded patients with confluent parenchymal haematomas, subdural haematomas, or subarachnoid haemorrhage.10 DECIMAL, DESTINY, Zhao et al. and DEMITUR excluded patients with space-occupying haemorrhagic transformation of the infarct.3–5,7,9 HAMLET, HeMMI, and Slezins et al. did not explicitly exclude these patients.6,8,11 There are no data or subgroup analyses of these patients available. It is unclear whether additional space-occupying haemorrhagic transformation affects outcome after surgical decompression.
In DECIMAL and DEMITUR comparatively unspecific recommendations concerning the size of the hemicraniectomy were given (“as large as possible including temporal, frontal, parietal, and some occipital bones”).3,5 The study protocols of DESTINY, HAMLET, Zhao et al., Slezins et al., and HeMMI demanded a size of the bone flap of at least 12 cm in diameter.4,6–9,11 HeADDFIRST gave more specific instructions (“minimal surgical decompression boundaries were anteriorly from the floor of the anterior cranial fossa at the mid pupillary line, posteriorly to 4 cm posterior to the external auditory canal, superiorly to 1 cm lateral to the superior sagittal sinus, and inferiorly to the floor of the middle cranial fossa”), similar to those of DESTINY II (“including the frontal (up to the middle pupillary line), parietal (up to 2 cm lateral of the sagittal superior sinus), temporal (down to the base of the middle cranial fossa), and parts of the occipital (up to 4 cm behind the outer ear canal) squamae”).10,29 None of the RCTs provided data on the size of the craniectomies or subgroup analyses concerning this issue. There are only few data from observational studies comparing different sizes of craniectomy.

Expert consensus statements.
There is consensus among the group members that in patients with space-occupying hemispheric infarction the benefit of surgical decompression does not depend on the absence or presence of aphasia.
Most group members agree that the benefit of surgical decompression does not depend on the presence of an infarct in the territory of the anterior or posterior cerebral artery in addition to that of the middle cerebral artery.
There is consensus among the group members that additional space-occupying haemorrhagic transformation should not be regarded as a contraindication to surgery.
There is consensus among the group members that in patients with space-occupying hemispheric infarction who will undergo decompressive surgery the diameter of the craniectomy should be at least 12 cm.
PICO 2: In patients with space-occupying hemispheric infarction aged 18 up to and including 60 years, does surgical decompression initiated later than 48 hours of stroke onset as compared to no surgical decompression reduce the risk of death or poor outcome?
HAMLET, HeADDFIRST, and HeMMI allowed treatment beyond 48 hours of symptom onset.6,10,11 No patient was treated beyond 96 hours. The pooled analysis of individual patient data including patients from DECIMAL, DESTINY, HAMLET, Zhao et al., Slezins et al., and DEMITUR identified 32 patients treated after 48 hours, 17 of whom were treated surgically and 15 conservatively. In this meta-analysis, no benefit of surgery was observed with regard of the risk of death or that of a poor outcome.3
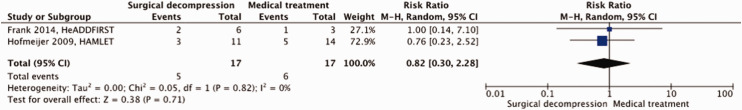
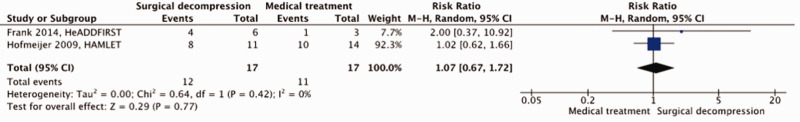
A total of 34 patients were included in our meta-analysis. For the risk of bias see Figure 1 above. Surgical decompression had no effect on the risk of death (RR, 0.82; 95% CI, 0.30 – 2.28]; p = 0.71; Figure 4) nor on the risk of a poor outcome (RR, 1.07; 95% CI, 0.67 – 1.72; p = 0.77; Figure 5). The quality of the evidence was rated as low (Table 2).
Figure 4.
Risk of death with surgical decompression vs. medical treatment in patients aged 18 up to and including 60 years with space-occupying hemispheric infarction who are treated 48 to 96 hours after stroke onset.
Figure 5.
Risk of a poor outcome (mRS ≥4) with surgical decompression vs. medical treatment in patients aged 18 up to and including 60 years with space-occupying hemispheric infarction who are treated 48 to 96 hours after stroke onset.
Table 2.
GRADE evidence profile table for PICO 2.
|
Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Surgical decompression | Medical management | Relative (95% CI) | Absolute (95% CI) | ||
| PICO 2: Death, Age 18-60 years, > 48 hr | ||||||||||||
| 2 | randomised trials | seriousa | not serious | not serious | seriousb | small numbers of trials and patients | 5/17 (29.4%) | 6/17 (35.3%) | RR 0.82(0.30 to 2.28) | 64 fewer per 1,000(from 247 fewer to 452 more) | ⨁⨁◯◯LOW | critical |
| PICO 2: Death or moderately severe disability (mRS 4-6), Age 18-60 years, > 48 hr | ||||||||||||
| 2 | randomised trials | seriousa,b | not serious | not serious | seriousb | small numbers of trials and patients | 12/17 (70.6%) | 11/17 (64.7%) | RR 1.07(0.67 to 1.72) | 45 more per 1,000(from 214 fewer to 466 more) | ⨁⨁◯◯LOW | critical |
CI: confidence interval; RR: risk ratio.
aTrials terminated prematurely.
bLack of blinding.
Additional information
We refer to the additional information under PICO 1 above.

Expert consensus statement
There is consensus among the module working group members that surgical decompression should also be considered later than 48 hours after stroke onset if based on clinical grounds death due to herniation appears likely.
PICO 3: In patients with space-occupying hemispheric infarction aged 61 years or older, does surgical decompression initiated within 48 hours of stroke onset as compared to no surgical decompression reduce the risk of death or poor outcome?
Five RCTs included adult patients up to and including 80 years: Zhao et al., Slezins et al., Hemicraniectomy And Durotomy Upon Deterioration From Infarction Related Swelling (HeADDFIRST), Hemicraniectomy for Malignant Middle cerebral Infarction (HeMMI), and Decompressive surgery for the treatment of Malignant Infarction of the middle cerebral artery: a randomised, controlled trial in a Turkish population (DEMITUR).7,8,10–12 The Decompressive Surgery for the Treatment of malignant infarction of the Middle Cerebral Artery II (DESTINY II) trial exclusively included patients > 60 years of age.9
A meta-analysis of individual patient data included 253 patients aged 61 years or older from DESTINY II, Zhao et al., Slezins et al., and DEMITUR.3 This meta-analysis found no statistically significant benefit with regard to the chance of a favourable outcome defined as mRS ≤3 in patients aged 61 years or older. On the other hand, the meta-analysis found no statistically significant heterogeneity in the effects of surgical decompression on functional outcome between patients aged 60 years or younger and those aged 61 years or older. HeADDFIRST enrolled 8 patients > 60 years of age, 5 in the surgical and 3 in the medical arm.10
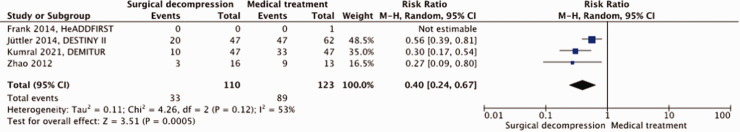
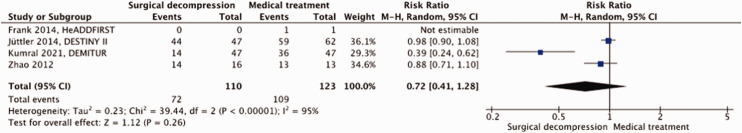
A total of 233 patients were included in our meta-analysis. Surgical decompression reduced the risk of death by an absolute 42% (RR, 0.40; 95% CI, 0.24 – 0.67; p = 0.0005; Figure 6) but had no effect on the risk of a poor outcome (RR, 0.72; 95% CI, 0.41 – 1.28; p = 0.26; Figure 7). Surgical decompression increased the chance of survival with moderately severe to severe disability (mRS 4 or 5) from 16% to 35% (RR, 2.35; 95% CI, 1.49 – 3.72; Supplemental Figure 3). The quality of the evidence was rated as low (Table 3).
Figure 6.
Risk of death with surgical decompression vs. medical treatment in patients aged ≥61 years with space-occupying hemispheric infarction who can be treated within 48 hours of stroke onset.
Figure 7.
Risk of a poor outcome (mRS ≥4) with surgical decompression vs. medical treatment in patients aged ≥61 years with space-occupying hemispheric infarction who can be treated within 48 hours of stroke onset.
Table 3.
GRADE evidence profile table for PICO 3.
|
Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Surgical decompression | Medical management | Relative (95% CI) | Absolute (95% CI) | ||
| PICO 3: Death, Age > 60 years | ||||||||||||
| 4 | randomised trials | seriousa | not serious | not serious | not serious | large effect | 33/110 (30.0%) | 89/123 (72.4%) | RR 0.40(0.24 to 0.67) | 434 fewer per 1,000(from 550 fewer to 239 fewer) | ⨁⨁⨁⨁HIGH | critical |
| PICO 3: Death or moderately severe disability (mRS 4-6), Age > 60 | ||||||||||||
| 4 | randomised trials | seriousa,b | serious | not serious | serious | none | 72/110 (65.5%) | 109/123 (88.6%) | RR 0.72(0.41 to 1.28) | 248 fewer per 1,000(from 523 fewer to 248 more) | ⨁⨁◯◯LOW | critical |
CI: confidence interval; RR: risk ratio.
aTwo trials terminated prematurely.
bLack of blinding.
Additional information
We refer to the additional information for PICO 1 above.

PICO 4: In patients with space-occupying cerebellar infarction, does surgical decompression as compared to no surgical decompression reduce the risk of death or a poor outcome?
Analysis of current evidence
The literature search did not identify any RCT comparing surgical decompression with no surgical decompression in patients with space-occupying cerebellar infarction.
Additional information
Space-occupying cerebellar infarction may lead to brainstem compression and obstructive hydrocephalus by compression of the fourth ventricle. Both brainstem compression, upward transtentorial herniation and downward herniation of part of the cerebellum through the foramen magnum may lead to death.30
In clinical practice, surgical decompression and drainage of cerebrospinal fluid (CSF) are established treatment options, which is reflected by numerous mostly retrospective case series and observational studies. Surgical decompression usually comprises suboccipital craniectomy with or without removal of infarcted brain tissue, and can be combined with CSF drainage. The widespread use of surgical decompression for treatment of space-occupying cerebellar infarction is further supported by the experience that even patients with large cerebellar infarcts can have a good outcome if the clinical course is not complicated by brainstem compression or herniation.31 Retrospective studies have suggested a lower risk of death and a better functional outcome if patients with a massive cerebellar infarct are treated with surgical decompression than conservatively.32,33 In a review of observational studies, mortality of space-occupying cerebellar infarction was 43% with conservative treatment and 18 to 27% with different techniques of surgical treatment.30 The effects of surgery on functional outcome of patients with space-occupying cerebellar infarction are even less clear, and reported rates of a favourable or independent outcome are heterogeneous. Altogether, no high-quality systematic observational studies comparing surgical treatment with conservative treatment in these patients are available. Thus, the available information that may guide expert opinion is limited.

Expert consensus statement
All group members suggest considering surgical decompression with or without CSF drainage in selected patients with space-occupying cerebellar infarction, such as in those with a reduced consciousness caused by brainstem compression. The precise selection of patients and the optimal timing of treatment remain uncertain. There is insufficient evidence to support its routine use.
PICO 5: In patients with space-occupying cerebellar infarction, does CSF drainage reduce mortality or improve functional outcome?
Analysis of current evidence
The literature search did not identify any RCT comparing CSF drainage with no CSF drainage in patients with space-occupying cerebellar infarction.
Additional information
Space-occupying cerebellar infarction may lead to brainstem compression and obstructive hydrocephalus by compression of the fourth ventricle.30 CSF drainage alone is considered in patients in whom the obstructive hydrocephalus is presumed to be the main cause of neurological deterioration. In addition, CSF drainage is sometimes used as a first step in surgical treatment, prior to surgical decompression.32,33 There are no high-quality systematic observational studies comparing CSF drainage with conservative treatment in space-occupying cerebellar infarction. Thus, the overall available information that may guide expert opinion is limited.

Expert consensus statement
All group members suggest considering CSF drainage alone or combined with surgical decompression in selected patients with space-occupying cerebellar infarction and signs of an obstructive hydrocephalus, such as in those with a reduced consciousness. The selection of patients and the optimal timing of treatment remain uncertain. There is insufficient evidence to support its routine use.
PICO 6: In patients with space-occupying hemispheric infarction, does monitoring of intracranial pressure (ICP) as compared with no monitoring of ICP reduce the risk of death or a poor outcome?
Analysis of current evidence
The literature search did not identify any RCT comparing ICP monitoring with no ICP monitoring in patients with space-occupying hemispheric infarction.
Additional information
ICP monitoring in patients with space-occupying hemispheric infarction has been assessed mainly in small prospective observational studies. The gold standard of ICP monitoring is via an external ventricular drain in the lateral ventricle.34 Alternatively, ICP can be monitored continuously with a parenchymal probe, preferably in the right frontal lobe.34 Clinical deterioration and outcomes cannot reliably be predicted with ICP monitoring, which has been explained in part by pressure gradients between various intracranial compartments and the location of herniation. Furthermore, ICP values in the right frontal lobe may not sufficiently reflect pressures in the left hemisphere or infratentorially.35,36 An early prospective observational study evaluated the clinical course of 48 patients with clinical signs of increased ICP due to large hemispheric infarction.35 Thirty nine patients died, and in all clinical signs of herniation preceded the increase in ICP. Another prospective observational study showed that in patients with space-occupying MCA infarction, pupillary abnormalities and severe brainstem compression may be present despite normal ICP values.37 Additional retrospective analysis of postoperative ICP monitoring in 12 patients with space-occupying MCA infarction showed that elevations in ICP are common after surgical decompression and that the monitoring of ICP influenced the postoperative management.38 A retrospective study in 25 patients with space-occupying MCA infarction who underwent ICP monitoring and had a brain CT within the first hour of surgical decompression showed that an increase in ICP early after surgical decompression was associated with a greater risk of death at six months.39

Expert consensus statement
A majority of group members suggest against routine ICP monitoring as a means to reduce the risk of death or poor outcome in patients with space-occupying hemispheric infarction.
PICO 7: In patients with space-occupying hemispheric infarction, does admission to an intensive care unit (ICU; i.e., the possibility for mechanical ventilation) as compared with no ICU admission reduce the risk of death or a poor outcome?
Analysis of current evidence
The literature search did not identify any RCT comparing ICU admission with no ICU admission in patients with space-occupying hemispheric infarction.
Additional information
Patients with large hemispheric infarctions who might become candidates for surgical decompression are prone to sudden and rapid deterioration requiring immediate therapeutic action. Therefore, these patients are in need for tight clinical and technical monitoring. This is to ensure immediate conservative treatment but also to assess the optimal timing of surgical decompression. Because clinical deterioration and cerebral herniation may occur up to at least seven days after stroke onset, patients may require intensive clinical and technical monitoring for at least several days, depending on their clinical condition and the development of ICP. In most cases, these requirements are best fulfilled on intensive care units.

Expert consensus statement
A majority of group members suggest admission to an ICU for clinical and technical monitoring and intensive care treatment including mechanical ventilation, if required, irrespective of treatment with surgical decompression.
PICO 8: In patients with space-occupying hemispheric infarction, does sedation as compared to no sedation reduce the risk of death or poor outcome?
Analysis of current evidence
The literature search did not identify RCTs comparing sedation vs. no sedation in patients with space-occupying infarction.
Additional information
Sedation has been advocated for patients with space-occupying hemispheric infarction because this may reduce ICP and reduce metabolic demands, comparable to patients with severe traumatic brain injury. In a German study of 60 patients with increased ICP due to space-occupying hemispheric infarction, ICP was indeed lowered in 50 patients after the start of high-dose barbiturates, but this came at the expense of a mean reduction in cerebral perfusion pressure of 9 mm Hg. Despite treatment, 55 patients (92%) died after transtentorial herniation. In addition, barbiturates were associated with serious complications in a quarter of the patients.40
Guidelines for patients with severe traumatic brain injury recommend administration of barbiturates or propofol to control elevated ICP refractory to medical and surgical management.41 However, there is also no convincing evidence that sedation improves functional outcomes in patients with severe traumatic brain injury.42,43
In addition to the lack of evidence of benefit, we discourage sedation because in patients with space-occupying hemispheric or cerebellar infarction the level of consciousness should be monitored closely, in addition to the loss of pupillary reaction to light, in order to detect deterioration at an early stage. For patients who require endotracheal intubation, short-acting sedatives may be used to avoid discomfort.

Expert consensus statement
In patients with space-occupying hemispheric infarction, a majority of group members suggest against the use of sedation as a means to reduce the risk of death or a poor outcome if this is not otherwise indicated.
Short-term sedation may be considered as a rescue procedure in patients with clinical signs of raised ICP or impending herniation.
PICO 9: In patients with space-occupying hemispheric infarction, does osmotic therapy as compared to no osmotic therapy reduce the risk of death or a poor outcome?
Analysis of current evidence
The literature search did not identify any RCT comparing osmotic therapy with no osmotic therapy in patients with space-occupying hemispheric infarction.
Additional information
Osmotic agents, such as mannitol or hypertonic saline, are presumed to draw water from the interstitial to the intravascular space by creating an osmotic gradient over the semi-permeable blood brain barrier.44 Other reported effects of osmotic therapy include reduction of blood viscosity with improved microvascular flow,45,46 reduction of cerebral blood volume by vasoconstriction,47 and scavenging of free radicals.48
In a randomised cross-over trial in 30 episodes of increased ICP in eight patients with space-occupying hemispheric infarction and one with putaminal haemorrhage with oedema, single doses of 200 mL of a 20% mannitol solution or 100 mL hypertonic saline solution (containing 75 g/L NaCl) prepared in 60 g/L hydroxyethyl starch were effective in temporarily reducing ICP by 10% or more with mannitol in 10 of 14 episodes and with hypertonic saline in all 16 episodes. The maximum effect occurred at the end of the bolus infusion and lasted over four hours.49 One prospective and one retrospective study from the 1970s did not suggest a statistically significant benefit of osmotic therapy in rather unselected stroke patients.50,51
With large hemispheric infarction, damage to the blood brain barrier may prevent the establishment of an adequate osmotic gradient. Leakage of osmotically active particles from the intravascular space to brain tissue may even lead to a reverse gradient, with the risk of increased oedema. Animal studies have shown an increase of oedema52 and aggravation of tissue shifts53 after osmotic therapy. An increased midline shift after a 1.5 g/kg bolus of mannitol was also found in six patients with space-occupying infarction.54 However, in a series of seven patients with space-occupying infarction, single doses of mannitol did not increase midline shift as measured by MRI.55 The risk of an increase in oedema is probably greater with repeated or prolonged administration of osmotic substances.56 Other adverse effects of osmotic therapy include hypovolemia, hypotension, electrolyte imbalance, and acute kidney injury.

Expert consensus statement
All group members suggest against the routine use of osmotic therapy as a means to reduce the risk of death or a poor outcome. Short-term osmotic therapy (i. e. a single or a few doses) may be considered as a rescue procedure in case of signs of an increased intracranial pressure or impending herniation. For short-term osmotic therapy with mannitol, we suggest the use of bolus administration rather than continuous infusion. The choice of mannitol or hypertonic saline depends on local preferences and expertise.
PICO 10: In patients with space-occupying hemispheric infarction, do corticosteroids as compared to no corticosteroids reduce the risk of death or a poor functional outcome?
Analysis of current evidence
The literature search did not identify any RCT comparing corticosteroids versus no corticosteroids in patients with space-occupying hemispheric infarction.
Additional information
A Cochrane meta-analysis of eight randomised trials performed in the 1970s or earlier including 466 patients with acute presumed or definite ischaemic stroke found no effect of treatment with corticosteroids on the rate of death at one year or earlier. Treatment did not appear to improve functional outcome in survivors.57 The probable absence of a response to corticosteroids could be explained by its predominant cytotoxic nature of oedema in patients with ischaemic stroke.58 This is comparable to the lack of benefit of corticosteroids in patients with traumatic brain injury.59 The largest trial of corticosteroids in patients with traumatic brain injury found an increase in the risk of death in patients treated with corticosteroids, but no difference in the risk of death or severe disability.60
Expert consensus statement
All group members suggest against the use of corticosteroids as a means to reduce the risk of death or a poor outcome.
PICO 11: In patients with space-occupying hemispheric infarction, does hyperventilation as compared to no hyperventilation reduce the risk of death or a poor outcome?
Analysis of current evidence
The literature search did not identify any RCT comparing hyperventilation with no hyperventilation in patients with space-occupying hemispheric infarction.
Additional information
Hyperventilation causes a rapid lowering of intracranial pressure by inducing cerebral vasoconstriction, with a short-time effect. A target partial pressure of carbon dioxide (PaCO2) of 30–35 mmHg has been suggested. More aggressive or prolonged hyperventilation may result in worsening ischemic injury from vasoconstriction.61 Very early studies of hyperventilation in patients with stroke showed no benefit on patient outcome.62,63
Transient hyperventilation has been extensively evaluated in clinical practice in patients with increased ICP due to traumatic brain injury64,65 with variable results. Improvements in pupil diameter, neurological status, and ICP have been reported. Data to support the use of hyperventilation as a means to improve long-term outcomes in patients with brain oedema are however limited and the quality of evidence is low.66

Expert consensus statement
All group members suggest against the use of routine hyperventilation.
Short-term hyperventilation may be considered as a rescue procedure in patients with clinical signs of brain herniation.
PICO 12: In patients with space-occupying hemispheric infarction, does hypothermia as compared to no hypothermia reduce the risk of death or a poor functional outcome?
Analysis of current evidence
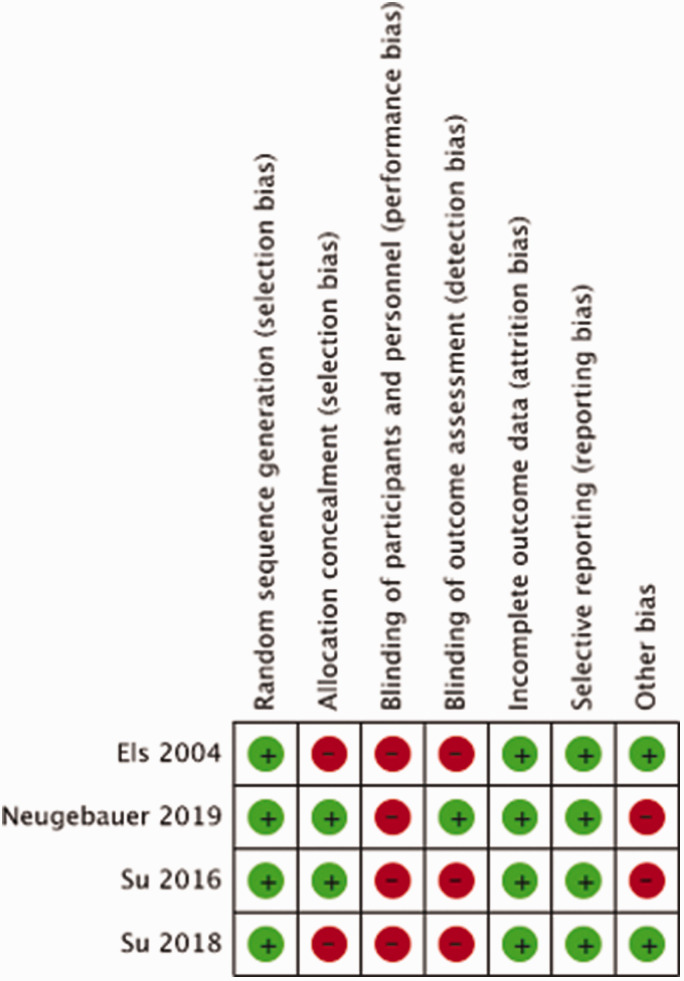
Small observational studies performed at the ICU have suggested that cooling to a body temperature of 33 °C might lower ICP and reduce the risk of death in patients with space-occupying hemispheric infarction.67,68 The effect of hypothermia on functional outcome after space-occupying hemispheric infarction treated with or without surgical decompression has been tested in four randomised trials (Supplemental Figure 4).
In a German study,69 25 patients aged 18 to 65 years with a large supratentorial infarct were randomised to receive surgical decompression immediately followed by endovascular or surface cooling to a target oesophageal temperature of 35 °C for 48 hours, or to surgical decompression and normothermia. It is unknown whether outcomes were assessed in a blinded fashion. The time to surgical decompression was 15 ± 6 hours in both groups. At six months, there were no differences in case fatality or score on the mRS, but data on the last were insufficient to include in a meta-analysis. There were no differences between the groups in the risk of complications such as pneumonia.
In one Chinese trial,70 patients aged 18 to 80 years with space-occupying infarction in the territory of the middle cerebral artery were randomised to endovascular cooling to a target bladder temperature of 33 or 34 °C for 24 to 72 hours or to standard treatment aimed at maintaining normothermia. Treatment was started within 48 hours of stroke onset. Shivering was suppressed with cotton-padded gloves, socks, and a quilt, and with oral buspirone, intravenous pethidine, midazolam, and the muscle relaxants atracurium and vecuronium. The primary outcomes were mortality and the score on the mRS at six months, assessed in an unblinded fashion. The original sample size was 168 patients, but the trial was terminated after the inclusion of 36 patients of whom 33 were included in the analyses. The time between stroke onset and initiation of hypothermia was 42.0 ± 14.9 hours. At six months, there were no differences in the death or score on the mRS. The risk of having any complication was greater in patients treated with hypothermia than in controls.
In another Chinese trial71 of which the results have been published in an abstract, patients with “large hemispheric infarction” who were treated with surgical decompression were randomised within 48 hours of symptom onset to head-surface cooling, endovascular cooling, or no cooling. Data of the two cooling groups were combined. The primary outcomes were mortality and the score on the mRS at six months. At six months, there were no differences in the death or score on the mRS.
In another German study,72 patients aged 18 to 60 years with space-occupying infarct in the territory of the middle cerebral artery were randomised to receive surgical decompression followed within 12 hours by endovascular or surface cooling to a target bladder temperature of 33.0 ± 1.0 °C for at least 72 hours, or to surgical decompression alone. In the control group, temperature drops below 36.5 °C were avoided. Patients were intubated and sedated during induction and maintenance of hypothermia. The target sample size was 324 patients, but the trial was stopped prematurely after inclusion of 50 patients because of safety concerns. The primary outcome was mortality at day 14. The score on the mRS at 12 months was a secondary outcome. Outcomes were assessed blinded to treatment allocation. There were no differences in the risk of death at 14 days or in the distribution of scores on the mRS at 12 months. At 12 months, 20 of 25 patients (80%) in the hypothermia group and 10 of 23 patients (43%) in the standard care group had had a serious adverse event (hazard ratio, 2.54; 95%CI, 1.29–5.00; P = 0.005).
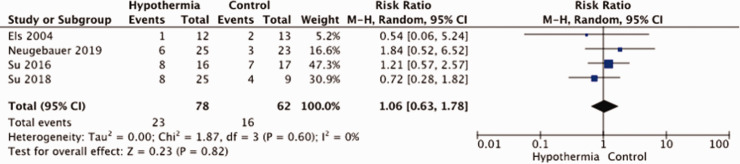
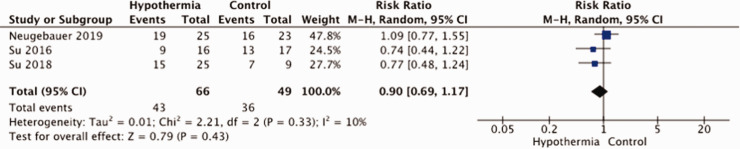
The meta-analysis of the results is hampered by small sample sizes of the three studies and by differences in study methodology and patient population, especially with regard to having a surgical decompression. In all trials, there was no blinding of patient or staff for treatment arm, and just in one trial outcomes were assessed masked to treatment allocation (Figure 8). Allocation concealment was another risk of bias in two of the four studies. A total of 140 patients were entered in the meta-analysis. There was no evidence that hypothermia reduces the risk of death (RR, 1.06; 95% CI, 0.63 – 1.78; Figure 9) or that of a poor outcome (RR, 1.28; 95% CI, 0.70 – 2.35; Figure 10). The quality of the evidence was rated as very low (Table 4).
Figure 8.
Risk of bias in each trial of hypothermia for space-occupying hemispheric infarction.
Figure 9.
Risk of death with hypothermia vs. no hypothermia in patients with space-occupying hemispheric infarction.
Figure 10.
Risk of a poor outcome (mRS ≥4) with hypothermia vs. no hypothermia in patients with space-occupying hemispheric infarction.
Table 4.
GRADE evidence profile table for PICO 12.
|
Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Hypothermia | Control | Relative (95% CI) | Absolute (95% CI) | ||
| PICO 12: Mortality (6-12 months) | ||||||||||||
| 4 | randomised trials | seriousa | not serious | not serious | seriousb | small numbers of patients and studies | 23/78 (29.5%) | 16/62 (25.8%) | RR 1.06(0.63 to 1.78) | 15 more per 1,000(from 95 fewer to 201 more) | ⨁◯◯◯VERY LOW | critical |
| PICO 12: Death or moderately severe disability (mRS 4-6) | ||||||||||||
| 3 | randomised trials | seriousa | not serious | not serious | seriousb | small numbers of patients and studies | 43/66 (65.2%) | 36/49 (73.5%) | RR 0.90(0.69 to 1.17) | 73 fewer per 1,000(from 228 fewer to 125 more) | ⨁◯◯◯VERY LOW | critical |
CI: confidence interval; RR: risk ratio.
aStudies were not always blinded; treatment allocation were not always concealed; two trials were terminated prematurely.
bFew events and wide confidence intervals.

PICO 13: In patients with space-occupying hemispheric infarction, does glyburide as compared to no glyburide reduce the risk of death or poor outcome?
Analysis of current evidence
Glyburide, also known as glibenclamide, is a second-generation sulfonylurea drug that inhibits inducible sulfonylurea receptor 1 (SUR1). In animal stroke studies, blockade of the SUR1-transient receptor potential melastatin 4 channel in neurons, astrocytes, and endothelium reduced brain oedema.
The literature search identified one RCT (Supplemental Figure 5). In this American, double-blind, randomised, placebo-controlled, phase 2 clinical trial, patients aged 18–80 years with large anterior circulation hemispheric infarction for less than 10 h and baseline diffusion-weighted MRI image lesion volume of 82 to 300 mL on MRI were randomised to intravenous glyburide or placebo.73 The primary efficacy outcome was the proportion of patients who achieved an mRS score of 0 to 4 at 90 days without surgical decompression. The primary analysis was per-protocol. The study was terminated prematurely because of funding reasons after inclusion of 86 patients, against a target of 240. The risk of bias was considered low (Figure 11). A total of 77 patients were included in our analysis. Glyburide had no effect on the risk of death or a poor outcome, defined as mRS 4 – 6. The quality of the evidence was rated as very low (Table 5).
Figure 11.
Risk of bias in the trial of glyburide for space-occupying hemispheric infarction.
Table 5.
GRADE evidence profile table for PICO 13.
|
Certainty assessment |
No. of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Glyburide | placebo | Relative (95% CI) | Absolute (95% CI) | ||
| PICO 13: Mortality, 3 months | ||||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | not serious | single study | 7/41 (17.1%) | 13/36 (36.1%) | RR 0.47(0.21 to 1.05) | 191 fewer per 1,000(from 285 fewer to 18 more) | ⨁⨁◯◯LOW | critical |
| PICO 13: Poor outcome (mRS 4-6) | ||||||||||||
| 1 | randomised trials | seriousa | not serious | not serious | serious | single study | 27/41 (65.9%) | 27/36 (75.0%) | RR 0.88(0.66 to 1.17) | 90 fewer per 1,000(from 255 fewer to 127 more) | ⨁◯◯◯VERY LOW | critical |
CI: confidence interval; RR: risk ratio.

Discussion
This guideline provides evidence-based recommendations for physicians treating patients with space-occupying hemispheric or cerebellar infarction. Where no sufficient scientific evidence was available, we have provided ‘expert consensus statements’ that are based on observational studies and on our own expertise.
One striking but not unexpected observation is that the evidence supporting all management options is very limited, with the exception of surgical decompression for hemispheric infarction within 48 hours. The last is supported by nine RCTs4–12 and two individual patient data meta-analyses.3,13 Although these trials were small and had the potential of bias, the large and consistent effects allowed a strong recommendation for patients aged up to 60 years who can be treated with surgical decompression within 48 hours of stroke onset.
The endpoints on which our recommendations are based are limited to survival and functional outcome, and do not include aspects that are highly relevant to patients as well, such as quality of life. Given the very large difference in survival between patients treated with surgical decompression and those treated conservatively, any randomised comparison of quality of life during follow-up will be strongly biased. However, observational studies have suggested that most patients treated with surgical decompression for space-occupying infarction have a reasonable quality of life at long-term follow-up and are satisfied with the treatment received.74
Because patients with space-occupying hemispheric infarction by common definition have very large infarcts and severe deficits and therefore probably require a longer rehabilitation period and more time to reach a ‘final’ functional outcome, our primary endpoints were at one year and not at 90 days as in most RCTs in patients with acute stroke. There is evidence from a single RCT that the effects of surgical decompression on survival and functional outcome observed at one year are sustained at three years.75
Early selection of candidates for surgical decompression is hampered by the paucity of methods to predict the course of oedema formation reliably. Large infarct size on CT or MRI in the first hours after stroke onset is the major determinant of the development of life-threatening oedema, but most single measurements lack sufficient predictive value to strongly support a decision to perform surgical decompression.76,77 In one study, a lesion volume >82ml on diffusion-weighted MRI within six hours of stroke onset had high positive and negative predictive values for the development of ‘malignant’ space-occupying MCA infarction.78 In this study, ‘malignant MCA infarction’ was defined as: 1. clinical signs of large MCA territory infarction with an NIHSS score >18 and a level of consciousness of 1 or higher on item 1a of the NIHSS either on admission or after secondary deterioration; 2. Large space-occupying MCA infarction on follow-up MRI or CT of at least two-thirds of the MCA territory with compression of ventricles or midline shift; and 3. No other obvious cause for neurological deterioration. This definition overlaps substantially with the inclusion criteria of most RCTs of surgical decompression, but it is uncertain whether each patient who fulfils this definition will benefit from surgery. In addition, in most centres MRI is not the first imaging modality in patients with acute ischaemic stroke. Based on the inclusion criteria of RCTs, we think that referral to a comprehensive stroke centre with neurosurgical facilities should be considered in patients with a combination of a severe focal neurological deficit; a reduction in consciousness; a space-occupying infarct in two thirds or more of the territory of the MCA; and no signs of irreversible brain stem damage, or in those with a DWI lesion volume > 82 mL in the first six hours after stroke onset. For patients with space-occupying cerebellar infarction referral could be considered in case of any substantial mass effect of the infarct or infarction of two thirds of one cerebellar hemisphere.
In our view, some of the management options discussed in this guideline deserve more and better scientific evaluation of their risks and benefits. Where randomisation may be considered unethical, for example when assessing the effects of an occipital craniectomy in patients with space-occupying cerebellar infarction and brainstem compression, large (international) registries could inform treatment decisions. We encourage colleagues to start well-designed studies assessing treatment options for patients with space-occupying brain infarction, so that a revision of the present guideline in a few years’ time can be based on more solid evidence. To make such studies better feasible, we also encourage the relevant policy makers and authorities to reduce regulatory barriers79–81 where appropriate.
Plain language summary
In the first few days after the onset of a brain infarct, the damaged brain tissue may swell. In patients with a very large infarct, the swelling can be life-threatening if this leads to major compression on healthy brain tissue or a large increase in intracranial pressure. Several treatment strategies for this severe complication are available, but the size and quality of the scientific evidence on which these strategies are based vary considerably. The aim of this guideline document is to assist physicians in their management decisions when treating patients with this so-called ‘space-occupying brain infarction.’
These Guidelines were developed based on the European Stroke Organisation (ESO) standard operating procedure and followed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach. A working group identified 13 relevant questions, performed systematic reviews and meta-analyses of the literature, assessed the quality of the available evidence, and wrote evidence-based recommendations. An expert consensus statement was provided if not enough evidence was available to provide recommendations based on the GRADE approach.
The primary treatment option for space-occupying infarction in one of the brain hemispheres is surgical decompression, consisting of the surgical removal of a large part of the skull and widening of the meninges on the side of the infarct. We found high-quality evidence to recommend surgical decompression to reduce the risk of death and to increase the chance of a favourable outcome in adult patients aged up to and including 60 years with space-occupying infarction in one of the brain hemispheres who can be treated within 48 hours of stroke onset, and low-quality evidence to support this treatment in older patients. There is continued uncertainty about the benefit and risks of surgical decompression in patients with space-occupying hemispheric infarction if this is done after the first 48 hours. There is also continued uncertainty about the selection of patients with space-occupying cerebellar infarction for surgical treatment.
Supplemental Material
Supplemental material, sj-pdf-1-eso-10.1177_23969873211014112 for European Stroke Organisation (ESO) guidelines on the management of space-occupying brain infarction by H Bart van der Worp, Jeannette Hofmeijer, Eric Jüttler, Avtar Lal, Patrik Michel, Paola Santalucia, Silvia Schönenberger, Thorsten Steiner and Götz Thomalla in European Stroke Journal
Acknowledgements
The authors acknowledge Luzia Balmer for her management assistance.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The potential conflicts of interest are listed in Supplemental Table 1.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Not applicable.
Ethical approval: Not applicable.
Guarantor: HBvdW.
Contributorship: HBvdW drafted the PICO questions, which were refined by all authors. All authors conducted the literature search. AL conducted data extraction and performed meta-analyses. All authors participated in the writing of the first draft of the manuscript. All authors reviewed and edited the manuscript for important intellectual content and approved the final version of the manuscript.
ORCID iD: H Bart van der Worp https://orcid.org/0000-0001-9891-2136
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Hacke W, Schwab S, Horn M, et al. Malignant middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 1996; 53: 309–315. [DOI] [PubMed] [Google Scholar]
- 2.Berrouschot J, Sterker M, Bettin S, et al. Mortality of space-occupying (malignant) middle cerebral artery infarction under conservative intensive care. Intensive Care Med 1998; 24: 620–623. [DOI] [PubMed] [Google Scholar]
- 3.Reinink H, Jüttler E, Hacke W, et al. Surgical decompression for space-occupying hemispheric infarction: a systematic review and individual patient Meta-analysis of randomized clinical trials. JAMA Neurol 2021; 78: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jüttler E, Schwab S, Schmiedek P, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke 2007; 38: 2518–2525. [DOI] [PubMed] [Google Scholar]
- 5.Vahedi K, Vicaut E, Mateo J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke 2007; 38: 2506–2517. [DOI] [PubMed] [Google Scholar]
- 6.Hofmeijer J, Kappelle LJ, Algra A, et al. Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after Middle cerebral artery infarction with life-threatening edema trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 2009; 8: 326–333. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Su YY, Zhang Y, et al. Decompressive hemicraniectomy in malignant middle cerebral artery infarct: a randomized controlled trial enrolling patients up to 80 years old. Neurocrit Care 2012; 17: 161–171. [DOI] [PubMed] [Google Scholar]
- 8.Slezins J, Keris V, Bricis R, et al. Preliminary results of randomized controlled study on decompressive craniectomy in treatment of malignant Middle cerebral artery stroke. Medicina 2012; 48: 521–524. [PubMed] [Google Scholar]
- 9.Jüttler E, Unterberg A, Woitzik J, et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med 2014; 370: 1091–1100. [DOI] [PubMed] [Google Scholar]
- 10.Frank JI, Schumm LP, Wroblewski K, et al. Hemicraniectomy and durotomy upon deterioration from infarction-related swelling trial: randomized pilot clinical trial. Stroke 2014; 45: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chua AE, Buckley BS, Lapitan MC, Jamora RD.Hemicraniectomy for malignant Middle cerebral artery infarction (HeMMI): a randomized controlled clinical trial of decompressive surgery with standardized medical care versus standardized medical care alone. Acta Med Philipp 2015; 49: 28–33. [Google Scholar]
- 12.Kumral E, Irin HA, Sagduyu A, et al. Decompressive surgery in patients with malignant middle cerebral artery infarction: a randomized, controlled trial in a Turkish population (DEMITUR trial). Int J Stroke. Epub ahead of print 2021. [DOI] [PubMed]
- 13.Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant Middle cerebral artery infarction: a pooled analysis of three randomised controlled trials. Lancet Neurol 2007; 6: 215–222. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Schunemann HJ, et al. Grade guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol 2011; 64: 380–382. [DOI] [PubMed] [Google Scholar]
- 15.Ntaios G, Bornstein NM, Caso V, et al. The European Stroke Organisation guidelines: a standard operating procedure. Int J Stroke 2015; 10: 128–135. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Back L, Nagaraja V, Kapur A, et al. Role of decompressive hemicraniectomy in extensive middle cerebral artery strokes: a meta-analysis of randomised trials. Intern Med J 2015; 45: 711–717. [DOI] [PubMed] [Google Scholar]
- 18.Yang MH, Lin HY, Fu J, et al. Decompressive hemicraniectomy in patients with malignant middle cerebral artery infarction: a systematic review and meta-analysis. Surgeon J R Coll Surgeons Edinburgh Ireland 2015; 13: 230–240. [DOI] [PubMed] [Google Scholar]
- 19.Alexander P, Heels-Ansdell D, Siemieniuk R, et al. Hemicraniectomy versus medical treatment with large MCA infarct: a review and meta-analysis. BMJ Open 2016; 6: e014390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streib CD, Hartman LM, Molyneaux BJ.Early decompressive craniectomy for malignant cerebral infarction: meta-analysis and clinical decision algorithm. Neurol Clin Pract 2016; 6: 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Hou M, Lu G, et al. Decompressive craniectomy for severe middle cerebral artery infarction: a meta-analysis of randomised controlled trials. Lancet 2016; 388: S92. [Google Scholar]
- 22.Qureshi AI, Ishfaq MF, Rahman HA, et al. Hemicraniectomy versus conservative treatment in large hemispheric ischemic stroke patients: a meta-analysis of randomized controlled trials. J Stroke Cerebrovasc Dis 2016; 25: 2209–2214. [DOI] [PubMed] [Google Scholar]
- 23.Li YP, Hou MZ, Lu GY, et al. Neurologic functional outcomes of decompressive hemicraniectomy versus conventional treatment for malignant middle cerebral artery infarction: a systematic review and Meta-Analysis. World Neurosurg 2017; 99: 709–725. [DOI] [PubMed] [Google Scholar]
- 24.Rajwani KM, Crocker M, Moynihan B.Decompressive craniectomy for the treatment of malignant middle cerebral artery infarction. Br J Neurosurg 2017; 31: 401–409. [DOI] [PubMed] [Google Scholar]
- 25.Gul W, Fuller HR, Wright H, et al. A systematic review and meta-analysis of the effectiveness of surgical decompression in treating patients with malignant middle cerebral artery infarction. World Neurosurg 2018; 120: e902–e920. [DOI] [PubMed] [Google Scholar]
- 26.Das S, Mitchell P, Ross N, et al. Decompressive hemicraniectomy in the treatment of malignant middle cerebral artery infarction: a meta-analysis. World Neurosurg 2019; 123: 8–16. [DOI] [PubMed] [Google Scholar]
- 27.Rahme R, Zuccarello M, Kleindorfer D, et al. Decompressive hemicraniectomy for malignant middle cerebral artery territory infarction: is life worth living? J Neurosurg 2012; 117: 749–754. [DOI] [PubMed] [Google Scholar]
- 28.Hofmeijer J, van der Worp HB, Kappelle LJ, et al. Cognitive outcome of survivors of space-occupying hemispheric infarction. J Neurol 2013; 260: 1396–1403. [DOI] [PubMed] [Google Scholar]
- 29.Jüttler E, Bösel J, Amiri H, et al. DESTINY II: DEcompressive surgery for the treatment of malignant INfarction of the Middle cerebral arterY II. Int J Stroke 2011; 6: 79–86. [DOI] [PubMed] [Google Scholar]
- 30.Neugebauer H, Witsch J, Zweckberger K, et al. Space-occupying cerebellar infarction: complications, treatment, and outcome. Neurosurg Focus 2013; 34: E8. [DOI] [PubMed] [Google Scholar]
- 31.Kelly PJ, Stein J, Shafqat S, et al. Functional recovery after rehabilitation for cerebellar stroke. Stroke 2001; 32: 530–534. [DOI] [PubMed] [Google Scholar]
- 32.Mostofi K.Neurosurgical management of massive cerebellar infarct outcome in 53 patients. Surg Neurol Int 2013; 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwalla PK, Stapleton CJ, Ogilvy CS.Craniectomy in acute ischemic stroke. Neurosurgery 2014; 74: S151–S162. [DOI] [PubMed] [Google Scholar]
- 34.Steiner LA, Andrews PJ.Monitoring the injured brain: ICP and CBF. Br J Anaesth 2006; 97: 26–38. [DOI] [PubMed] [Google Scholar]
- 35.Schwab S, Aschoff A, Spranger M, et al. The value of intracranial pressure monitoring in acute hemispheric stroke. Neurology 1996; 47: 393–398. [DOI] [PubMed] [Google Scholar]
- 36.Slavin KV and, Misra M.Infratentorial intracranial pressure monitoring in neurosurgical intensive care unit. Neurol Res 2003; 25: 880–884. [DOI] [PubMed] [Google Scholar]
- 37.Poca MA, Benejam B, Sahuquillo J, et al. Monitoring intracranial pressure in patients with malignant middle cerebral artery infarction: is it useful? J Neurosurg 2010; 112: 648–657. [DOI] [PubMed] [Google Scholar]
- 38.Paldor I, Rosenthal G, Cohen JE, et al. Intracranial pressure monitoring following decompressive hemicraniectomy for malignant cerebral infarction. J Clin Neurosci 2015; 22: 79–82. [DOI] [PubMed] [Google Scholar]
- 39.Jeon SB, Park JC, Kwon SU, et al. Intracranial pressure soon after hemicraniectomy in malignant middle cerebral artery infarction. J Intensive Care Med 2018; 33: 310–316. [DOI] [PubMed] [Google Scholar]
- 40.Schwab S, Spranger M, Schwarz S, et al. Barbiturate coma in severe hemispheric stroke: useful or obsolete? Neurology 1997; 48: 1608–1613. [DOI] [PubMed] [Google Scholar]
- 41.Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017; 80: 6–15. [DOI] [PubMed] [Google Scholar]
- 42.Roberts I, Sydenham E.Barbiturates for acute traumatic brain injury. Cochrane Database Syst Rev 2012; CD000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts DJ, Hall RI, Kramer AH, et al. Sedation for critically ill adults with severe traumatic brain injury: a systematic review of randomized controlled trials. Crit Care Med 2011; 39: 2743–2751. [DOI] [PubMed] [Google Scholar]
- 44.Schell RM, Applegate RL, Cole DJ.Salt, starch, and water on the brain. J Neurosurg Anesthesiol 1996; 8: 178–182. [DOI] [PubMed] [Google Scholar]
- 45.Burke AM, Quest DO, Chien S, et al. The effects of mannitol on blood viscosity. J Neurosurg 1981; 55: 550–553. [DOI] [PubMed] [Google Scholar]
- 46.Jafar JJ, Johns LM, Mullan SF.The effect of mannitol on cerebral blood flow. J Neurosurg 1986; 64: 754–759. [DOI] [PubMed] [Google Scholar]
- 47.Rosner MJ, Coley I.Cerebral perfusion pressure: a hemodynamic mechanism of mannitol and the postmannitol hemogram. Neurosurgery 1987; 21: 147–156. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki J, Imaizumi S, Kayama T, et al. Chemiluminescence in hypoxic brain – the second report: cerebral protective effect of mannitol, vitamin E and glucocorticoid. Stroke 1985; 16: 695–700. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz S, Schwab S, Bertram M, et al. Effects of hypertonic saline hydroxyethyl starch solution and mannitol in patients with increased intracranial pressure after stroke. Stroke 1998; 29: 1550–1555. [DOI] [PubMed] [Google Scholar]
- 50.Candelise L, Colombo A, Spinnler H.Therapy against brain swelling in stroke patients. A retrospective clinical study on 227 patients. Stroke 1975; 6: 353–356. [DOI] [PubMed] [Google Scholar]
- 51.Santambrogio S, Martinotti R, Sardella F, et al. Is there a real treatment for stroke? Clinical and statistical comparison of different treatments in 300 patients. Stroke 1978; 9: 130–132. [DOI] [PubMed] [Google Scholar]
- 52.Paczynski RP, Venkatesan R, Diringer MN, et al. Effects of fluid management on edema volume and midline shift in a rat model of ischemic stroke. Stroke 2000; 31: 1702–1708. [DOI] [PubMed] [Google Scholar]
- 53.Paczynski RP, He YY, Diringer MN, et al. Multiple-dose mannitol reduces brain water content in a rat model of cortical infarction. Stroke 1997; 28: 1437–1443. [DOI] [PubMed] [Google Scholar]
- 54.Videen TO, Zazulia AR, Manno EM, et al. Mannitol bolus preferentially shrinks non-infarcted brain in patients with ischemic stroke. Neurology 2001; 57: 2120–2122. [DOI] [PubMed] [Google Scholar]
- 55.Manno EM, Adams RE, Derdeyn CP, et al. The effects of mannitol on cerebral edema after large hemispheric cerebral infarct. Neurology 1999; 52: 583–587. [DOI] [PubMed] [Google Scholar]
- 56.Kaufmann AM, Cardoso ER.Aggravation of vasogenic cerebral edema by multiple-dose mannitol. J Neurosurg 1992; 77: 584–589. [DOI] [PubMed] [Google Scholar]
- 57.Sandercock PA, Soane T.Corticosteroids for acute ischaemic stroke. Cochrane Database Syst Rev 2011; CD000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forbes KP, Pipe JG, Heiserman JE.Evidence for cytotoxic edema in the pathogenesis of cerebral venous infarction. AJNR Am J Neuroradiol 2001; 22: 450–455. [PMC free article] [PubMed] [Google Scholar]
- 59.Alderson P, Roberts I.Corticosteroids for acute traumatic brain injury. Cochrane Database Syst Rev 2005; CD000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 2005; 365: 1957–1959. [DOI] [PubMed] [Google Scholar]
- 61.Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg 1991; 75: 731. [DOI] [PubMed] [Google Scholar]
- 62.Yundt KD, Diringer MN.The use of hyperventilation and its impact on cerebral ischemia in the treatment of traumatic brain injury. Crit Care Clin 1997; 13: 163. [DOI] [PubMed] [Google Scholar]
- 63.Simard D, Paulson OB.Artificial hyperventilation in stroke. Trans Am Neurol Assoc 1973; 98: 309–310 [PubMed] [Google Scholar]
- 64.Christensen MS, Paulson OB, Olesen J, et al. Cerebral apoplexy (stroke) treated with or without prolonged artificial hyperventilation. Cerebral cirdulation, clinical course, and cause of death. Stroke 1973; 4: 568–631 [DOI] [PubMed] [Google Scholar]
- 65.Stocchetti N, Maas AI, Chieregato A, et al. Hyperventilation in head injury: a review. Chest 2005; 127: 1812. [DOI] [PubMed] [Google Scholar]
- 66.Torbey MT, Bösel J, Rhoney DH, et al. Evidence-based guidelines for the management of large hemispheric infarction. A statement for health care professionals from the neurocritical care society and the German Society for the neuro-intensive care and emergency medicine. Neurocrit Care 2015; 22: 146–164. [DOI] [PubMed] [Google Scholar]
- 67.Schwab S, Schwarz S, Spranger M, et al. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke 1998; 29: 2461–2466. [DOI] [PubMed] [Google Scholar]
- 68.Schwab S, Georgiadis D, Berrouschot J, et al. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke 2001; 32: 2033–2035. [DOI] [PubMed] [Google Scholar]
- 69.Thomas Els, Oehm E, Voigt S, et al. Safety and therapeutical benefit of hemicraniectomy combined with mild hypothermia in comparison with hemicraniectomy alone in patients with malignant ischemic stroke. Cerebrovasc Dis 2006; 21: 79–85. [DOI] [PubMed] [Google Scholar]
- 70.Su Y, Fan L, Zhang Y, et al. Improved neurological outcome with mild hypothermia in surviving patients with massive cerebral hemispheric infarction. Stroke 2016; 47: 457–463. [DOI] [PubMed] [Google Scholar]
- 71.Su Y.Decompressive craniectomy combined with hypothermia may improve neurological outcomes in patients with large hemispheric infarction. Cerebrovasc Dis 2018; 45: 291. [Google Scholar]
- 72.Neugebauer H, Schneider H, Bösel J, et al. Outcomes of hypothermia in addition to decompressive hemicraniectomy in treatment of malignant middle cerebral artery stroke: a randomized clinical trial. JAMA Neurol 2019; 76: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sheth KN, Elm JJ, Molyneaux BJ, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemisferic infarction: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2016; 15: 1160–1169. [DOI] [PubMed] [Google Scholar]
- 74.van Middelaar T, Nederkoorn PJ, van der Worp HB, et al. Quality of life after surgical decompression for space-occupying middle cerebral artery infarction: systematic review. Int J Stroke 2015; 10: 170–176 [DOI] [PubMed] [Google Scholar]
- 75.Geurts M, van der Worp HB, Kappelle LJ, et al. Surgical decompression for space-occupying cerebral infarction: outcomes at 3 years in the randomized HAMLET trial. Stroke 2013; 44: 2506–2508. [DOI] [PubMed] [Google Scholar]
- 76.Hofmeijer J, Algra A, Kappelle LJ, et al. Predictors of life-threatening brain edema in middle cerebral artery infarction. Cerebrovasc Dis 2008; 25: 176–184. [DOI] [PubMed] [Google Scholar]
- 77.Wu S, Yuan R, Wang Y, et al. Early prediction of malignant brain edema after ischemic stroke. Stroke 2018; 49: 2918–2927. [DOI] [PubMed] [Google Scholar]
- 78.Thomalla G, Hartmann F, Juettler E, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: a prospective multicenter observational study. Ann Neurol 2010; 68: 435–445. [DOI] [PubMed] [Google Scholar]
- 79.Berge E, Al-Shahi Salman R, van der Worp HB, et al. Increasing value and reducing waste in stroke research. Lancet Neurol 2017; 16: 399–408. [DOI] [PubMed] [Google Scholar]
- 80.Al-Shahi Salman R, Beller E, Kagan J, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014; 383: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Jonge JC, Reinink H, Colam B, et al. Regulatory delays in a multinational clinical stroke trial. Eur Stroke J, in press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-eso-10.1177_23969873211014112 for European Stroke Organisation (ESO) guidelines on the management of space-occupying brain infarction by H Bart van der Worp, Jeannette Hofmeijer, Eric Jüttler, Avtar Lal, Patrik Michel, Paola Santalucia, Silvia Schönenberger, Thorsten Steiner and Götz Thomalla in European Stroke Journal